Abstract
Phenolic compounds of pistachio green hull extract (PGHE) were incorporated into nanoliposomes (NLs). The NLs were prepared with different concentrations of phenolic compounds of PGHE (500, 750 and 1000 ppm) and particle size, polydispersity index (PDI), zeta potential and encapsulation efficiency (EE) were investigated. The antioxidant activity of free and incorporated phenolic compounds of PGHE were evaluated in soybean oil during 39 days of storage by measuring peroxide (PV), thiobarbituric acid (TBA) values and color. The total phenolic content and IC50 (DPPH assay) of PGHE were 614.91 ± 0.45 mg gallic acid equivalent/g fresh weight of extract and 10 ± 0.05 μg/ml extract, respectively. The prepared NLs had 101.86–105.81 nm size and PDI = 0.202–0.235. The zeta potential value of NLs varied between −47.7 and −52.3 mV. The highest EE (32.47%) was observed for NLs containing 1000 ppm of phenolic compounds. The lowest PV and TBA values were related to free phenolic compounds at 500 mg/kg oil. In comparison with free phenolic compounds, loaded NLs had lower antioxidant activity, but encapsulation could improve the stability, gradual release and solubility of phenolic compounds in soybean oil. The color of NLs containing oil samples remained constant during the storage, but free phenolic compounds changed the oil`s color. All concentrations of free and incorporated phenolic compounds had also higher antioxidant activity than BHT. Finally, 500 ppm of phenolic compounds of PGHE in its incorporated forms could be recommended as a substitute for synthetic antioxidant in soybean oil.
Keywords: Encapsulation, Nanoliposome, Oil stability, Phenolic compounds, Pistachio green hull
Introduction
Lipid oxidation is one of the main causes of quality deterioration in edible oils, fats and lipid containing foods, which results in substantial economic losses for food industry (Habeebullah et al. 2010). There are different strategies for controlling lipid oxidation, but adding antioxidants to lipid bearing foods is the most efficient, economical and suitable approach. Antioxidants can retain edible oils, fats and lipid bearing foods quality and extend their shelf-life by decreasing rancidity, maintaining nutritional values (specially unsaturated fatty acids) and hindering the formation of toxic oxidation products. Furthermore, antioxidants have quenching effects on free radicals and protect the human body against oxidative stress and its related diseases which make them considered as health-promotive additives (Zhong and Shahidi 2012). Antioxidants are available in natural and synthetic forms in the market, but using synthetic ones such as tert-butyl hydroquinone (TBHQ), butylated hydroxyl anisole (BHA), and butylated hydroxyl toluene (BHT) have been limited due to their toxicity and carcinogenic effects. However, natural antioxidants as alternative for synthetic ones are safe for application in food products. Recently, the tendency for natural antioxidants, especially the plant-based ones, has increased. Regardless of the antioxidant source, the antioxidant activity of plant materials can be attributed to simple phenols, phenolic acid derivatives, flavonoids, and polyphenolic compounds (Habeebullah et al. 2010; Taghvaei and Jafari 2015). Due to the antioxidant and quenching activity of phenolic compounds, they are able to mitigate the risk of serious health problems. Nevertheless, there are some limitations for using phenolic compounds in foods, such as interaction with food components, low bioavailability, undesirable tastes, degradation during food processing and storage, or in the gastrointestinal tract (Fang and Bhandari 2010). As many phenolic compounds have low solubility in lipophilic media, it is difficult to use them in lipophilic systems including fats, oils, lipid containing foods, emulsions, cosmetic formulations and biological environments (Zhong and Shahidi 2012). Encapsulation is a suitable approach to overcome these problems. Liposome is one of the most effective encapsulation methods to protect phenolic compounds due to its small size, capability to incorporate water-soluble and water-insoluble compounds, high biodegradability and biocompatibility as well as non-toxicity. Liposome is a spherical bilayer vesicle made of polar lipids which include an aqueous core enclosed through lipid bilayers (Rashidinejad et al. 2014; Lu et al. 2014). Consequently, liposomes cover incorporated compounds and protect them against external adverse conditions such as pH, oxygen, light or enzymes and digestion in the stomach and increase their absorption in the gastrointestinal tract which can improve bioavailability, bioactivity and release at particular targets (Hasan et al. 2014). Lecithin is a natural and inexpensive source of phospholipids with a wide accessibility and high safety. Thus, it can be used for producing liposomes in commercial applications. On the other hand, phenolic compounds encapsulated into liposomes using lecithin, are able to provide the possibility for novel foods and pharmaceutical applications (Rashidinejad et al. 2014).
Pistachio (Pistachia vera L.) is extensively cultivated in Iran. Iran is the largest producer and exporter of pistachio nut in the world with total production of 478,600 tons in 2013 (FAO 2013). Pistachio green hull is produced in large quantities during processing of pistachio nut as waste, but studies showed that, pistachio green hull extract (PGHE) has high content of phenolic compounds and remarkable antioxidants, antimutagenicity and antimicrobial activities (Goli et al. 2005; Rajaei et al. 2010; Behgar et al. 2011; Garavand et al. 2017). Goli et al. (2005) reported that PGHE precipitated in soybean oil during the storage because of its low solubility, especially in higher concentrations which resulted in decrease of its antioxidant activity. Furthermore, PGHE significantly changed the color and taste of oil samples. For mentioned limitations, using free PGHE will be almost impossible and useless in edible oils and lipid containing foods. Therefore, encapsulation of PGHE, as an inexpensive and natural source of phenolic compounds in liposomes, may be an efficient approach to solve the problems related to their direct application in food products and produce appropriate food grade antioxidants.
Although many works have reported on antioxidant activities of plant phenolic extracts, only a few described the application of encapsulated phenolic compounds in food systems (Taghvaei et al. 2014; Rashidinejad et al. 2014; Mohammadi et al. 2016a).
The objective of this study was to investigate the incorporation of phenolic compounds of PGHE in liposomes and to evaluate their characteristics (particle size, size distribution, zeta potential and entrapment efficiency). The effect of incorporation of phenolic compounds on the oxidative stability of soybean oil was also evaluated during the storage.
Materials and methods
Plant material and chemicals
Pistachio green hulls (Ahmad aghaei variety) were provided from Agricultural Research Center of Yazd in Iran. RBD (refined, bleached, deodorized) soybean oil was purchased from Pars Ghou factory (Tehran, Iran). l-α-granular lecithin of soybean (purity of 99%) was supplied by Acros Organics Company (New Jersey, USA). SPE (solid phase extraction) cartridge was obtained from Membrane Solution (Dallas, USA). Other chemicals and reagents of analytical grade were purchased from Sigma-Aldrich (St. Louis, USA) and Merck (Darmstadt, Germany) companies.
Extraction and purification of PGHE
The pistachio green hulls were dried in shade, ground and sieved (particle size of 0.5–2 mm). The powder sample (1 g) was mixed with distilled water (15 ml) and agitated for 8 h at ambient temperature. Then, the extract was filtered through filter paper (Whatman No. 1). In order to obtain pure phenolic extract, the filtrate was purified using C18 SPE cartridges according to the method proposed by Rajaei et al. (2010).
Determination of total phenolic content (TPC)
The TPC of extracts was evaluated with the Folin–Ciocalteu reagent according to the method of Rafiee et al. (2012). Briefly, 20 µl of the extract was mixed with 1.16 ml distilled water and 100 µl of Folin–Ciocalteu reagent. After at least 1 min (but not exceeding 8 min), 300 µl of Na2CO3 solution (20% w/v) was added to the mixture and stored in a shaking incubator (Memmert WB14, Germany) at 40 °C for 30 min. The absorbance was measured at 760 nm by UV–Vis spectrometer (Agilent Cary 60, USA) and the amount of phenolic compounds was expressed as mg gallic acid equivalent (GAE)/gram fresh weight (gfw).
Antioxidant activity of PGHE
The antioxidant activity of the PGHE was measured according to the procedure described by Rafiee et al. (2012). The free radical scavenging capacity was determined using DPPH (2,2-diphenyl-1-picrylhydrazyl) assay. The DPPH radical scavenging activity (%) of the sample was calculated using the following equation:
where A and B represent the absorbance of control (ethanolic solution of DPPH) and the sample at 517 nm, respectively. The antioxidant activity was evaluated with IC50 value. The concentration of the extract which is required to scavenge 50% of the DPPH free radicals was considered as IC50.
Preparation of nanoliposomes
First, 2 g of the lecithin was added to 98 g of water and agitated (500 rpm) for 1 h to prepare 2% (w/w) lecithin solution. Then, PGHE was added to the mixture to obtain the final concentrations of 500, 750 and 1000 ppm of phenolic compounds and agitated for 4 h by stirrer at 500 rpm (IKA, Staufen im Breisgau, Germany). Finally, the samples were sonicated at operating frequency of 24 kHz and 50% of total power (UP400S, Hielscher, Germany) for 5 min. The empty liposomes were also produced using the same procedure except for adding PGHE to the lecithin solution (Arab Tehrany et al. 2012). The following formulations were used to produce nanoliposomes: ENL (empty nanoliposomes), LNL-500 (loaded nanoliposomes containing 500 ppm of phenolic compounds), LNL-750 (loaded nanoliposomes containing 750 ppm of phenolic compounds), and LNL-1000 (loaded nanoliposomes containing 1000 ppm of phenolic compounds).
Particle size and zeta potential
The average particle size and surface charge of produced nanoliposomes were determined by dynamic light scattering (DLS) using a Malvern Zetasizer Nano ZS (Malvern instruments, UK). Before the measurement, liposomal dispersions were diluted (1:20) with deionized water to reduce particle aggregation and prevent multiple scattering effects (Arab Tehrany et al. 2012).
Entrapment efficiency (EE)
The EE was estimated according to the procedure described by Madrigal-Carballo et al. (2010). In order to separate unloaded phenolic compounds, liposome dispersion was centrifuged at 65,000 g for 1 h at 4 °C (3–30 k, Sigma, Germany). The content of phenolic compounds was determined in initial dispersion and supernatant by the Folin–Ciocalteau assay. The EE (%) was calculated using the following equation:
where Cinitial is the content of initial phenolic compounds used for preparing nanoliposomes and Csup represents unloaded phenolic compounds in the supernatant.
Fatty acid composition of soybean oil
The fatty acid profiles of soya bean oil were determined by gas chromatography (GC). The fatty acid methyl esters (FAME) were prepared and analyzed using a GC (Shimadzu 17A, Kyoto, Japan) equipped with a flame ionization detector and a fused silica capillary column (50 cm × 0.25 mm and 0.20 µm of Carbowax 20 M). Lastly, the identification of the FAME peaks was performed with FAME standards (Abedi et al. 2015).
Oxidative stability of soybean oil during the storage
Free and nanoliposome loaded PGHE were individually added to 1 kg of soybean oil at different concentrations (three concentrations of 500, 750 and 1000 mg phenolic compounds). Sample oils containing BHT (100 and 200 mg/kg oil) were also used for comparison. Soybean oil bearing no antioxidant was also considered as control. Totally, the oil was treated with Ex-500, Ex-750 and Ex-1000 (containing 500, 750 and 1000 mg free phenolic compounds of PGHE/kg oil, respectively); NL-500, NL-750 and NL-1000 (nanoliposomes containing 500, 750 and 1000 mg incorporated phenolic compounds of PGHE/kg oil, respectively); BHT-100 and BHT-200 (containing 100 and 200 mg BHT/kg oil, respectively). The oil samples were transferred into glass bottles (25 ml oil in each bottle) and were stored in an oven (Memmert, Germany) at 35 °C for 39 days. The oxidative stability of oil samples was evaluated by the measurement of peroxide value (PV) and thiobarbituric acid value (TBA) during the storage, according to AOCS methods (AOCS 2007).
Color analysis
The color of the soybean oil samples were determined by a Lovibond (5¼ Tintometer model F, England) using glass cell with an optical path length of 10 mm. Also, the comparison of the colors were carried out with CIE L*, a* and b* color (Abedi et al. 2015).
Statistical analysis
A completely randomized design and spilt plot in time design were employed for the nanoliposomes preparing experiments (0, 500, 750, and 1000 ppm of phenolic compounds used for encapsulation) and oxidative stability tests were carried out on the 9 treatments with three replicates (27 samples), respectively. The statistical analyses (ANOVA) were carried out by SAS version 9.1. Significant differences among means were determined with the least significant difference (LSD) test at p < 0.05. All experiments were carried out in triplicates.
Results and discussion
TPC and antioxidant activity of PGHE
SPE has been utilized to remove impurities such as carbohydrates, organic acids, fatty substances etc., from phenolic compounds of plant extracts (Rajaei et al. 2010). TPC and IC50 of PGHE were 614.91 ± 0.45 mg GAE/gfw extract and 10.00 ± 0.05 µg/ml extract, respectively. Different TPC and IC50 of PGHEs were reported in other studies. Rajaei et al. (2010) demonstrated that the highest TPC (49.32 mg GAE/gfw sample) and IC50 (1.94 µg/ml extract) of pistachio green hull extract (Ahmad aghaei variety) were achieved by water as solvent. Water extract of Fandoghi variety of pistachio green hulls had also higher TPC (34.7 mg TAE/g dry weight sample) in comparison to methanol and ethyl acetate extracts (Goli et al. 2005). This difference in TPC of PGHEs can be related to variety, harvest stage, extraction procedure (solvent type, time and temperature), geographic location, agricultural conditions and so on.
Liposome characterization
The characteristics of empty nanoliposomes and nanoliposomes containing different concentrations of phenolic compounds of PGHE (500, 750 and 1000 ppm) such as particle size, polydispersity index (PDI), zeta potential and entrapment efficiency are shown in Table 1.
Table 1.
Particle size, PDI, zeta potential and entrapment efficiency of produced nanoliposomes
| Conc. of phenolic compounds (ppm) | Particle size (nm) | PDI | Zeta potential (mV) | Entrapment efficiency (%) |
|---|---|---|---|---|
| 0 | 105.81 ± 1.08a | 0.202 ± 0.020c | −47.7 ± 0.4c | – |
| 500 | 101.86 ± 0.85b | 0.224 ± 0.020b | −49.9 ± 0.7b | 24.83 ± 0.89b |
| 750 | 102.09 ± 0.88b | 0.228 ± 0.040ab | −50.1 ± 0.9b | 25.36 ± 0.95b |
| 1000 | 102.37 ± 0.59b | 0.235 ± 0.050a | −52.3 ± 1.0a | 32.47 ± 0.82a |
Values are the means ± standard deviations (n = 3). Different letters in the same column indicates significant differences among means (LSD test, p < 0.05)
Particle size has great influence on characteristics of nanoliposomes such as stability, bioavailability and release behavior. The results of particle size analysis showed that all of samples had nanometer size which was an attractive feature regarding their applications. The maximum average size (105.81 ± 1.08 nm) belonged to ENL. As illustrated in Table 1, the incorporation of phenolic compounds reduced the nanoliposome size, but the increase of phenolic compounds concentration had no significant effect on the size of nanoliposome (p > 0.05). The physicochemical properties of phenolic compounds such as polarity, solubility and the size of the molecules may have influences on size of liposomes. On the other hand, the entrapment of phenolic compounds can lead to conformational changes in bilayers, because of several phenolic hydroxyl groups in their structure. The interaction between certain phenolic compounds and the acyl chains of phospholipids may also decrease the liposome size. Moreover, the size reduction of liposomes can be associated with the effect of phenolic compounds on the acyl chain order and the membrane fluidity of bilayers (Wink 2010; Maherani et al. 2013). Principally, hydrophobic substances are incorporated in the lipid bilayers, while hydrophilic compounds move toward the aqueous interior core. Therefore, it was concluded that since most phenolic compounds of the extract were polar compounds, they were located inside the aqueous part and consequently, the particle size change was not significantly different (p < 0.05) by increasing phenolic compounds concentration.
As illustrated in Table 1, PDI values of all samples were lower than 0.3 which indicates homogenous dispersions (Hasan et al. 2014). There was significant difference between PDI value of ENL and LNLs. It was observed that the addition of phenolic compounds led to increase of PDI and the highest PDI value was related to LNL-1000 (0.235 ± 0.050).
The zeta potential values indicate the electric potential in the interface or particle surface and are usually used to predict the stability of colloidal systems. As zeta potential values are lower than −30 mV, the system is considered stable. It is due to increase of repulsion between particles which prevent the aggregation, coagulation or flocculation of particles (Lu et al. 2014). As illustrated in Table 1, the zeta potential value of samples varied between −47.7 and −52.3 mV (Table 1). The highest and the lowest negative surface charges were related to LNL-1000 and ENL, respectively. There was no significant difference between LNLs produced by 500 and 750 ppm of phenolic compounds. LNLs had higher zeta potential than ENL which showed the high physical stability of LNLs. It could be concluded that the addition of phenolic compounds significantly influenced the zeta potential values of LNLs (p < 0.05) and liposomes containing PGHE have good stability. The presence of anionic phospholipids like phosphatidylserine, phosphatidylglycerol, phosphatidic acid and phosphatidylinositol in lecithin can be responsible for negative surface charge of the produced nanoliposomes (Rafiee et al. 2017). Furthermore, higher zeta potential of LNLs may be attributed to the negatively charged carboxyl group of phenolic acids which are one of the most important groups of phenolic compounds in PGHE. Consequently, it could be stated that, the increase of surface charge of LNLs in higher concentrations of phenolic compounds may be related to the increase of phenolic acids concentrations. High zeta potential values have been reported in earlier studies (Hasan et al. 2014; Rashidinejad et al. 2014).
The entrapment efficiency of prepared samples is illustrated in Table 1. According to Table 1, LNL-1000 had the highest EE (32.47%). There was no significant difference between 500 and 750 ppm of phenolic compounds regarding EE. Similar results were reported by Isailović et al. (2013) regarding particle size, PDI and EE for resveratrol.
Fatty acid profile of soybean oil
The fatty acid profile of soybean oil is presented in Table 2. The most abundant unsaturated fatty acids (UFAs) in soybean oil were linoleic acid (52.55%), oleic acid (22.86%) and linolenic acid (6.63%). Regarding saturated fatty acids, palmitic acid (12.35%) and stearic acid (4.72%) had higher amounts. As demonstrated in Table 2, the total UFAs content in soybean oil was around 82% which made it suitable for oxidative stability evaluation.
Table 2.
Fatty acids composition of studied soybean oil
| Fatty acids | Content (%) |
|---|---|
| Myristic (C14:0) | 0.079 |
| Palmitic (C16:0) | 12.350 |
| Palmitoleic (C16:1) | 0.061 |
| Heptadecanoic (C17:0) | 0.077 |
| Stearic (C18:0) | 4.720 |
| Oleic (C18:1) | 22.860 |
| Linoleic (C18:2) | 52.550 |
| Linolenic (C18:3) | 6.630 |
| Eicosanoic (C20:0) | 0.300 |
| Arachidonic (C20:4) | 0.330 |
| Total | 99.975 |
Oxidative stability
PV
PV is usually used for the measurement of hydroperoxides which are the main initial products of lipid oxidation. The effects of different treatments on PV of soybean oil during the storage are illustrated in Table 3. As demonstrated in Table 3, the statistical results showed that time and treatment interaction (time × treatment) had significant effect on PV value and the PV of all samples significantly increased during the storage time (p < 0.05). The highest increase of PV during the storage period belonged to control sample (from 2.06 on the 4th day to 115.53 meq kg−1 of oil on the 39th day).
Table 3.
Effect of free and capsulated antioxidants on PV values of soy bean oil (meq kg−1 of oil) during 39 days of storage
| Treatment | Day | |||||
|---|---|---|---|---|---|---|
| 4 | 11 | 18 | 25 | 32 | 39 | |
| Control | 2.06 ± 0.05eF | 3.26 ± 0.05cE | 12.33 ± 0.41aD | 43.90 ± 0.36aC | 72.96 ± 0.50aB | 115.53 ± 0.55aA |
| BHT-100 | 1.91 ± 0.07fF | 2.78 ± 0.07eE | 7.36 ± 0.41dD | 20.93 ± 1.00bC | 35.53 ± 0.55bB | 45.00 ± 0.20bA |
| BHT-200 | 2.58 ± 0.07cE | 2.89 ± 0.03edE | 8.43 ± 0.05cD | 19.43 ± 0.50cC | 29.40 ± 0.50cB | 43.56 ± 0.55cA |
| Ex-500 | 1.76 ± 0.05gF | 2.79 ± 0.01eE | 3.38 ± 0.16hD | 4.20 ± 0.10gC | 5.93 ± 0.05iB | 8.43 ± 0.20iA |
| Ex-750 | 2.35 ± 0.05dF | 3.05 ± 0.04eE | 5.63 ± 0.15fD | 9.56 ± 0.05eC | 13.10 ± 0.30fB | 16.90 ± 0.79fA |
| Ex-1000 | 3.55 ± 0.05bE | 3.61 ± 0.07bE | 7.20 ± 0.16dD | 13.03 ± 0.15dC | 18.43 ± 0.20 dB | 24.76 ± 0.25dA |
| NL-500 | 1.96 ± 0.05feF | 3.43 ± 0.05cE | 4.86 ± 0.05gD | 7.13 ± 0.15fC | 8.90 ± 0.10hB | 12.70 ± 0.20hA |
| NL-750 | 2.28 ± 0.07dF | 3.30 ± 0.26cE | 6.20 ± 0.36eD | 7.40 ± 0.17fC | 10.40 ± 0.20gB | 13.36 ± 0.40gA |
| NL-1000 | 3.74 ± 0.04aF | 6.06 ± 0.05aE | 9.03 ± 0.15bD | 12.90 ± 0.10dC | 16.90 ± 0.10eB | 22.85 ± 0.15eA |
Values are the means ± standard deviations (n = 3). Different capital letters (A, B, C,…) in the same row shows significant difference and different lowercase letters (a, b, c …) in the same column shows significant difference among different treatments (LSD test, p < 0.05). Control (without phenolic compounds); Ex-500, Ex-750 and Ex-1000 (500, 750 and 1000 mg of free phenolic compounds of PGHE/kg oil, respectively); NL-500, NL-750 and NL-1000 (500, 750 and 1000 mg of incorporated phenolic compounds of PGHE/kg oil, respectively); BHT-100 and BHT-200 (100 and 200 mg of BHT/kg oil, respectively)
The results showed an increase of phenolic concentration in Ex and NL forms intensified soybean oil oxidation and significantly increased PV in all days except for NLs on the 11th and 25th days (p < 0.05). It was obvious that the lowest concentration of PGHE (500 mg/kg) in both Ex and NL forms had better antioxidant activity than 750 and 1000 mg/kg. Totally, 1000 mg/kg showed the lowest potency for retarding oxidation in free and incorporated phenolic compounds. This can be due to the pro-oxidant effect of phenolic compounds of PGHE in concentrations higher than 500 mg/kg. The results were comparable with Gortzi et al. (2008), who reported that there is no linear correlation between the antioxidant activities and the concentration of some antioxidants. They also reported that the antioxidants showed pro-oxidant effect at high concentrations. However, PV values of Ex were lower than NL at 500 mg/kg in all days (p < 0.05) due to slow and controlled release of phenolic compounds from NLs. Zou et al. (2014a) also indicated the lower ferric reducing activities of tea polyphenols loaded liposomes in comparison to free ones, because of their controlled release behavior.
Furthermore, Exs at 750 and 1000 mg/kg showed better antioxidant activity than NLs with the same concentrations until 18th day, but reverse trend was observed in subsequent days. This could be attributed to the high decomposition rate of phenolic compounds in Ex during the storage, but phenolic compounds were more retained in NLs, because of the protective effect of liposomes. Similarly, Zou et al. (2014b) observed that incorporation of Epigallocatechin gallate (EGCG) in nanoliposomes significantly enhanced stability of EGCG in simulated intestinal fluid (SIF) compared to EGCG solutions. Also, nanoliposomes containing fish oil showed higher ability for retention of omega 3 fatty acids in yoghurt samples than free ones (Ghorbanzade et al. 2017).
As seen in Table 3, NLs and Exs exhibited significantly lower PV than BHT (at 100 and 200 mg/kg) in most cases. The results also demonstrated that the PV of NL-500 and Ex-500 remained below the maximum permitted limit (≤10 meq kg−1 oil) stated by Codex Alimentarius Commission (1999) after 32 and 39 days of storage, respectively, but the PV of BHT samples exceeded the permitted limit after 18 days of storage. It could be concluded that, liposomes protect phenolic compounds against interactions with other food ingredients and retain their bioactivity and stability during food processing, storage and digestion. Also, NLs had more antioxidant capability than Exs in longer storage times.
TBA
As PV can not completely indicate oxidation state of edible oils, TBA value was measured to evaluate secondary oxidation products which are formed along with the decomposition of initial oxidation products. The TBA values of different soybean oil samples were measured on the 18th, 25th, 32th and 39th days and expressed as malonaldehyde equivalents kg−1 oil (Table 4). The statistical results showed that time and treatment interaction (time × treatment) had significant effects on TBA values (p < 0.05). TBA values followed an increasing trend with increment of formation and decomposition of hydroperoxides during the storage time in all samples.
Table 4.
TBA values of different oil samples (mg malonaldehyde equivalent kg−1 oil) during 39 days of storage
| Treatment | Day | |||
|---|---|---|---|---|
| 18 | 25 | 32 | 39 | |
| Control | 0.408 ± 0.005aD | 0.927 ± 0.010aC | 1.426 ± 0.010aB | 3.794 ± 0.010aA |
| BHT-100 | 0.101 ± 0.007dD | 0.378 ± 0.010bC | 0.542 ± 0.005bB | 1.638 ± 0.010bA |
| BHT-200 | 0.118 ± 0.010cD | 0.355 ± 0.010cC | 0.480 ± 0.010cB | 1.628 ± 0.006bA |
| Ex-500 | 0.028 ± 0.006gD | 0.045 ± 0.001gC | 0.0723 ± 0.001hB | 0.212 ± 0.001hA |
| Ex-750 | 0.078 ± 0.006efD | 0.141 ± 0.006eC | 0.165 ± 0.004fB | 0.514 ± 0.002eA |
| Ex-1000 | 0.120 ± 0.009cD | 0.245 ± 0.007dC | 0.309 ± 0.006 dB | 0.909 ± 0.006cA |
| NL-500 | 0.067 ± 0.006fD | 0.101 ± 0.003fC | 0.121 ± 0.010gB | 0.373 ± 0.010gA |
| NL-750 | 0.090 ± 0.006deD | 0.112 ± 0.010fC | 0.156 ± 0.007fB | 0.394 ± 0.005fA |
| NL-1000 | 0.173 ± 0.010bD | 0.244 ± 0.006dC | 0.283 ± 0.007eB | 0.871 ± 0.010dA |
Values are the means ± standard deviations (n = 3). Different capital letters (A, B, C,…) in the same row shows significant difference among means and different lowercase letters (a, b, c…) in the same column shows significant difference among means (LSD test, p < 0.05). All abbreviations defined in Table 3
As seen in Table 4, the control and Ex-500 had the highest and lowest TBA values, respectively. TBA value of control sample increased from 0.4 (malonaldehyde equivalent kg−1 oil) on the 18th day to 3.79 (malonaldehyde equivalent kg−1 oil) on the 39th day, because there was no antioxidant ingredient applied in order to prevent oxidation.
Similar to PV results, Ex-500 had significantly higher antioxidant activity and lower TBA value in comparison to Ex-750 and Ex-1000 in all days (p < 0.05). In the case of NLs, there were significant differences among concentrations in all days except for NL-500 and NL-750 on the 25th day. Likewise, Ex-500 and NL-500 had higher antioxidant activity and lower TBA values in comparison to NL-750 and NL-1000 in all days. It was also observed that, Ex-500 had more potent antioxidant activity in comparison to NL-500. As demonstrated in Table 4, Ex-750 and Ex-1000 could efficiently inhibit the formation of malonaldehyde on the 18th day, but NL-750 and NL-1000 were superior on the subsequent days. Because of higher rate of hydroperoxide decomposition than their formation in longer storage times, higher amounts of phenolic compounds are necessary for reducing TBA value. Therefore, due to the slow release of phenolic compounds from NLs, they were not able to control the formation of malonaldehyde on the 18th day. Contradictorily, Exs had sufficient amounts of phenolic compounds and could monitor the malonaldehyde formation. However, the results indicated that the efficiency of NLs improved in next days, because of the increasing release rate of phenolic compounds and probably decomposition of Exs under environmental conditions.
The antioxidant activity of incorporated phenolic compounds depends on interaction with bilayers and location within liposomes. Hydrophobic phenolic compounds place in non-polar part of lipid bilayers, while hydrophilic phenolic compounds locate inside aqueous region of liposomes which can decrease the release rate (Gosangari and Watkin 2012; Paiva-Martins et al. 2003). The slow release can be associated with interaction between bilayers and phenolic compounds of PGHE by formation of hydrogen bonds between the polar zone of phospholipids and the OH groups of these compounds (Rafiee et al. 2017). As mentioned before, phenolic compounds concentration had no significant effect on the particle size of NLs. Consequently, it can be found that, phenolic compounds successfully encapsulated in nanoliposomes and were released over a period of time. Therefore, the lower antioxidant activity of NLs can be justified. Similarly, the controlled release and gradual accessibility to antioxidant effect of olive leaf phenolic compounds have been stated by Mohammadi et al. (2016b).
The controlled release of the phenolic compounds also leads to masking unpleasant flavors, such as bitterness. Moreover, loading bioactive compounds in nanoliposomes slows down degradation in the gastrointestinal tract, increases their surface area and enhances their bioavailability and bioactivity (Fang and Bhandari 2010).
In comparison to Exs, no precipitations were observed for different nanoliposomes formulations during the storage time due to the amphipathic nature of phospholipids which enhanced the solubility of the extract in oil. The precipitation of Exs (particularly in higher concentrations) can be attributed to their low lipid solubility. Thus, nanoliposomes could not only enhance the stability of phenolic compounds, but also improve their solubility.
Furthermore, lecithin has antioxidant activity and improves the oxidative stability of oils and fats due to the presence of phospholipids in lecithin composition. Phospholipids have different antioxidative mechanisms such as amino actions of phosphatidylcholine (PC), phosphatidylethanolamine (PE) or phosphatidylserine (PS) and metal-chelating activities of the sugar part of phosphatidylinositol (PI). The presence of phospholipids in the oil/water interface results in an oxygen barrier activity, protecting lipids against atmospheric oxygen. Additionally, lecithin facilitates the dispersion of other antioxidants and inhibits the propagation of free radicals in the food systems. Lecithin retards lipid oxidation by scavenging free radical and converting hydroperoxides into stable products. The reaction of PC with peroxy radicals leads to forming trimethylammonium oxides, while PE yields imines by reaction with hydroperoxides in the non-radical pathway. Lecithin also has a synergistic effect with phenolic compounds, because of donating a hydrogen atom by amino group of phospholipids and regenerating oxidized phenolic compounds. It has been reported that, the addition of lecithin (1% w/w) to rapeseed oil resulted in an increment of Rancimat induction time and a reduction of PV (Judde et al. 2003; Cardenia et al. 2011). Also, according to our previous work, the incorporation of phenolic compounds of PGHE into nanoliposomes could improve their oxidative stability in comparison to free ones (Rafiee et al. 2017).
The TBA values of Exs and NLs were significantly lower in comparison to those of BHT expect for similar TBA value of BHT-100 and NL-500 on the 18th day. Therefore, a synthetic antioxidant such as BHT can be replaced by capsulated PGHE. Generally, TBA values followed the same trend with PV results and confirmed them. Finally, it could be concluded that, Ex-500 was the most potent antioxidant for retarding oxidation followed by NL-500, NL-750 and NL-1000. But for avoiding inverse effects of free extract in soy bean oil (as mentioned before), using NL-500 is recommended as a natural antioxidant in oil system.
The findings were in accordance with the results of Taghvaei et al. (2014) who studied the antioxidant capability of olive leaf extract. They reported that, incorporated olive leaf extract had higher stability than free ones, but encapsulation by freeze-drying method led to a decrease in the antioxidant activity of the extract in soybean oil. Besides, Chatterjee and Bhattacharjee (2013) suggested using encapsulated clove extracts instead of un-encapsulated form in frying oil, due to their controlled release. Spigno et al. (2013) reported that grape marc extract addition significantly retarded the oxidation of hazelnut paste under accelerated shelf-life test at 60 °C. They also observed that the enclosing of phenolic compounds improved the dispersion of extract in the medium and maintained their antioxidant activity. Different results have been reported by researchers about the effectiveness of encapsulated phenolic compounds. Mohammadi et al. (2016a) prepared nano-encapsulated olive leaf extract (NOLE) by W/O nano-emulsions and W/O/W double emulsions and added it to the soybean oil. They demonstrated, NOLE was more effective for retarding oxidation in comparison with non-encapsulated ones, but NOLE had a lower thermal stability than that of free form. Additionally, the encapsulation of the Myrtuscommunis extract in liposomes led to an increment of antioxidant effect in comparison to the free form of the extract (Gortzi et al. 2008). The dissimilarity can be attributed to type of encapsulation method, type and concentration of phenolic compounds, antioxidant activity assay, storage conditions, the nature of food systems, release profile, and other factors.
Color analysis
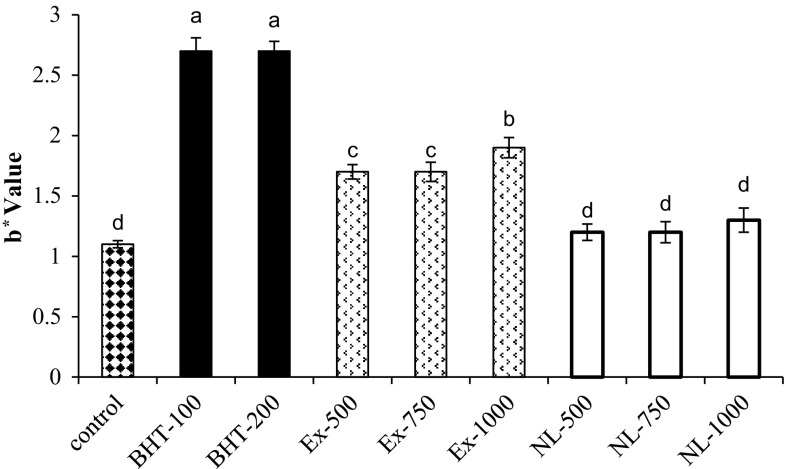
L*, a* and b* are indicators of lightness, redness, and yellowness of samples. L* and a* parameters of all samples were statistically constant after 39 days (data not shown). Figure 1 illustrates the b* values of oil samples after 39 days of storage at 35 °C. Most color changes were related to BHT. The yellowness of oil samples containing Exs and BHT enhanced significantly in comparison to that of the control, but no significant changes were observed for NLs. Hence, encapsulated phenolic compounds could maintain color by preventing the direct contact of color compounds of PGHE with soybean oil. There was also a positive correlation between phenolic compounds and b*value.
Fig. 1.
b* values of different samples on the 39th day of storage. Control (without phenolic compounds); Ex-500, Ex-750 and Ex-1000 (500, 750 and 1000 mg of free phenolic compounds of PGHE/kg oil, respectively); NL-500, NL-750 and NL-1000 (500, 750 and 1000 mg of incorporated phenolic compounds of PGHE/kg oil, respectively); BHT-100 and BHT-200 (100 and 200 mg of BHT/kg oil, respectively)
The increase of yellowness in RBD oil mostly occurs in soybean oil during the storage and it is known as “color reversion”. Color reversion is associated with oxidation and conversion of γ-tocopherol to tocored (2,7,8-trimethyl-2 (40,80,120-tridecyl 2)-chromane, 5, 6-quinone). Lipid peroxide radicals enhance these reactions (Shahidi 2005).
Similarly, Taghvaei et al. (2014) demonstrated that the yellowness of encapsulated olive leaf extract by Arabic gum and maltodextrin was lower than free olive leaf extract in soybean oil after 20 days of storage at 55 °C. It was concluded that the incorporation of the phenolic compounds in nanocarriers such as nanoliposomes has good potential for protecting them from adverse reactions, enhancing of their bioactivity and bioavailability and reducing their influence on the sensory properties of the food products by masking their undesirable effects (Spigno et al. 2013).
Conclusion
In the present study, the phenolic compounds of PGHE were incorporated into liposomes and their effect on oxidative stability of soybean oil was evaluated. According to the results, all produced liposomes had nanometer size, low PDI values and high zeta potential, indicating homogenous and stable systems. Average size showed significant change after incorporation of phenolic compounds. Moreover, the highest EE was observed for LNL-1000. The application of PGHE to retard soybean oil oxidation demonstrated that PV and TBA values of all samples increased in longer storage times. Ex-500 showed most potent antioxidant activity, but in higher concentrations, PGHE had pro-oxidant effect. Exs could retard oxidation more effectively in comparison to LNs, but using LNs enhanced the solubility and stability of phenolic compounds in soybean oil. Besides, the color of LNs containing oil samples was constant during the storage time. It was concluded that LN-500 could be used as a substitute for synthetic antioxidants to control oxidation in soybean oil. Finally, it could also be concluded that, the encapsulation of PGHE as an inexpensive and natural source of phenolic compounds in liposomes was an appropriate approach to solve the problems related to their direct application in food products and produce good food grade antioxidants.
Acknowledgements
This work was supported by the Research Council of Tarbiat Modares University.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Abedi E, Sahari MA, Barzegar M, Azizi MH. Optimisation of soya bean oil bleaching by ultrasonic processing and investigate the physico-chemical properties of bleached soyabean oil. Int J Food Sci Technol. 2015;50:857–863. doi: 10.1111/ijfs.12689. [DOI] [Google Scholar]
- AOCS . Official methods and recommended practices of the American Oil Chemist’s Society. 5. Champaign: AOCS; 2007. [Google Scholar]
- Arab Tehrany E, Kahn CJF, Baravian C, Maherani B, Belhaj N, Wang X, Linder M. Elaboration and characterization of nanoliposome made of soya; rapeseed and salmon lecithins: application to cell culture. Colloids Surf B. 2012;95:75–81. doi: 10.1016/j.colsurfb.2012.02.024. [DOI] [PubMed] [Google Scholar]
- Behgar M, Ghasemi S, Naserian A, Borzoie A, Fatollahi H. Gamma radiation effects on phenolics, antioxidants activity and in vitro digestion of pistachio (Pistachia vera) hull. Radiat Phys Chem. 2011;80:963–967. doi: 10.1016/j.radphyschem.2011.04.016. [DOI] [Google Scholar]
- Cardenia V, Waraho T, Rodriguez-Estrada MT, McClements DJ, Decker EA. Antioxidant and prooxidant activity behavior of phospholipids in stripped soybean oil-in-water emulsions. J Am Oil Chem Soc. 2011;88:1409–1416. doi: 10.1007/s11746-011-1807-y. [DOI] [Google Scholar]
- Chatterjee D, Bhattacharjee P. Comparative evaluation of the antioxidant efficacy of encapsulated and un-encapsulated eugenol-rich clove extracts in soybean oil: shelf-life and frying stability of soybean oil. J Food Eng. 2013;117:545–550. doi: 10.1016/j.jfoodeng.2012.11.016. [DOI] [Google Scholar]
- Codex Alimentarius Commission (1999) Report of the sixteenth session of the Codex Committee on fats and oils. http://www.codexalimentarius.org/codexhome/en/. Accessed 04 Dec 2013
- Fang Z, Bhandari B. Encapsulation of polyphenols—a review. Trends Food Sci Technol. 2010;21:510–523. doi: 10.1016/j.tifs.2010.08.003. [DOI] [Google Scholar]
- FAO (2013) The statistics division of the FAO. http://faostat3.fao.org. Accessed 12 July 2015
- Garavand F, Madadlou A, Moini S. Determination of phenolic profile and antioxidant activity of pistachio hull using HPLC-DAD-ESI-MS as affected by ultrasound and microwave. Int J Food Prop. 2017;20(1):19–29. doi: 10.1080/10942912.2015.1099045. [DOI] [Google Scholar]
- Ghorbanzade T, Jafari SM, Akhavan S, Hadavi R. Nano-encapsulation of fish oil in nano-liposomes and its application in fortification of yogurt. Food Chem. 2017;216:146–152. doi: 10.1016/j.foodchem.2016.08.022. [DOI] [PubMed] [Google Scholar]
- Goli AH, Barzegar M, Sahari MA. Antioxidant activity and total phenolic compounds of pistachio (Pistachia vera) hull extracts. Food Chem. 2005;92:521–525. doi: 10.1016/j.foodchem.2004.08.020. [DOI] [Google Scholar]
- Gortzi O, Lalas S, Chinou I, Tsaknis J. Reevaluation of bioactivity and antioxidant activity of Myrtus communis extract before and after encapsulation in liposomes. Eur Food Res Technol. 2008;226:583–590. doi: 10.1007/s00217-007-0592-1. [DOI] [Google Scholar]
- Gosangari SL, Watkin KL. Effect of preparation techniques on the properties of curcumin liposomes: characterization of size, release and cytotoxicity on a squamous oral carcinoma cell line. Pharm Dev Technol. 2012;17(1):103–109. doi: 10.3109/10837450.2010.522583. [DOI] [PubMed] [Google Scholar]
- Habeebullah SFK, Nielsen NS, Jacobsen C. Antioxidant activity of potato peel extracts in a fish-rapeseed oil mixture and in oil-in-water emulsions. J Am Oil Chem Soc. 2010;87:1319–1332. doi: 10.1007/s11746-010-1611-0. [DOI] [Google Scholar]
- Hasan M, Belhaj N, Benachour H, Barberi-Heyob M, Kahn CJF, Jabbari E, Linder M, Arab-Tehrany E. Liposome encapsulation of curcumin: physicochemical characterizations and effects on MCF7 cancer cell proliferation. Int J Pharm. 2014;461:519–528. doi: 10.1016/j.ijpharm.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Isailović BD, Kostić IT, Zvonar A, Đorđević VB, Gašperlin M, Nedović VA, Bugarski BM. Resveratrol loaded liposomes produced by different techniques. Innov Food Sci Emerg. 2013;19:181–189. doi: 10.1016/j.ifset.2013.03.006. [DOI] [Google Scholar]
- Judde A, Villeneuve P, Rossignol-Castera A, Le Guillou A. Antioxidant effect of soy lecithins on vegetable oil stability and their synergism with tocopherols. J Am Oil Chem Soc. 2003;80:1209–1215. doi: 10.1007/s11746-003-0844-4. [DOI] [Google Scholar]
- Lu Q, Lu PM, Piao JH, Xu XL, Chen J, Zhu L, Jiang JG. Preparation and physicochemical characteristics of an allicin nanoliposome and its release behavior. LWT Food Sci Technol. 2014;57:686–695. doi: 10.1016/j.lwt.2014.01.044. [DOI] [Google Scholar]
- Madrigal-Carballo S, Lim S, Rodriguez G, Vila AO, Krueger CG, Gunasekaran S, Reed JD. Biopolymer coating of soybean lecithin liposomes via layer-by-layer self-assembly as novel delivery system for ellagic acid. J Funct Foods. 2010;2:99–106. doi: 10.1016/j.jff.2010.01.002. [DOI] [Google Scholar]
- Maherani B, Arab Tehrany E, Kheirolomoom A, Geny D, Linder M. Calcein release behavior from liposomal bilayer; influence of physiochemical/mechanical/structural properties of lipids. Biochimie. 2013;95:2018–2033. doi: 10.1016/j.biochi.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Mohammadi A, Jafari SM, Faridi Esfanjani A, Akhavan S. Application of nano-encapsulated olive leaf extract in controlling the oxidative stability of soybean oil. Food Chem. 2016;190:513–519. doi: 10.1016/j.foodchem.2015.05.115. [DOI] [PubMed] [Google Scholar]
- Mohammadi A, Jafari SM, Assadpour E, Faridi Esfanjani A. Nano-encapsulation of olive leaf phenolic compounds through WPC-pectin complexes and evaluating their release rate. Int J Biol Macromol. 2016;82:816–822. doi: 10.1016/j.ijbiomac.2015.10.025. [DOI] [PubMed] [Google Scholar]
- Paiva-Martins F, Gordon MH, Gameiro P. Activity and location of olive oil phenolic antioxidants in liposomes. Chem Phys Lipids. 2003;124(1):23–36. doi: 10.1016/S0009-3084(03)00032-X. [DOI] [PubMed] [Google Scholar]
- Rafiee Z, Jafari SM, Alami M, Khomeiri M. Antioxidant effect of microwave-assisted extracts of olive leaves on sunflower oil. J Agric Sci Technol. 2012;14:1497–1509. [Google Scholar]
- Rafiee Z, Barzegar M, Sahari MA, Maherani B. Nanoliposomal carriers for improvement the bioavailability of high-valued phenolic compounds of pistachio green hull extract. Food Chem. 2017;220:115–122. doi: 10.1016/j.foodchem.2016.09.207. [DOI] [PubMed] [Google Scholar]
- Rajaei A, Barzegar M, Mohabati Mobarez A, Sahari MA, Hamidi Esfahani Z. Antioxidant, anti-microbial and antimutagenicity activities of pistachio (Pistachia vera) green hull extract. Food Chem Toxicol. 2010;48:107–112. doi: 10.1016/j.fct.2009.09.023. [DOI] [PubMed] [Google Scholar]
- Rashidinejad A, Birch EJ, Sun-Waterhouse D, Everett DW. Delivery of green tea catechin and epigallocatechin gallate in liposomes incorporated into low-fat hard cheese. Food Chem. 2014;156:176–183. doi: 10.1016/j.foodchem.2014.01.115. [DOI] [PubMed] [Google Scholar]
- Shahidi F. Bailey’s industrial oil and fat products. New York: Wiley; 2005. [Google Scholar]
- Spigno G, Donsì F, Amendola D, Sessa M, Ferrari G, Faveri DMD. Nanoencapsulation systems to improve solubility and antioxidant efficiency of a grape marc extract into hazelnut paste. J Food Eng. 2013;114:207–214. doi: 10.1016/j.jfoodeng.2012.08.014. [DOI] [Google Scholar]
- Taghvaei M, Jafari SM. Application and stability of natural antioxidants in edible oils in order to substitute synthetic additives. J Food Sci Technol. 2015;52(3):1272–1282. doi: 10.1007/s13197-013-1080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghvaei M, Jafari SM, Sadeghi Mahoonak A, Mehregan Nikoo A, Rahmanian N, Hajitabar J, Meshginfar N. The effect of natural antioxidants extracted from plant and animal resources on the oxidative stability of soybean oil. LWT Food Sci Technol. 2014;56:124–130. doi: 10.1016/j.lwt.2013.11.009. [DOI] [Google Scholar]
- Wink M. Functions and biotechnology of plant secondary metabolites. 2. London: Blackwell Publishing Ltd; 2010. [Google Scholar]
- Zhong Y, Shahidi F. Lipophilised epigallocatechin gallate (EGCG) derivatives and their antioxidant potential in food and biological systems. Food Chem. 2012;131:22–30. doi: 10.1016/j.foodchem.2011.07.089. [DOI] [Google Scholar]
- Zou L, Liu W, Liu W, Liang R, Li T, Liu C, Cao Y, Niu J, Liu Z. Characterization and bioavailability of tea polyphenol nanoliposome prepared by combining an ethanol injection method with dynamic high-pressure microfluidization. J Agric Food Chem. 2014;62:934–941. doi: 10.1021/jf402886s. [DOI] [PubMed] [Google Scholar]
- Zou L, Peng S, Liu W, Gan L, Liu W, Liang R, Liu C, Niu J, Cao Y, Liu Z, Chen X. Improved in vitro digestion stability of (−)-epigallocatechin gallate through nanoliposome encapsulation. Food Res Int. 2014;64:492–499. doi: 10.1016/j.foodres.2014.07.042. [DOI] [PubMed] [Google Scholar]