Abstract
Pectic oligosaccharides (POS) have been indicated as novel candidate prebiotics. Traditionally, POS are produced from pectin-rich by-products using a two-step process involving extraction of the pectin, followed by its hydrolysis into POS. A one-step approach, in which the POS is directly produced from the raw material, might provide a more efficient alternative. Thus, the main aim of this paper was to investigate a one-step enzymatic hydrolysis approach to directly produce POS from sugar beet pulp (SBP). The POS yield was investigated as a function of the process parameters, as well as raw material characteristics. A statistically-based response surface methodology, using a central composite design was applied, to investigate the individual as well as the combined influences of the diverse parameters. The model was confirmed by a validation experiment, carried out at 135 g/l substrate concentration, 0.75 FPU/g SBP enzyme concentration, 0.8 mm particle size and 3 h hydrolysis time. Under these conditions, a POS-rich hydrolysate was obtained, containing rhamnose, arabinose, galactose, xylose and galacturonic acid, at 0.9, 15.2, 5.1, 1.4, and 13.2 g/l, respectively, enzymes were added each at 20 FPU/g dry matter (DM).
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2835-x) contains supplementary material, which is available to authorized users.
Keywords: Pectic oligosaccharides, Sugar beet pulp, One-step hydrolysis, Response surface methodology, Central composite design, Yield optimization
Introduction
Sugar beet (Beta vulgaris) is a temperate crop abundantly produced in Europe. After the sugar extraction, a by-product in the form of pulp, is generated, which is mainly used as animal feed. Approximately 5 million tonnes of sugar beet pulp (SBP) by-product is produced per annum in the EU-28, on a dry matter basis (CIBE 2014). The major components are hemicellulose (30%), followed by cellulose (22–24%), pectin (15–25%) and other compounds (9–10%) (Concha and Zuniga 2012). Cellulosic and hemicellulosic fractions of SBP have been extensively exploited for the production of bioethanol and biogas (Zheng et al. 2012). More recent research is fostered on the valorisation of the pectin fraction in the form of pectic oligosaccharides (POS) (Babbar et al. 2016a; Leijdekkers et al. 2013; Martinez et al. 2009a; Westphal et al. 2010). Pectin is a complex and heterogeneous group of polysaccharides, which is composed of distinctive domains covalently linked to one another (Zykwinska et al. 2008). The main structural domains in pectin are homogalacturonan (HG), comprised of long chains of galacturonic acid (GalA) and rhamnogalacturonan-I (RG-I) and -II (RG-II), often described as the smooth and hairy regions, respectively (Westphal et al. 2010). HG is composed of α-1,4-linked-d-GalpA residues that can be partly methyl-esterified at C-6 and possibly partly acetyl esterified at O-2 and O-3 (Ralet et al. 2001). RG-I consists of chains, with alternate units of GalA and rhamnose (Rha), having branched arabinan, galactan or even arabinogalactan chains at O-4 of Rhap residues. The arabinan side chains are composed of α-(1-5)-linked-Araf residues, which can be further branched at α-l-Araf units at O-2 and O-3, whereas β-1-4-linked-d-Galp units constitute the galactan side chains (Zykwinska et al. 2008). RG-II is present in a minor amount and consists of GalA, Rha and neutral sugars (Martinez et al. 2009a). In addition, xylogalacturonan is also a part of pectin (Westphal et al. 2010). These acidic and neutral sugars are arranged such that the use of specific hydrolysis processes, generate an oligosaccharide mixture that can contain arabino-galactooligosaccharides, arabino-xylooligosaccharides, arabino-oligosaccharides, galacto-oligosaccharides, oligo-galactouronides, and rhamno-galacturonan oligosaccharides (Concha and Zuniga 2012).
The major interest in POS lies in their physicochemical and physiological properties (Babbar et al. 2016b). Such properties include the selective stimulation of growth of beneficial bacteria in the colon (Al-Tamimi et al. 2006; Olano et al. 2002). Thus, pectin-derived oligosaccharides are considered “novel candidate prebiotics” and “emerging prebiotics”. In addition, POS have also been investigated for other health-related effects, such as apoptosis of colon cancer cells, inhibition of Campylobacter jejuni on human Caco-2 cells, protection against various pathogens, and repression of lipid accumulation (Ganan et al. 2010; Iwasaki et al. 1998; Olano et al. 2003).
Although the enzymatic hydrolysis of SBP for the production of POS has already been investigated to some extent by Martinez et al. (2009b), Concha and Zuniga (2012) and Leijdekkers et al. (2013), an efficient and economically viable enzymatic production of POS directly from the pulp, still requires the optimisation of the various process parameters, as well as the physical state of the pulp raw material. The conventional approach, in which one or a small set of parameters is optimised separately, while the other parameters are kept fixed, is typically long and cumbersome. In contrast, optimisation through a statistically-based design-of-experiment approach allows the efficient and simultaneous optimisation of the various parameters. Response surface methodology (RSM) is a statistically-based methodology that explores the impact of several variables (such as the process parameters) on one or more responses (such as the yield) in a pre-chosen parameter range. Through a series of designed experiments, and the statistically-based interpretation of the corresponding results, according to the chosen design, predictions can be made of the optimal result for the various responses within the parameter range selected. Depending on the exact design used, not only the effect of the individual parameters but also of their combined action can be assessed (Myers et al. 2016; Oberoi et al. 2010). The most commonly applied design is the central composite design (CCD). This design is based on the results for the corner, centre, and specific external points of the pre-selected parameter range. It allows the determination of the impact of the individual effects of the parameters, as well as their interaction up to the second degree (quadratic), without the need to use a complete three-level factorial experiment. There is very limited literature available on the statistical optimisation of the use of enzymes, and their relation to other operational parameters, for POS production from SBP. Martinez et al. (2009b) presented a mathematical model of the process, calculating the yield of GalA oligomers and arabino-oligosaccharides but no optimisation was done and no other POS structures, eventually present in the mixtures, were characterised.
Thus, the present study was undertaken to investigate the efficiency of a one-step hydrolysis approach, as an alternative to the traditional two-step approach, in which the pectin is first extracted before being hydrolysed to POS. The SBP was enzymatically treated, and the liquefied POS was separated and analytically analysed. In order to optimise the particle size, the enzyme concentration, as well as the time required to convert the SBP pectin to POS, at high yield, a design-of-experiment was performed based on RSM, with the CCD-model consisting of 20 individual experiments. Two enzymes, Celluclast (to solubilise the pectin) and Viscozyme (to cleave the side chains of the pectin), were studied. The oligosaccharide mixtures obtained, were characterised for their composition in terms of GalA, Rha, arabinose (Ara) and galactose (Gal), and the optimisation, was performed, taking into consideration the different POS structures, independently. Based on the modelling results, the optimised parameters were then selected and used for a validation experiment, to establish the correlation between the actual yield and the yield predicted by the Design Expert™ software.
Materials and methods
Raw material and reagents
SBP with a dried matter content of 94.7% (wt/wt) was provided by the Institut fur Getreideverarbeitung (IGV GmbH, Germany). The dried SBP was ground using a Robot coupe R 20.V.V mixer at room temperature and 3500 rpm for 30 min, sieved to different particle sizes (as given by the design) and stored in ziplock bags at room temperature until use. The polygalacturonic acid, 4-nitrophenyl-β-d-glucopyranoside and 4-nitrophenyl-α-l- arabinofuranoside and standards for monomeric sugars were procured from Sigma Aldrich, USA, while linear arabinan was bought from Megazyme, Ireland. The enzymes Viscozyme L (Viscozyme) and Celluclast 1.5L (Celluclast) were obtained from Sigma Aldrich, USA. All other analytical chemicals were purchased from Sigma Aldrich, USA or Merck, Germany.
Pectin composition of the raw material
The total sugars present in the pectin part of sugar beet pulp were estimated by following the protocol optimized in our previous study (Babbar et al. 2016c). In particular, after complete hydrolysis of the pectin of the biomass, the supernatant (containing the pectin-derived monosaccharides) was assayed for its monosaccharide composition by high performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD). The hydrolysis was performed by subjecting the sugar beet pulp (1 g) to an enzyme cocktail of Celluclast and Viscozyme at a substrate concentration of 5% wt/vol in a 50 mmol/L citrate buffer (pH 4.8) in a 100 mL erlenmeyer flask. The enzymes were added each at 20 FPU/g DM, whereby the filter paper activity (FPU) of the enzyme was determined using laboratory analytical procedure of the National Renewable Energy Laboratory (Adney and Baker 2008). The flasks were incubated in an incubator shaker (Innova, New Bunswick, NJ, USA) at 48 °C and 150 rpm for 48 h. The hydrolysed biomass was centrifuged at 5000×g for 10 min. The supernatant (containing the monosaccharides after hydrolysis) was assayed by HPAEC-PAD according to the analysis of total sugars described elsewhere in the article.
Enzyme activity assays of the enzymes
The different enzyme activities present in Viscozyme and Celluclast were determined using appropriate substrates. The activities tested were selected based on the approximate composition of SBP. The activities of polygalacturonase, endo arabinase, β-d-glucosidase and α-l-arabinofuranosidase were respectively determined by measuring the amount of reducing sugars released from the hydrolysis of poly-galacturonic acid, linear arabinan, 4-nitrophenyl-β-d-glucopyranoside and 4-nitrophenyl-α-l-arabinofuranoside according to the method of Kuhnel et al. (2010, 2011). The FPU of the enzymes was analysed using the analytical procedure of National Renewable Energy Laboratory (Adney and Baker 2008).
Experimental procedure and design for optimization
The main aim of this work was to investigate the direct production of POS from SBP as an alternative to the conventional two-step approach in which the pectin is first extracted from the SBP before being converted to POS. To investigate the influence of the various parameters in an efficient way, the process was optimized using the statistically based RSM.
A three-factor CCD consisting of 20 experimental runs with three replications at the central point (Table 1) was used to optimize the independent variables, i.e. particle size (X1), Viscozyme concentration (X2) and incubation time (X3) on pectic oligosaccharides (POS) containing arabinose (AOS), galactose (GalOS), rhamnose (RhOS), xylose (XOS) and galacturonic acid (GalAOS). The selected ranges for the Viscozyme concentration, time and particle size were 0.75–2.0 filter paper units per gram dried substrate FPU/g DM, 1–3 h, and 0.3–0.8 mm respectively.
Table 1.
RSM design employed during the optimization study for three independent variables and the resulting concentration of POS obtained
| Run | Variables | POS concentrationb | ||||||
|---|---|---|---|---|---|---|---|---|
| Particle size (mm) X1 | Enzyme Concentrationa (FPU/g DM) X2 | Time (h) X3 | GalA (g/l) | Ara (g/l) | Gal (g/l) | Rha (g/l) | Xyl (g/l) | |
| 1 | 1.0 | 1.4 | 2.0 | 10.4 | 18.0 | 5.5 | 1.0 | 1.7 |
| 2 | 0.5 | 1.4 | 2.0 | 9.7 | 17.0 | 5.3 | 0.9 | 1.7 |
| 3 | 0.8 | 0.7 | 3.0 | 12.1 | 18.0 | 5.3 | 1.0 | 1.6 |
| 4 | 0.5 | 1.4 | 2.0 | 9.8 | 18.0 | 5.7 | 1.0 | 1.8 |
| 5 | 0.5 | 1.4 | 2.0 | 10.0 | 18.3 | 5.7 | 1.0 | 1.6 |
| 6 | 0.3 | 0.7 | 1.0 | 12.3 | 15.0 | 4.9 | 0.9 | 1.5 |
| 7 | 0.3 | 0.7 | 3.0 | 12.0 | 16.0 | 5.2 | 1.0 | 1.5 |
| 8 | 0.5 | 1.4 | 2.0 | 9.8 | 18.4 | 5.7 | 1.0 | 1.7 |
| 9 | 0.5 | 1.4 | 3.7 | 8.7 | 19.9 | 5.7 | 1.0 | 1.8 |
| 10 | 0.8 | 2.0 | 3.0 | 7.2 | 20.2 | 5.9 | 1.1 | 1.9 |
| 11 | 0.5 | 1.4 | 2.0 | 8.2 | 16.7 | 5.8 | 0.8 | 1.7 |
| 12 | 0.8 | 2.0 | 1.0 | 9.8 | 17.0 | 5.3 | 0.8 | 1.6 |
| 13 | 0.3 | 2.0 | 1.0 | 10.0 | 17.7 | 5.5 | 0.8 | 1.6 |
| 14 | 0.1 | 1.4 | 2.0 | 12.4 | 18.9 | 5.8 | 0.9 | 1.4 |
| 15 | 0.5 | 0.5 | 0.3 | 8.3 | 13.1 | 4.7 | 0.7 | 1.3 |
| 16 | 0.8 | 0.7 | 1.0 | 13.3 | 17.5 | 4.9 | 0.7 | 1.4 |
| 17 | 0.5 | 0.3 | 2.0 | 13.5 | 13.5 | 4.3 | 0.8 | 1.3 |
| 18 | 0.5 | 1.4 | 2.0 | 11.3 | 18.8 | 5.9 | 0.9 | 1.8 |
| 19 | 0.5 | 2.4 | 2.0 | 7.9 | 20.3 | 6.0 | 1.0 | 2.0 |
| 20 | 0.3 | 2.0 | 3.0 | 7.2 | 19.9 | 6.0 | 0.9 | 1.9 |
aConcentration of Viscozyme was varied. Celluclast was kept constant at 10 FPU/g DM
bPOS GalA, Ara, Gal, Rha, Xyl refers to the corresponding sugars present in solubilised oligosaccharides and not as free form. The experiment was conducted at a substrate concentration of 13.5% w/v
The experiment was performed in capped fermentation flasks, each containing 1 g of dried SBP. Hydrolysis was conducted at a substrate concentration of 13.5% (wt/vol) and a temperature of 45 °C in an incubator shaker at 150 rpm. The enzyme concentrations, particle size and time of hydrolysis were selected according to data generated by the RSM software (Table 1). After hydrolysis the flasks were heated to 100 °C for 5 min to inactivate the enzyme. At the end of the process, the hydrolysate was separated by centrifugation (5000×g for 10 min) and analysed for monosaccharide and oligosaccharide content. For the optimization, only the oligosaccharide content were considered, and not the free monosaccharides.
Experimental data from the CCD were analysed with RSM algorithm Design Expert 8.1 and fitted according to Eq. (1) as a second order polynomial equation including the main effects and interaction effects of each variable.
| 1 |
With Y the predicted response (oligosaccharide amount), X the considered parameters (see above), β o the constant coefficient, βi the linear coefficient, βii the quadratic coefficient, and βij the interaction coefficient.
Statistical analysis
The analysis of variance (ANOVA) and surface plots were generated using Design Expert 8.0, and the optimized values of three independent variables for maximum response were determined using the numerical optimization package of the same software.
Analysis of pectic mono-, oligo- and polysaccharides by HPAEC-PAD
In order to determine the total amount of pectic sugars, the extraction fluid was post-hydrolysed by digestion with 5% (vol/vol) of Viscozyme at 45 °C for 24 h based on the method of Martinez et al. (2009b) modified according to Babbar et al. (2016c). After hydrolysis, the enzyme was inactivated by a thermal treatment at 100 °C for 5 min, and the liquid was centrifuged at 5000×g for 10 min to get a clear supernatant. Samples of the extraction fluid were adequately diluted and injected into a high performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD).
The HPAEC-PAD used for analytical purpose was a Dionex ICS-5000 model (Thermo Scientific, Inc., USA) equipped with an ED-5000 electrochemical detector. The separation of monosaccharides was carried out with a Carbopac PA-1 (4 mm × 250 mm) column coupled to a guard Carbopac PA-1 (4 mm × 50 mm) column. The analyses were performed using a gradient of deionized water (eluent A and D), 250 mM sodium hydroxide (eluent B) and 1 M sodium acetate (eluent C). The elution conditions were: at time zero, A:B at 25:75 (start clean-up); at 10 min, B:C:D at 6:0:47 (re-equilibration); at 30 min, B:C:D at 6:15:39.50 (stop re-equilibration and start acquisition); at 35 min, B:C:D at 50:50:0; at 36 min, B:C:D at 6:0:47; and at 46 min B:C:D at 6:0:47 (stop acquisition). The mobile phase was set at a flow rate of 1 ml/min. The monosaccharides were quantified by comparison with the concentration of known standard solutions of GalA, Rha, Gal and Xyl (ranging from 10 to 1000 mg/l).
Results and discussion
Pectin composition of SBP
In order to determine the pectin composition of the biomass, SBP was hydrolysed with Viscozyme and analysed for the monosaccharides representative of the pectin. These included GalA (an acidic sugar) and Ara, Gal and Rha (as neutral sugars) present in pectin, plus xylose (Xyl), present in hemicellulose, which is also hydrolysed by Viscozyme L. The results were expressed as a percentage of the dry substrate, on a weight-for-weight basis [% (wt/wt) DM]. The GalA content of SBP was 16.5 ± 0.8%. The Ara content was the highest amongst the neutral sugars, accounting for 16.0 ± 0.6%. The Rha and Gal contents of SBP were 1.1 ± 0.3% and 5.6 ± 0.2%, respectively. This is consistent with the known pectin composition of SBP, which consists primarily of GalA and arabinose (Müller-Maatsch et al. 2016), even if the relative amount of Ara was relatively high compared to GalA. This might derive from concomitant hydrolysis of hemicellulose, which is consistent with the determined Xyl content of 2.1 ± 0.2%. In general, the compositional data fall within the range reported in the literature (Martinez et al. 2009b). Note, the commonly used acid hydrolysis was not suitable for the total hydrolysis of these pectin-rich samples because, in all instances, low amounts of GalA were found, due to degradation.
Enzymatic hydrolysis of SBP for POS production
Preliminary experiments showed that the combination of Celluclast and Viscozyme provided the highest performance for direct pectin hydrolysis from SBP. Celluclast, with cellulase activity, improves the solubilisation of cellulose and, thus, pectin release, while Viscozyme, with multiple pectin degrading activities, allows the actual hydrolysis of the pectin. Viscozyme is a multienzyme complex composed of pectinases, hemicellulases and arabinases (Foulk et al. 2008). Thus, a mixture of these two enzymes was used to hydrolyse SBP. The various enzyme activities present in Viscozyme and Celluclast were preliminarily determined, using appropriate substrates, as described in the experimental section (Table 2). Based on the optimisation of the enzyme units in preliminary experiments, the Celluclast concentration was not taken as a variable in the RSM and fixed at a concentration of 10.0 FPU/g DM. The POS production by enzymatic hydrolysis, was also assessed in a set of preliminary experiments performed to identify the most influential parameters and their range of practical interest (data not shown). Following these preliminary experiments, particle size (X1), Viscozyme concentration (X2) and incubation time (X3) were chosen as independent variables. The selected ranges for the Viscozyme concentration, incubation time and particle size were 0.75–2.0 filter paper units per gram of dried substrate (FPU/g DM), 1–3 h, and 0.3–0.8 mm, respectively. Considering this study aimed to obtain high oligosaccharide yields and low monosaccharide yields, the selected time range was lower than that used by previous researchers (Martinez et al. 2009b).
Table 2.
Activities of viscozyme and celluclast on different substrates (U/ml)
| Substrate | Activity measured | Viscozyme | Celluclast |
|---|---|---|---|
| U/ml | |||
| Polygalacturonic acid | Polygalacturonase | 6516 | 66 |
| Filter paper | Total cellulase | 29.4 | 70 |
| 4-nitrophenyl-β-d- glucopyranoside | β-d-glucosidase | 516 | 855 |
| Arabinan | Endo-arabinase | 180 | 64 |
| 4-nitrophenyl-α-l- arabinofuranoside | α-l-arabinofuranosidase | 0.18 | 0.27 |
The concentrations of Ara, Gal, Rha, Xyl and GalA included in POS, were taken as dependent variables. Given the hydrolysis was optimised for oligosaccharide production, the monosaccharide concentration was not considered as a response. In order to exclusively determine the sugar content associated with oligosaccharides, and not the free monosaccharides, all extracts after SBP hydrolysis were analysed by HPAEC-PAD, both after complete post-digestion hydrolysis, to determine the total amount of GalA, Ara, Gal, Rha and Xyl, and the free sugars. The amount of dissolved POS, and not of the free monosaccharides possibly released by the action of Viscozyme, was then readily determined by subtracting, from the total amount of monosaccharides detected after hydrolysis, the amount of monosaccharides already present in the extract.
Table 1 depicts the design used in the optimisation experiment involving the three independent variables and their effect on the production of various oligosaccharides from SBP. The highest concentrations of POS-derived GalA were 13.3 and 13.5 g/l, obtained at 0.7 and 0.3 FPU/g DM of Viscozyme, respectively (runs 16 and 17). When the enzyme concentration was increased above 0.7 FPU/g DM, while keeping constant the other two parameters, a lower POS-derived GalA concentration (runs 2, 4, 12 and 19) was obtained. This indicates that low enzyme concentrations (≤ 0.7 FPU/g DM) are required to produce POS containing GalA, likely due to a further hydrolysis of this sugar to free monosaccharides at higher enzyme concentrations. Table 1S (supplementary material) shows the corresponding conversion of pectic-GalA to GalA-containing POS and free GalA. Generally, an increase in the concentration of monosaccharides is observed with an increase in enzyme concentration. This effect is due to the exo-activity of Viscozyme (Combo et al. 2012).
Interestingly, a different effect was observed for the neutral sugars contained in the POS. In the case of Ara, the highest production of 20.3 and 20.2 g/l was obtained with 2.4 and 2.0 FPU/g DM of Viscozyme, respectively. Moreover, its solubilisation into POS remained at more than 15 g/l, even when higher doses of Viscozyme (1.4 FPU/g DM) were applied (runs 1, 2, 4, 5, 8, 9, 11, 14 and 18). The results obtained from run 17, suggests that if the enzyme concentration is lowered but the other two parameters are kept constant, the POS containing Ara significantly decrease. This observation is contrary to what Martinez et al. (2009b) found. The disparity could be due to the different range of enzyme loading in the present study because lower units of Viscozyme will have less Ara degrading activity, which eventually resulted in a lower concentration of POS containing Ara. In contrast, comparatively higher enzyme doses were used in the study by Martinez et al. (2009b). Table 1S shows the conversion of arabinan to Ara containing POS and free Ara. The Ara monosaccharide remains low compared to free GalA. Conversely, because a higher enzyme concentration leads to a more rapid hydrolysis of POS oligomers containing GalA, as described above, a balance is needed to obtain POS having both GalA and Ara. This can also indicate that the two structures mostly present in SBP, namely HG and arabinogalacturonan, are differentially degraded by the action of the enzyme. Thus, HG is more easily hydrolysed but is then, also more easily degraded to monomers, whereas arabinogalacturonan is less easily hydrolysed.
Data in Table 1 show that a higher concentration of enzyme is needed to produce oligosaccharides containing Xyl. The Xyl is partly from the hemicellulose fraction and a high xylanase activity is needed to degrade it, whereas the enzymes used in the present study possess a lower xylanase activity (Table 2). The highest concentration of xylose in oligomers (2 g/l) was obtained when 2.4 FPU/g DM of Viscozyme was applied (run 19). Finally, the amounts of Rha and Gal in the POS were not much affected by the variation in enzyme concentration.
As shown in Table 1, the incubation time is also a critical parameter for the production of oligosaccharides. A longer time was needed when higher amounts of POS containing the neutral sugars were desired but comparatively less time is necessary for the formation of POS containing GalA (runs 2 and 9, runs 3 and 16, runs 6 and 7, runs 10 and 11, and runs 13 and 20), again confirming the easiest degradation of the HG part of pectin by Viscozyme. The change in particle size did not significantly affect POS production from SBP.
Effect of different independent variables on the production of oligosaccharides
The results of the RSM experiment were analysed with Design expert 8.0 evaluation software, using a quadratic model. For all responses, the factors and second-order interactions were selected that were significant in the 95% confidence range. Thus, p ≤ 0.05 was considered significant in the present study. The final response function to predict GalA, Ara, Gal, Rha, and Xyl oligosaccharide concentrations (calculated only in POS and not as a free form), after eliminating the nonsignificant terms, are presented in Eqs. (2–6).
| 2 |
| 3 |
| 4 |
| 5 |
| 6 |
Y, X1, X2 and X3 represent the concentration, particle size, enzyme concentration and time, respectively. The overall quadratic model was significant. The R2 values for GalA, Ara, Gal, Rha and Xyl were 0.85, 0.79, 0.90, 0.82, and 0.83, respectively. This indicates 85, 79, 90, 82, and 83% of the total variation around the average, could be explained by the regression analysis performed for the GalA, Ara, Gal, Rha and Xyl production. In all cases, the lack of fit was not significant, indicating the fitness of the model for all five responses. The models show that the responses depend on the three parameters, i.e. size of the particles, the enzyme concentration and incubation time. However, the extent and the details of the effect, differ from the responses analysed. Also, in some instances, the interaction between the parameters, as well as second-order effects, were found to be relevant, as discussed in the next section.
Model graphs and numeric optimisation
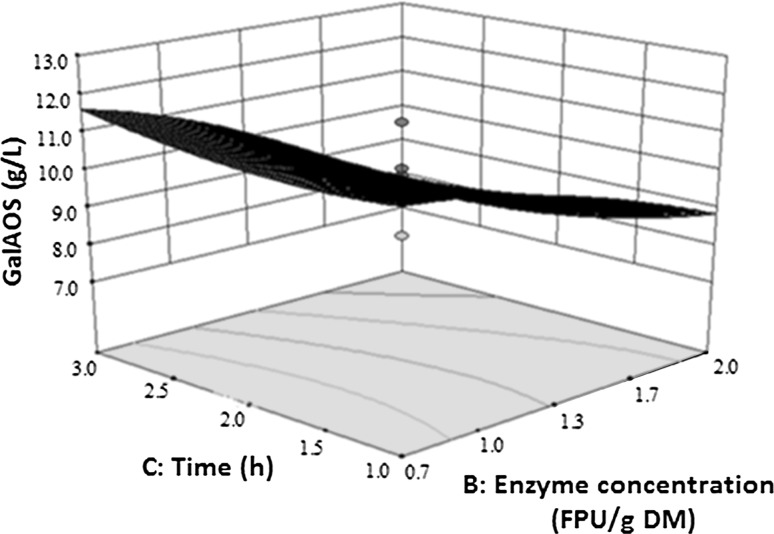
The model graphs were plotted according to the model Eqs. (2–6), to investigate the interaction between the independent variables and to determine the optimal value of each variable for the desired response. The response surfaces shown in Figs. 1, 2, 3, 4, were based on the final model, in which two variables were kept constant at their optimum values and the other two were varied within their experimental range. It was evident from the model graphs responses that the interaction between time and enzyme concentration had a significant effect on the GalA concentration. As shown in Fig. 1, as time progressed, GalA production in POS also increased (at a minimum enzyme concentration of 0.7 FPU/g DM). However, at a higher enzyme concentration of 2.0 FPU/g DM, the GalA content in POS decreased with increasing incubation time (Fig. 1), which corroborated with the increased free GalA content of the hydrolysates (Table 1S), as already mentioned above.
Fig. 1.
Response surface and contour plot showing the effect of interaction between enzyme concentration and time on the production of GalA-containing oligosaccharides (GalAOS) from SBP
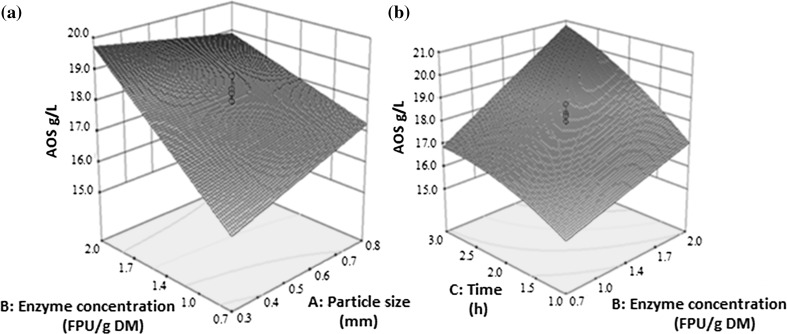
Fig. 2.
Response surface and contour plot showing effect of interaction between a particle size and enzyme concentration and b enzyme concentration and time on Ara-containing oligosaccharides (AOS) from SBP
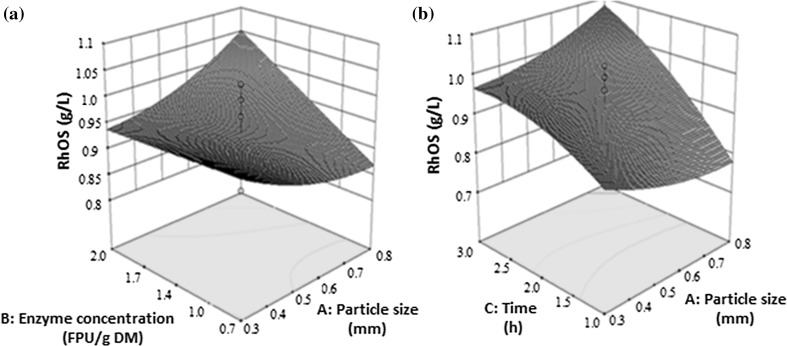
Fig. 3.
Response surface and contour plot showing the effect of interaction between a particle size and enzyme concentration and b particle size and time on Rha-containing oligosaccharides (RhOS) from SBP
Fig. 4.
Response surface and contour plot showing effect of interaction between enzyme concentration and time on Xyl-containing oligosaccharides (XOS) from SBP
In the case of Ara, at the lowest particle size of 0.3 mm, a sharp increase in the Ara content of POS occurred as the enzyme concentration was increased from 0.7 to 2.0 FPU/g DM. Also, at the maximum enzyme concentration of 2.0 FPU/g DM, a slight increase in Ara content of the POS production was seen with increased particle size (Fig. 2a). The results in Fig. 2b shows that the interaction between incubation time and enzyme concentration is significantly affecting the Ara production in POS (Fig. 2b). When both were increased, Ara production in POS increased. The maximum Ara in POS was produced at 2.0 FPU/g DM enzyme concentration and 3 h hydrolysis time.
In the case of Rha in POS, an increase in the enzyme concentration at the lowest particle size i.e. 0.3 mm, had a non-significant effect on the production. However, when the particle size and enzyme concentration were both increased, Rha in POS also increased from 0.94 to 1.05 g/l (Fig. 3a). For the interaction between time and particle size, at a particle size between 0.3–0.8 mm, an increase in time lead to an increase in Rha production in POS. A maximum Rha in POS of 1.08 g/l was observed at 3 h and a particle size of 0.8 mm (Fig. 3b).
In the case of Xyl production, at the lowest enzyme concentration (0.7 FPU/g DM), Xyl production in oligosaccharides increased with increased time, while maximum production was seen at 3 h and an enzyme concentration of 2.0 FPU/g DM (Fig. 4).
Validation using optimised parameters
The model was used to determine the optimal reaction conditions, to achieve maximal POS-yield, regarding the time, enzyme concentration and particle size. These conditions were found to correspond to a particle size of 0.8 mm, a Viscozyme activity of 0.75 FPU/g DM, and a reaction time of 3 h, with a predicted oligosaccharide concentration of 12.0 g/l for GalA, 17.7 g/l for Ara, 5.1 g/l for Gal, 0.9 g/l for Rha and 1.6 g/l for Xyl.
In order to confirm the model, a validation experiment was performed under these optimised conditions. The experiment yielded concentrations of 13.2 (GalA), 15.2 (Ara), 5.1 (Gal), 0.9 (Rha) and 1.4 (Xyl) g/l, respectively, corresponding to respective yields of 9.8, 11.2, 3.8, 0.6 and 1.0%, expressed as wt/wt per DM of SBP.
The validation experiment confirms that under these conditions, a high POS yield is achieved. The deviations between the predicted and the observed values of the various responses were less than 15%, indicating the suitability of the model (Myers et al. 2016). Moreover, the model can also be used to determine the conditions of production for POS enriched in a specific class, for instance, more shifted towards neutral sugars. Notwithstanding that the selected parameters for the model are considered to be key in the hydrolysis of SBP for POS production, other parameters might still need to be optimised for further fine-tuning of the process. Particularly, when upscaling the process, the optimisation of other, more engineering-based parameters, may still be required, to fulfil the needs of an industrial production.
Conclusion
In this study, a one-step enzymatic hydrolysis approach was successfully applied to directly produce POS from SBP.
Based on numerical optimisation, the optimal production condition to achieve a maximal total POS-yield at a substrate concentration of 135 g/l was found to correspond to an enzyme concentration of 0.75 FPU/g DM SBP, an hydrolysis time of 3 h and a SBP particle size of 0.8 mm. A validation experiment under these conditions, resulted in a POS-rich hydrolysate, containing oligomers formed by Rha, Ara, Gal, Xyl and GalA at a concentration of 0.9, 15.2, 5.1, 1.4 and 13.2 g/l respectively, with a notable high POS-yield of 26.5% (wt/wt) on SBP DM-base. The deviations with the predicted concentrations were found to be less than 15%, confirming the RSM-model.
Thus, this study demonstrated that the statistical design based on RSM allows defining the conditions for an efficient and direct hydrolysis of SBP with high yield. The combination of this approach with the independent analysis of the various POS might also allow, if required, to rapidly tailor the conditions for specific POS production. The POS obtained by the methodology might find an application as prebiotics in food and feed, thus allowing to obtain high added value compounds from the lower value agricultural sugar beet by-product. The POS will be tested for their ability to act as prebiotics, both in humans and animals, in future work, to substantiate this commercialization path.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table 1 s: Conversion of pectin to various oligosaccharides and monosaccharides (DOCX 27 kb)
Acknowledgement
The authors acknowledge the work supported by the European commission (FP7, NOSHAN, contract no. 312140). The authors also acknowledge IGV for providing the raw material. Neha Babbar gratefully acknowledges the PhD scholarship grant from VITO and University of Parma, Italy.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2835-x) contains supplementary material, which is available to authorized users.
References
- Adney B, Baker J (2008) Measurement of cellulase activities. Technical Report, NREL/TP-510-42628, Laboratory Analytical Procedure, National renewable Energy Laboratory, Golden, Colorado, USA
- Al-Tamimi MAHM, Palframan RJ, Cooper JM, Gibson GR, Rastall RA. In vitro fermentation of sugarbeet arabinan and arabino-oligosaccharides by the human gut microflora. J of Appl Microbiol. 2006;100:407–414. doi: 10.1111/j.1365-2672.2005.02780.x. [DOI] [PubMed] [Google Scholar]
- Babbar N, Baldassare S, Maesen M, Prandi B, Dejonghe W, Sforza S, Elst K. Enzymatic production of pectic oligosaccharides from onion skins. Carbohydr Polym. 2016;146:245–252. doi: 10.1016/j.carbpol.2016.03.011. [DOI] [PubMed] [Google Scholar]
- Babbar N, Dejonghe W, Gatti M, Sforza S, Elst K. Pectic oligosaccharides from agricultural by-products: production, characterization and health benefits. Crit Rev Biotechnol. 2016;36:594–606. doi: 10.3109/07388551.2014.996732. [DOI] [PubMed] [Google Scholar]
- Babbar N, Roy VS, Wijnants M, Dejonghe W, Caligiani A, Sforza S, Elst K. Effect of extraction conditions on the saccharide (neutral and acidic) composition of the crude pectic extract from various agro-industrial residues. J Agric Food Chem. 2016;64:268–276. doi: 10.1021/acs.jafc.5b04394. [DOI] [PubMed] [Google Scholar]
- Combo AMM, Aguedo M, Goffin D, Wathelet B, Paquot M. Enzymatic production of pectic oligosaccharides with commercial pectinase preparations. Food Bioprod Process. 2012;90:588–596. doi: 10.1016/j.fbp.2011.09.003. [DOI] [Google Scholar]
- Concha OJ, Zuniga HME. Enzymatic depolymerization of sugar beet pulp: production and characterization of pectin and pectic-oligosaccharides as a potential source for functional carbohydrates. Chem Eng J. 2012;192:29–36. doi: 10.1016/j.cej.2012.03.085. [DOI] [Google Scholar]
- Foulk J, Akin D, Dodd R. Influence of pectinolytic enzymes on retting effectiveness and resultant fiber properties. BioResources. 2008;3:155–169. [Google Scholar]
- Ganan M, Collins M, Rastall R, Hotchkiss AT, Chau HK, Carrascosa AV, Martinez RAJ. Inhibition by pectic oligosaccharides of the invasion of undifferentiated and differentiated Caco-2 cells by Campylobacter jejuni. Int J Food Microbiol. 2010;137:181–185. doi: 10.1016/j.ijfoodmicro.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Iwasaki KI, Inoue M, Matsubaro Y. Continuous hydrolysis of pectate by immobilized endo-polygalacturonase in a continuously stirred reactor. Biosci Biotechnol Biochem. 1998;62:262–272. doi: 10.1271/bbb.62.262. [DOI] [PubMed] [Google Scholar]
- Kuhnel S, Hinz SWA, Pouvreau L, Wery J, Schols HA, Gruppen H. Chrysosporium lucknowense arabinohydrolases effectively degrade sugar beet arabinan. Biores Technol. 2010;101:8300–8307. doi: 10.1016/j.biortech.2010.05.070. [DOI] [PubMed] [Google Scholar]
- Kuhnel S, Schols HA, Gruppen H. Aiming for the complete utilization of sugar beet pulp: examination of the effects of mild acid and hydrothermal pretreatment followed by enzymatic digestion. Biotechnol for biofuels. 2011;4:14. doi: 10.1186/1754-6834-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijdekkers AGM, Bink JPM, Geuthes S, Schols HA, Gruppen H. Enzymatic saccharification of sugar beet pulp for the production of galacturonic acid and arabinose, a study on the impact of the formation of recalcitrant oligosaccharides. Biores Technol. 2013;128:518–525. doi: 10.1016/j.biortech.2012.10.126. [DOI] [PubMed] [Google Scholar]
- Martinez M, Gullon HA, Schols HA, Alonso JL, Parajo JC. Assessment of the production of oligomeric compounds from sugar beet pulp. Ind Eng Chem Res. 2009;48:4681–4687. doi: 10.1021/ie8017753. [DOI] [Google Scholar]
- Martinez M, Gullon B, Yanez R, Alonso JL, Parajo JC. Direct enzymatic production of oligosaccharides mixtures from sugar beet pulp: experimental evaluation and mathematical modelling. J Agric Food Chem. 2009;57:5510–5517. doi: 10.1021/jf900654g. [DOI] [PubMed] [Google Scholar]
- Müller-Maatsch J, Bencivenni M, Caligiani A, Tedeschi T, Bruggeman G, Bosch M, Petrusan J, Van Droogenbroeck B, Elst K, Sforza S. Pectin content and composition from different food waste streams. Food Chem. 2016;201:37–45. doi: 10.1016/j.foodchem.2016.01.012. [DOI] [PubMed] [Google Scholar]
- Myers RH, Montgomery DC, Anderson-Cook CM. Response Surface Methodology: process and product optimization using designed experiments. 4. New York: Wiley; 2016. [Google Scholar]
- Oberoi HS, Vadlani PV, Madl RL, Saida L, Abeykoon JP. Ethanol production from orange peels: two-stage hydrolysis and fermentation studies using optimized parameters through experimental design. J Agric Food Chem. 2010;58:3422–3429. doi: 10.1021/jf903163t. [DOI] [PubMed] [Google Scholar]
- Olano ME, Gibson GR, Rastall RA. Comparison of the in- vitro bifidogenic properties of pectins and pectic-oligosaccharides. J Appl Microbiol. 2002;93:505–511. doi: 10.1046/j.1365-2672.2002.01719.x. [DOI] [PubMed] [Google Scholar]
- Olano ME, Rimbach GH, Gibson GR, Rastall RA. Pectin and pectic-oligosaccharides induce apoptosis in in vitro human colonic adenocarcinoma cells. Anticancer Res. 2003;23:341–346. [PubMed] [Google Scholar]
- Ralet MC, Bonnin E, Thibault JF. Pectins. In: Baets S, Vandamme E, Steinbüchel A, editors. Biopolymers. Weinhein: Wiley; 2001. pp. 345–380. [Google Scholar]
- Westphal Y, Kuhnel S, Waard P, Hinz S, Schols HA, Voragen AGJ, Gruppen H. LC/CE-MS tools for the analysis of complex arabino-oligosaccharides. Carbohydr Res. 2010;345:2239–2251. doi: 10.1016/j.carres.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Yu C, Cheng YS, Lee C, Simmons CW, Dooley TM, Zhang R, Jenkins BM, VanderGheynst JS. Integrating sugar beet pulp storage, hydrolysis and fermentation for fuel ethanol production. Appl Energy. 2012;9:168–175. doi: 10.1016/j.apenergy.2011.12.084. [DOI] [Google Scholar]
- Zykwinska A, Boiffard MH, Kontkanen H, Buchert J, Thibault JF, Bonnin E. Extraction of green labeled pectins and pectic oligosaccharides from plant by products. J Agric Food Chem. 2008;56:8926–8935. doi: 10.1021/jf801705a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1 s: Conversion of pectin to various oligosaccharides and monosaccharides (DOCX 27 kb)