Abstract
Present study was carried out to assess the significances of solid state fermentation of peanut oil cakes (POC) by Aspergillus oryzae on in vitro bioavailability of minerals (iron, zinc and calcium) and cellular transport, retention and uptake from POC through Caco-2 cells. Bioavailability of iron, zinc and calcium of POC was examined by means of a combined simulated gastrointestinal digestion/Caco-2 cell system. Bio-augmentation of minerals of fermented POC attributed a positive, statistically significant increased influence on minerals retention, transport and uptake values when compared with that of respective inorganic salts as reference. Results revealed increased cellular ferritin content from fermented POC digests than the digests of free form of respective inorganic salt. In prospect of the present investigation the fermented POC samples showed significantly higher iron, zinc and calcium bioavailability and enormous possible health benefits.
Keywords: Solid state fermentation, Caco 2 cell, In vitro, Mineral bioavailability, Zinc, Ferritin
Introduction
Ingredients of natural origin gained better acceptability by the global community over their synthetic counterparts (Górnas and Rudzinska 2016). Therefore, organic residues of various agricultural products are utilized as potential raw materials in various bioprocesses as they are excellent substratum for the growth of microorganisms and they also supply essential nutrients for the growth and nourishment of the microorganisms (Ramachandran et al. 2007). Peanut (Arachis hypogaea L.) is one of the widely utilized, highly valuable principle oil seed crop which possess rich nutritional constituents for human as well animal nutrition (Wang et al. 2017; Zhang 2016). The oil extracted peanut press oil cake (POC) or meals are highly nutritionally valuable due to its high protein content (47–55%). However, it is currently underutilized due to its inferior functional properties (Shi et al. 2014). POC obtained from peanut oil extraction are auspicious source of food proteins due to their prevalent accessibility, high protein content, low amounts of antinutritive compounds and no toxicity (Pickardt et al. 2015). Being a rich source of protein, oil cakes also have immense potential in several food based applications as an ideal candidate for food supplementation. Besides from the major source of proteins, peanut seeds and obtained oil press cakes are also rich in various micronutrients which are well known and reported by several authors (Martin et al. 2016; Arya et al. 2016; Zhang 2016).
Iron, zinc and calcium are fundamental transition metals and these elements are essential for all life and vital for cellular survival (Lane et al. 2015). Peanuts and obtained meals are the rich source of trace elements including iron, zinc and calcium which are responsible for the intake of these minerals (Shi et al. 2014). Bioavailability of these minerals is directly affected by the presence of anti-nutritional factors such as phytate, phytic acid, oxalic acid and complex polysaccharides (Gupta et al. 2015). It is well known and reported that solid state fermentation (SSF) using filamentous fungi (mainly Aspergillus oryzae) helps to reduce the anti-nutritional and toxic factors in the raw materials by making the proteins and minerals complex with phytochemicals readily available (Guan et al. 2015; Tokuoka et al. 2010; Chancharoonpong et al. 2012; Adegbehingbe 2015). Also, it is an alternative process to improve the nutritional aspects for a great variety of legumes and cereals, or combination of them, and helpful to obtain edible products with palatable sensorial characteristics and formation of shorter chain compounds with lower molecular mass (Xiao et al. 2015). Therefore, SSF widely used to increase functional properties as well as food applications of many cereals, legumes and their byproducts (Chawla et al. 2017).
For studies of intestinal mineral uptake, Caco-2 cell line in combination with simulated gastro-intestinal digestion is widely used as an alternative to human and animal studies. Caco-2 characteristics of small intestinal absorptive enterocytes (Hidalgo et al. 1989). Estimation of cellular iron stores in the form of a protein–iron complex known as ferritin is a sensitive and proportional measure of iron uptake by the cell (Glahn et al. 2000).
However, no attempt has been made so far to evaluate in vitro bioavailability of minerals of fermented POC through Caco-2 cell culture model. Therefore, the present study was designed with following objectives. (i) Preparation of fermented POC samples (ii) Mineral estimation of fermented POC samples and (iii) Estimation of in vitro bioavailability of minerals (i.e. iron, zinc and calcium) and ferritin content of fermented POC samples using Caco-2 cell culture in comparison with free form of salt.
Materials and methods
Materials
Peanut (HNG-10) was obtained from Pilimandori, Fatehabad, Haryana (India). Aspergillus oryzae (MTCC 3107) was procured from Microbial Type Culture Collection (MTCC), Institute of Microbial Technologies Chandigarh, India. Xylan (from Birchwood), ferrous sulphate heptahydrate, zinc sulphate heptahydrate, calcium carbonate, α-amylase, p-nitrophenol-b-D-glucoside, β-glucosidase, potato dextrose agar and Czapek-dox medium were procured from Sigma Aldrich Co. (St. Louis, USA).
Gastrointestinal digestion
Alpha-amylase, human pancreatic lipase, colipase, cholesterol esterase, phospholipase A2, mucin, bovine serum albumin, pepsin (2950 units mg/L of protein), pancreatin, glucuronic acid, glucosamine hydrochloride, ammonium sulphate and taurocholic acid sodium salt hydrate were purchased from Sigma Chemical Co (Madrid, Spain). Disodium hydrogen phosphate, monosodium dihydrogen phosphate, calcium chloride, sodium chloride, trichloroacetic acid, glycine and ammonium persulphate were purchased from Hi-Media Laboratories Pvt. Ltd., Mumbai, India. Chemicals used were of AR grade. Triple distilled, cellular grade water and acid washed glassware were used throughout the experiments.
Cell culture
Human colon adenocarcinoma, Caco-2 cells were obtained from National Centre for Cell Science, Pune (India). Dulbeccos Modified Eagles Medium DMEM, l-glutamine, dimethyl sulfoxide (DMSO), Trypsin EDTA solution (0.25%), phenol red and streptomycin sulfate salt were procured from Sigma Aldrich, St. Louis, MO, USA. Human ferritin ELISA kit (SEA518Hu 96 Tests) from Cloud Clone-Corp. Houston, USA. Plastic dishes, plates and transwell were obtained from Corning (Corning, NY, USA).
Preparation of peanut oil cakes (POC)
Peanut variety HNG-10 samples were brought to the expeller of local market of Sirsa, India for preparation of peanut oil cakes. Peanut cakes were grinded using blender (Braun AG Frankfurt A.M. Mx 32, Germany) to obtain fine powder and stored in air tight container at 4–7 °C temperature.
Microbial strain and substrate
Fungal strain i.e. Aspergillus oryzae (MTCC 3107) was used for solid state fermentation in present study. This fungal strain is generally recognized as safe (GRAS), was cultivated and maintained on potato dextrose agar (PDA) plates. Peanut oil cakes of variety HNG-10 were used as the substrate for the fungi.
Methods
Preparation of inoculum
The fungal culture of Aspergillus oryzae, maintained on slants of potato dextrose agar were transferred to fresh PDA plates before starting of each experiment. The inoculated plates were incubated at 30 °C for 144 h. Spore suspension was prepared in sterilized cellular grade water having a spore count of approximately 1 × 106 spores/ml.
Fermentation conditions
Substrate was first washed and dried in a hot air oven (Narang Scientific Instruments, NSW 143, Ambala, India) at 30 °C before use, then it was grinded to fine powder in a grinder to make suitable for fermentation. Fifty gram of grinded sample was taken in 500 ml Erlenmeyer flasks and then soaked in 50 ml Czapek-dox medium [NaNO3 (2.5 g/L), KH2PO4 (1.0 g/L), KCl (0.5 g/L) and MgSO4·2H2O (0.5 g/L)] at room temperature (25–30 °C) overnight. After decanting the excess media if any, the substrate was autoclaved (Vertical autoclave, Calton, NSW-227, India) and then subsequently cooled at room temperature before inoculation. The autoclaved substrate was inoculated with 5.0 ml spore suspension (1 × 106 spores/ml) of fungal strain, mixed properly and incubated in BOD incubator (Calton, NSW-152, India) for 0, 48, 72, 96, 120 and 144 h, respectively at 30 °C. The non-fermented substrate as raw material was prepared without the addition of spore suspension.
Mineral estimation
Calcium, iron and zinc content of fermented POC samples were estimated in atomic absorption mode using Atomic Absorbance Spectrophotometer (AAS) (AA-7000, Shimadzu, Tokyo, Japan) as described by AOAC (2005). Samples were subjected to ashing (at 550 °C for 8 h), solubilized in tri acid mixture and heated for complete dissolution. All the samples were diluted to a suitable dilution before analysis by AAS.
In-vitro bioavailability of minerals
Method described by Chawla et al. (2017) was applied to evaluate the in vitro bio accessibility of iron, zinc and calcium in fermented POC samples in comparison with free form of inorganic salts (Table 1). The simulated gastro-intestinal model consisted of two phases: gastric and intestinal. Compositions and concentrations of various inorganic and organic solutions of the in vitro digestion are given in table (Fernando et al. 2007).
Table 1.
Composition and concentration of the various synthetic juices utilized during in vitro digestion
| Solution↓ | Saliva | Gastric juice | Duodenal juice | Bile |
|---|---|---|---|---|
| Inorganic solution | 1 ml KCl (89.6 g/L) | 1.57 ml NaCl (175.3 mg/L) | 4 ml NaCl (175.3 mg/L) | 3.0 ml NaCl (175.3 g/L) |
| 1 ml KSCN (20 g/L) | 0.3 ml NaH2PO4 (88.8 mg/L) | 1 ml NaHCO3 (84.7 mg/L) | 6.83 ml NaHCO3 (84.7 g/L) | |
| 1 ml NaH2PO4 (88.8 g/L) | 0.92 ml KCl (89.6 g/L) | 1 ml KH2PO4 (8 g/L) | 0.42 ml KCl (89.6 g/L) | |
| 1 ml Na2HPO4 (57 g/L) | 0.18 ml CaCl2·2H2O (22.2 | 0.63 ml KCl (89.6 g/L) | 20.0 μl HCl (37%) | |
| 0.17 ml NaCl (175.3 g/L) | 1.0 ml NH4Cl (30.6 g/L) | 1 ml MgCl2 (5 mg/L) | ||
| 0.18 ml NaOH (40 g/L) | 0.83 ml HCl (37%) | 18.0 μl HCl (37%) | ||
| Organic solution | 0.8 ml urea (25 g/L) | 1 ml glucose (65 g/L) | 0.4 ml urea (25 g/L) | 1 ml urea (25 g/L) |
| 1 ml glucuronic acid (2 g/L) | ||||
| 0.34 ml urea (25 g/L) | ||||
| 1 ml glucosamine hydrochloride (33 g/L) | ||||
| Add to mixture organic + inorganic solution | 14.5 mg α-amylase | 0.1 g BSA | 0.9 ml CaCl2·2H2O (22.2 g/L) | 1 ml CaCl2·2H2O (22.2 g/L) |
| 1.5 mg uric acid | 0.1 g pepsin | 0.1 g BSA | 0.18 g BSA | |
| 5.0 mg mucin | 0.3 g mucin | 0.3 g pancreatin | 0.6 g bile | |
| Pancreatic lipase (1 units) | 40 mg trypsin | |||
| 1.25 μg colipase | 40 mg chymotrypsin | |||
| Cholesterol esterase (1 units) | ||||
| 5.0 μl phospholipase A2 | ||||
| 1.99 mg sodium taurocholate | ||||
| pH | 6.5 ± 0.2 | 1.07 ± 0.07 | 7.8 ± 0.2 | 8.0 ± 0.2 |
The inorganic and organic solutions were augmented to 50 ml with distilled water
Simulated gastrointestinal digestion
Briefly, 5 g of each POC samples were transferred to a flask with saliva solution (9 ml, pH 6.5) containing organic, inorganic components and α-amylase (700 mg/L of saliva solution) after which the samples were incubated in water bath at 37 °C, 95 rpm for 5 min. Gastric juice (13.5 ml) with organic, inorganic solutions, mucin (6 g/L of gastric juice), bovine serum albumin (2 g/L of gastric juice), and pepsin (2 g/L of gastric juice) from porcine stomach was added to the flask, pH was adjusted to 1.1 with HCl and subsequently incubated for 1 h at 37 °C. Duodenal juice (25 ml, organic and inorganic solutions containing porcine pancreatin 6 g/L of duodenal juice) and bile solutions (9 ml, containing bile salt 12 g/L of bile solution), prepared fresh, were supplemented after neutralization of the pH (7.8) and the human pancreatic lipase (5 units), colipase (25 mg), cholesterol esterase (10 units), phospholipase A2 (50 ml) and taurocholate salts (39.8 mg) were added. Final volume of approximately 15 ml was then incubated up to 3 h at 37 °C and subjected to centrifugation at 5000 rpm/30 min in Amicon UF centrifuge tube (MW cutoff 10 kDa).Inorganic salts were also treated same and the amount used was as equal to mineral content (i.e. iron, zinc and calcium) present in POC sample taken. The % bio accessibility of minerals under simulated gastro-intestinal conditions in permeate was determined by AAS. Bioavailability of minerals (iron, zinc and calcium) was calculated from the amount of the nutrient (iron) that had passed the ultrafiltration membrane in proportion to the total mineral content of the sample. Bioavailability was calculated as:
where,
D = Mineral content in the dialysate (permeate) and
C = Mineral content of sample
Caco-2 cell culture (transwell assay)
The selection of medium and the procedure to maintain the cell cultures and cell seeding were performed according to method as described by Chawla et al. (2017). Cells were cultured in growth medium containing antibiotic-free Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% v/v heat inactivated FBS, 2 mM l-Glutamine, 1% v/v non-essential amino acids and antibiotics (penicillin 100 U/ml, streptomycin 30 μg/ml, amphotercine 25 μg/ml). They were then incubated in CO2 incubator (Thermo Fisher Scientific, Mumbai, India) at 37 °C in humidified atmosphere (95%) containing 5% CO2. The growth medium was changed at every alternate day, and the cells were passaged at nearly 80% confluence, achieved 6–8 days after seeding a density of 1 × 106 cells/flasks (25 cm2 flasks). Cell sub culturing was executed at split ratio of 1:3 by trypsinization (0.5% trypsin and 0.05% EDTA). Cells used for further assay were of 43 passages.
Preparation of the 6-well culture plates with cell monolayers
For transport studies, cells at a density of 50,000 cells/well were seeded into 24 mm polyester membrane (pore size of 0.4 µm) inserts (six welled sterile transwell polystyrene plates) dividing an apical or a donor-like compartment from a basal or acceptor compartment. The cells were allowed to differentiate in CO2 incubator at 37 °C, 5% CO2 and 95% relative humidity. Spent culture medium was aspirated every second day and cells were washed three times with PBS till usage. The monolayer became confluent after 4–5 days and the cells were allowed to differentiate for another 11 days before performing trans epithelial transport experiments.
Assessment of cell monolayer integrity
The integrity of the monolayer was monitored before and after completion of the experiment using microscopic examination and phenol red test as per the method of Jovov et al. (1991). The morphology, surface area covered, presence of mucus or contamination were checked repeatedly. After attaining 95–100% confluency the integrity of the Caco2 monolayer was confirmed by phenol red dye test using the method of Szlapka et al. (2009). Following washing, 2 ml each of DMEM with phenol red and PBS without phenol red were placed in the apical and basal chambers respectively. After incubation at 37 °C in CO2 incubator for 1 h, 100 µl solutions were collected from both the chambers and the amount of phenol red leakage in the basolateral medium through intercellular spaces was checked automated ELISA plate reader (Epoch Bio Tek, Winooski, USA) at 558 nm. The experiments were conducted only after the culture has attained 95–100% confluent.
Transport studies of minerals
Previously washed Caco-2 cells were treated at the apical surface with 2 ml each of growth medium with ferrous sulphate and growth medium with dialysate (iron concentration adjusted to 50 μM) into different culture wells. Likewise, 2 ml of growth medium was added into the basal compartment. After incubation (3 h at 37 °C in 5% CO2 with 95% relative humidity), the basal medium was aspirated off for determination of iron transport across the monolayer and the cells were returned to the incubator in fresh medium for additional 22 h to allow ferritin synthesis. Mineral retention, transport and uptake (retention plus transport) by the cells were determined by measuring calcium, iron and zinc contents in blank, soluble mineral fraction, cell monolayer and basolateral content using AAS.
Mineral estimation
Iron, zinc and calcium content of the cell monolayer (retention) and basolateral chamber (transport) were measured using AAS. For determination of mineral content in the sample (before digestion), soluble fraction (apical solution), blank (HBSS), basal solution and in cell homogenates, all samples were subjected to ashing (550 °C for 8 h). Suitable dilutions of the digests were made using triple distilled water for analysis by AAS.
Ferritin content estimation
Intracellular ferritin formation in the Caco-2 cells was measured by sandwich ELISA using human ferritin ELISA kit (DRG, GmbH, Germany). After incubation, monolayer was washed with PBS and harvested using trypsin–EDTA solution. Subsequently, cells were collected using 2 ml of de-ionized water for 3 min and then subjected to sonication at 4 °C for 2 min with a pulse rate of 5 s. 100 µl of aliquots of the sonicated caco-2 monolayer were used for ferritin determination.
An anti-ferritin immunoglobulin-G-coated plate was incubated for 1 h with samples, standards and controls. Avidin conjugated to Horseradish Peroxidase (HRP) was added to each microplate well. Following washing, the TMB substrate was added and the plate was incubated for additional 10 min (in the dark). The reaction was terminated by addition of HCl, and the change in colour in case of wells containing ferritin was measured spectrophotometrically by means of a microplate reader (Tecan, GmbH) at a wavelength of 450 nm ± 10 nm. Ferritin concentration in the samples was determined by comparing the O.D. of the samples to that of the standard curve.
Statistical analysis
Means and standard error mean (SEM) were calculated using Microsoft Excel, 2013 (Microsoft Corp., Redmond, WA). Significant difference between values was verified by one way or two way analysis of variance and comparison between means was made by critical difference value (Snedecor and Cochran 1994).
Results and discussion
Mineral content of fermented POC samples
Mineral content (iron, zinc and calcium) of fermented POC samples was estimated using AAS and the results are depicted in Table 2. Fermentation significantly (P < 0.05) increased the mineral content of POC samples in comparison with raw POC. Our results were in line with the findings of Chawla et al. (2017) who reported increased mineral content in bio-augmented black eyed pea seed flour.
Table 2.
Mineral content of control (unfermented) and fermented POC sample during solid state fermentation
| Fermentation time (h) | Fe | Zn | Ca |
|---|---|---|---|
| Control (0 h) | 34.2896 ± 0.71a | 42.1851 ± 0.63b | 887.7419 ± 5.96c |
| 48 h | 36.5967 ± 0.11a | 42.3936 ± 0.96b | 890.0864 ± 1.82c |
| 72 h | 36.8632 ± 0.36a | 42.2415 ± 0.74b | 890.0869 ± 4.82c |
| 96 h | 36.8963 ± 0.39a | 42.8754 ± 0.43b | 890.2357 ± 3.89c |
| 120 h | 36.2859 ± 0.74a | 42.4967 ± 0.21b | 890.7267 ± 3.96c |
| 144 h | 36.5747 ± 0.16a | 42.6529 ± 0.39b | 890.6344 ± 3.75c |
Data are presented as mean ± SEM (n = 3)
a–cMeans within rows with different lowercase superscript are significantly different (P < 0.05) from each other
In vitro bioavailability of fermented POC samples
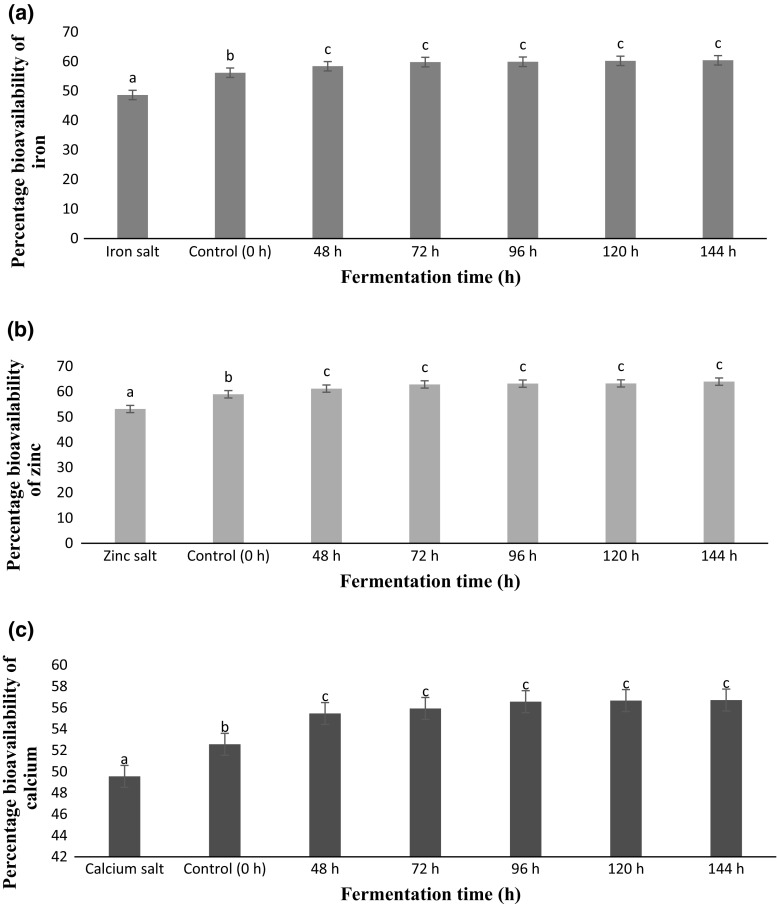
Mineral (iron, zinc and calcium) bioavailability of food and food products always depends upon the intrinsic components of the food (i.e. fat, proteins, carbohydrates, fibers etc.), interactions of these components with minerals and the level of digestion of the ingested food. In-vitro bioavailability of minerals (iron, zinc and calcium) of fermented POC samples (0–144 h) in comparison with free form of inorganic salts (i.e. FeSO4, ZnSO4 and CaCO3) was determined using simulated gastrointestinal digestion model system and results are depicted in Fig. 1a–c, respectively. Results obtained after simulated gastrointestinal digestion clearly revealed that fermentation of POC samples with A. oryzae increased the mineral bioavailability. Significant (P < 0.05) increase in terms of mineral bioavailability and digestibility was observed in all the fermented samples in comparison with free inorganic salts. Low mineral bioavailability of free inorganic salts was due to co-precipitation at intestinal pH thereby, further reducing its bioavailability (Chawla et al. 2017). Reduction in digestibility of free form of mineral salts is in line with the findings of Hoz et al. (2014) who suggested that proteins have the inherent ability to bind minerals with them. It has also been reported that plants have a great affinity to absorb minerals from the soil and form the mineral complexes. For instance, phytosiderophore, a hexadentate ligand with amino and carboxy groups co-ordinates with iron absorbed from the soil to form phytosiderophore-ferric complex (Wiren et al. 2000) contributing to significant increase in iron bioavailability.
Fig. 1.
In vitro bioavailability of a iron, b zinc and c calcium from fermented POC samples in comparison mineral salts. Data are presented as mean ± SEM (n = 3). a–cMeans within column with different lowercase superscript are significantly different (P < 0.05) from each other. *Uptake = transport plus retention
Iron retention, transport and uptake by Caco-2 cells (transwell assay)
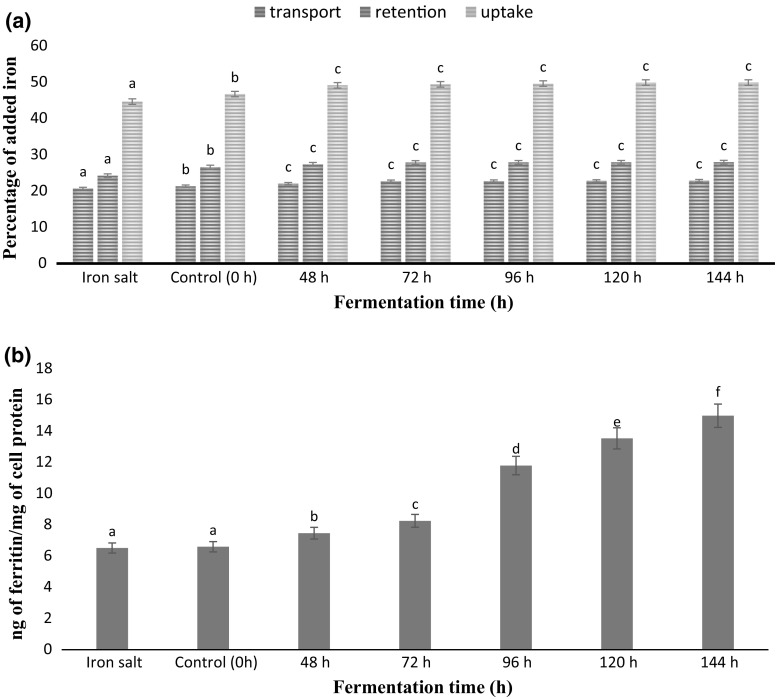
According to Camara et al. (2005) the percentage efficiency of mineral retention, transport and uptake can be used as bioavailability predictors for the trace elements. Peanuts are a fairly rich source of dietary iron (Fig. 2a). The iron content in dialysate filtrates obtained after simulated gastrointestinal digestion was measured by AAS. Final volume of filtrate equivalent to 50 μM concentration of iron was loaded in the upper chamber of transwell plates to carry out retention, transport and uptake studies by Caco-2 cells. Results of mineral retention, transport and uptake clearly revealed that bio-augmentation significantly (P < 0.05) increased in the value of all the parameters studied in comparison with free form of salt. This decrease in retention, transport and uptake of inorganic mineral salts might be due to precipitation of non heme iron (as ferrous or ferric form) which restricts their solubility at intestinal pH 7–8 and hence subsequent absorption in the duodenum. Our results were in line with the results of Conrad and Umbreit (2002), Chawla et al. (2017) who reported the limited solubility of iron salts at intestinal pH makes them unavailable for absorption. The improved absorption of iron in the form of phytosiderophore-ferric complex already existing in POC powder could be the contributor for its increased transport, retention and uptake. May et al. (1978) also discovered that complexing iron with organic ligands such as amino acids, carbohydrates, proteins, etc. enhances its solubility and hinders precipitation at intestinal pH. Similar findings were reported by Chawla et al. (2017) in case of fermented blackeyed pea seed flour according to which the presence of proteins in the intestine increased the uptake of iron. Laparra et al. (2008) also reported that the low molecular weight peptides generated on digestion could bind mineral ions which keep them in solution and eventually enhance their uptake by Caco-2 cells.
Fig. 2.
a Iron transport, retention and uptake* in Caco-2 cells from fermented POC samples with increasing time of fermentation in comparison with free iron salt b Ferritin synthesis in Caco-2 cells from fermented POC samples with increasing time of fermentation in comparison with free iron salt. Data are presented as mean ± SEM (n = 3). a–fMeans within column with different lowercase superscript are significantly different (P < 0.05) from each other. *Uptake = transport plus retention
Ferritin synthesis
According to study of Nikolaus and Peter (2015) and Chawla et al. (2017) increase in ferritin content of intestinal cells was a significant characteristic to increased intracellular iron levels as the cells are able to synthesize ferritin in response to the iron entering into the intestinal cells. Therefore, ferritin formation in the cells was used as an indicator of iron bioavailability and ferritin content of intestinal cells was estimated using human ferritin ELISA kit. Ferritin concentration in cells added with dialysates of digested fermented POC samples in comparison to dialysate containing iron in its free form was expressed in terms of iron uptake which is the ratio of ferritin and cell protein (ng ferritin/mg cell protein) (Fig. 2b). Results clearly shown that the ferritin content/cell protein ratios in Caco-2 cells in the presence of iron from fermented POC significantly increased (P < 0.05) than that from free form of iron. Therefore, the findings of present research clearly revealed that the digestion product of fermented POC improved iron uptake by increasing ferritin content synthesis in intestinal cells. All the POC samples showed significantly (P < 0.05) increased ferritin content with increasing days of fermentation, respectively. Moreover, according to study of Etcheverry et al. (2004) it was confirmed that the effect of sample composition, such as the presence of protein enhanced the iron digestibility which resulted in enhanced cellular iron uptake as well as ferritin synthesis. This increase might be due to the formation of peptides by the action of filamentous fungi used for the fermentation on the protein component of POC which could have eventually complexed with the available minerals. Therefore, it could be inferred from the results obtained that the developed product carry higher amount of iron per gram POC sample. Similar findings were reported by Chawla et al. (2017) in case of fermented blackeyed pea seed flour samples.
Zinc uptake and transport by Caco-2 cells
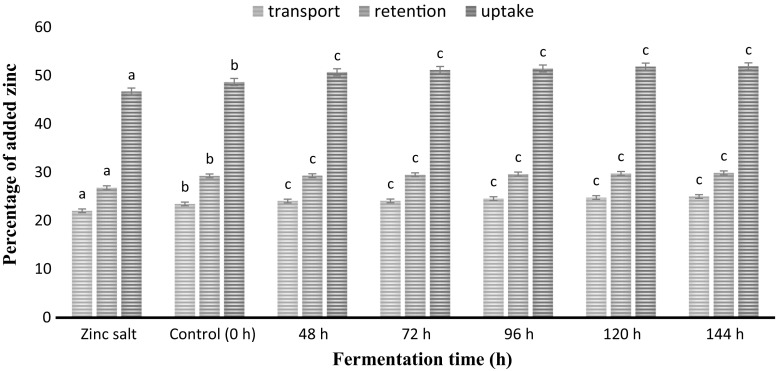
Similarly the zinc content in obtained filtrate was measured and the volume equivalent to 50 μM of zinc concentration was loaded in the upper chamber of transwell plates and results of cellular zinc transport, retention and uptake were depicted in Fig. 3. Obtained results clearly showed that there was significant (P < 0.05) increase in the values of cellular zinc transport, retention and uptake of fermented POC samples as compared with free form of zinc salt. Improved cellular zinc uptake was in concordance with the results obtained for iron concluding that bioavailability and intestinal absorption of zinc is potentially affected by protein content of fermented POC samples. Moreover, dialysates of fermented POC sample showed the presence of of higher amount of digested protein (19.76 μg protein/ml of filtrate) as compared to zinc salt fortified SYC (5.75 μg protein/ml of filtrate which could be the reason for improved cellular zinc transport, retention and uptake, respectively. This finding is well supported by the findings of Garcia-Nebot et al. (2013) who reported that cellular protein has significant role in mineral bioavailability and intestinal absorption. Also, Fleming et al. (1997) reported that the proteins were able to transport ions such as iron, zinc, copper and magnesium in the divalent state which ensured entry of the element into the cells by a non-receptor-mediated uptake system. Similar findings for increased cellular zinc uptake were reported by Chawla et al. (2017) in case of fermented blackeyed pea seed flour samples.
Fig. 3.
Zinc transport, retention and uptake* in Caco-2 cells from fermented POC samples with increasing time of fermentation in comparison with free zinc salt. a–cMeans within column with different lowercase superscript are significantly different (P < 0.05) from each other. *Uptake = transport plus retention
Calcium uptake and transport by Caco-2 cells
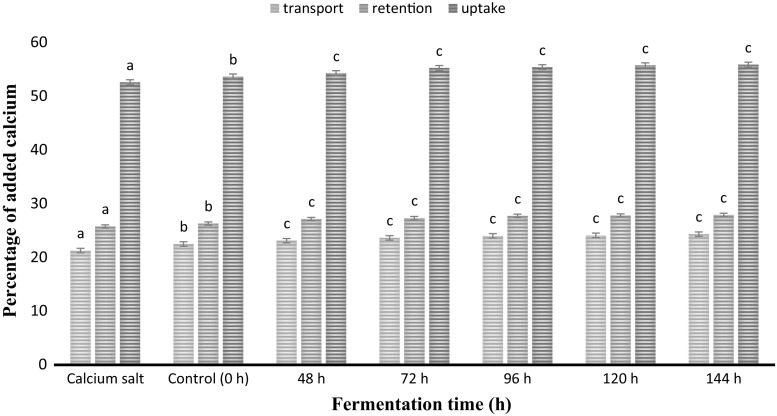
Calcium is a vital element for functioning of human body system. Garcia-Nebot et al. (2013) revealed that two important mechanisms are involved for the calcium absorption in human small intestine i.e. a transcellular active transport process which is situated largely in the duodenum and upper jejunum and a paracellular which is a passive process that functions throughout the length of the intestine. Despite this transcellular process consist of three major phases which are entry across the brush border, intracellular diffusion, and extrusion, respectively. Hence, Intestinal calcium absorption is accomplished through paracelluar pathway facilitated by generation of calcium gradient between plasma and lumen by passive diffusion across the intercellular tight junction (Kaushik et al., 2014). Also, it has been well described that vigorous absorption of minerals by the plants and seeds includes the action of a specific carrier compound present cells’ plasma membranes of plant body across which mineral transportation occurs. These carrier compounds are the transport proteins that are known as permesses. Carrier molecules present in the membrane forms bond with the minerals present outside in form of ions, resulting a carrier-ion-complex, which can move across the membrane (Pendias, 2004). Results clearly illustrated that fermented POC showed significantly (P < 0.05) increased transport, retention and uptake of calcium in comparison with free form of calcium salt (Fig. 4). As reported earlier in case of cellular zinc uptake, food protein and cellular protein play a potential role during calcium transport, retention and uptake. During simulated gastrointestinal digestion, proteins of the fermented POC samples might have broken down into smaller peptides, bind calcium to form complexes which ultimately improved the solubility of calcium at intestinal pH (Miller and Berner 1989).
Fig. 4.
Calcium transport, retention and uptake* in Caco-2 cells from fermented POC samples with increasing time of fermentation in comparison with free calcium salt. a–cMeans within column with different lowercase superscript are significantly different (P < 0.05) from each other
Conclusion
There is no doubt that SSF attributes considerably improvement in nutritional values of products. Fungal strain was incubated with substrate (POC) for 144 h at 30 °C and effect of SSF on mineral content and in vitro mineral (iron, zinc and calcium) bioavailability of POC has been studied. Combination of simulated gastrointestinal method and in vitro Caco-2 cell culture model (transwell assay) was used for the estimation of percentage bioavailability and cellular transport retention and uptake of minerals, respectively. Findings from present research revealed significant (P < 0.05) increased digestibility and mineral bioavailability in SSF fermented POC samples in comparison with unfermented samples. SSF also acquire part of credit in significant (P < 0.05) increased mineral uptake, retention and transport through Caco-2 cells as compared to respective inorganic mineral salts. Hence, fermentation of POC attributed a positive statistically significant increased influence on minerals retention, transport and uptake values when compared with that of respective inorganic salts as reference. Results revealed increased cellular ferritin content from fermented POC digests than from digests of free form of respective inorganic salt
Acknowledgements
The support by Department of Biotechnology, Chaudhary Devi Lal University, Sirsa, Haryana, India is gratefully acknowledged.
Abbreviations
- SSF
Solid state fermentation
- POC
Peanut oil cakes
- AAS
Atomic absorption spectrophotometer
- DMEM
Dulbecco’s Modified Eagles Medium
- PBS
Phosphate buffer saline
Contributor Information
Pardeep Kumar Sadh, Phone: +91-9728555388, Email: pardeep.sadh@gmail.com.
Prince Chawla, Phone: +91-9416547143, Email: princefoodtech@gmail.com.
Latika Bhandari, Phone: +91-8607706515, Email: latikabhandari1990@gmail.com.
Ravinder Kaushik, Phone: +91-9416962729, Email: ravinder_foodtech2007@rediffmail.com.
Joginder Singh Duhan, Phone: +91-1666 243147, Email: duhanjs68@gmail.com.
References
- Adegbehingbe KT. Effect of starter cultures on the anti-nutrient contents, minerals and viscosity of ogwo, a fermented sorghum–Irish potato gruel. Int Food Res J. 2015;22:1247–1252. [Google Scholar]
- AOAC International . Official methods of analysis. 18. Gaithersburg: AOAC International; 2005. [Google Scholar]
- Arya SS, Salve AR, Chauhan S. Peanuts as functional food: a review. J Food Sci Technol. 2016;53:31–41. doi: 10.1007/s13197-015-2007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camara F, Amaro MA, Barbera R, Clemente G. Bioaccessibility of minerals in school meals: comparison between dialysis and solubility methods. Food Chem. 2005;92:481–489. doi: 10.1016/j.foodchem.2004.08.009. [DOI] [Google Scholar]
- Chancharoonpong C, Hsieh PC, Sheu CS. Production of enzyme and growth of Aspergillus oryzae S. on soybean koji. Int J Biosci Biochem Bioinforma. 2012;2:228–231. [Google Scholar]
- Chawla P, Bhandari L, Sadh PK, Kaushik R. Impact of solid state fermentation (Aspergillus oryzae) on functional properties and mineral bioavailability of black eyed pea (Vigna unguiculata) seed flour. Cereal Chem. 2017;94(3):437–442. doi: 10.1094/CCHEM-05-16-0128-R. [DOI] [Google Scholar]
- Conrad M, Umbreit J. Pathways of iron absorption. Blood Cells Mol Dis. 2002;29:336–355. doi: 10.1006/bcmd.2002.0564. [DOI] [PubMed] [Google Scholar]
- Etcheverry P, Miller DD, Glahn RP. A low molecular weight factor in human milk whey promotes iron uptake by Caco-2 cells. J Nutr. 2004;134:93–98. doi: 10.1093/jn/134.1.93. [DOI] [PubMed] [Google Scholar]
- Fernando GL, Begon˜a OA, Carmen HB, Inmaculada BN, Bele´n PS, Silvia BG. In vitro bioaccessibility of carotenoids and tocopherols from fruits and vegetables. Food Chem. 2007;102:641–648. doi: 10.1016/j.foodchem.2006.05.043. [DOI] [Google Scholar]
- Fleming MD, Trenor CC, Su MA, Foernzler D, Beier DR, Dietrich WF. Microcytic anemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nature Genet. 1997;16:383–386. doi: 10.1038/ng0897-383. [DOI] [PubMed] [Google Scholar]
- Garcia-Nebot MJ, Barbera R, Alegria A. Iron and zinc bioavailability in Caco-2 cells: influence of caseinophosphopeptides. Food Chem. 2013;138:1298–1303. doi: 10.1016/j.foodchem.2012.10.113. [DOI] [PubMed] [Google Scholar]
- Glahn RP, Rassier M, Goldman MI, Lee OA, Cha J. A comparison of iron availability from commercial iron preparations using an in vitro digestion/Caco-2 cell culture model. J Nutr Biochem. 2000;11:62–68. doi: 10.1016/S0955-2863(99)00078-9. [DOI] [PubMed] [Google Scholar]
- Górnaś P, Rudzińska M. Seeds recovered from industry by-products of nine fruit species with a high potential utility as a source of unconventional oil for biodiesel and cosmetic and pharmaceutical sectors. Ind Crop Prod. 2016;83:329–338. doi: 10.1016/j.indcrop.2016.01.021. [DOI] [Google Scholar]
- Guan G, Zhang Z, Ding H, Li M, Shi D, Zhu M, Xia L. Enhanced degradation of lignin in corn stalk by combined method of Aspergillus oryzae solid state fermentation and H2O2 treatment. Biomass Bioenergy. 2015;81:224–233. doi: 10.1016/j.biombioe.2015.07.008. [DOI] [Google Scholar]
- Gupta RK, Gangoliya SS, Singh NK. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J Food Sci Technol. 2015;52:676–684. doi: 10.1007/s13197-013-0978-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo IJ, Borchardt RT, Raub TJ. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterol. 1989;96:736–749. doi: 10.1016/S0016-5085(89)80072-1. [DOI] [PubMed] [Google Scholar]
- Hoz L, Ponezi AN, Milani RF, Silva VSN, Souza SA, Bertoldo-Pacheco MT. Iron-binding properties of sugar cane yeast peptides. Food Chem. 2014;142:166–169. doi: 10.1016/j.foodchem.2013.06.133. [DOI] [PubMed] [Google Scholar]
- Jovov B, Wills NK, Lewis SA. A spectroscopic method for assessing confluence of epithelial cell cultures. Am J Physiol. 1991;261:C1196–C1203. doi: 10.1152/ajpcell.1991.261.6.C1196. [DOI] [PubMed] [Google Scholar]
- Kaushik R, Sachdeva B, Arora S, Kapila S, Wadhwa BK. Bioavailability of vitamin D2 and calcium from fortified milk. Food Chem. 2014;147:307–311. doi: 10.1016/j.foodchem.2013.09.150. [DOI] [PubMed] [Google Scholar]
- Lane DJR, Merlot AM, Huang MLH, Bae DH, Jansson PJ, Sahni S, Kalinowski DS, Richardson DS. Cellular iron uptake, trafficking and metabolism: key molecules and mechanisms and their roles in disease. Biochim Biophys. 2015;1853:1130–1144. doi: 10.1016/j.bbamcr.2015.01.021. [DOI] [PubMed] [Google Scholar]
- Laparra JM, Glahn RP, Miller DD. Bioaccessibility of phenols in common beans (Phaseolus vulgaris L.) and iron (Fe) availability to Caco-2 cells. J Agri Food Chem. 2008;56:10999–11005. doi: 10.1021/jf802537t. [DOI] [PubMed] [Google Scholar]
- Martin MP, Nepote V, Grosso NR. Chemical, sensory, and microbiological stability of stored raw peanuts packaged in polypropylene ventilated bags and high barrier plastic bags. LWT Food Sci Technol. 2016;68:174–182. doi: 10.1016/j.lwt.2015.12.031. [DOI] [Google Scholar]
- May PM, Williams DR, Linder PW. Biological significance of low molecular weight iron (III) complexes. In: Sigel H, editor. Metal ions in biological systems. New York: Marcel Dekker; 1978. pp. 30–37. [Google Scholar]
- Miller DD, Berner LA. Is solubility in vitro a reliable predictor of iron bioavailability? Biol Trace Elem Res. 1989;19:11–24. doi: 10.1007/BF02925446. [DOI] [PubMed] [Google Scholar]
- Nikolaus B, Peter ME. Oxidative stress and the homeodynamics of iron metabolism. Biomolecules. 2015;5:808–847. doi: 10.3390/biom5020808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendias AK. Soil plant transfer of trace elements-an environmental issue. Geoderma. 2004;122(2–4):143–149. doi: 10.1016/j.geoderma.2004.01.004. [DOI] [Google Scholar]
- Pickardt C, Eisner P, Kammerer RD, Carle R. Pilot plant preparation of light coloured protein isolates from de-oiled sunflower (Helianthus annuus L.) press cake by mild-acidic protein extraction and polyphenol adsorption. Food Hyd. 2015;44:208–219. doi: 10.1016/j.foodhyd.2014.09.020. [DOI] [Google Scholar]
- Ramachandran S, Larroche S, Pandey A. Oil cakes and their biotechnological applications–a review. Biores Technol. 2007;98:2000–2009. doi: 10.1016/j.biortech.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Shi A, Liu H, Liu L, Hu H, Wang Q, Adhikari B. Isolation, purification and molecular mechanism of a peanut protein-derived ACE-inhibitory peptide. PLoS ONE. 2014;9:e111188. doi: 10.1371/journal.pone.0111188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. Statistical methods. 8. Ames: Affiliated East-West Press, Iowa State University Press; 1994. [Google Scholar]
- Szlapka SE, Jarmolowska B, Krawczul S, Kostrya H. Transport of bovine milk-derived opioid peptides across a Caco-2 monolayer. Int Dairy J. 2009;19:251–257. [Google Scholar]
- Tokuoka M, Sawamura N, Kobayashi K, Mizuno A. Simple metabolite extraction method for metabolic profiling of the solid-state fermentation of Aspergillus oryzae. J Biosci Bioeng. 2010;110:665–669. doi: 10.1016/j.jbiosc.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Wang L, Hongzhi L, Liu L, Wang Q, Li S, Li Q. Prediction of peanut protein solubility based on the evaluation model established by supervised principal component regression. Food Chem. 2017;218:553–560. doi: 10.1016/j.foodchem.2016.09.091. [DOI] [PubMed] [Google Scholar]
- Wiren N, Khodr H, Hider RC. Hydroxylated phytosiderophore species possess an enhanced chelate stability and affinity for iron (III) Plant Physiol. 2000;124:1149–1158. doi: 10.1104/pp.124.3.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Yu, Xing G, Rui X, Li W, Chen X, Jiang M, Dong M. Effect of solid-state fermentation with Cordyceps militaris SN-18 on physicochemical and functional properties of chickpea (Cicer arietinum L.) flour. LWT Food Sci Technol. 2015;63:1317–1324. doi: 10.1016/j.lwt.2015.04.046. [DOI] [Google Scholar]
- Zhang BS. In vitro antithrombotic activities of peanut protein hydrolysates. Food Chem. 2016;201:1–8. doi: 10.1016/j.foodchem.2016.01.108. [DOI] [PubMed] [Google Scholar]