Abstract
The aim of this study was to evaluate the nutritional content and antioxidant capacity of the tubers, leaves and, flowers of the species Tropaeolum pentaphyllum Lam. The three parts of the plant were analyzed by physicochemical methods, atomic absorption spectrometry, spectrophotometric and chromatographic techniques. The tubers, leaves, and flowers exhibited significant differences in all parameters evaluated. The leaves showed significantly higher values of protein (16.28 ± 0.02 g/100 g), total dietary fiber (27.78 ± 0.15 g/100 g) and quercetin (3798.61 ± 37.57 µg/g) when compared to the tubers and flowers. The study revealed a potential content of the protein, dietary fiber, and flavonoids the species Tropaeolum pentaphyllum, when compared with the sweet potatoes leaves (Ipomoea batatas L.). In addition, the antioxidant activities of leaves and flowers were also higher measured by ABTS (2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid), DPPH (2,2-diphenyl-1-picrylhydrazyl), and TRAP (total radical-trapping antioxidant potential) methods. Tropaeolum pentaphyllum have high nutritional potential that can be exploited to improve nutritional value of various food products.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2817-z) contains supplementary material, which is available to authorized users.
Keywords: Tropaeolum pentaphyllum Lam., Dietary constituents, Protein, Dietary fibers, Flavonoids, Antioxidant capacity
Introduction
Tropaeolum pentaphyllum Lam., known as crem, is a native plant of southern Brazil that belongs to the family Tropaeolaceae. The main and traditional use of its tubers is a condiment, usually consumed as a side dish with soups and meats. The aerial portions (leaves and flowers) of the plant are known for their ornamental properties, besides being edible and used in salads, in which these parts can add a peppery flavor similar to the flavor of the tuber. On the other hand, leaves and flowers are being sold by agroecological producers for their inclusion into the traditional diet. At the same time, this species is in the List of Endangered Species of Rio Grande do Sul state in the vulnerable category due to the risk of habitat destruction, which warns for the beginning of the cultivation of this species in order to not be extinguished. Some of these edible plants are underutilized because their features are unknown, thus neglecting their role in improving the food security and nutrition of the population. In order to change this scenario, studying the nutritional composition of this species is fundamental.
Leaves and flowers of Tropaeolum majus (Nasturtium) have been reported to have bioactive properties. However, there is no data in the literature regarding T. pentaphyllum, hence this is the first nutritional characterization of this species.
Many epidemiological studies indicate an inverse association between the consumption of appropriate amounts of vegetables and the risk of developing chronic disorders such as cardiovascular diseases, some forms of cancer, and neurodegenerative disorders, among others (Block et al. 1992; Donaldson 2004).
The protective effect of diets rich in vegetables has been frequently associated with their contents of vitamins, minerals, fibers, and other compounds commonly referred to collectively as bioactive compounds, which are directly associated with antioxidant capacity. The contribution of Tropaeolaceae vegetables to health may be related to their antioxidant capacity due to the high contents of bioactive compounds such as carotenoids and flavonoids. Carotenoids, tocopherols, flavonoids, vitamin C, and certain minerals, among others, may protect human cell systems from oxidative damage through a variety of complementary and synergic mechanisms (Zhang et al. 2014).
The antioxidant capacity of vegetables is directly related to the presence of bioactive compounds, and the estimation of bioactive compounds in order to investigate antioxidant activity is of supreme importance. Therefore, the aim of this study was to analyze the nutritional composition and antioxidant capacity of the tubers, leaves, and flowers of T. pentaphyllum species native of southern Brazil. This study highlights the potential of this species as an important nutritional source; in addition, it may support the inclusion of this species on the daily diet of the population, improving the nutritional status of the population and valuing local biodiversity.
Materials and methods
Plant materials
The three parts (tubers, leaves, and flowers) of the plant Tropaeolum pentaphyllum Lam. were obtained in the region of Lami, Porto Alegre, Rio Grande do Sul, Brazil (latitude 30° 13′ 19″ S and longitude 51° 5′ 5″ W). The vouchers were deposited in the herbarium of the Institute of Natural Sciences (Instituto de Ciências Naturais—ICN) (UFRGS, Porto Alegre, Rio Grande do Sul, Brazil) under number 157404.
Physicochemical analysis
All analyses were performed according to AOAC International (1997). Protein content was determined by the Kjeldahl method using a conversion factor of 5.75. The content of lipids was determined by the Soxhlet extraction method, and the dietary fiber concentration was determined using an enzymatic gravimetric method. The ash was determined in a muffle furnace at 550 °C and the moisture content by the gravimetric method. Total carbohydrate content was determined by the concentration difference. Total soluble solids were determined by the refractometer °Brix reading (Atago Co., Taiwan, China). pH was measured using a pH meter (Quimis, model Q-400A). Titratable acidity was performed with 0.1N NaOH, expressing the results in g of malic acid/100 g. Total sugars and reducing sugars were determined by the Eynon–Lane method.
Minerals analysis
Instrumentation
The mineral content analysis was carried in an instrument of high-resolution atomic absorption spectrometer of continuum source equipped with a graphite furnace (HR-CS GF AAS) and a flame atomizer (HR-CS FAAS). Cadmium and chromium were analyzed in the graphite furnace atomizer compartment at 228.802 and 357.869 nm, respectively (Duarte et al. 2013). An air-acetylene flame was used for the determination of Cu (324.754 nm), Fe (248.327 nm), K (404.720 nm), Mg (202.582 nm), Na (589.592 nm), Rb (780.027 nm), and Zn (231.857 nm) content, while a nitrous oxide-acetylene flame was used to determine Ca (239.856 nm) content. The instrumental parameters were optimized, and the method was adapted according to Boschetti et al. (2013). Additionally, both samples underwent microwave digestion.
Sample preparation for mineral composition analysis
The freeze-dried tuber samples underwent two different pre-treatment procedures for mineral analysis. For the direct sampling analysis, used to determine trace elements the samples were ground in an A-11 Basic micro-mill (IKA-Werke, Germany) and after that sieved through a 200 µm polyester sieve and kept in sealed plastic vials until further processing.
For the macro and micro element analysis, the samples were digested by a MARS-6 microwave oven (CEM, Matthews, EUA). The digestion procedure included the addition of purified nitric acid followed by the microwave program: step 1–20 min ramp with 900 W of power; step 2–10 min hold time with 900 W of power; and step 3–15 min cooling time with 0 W of power.
Bioactive compound analysis
Equipment
The spectrophotometer used for bioactive compound analysis and antioxidant capacity analysis was a Shimadzu UV-1800 (Tokyo, Japan). The HPLC system used was an Agilent 1100 Series (Santa Clara, CA, USA) equipped with an online degasser, a quaternary pump, an automatic injector, and the spectra analyzed (UV–Visible Agilent 8453) were carotenoids and tocopherol. The carotenoids were separated in a C30 polymeric reverse-phase column (YMC, model CT99SO3-2546WT, Tokyo, Japan) (250 mm × 4.6 mm i.d., 3 µm) set to 33 °C. The tocopherols were separated in a 218TP54 Vydac C18 polymeric column (Columbia, Maryland, USA) (250 mm × 4.6 mm i.d., 5 μm.) set to 30 °C. Flavonoids and vitamin C analyses were performed with a Shimadzu CBM 20A HPLC unit (Shimadzu Corporation, Tokyo, Japan) equipped with an online degasser, a ternary pump, an automatic injector (SIL-20AHT), and a photodiode array detector (SPD-M20A) and coupled to a C18 polymeric column (Vydac, model 218TP54) (250 mm × 4.6 mm i.d., 5 μm.) set to 28 °C. The TRAP profile was obtained by measuring chemiluminescence (CL) emissions in a 1450 MicroBeta TriLux liquid scintillation counter (PerkinElmer Wallac, Turku, Finland).
Chlorophyll content
The freeze-dried samples of leaves and flowers (1 g) were homogenized with acetone:water (80:20) and centrifuged at 5000×g for 15 min. The supernatant was collected, and absorbance was measured followed by the absorbance of chlorophyll “a,” which was 663 nm, and that of chlorophyll “b”, 647 nm (Lichtenthaler 1987).
Analysis of phenolic compounds
Folin–Ciocalteu method was used for determination of phenolics (Swain and Hillis 1959) using gallic acid as standard (30–300 mg/L), and the results were expressed as gallic acid equivalent (GAE)/g dry sample.
Carotenoid profile
The analysis of carotenoids was performed according to the procedure described by Mercadante and Rodriguez-Amaya (1998). The mobile phase gradient elution began with water/methanol/MTBE at 5:90:5 and reached 0:95:5 after 12 min, 0:89:11 after 25 min, 0:75:25 after 40 min, and 00:50:50 after 60 min with a flow rate of 1 mL/min and injection volume of 5 μL. The spectra were collected between 250 and 600 nm, and the chromatograms were processed at a fixed wavelength of 450 nm for the carotenoids.
For quantification, a standard curve was constructed for carotenoids in the following concentration ranges for the carotenoids: β-carotene 3–700 µg/mL; α-carotene 0.05–300 µg/mL; lutein 0.1–850 µg/mL; β-cryptoxanthin 2–100 µg/mL; and zeaxanthin 1–40 µg/mL. The limits of detection (LOD) and quantification (LOQ) were as follows: β-carotene 2.53 × 10−2 and 5.42 × 10−2 µg/g; lutein 6.9 × 10−2 and 2.15 × 10−2 µg/g; β-cryptoxanthin 2.11 × 10−2 and 4.51 × 10−2 µg/g; zeaxanthin 9.56 × 10−2 and 1.59 µg/g; α-carotene 1.97 × 10−2 and 6.28 × 10−2 µg/g.
Tocopherol analysis
Tocopherol determination was based on the methodology proposed by Freitas et al. (2008) with some modifications. Freeze-dried samples were macerated with absolute methanol until pigments were completely removed, concentrated under reduced pressure in a rotary evaporator, and dried under nitrogen flow. For quantification by HPLC, a standard curve was constructed in the following concentration ranges: 0.05–50 µg/mL and 0.01–30 µg/mL for α-tocopherol and γ-tocopherol, respectively. The LOD and LOQ were as follows: α-tocopherol 10.53 × 10−2–1.37 × 102 µg/g and γ-tocopherol 1.23 × 10−2–2.65 × 10−2 µg/g.
Flavonoid analysis
The extract and mobile phase of the flavonoids were determined according to the methodology by Huber et al. (2009). The quantification analysis performed by HPLC used a standard curve in the following concentration ranges: luteolin 10–1200 µg/mL; quercetin 10–4000 µg/mL; kaempferol 1–80 µg/mL; and myricetin 1–50 µg/mL. The LOD and LOQ were as follows: luteolin 5.8 × 10−2–1.77 µg/g; quercetin 5.5 × 10−2–1.67 × 10−2 µg/g; kaempferol 8.3 × 10−3–2.7 × 10−3 µg/g; and myricetin 6.9 × 10−3–2.3 × 10−3 µg/g.
Vitamin C analysis
The determination of vitamin C was based on the methodology proposed by Rosa et al. (2007) with some modifications. The extraction was performed with freeze-dried samples and each 5 g was ground in an Ultra-Turrax (T25, IKA, China) with 20 mL of 0.05 M suprapure 96% sulfuric acid (Merck, Darmstandt, Germany) for 1 min, centrifuged at 22,000 g for 20 min in a refrigerated centrifuge at 4 °C and then filtered (Millex LCR 0.45 lm, 13 mm) for injection in the chromatography system. The analyses were performed by HPLC and the vitamin C was eluted isocratically using 0.05 M supra pure sulfuric acid as the mobile phase with a flow rate of 1 mL/min and an injection volume of 10 µL. For quantification, a standard curve was constructed for vitamin C in the 10–1000 µg/mL concentration range. The LOD and LOQ were 8.70 × 10−2 and 2.64 × 10−2 µg/g, respectively.
Antioxidant capacity analysis
ABTS+ (Radical cation scavenging) and DPPH (Radical scavenging assay)
Methodologies based on sequestering the 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic (ABTS) radical (Kuskoski et al. 2005) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical (Brand-Wiliams et al. 1995) adapted by Pereira et al. (2013) were used to determine the antioxidant capacity. The results of ABTS analysis were expressed as µM Trolox equivalent/g dry sample and DPPH were expressed as the concentration of antioxidant required to reduce the original amount of free radicals by 50% (EC50) and the values expressed as g dry sample/g DPPH.
TRAP (Total radical-trapping antioxidant potential) and total antioxidant reactivity (TAR)
TRAP and TAR were calculated and measured as previously described by Beretta et al. (2006) and Dresch et al. (2009), respectively. This assay is based on peroxyl radical quenching ability by sampled antioxidant compounds. In brief, the reaction mixture, containing AAPH (10 mM) and luminol (4 mM) in glycine buffer (0.1M, pH 8.6), was incubated at 21 °C for 2 h (the time required to stabilize the reaction). AAPH [2,2′-azobis(2-amidinopropane) dihydrochloride] is a source of peroxyl radicals that reacts with luminol, yielding chemiluminescence (CL). After 2 h incubation, 180 µL of the radical generator system were mixed in a 96-well plate with 20 µL of sample (18.75 µg/mL final concentration), Trolox (1.5 µM final concentration), or vehicle (water), which was used to represent the maximum radical generation. The TRAP profile was obtained by measuring CL emissions in a liquid scintillation counter, and the CL intensity was monitored for 60 min after the addition of the extracts or Trolox. The results were transformed into a percentile rank to calculate the area under the curve (AUC), which is inversely proportional to TRAP. The TAR index was calculated using I0/I, where (I0) is the initial CL emission of the vehicle and (I) is the initial CL emission of the sample or the reference antioxidant compound (Trolox).
Determination of bioactive compounds that contribute to antioxidant activity
The antioxidant extracts used in the assays (describe above) were injected under the same chromatographic conditions as all compounds analyzed by HPLC in this study, aiming to determine which bioactive compounds provides higher antioxidant capacity for the two different extracts.
Statistical analysis
The results were evaluated by one-way analysis of variance (ANOVA), and the mean values were analyzed by Tukey’s test using the software STATISTICA® 12.0 (StatSoft Inc, Tolson, USA).
Results and discussion
Proximate composition and physicochemical properties
The composition of tubers, leaves and flowers of T. pentaphyllum is presented in Table 1. Tubers, leaves and flower samples before frreze drying showed moisture content of 69.5 ± 0.42; 81.0 ± 0.56; and 85.0 ± 0.32%, respectively. The leaves showed higher contents of ash, protein, and fibers (p < 0.05) as compared to flowers and tubers. However, the flowers showed higher contents of lipids and carbohydrates (p < 0.05) as compared to leaves and tubers. The ash analysis provides information concerning the nutritional value of food, primarily its mineral content.
Table 1.
Proximate composition and physicochemical analysis results of tuber, leaf and flower Tropaeolum pentaphyllum Lam freeze-dried (g/100 g dw)
| Parameters | T. pentaphyllum | T. pentaphyllum | T. pentaphyllum |
|---|---|---|---|
| Tuber | Leaf | Flower | |
| Moisture | 5.74 ± 0.08c | 7.87 ± 0.23b | 8.13 ± 0.39a |
| Dry matter | 94.26 ± 0.42a | 92.13 ± 0.32b | 91.87 ± 0.32c |
| Ash | 3.53 ± 0.22b | 14.55 ± 0.86a | 1.67 ± 0.01c |
| Protein | 6.56 ± 0.02b | 16.28 ± 0.02a | 3.63 ± 0.26c |
| Lipid | 0.17 ± 0.00c | 0.90 ± 0.02b | 1.39 ± 0.02a |
| Carbohydrate | 84.00 | 61.46 | 85.44 |
| Total dietary fibre | 8.94 ± 0.10b | 27.78 ± 0.15a | 5.22 ± 0.76c |
| Insoluble dietary fibre | 8.37 ± 0.03b | 25.76 ± 0.60a | 3.34 ± 0.10c |
| Soluble dietary fibre | 0.57 ± 0.05b | 2.03 ± 0.01a | 1.88 ± 0.06a |
| pH | 5.12 ± 0.03c | 5.76 ± 0.01b | 6.11 ± 0.01a |
| Acidity | 1.28 ± 0.27b | 1.80 ± 0.63a | 0.60 ± 0.00c |
| Soluble solids (°Brix) | 5.60 ± 0.38a | 0.88 ± 0.00b | 0.14 ± 0.05c |
Mean ± SD (standard deviation) of three replicates (n = 3)
Different letters on the same line represent significant difference p < 0.05 by Tukey’s test
Comparing the parameters found in the literature, the results were consistent with Sun et al. (2014), who evaluated the nutritional composition of leaves from 40 sweet potatoes (Ipomoea batatas L.). The crude protein, crude fiber, crude fat, carbohydrate and ash contents ranged between 16.69 and 31.08, 9.15 and 14.26, 2.08 and 5.28, 42.03 and 61.36, and 7.39 and 14.66 g/100 g, respectively. Concerning the nutritional compositions, the values found for T. pentaphyllum leaves are significantly comparable with sweet potatoes leaves. Dincer et al. (2011) analyzed the protein, crude fiber and ash contents of tuber from 3 sweet potatoes (Ipomoea batatas L.) that ranged between 3.67 and 5.08, 2.11 and 2.76 and 2.13 and 2.62 g/100 g, respectively. These results demonstrated that the species T. pentaphyllum was important source of nutritional compounds available in the native Brazilian. Significant differences were observed between the mean concentrations of all the physicochemical parameters analyzed in T. pentapyllum tubers, leaves and flowers.
Galdón et al. (2012) found pH of 5.7 ± 0.2 for the potato species Solanum tuberosum ssp. tuberosum, which was similar to T. pentaphyllum tubers. Higher acidity was detected of T. pentaphyllum tubers compared with S. tuberosum ssp. tuberosum, which presented acidity 0.28 ± 0.02 g malic acid/100 g.
Jung et al. (2013) analyzed the acidity and °Brix values of Hibiscus Sabdariffa L. flowers and found higher values 2.62 ± 0.01 g malic acid/100 g and 1.80 ± 0.01 °Brix, respectively compared to the flowers of T. pentaphyllum.
The pH of T. pentaphyllum leaves was similar to those presented by conventional rocket Eruca sativa L., with pH of 5.68. Higher acidity and lower °Brix were observed compared with the acidity of 0.37 g malic acid/100 g and °Brix of 4.92 of E. sativa L. (Vasconcelos et al. 2011).
Minerals
The mineral content present in food can be considered beneficial or toxic to human health, depending on its concentration. This study (Table 2) showed that the T. pentaphyllum tuber samples was lower in Fe and Cu, with concentrations under the LOQ: 46 µg/g and 5.2 µg/g, respectively. Based on the results obtained for K, Mg, and Ca—the major elements present in the sample—and for Zn, Na, and Rb, it can be stated that this root may be used as a nutritional complement in the human diet. The potentially toxic elements Cd and Cr were found at low concentrations in the tuber samples.
Table 2.
Mineral contents of tuber of the Tropaeolum pentaphyllum Lam freeze-dried
| Element | T. pentaphyllum |
|---|---|
| Tuber | |
| *Cd (ng/g) | 24 ± 2 |
| *Cr (ng/g) | 554 ± 27 |
| **Zn (µg/g) | 23 ± 1 |
| **Na (µg/g) | 74 ± 3 |
| **Rb (µg/g) | 8.8 ± 0.5 |
| **Cu (µg/g) | Nd |
| **Fe (µg/g) | Nd |
| **K (%) | 1.14 ± 0.05 |
| **Mg (%) | 0.092 ± 0.006 |
| ***Ca (%) | 0.158 ± 0.009 |
Mean ± SD (standard deviation) of five replicates (n = 5) by HR-CS GF AAS and mean ± SD of three replicates (n = 3) by HR-CS FAAS
Nd not detected (FeLOQ = 46 µg/g; CuLOQ = 5.2 µg/g)
* Analyzed by HR-CS GF AAS, ** Analyzed by HR-CS FAAS with air-acetylene flame, *** Analyzed by HR-CS FAAS with nitrous oxide-acetylene flame
Chlorophyll and carotenoids
The chlorophyll and carotenoid contents of T. pentaphyllum tubers, leaves, and flowers are shown in Table 3.
Table 3.
Analysis of bioactive compounds of tuber, leaf and flower Tropaeolum pentaphyllum Lam freeze-dried
| Parameters | T. pentaphyllum | T. pentaphyllum | T. pentaphyllum |
|---|---|---|---|
| Tuber | Leaf | Flower | |
| Chlorophyll “a” (mg/g dw) | Na | 4.25 ± 0.44a | 0.51 ± 0.02b |
| Chlorophyll “b” (mg/g dw) | Na | 1.62 ± 0.20a | 0.44 ± 0.01b |
| Total Chlorophyll (mg/g dw) | Na | 5.87 ± 0.61a | 0.95 ± 0.02b |
| Lutein (µg/g dw) | 0.22 ± 0.02c | 642.50 ± 4.56a | 243.23 ± 0.89b |
| Zeaxanthin (µg/g dw) | Nq | 23.20 ± 0.82a | 14.23 ± 0.04b |
| β-cryptoxanthin (µg/g dw) | Nq | 21.71 ± 0.09a | 2.58 ± 0.03b |
| α-carotene (µg/g dw) | 0.063 ± 0.00c | 8.14 ± 0.02a | 3.61 ± 0.02b |
| β-carotene (µg/g dw) | 4.20 ± 0.02c | 467.74 ± 2.39a | 132.06 ± 0.76b |
| Total carotenoids (µg/g dw) | 4.48 | 1163.29 | 395.71 |
| α-tocopherol (µg/g dw) | Nd | 0.09 ± 0.05b | 0.28 ± 0.07a |
| γ-tocopherol (µg/g dw) | Nd | 0.03 ± 0.02b | 0.10 ± 0.04a |
| Vitamin C (µg/g dw) | 26 ± 0.01c | 160 ± 0.30a | 82 ± 0.10b |
| Phenolic compounds | |||
| (GAE/g dry sample) | 2.79 ± 0.02c | 6.83 ± 0.09b | 21.30 ± 0.01a |
| Luteolin (µg/g dw) | Nd | 1124.06 ± 9.75a | 408.95 ± 0.14b |
| Myricetin (µg/g dw) | Nd | 41.87 ± 0.96a | 5.43 ± 0.01b |
| Quercetin (µg/g dw) | 30 ± 0.10c | 3798.61 ± 37.57a | 840.39 ± 10.99b |
| Kaempferol (µg/g dw) | Nd | Nd | 20 ± 0.09b |
Mean ± SD (standard deviation) of three replicates (n = 3)
(ZeaxanthinLOQ = 1.59 µg/g; β-CryptoxanthinLOQ = 0.0451 µg/g; α-tocopherolLOQ = 0.0137 µg/g; γ-tocopherolLOQ = 0.0265 µg/g; LuteolinLOQ = 1.77 µg/g; MyricetinLOQ = 0.0023 µg/g; KaempferolLOQ = 0.0027 µg/g)
Na not applied, Nd not detected
Different letters on the same line represent significant difference p < 0.05 by Tukey’s test
Hue et al. (2011) analyzed the chlorophyll “a” values for sweet potato leaves (Ipomoea batatas) of six varieties on the first to 18th days of the development stage and found values between 0.55 ± 0.01 mg/g and 1.61 ± 0.03 mg/g. The chlorophyll “b” values ranged between 0.246 ± 0.01 mg/g and 0.780 ± 0.03 mg/g, with both values lower than those found in T. pentaphyllum leaves.
Flowers of T. pentaphyllum showed lower chlorophyll a value as compared to that of leaves and buds of Camellia sinennis L. cv. Anji Baicha originated from Anji, with content of 0.69 ± 0.01 mg/g. However, chlorophyll “b,” content of T. pentaphyllum flowers was higher compared with C. sinensis of the same cultivar and location, which was 0.05 ± 0.00 mg/g (Wei et al. 2012).
Five carotenoids were identified (lutein, zeaxanthin, β-cryptoxanthin, β-carotene, and α-carotene); however, the zeaxanthin and β-cryptoxanthin quantification levels found were below the <1.0 µg/g for T. pentaphyllum tuber samples (Fig. S1).
The tree parts of T. pentaphyllum were significantly different (p < 0.05), with lutein and β-carotene identified as the major compounds in all samples (except lutein for the tuber sample), although their concentrations in leaves were significantly higher compared with the flowers. Isabelle et al. (2010) evaluated the carotenoid contents of sweet potato (I. batatas L.) and lutein content. T. pentaphyllum tuber, 0.22 µg/g of lutein. Sweet potato leaves showed lutein content of 61.84 which was almost 11 times lower than that found in T. pentaphyllum leaves. The β-carotene contents of T. pentaphyllum tuber and leaf samples were also higher as compared to 0.30 µg/g for the tuber of sweet potato and 48.97 µg/g for sweet potato leaves. Rodriguez-Amaya et al. (2008) evaluated the lutein and β-carotene contents in leaves of T. majus and found 69 µg/g and 136 µg/g, respectively, which were also lower in comparison with the T. pentaphyllum leaf values. On the other hand, Rodriguez-Amaya et al. (2008) evaluated lutein in yellow and orange flowers of T. majus and found 450 µg/g and 350 µg/g of lutein, respectively, which were lower as compared to T. pentaphyllum flowers.
Concerning α-carotene and zeaxanthin, the values found for the leaves and flowers were significantly higher when compared with those found in studies on common vegetables. In the study of Isabelle et al. (2010), the amount of zeaxanthin in T. pentaphyllum flowers was lower as compared with the Chinese kale sample, 15.16 µg/g and T. pentaphyllum leaves when compared with seaweed 33.67 µg/g. T. pentaphyllum leaves and flowers, the α-carotene content as compared to carrot (14.49 µg/g). Zeaxanthin and α-carotene contents in the T. pentaphyllum leaf samples were significantly higher than sweet potato leaves, which presented content of 3.33 µg/g and 0.30 µg/g, respectively.
The β-cryptoxanthin content of T. pentaphyllum flowers was higher when compared with acerola 2.1 µg/g, while the leaves showed higher values as compared with candied papaya 20 µg/g (Rodriguez-Amaya et al. 2008).
Tocopherols and vitamin C
Table 3 shows tocopherol and vitamin C contents of T. pentaphyllum. Tocopherols (α-tocopherol and γ-tocopherol) were identified in T. pentaphyllum leaf and flower samples, but were absent inn tuber samples (Fig. S2).
The values found for α-tocopherol and γ-tocopherol were significantly different for leaves and flowers. The α-tocopherol contents in the leaf and flower samples were higher when compared with the γ-tocopherol contents. Sanchéz-Machado et al. (2006) analyzed the flowers of Moringa oleifera and found higher α-tocopherol and γ-tocopherol contents, (305.7 ± 23.9 µg/g and 9.1 ± 0.90 µg/g, respectively) as compared to T. pentaphyllum flower. In addition, the α-tocopherol and γ-tocopherol contents of T. pentaphyllum leaves were significantly lower in comparison to sweet potato leaves, which presented contents of 7.23 µg/g and 0.56 µg/g, respectively (Isabelle et al. 2010).
Vitamin C was identified in freeze-dried T. pentaphyllum tuber and leaf samples, whereas for T. pentaphyllum flowers the separation could only be performed using a fresh sample (Fig. S3).
Isabelle et al. (2010) found vitamin C contents of 1.0 µg/g in both sweet potato tubers leaves, which were significantly lower than the results found for T. pentaphyllum tuber and leaf samples. According to Garzón and Wrolstad (2009), nasturtium (Tropaeolum majus) flowers contain 715 µg/g of vitamin C, which was higher than the content of T. pentaphyllum flowers.
Phenolic compounds and flavonoids
Table 3 shows values obtained for phenolic compounds and flavonoids of T. pentaphyllum.
Significant differences (p < 0.05) were observed among the mean contents in T. pentaphyllum tubers, leaves, and flowers. Earlier study of Isabelle et al. (2010) investigated the phenolic compounds of sweet potato tubers and leaves. The values found were significantly lower, (0.59 mg GAE/g and 3.97 mg GAE/g, respectively) compared with T. pentaphyllum tubers and leaves. For T. pentaphyllum flowers, the values found were also significantly higher when compared with phenolic compound contents in the flowers of H. Sabdariffa, which presented 7.27 ± 0.20 mg GAE/g (Jung et al. 2013).
Four flavonoids (luteolin, myricetin, quercetin, and kaempferol) were identified in T. pentaphyllum flowers, three except kaempferol flavonoids in leaves and only quercitin was identified in tubers.
Using a previously optimized and validated method proposed by Huber et al. (2009), the findings presented above indicate that the optimum conditions vary with the different parts of the T. pentaphyllum plant (tuber, leaf, and flower). Before initiating flavonoid analyses, the extraction/hydrolysis conditions were optimized for each part of the plant compared with conventional vegetables. The extraction/hydrolysis procedures were repeated six times (n = 6) for tuber samples, four times (n = 4) for leaf samples, and two times (n = 2) for flower samples. The following items were verified: sample weight; lyophilized or fresh sample; HCl of 1,0 M and 1.2 M; methanol concentration (50 or 62.5%); reflux time (90 °C); the use of ascorbic acid or 3,5-Di-tert-4-butylhydroxytoluene (BHT) as antioxidant, the volume of methanol used, and samples unfiltered and filtered with PTFE filters (Millex LCR 0.45 µm, 13 mm) and Solid Phase Extraction (SPE). Based on this investigation, the best extraction parameters were defined, and the use of 50% methanol and PTFE filters was established. The antioxidant (ascorbic acid) content was also recognized; however, no difference was found compared with BHT.
For T. pentaphyllum tuber and leaf samples, the extraction/hydrolysis procedures were more effective with (20 g) freeze-dried samples of tuber and (1 g) freeze-dried samples of leaves, which were both homogenized with 50% methanol and 1.2M HCl for 2 h at 90 °C. The volumes used were 110 mL for tuber samples and 20 mL for leaf samples. Chu et al. (2000) used extraction/hydrolysis procedures for potato and sweet potato leaves (green) with 60% methanol 6M HCl for 2 h at 90 °C. The values found were 0.01 ± 0.00 µg/g for myricetin; 0.05 ± 0.00 µg/g for quercetin; 0.50 ± 0.00 µg/g for kaempferol, and undetermined for luteolin. The quercetin content in T. pentaphyllum tuber was significantly lower in comparison with those reported by those authors. In contrast, values found in this study for T. pentaphyllum leaves were significantly higher when compared with the quercetin value in sweet potato leaves (green) 143.78 ± 5.06 µg/g and similar to the myricetin content 38.88 ± 1.79 µg/g. In addition, this study also identified high amounts of luteolin and kaempferol.
For T. pentaphyllum flowers, the extraction/hydrolysis procedure was more effective with 1 g fresh sample, which was homogenized with 50% methanol and 1.2 M HCl for 2 h at 90 °C. The volume used was 32.5 mL for flowers. Kaisoon et al. (2011) used extraction/hydrolysis for 12 edible flowers from Thailand with methanol/HCl (100:1 v/v), which contained 2% tert-butylhydroquinone, for 12 h at 35 °C. The values found for Tagetes erecta sample were 4.0 ± 0.60 µg/g for myricetin; 145.6 ± 2.4 µg/g for quercetin, and 23.7 ± 2.3 µg/g for kaempferol. The contents of myricetin and quercetin in T. pentaphyllum flower samples were higher, while the kaempferol content was lower compared with the values reported by those authors.
Antioxidant capacity
The results of ABTS and DPPH, expressed in µM Trolox equivalents/g of dry sample and g dry sample/g DPPH, respectively, are shown in (Table 4).
Table 4.
Antioxidant properties of tuber, leaf and flower of Tropaeolum pentaphyllum Lam freeze-dried
| Parameters | T. pentaphyllum | T. pentaphyllum | T.pentaphyllum |
|---|---|---|---|
| Tuber | Leaf | Flower | |
| ABTS (µM Trolox Eq./g dry sample) | 162.80 ± 0.04c | 266.12 ± 0.21a | 273.83 ± 0.87a |
| DPPH (g dry sample/g DPPH) | 585.05 ± 1.03c | 126.00 ± 0.86a | 249.15 ± 0.17b |
| TRAP–AUC (104) | 270.85 ± 10.53c | 436.80 ± 9.21a | 348.01 ± 12.90b |
| TAR | 21.88 ± 1.28c | 43.08 ± 2.02b | 49.24 ± 4.65a |
Mean ± SD (standard deviation) of three replicates (n = 3)
Different letters on the same line represent significant difference p < 0.05 by Tukey’s test
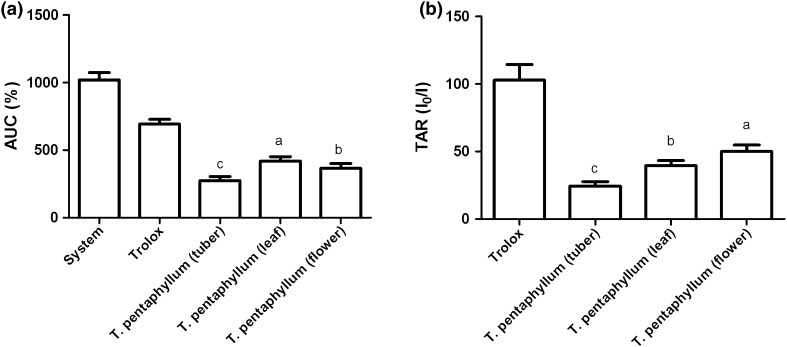
The results of the TRAP method are expressed by the area under the curve (AUC) (Fig. 1a) and (Fig. 1b) as total antioxidant reactivity (TAR–I0/I).
Fig. 1.
Analyses of (TRAP) (a) and (TAR) (b) of tuber, leaf and flower of Tropaeolum pentaphyllum Lam. Mean of three replicates (n = 3) and the values are expressed as the mean ± SD (p < 0.05)
The loss of chemiluminescence can be assessed by measuring the AUC. TRAP is one of the most commonly used assays to test antioxidant activity using chemiluminescence, whereas the parameter measured to indicate the plant antioxidant activity is the AUC (Dresch et al. 2009).
In the ABTS analysis, the value for T. pentaphyllum tubers was significantly different (p < 0.05) when compared with leaves and flowers, while no statistical difference was found between leaves and flowers (p > 0.05). The ABTS analysis results showed higher natural antioxidant content for T. pentaphyllum flowers and leaves comparing with the tubers.
DPPH analysis, results of tubers, leaves, and flowers differed significantly (p < 0.05). The results of the DPPH analysis revealed that antioxidant content of leaves were higher than those of the tubers and flowers.
Regarding TRAP–AUC (Fig. 1a), tubers showed higher antioxidant content and statistically differed (p < 0.05) from the leaves and flowers. However, TAR method revealed that flowers had higher quality antioxidants, followed by the leaves. According to Rossato et al. (2009) reported that TRAP and TAR were not proportional because these indices depend on the content of antioxidants in the extract and sometimes relatively low reactant antioxidant molecules may be present.
The results of TAR (Fig. 1b) analysis revealed that flowers obtained the highest TAR (high extract reactivity), and were also statistically different (p < 0.05) from the tubers and the leaves. The flowers had higher amounts of total phenolic compounds, α-tocopherol, and γ-tocopherol when compared with the leaves. On the other hand, flowers was more efficient as antioxidants. Tubers had a lower content of bioactive compounds and free-radical scavenging activity when compared to leaves and flowers measured by ABTS and DPPH, which revealed consistency between the antioxidant assays. That indicated the antioxidant capacity was directly related to the presence of bioactive compounds.
Determination of bioactive compounds that contribute to antioxidant activity
In order to estimate which of the bioactive compounds analyzed provides antioxidant activity, the ABTS, DPPH, and TRAP extract samples were evaluated under the same chromatographic conditions studied in this research (carotenoids, tocopherols, vitamin C, and flavonoids).
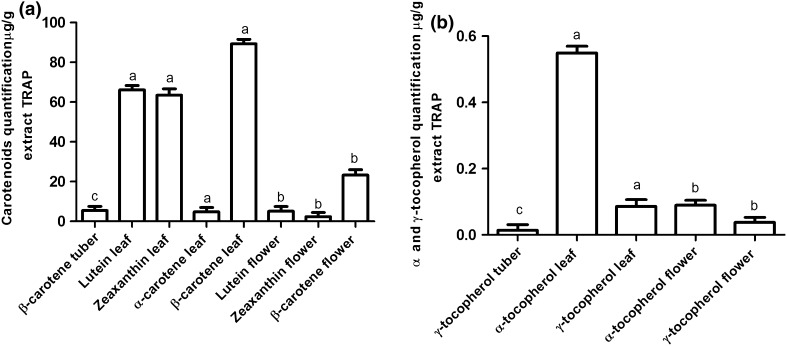
The leaves and flowers showed carotenoid content of 64.61 ± 0.10 lg/g and 4.59 ± 0.24 lg/g, respectively and lutein of 60.94 ± 0.35 lg/g and 0.84 ± 0.20 lg/g, zeaxanthin of 2.31 ± 0.10 lg/g for α-carotene (only in the leaves); and 89.79 ± 2.99 µg/g and 10.27 ± 0.14 µg/g for β-carotene, respectively (Fig. 2a). However, the zeaxanthin content in the leaf sample, which increased by nearly three-folds in the extract, was not saponified. According to Kimura et al. (1990) and Rodriguez-Amaya (1989), some carotenoids (α-carotene, β-carotene, γ-carotene, and β-cryptoxanthin) can resist saponification. Moreover, lutein, violaxanthin, and other dihydroxy-, trihydroxy-, and epoxycarotenoids are considerably reduced during saponification and during the subsequent washing step (Riso and Porrini, 1997; Kimura et al. 1990; Khachik et al. 1986).
Fig. 2.
Alcoholic extracts (TRAP) injected into the HPLC conditions to carotenoids (a) and tocopherols (b) of tuber, leaf and flower of Tropaeolum pentaphyllum Lam. Mean of three replicates (n = 3) and the values are expressed as the mean ± SD (p < 0.05)
The α- tocopherol and γ-tocopherol content in leaves and flowers samples ranged from 0.09 ± 0.18 to 0.03 ± 0.04 lg/g and 0.28 ± 0.03 to 0.10 ± 0.01 lg/g, respectively (Fig. 2b). Regarding α- tocopherol and γ-tocopherol, an increase was observed only in the T. pentaphyllum leaf sample. With the ethanol extract, the content increased by 0.09 ± 0.18, with a value of 0.53 ± 0.01 µg/g for α-tocopherol, and by 0.03 ± 0.04 µg/g, with a value of 0.09 ± 0.01 µg/g for γ-tocopherol, when compared with the methanol extract used in the quantification of tocopherol.
In the ABTS and DPPH extracts, only lutein was detected in the T. pentaphyllum leaf and flower samples, with concentrations of 5.48 ± 0.12 and 9.34 ± 1.07 µg/g lutein, respectively. Water was used to extract polar compounds, which may, in fact, have impacted the ABTS and DPPH carotenoid extraction analysis. Moreover, in contrast to the extract for the TRAP analysis, the ABTS and DPPH extracts had not been macerated, which influences the release of carotenoids.
Conclusion
The study of species Tropaeolum pentaphyllum Lam. showed nutritional quality and antioxidant potential when compared with sweet potato and sweet potato leaves, with values of protein (16.28 ± 0.02 g/100 g), total dietary fiber (27.78 ± 0.15 g/100 g), and quercetin (3798.61 ± 37.57 µg/g) found on leaves of this species. The T. pentaphyllum tubers also exhibited a significant amount of proteins (6.56 ± 0.02 g/100 g), total dietary fiber (8.94 ± 0.10 g/100 g) and quercetin (30 ± 0.10 µg/g), when compared with sweet potatoes. In addition, the tubers, leaves, and flowers showed antioxidant activity by ABTS, DPPH, and TRAP methods. This study highlights the potential of this species as an important source of nutritional compounds that are available in the native Brazilian flora, valuing local biodiversity and the small producer, which are trends that are growing in the food industry globally. Besides its use as a condiment (tuber) can be consumed as a side dish with soups and meats, as the (leaves and flowers) can be traditionally consumed in salads; parts of this plant can also be introduced into various food preparations, as in bakery, in order to nutritionally enrich and add value to the final product. The outcome of this study, show that T. pentaphyllum could significantly contribute to improve nutrition, as it supports the inclusion of this species on the menu of the local population, allied to ecological sustainability.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to the CNPq (National Scientific and Technological Development Council) and Capes (Coordinator for Upgrading Graduate-level Personnel) for the financial support.
Compliance with ethical standards
Conflict of interest
The authors declare that there are no conflicts of interest.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2817-z) contains supplementary material, which is available to authorized users.
References
- AOAC International . Official methods of analysis of AOAC International. 16. Gaithersburg: Association of Analytical Communities; 1997. [Google Scholar]
- Beretta G, Aldini G, Facino RM, Russel RM, Krinsky NI, Yeum K-J. Total antioxidant performance: a validated fluorescence assay for the measurement of plasma oxidizability. Anal Biochem. 2006;354:290–298. doi: 10.1016/j.ab.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- Boschetti W, Rampazzo RT, Dessuy MB, Vale MGR, Rios AO, Hertz P, Manfroi V, Celso PG, Ferrão MF. Detection of the origin of Brazilian wines based on the determination of only four elements using high-resolution continuum source flame AAS. Talanta. 2013;111:147–155. doi: 10.1016/j.talanta.2013.02.060. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Food Sci Technol. 1995;28:25–30. [Google Scholar]
- Chu YH, Chang CL, Hsu HF. Flavonoid content of several vegetables and their antioxidant activity. J Sci Food Agric. 2000;80:561–566. doi: 10.1002/(SICI)1097-0010(200004)80:5<561::AID-JSFA574>3.0.CO;2-#. [DOI] [Google Scholar]
- Dincer C, Karaoglan M, Erden F, Tetik N, Topuz A, Ozdemir F. Effects of baking and boiling on the nutritional and antioxidant properties of sweet potato [Ipomoea batatas (L.) Lam.] cultivars. Plant Food Hum Nutr. 2011;66:341–347. doi: 10.1007/s11130-011-0262-0. [DOI] [PubMed] [Google Scholar]
- Donaldson MS. Nutrition and cancer. A review of the evidence for an anti-cancer diet. Nutr J. 2004;3:19. doi: 10.1186/1475-2891-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos Freitas L, Jacques RA, Richter MF, Silva AL, Camarão EB. Pressurized liquid extraction of vitamin E from Brazilian grape seed oil. J Chromatogr A. 2008;1200:80–83. doi: 10.1016/j.chroma.2008.02.067. [DOI] [PubMed] [Google Scholar]
- Dresch MT, Rossato SB, Kappel VD, Biegelmeyer R, Hoff ML, Mayorga P, Zuanazzi JA, Henriques AT, Moreira JC. Optimization and validation of an alternative method to evaluate total reactive antioxidant potential. Anal Biochem. 2009;385:107–114. doi: 10.1016/j.ab.2008.10.036. [DOI] [PubMed] [Google Scholar]
- Duarte AT, Dessuy MB, Vale MGR, Welz B, Andrade JB. Sequential determination of Cd and Cr in biomass samples and their ashes using high-resolution continuum source graphite furnace atomic absorption spectrometry and direct solid sample analysis. Talanta. 2013;115:55–60. doi: 10.1016/j.talanta.2013.04.036. [DOI] [PubMed] [Google Scholar]
- Galdón BR, Rodríguez LH, Mesa DR, León HL, Pérez NL, Rodríguez EMR, Romero CD. Differentiation of potato cultivars experimentally cultivated based on their chemical composition and by applying linear discriminant analysis. Food Chem. 2012;133:1241–1248. doi: 10.1016/j.foodchem.2011.10.016. [DOI] [Google Scholar]
- Garzón GA, Wrolstad RE. Major anthocyanins and antioxidant activity of Nasturtium flowers (Tropaeolum majus) Food Chem. 2009;114:44–49. doi: 10.1016/j.foodchem.2008.09.013. [DOI] [Google Scholar]
- Huber SL, Hoffmann-Ribani R, Rodriguez-Amaya BD. Quantitative variation in Brazilian vegetable sources of flavonols and flavones. Food Chem. 2009;113:1278–1282. doi: 10.1016/j.foodchem.2008.08.030. [DOI] [Google Scholar]
- Hue SM, Boyce AN, Somasundram C. Influence of growth stage and variety on the pigment levels in Ipomoea batatas (sweet potato) leaves. Afr J Agric Res. 2011;6:2379–2385. [Google Scholar]
- Isabelle M, Lee BL, Lim MT, Koh WP, Huang D, Ong CN. Antioxidant activity and profiles of common vegetables in Singapore. Food Chem. 2010;120:993–1003. doi: 10.1016/j.foodchem.2009.11.038. [DOI] [Google Scholar]
- Jung E, Kim Y, Joo N. Physicochemical properties and antimicrobial activity of Roselle (Hibiscus sabdariffa L.) J Sci Food Agr. 2013;93:3769–3776. doi: 10.1002/jsfa.6256. [DOI] [PubMed] [Google Scholar]
- Kaisoon O, Siriamornpun S, Weerapreeyakul N, Meeso N. Phenolic compounds and antioxidant activities of edible flowers from Thailand. J Funct Foods. 2011;3:88–99. doi: 10.1016/j.jff.2011.03.002. [DOI] [Google Scholar]
- Khachik F, Beecher GR, Whittaker NF. Separation, identification, and quantification of the major carotenoid and chlorophyll constituents in extracts of several green vegetables by liquid chromatography. J Agric Food Chem. 1986;34:603–616. doi: 10.1021/jf00070a006. [DOI] [Google Scholar]
- Kimura M, Rodriguez-Amaya DB, Godoy HT. Assessment of the saponification step in the quantitative determination of carotenoids and provitamins A. Food Chem. 1990;35:187–195. doi: 10.1016/0308-8146(90)90032-Y. [DOI] [Google Scholar]
- Kuskoski EM, Asuero AG, Troncoso AM, Mancini-Filho J, Fett R. Aplicatíon de diversos métodos químicos para determinar actividad antioxidante em pulpa de frutos. Ciênc Tecnol Aliment. 2005;25:726–732. doi: 10.1590/S0101-20612005000400016. [DOI] [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–382. doi: 10.1016/0076-6879(87)48036-1. [DOI] [Google Scholar]
- Mercadante AZ, Rodriguez-Amaya DB. Effects of ripening, cultivar differences, and processing on the carotenoid composition of mango. J Agric Food Chem. 1998;46:128–130. doi: 10.1021/jf9702860. [DOI] [PubMed] [Google Scholar]
- Pereira MC, Steffens RS, Jablonski A, Hertz PF, Rios AO, Vizzotto M, Flôres SH. Characterization, bioactive compounds and antioxidant potential of three Brazilian fruits. J Food Compos Anal. 2013;29:19–24. doi: 10.1016/j.jfca.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Riso P, Porrini M. Determination of carotenoids in vegetable foods and plasma. Int J Vitam Nutr Res. 1997;67:47–54. [PubMed] [Google Scholar]
- Rodriguez-Amaya DB. Critical review of provitamin A determination in plant foods. J Micronutr Anal. 1989;5:191–225. [Google Scholar]
- Rodriguez-Amaya DB, Kimura M, Amaya-Farfan J. Fontes Brasileiras de carotenoides/Tabela Brasileira de composição de carotenoides em alimentos. Brasília: Ministério do Meio Ambiente; 2008. [Google Scholar]
- Rosa JS, Godoy RLO, Neto JO, Campos RS, Matta VM, Freire CA, Silva AS, Souza RS. Desenvolvimento de um método de análise de vitamina C em alimentos por cromatografia líquida de alta eficiência e exclusão iônica. Ciênc Tecnol Aliment. 2007;27:837–846. doi: 10.1590/S0101-20612007000400025. [DOI] [Google Scholar]
- Rossato SB, Hass C, Raseira MCB, Moreira JCF, Zuanazzi JAS. Antioxidant potential of peels and fleshes of peaches from different cultivars. J Med Food. 2009;12:1119–1126. doi: 10.1089/jmf.2008.0267. [DOI] [PubMed] [Google Scholar]
- Sánches-Machado DI, López-Cervantes J, Ríos Vázquez NJ. High-performance liquid chromatography method to measure α- and γ-tocopherol in leaves, flowers and fresh beans from Moringa oleifera. J Chromatogr A. 2006;1105:111–114. doi: 10.1016/j.chroma.2005.07.048. [DOI] [PubMed] [Google Scholar]
- Sun H, Mu T, Xi L, Zhang M, Chen J. Sweet potato (Ipomoea batatas L.) leaves as nutritional and functional foods. Food Chem. 2014;156:380–389. doi: 10.1016/j.foodchem.2014.01.079. [DOI] [PubMed] [Google Scholar]
- Swain T, Hillis WE. The phenolic constituents of Prunus domestica I. The quantitative analysis of phenolic constituents. J Sci Food Agric. 1959;10:63–68. doi: 10.1002/jsfa.2740100110. [DOI] [Google Scholar]
- Vasconcelos RL, Freitas MPN, Brunini MA. Características físico-químicas da rúcula cv. cultivada produzida no sistema convencional e no baby leaf. Nucleus. 2011;8:7–14. doi: 10.3738/1982.2278.607. [DOI] [Google Scholar]
- Wei K, Wang LY, Zhou J, He W, Zeng JM, Jiang YW, Cheng H. Comparison of catechins and purine alkaloids in albino and normal green tea cultivars (Camellia sinensis L.) by HPLC. Food Chem. 2012;130:720–724. doi: 10.1016/j.foodchem.2011.07.092. [DOI] [Google Scholar]
- Zhang J, Hou X, Ahmad H, Zhang H, Zhang L, Wang T. Assessment of free radicals scavenging activity of seven natural pigments and protective effects in AAPH-challenged chicken erythrocytes. Food Chem. 2014;145:57–65. doi: 10.1016/j.foodchem.2013.08.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.