Abstract
Culture broth of Ganoderma lucidum was determined for antioxidant, antibacterial and α-amylase inhibitory activities. The culture broth contained protein as determined by Bradford method equaled to 0.2 mg/ml and total phenol content as 0.078 mg GAE/mg protein (0.557 mg GAE/g extract). It exhibited radicals scavenging activities against ABTS+· and DPPH· radicals with a half maximal inhibitory concentration (IC50) less than 1.70 ± 0.02 and 2.28 ± 0.02 µg protein/ml, respectively and reducing power equaled to 4.38 ± 0.02 µmol Trolox/µg protein as investigated by ferric ion reducing antioxidant power method. The culture broth experimented into two approached; (1) treated with pronase and (2) filtered through a membrane with 10 kDa molecular weight cut-off (MWCO). The pronase-treated culture broth exhibited insignificant lower antioxidant activities, but the retained culture broth 10 kDa MWCO resulted in significant decrease in antioxidant activities suggesting that the small proteins might play the key role in antioxidant activity. The culture broth could protect DNA damage from hydroxyl radicals (·OH) generated by Fenton’s reaction. This culture broth showed antibacterial activity towards pathogenic strains Staphylococcus epidermidis and Pseudomonas aeruginosa and also had an interesting α-amylase inhibitory activity. This study suggested that apart from the fruiting bodies and the mycelial of G. lucidum, its culture broth also had potential applications as a value-added ingredient in the product such as in cosmetics and in nutraceuticals.
Keywords: Antimicrobial, Anti-radical, Cultured filtrate, DNA protection, Ganoderma lucidum, Reducing power
Introduction
Ganoderma lucidum is well-known mushrooms in the issue of medicinal properties for centuries, especially in Asian countries. Many works have been shown that bioactive molecules from fruiting body exhibited several bioactivities including anticancer, antioxidant, antibacterial activity (Russell and Paterson 2006). However, at least 3 or 4 months have to be waited to get the fruit bodies. This time-consuming period is one of the obstacles in using the fruiting body of this mushroom. Apart from fruiting body, mycelia of G. lucidum had been shown containing molecules with bioactivities (Li et al. 2013). There were also reports that mycelia free culture filtrates of these mushrooms contained anticancer activity (Chung et al. 2001), antioxidant activity (Lung et al. 2010; Lung and Chang 2011) and antimicrobial properties (Siriwattanametanon et al. 2014). The advantage of using mycelia and culture filtrate is a time-consuming shortcut. Therefore, in this research, we reported the antioxidant and antibacterial activity of G. lucidum G2 mycelia free filtrate. The benefit of this culture broth would also support a zero waste process.
Materials and methods
Fungal cultivation
Ganoderma lucidum G2 cultured in Rujira Mushroom Farm in Ka La Sin province (Northeast, Thailand), originated from Department of Agriculture, Thailand was cultured in potato dextrose agar (PDA) for 7 days, maintained at 4 °C and sub-cultured periodically. The fungal active growing mycelia plugs were transferred to a 125 ml-flask containing (30 ml) Glucose Yeast Peptone (GYP) liquid medium composed of glucose (10 g/l), yeast extract (5 g/l), peptone Type-I (5 g/l) and MgSO4 (1 g/l), and cultured for 14 days at ambient temperature with 120 revolutions per minute (rpm) shaking. The fungal mycelia were removed by filtering through a filter paper. The culture broth was assayed for protein content, antioxidant and antibacterial activities. The abiotic culture medium was used in control reaction.
Protein determination and total phenol content
Protein concentration was determined according to Bradford method (Bradford 1976) using a Bio-Rad protein assay reagent (Bio-Rad, USA) and bovine serum albumin (BSA) was used to create a standard curve. Total phenol content was determined according to Singleton and Rossi (1965) with some modifications, using Folin–Ciocalteu reagent and gallic acid as the standard phenol compound as previously described (Khammuang and Sarnthima 2011).
Antioxidant activity assays
ABTS+· radical scavenging activity has experimented as previously described (Thetsrimuang et al. 2011). The ABTS·+ radicals’ solution at the initial optical density at 734 nm (OD734nm) at around 0.7, was mixed with samples at various concentrations. After incubation in the dark at room temperature for 5 min, the decreasing absorbance at 734 nm was measured by a visible spectrophotometer. The total antioxidant activity was expressed as TEAC value (Trolox equivalent antioxidant capacity, µmol Trolox/µg protein) and as the half maximum inhibitory concentration (IC50). DPPH· radical scavenging activity was measured using a modified Yamaguchi et al. (1998) as previous described (Thetsrimuang et al. 2011) and incubation time was 5 h instead of 5 min.
Ferric reducing antioxidant power (FRAP)
FRAP was experimented as previously described by Thetsrimuang et al. (2011) as a modified method according to Benzie and Strain (1999). Briefly to prepare fresh FRAP reagent, mixing 1 volume of FeCl3·6H2O solution (20 mM), 1 volume of TPTZ (2,4,6-tripyridyl-s-triazine) solution and 10 volumes of acetate buffer (0.5 M, pH 3.6) and warmed to 37 °C. Reaction was consisted of 980 µl of warmed reagent and 20 µl of sample. The mixture was measured at absorbance 593 nm at initial mixing and at 90 min of reaction. Reducing power was expressed as µmol Trolox/µg protein of sample using a calibration curve of the FeSO4·7H2O solution.
Pronase digestion
Extracellular proteins from G. lucidum culture broth were treated with pronase (Roche, USA) in a ratio of enzyme to the protein of 1:5 (by mass) in 50 mM Tris–HCl buffer (pH 7.5) containing 10 mM CaCl2 at 40 °C for 30 min, 1 h, and 2 h. Reactions were stopped by deactivating the enzyme in boiling water for 10 min. The supernatant was obtained by centrifugation and used for antioxidant activity assay as described. To analyze its protein pattern, SDS-PAGE was performed according to the method of Laemmli (1970) using 15% separating gel and a 5% stacking gel compared to untreated samples. Electrophoresis was run for 1 h at a constant voltage of 100 V/gel. The gel was stained with a silver solution.
DNA protection assay
Protective effect of the extracellular protein of G. lucidum on DNA damaging induced by hydroxyl radicals via Fenton’s reaction was performed as described by Siswoyo et al. (2011) with some modifications. Briefly, the reactions were done in a 1.5 ml microcentrifuge tube at the total volume of 20 µl containing 0.5 µg of pGEX DNA in a mixing solution (containing 15 mM H2O2, 25 µM ascorbic acid and 40 µM FeCl3) and tested protein samples at various concentrations. Trolox (200 µM), a reference antioxidant compound was also experimented. The reactions were incubated at 37 °C for 15 min and then analyzed by 1% agarose gel electrophoresis and stained with GelStar™ (Lonza, USA) and visualized under UV light.
Antibacterial activity
Gram positive bacteria included Staphylococcus epidermidis TISTR 518, Staphylococcus aureus TISTR 1466, Bacillus subtilis TISTR 008, Bacillus cereus TISTR 687 and gram negative bacterial included Escherichia coli TISTR 780 and Pseudomonas aeruginosa TISTR 781 were obtained from Thailand Institute of Scientific and Technological Research (TISTR). Luria–Bertani (LB) medium was used for culture all bacteria.
Disc diffusion assay was performed as described (Phansri et al. 2011). Each bacterial strain was grown in LB broth overnight, then transferred 50 µl to 5 ml of a new LB broth tube and incubated with shaking at 180 rpm at 37 °C for 3–4 h. Appropriated diluted of one milliliter (containing around 1 × 106 colony) of this culture was added to 5 ml warm melted LB agar, mixing briefly and poured on petri dish plate containing underlay LB agar. The sterile discs were then placed on plates, positive control (500 µg of kanamycin) and protein samples were applied onto each different disc and fresh LB broth was used as negative control. The plates were incubated at 37 °C for 15–17 h before the inhibition zone diameters surrounding the disc were measured. All experiments were carried out in a triplicate.
Alpha-amylase inhibitory activity assay (aAI)
The α-amylase inhibitory activity assay was modified from Bernfeld method (Bernfeld 1955) and Wisessing et al. (2010). To begin the reaction, 100 µl of culture broth was pre-incubated with 50 μl of porcine α-amylase in 0.05 M Phosphate buffer pH 6.9 which contains 0.2 mM CaCl2 and 20 mM NaCl at 37 °C for 20 min. After that 200 µl of 1% starch solution was added into the reaction and further incubated for 15 min, the reaction was then terminated by addition of 200 µl of 3,5-dinitrosalicylic acid reagent, followed by incubation in boiling water for 10 min. The reaction mixture was diluted with 2 ml of distilled water, and the absorbance was measured at 540 nm by visible-spectrophotometer. Controls are reactions without culture broth addition. The α-amylase inhibitory activity was reported in percentage calculated in the comparison between the inhibitor test set (with inhibitor) and the enzyme control set (without inhibitor) according to Eq. 1.
| 1 |
where AE is amylase activity of enzyme control set, AI is amylase activity of inhibitor test set.
Data were reported as the mean ± standard deviation (SD). Differences between groups were tested by one-way ANOVA and T test was used for comparisons. P ≤ 0.05 was considered as statistically significant.
Results and discussion
Soluble protein in culture broth
After cultivation of G. lucidum G2 for 2 weeks, the culture broth contained extracellular protein around 0.2 mg/ml (0.02%). The cultivation of G. lucidum G2 for 2 weeks obtained extracellular protein less than the production of extracellular protein from G. lucidum 447 cultured in submerged fermentation of wheat bran with different nitrogen source (Songulashvili et al. 2007). In their experiment, the protein accumulated ranging between 41 and 66 mg/100 ml and around 65–91 mg/100 ml obtained when cultured in submerged fermentation of food industry wastes. The same species G. lucidum HAI 447 cultured in solid-state fermentation yield protein around 0.12 mg/ml (Stajić et al. 2010). The difference in protein production by any fungus is probably due to differences in many factors such as fungal strain, type of cultivation, culture medium, the effect of carbon and nitrogen sources and ratios as well as culture condition.
Antioxidant activities
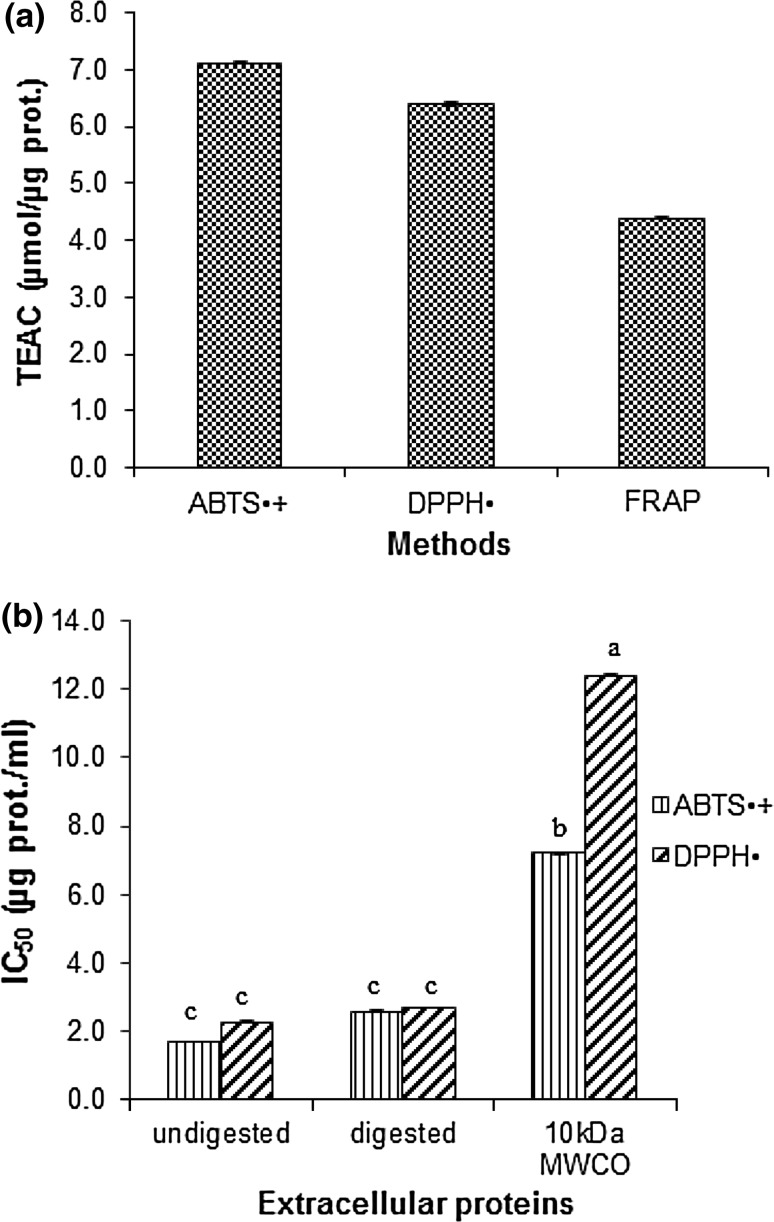
The scavenging effect of culture broth of G. lucidum was measured using ABTS+· and DPPH· radicals. Results are expressed as Trolox equivalent antioxidant capacity (TEAC) values (Fig. 1a). It was found that the culture broth showed scavenging activities against both radicals. It also had reducing ability as measured by FRAP method (Fig. 1a).
Fig. 1.
The antioxidant activities of culture broth measured as Trolox Equivalents (TEAC) (µmol trolox/µg protein) values (a), and IC50 (µg/ml) against ABTS+· and DPPH· radicals of undigested and digested protein samples and dialysate of 10 kDa MWCO (b). Each value is expressed as mean ± standard deviation (n = 3). Mean in different letter of each sample is significantly different (P < 0.05)
In order to prove that the soluble protein in the culture broth plays an important role in radical scavenging activity, the culture broth after treated with protease (pronase) was assayed for radicals scavenging activity against both radicals. It was found that insignificantly higher in IC50 values were observed (Fig. 1b). In this work, the IC50 values of culture broth of G. lucidum G2 on DPPH· radicals were obtained as low as 2.28 ± 0.02 µg protein/ml (or 0.32 mg extract/ml) and as low as 1.70 ± 0.02 µg protein/ml (or 0.24 mg extract/ml) in case of ABTS+· radicals. The mycelia protein extract of this mushroom species had IC50 against ABTS+· and DPPH· radicals in higher values (Sa-ard et al. 2015). The culture broth after treated with pronase had insignificant fewer radicals scavenging activity than its crude culture broth. This might be due to proteins after digested into shortening peptides, some of those peptides might have antioxidant activity whereas some of the antioxidant proteins are inactive. When the culture broth was dialyzed against distilled water using 10 kDa MW cut-off, it was found that the dialysate still remained antioxidant activity, but with pretty much higher IC50 against ABTS+· and DPPH· radicals equaled to 7.2 ± 0.02 µg protein/ml (4.2-time higher) and 12.4 ± 0.02 µg protein/ml (5.4-time higher), respectively (Fig. 1b). This result suggested that small proteins with MW less than 10 kDa might be key compounds that have antiradical’s activities by this species. The G. lucidum peptide (GLP) has been reported as the major antioxidant component of fermented G. lucidum (Sun et al. 2004). The peptide isolated from G. lucidum fruiting body with molecular weight 2.8, 3.34 and 3.35 kDa which are rich in phenylalanine, aspartic acid, proline, histidine, and isoleucine exhibited antioxidant properties (Girjal et al. 2012). Huang et al. (2013) reported that protein hydrolysate derived from enzymatic digestion of G. lucidum proteins with commercial proteases, Alcalase and Protamex, showed higher hydroxyl radical scavenging ability than undigested proteins. However, due to the culture broth of G. lucidum in this research contained a small amount of phenol compounds (0.557 mg GAE/g extract or 0.078 mg GAE/mg protein) which might also synergistic in these antioxidant activities.
SDS-PAGE
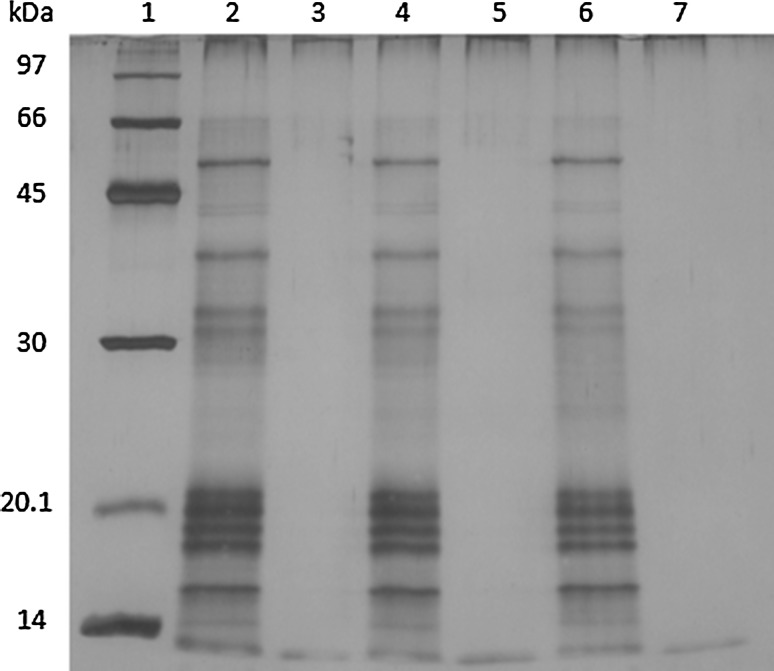
The G. lucidum culture broth proteins were analyzed by SDS-PAGE staining with the silver solution. The result showed one protein band with the molecular weight (MW) between 45 and 66 kDa, about three protein bands with MW between 30 and 45 kDa, about five protein bands with MW between 14 and 20.1 kDa, and at least one protein band showed MW less than 14 kDa (as shown in Fig. 2). The G. lucidum culture broth proteins showed several major protein bands with different molecular weight (MW) between less than 14–66 kDa according to SDS-PAGE. The protein patterns of culture broth proteins are clearly different from its mycelia and fruiting bodies protein extracts (Sa-ard et al. 2015). A novel water-soluble fungi Se-containing protein, named as Se-GL-P, was purified and identified as a monomer of 36,600 Da. It has stronger scavenging activities on superoxide and hydroxyl radicals (Du et al. 2007).
Fig. 2.
15% SDS-PAGE of culture broth protein and after digested with pronase. Lane 1 protein molecular weight markers, lanes 2, 4, 6 control protein at 30 min, 1, 2 h, lanes 3, 5, 7 protein treated with pronase for 30 min, 1 h and 2 h, respectively
Polysaccharide–protein (PSP) complexes with antioxidant activities prepared by ultrasound-assisted extraction from three medicinal mushrooms (Grifola frondosa, Coriolus versicolor and Lentinus edodes) contained 3–4 distinct protein bands between 10 and 130 kDa according to SDS-PAGE (Cheung et al. 2012). The complete digestion of culture broth proteins in this work accompanied with a significant decreased in radicals scavenging activities strongly supported that proteins are responsible for antioxidant properties. This result also suggested that antioxidant proteins might be small proteins with MW less than 10 kDa. Therefore, successful purification of the antioxidant proteins or bioactive proteins from the culture broth of this mushroom is still needed.
Antibacterial activity
The culture broth of G. lucidum G2 at the amount of about 100 µg protein (or 14 mg extract) per a paper disc exhibited antibacterial activities. It exhibited a various degree of inhibition against all tested bacteria including gram positive and gram negative bacteria (Table 1). Our result revealed that the most susceptible microorganism was gram positive bacterium S. epidermidis (zone of inhibition 7.67 ± 0.60 mm), followed by gram negative bacterium P. aeruginosa (zone of inhibition 7.33 ± 0.60 mm).
Table 1.
Antibacterial activity of Ganoderma lucidum culture broth
| Sample | Inhibition zone (mm) | |||||
|---|---|---|---|---|---|---|
| S. epidermidis | S. aureus | B. subtilis | B. cereus | E. coli | P. aeruginosa | |
| Culture broth | 7.67 ± 0.6 | 4.67 ± 0.6 | 6.67 ± 0.6 | 0.67 ± 0.6 | 2.67 ± 0.6 | 7.33 ± 0.6 |
| Kanamycin | 25 ± 0.0 | 18 ± 0.0 | 26 ± 0.2 | 19 ± 0.0 | 16 ± 0.0 | 25 ± 0.0 |
Note: Inhibition zone (mm) after subtracted from negative control (n = 3)
Loaded protein 100 μg/disc (14 mg extract). Kanamycin is positive control antibiotics (500 μg/disc)
Culture broth proteins of G. lucidum G2 exhibited fairly broad range antibacterial including gram-positive and gram-negative bacteria, especially S. epidermidis. This antibacterial profile is also different from those of mycelia and fruiting bodies protein extract (Sa-ard et al. 2015). Submerged fermentation of Russula sp. and Pycnoporus cinnabarinus for 7 days, metabolites extracted from culture broth also exhibited antibacterial activities (Shittu et al. 2005). Several peptides and proteins with antimicrobial activities from mushrooms have been reviewed. L. edodes is the most studied species and seems to have a broad antimicrobial action against both gram-positive and gram-negative bacteria. Plectasin peptide (Pseudoplectania nigrella) is the isolated compound with the highest antimicrobial activity against gram-positive bacteria (Alves et al. 2012).
DNA protection assay
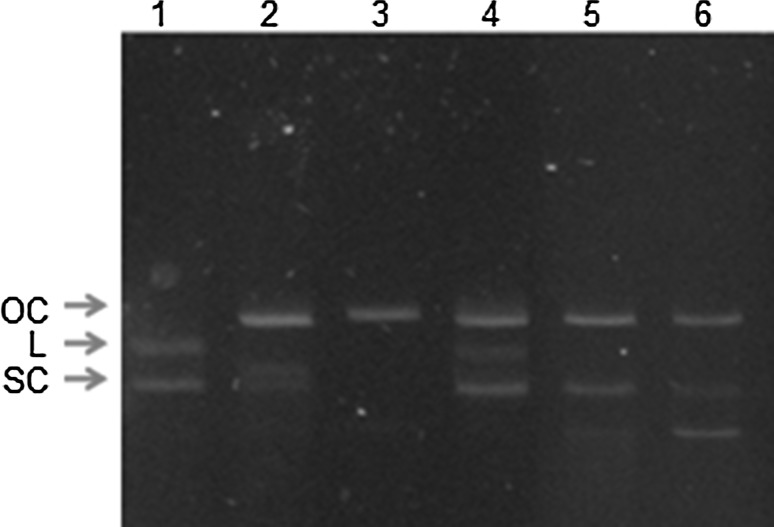
In this experiment, Fenton’s reaction was used to generate hydroxyl radicals that leading to a single-strand break in supercoiled plasmid DNA (SC) to the formation of open circular DNA (OC) and probably to be in a linear form (L). Our work revealed that the culture broth protein of G. lucidum G2 at concentration 0.5 µg protein/0.5 µg plasmid DNA (25 µg protein/ml) could protect DNA damage from hydroxyl radicals, but when amounts of the protein increased, the protection of DNA damage seemed to decrease as observed from the decreasing in intensity of supercoiled DNA band in agarose gel (Fig. 3). However, the protective effect of DNA damage has not been observed with Trolox which normally uses as reference antioxidant compound.
Fig. 3.
Inhibitory effect of Ganoderma lucidum culture broth on DNA damage caused by hydroxyl radicals from Fenton’s reaction. Lane 1 control plasmid DNA (0.05 µg); lane 2 plasmid DNA exposed Fenton reaction; lane 3 plasmid DNA with Fenton reaction plus 200 µM trolox; lanes 4, 5, 6 plasmid DNA with Fenton reaction plus culture broth 0.5, 1, 4 µg protein (70, 140, 560 µg extract), respectively. OC open chain, L linear, SC supercoil DNA
Our work revealed that the culture broth of G. lucidum G2 could protect DNA damage caused by hydroxyl radicals which generated from Fenton’s reaction at a low protein concentration. This property is similar to its fruiting body as well as its mycelia protein extract as report previously (Sa-ard et al. 2015). Pro-oxidant activity of Trolox has been reported (Ko et al. 1994). This property could make Trolox become an oxidant, therefore, loss the ability to protect damage of plasmid DNA from destroying by hydroxyl radicals. From our result, at the higher amount of sample added showed less DNA protection. There are probably some agents apart from protein in the broth culture that might have a pro-oxidant activity like Trolox. The addition of purification step might clarify the cause. The difference of ability in protecting against DNA strand break induced by hydroxyl radicals between previous reported (Kim and Kim 1999) and our investigation may due to the difference in composition of fruiting bodies extract which contained mainly proteins.
Protein from other sources apart from mushroom had been reported, two antioxidant protein fractions were isolated from Gnetum gnemon seed with molecular weights of approximately 30 kDa (Gg-AOPI) and 12 kDa (Gg-AOPII) at 25 μg to the DNA and Fenton’s reagent mixture significantly decreased the conversion of supercoiled DNA (SC) to open chain (OC) (Siswoyo et al. 2011).
Alpha-amylase inhibitory activity
One mechanism to lower serum glucose is the inhibition of one of carbohydrate degrading enzymes, α-amylase and/or α-glucosidase. In our work, the culture broth of the G. lucidum G2 species was tested for α-amylase inhibitory activity. It was found that when 20 µg proteins of culture broth (2.8 mg extract) used in the reaction, the inhibitory activity observed 78.00 ± 0.03%, thus specific activity was about 3.90% αAI per 1 µg protein. This biological activity is similar to its mycelia protein extract (65.08 ± 0.02% inhibition, 4.35% αAI per 1 µg protein).
In this work also reported that the culture broth of the G. lucidum G2 species with α-amylase inhibitory activity. Kim and Nho (2004) had reported non-proteinaceous α-glucosidase inhibitor (SKG-3) from dried fruiting bodies of G. lucidum methanolic extract. Ganoderol B exhibited a high α-glucosidase inhibition (IC50 48.5 µg/ml), has been reported as active compounds in the fruiting body of G. lucidum (Fatmawati et al. 2011). The nonpolar fraction of Grifola frondosa (GF) has shown to have the inhibitory effect on α-amylase and α-glucosidase (Su et al. 2013). A water-soluble polysaccharide from Inonotus obliquus (IOPS), an acid protein-bound polysaccharide, with a molecular weight of 1.7 × 104 Da exhibited an inhibitory activity against α-glucosidase with the IC50 value of 93.3 µg/ml, whereas it had no effective inhibition on α-amylase (Chen et al. 2010). These antihyperglycemic activities of mushrooms could be beneficial for blood sugar control or anti-diabetes management. Our result also reveals a promise of G. lucidum culture broth as a source of anti-glycemic agent. However, due to in the culture broth of G. lucidum in our research contained other compounds apart from soluble protein that might also contribute in this bioactivity. Therefore, further isolation and identify the bioactive compound in the culture broth as well as gastrointestinal digestion simulation of these bioactive proteins are still needed in order to apply in food supplement products.
Conclusion
The culture broth of G. lucidum G2 showed interesting antioxidant activities and antibacterial activity against both gram-negative and gram-positive bacteria. Pronase digestion of the protein in culture broth showed completely digested and antioxidant activity of its protein hydrolysates was decreased insignificantly, while of the protein with MW >10 kDa showed significantly decreased. The result indicates the major role of small proteins in antioxidant activity. Moreover, the culture broth protected hydroxyl radical-induced oxidative DNA damage and also exhibited α-amylase inhibitory activity. These data established the potential uses of culture broth of G. lucidum G2 for being a source of bioactive agents. However, major bioactive compounds are still needed to elucidate their structure and function.
Acknowledgements
The Thailand Research Fund-Master Research Grants (TRF-MAG window I, Grant Number MRG545S046), Mahasarakham University (Grant No. 5604012/2556) and Protein and Enzyme Technology Research Unit, Center of Excellence for Innovation in Chemistry (PERCH-CIC), Department of Chemistry, Faculty of Science, Mahasarakham University are acknowledged for research grants and partial financial supports. The authors thank W. Kanchanarach, Department of Biology, Faculty of Science, Mahasarakham University for suggestion in bacterial culture.
Authors’ contributions
PS carried out all experiments and analysis. SK and RS conceived of the study, participated in its experimental design and coordination, drafted the manuscript and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they do not have any competing interests.
References
- Alves MJ, Ferreira ICFR, Dias J, Teixeira V, Martins A, Pintado M. A review on antimicrobial activity of mushroom (Basidiomycetes) extracts and isolated compounds. Planta Med. 2012;78:1707–1718. doi: 10.1055/s-0032-1315370. [DOI] [PubMed] [Google Scholar]
- Benzie FF, Strain JJ. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999;299:15–23. doi: 10.1016/S0076-6879(99)99005-5. [DOI] [PubMed] [Google Scholar]
- Bernfeld P. Amylases, α and β. In: Colowich SP, Kaplan NO, editors. Methods in enzymology. New York: Academic Press; 1955. pp. 149–158. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen H, Lu X, Qu Z, Wang Z, Zhang L. Glycosidase inhibitory activity and antioxidant properties of a polysaccharide from mushroom Inonotus obliquus. J Food Biochem. 2010;34:78–191. doi: 10.1111/j.1745-4514.2009.00265.x. [DOI] [Google Scholar]
- Cheung YC, Siu KC, Liu YS, Wu JY. Molecular properties and antioxidant activities of polysaccharide–protein complexes from selected mushrooms by ultrasound-assisted extraction. Process Biochem. 2012;47:892–895. doi: 10.1016/j.procbio.2012.02.004. [DOI] [Google Scholar]
- Chung WT, Lee SH, Kim JD, Park YS, Hwang B, Lee SY, Lee HY. Effect of mycelial culture broth of Ganoderma lucidum on the growth characteristics of human cell lines. J Biosci Bioeng. 2001;92(6):550–555. doi: 10.1016/S1389-1723(01)80314-5. [DOI] [PubMed] [Google Scholar]
- Du M, Zhao L, Li C-R, Zhao G-H, Hu X-S. Purification and properties of a novel Se-containing protein from Se-enriched Ganoderma lucidum and its antioxidant activity. Gaodeng Xuexiao Huaxue Xuebao/Chem J Chin Univ. 2007;28:75–78. [Google Scholar]
- Fatmawati S, Shimizu K, Kondo R. Ganoderol B: a potent α-glucosidase inhibitor isolated from the fruiting body of Ganoderma lucidum. Phytomedicine. 2011;18:1053–1055. doi: 10.1016/j.phymed.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Girjal VU, Neelagund S, Krishnappa M. Antioxidant properties of the peptides isolated from Ganoderma lucidum fruiting bodies. Int J Pept Res Ther. 2012;18:319–325. doi: 10.1007/s10989-012-9303-2. [DOI] [Google Scholar]
- Huang S-Q, Ao H, Zeng F-K, Yang B. Analysis of enzymetic hydrolysis of Ganoderma lucidum protein and antioxidation of its hydrolysates. Mod Food Sci Technol. 2013;29:24–28. [Google Scholar]
- Khammuang S, Sarnthima R. Antioxidant and antibacterial activities of selected varieties of Thai mango seed extract. Pak J Pharm Sci. 2011;24:37–42. [PubMed] [Google Scholar]
- Kim KC, Kim IG. Ganoderma lucidum extract protects DNA from strand breakage caused by hydroxyl radical and UV irradiation. Int J Mol Med. 1999;4:273–277. [PubMed] [Google Scholar]
- Kim SD, Nho HJ. Isolation and characterization of α-glucosidase inhibitor from the fungus Ganoderma lucidum. J Microbiol. 2004;42:223–227. [PubMed] [Google Scholar]
- Ko KM, Yick PK, Poon MK, Ip SP. Prooxidant and antioxidant effects of Trolox on ferric ion-induced oxidation of erythrocyte membrane lipids. Mol Cell Biochem. 1994;141:65–70. doi: 10.1007/BF00935592. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li Y-B, Liu R-M, Zhong J-J. A new ganoderic acid from Ganoderma lucidum mycelia and its stability. Fitoterapia. 2013;84:115–122. doi: 10.1016/j.fitote.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Lung MY, Chang YC. Antioxidant properties of the edible Basidiomycete Armillaria mellea in submerged cultures. Int J Mol Sci. 2011;12:6367–6384. doi: 10.3390/ijms12106367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung MY, Tsai JC, Huang PC. Antioxidant properties of edible BasidiomycetePhellinus igniariusin submerged cultures. J Food Sci. 2010;75:E18–E24. doi: 10.1111/j.1750-3841.2009.01384.x. [DOI] [PubMed] [Google Scholar]
- Phansri K, Sarnthima R, Thammassirirak S, Boonchalee P, Khammuang S. Antibacterial activity of Bauhinia acuminata L. Seed protein extract with low hemolytic activity against human erythrocytes. Chiang Mai J Sci. 2011;38:242–251. [Google Scholar]
- Russell R, Paterson M. Ganoderma—a therapeutic fungal biofactory. Phytochemistry. 2006;67:1985–2001. doi: 10.1016/j.phytochem.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Sa-ard P, Sarnthima R, Khammuang S, Kanchanarach W. Antioxidant, antibacterial and DNA protective activities of protein extracts from Ganoderma lucidum. J Food Sci Technol. 2015;52:2966–2973. doi: 10.1007/s13197-014-1343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shittu OB, Alofe FV, Onawunmi GO, Ogundaini AO, Tiwalade TA. Mycelial growth and antibacterial metabolite production by wild mushrooms. Afr J Biomed Res. 2005;8:157–162. [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic acid-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Siriwattanametanon W, Kanchanarach W, Thiwthong R, Dodgson JLA. Culture filtrates from laboratory grown Phellinus mushrooms for use as antibacterial agents. Chiang Mai J Sci. 2014;41:243–247. [Google Scholar]
- Siswoyo TA, Mardiana E, Lee KO, Hoshokawa K. Isolation and characterization of Antioxidant protein fractions from Melinjo (Gnetum gnemon) seeds. J Agric Food Chem. 2011;59:5648–5656. doi: 10.1021/jf2000647. [DOI] [PubMed] [Google Scholar]
- Songulashvili G, Elisashvili V, Wasser SP, Nevo E, Hadar Y. Basidiomycetes laccase and manganese peroxidase activity in submerged fermentation of food industry wastes. Enzyme Microb Technol. 2007;41:57–61. doi: 10.1016/j.enzmictec.2006.11.024. [DOI] [Google Scholar]
- Stajić M, Kukavica B, Vukojević J, Simonić J, Veljović-Jovanović S, Duletić-Laušević S. Wheat straw conversion by enzymatic system of Ganoderma lucidum. BioResources. 2010;5:2362–2373. [Google Scholar]
- Su C-H, Lu T-M, Lai M-N, Ng L-T. Inhibitory potential of Grifola frondosa bioactive fractions on α-amylase and α-glucosidase for management of hyperglycemia. Biotechnol Appl Biochem. 2013;60:446–452. doi: 10.1002/bab.1105. [DOI] [PubMed] [Google Scholar]
- Sun J, He H, Xie BJ. Novel antioxidant peptides from fermented mushroom Ganoderma lucidum. J Agric Food Chem. 2004;52:6646–6652. doi: 10.1021/jf0495136. [DOI] [PubMed] [Google Scholar]
- Thetsrimuang C, Khammuang S, Chiablaem K, Srisomsap C, Sarnthima R. Antioxidant properties and cytotoxicity of crude poly saccharides from Lentinus polychrous Lév. Food Chem. 2011;128:634–639. doi: 10.1016/j.foodchem.2011.03.077. [DOI] [Google Scholar]
- Wisessing A, Engkagul A, Wongpiyasatid A, Choowongkomon K. Biochemical characterization of the α-amylase inhibitor in mungbeans and its application in inhibiting the growth of Callosobruchus maculatus. J Agric Food Chem. 2010;58:2131–2137. doi: 10.1021/jf903411x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Takamura H, Matoba T, Terao J. HPLC method for evaluation of the free radical-scavenging activity of foods by using 1,1-diphenyl-2-picrylhydrazyl. Biosci Biotechnol Biochem. 1998;62:1201–1204. doi: 10.1271/bbb.62.1201. [DOI] [PubMed] [Google Scholar]