Knowledge about the impact of obesity and diabetes on the biophysical properties of motoneurons is lacking. We found that diabetes reduces the duration of the afterhyperpolarization and that motoneuron function is unchanged by obesity. A reduced afterhyperpolarization may impact discharge characteristics and may be the first sign of change to motoneuron function.
Keywords: motoneuron, obesity, diabetes, electrophysiology, neurophysiology
Abstract
Small-diameter sensory dysfunction resulting from diabetes has received much attention in the literature, whereas the impact of diabetes on α-motoneurons (MN) has not. In addition, the chance of developing insulin resistance and diabetes is increased in obesity. No study has examined the impact of obesity or diabetes on the biophysical properties of MN. Lean Zucker rats and Zucker diabetic fatty (ZDF) rats were separated into lean, obese (ZDF fed standard chow), and diabetic (ZDF fed high-fat diet that led to diabetes) groups. Glass micropipettes recorded hindlimb MN properties from identified flexor and extensor MN. MN were separated within their groups on the basis of input conductance, which created high- and low-input conductance subpopulations for each. A significant shorter (20%) afterhyperpolarization half-decay (AHP1/2) was found in low-conductance MN for the diabetic group only, whereas AHP½ tended to be shorter in the obese group (19%). Significant positive correlations were found among rheobase and input conductance for both lean and obese animals. No differences were found between the groups for afterhyperpolarization amplitude (AHPamp), input conductance, rheobase, or any of the rhythmic firing properties (frequency-current slope and spike-frequency adaptation index). MN properties continue to be heterogeneous in obese and diabetic animals. Obesity does not seem to influence lumbar MN. Despite the resistance of MN to the impact of diabetes, the reduced AHP1/2 decay and the tendency for a reduction in AHPamp may be the first sign of change to MN function.
NEW & NOTEWORTHY Knowledge about the impact of obesity and diabetes on the biophysical properties of motoneurons is lacking. We found that diabetes reduces the duration of the afterhyperpolarization and that motoneuron function is unchanged by obesity. A reduced afterhyperpolarization may impact discharge characteristics and may be the first sign of change to motoneuron function.
health concerns related to obesity and diabetes are growing. In Canada, adults that are overweight or obese (body mass index >25) constitute 54% of the population (Statistics Canada 2014). Furthermore, obesity is associated with increased risk of developing diabetes in persons greater than 18 yr of age (Millar and Young 2003). It has been shown by many that diabetes disrupts the normal physiological function of small-diameter sensory nerves over time, leading to increased pain and/or loss of sensation (see review, Duby et al. 2004). However, there are few studies that have examined how diabetes affects α-motoneurons (hereafter referred to as motoneuron) in the lumbar spinal cord.
A properly functioning sensory system seems to be necessary for producing optimal motor output. In a spinal transected animal model, with either reduced or eliminated sensory feedback, basic and rhythmic lumbar motoneuron properties show less excitability (Beaumont et al. 2004; Button et al. 2008). However, when spinal transected rats go through a passive cycling exercise regime, proper spinal motoneuron function is restored (Beaumont et al. 2004). Part of this restoration in function may be due to the sensory feedback created by the movement of the lower limbs. As such, any change in small-diameter sensory afferents via diabetes may impact motoneuron output.
In humans, studies have shown that motor units from limb muscles in both type I and type II diabetics show deficits compared with those in nondiabetics. Motor axons were shown to have a decreased conduction velocity, whereas compound muscle action potentials were smaller in amplitude and longer in duration in diabetics (Brown and Feasby 1974; Hansen and Ballantyne, 1977). In addition, motor unit number estimates showed estimated decreases in the thenar and extensor digitorum brevis muscles of adult diabetics (Brown and Feasby 1974), a finding that has been confirmed in additional muscles in adults (Allen et al. 2013; Hansen and Ballantyne 1977) and type I diabetic children (Toth et al. 2014). Functionally, motor unit firing rates, peak force production, and time to fatigue parameters were reduced in human type I diabetics (Almeida et al. 2008), whereas in type II diabetics, both motor unit firing rate and the stability of the force signal were reduced during isometric contraction (Watanabe et al. 2013). Finally, a redistribution of muscle fiber type to a fast-twitch phenotype has been shown to occur as a result of diabetes (Oberbach et al. 2006).
In type I diabetic rodent models, similar changes in the neuromuscular system have been found. Following streptozotocin (STZ) injection (type I diabetes model), mice showed a decrease in motor unit number estimates, increased single motor unit potentials (Souayah et al. 2009), decreased motor axonal conduction velocity, and reduced compound muscle action potential (CMAP) amplitude (Ramji et al. 2007). In addition, neuromuscular junction numbers were reduced by 60% (Ramji et al. 2007), and miniature endplate current amplitudes and acetylcholine quantal release were reduced in response to 1-Hz nerve stimulation (Souayah et al. 2009). In type II diabetic rodents, the Zucker diabetic fatty rat (ZDF) model suffers from slowed motor nerve (Coppey et al. 2002) and sensory nerve conduction velocity, as well as decreased CMAP and sensory nerve action potential amplitudes (Russell et al. 2008). Whereas type I myosin heavy chain (MHC) content decreases, fast type II MHC content increases (Kim et al. 2015). Finally, changes to thermal and mechanical nociception occur (Sugimoto et al. 2008).
At the spinal motoneuron and motor nucleus level, available literature suggests that diabetes may impact motor nuclei size and number. Dorfman et al. (2004) found a decrease in spinal nucleus volume and a shift toward smaller nucleus area in the bulbocavernosus motor nucleus of induced diabetic rats (4 wk post-STZ). However, Ramji et al. (2007) found no difference in either the number or morphological characteristic of motor nuclei in the lumbar spinal cord of mice (8 mo post-STZ) but did find an increase in cellular markers of neuronal stress and protection. In addition, Muramatsu et al. (2012) found that both size and number of presumed γ-motoneurons were reduced (22 wk post-STZ) in rats but later reported (Muramatsu et al. 2017) that large (presumed alpha) and small motoneurons of the medial gastrocnemius nucleus had smaller cross-sectional area (12 wk post-STZ) and were fewer in number (12 and 24 wk post-STZ). Although slightly variable, the balance of results from the available literature suggests motoneurons are susceptible to the effects of diabetes in a type I diabetic model.
A strong association exists between obesity and insulin resistance that leads to type II diabetes, which is likely mediated by widespread chronic inflammation (Hotamisligil et al. 1993; Hotamisligil 2006). Given the association between obesity and diabetes, any change in motoneuron function may exist along a continuum and therefore be evident in obese nondiabetics. In the ZDF model, decreased oxidative capacity and increase in fast type II muscle fibers have been found compared with control rats (Acevedo et al. 2017). In humans, with regard to obesity and the neuromuscular system, the reported impact of being overweight or obese on motoneuron function is limited to voluntary activation assessed via the twitch interpolation technique and muscular strength. Blimkie et al. (1990) showed voluntary activation in adolescent obese males to be lower during isometric knee extensor contractions compared with that in age-matched lean adolescents. In contrast, Garcia-Vicencio and colleagues have shown that adolescent girls have increased voluntary activation of knee extensors during fatiguing isometric contractions (Garcia-Vicencio et al. 2015) and during isometric contractions (Garcia-Vicencio et al. 2016) in both knee extensor and plantar flexor muscle groups. In addition, greater absolute knee extensor strength has been shown in obese adolescent males (Abdelmoula et al. 2012) and females (Garcia-Vicencio et al. 2015) compared with lean individuals, whereas relative strength has been shown to be higher (Abdelmoula et al. 2012), lower (Maffiuletti et al. 2008), or similar (Blimkie et al. 1990) compared with lean adolescents. Relative plantar flexor strength has been evaluated only in females and was recently shown to be greater in obese adolescent girls (Garcia-Vicencio et al. 2016). Although variable, these results may imply that increased body mass may confer a neuromuscular overload effect consistent with changes seen in the neuromuscular system as a result of increased activity in an animal model (wheel and treadmill running; Beaumont and Gardiner 2002, 2003).
Healthy motoneurons have distinct heterogeneous properties that operate over a continuum and relate to the type of muscle fiber innervated. For example, motoneuron afterhyperpolarization amplitude and duration are greater, rheobase current is less, and input resistance is larger in motoneurons that innervate slow-twitch muscle fibers compared with those that innervate fast-twitch muscle fibers (Eccles et al. 1958; Fleshman et al. 1981; Gardiner and Kernell 1990; Zengel et al. 1985). Motoneuron biophysical properties are also not static and respond to exercise (Beaumont and Gardiner 2002, 2003; Beaumont et al. 2004; MacDonell et al. 2012) and sedentary activity (Cormery et al. 2005), as well as eliminating descending and afferent input (Button et al. 2008). To our knowledge, no studies have examined the impact of diabetes or obesity on motoneuron biophysical function. The purpose of this investigation was to establish the impact of diabetes and obesity on flexor and extensor lumbar motoneurons in diabetic and obese rats. Given the reported changes in motor units and reduced motoneuron number and area (Dorfman et al. 2004; Muramatsu et al. 2017), altered electrophysiological properties mentioned above should be evident from the sampled motoneuron pool. In addition, with obesity, the extra mass may confer a training overload effect that would translate to changes in motoneuron properties similar to those seen with exercise trained rats (Beaumont and Gardiner 2002, 2003).
METHODS
Experimental Animals
Female Zucker diabetic fatty (ZDF) and Zucker lean rats were received from Charles River at 6 wk of age. The animals were divided into three groups: 1) Zucker lean (lean) group that were fed standard chow, 2) Zucker obese (obese) group that were of the ZDF strain but were fed standard chow, and 3) Zucker obese-diabetic (diabetic) group that were fed a high-fat diet (D12468; Research Diets, New Brunswick, NJ) that has been shown to consistently induce a diabetic phenotype (Mulder et al. 2010). Rats were caged in pairs in the animal care facility at the University of Manitoba.
A subset of animals had their blood glucose tested (Table 1) at the beginning of the experiment, immediately after induction of a surgical plane using a mixture of isoflurane (5%) and pure oxygen. Following induction, anesthesia was maintained (1–2.5% isoflurane) and was verified by monitoring heart rate and testing bilateral toe-pinch and eye-lash reflexes. Isoflurane delivery occurred until the completion of a precollicular-postmamillary decerebration, after which ventilation of the animal occurred with pure oxygen until termination of the experiment. The animals had a mean age of 9.2 mo at the time of data collection. In accordance with the University of Manitoba animal ethics, at the termination of data collection, animals were killed by an intravenous injection of potassium chloride and a bilateral pneumothorax. The present research was carried out with the approval of the animal ethics committee for the University of Manitoba, which met the guidelines set forth by the Canadian Council of Animal Care.
Table 1.
Zucker rat mass and blood glucose level
| Measure | Mass, g | Glucose, mM |
|---|---|---|
| Lean | 249.2 (24.2) | 7.26 (0.9) |
| Obese | 358.6 (70.2) | 12.0 (6.4) |
| Diabetic | 367.6 (43.9) | 20.5 (4.9) |
Values are means (SD). Bold type indicates a significant difference from other groups.
Surgical Procedures
Immediately following the induction of anesthesia and glucose testing, an intraperitoneal injection of atropine (0.05 mg/kg atropine within 5% dextrose physiological saline) was administered to minimize airway secretions. Following atropine administration, bilateral toe-pinch reflexes were reassessed to ensure the animal was in a surgical plane of anesthesia. The surgical procedures, in order, included 1) exposure of left tibial (extensor) and peroneal (flexor) nerves for antidromic stimulation, 2) insertion of a tracheal tube for ventilation (Harvard Apparatus; rate: 60–80 strokes/min; tidal volume range: 2.0–2.5 ml) and expired CO2 monitoring (levels ranged 3–4%; CAPSTAR 100 CO2 analyzer, CWE, Ardmore, PA); 3) cannulization of the right carotid artery to monitor mean arterial blood pressure (MAP) and provide an infusion port; 4) dexamethasone administration (0.1 ml) via the carotid artery cannula to reduce swelling of the brain; 5) occlusion of left carotid artery with a suture (2-0) before dissection of the back musculature in preparation for a laminectomy (T12 to S1); and 6) laminectomy within a stereotaxic frame. On completion of the laminectomy, the dorsal roots were brushed aside, a mineral oil bath was formed, and the parietal bones of the skull were exposed and excised to prepare for a precollicular-postmamillary decerebration.
In an attempt to reduce bleeding, both carotid arteries were previously tied off. The skull was removed bilaterally, leaving the central and interparietal sutures intact. This resulted in small oval holes in the skull superior to the zygomatic arches with the interparietal suture, the central suture, and 2 mm rostral of bregma as the borders. The sutures and dura were cauterized to reduce bleeding. Under low suction the cortex was removed in the parietal regions while bleeding was controlled with a combination of Surgicel (Johnson and Johnson), Instat (Johnson and Johnson); Gelfoam (Pharmacia and Upjohn); and Surgi absorbent swabs (Kettenbach, Eschenburg, Germany). The remaining skull along the central suture was removed, and the remaining tissue was aspirated until the superior colliculi and thalamus were exposed. At this point, a precollicular cut was made and the hypothalamus, thalamus, and forebrain were removed. Absorbable hemostat was applied to control bleeding throughout the procedure. Typically, MAP decreased to 50 mmHg during the procedure but rebounded to 80 mmHg or more within minutes. However, if MAP did not restore due to excessive hemorrhaging, a saline-alginate (0.07%) solution was administered intravenously to expand plasma volume and restore MAP (Cabrales et al. 2005). Following decerebration, isoflurane delivery was discontinued and the animal ventilated with pure oxygen for data collection. To eliminate movement of the animal from antidromic stimulation of the peripheral nerves, a neuromuscular junction blocker (pancuronium bromide, 2 mg/ml) was administered to paralyze the animal and a unilateral pneumothorax helped reduce movement of the spinal cord related to ventilation. Following the pneumothorax, both tibial and peroneal nerves were mounted using silver chloride hook electrodes, and the micropipette was positioned along the L3-L4 dorsal root.
Intracellular Recordings
Glass micropipettes (1.0-mm thin-walled; World Precision Instruments) were formed with a 1- to 2-μm-diameter Kopf vertical pipette puller (resistance 7–12 MΩ; David Kopf Instruments) and filled with a 2 M solution of potassium citrate. The use of bilateral flexor (peroneal) and extensor (tibial) silver chloride hook electrodes allowed for peripheral nerve stimulation to identify spinal motoneurons antidromically. Stimulation of the peroneal and tibial nerves occurred at a frequency of 2–3 Hz (0.1–0.2 mA for 0.1 ms) whereby the field potentials produced were monitored continuously during micropipette advancement through the spinal cord. Intracellular motoneuron records were collected at 20 kHz by an Axoclamp intracellular amplifier system (Axoclamp 2B; Axon Instruments) used in either bridge or discontinuous current-clamp mode (DCC; 3–10 kHz switching), with capacitance maximally compensated. Evidence of successful motoneuron impalement included a sudden increase in membrane potential to at least 50 mV, an antidromic action potential (AP) spike amplitude >55 mV with a positive overshoot, and a reproducible latency of <2.5 ms from the stimulation artifact. On completion of data collection from a motoneuron, confirmation of the resting membrane potential occurred by backing the micropipette out of the cell using steps of 5 μm.
Intracellular Data
The following intracellular data were collected in DCC mode (3–8 kHz) from antidromically identified hindlimb motoneurons: 1) rheobase, defined as the current amplitude of a 50-ms depolarizing pulse that caused an action potential 50% of the time; 2) input conductance (IC), defined as the reciprocal of the motoneuron input resistance calculated from the average membrane response to 25 or more 150-ms, 1-nA hyperpolarizing current pulses; 3) the discharge response to a 10-s triangular depolarizing ramp current injection, to calculate the frequency-current (F/I) slope to slow input; 4) a series of 500-ms depolarizing current pulse injections to calculate the F/I slope to a fast input; and 5) an adaptation index from the F/I slope calculated from the reciprocal of the averaged last 3 ISI to the first ISI (see below). In addition, resting membrane potential was measured before a short 0.5-ms intracellular depolarizing pulse in bridge mode. An average of at least 30 of the resulting action potentials, the afterhyperpolarization (AHP) amplitude (AHPamp), AHP half-decay (AHP1/2), spike height, and spike duration were measured. Except for the adaptation index and F/I slope relationship (described below), the groups were subdivided into high- and low-conductance subpopulations based on the 50th percentile value of each group (Fig. 1). Those motoneurons with an IC greater than the 50th percentile were designated as high-input conductance motoneurons, whereas those less than or equal to the 50th percentile were designated as low-input conductance motoneurons.
Fig. 1.
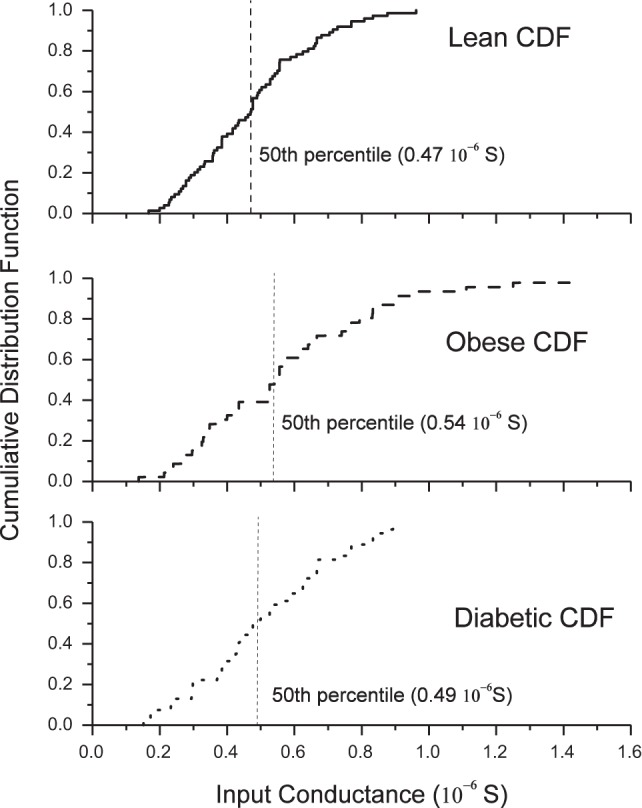
Cumulative distribution function (CDF) of input conductance (IC) for the groups. IC for lean (top), obese (middle), and diabetic groups (bottom) are shown. The 50th percentile for each group, indicated by the vertical hatched line, was used to separate motoneurons into high-input conductance (H-IC) and low-input conductance (L-IC) cells. Those motoneurons with an IC greater than the 50th percentile were designated as high-IC motoneurons. No difference existed between the distributions of IC values between each group.
Frequency-Current Relationship Slope
The slope of the F/I relationship was calculated by applying linear regression to the data obtained from a slow-depolarizing triangular (5-s ascending and 5-s descending) intracellular current injection (slow F/I) and a fast-depolarizing (500-ms) intracellular current injection (fast F/I).
Slow-depolarizing triangular current injection.
The reciprocals of the ISI from action potentials produced by current injection were plotted against current amplitude to obtain the slow F/I relationship, wherefrom the slow F/I slope was calculated.
Fast (500 ms)-depolarizing current injection.
A series of increasing-amplitude 500-ms depolarizing current steps were delivered (0.3 Hz) until the motoneuron failed to discharge the entire 500-ms epoch. The reciprocal of the first ISI produced from each current step (with exception of the step where discharge failed) was plotted against the current amplitude to obtain the F/I relationship, wherefrom the fast F/I slope was calculated.
Spike-Frequency Adaptation Index
Spike-frequency adaptation (SFA) is the time-dependent decrease in discharge rate during a constant depolarizing current injection (Granit et al. 1965). SFA was assessed by creating an index of the F/I slopes calculated from the initial firing frequency and steady-state firing frequency of the action potentials collected during a series of 500-ms depolarizing current injections. The initial firing frequency comprised the first two spikes (initial), whereas the steady-state firing frequency (SSFF) contained the last four spikes. As done previously (MacDonell et al. 2012), the ratio of the SSFF F/I slope to the initial F/I slope yielded the SFA adaptation index (AI).
Statistics
All statistical tests were computed in MATLAB (R2013a; The MathWorks, Natick, MA), and figures were created with Origin (version 7; OriginLab, Northampton, MA). The first step in statistical analysis was to determine whether the dependent variables were distributed normally. For the normality test and subsequent analyses, a P value of ≤0.05 determined significance.
The following variables were found to be not normally distributed according to the Kolmogorov-Smirnov normality test: AHP1/2, AHPamp, AI, IC, rheobase, fast F/I, and slow F/I (P values <0.0001). Because of the decision to separate the motoneurons into high- and low-conductance groups, the Kolmogorov-Smirnov two-sample test was used to determine if the distribution of IC values was different between the groups. The Kolmogorov-Smirnov test returned nonsignificant probability values for each comparison (lean-obese, P = 0.184; lean-diabetic, P = 0.452; obese-diabetic, P = 0.1334).
Given the above results, nonparametric statistics were chosen to determine if differences existed between lean, obese and diabetic hindlimb motoneuron properties. Kruskal-Wallis analysis of variance (KW-ANOVA) on ranks was used to test whether the dependent variables (see above) were significantly different between the groups (lean, obese, and diabetic) but is limited to one level. Rank-sum tests were used to evaluate which groups differed following a significant KW-ANOVA test and also to determine significance between two groups. Spearman's rho (ρ) was used to test the magnitude and direction of any correlation between variables.
Differences between groups for mass and blood glucose levels were tested with parametric tests. Significant differences were tested with separate one-way independent ANOVA. If a significant result was found, a Student’s t-test was used to test for any differences in means between the groups. Because the variances between the groups were unequal, the unequal variance t-test was used to determine the t-critical value.
The increase in familywise error rate due to multiple comparisons was not corrected for because it was deemed that avoiding a type II error (failing to reject the null hypothesis when it should have been rejected) was more important than inflating the type I error rate (rejecting the null hypothesis instead of failing to reject the null hypothesis). Values are presented as the median with the interquartile range (IQR) in brackets for nonparametric data and as mean (SD) for parametric data.
RESULTS
In total, data were collected from 195 hindlimb motoneurons from 50 animals (17 lean, 17 obese, and 16 diabetic). Motoneuron properties were collected from both flexor and extensor motoneuron pools. No significant difference between motoneuron pools was evident. Therefore, flexor and extensor data was pooled. Average mass and glucose levels for each group are displayed in Table 1. Significant main effects for mass [F(2,45) = 3.2, P < 0.00001] and blood glucose levels [F(2,18) = 3.5, P = 0.00013] were found. For blood glucose, all groups had a significantly different blood glucose level, where lean (7.3 mM) < obese (10.2 mM) < diabetic (20.5 mM) animals (P < 0.001). For mass, obese (359 g) and diabetic (367 g) animals had a similar mean mass, but both were heavier than lean (249 g) animals (P < 0.00001).
Figure 1 shows the cumulative distribution of the input conductance for each group and the 50th percentile cutoff that separated the data into high- and low-conductance motoneuron groups. Those cells with an IC above the 50th percentile were categorized as high-IC cells, whereas those at or below the 50th percentile were categorized as low-IC cells. Table 2 contains median (IQR) biophysical properties for hindlimb motoneurons. Median values for IC, AI, fast F/I, slow F/I, and rheobase were found to be similar, whereas AHP1/2 was shorter in low-conductance cells in diabetic animals compared with controls.
Table 2.
Motoneuron properties separated by group
| Lean | Obese | Diabetic | |
|---|---|---|---|
| Input conductance, 10−6 S | 0.48 (0.2); n = 66 | 0.53 (0.42); n = 43 | 0.48 (0.28); n = 54 |
| High IC, 10−6 S | 0.56 (0.16); n = 34 | 0.78 (0.29); n = 20 | 0.67 (0.18); n = 27 |
| Low IC, 10−6 S | 0.33 (0.11); n = 32 | 0.35 (0.16); n = 23 | 0.39 (0.19); n = 27 |
| Rheobase, mV | 7.2 (7.3); n = 58 | 7.8 (8.1); n = 40 | 7.5 (6.6); n = 49 |
| Rheobase–high IC, mV | 10.25(5.8); n = 29 | 10.7 (5.8); n = 19 | 8.5 (5.3); n = 25 |
| Rheobase–low IC, mV | 4.75 (3.0); n = 29 | 4.5 (6.8); n = 21 | 5.6 (7.8); n = 24 |
| AHPAMP–high IC, mV | 1.22 (0.55); n = 34 | 1.25 (0.60); n = 20 | 1.19 (0.70); n = 26 |
| AHP1/2–high IC, ms | 12.20 (2.8); n = 34 | 11.3 (2.8); n = 20 | 12.0 (2.4); n = 26 |
| AHPAMP–low IC, mV | 1.8 (2.8); n = 32 | 1.5 (1.5); n = 22 | 1.2 (0.9); n = 25 |
| AHP1/2–low IC, ms | 15.35 (6.7); n = 32 | 12.5 (8.0); n = 22 | 12.3 (3.8); n = 25 |
| Adaptation index, a.u. | 0.21 (0.25); n = 25 | 0.15 (0.27); n = 21 | 0.19 (0.14); n = 27 |
| Fast F/I | One linear range = 9 | One linear range = 9 | One linear range = 12 |
| Two linear ranges = 15 | Two linear ranges = 15 | Two linear ranges = 16 | |
| F/I slope, Hz⋅nA | 29.75 (12.43) | 30.12 (24.33) | 34.77 (16.35) |
| IPK, nA | 12.87 (10.70) | 11.10 (11.00) | 14.96 (11.01) |
| IMN, nA | 7.03 (7.04) | 9.20 (9.59) | 8.26 (11.12) |
| RatePK, Hz | 246.31 (194.84) | 187.34 (200.15) | 206.19 (187.34) |
| RateMN, Hz | 38.74 (56.27) | 59.99 (84.22) | 56.98 (60.88) |
| Slow F/I | n = 43 | n = 30 | n = 40 |
| F/I slope, Hz⋅nA | 9.71 (8.15) | 11.05 (4.89) | 10.38 (6.2) |
| IPK, nA | 10.08 (9.76) | 10.5 (8.93) | 14.08 (9.14) |
| IMN, nA | 7.17 (9.72) | 7.75 (8.77) | 11.28 (9.2) |
| RatePK, Hz | 48.40 (19.34) | 50.53 (29.64) | 56.61 (22.30) |
| RateMN, Hz | 10.87 (13.69) | 9.54 (10.9) | 9.23 (12.71) |
Data are the median value and interquartile range (IQR) in parentheses; n = no. of motoneurons. Bold type indicates a significant difference from the lean group (P < 0.05). Italic type indicates a tendency to differ from the lean group (P < 0.07). IPK, mean peak depolarizing current; IMN, mean minimum depolarizing current; RatePK, mean peak motoneuron discharge rate, RateMN, mean minimum motoneuron discharge rate. For the fast F/I relationship, “one linear range” indicates that a single linear relationship occurred between motoneuron discharge rate and depolarizing current amplitude, whereas “two linear ranges” indicates that a primary and secondary range of firing with different F/I slopes existed. A motoneuron displaying two linear ranges had a primary range with a lower slope than the secondary slope. The F/I slope for the primary range was used for the mean comparisons between the groups.
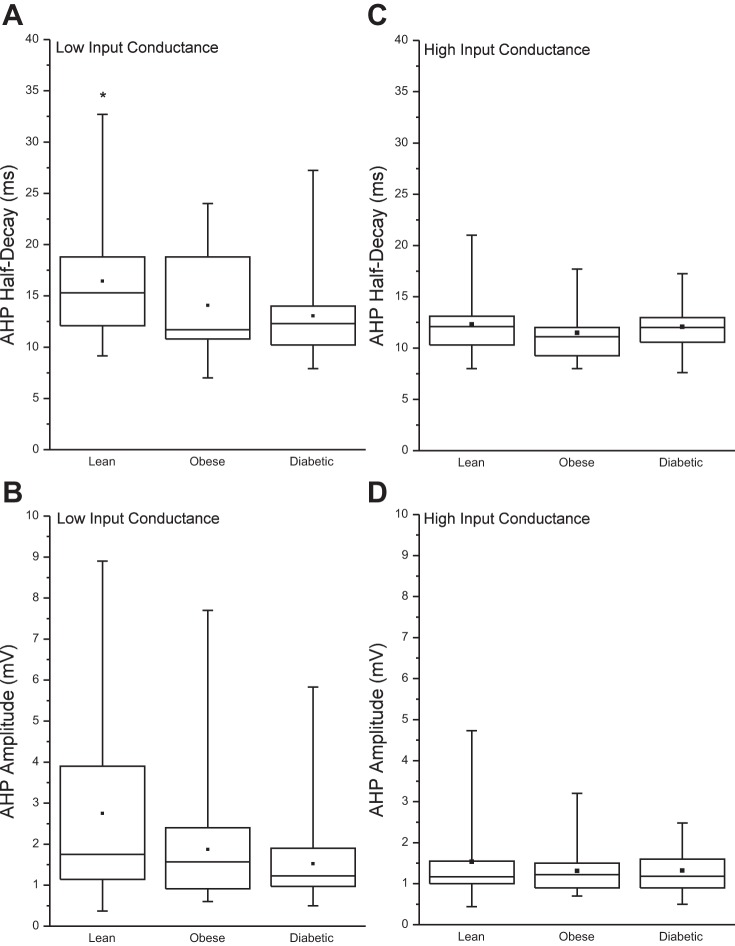
Figure 2 illustrates the AHP1/2 and AHPamp for high-IC and low-IC motoneurons in each of the three groups. The AHPamp and AHP1/2 for high-IC cells showed no difference, and the AHPamp of low-IC cells tended to be smaller in amplitude (P = 0.067). Significant main effects were found for AHP1/2 [χ2(2, 76) = 7.73, P = 0.021] in low-IC cells (Fig. 3), whereby a longer duration AHP1/2 was found in the lean group (15.35 ± 6.9 ms) compared with the diabetic group (12.3 ± 3.8 ms; Z = 2.67, P = 0.0076). The AHP1/2 of the obese group (12.5 ± 8 ms) tended to be shorter than that of the lean group (P = 0.065) but did not differ from that of the diabetic group. When motoneurons are separated into low IC and high IC regardless of experimental group, the low-IC group had a greater AHPamp (1.7 ± 3.1 vs. 1.2 ± 0.55 mV; P = 0.018) and AHP1/2 duration (15.3 ± 6.9 vs. 12.2 ± 2.8 ms; P = 0.0009) than those of high-IC cells.
Fig. 2.
Post-spike afterhyperpolarization (AHP) amplitude and half-decay time. Data were separated into high- and low-input conductance (IC) categories according to the 50th percentile of the IC for each group (see Fig. 1). A and B: AHP half-decay time (A) and AHP amplitude (B) for the low-IC category. C and D: AHP half-decay time (C) and AHP amplitude (D) for the high-IC category. Whiskers represent the range of values, the 25th and 75th percentiles are indicated by the top and bottom of the box, and the median is the horizontal line within the box, whereas the symbol in the center indicates the mean. *P = 0.0026 denotes a significant difference from the diabetic group.
Fig. 3.
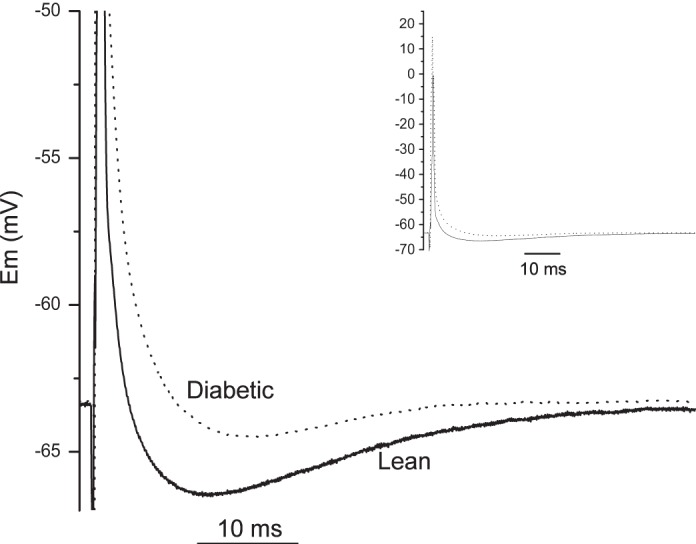
Post-spike afterhyperpolarization (AHP) comparison between lean and diabetic groups. Magnified AHP tracings are representative of the median value from the significantly different (P < 0.007) lean (solid line) and diabetic (dotted line) groups of low-conductance cells. Inset shows the full action potentials of each group (solid line, lean; dotted line, diabetic) generated from a supramaximal orthodromic depolarizing pulse (0.5 ms). Em, membrane potential.
Moderate positive correlations (Fig. 4) between IC and rheobase were found for lean (ρ = 0.65, P < 0.00001), obese (ρ = 0.46, P = 0.003), and diabetic animals (ρ = 0.51, P = 0.0002), indicating that the relationship between excitability and ion conductance was not appreciably altered in the experimental groups. Significant moderate negative correlations were also shown for lean (ρ = −0.51, P = 0.00001) and obese animals (ρ = −0.39, P = 0.0109) for the relationship between AHP1/2 and IC, whereas diabetic animals (ρ = −0.14, P = 0.3257) showed no correlation between AHP1/2 and IC (Fig. 5). Diabetic animals seem to lack the same number of long-duration AHP1/2, compared with that seen in the lean and obese animals, despite having a similar range of IC.
Fig. 4.
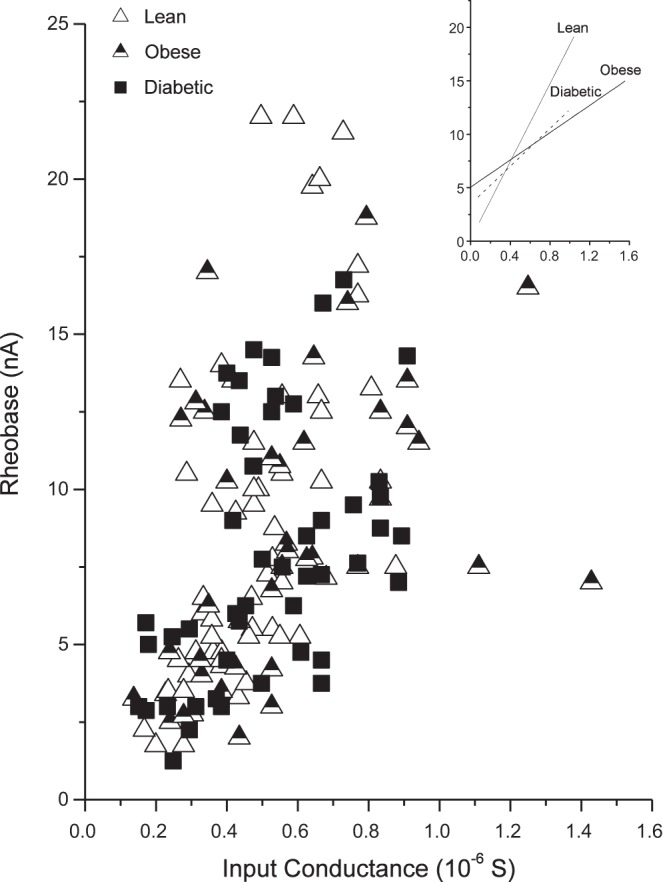
Rheobase vs. input conductance according to groups. Rheobase is shown as a function of input conductance for lean (open triangles; ρ = 0.65, P < 0.00001), obese (filled triangles; ρ = 0.47, P = 0.0028), and diabetic groups (squares; ρ = 0.51, P = 0.000217). Inset shows the lines of best fit for each group.
Fig. 5.
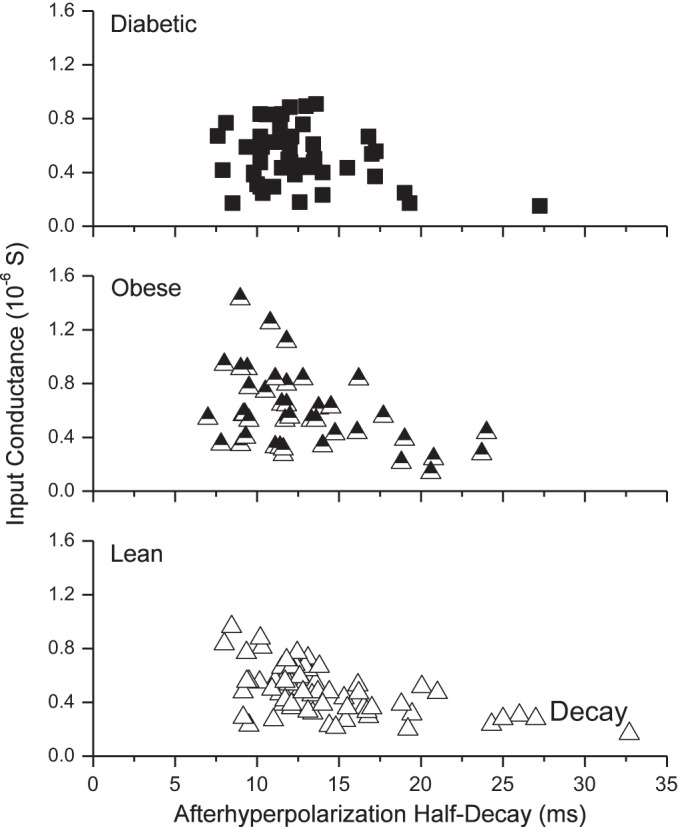
Input conductance as a function of the afterhyperpolarization half-decay. Spearman's rank correlation coefficients were calculated for lean (ρ = −0.51, P < 0.0001), obese (ρ = −0.39, P = 0.01), and diabetic groups (ρ = −0.14, no significance).
DISCUSSION
This investigation is the first to report on biophysical motoneuron properties in diabetic and obese animals. The main finding of this investigation is that spinal lumbar motoneurons are not appreciably impacted by diabetes or obesity, showing that motoneurons maintain their biophysical heterogeneity. A decrease in the half-decay duration of the afterhyperpolarization (AHP) in diabetic animals was the only difference found among the animals and is discussed below. The finding that all other properties measured were similar across groups suggests that changes in motor unit physiology are not necessarily due to a widespread change in function of motoneurons, at least at the level of the lumbar spinal cord. These findings do not preclude changes to motoneuron morphology but does indicate that most electrophysiological parameters remain intact in the Zucker type II diabetes model. As for obese rats, motoneurons were not impacted by increased body mass.
Afterhyperpolarization
This investigation found the AHP half-decay to be reduced in diabetic rats (3-ms median reduction) and a tendency for the amplitude to be reduced in diabetic animals (0.6-mV median reduction). When the motoneuron is driven to fire action potentials via depolarizing current injections, the AHP duration is correlated to the cells minimum rate of discharge (Kernell 1965) and to the type (fast or slow) of motoneuron (Eccles et al. 1958; Gardiner 1993). When the motoneuron is driven to fire by way of mesencephalic locomotor region stimulation, the AHP is largely reduced (cats and rats) and motoneuron firing is more variable (Brownstone et al. 1992; MacDonell et al. 2015). These two scenarios, discharge during quiescence and discharge during motor output, represent two highly different states of synaptic drive, but the average discharge rate between the two scenarios has been found to be similar (Brownstone et al. 1992), suggesting that the AHP may not govern discharge during motor behavior. The faster AHP1/2 in diabetic motoneurons found in this study may represent a transition to more excitable state. This could allow for faster motoneuron discharge at lower levels of drive (a reduced AHP is known to increase the gain of the motoneuron; Lape and Nistri 2000; Miles et al. 2007) and impact the ability of the motoneuron to maintain low rates of firing during fine motor movements/low synaptic drive.
Another possibility is that muscle remodeling influenced the AHP duration of the innervated muscle unit. Although fiber-type distribution was not measured in this study, a shift in fiber-type distribution in human and animals has been shown. Humans with non-insulin-dependent type II diabetes show increased type IIx muscle fibers and decreased type I muscle fibers compared with controls (Mårin et al. 1994; Oberbach et al. 2006). In both ZDF (Kim et al. 2015) and STZ rats (Snow et al. 2005), increases in type II MHC (associated with fast-type muscle fibers) and decreases in the type I MHC (associated with slow-type muscle fibers) compared with control rats have been shown to occur by 12 (STZ) and 13 wk (ZDF). Finally, Russell et al. (2008) showed decreased sensory nerve conduction velocities, CMAP amplitudes, and sensory nerve synaptic potential amplitudes following 10 wk of hyperglycemia. Muscle and sensory changes, documented above, all occurred before the mean age (36.8 wk) of the ZDF rat used in the current study.
If there were a similar reorganization of fiber type in the diabetic animals, motoneurons might have adapted to the change in phenotype due to the speed match that exists between motoneurons and the muscle fibers they innervate (Gardiner and Kernell 1990; MacDonell et al. 2008). A retrograde influence of muscle on motoneuron AHP following muscle denervation/reinnervation has been demonstrated (Foehring and Munson 1990). In these experiments, Foehring and Munson (1990) cross-innervated the medial gastrocnemius and soleus nerves. The electrical properties of the medial gastrocnemius motoneuron pool innervating the soleus muscle changed and became more “slow-like.” That is, the AHP duration and input resistance increased and rheobase decreased. In addition, Cormery et al. (2000) showed that AHP durations in slow motoneurons are shorter in rats after 4 wk of tetrodotoxin-induced hindlimb paralysis. A change in muscle phenotype, therefore, may explain the change in AHP, if a change in fiber-type distribution occurred.
We used the input conductance 50th percentile value to separate the groups into high- and low-input conductance subpopulations. This was done to ensure a change in the AHP parameters was not missed, given that the AHP is mutable (Cormery et al. 2005; Beaumont and Gardiner 2002, 2003). Had we used the AHP1/2 duration criteria to separate putative fast and slow motoneuron types used by Gardiner (1993), any change in AHP might have been masked. Because the cumulative distribution of the input conductance between lean, obese, and diabetic groups did not differ (Fig. 1), and input conductance is a robust measure that relates well with motoneuron type (Zengel et al. 1985), it provided a way to compare motoneurons without missing any potential change in AHP parameters.
Unchanged Motoneuron Properties in Diabetic Animals
In a type I diabetic animal model, Ramji et al. (2007) showed little change to motoneuron morphology or number in the lumbar spinal cord but showed that the tibialis anterior muscle and neuromuscular junction were adversely affected by diabetes. Similarly in a type I diabetes model, Muramatsu et al. (2012) also reported that STZ did not alter α-motoneuron numbers but did suggest that gamma motoneurons were lost. However, a follow-up study by the same group (Muramatsu et al. 2017) showed evidence for reduced motoneuron numbers in the gastrocnemius motor nuclei at 12 and 24 wk, with both the smallest and largest motoneurons being reduced in number, demonstrating variability in assessing motoneuron changes in diabetic models. The ZDF rat is an animal model for type II diabetes. The ZDF rat has been shown to have elevated levels of blood glucose at 8 wk that continue to increase up to 20 wk before plateauing (Sugimoto et al. 2008). In addition, at 16 wk, the ZDF rat developed an increased sensitivity to thermal nociceptive stimuli, and at 18 wk, a decreased response to mechanical nociceptive stimuli occurred (Sugimoto et al. 2008). Other deficits, such as a decreased motor nerve conduction velocity, decreased endoneural blood flow (Coppey et al. 2002), and, as mentioned above, changes in muscle phenotype, occur as early as 13 wk in ZDF rats.
In relation to this, three reasons may be considered for the lack of widespread difference in the biophysical properties of motoneurons in our study. First, our investigation used a type II model of diabetes, whereas the investigations noted above used a type I model of disease. Second, the change in cross-sectional area and motoneuron number found by Muramatsu et al. (2017), although significant, may be too modest to effect biophysical properties. Gardiner (1993) showed that rat motoneurons have a considerable range of properties that include both slow and fast motor units. Given this wide range, the effect of type II diabetes on motoneuron numbers may be too modest to detect, and thus a sampling of the available motor pool reveals little difference. Third, the full impact of diabetes on spinal cord motoneurons might not be fully realized until much later. Diabetic neuropathy is polymodal, and the progression of diabetic neuropathy includes changes to both peripheral nerve fibers (Dyck et al. 1986) and ion channel changes at the DRG (Hong et al. 2004). The progression of neuronal dysfunction in efferent somatic neurons may not be realized at a mean age of 9.2 mo. As such, the reduced AHP1/2 decay and the tendency for a reduction in AHPamp shown in this study may be the first sign of change.
A probability value of 0.05 set the level of significance (i.e., type I error rate); despite the performance of multiple comparisons, no adjustment to the probability value was made. As the number of comparisons increase, so does the likelihood of incorrectly rejecting the null hypothesis (false discovery). Given that this is the first report examining motoneuron biophysical properties in obese and diabetic animals, we chose a more liberal level of significance. Had we adjusted for the false discovery using the equation described by Hassard and Becker (1986), an adjusted P value of 0.026 (20 comparisons) would have been set. In relation to the data presented in this study, this would have impacted only the tendency of the AHP1/2 decay to be different between the obese and lean groups. Although adjusting the false discovery rate is an important practice, its use needs to be evaluated with the type of study being conducted.
Lack of Change in Obese Zucker Rats
To our knowledge, this is the first study to examine the effects of obesity on motoneuron properties. We hypothesized that the sampling distribution of electrophysiological properties may be shifted toward those properties consistent with exercised (running) motoneurons (Beaumont and Gardiner 2002, 2003). The only relevant literature known to the us that examined how the neuromuscular system responds to obesity looked at adolescent boys and girls. These studies suggest that the neuromuscular system adapts to obesity (Abdelmoula et al. 2012; Garcia-Vicencio et. al. 2015; 2016). In this, adolescent boys and girls tend to have increased muscle strength and total muscle activation, although there is discrepancy in the literature (Blimkie et al. 1990; Maffiuletti et al. 2008). Testing whether obesity changed motoneuron properties was important because of the association between obesity and development of type II diabetes (Hotamisligil 2006). The lack of significant results found in this investigation may indicate that obesity simply does not confer any benefit/detriment to motoneurons.
Conclusion
Motoneuron properties continue to be heterogeneous in obese and diabetic animals. Although obesity did not influence motoneuron properties significantly, a tendency toward an altered AHP existed. Despite the motoneurons’ overall resilience, for diabetic neurons the reduced AHP1/2 decay and the tendency for a reduction in AHPamp shown in this study may be the first signs of change to motoneuron function.
GRANTS
This research was supported by a Canadian Institutes of Health Research (CIHR) Team NERVE grant and the Canada Research Chairs program. Financial support was provided by a CIHR Doctoral Award (to J. W. Chopek).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.F.G. conceived and designed research; C.W.M., J.W.C., and K.R.G. performed experiments; C.W.M. analyzed data; C.W.M., J.W.C., and P.F.G. interpreted results of experiments; C.W.M. prepared figures; C.W.M. drafted manuscript; C.W.M., J.W.C., K.R.G., and P.F.G. edited and revised manuscript; C.W.M., J.W.C., K.R.G., and P.F.G. approved final version of manuscript.
REFERENCES
- Abdelmoula A, Martin V, Bouchant A, Walrand S, Lavet C, Taillardat M, Maffiuletti NA, Boisseau N, Duché P, Ratel S. Knee extension strength in obese and nonobese male adolescents. Appl Physiol Nutr Metab 37: 269–275, 2012. doi: 10.1139/h2012-010. [DOI] [PubMed] [Google Scholar]
- Acevedo LM, Raya AI, Ríos R, Aguilera-Tejero E, Rivero JL. Obesity-induced discrepancy between contractile and metabolic phenotypes in slow- and fast-twitch skeletal muscles of female obese Zucker rats. J Appl Physiol (1985) 123: 249–259, 2017. doi: 10.1152/japplphysiol.00282.2017. [DOI] [PubMed] [Google Scholar]
- Allen MD, Choi IH, Kimpinski K, Doherty TJ, Rice CL. Motor unit loss and weakness in association with diabetic neuropathy in humans. Muscle Nerve 48: 298–300, 2013. doi: 10.1002/mus.23792. [DOI] [PubMed] [Google Scholar]
- Almeida S, Riddell MC, Cafarelli E. Slower conduction velocity and motor unit discharge frequency are associated with muscle fatigue during isometric exercise in type 1 diabetes mellitus. Muscle Nerve 37: 231–240, 2008. doi: 10.1002/mus.20919. [DOI] [PubMed] [Google Scholar]
- Beaumont E, Gardiner P. Effects of daily spontaneous running on the electrophysiological properties of hindlimb motoneurones in rats. J Physiol 540: 129–138, 2002. doi: 10.1113/jphysiol.2001.013084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont E, Gardiner PF. Endurance training alters the biophysical properties of hindlimb motoneurons in rats. Muscle Nerve 27: 228–236, 2003. doi: 10.1002/mus.10308. [DOI] [PubMed] [Google Scholar]
- Beaumont E, Houlé JD, Peterson CA, Gardiner PF. Passive exercise and fetal spinal cord transplant both help to restore motoneuronal properties after spinal cord transection in rats. Muscle Nerve 29: 234–242, 2004. doi: 10.1002/mus.10539. [DOI] [PubMed] [Google Scholar]
- Blimkie CJ, Sale DG, Bar-Or O. Voluntary strength, evoked twitch contractile properties and motor unit activation of knee extensors in obese and non-obese adolescent males. Eur J Appl Physiol Occup Physiol 61: 313–318, 1990. doi: 10.1007/BF00357619. [DOI] [PubMed] [Google Scholar]
- Brown WF, Feasby TE. Estimates of functional motor axon loss in diabetics. J Neurol Sci 23: 275–293, 1974. doi: 10.1016/0022-510X(74)90231-7. [DOI] [PubMed] [Google Scholar]
- Brownstone RM, Jordan LM, Kriellaars DJ, Noga BR, Shefchyk SJ. On the regulation of repetitive firing in lumbar motoneurones during fictive locomotion in the cat. Exp Brain Res 90: 441–455, 1992. doi: 10.1007/BF00230927. [DOI] [PubMed] [Google Scholar]
- Button DC, Kalmar JM, Gardiner K, Marqueste T, Zhong H, Roy RR, Edgerton VR, Gardiner PF. Does elimination of afferent input modify the changes in rat motoneurone properties that occur following chronic spinal cord transection? J Physiol 586: 529–544, 2008. doi: 10.1113/jphysiol.2007.141499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrales P, Tsai AG, Intaglietta M. Alginate plasma expander maintains perfusion and plasma viscosity during extreme hemodilution. Am J Physiol Heart Circ Physiol 288: H1708–H1716, 2005. doi: 10.1152/ajpheart.00911.2004. [DOI] [PubMed] [Google Scholar]
- Coppey LJ, Gellett JS, Davidson EP, Dunlap JA, Yorek MA. Changes in endoneurial blood flow, motor nerve conduction velocity and vascular relaxation of epineurial arterioles of the sciatic nerve in ZDF-obese diabetic rats. Diabetes Metab Res Rev 18: 49–56, 2002. doi: 10.1002/dmrr.257. [DOI] [PubMed] [Google Scholar]
- Cormery B, Beaumont E, Csukly K, Gardiner P. Hindlimb unweighting for 2 weeks alters physiological properties of rat hindlimb motoneurones. J Physiol 568: 841–850, 2005. doi: 10.1113/jphysiol.2005.091835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormery B, Marini JF, Gardiner PF. Changes in electrophysiological properties of tibial motoneurones in the rat following 4 weeks of tetrodotoxin-induced paralysis. Neurosci Lett 287: 21–24, 2000. doi: 10.1016/S0304-3940(00)01110-1. [DOI] [PubMed] [Google Scholar]
- Dorfman VB, Vega MC, Coirini H. Reduction of the spinal nucleus of the bulbocavernosous volume by experimental diabetes. Brain Res 1019: 265–269, 2004. doi: 10.1016/j.brainres.2004.05.073. [DOI] [PubMed] [Google Scholar]
- Duby JJ, Campbell RK, Setter SM, White JR, Rasmussen KA. Diabetic neuropathy: an intensive review. Am J Health Syst Pharm 61: 160–176, 2004. [DOI] [PubMed] [Google Scholar]
- Dyck PJ, Lais A, Karnes JL, O’Brien P, Rizza R. Fiber loss is primary and multifocal in sural nerves in diabetic polyneuropathy. Ann Neurol 19: 425–439, 1986. doi: 10.1002/ana.410190503. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. The action potentials of the alpha motoneurones supplying fast and slow muscles. J Physiol 142: 275–291, 1958. doi: 10.1113/jphysiol.1958.sp006015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshman JW, Munson JB, Sypert GW, Friedman WA. Rheobase, input resistance, and motor-unit type in medial gastrocnemius motoneurons in the cat. J Neurophysiol 46: 1326–1338, 1981. [DOI] [PubMed] [Google Scholar]
- Foehring RC, Munson JB. Motoneuron and muscle-unit properties after long-term direct innervation of soleus muscle by medial gastrocnemius nerve in cat. J Neurophysiol 64: 847–861, 1990. [DOI] [PubMed] [Google Scholar]
- Garcia-Vicencio S, Coudeyre E, Kluka V, Cardenoux C, Jegu AG, Fourot AV, Ratel S, Martin V. The bigger, the stronger? Insights from muscle architecture and nervous characteristics in obese adolescent girls. Int J Obes 40: 245–251, 2016. doi: 10.1038/ijo.2015.158. [DOI] [PubMed] [Google Scholar]
- Garcia-Vicencio S, Martin V, Kluka V, Cardenoux C, Jegu AG, Fourot AV, Coudeyre E, Ratel S. Obesity-related differences in neuromuscular fatigue in adolescent girls. Eur J Appl Physiol 115: 2421–2432, 2015. doi: 10.1007/s00421-015-3222-9. [DOI] [PubMed] [Google Scholar]
- Gardiner PF. Physiological properties of motoneurons innervating different muscle unit types in rat gastrocnemius. J Neurophysiol 69: 1160–1170, 1993. [DOI] [PubMed] [Google Scholar]
- Gardiner PF, Kernell D. The “fastness” of rat motoneurones: time-course of afterhyperpolarization in relation to axonal conduction velocity and muscle unit contractile speed. Pflugers Arch 415: 762–766, 1990. doi: 10.1007/BF02584018. [DOI] [PubMed] [Google Scholar]
- Granit R, Kernell D, Smith RS. Delayed depolarization and the repetitive response to intracellular stimulation of mammalian motorneurones. J Physiol 168: 890–910, 1963. doi: 10.1113/jphysiol.1963.sp007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S, Ballantyne JP. Axonal dysfunction in the neuropathy of diabetes mellitus: a quantitative electrophysiological study. J Neurol Neurosurg Psychiatry 40: 555–564, 1977. doi: 10.1136/jnnp.40.6.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassard T, Becker AB. Faulty statistical analysis. J Pediatr 109: 1075–1076, 1986. doi: 10.1016/S0022-3476(86)80306-7. [DOI] [PubMed] [Google Scholar]
- Hong S, Morrow TJ, Paulson PE, Isom LL, Wiley JW. Early painful diabetic neuropathy is associated with differential changes in tetrodotoxin-sensitive and -resistant sodium channels in dorsal root ganglion neurons in the rat. J Biol Chem 279: 29341–29350, 2004. doi: 10.1074/jbc.M404167200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature 444: 860–867, 2006. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science 259: 87–91, 1993. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Kernell D. The limits of firing frequency in cat lumbosacral motoneurones possessing different time course of afterhyperpolarization. Acta Physiol Scand 65: 87–100, 1965. doi: 10.1111/j.1748-1716.1965.tb04252.x. [DOI] [Google Scholar]
- Kim JY, Choi MJ, So B, Kim HJ, Seong JK, Song W. The preventive effects of 8 weeks of resistance training on glucose tolerance and muscle fiber type composition in Zucker rats. Diabetes Metab J 39: 424–433, 2015. doi: 10.4093/dmj.2015.39.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lape R, Nistri A. Current and voltage clamp studies of the spike medium afterhyperpolarization of hypoglossal motoneurons in a rat brain stem slice preparation. J Neurophysiol 83: 2987–2995, 2000. [DOI] [PubMed] [Google Scholar]
- MacDonell CW, Button DC, Beaumont E, Cormery B, Gardiner PF. Plasticity of rat motoneuron rhythmic firing properties with varying levels of afferent and descending inputs. J Neurophysiol 107: 265–272, 2012. doi: 10.1152/jn.00122.2011. [DOI] [PubMed] [Google Scholar]
- MacDonell CW, Ivanova TD, Garland SJ. Afterhyperpolarization time-course and minimal discharge rate in low threshold motor units in humans. Exp Brain Res 189: 23–33, 2008. doi: 10.1007/s00221-008-1400-2. [DOI] [PubMed] [Google Scholar]
- MacDonell CW, Power KE, Chopek JW, Gardiner KR, Gardiner PF. Extensor motoneurone properties are altered immediately before and during fictive locomotion in the adult decerebrate rat. J Physiol 593: 2327–2342, 2015. doi: 10.1113/JP270239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffiuletti NA, Jubeau M, Agosti F, De Col A, Sartorio A. Quadriceps muscle function characteristics in severely obese and nonobese adolescents. Eur J Appl Physiol 103: 481–484, 2008. doi: 10.1007/s00421-008-0737-3. [DOI] [PubMed] [Google Scholar]
- Mårin P, Andersson B, Krotkiewski M, Björntorp P. Muscle fiber composition and capillary density in women and men with NIDDM. Diabetes Care 17: 382–386, 1994. doi: 10.2337/diacare.17.5.382. [DOI] [PubMed] [Google Scholar]
- Miles GB, Hartley R, Todd AJ, Brownstone RM. Spinal cholinergic interneurons regulate the excitability of motoneurons during locomotion. Proc Natl Acad Sci USA 104: 2448–2453, 2007. doi: 10.1073/pnas.0611134104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar WJ, Young TK. Tracking diabetes: prevalence, incidence and risk factors. Health Rep 14: 35–47, 2003. [PubMed] [Google Scholar]
- Mulder GB, Luo S, Gramlich P. Diet Evaluation Study for the Induction of Type 2 Diabetes in Obese Female ZDF Rats. (The Zucker Diabetic Fatty (ZDF) Rat Technical Sheet: Charles River Research Models and Services). Wilmington, MA: Charles River Laboratories International, 2010. [Google Scholar]
- Muramatsu K, Niwa M, Nagai M, Kamimura T, Sasaki S, Ishiguro T. The size of motoneurons of the gastrocnemius muscle in rats with diabetes. Neurosci Lett 531: 109–113, 2012. doi: 10.1016/j.neulet.2012.10.031. [DOI] [PubMed] [Google Scholar]
- Muramatsu K, Niwa M, Tamaki T, Ikutomo M, Masu Y, Hasegawa T, Shimo S, Sasaki SI. Effect of streptozotocin-induced diabetes on motoneurons and muscle spindles in rats. Neurosci Res 115: 21–28, 2017. doi: 10.1016/j.neures.2016.10.004. [DOI] [PubMed] [Google Scholar]
- Oberbach A, Bossenz Y, Lehmann S, Niebauer J, Adams V, Paschke R, Schön MR, Blüher M, Punkt K. Altered fiber distribution and fiber-specific glycolytic and oxidative enzyme activity in skeletal muscle of patients with type 2 diabetes. Diabetes Care 29: 895–900, 2006. doi: 10.2337/diacare.29.04.06.dc05-1854. [DOI] [PubMed] [Google Scholar]
- Ramji N, Toth C, Kennedy J, Zochodne DW. Does diabetes mellitus target motor neurons? Neurobiol Dis 26: 301–311, 2007. doi: 10.1016/j.nbd.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Russell JW, Berent-Spillson A, Vincent AM, Freimann CL, Sullivan KA, Feldman EL. Oxidative injury and neuropathy in diabetes and impaired glucose tolerance. Neurobiol Dis 30: 420–429, 2008. doi: 10.1016/j.nbd.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow LM, Sanchez OA, McLoon LK, Serfass RC, Thompson LV. Myosin heavy chain isoform immunolabelling in diabetic rats with peripheral neuropathy. Acta Histochem 107: 221–229, 2005. doi: 10.1016/j.acthis.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Souayah N, Potian JG, Garcia CC, Krivitskaya N, Boone C, Routh VH, McArdle JJ. Motor unit number estimate as a predictor of motor dysfunction in an animal model of type 1 diabetes. Am J Physiol Endocrinol Metab 297: E602–E608, 2009. doi: 10.1152/ajpendo.00245.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistics Canada. Body mass index, overweight or obese, self-reported, adult, by sex, provinces and territories (percent) (Online). http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/health82b-eng.htm?sdi=body%20mass%20index. [Modified March 7, 2016].
- Sugimoto K, Rashid IB, Kojima K, Shoji M, Tanabe J, Tamasawa N, Suda T, Yasujima M. Time course of pain sensation in rat models of insulin resistance, type 2 diabetes, and exogenous hyperinsulinaemia. Diabetes Metab Res Rev 24: 642–650, 2008. doi: 10.1002/dmrr.903. [DOI] [PubMed] [Google Scholar]
- Toth C, Hebert V, Gougeon C, Virtanen H, Mah JK, Pacaud D. Motor unit number estimations are smaller in children with type 1 diabetes mellitus: a case-cohort study. Muscle Nerve 50: 593–598, 2014. doi: 10.1002/mus.24212. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Gazzoni M, Holobar A, Miyamoto T, Fukuda K, Merletti R, Moritani T. Motor unit firing pattern of vastus lateralis muscle in type 2 diabetes mellitus patients. Muscle Nerve 48: 806–813, 2013. doi: 10.1002/mus.23828. [DOI] [PubMed] [Google Scholar]
- Zengel JE, Reid SA, Sypert GW, Munson JB. Membrane electrical properties and prediction of motor-unit type of medial gastrocnemius motoneurons in the cat. J Neurophysiol 53: 1323–1344, 1985. [DOI] [PubMed] [Google Scholar]