Levya-Díaz et al. identify a Caenorhabditis elegans gene involved in transgene silencing and RNA interference.
Keywords: Caenorhabditis elegans, transgene silencing, RNA interference
Abstract
Repetitive DNA sequences are subject to gene silencing in various animal species. Under specific circumstances repetitive DNA sequences can escape such silencing. For example, exogenously added, extrachromosomal DNA sequences that are stably inherited in multicopy repetitive arrays in the nematode Caenorhabditis elegans are frequently silenced in the germline, whereas such silencing often does not occur in the soma. This indicates that somatic cells might utilize factors that prevent repetitive DNA silencing. Indeed, such “antisilencing” factors have been revealed through genetic screens that identified mutant loci in which repetitive transgenic arrays are aberrantly silenced in the soma. We describe here a novel locus, pals-22 (for protein containing ALS2CR12 signature), required to prevent silencing of repetitive transgenes in neurons and other somatic tissue types. pals-22 deficiency also severely impacts animal vigor and confers phenotypes reminiscent of accelerated aging. We find that pals-22 is a member of a large family of divergent genes (39 members), defined by homology to the ALS2CR12 protein family. While gene family members are highly divergent, they show striking patterns of chromosomal clustering. The family expansion appears C. elegans-specific and has not occurred to the same extent in other nematode species for which genome sequences are available. The transgene-silencing phenotype observed upon loss of PALS-22 protein depends on the biogenesis of small RNAs. We speculate that the pals gene family may be part of a species-specific cellular defense mechanism.
Over half the human genome consists of repetitive DNA elements (Lander et al. 2001; de Koning et al. 2011). The view of the role of repetitive DNA has evolved over the last few decades from considering it as “junk DNA” to the recognition of repetitive DNA as being essential for genome function (Doolittle and Sapienza 1980; Orgel and Crick 1980; Lynch and Conery 2003; Shapiro and von Sternberg 2005). The main constituents of these repetitive DNA elements are retrotransposons, a large family of transposable elements capable of copying themselves and reinserting into the host genome (Kazazian 2004; Goodier and Kazazian 2008; Cordaux and Batzer 2009). Retrotransposons and other elements with the ability to copy themselves pose a threat to genome integrity due to the potential deleterious effects of landing in coding or regulatory regions (Friedli and Trono 2015). The activation of proto-oncogenes in some leukemias represent an example of such harmful consequences (Hacein-Bey-Abina et al. 2003). However, repetitive DNA elements have also been found to play beneficial roles in a number of processes, ranging from the regulation of gene expression to interaction with nuclear structures for genome packaging, to DNA repair and restructuring (Shapiro and von Sternberg 2005; Goke and Ng 2016). Hence, transposable elements have been postulated as a powerful genetic force involved in the evolution of organismal complexity (Kazazian 2004; Friedli and Trono 2015).
To balance the deleterious and beneficial features of repetitive sequences, organisms have evolved ways to finely tune the regulation of repetitive DNA elements (Schlesinger and Goff 2015; Chuong et al. 2017). For example, endogenous silencing mechanisms have evolved to prevent genome damage by the spread of mobile repetitive DNA elements. Silencing can be directed by sequence-specific transcription factors and by small RNAs. For example, retrotransposons are extensively recognized by the Krueppel-associated box-zinc finger (KRAB-ZFP) proteins (Rowe et al. 2010; Quenneville et al. 2012; Jacobs et al. 2014), which form a large family of repressive transcription factors. These transcription factors are among the fastest evolving group of genes in the human genome and their diversity facilitates their ability to recognize a large number of retrotransposons (Nowick et al. 2010). Sequence-specific binding to retrotransposons by KRAB-ZFP factors triggers a cascade leading to chromatin-based silencing mechanisms (Wolf and Goff 2009).
RNAs of retrotransposons that escape transcriptional silencing are targeted and destroyed by the small RNA pathways in the cytoplasm (Toth et al. 2016). RNA-based mechanisms represent the most ancient defense against the genomic spread of repetitive DNA elements (Friedli and Trono 2015). These mechanisms comprise the action of small RNA molecules, including small interfering RNAs (siRNAs), PIWI-interacting RNAs (piRNAs), and microRNAs (miRNAs), which guide repressor protein complexes to particular targets in a sequence-specific manner. In addition to intervening at the post-transcriptional level, small RNAs can also intervene at the transcriptional level by directing deposition of repressive histone marks and DNA methylation to copies of retrotransposons and other elements (Le Thomas et al. 2013). piRNAs and siRNAs can be produced from repetitive DNA elements, which they silence in return (Law and Jacobsen 2010). Thus, in many organisms, repeated sequences can be particularly sensitive to gene silencing.
Repetitive DNA elements are not only abundant in vertebrate genomes. Repetitive DNA elements also abound in model organisms with smaller genome sizes; repetitive DNA accounts for 34–57% of the total genome in Drosophila melanogaster (Celniker et al. 2002), and at least 17% of the Caenorhabditis elegans genome (Stein et al. 2003). Repetitive DNA elements can also be generated experimentally. DNA transformation techniques in C. elegans produce repetitive extrachromosomal DNA arrays (“simple” arrays) (Stinchcomb et al. 1985). Several studies have shown that expression of transgenes organized in these repetitive arrays is silenced both in somatic cells and in the germline through heterochromatin formation, involving several chromatin factors [reviewed in Cui and Han (2007)]. Somatic and especially germline transgene expression can be improved when the transgenic DNAs are cotransformed with an excess of carrier DNA, producing a less repetitive, more “complex” array (Kelly et al. 1997).
Importantly, gene expression from repetitive genomic regions can still be observed, suggesting that there are mechanisms that can antogonize silencing effects (Tseng et al. 2007). Multiple forward genetic screens in C. elegans have indeed identified factors that act to counter silencing of genes contained in repetitive sequences, based on screens for mutations that alter gene expression from transgenes present in tandemly repeated arrays (Hsieh et al. 1999; Grishok et al. 2005; Tseng et al. 2007; Fischer et al. 2013). In a classic study, mutations in tam-1 (a RING finger/B-box factor) were found to reduce the expression of transgenes organized in simple but not complex repetitive arrays (Hsieh et al. 1999). Therefore, tam-1 is an “antisilencing factor” that attenuates the context-dependent silencing mechanism affecting multicopy tandem-array transgenes in C. elegans. A subsequent study identified mutations in another gene important for expression of repetitive sequences, lex-1, which genetically interacts with tam-1 (Tseng et al. 2007). LEX-1 encodes a protein containing an ATPase domain and a bromodomain, both of which suggest that LEX-1 associates with acetylated histones and modulates chromatin structure. Hence, TAM-1 and LEX-1 are antisilencing factors that function together to influence chromatin structure and to promote expression from repetitive sequences.
Further studies found that tandem-array transgenes become silenced in most mutants that cause enhanced exogenous RNA interference (RNAi) (Simmer et al. 2002; Kennedy et al. 2004; Fischer et al. 2013). Examples of gene inactivation known to cause increased transgene silencing and enhanced RNAi include the retinoblastoma-like gene lin-35 (Hsieh et al. 1999; Wang et al. 2005; Lehner et al. 2006), the RNA-dependent RNA polymerase rrf-3 (Simmer et al. 2002), and the helicase gene eri-6/7 (Fischer et al. 2008). Some of the silencing acting on repetitive DNA elements (multicopy transgenes) depends on a complex interaction between different small RNA pathways (Fischer et al. 2013).
Here, we identify a novel locus, pals-22 (protein containing ALS2CR12 signature), whose loss confers a transgene-silencing phenotype. pals-22 mutants display context-dependent array silencing, affecting the expression of highly repetitive transgenes but not single-copy reporters. Animals lacking pals-22 show locomotory defects and premature aging. pals-22 is a member of a large family of divergent genes defined by homology to the ALS2CR12 protein family (Interpro, IPR026674; Panther, PTHR21707). The ALS2CR12 protein family is specifically expanded in C. elegans, and pals gene family members are clustered in the genome. We found that transgene silencing on pals-22 mutants depends on a component of the RNAi pathway, indicating that pals-22 might act as regulator of small RNA-dependent gene silencing.
Materials and Methods
Mutant strains
Strains were maintained by standard methods (Brenner 1974). The C. elegans mutant alleles used in this study were: pals-22(ot723), pals-22(ot810), pals-22(ot811), rde-4(ne301) (Tabara et al. 1999), and tam-1(cc567) (Hsieh et al. 1999).
Reporter and transgenic strains
The C. elegans transgenic strains used in this study were otIs381[ric-19prom6::NLS::gfp], otIs380[ric-19prom6::NLS::gfp], ccIs4251[myo-3prom::gfp], otIs251[cat-2prom::gfp], otIs355[rab-3prom1::NLS::tagrfp], otIs447[unc-3prom::mChOpti], otEx6944 [ric-4prom26::NLS::yfp], otIs620[unc-11prom8::NLS::gfp], otIs353[ric-4fosmid::SL2::NLS-YFP-H2B], otIs534[cho-1fosmid::SL2::NLS-YFP-H2B], otTi32[lin-4prom::yfp], ieSi60[myo-2prom::TIR1::mRuby], otEx7036[pals-22prom::gfp], otEx7037[pals-22::gfp], and jySi37[pals-22::gfp]. pals-22::gfp reporters (otEx7036 and otEx7037) were generated using a PCR fusion approach (Hobert 2002). Genomic fragments were fused to the GFP coding sequence, which was followed by the unc-54 3′-UTR. See Supplemental Material, Table S1 in File S1 for transgenic strain names and microinjection details.
Forward genetic screens
Standard ethyl methanesulfonate (EMS) mutagenesis was performed on the fluorescent transgenic reporter strain, otIs381[ric-19prom6::NLS::gfp], and ∼60,000 haploid genomes were screened for expression defects with an automated screening procedure (Doitsidou et al. 2008) using the Union Biometrica COPAS FP-250 system. The mutant allele pals-22(ot723) was identified in an independent manual clonal screen for changes in reporter expression in neurons of otIs381[ric-19prom6::NLS::gfp] after EMS mutagenesis. ot723, ot810, and ot811 were the only three mutations derived from screens with otIs381[ric-19prom6::NLS::gfp]. To identify the causal genes of the mutants obtained, we performed Hawaiian single-nucleotide polymorphism mapping and a whole-genome sequencing pipeline (Doitsidou et al. 2010; Minevich et al. 2012).
Microscopy
Worms were anesthetized using 100 mM sodium azide (NaN3) and mounted on 5% agarose pads on glass slides. All images (except Figure 3, B, C, and E and Figure S1C in File S1) were acquired as Z-stacks of ∼1 μm-thick slices with the Micro-Manager software (Edelstein et al. 2010) using the Zeiss Axio Imager.Z1 automated fluorescence microscope (Zeiss [Carl Zeiss], Thornwood, NY). Images were reconstructed via maximum intensity Z-projection of 2–10 μm Z-stacks using the ImageJ software (Schneider et al. 2012). Images shown in Figure 3, B, C, and E and Figure S1C in File S1 were acquired using a Zeiss confocal microscope (LSM880). Several Z-stack images (each ∼0.4 μm thick) were acquired with the ZEN software. Representative images are shown following orthogonal projection of 2–10 μm z-stacks.
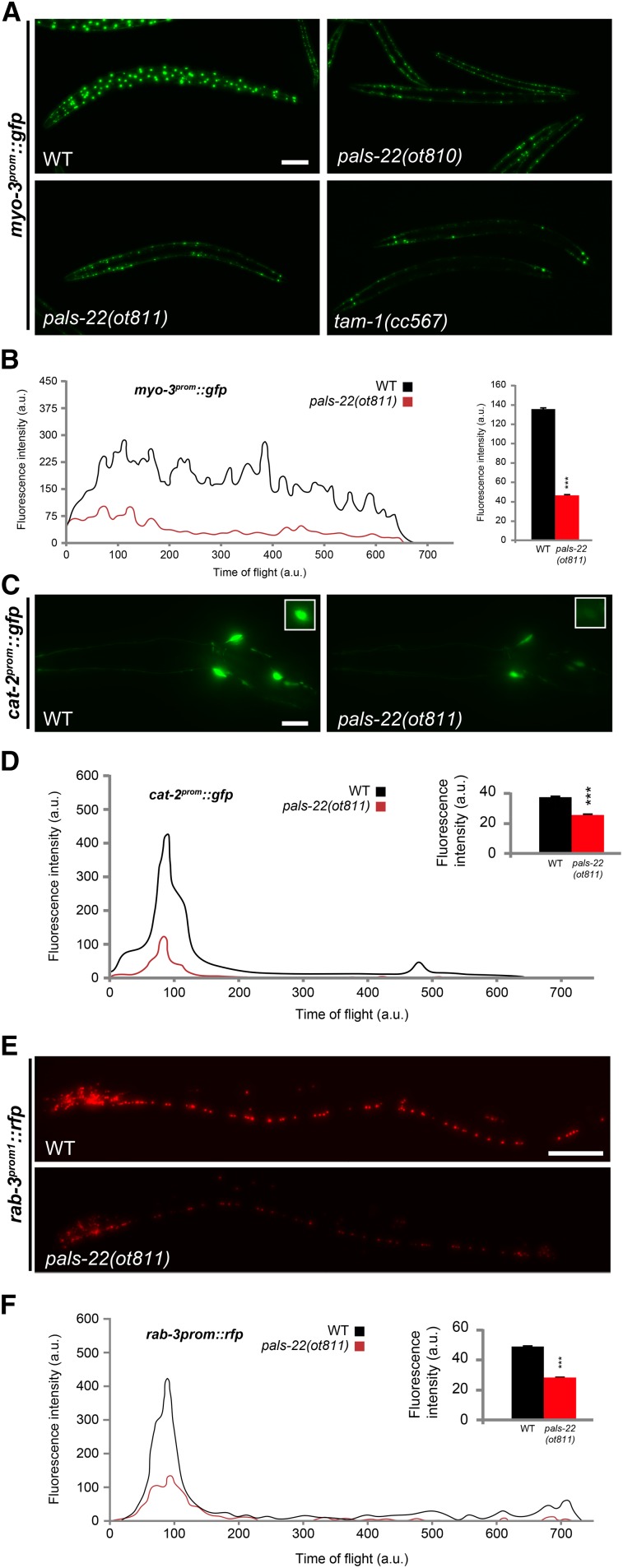
Figure 3.
pals-22 mutants show silencing of several multicopy arrays. (A) The myo-3prom::gfp transcriptional reporter is brightly expressed in all body muscles in wild-type (WT) N2 worms, with a combination of mitochondrial and nuclear localization. In pals-22(ot810), pals-22(ot811), and tam-1(cc567) mutants, a generalized reduction in GFP fluorescence is observed. All images correspond to L4 worms. Bar, 50 µm. (B) Fluorescence profile of a representative individual day 1 adult worm expressing myo-3prom::gfp in WT (black line) or pals-22(ot811) mutant background (red line) obtained with a COPAS FP-250 system. Time of flight indicates worm length, with lower values corresponding to the head of the worms. a.u., arbitrary units. Inset bar graph displays quantification of total fluorescence intensity averaged over 1000 animals analyzed by COPAS. The data are presented as mean + SEM. Unpaired t-tests were performed for pals-22(ot811) compared to WT; *** P < 0.001. (C) GFP images showing silencing of cat-2prom::gfp expression in the head of pals-22(ot811) mutants (right) compared to WT L4 worms (left). The cat-2prom::gfp transcriptional reporter is expressed in all dopamine neurons: CEPD, CEPV, and ADE in the head, and PDE in the posterior midbody (top right insets). Bar, 10 µm. (D) Fluorescence profile of a representative individual day 1 adult worm expressing cat-2prom::gfp in WT (black line) or pals-22(ot811) mutant background (red line) obtained with a COPAS FP-250 system. Time of flight indicates worm length, with lower values corresponding to the head of the worms. a.u., arbitrary units. Inset bar graph displays quantification of total fluorescence intensity averaged over 1000 animals analyzed by COPAS. The data are presented as mean + SEM. Unpaired t-tests were performed for pals-22(ot811) compared to WT; *** P < 0.001. (E) Red fluorescent protein images showing silencing of rab-3prom1::rfp expression in the head of pals-22(ot811) mutants (right) compared to WT L4 worms (left). (F) Fluorescence profile of a representative individual day 1 adult worm expressing rab-3prom1::rfp in WT (black line) or pals-22(ot811) mutant background (red line) obtained with a COPAS FP-250 system. Time of flight indicates worm length, with lower values corresponding to the head of the worms. a.u., arbitrary units. Inset bar graph displays quantification of total fluorescence intensity averaged over 1000 animals analyzed by COPAS. The data are presented as mean + SEM. Unpaired t-tests were performed for pals-22(ot811) compared to WT; *** P < 0.001.
Single-molecule FISH (smFISH)
smFISH was done as previously described (Ji and van Oudenaarden 2012). Samples were incubated overnight at 37° during the hybridization step. The ric-19 and gfp probes were designed using the Stellaris RNA FISH probe designer and were obtained conjugated to Quasar 670 from Biosearch Technologies.
Fluorescence quantification
Synchronized day 1 adult worms were grown on NGM plates seeded with OP50 and incubated at 20°. The COPAS FP-250 system (Union Biometrica) was used to measure the fluorescence of 200–1000 worms for each strain. In Figures S2 and S3 in File S1, ImageJ software was used to measure the fluorescence intensity in the head of L4 worms (images obtained in the Zeiss Axio Imager.Z1 automated fluorescence microscope as described in Microscopy).
Bioinformatic analysis
The ALS2CR12 family protein phylogenetic tree was generated using MrBayes (Huelsenbeck and Ronquist 2001; Ronquist and Huelsenbeck 2003). Default setting for MrBayes were as follows: lset nst = 6, rates = invgamma, and ngen increased until the standard deviation of split frequencies was < 0.05. Input protein coding sequences for ALS2CR12 protein family proteins were aligned with M-Coffee (Wallace et al. 2006) using default settings. The MrBayes tree figure was rendered with FigTree (http://tree.bio.ed.ac.uk/software/figtree/). The ALS2CR12 signature logo was generated using Skylign.org (Wheeler et al. 2014) from the PANTHER database (http://www.pantherdb.org/) (Mi et al. 2013, 2017) HMM profile PTHR21707.SF42.
RNAi by feeding
RNAi was performed as previously described with minor adaptations (Kamath and Ahringer 2003). L4-stage hermaphrodite worms were placed onto NGM plates containing seeded bacteria expressing dsRNA for each assayed gene. After 24 hr at 20°, adults were removed. After a further 36–40 hr at 20°, phenotypes were scored blindly.
Swim analysis
The swimming assay was performed using the CeleST program as previously described (Restif et al. 2014). In brief, we transferred five (day 1) adult hermaphrodites into 50 µl M9 buffer located in a 10-mm staggered ring on a glass slide. A 30-sec dark-field video (18 frames per second) was immediately recorded via StreamPix 7. Multiple features of the swim behavior were then analyzed using CeleST (Restif et al. 2014). Graphpad Prism 6 was used for data plotting and statistics.
Crawling assay
We washed ∼30 day 1 adult hermaphrodites into M9 buffer (containing 0.2% BSA to prevent worms sticking to plastic tips) via low-speed centrifuging. We transferred these worms (in 20 µl volume) to a NGM agar plate (60 mm). After the liquid was completely absorbed and most animals were separated from each other, we started a 30-sec video recording (20 frames per second). The video was processed on ImageJ and analyzed via wrMTrck plugin (Nussbaum-Krammer et al. 2015). The crawling paths were generated in ImageJ.
Age pigment assay
Age pigments of day 5 adult hermaphrodite (Gerstbrein et al. 2005) were captured via Zeiss LSM510 Meta Confocal Laser Scanning Microscope (excitation: Water cooled Argon laser at 364 nm; emission: 380–420 nm). The auto-fluorescence intensity was quantified in ImageJ.
Life span
Synchronized worms were picked at the L4 stage, and fed with OP50-1 bacteria on a 35 mm NGM agar plate (12 worms per plate, ∼100 animals initiating each trial). Before the end of reproductive phase, animals were transferred into a new plate every 2 days to keep adults separated from progeny. Immobile animals without any response to touch were counted as dead; bagged worms were also counted as deaths; and animals crawling off the NGM agar were counted as lost and were excluded from analysis.
Data availability
Strains are available upon request. File S1 contains four supplemental figures, supplemental figure legends, and one supplemental table.
Results
pals-22 mutants show a transgene-silencing phenotype
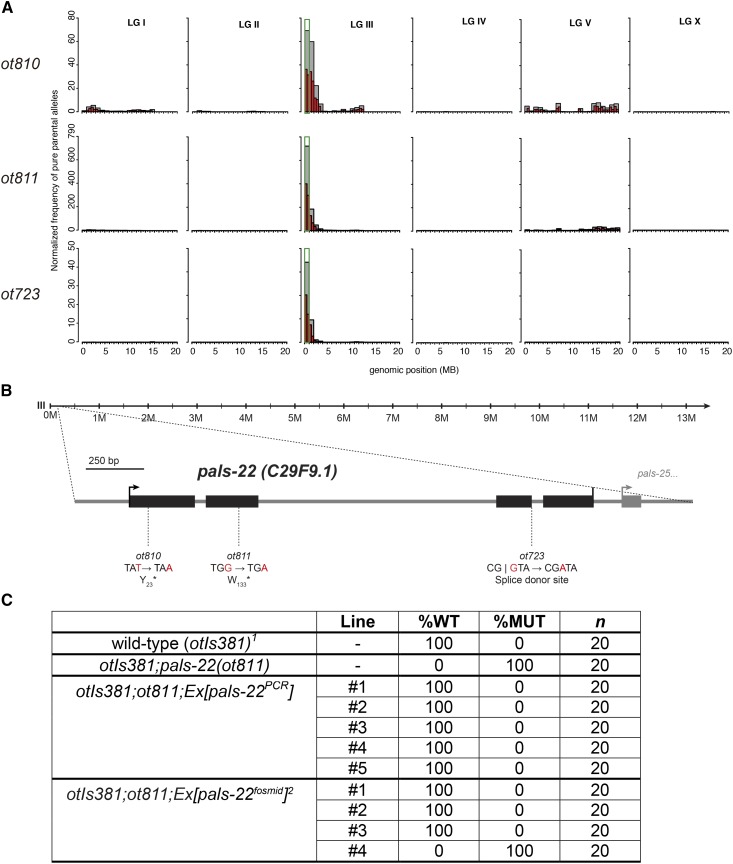
Based on our long-standing interest in studying the regulation of pan-neuronal gene expression (Stefanakis et al. 2015), we sought to use genetic mutant screens to isolate factors that control the expression of pan-neuronally-expressed reporter transgenes. One screen that we undertook used a regulatory element from the pan-neuronally-expressed ric-19 locus, fused to gfp (otIs381[ric-19prom6::NLS::gfp]) (Stefanakis et al. 2015). We identified three independent mutant alleles, ot723, ot810, and ot811, in which expression of ric-19prom6::NLS::gfp was reduced throughout the nervous system in all animals examined (Figure 1, A and B). Using our previously described whole-genome sequencing and mapping pipeline (Doitsidou et al. 2010; Minevich et al. 2012) we found that all three mutations affect the same locus, C29F9.1 (Figure 2, A and B), which we named pals-22 for reasons that we explain further below. The ric-19prom6::NLS::gfp expression defect of pals-22(ot811) can be rescued by a fosmid (WRM0616DC09) encompassing the pals-22 locus plus neighboring genes, as well as a genomic fragment that only contains the pals-22 locus (791 bp upstream of the start codon to the stop codon and its 3′-UTR) (Figure 2C). Both ot810 and ot811 alleles carry early nonsense mutations and are therefore predicted to be null alleles (Figure 2B).
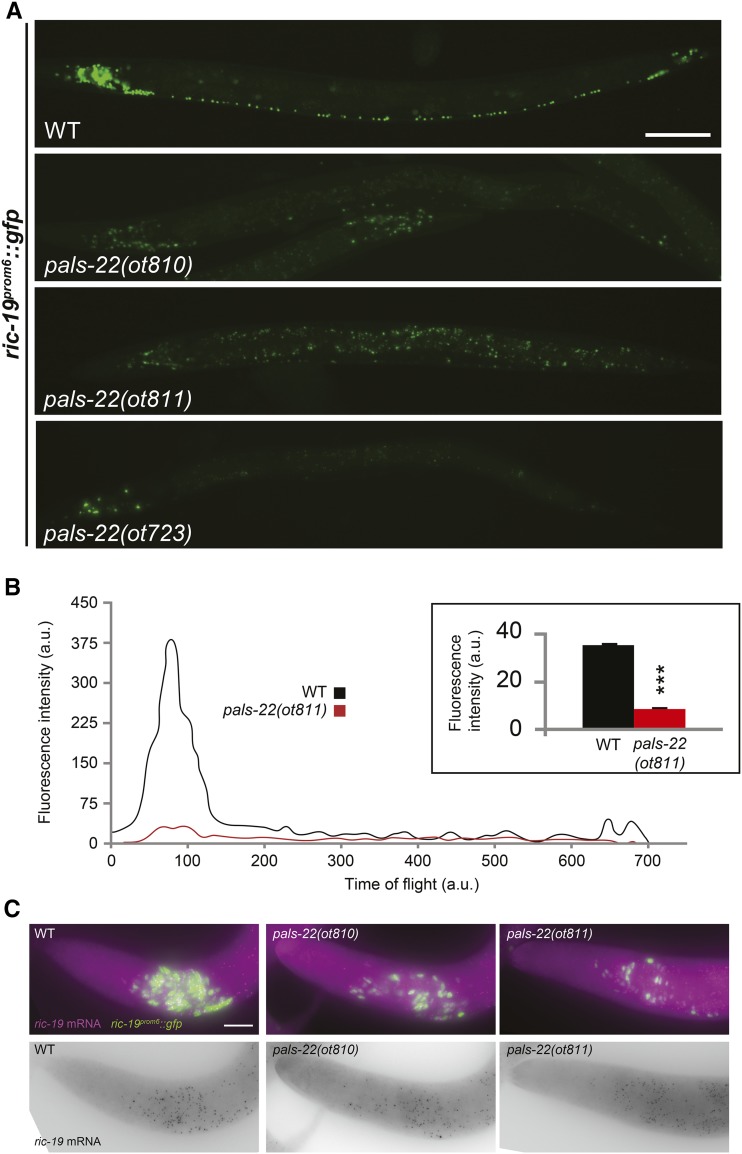
Figure 1.
Loss of pan-neuronal reporter gene expression in pals-22 mutants. (A) The ric-19prom6::NLS::gfp transcriptional reporter is brightly expressed in all neurons in wild-type (WT) N2 worms, with a nuclear localization. In pals-22(ot723), pals-22(ot810), and pals-22(ot811) mutants, expression is reduced throughout the nervous system. All images correspond to L4 worms. Bar, 50 µm. (B) Fluorescence profiles of a representative individual day 1 adult worm expressing ric-19prom6::NLS::gfp in WT (black line) or pals-22(ot811) mutant background (red line) obtained with a COPAS FP-250 system. Time of flight indicates worm length, with lower values corresponding to the head of the worms. a.u., arbitrary units. Inset bar graph displays quantification of total fluorescence intensity averaged over 500 animals analyzed by COPAS. The data are presented as mean + SEM. Unpaired t-tests were performed for pals-22(ot811) compared to WT; *** P < 0.001. (C) WT (left), pals-22(ot810) (middle), and pals-22(ot811) (right) images of the anterior part of L3 worms showing equal ric-19 mRNA levels in control and mutant worms as assessed by single-molecule fluorescence in situ hybridization. Individual transcripts shown as purple dots in top and as black dots in bottom panels. GFP expression of the reporter transgene is shown in green. At least 20 animals examined for each genotype displayed indistinguishable staining. Bar, 10 µm.
Figure 2.
pals-22 codes for a protein with an ALS2CR12 signature. (A) Single-nucleotide polymorphisms from Hawaiian wild C. elegans strain CB4856 mapping plots obtained from whole-genome sequencing of the following mutant alleles: pals-22(ot810) (top row), pals-22(ot811) (middle row), and pals-22(ot723) (bottom row). (B) Schematic of pals-22 gene locus with mutant allele annotation. (C) pals-22 rescue data. %WT = % percent animals that express the ric-19 reporter strongly (as in wild-type animals). %MUT = % percent animals that express the ric-19 reporter more weakly than wild-type.1 otIs381 = ric-19prom6::NLS::gfp. 2 WRM0616DC09 fosmid.
pals-22 mutants display reduced GFP expression of two separate ric-19prom6::NLS::gfp integrated reporter transgenes (Table 1). However, smFISH against endogenous ric-19 transcripts failed to detect effects on the endogenous ric-19 expression (Figure 1C). Since the two ric-19 reporter transgenes that are affected by pals-22 are repetitive, “simple” arrays, we considered the possibility that pals-22 may encode a transgene-silencing activity. To test this notion, we examined the expression of a wide range of reporter transgenes in pals-22-deficient mutants (summarized in Table 1). Six additional simple arrays with widely different cellular specificities of expression (pan-neuronal, dopaminergic neurons, ventral cord motorneurons, and muscle) are also silenced in pals-22 mutants (Figure 3 and Table 1). Two of these arrays, myo-3::gfp (ccIs4251) and cat-2prom::gfp (otIs251), were previously shown to be silenced by loss of tam-1, a “classic” transgene silencer mutation (Hsieh et al. 1999) (M. Doitsidou and O. Hobert, unpublished data) (Figure 3). We quantified the magnitude of the pals-22(ot811) effect on expression of simple array reporters by acquiring fluorescence intensity information from a synchronized worm population using a COPAS FP-250 system (Union Biometrica; “worm sorter”). At the L4 larval stage, we observed a 76% reduction in green fluorescence intensity for ric-19prom6::NLS::gfp, 66% reduction for myo-3::gfp, 32% reduction for cat-2prom::gfp, and 42% reduction in red fluorescence intensity for rab-3prom1::NLS::rfp (Figure 1B and Figure 3, B, D, and E).
Table 1. pals-22 effects on transgene reporter expression.
| Array Type | Construct | Expression Pattern | Relative Intensities of Reporters | |
|---|---|---|---|---|
| Wild-Type | pals-22a | |||
| Simple array | ric-19prom6::gfpb | Pan-neuronal | +++ | + |
| myo-3p::gfp | Body-wall muscle | ++++ | + | |
| cat-2prom::gfp | Dopaminergic neurons | ++++ | ++ | |
| rab-3prom1::rfp | Pan-neuronal | +++ | + | |
| unc-3p::mCherry | Cholinergic neurons | ++++ | ++ | |
| tdc-1p::yfp | RIC and RIM neurons | ++ | + | |
| ric-4p26::yfpc | Neuronal | ++++ | + | |
| Complex array | unc-11p8::gfp | Pan-neuronal | +++ | + |
| cho-1fosmid::yfp | Cholinergic neurons | ++ | ++ | |
| ric-4fosmid::yfp | Pan-neuronal | ++ | ++ | |
| Single copy | lin-4p::yfp | Ubiquitous | ++ | ++ |
| myo-3p::mRuby | Pharyngeal muscle | ++ | ++ | |
| None (endogenous tag) | che-1::gfp | ASE neurons | + | + |
Transgene expression in wild-type and pals-22 mutants. See Table S1 in File S1 for details on transgenic arrays. Number of plus signs (+) indicates the relative intensity of GFP fluorescence. At least 50 animals examined for each genotype. Unless otherwise indicated, all simple and complex arrays correspond to stable genome integrated transgenes. Single-copy reporters were generated by miniMos. GFP tagging of the che-1 locus was achieved using clustered regularly interspersed short palindromic repeats/Cas9 technology.
pals-22(ot811) mutant background.
otIs381 and otIs380 strains (independently integrated lines).
Extrachromosomal array.
We also analyzed the expression of complex arrays (tandemly repeated transgenes with a less repetitive structure) and single-copy reporter transgenes in pals-22 mutants (summarized in Table 1). One complex array transgene was silenced (unc-11p8::gfp, Table 1) but others were not (ric-4fosmid::yfp and cho-1fosmid::yfp, Table 1 and Figure S1A in File S1). Perhaps the much smaller reporter fragment unc-11p8::gfp generated more repetitive structures even in the context of “complex” arrays than the larger fosmid-based reporters. Two single-copy insertions and one endogenously-tagged gene were not affected by mutations in pals-22 (Figure S1, B and C in File S1).
Similar to previously characterized transgene-silencing mutants, such as tam-1 (Hsieh et al. 1999), the reduction of reporter expression is temperature sensitive. However, the direction of the sensitivity is inverted as compared to the tam-1 case: the decrease in ric-19prom6::NLS::gfp expression is most pronounced at 15°, while the effect is milder at 25° (Figure S2 in File S1). We also found stage-dependent variability: the decrease in transgene reporter expression is stronger as the animals develop, from mild differences in expression in early stages of development to more obvious defects at later stages (Figure S3 in File S1).
PALS-22 is a broadly expressed, cytoplasmically located protein
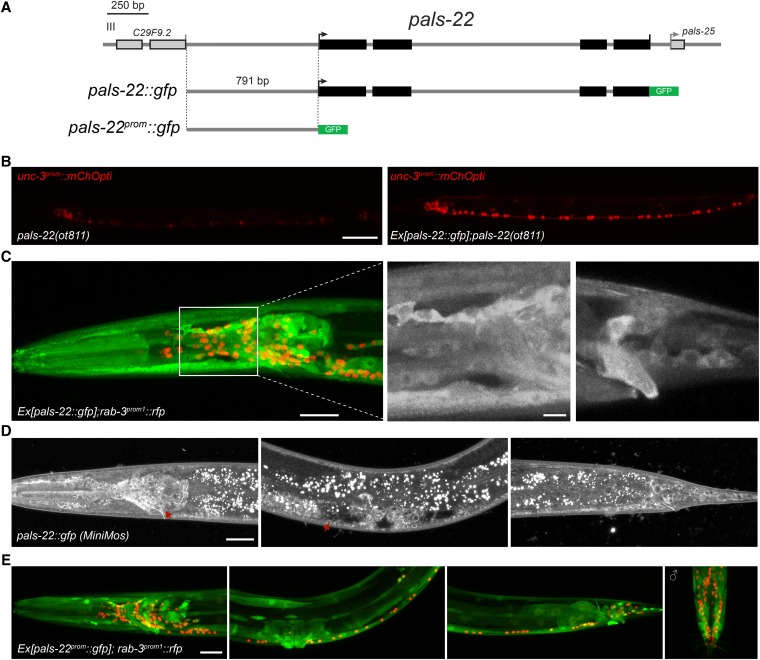
To analyze the expression pattern of pals-22, we fused the entire locus (including 791 bp of all intergenic 5′ sequences and all exons and introns) to gfp (Figure 4A). This reporter construct fully rescues the transgene-silencing phenotype of pals-22(ot811) mutants (Figure 4B). The pals-22 gene is expressed in a broad set of tissues: nervous system (pan-neuronal), body wall and pharyngeal muscle, gut, and hypodermal cells (Figure 4C). A single copy, chromosomal integrant of this translational reporter, generated by MosSCI (kindly provided by E. Troemel), shows a similar expression pattern (Figure 4D). Both the rescuing, translational multicopy reporter transgene, as well as the single-copy integrant, revealed a strong if not exclusive enrichment in the cytoplasm and appears to be excluded from the nucleus in most tissues (Figure 4, C and D). However, we cannot exclude the possibility that the nucleus contains a low amount of functional PALS-22 protein. The cis-regulatory information that controls broad pals-22 gene expression appears to be located in the 5′ intergenic regions, since a transcriptional reporter that only contains 791 bp of the 5′ intergenic region of pals-22, fused to GFP, displays the same broad tissue distribution as the translational reporter (Figure 4E).
Figure 4.
PALS-22 is a broadly expressed, cytoplasmically enriched protein. (A) Schematic of pals-22 transcriptional and translational GFP reporters. The gfp reporter is followed by the 3′-UTR from the unc-54 gene. (B) PALS-22::GFP (right) rescues the expression of unc-3prom::mChOpti in pals-22(ot811) mutants (left). At least 50 animals were examined for each genotype. (C) Expression of the pals-22::gfp translational reporter (otEx7037) is shown in green in the head (left) in a representative hermaphrodite L4 worm. High-magnification images in black and white for the head (middle) and tail (right) show PALS-22 cytoplasmic enrichment. Nuclear pan-neuronal rab-3prom1::rfp expression is shown in red on the background. Two distinct extrachromosomal arrays show the same pattern. (D) Expression of the pals-22::gfp MosSCI insertion (jySi37) is shown in black and white in the head (left panel), midbody (second panel), and tail (third panel) in a representative hermaphrodite L4 worm. Red arrows point to individual, isolated neuron cell bodies where cytoplasmic localization is particularly evident. (E) Expression of the pals-22prom::gfp transcriptional reporter (otEx7036) is shown in green in the head (left panel), midbody (second panel), and tail (third panel) in a representative hermaphrodite L4 worm; and in the male tail (right panel). Nuclear pan-neuronal rab-3prom1::rfp expression is shown in red on the background. Three distinct extrachromosomal arrays show the same pattern. Bar, 20 µm (B, C, and E), 5 µm ((B), high-magnification images), and 50 µm (D).
pals-22 is a member of an unusual C. elegans gene family
Analysis of the PALS-22 protein sequence revealed that it is a member of the previously completely uncharacterized ALS2CR12 protein family. This family is defined by a sequence signature in the InterPro (https://www.ebi.ac.uk/interpro/; family IPR026674) (Finn et al. 2017) and Panther (http://www.pantherdb.org/; family PTHR21707) (Mi et al. 2013, 2017) databases, hence the name “PALS” for protein containing ALS2CR12 signature. The ALS2CR12 sequence signature is comprised of a 404 amino acid position weight matrix modeled from an alignment of the human ALS2CR12 protein (Hadano et al. 2001) and 27 homologs (Figure S4A in File S1). No biochemical or cellular function has yet been assigned to the human ALS2CR12 protein or any of its homologs in other organisms.
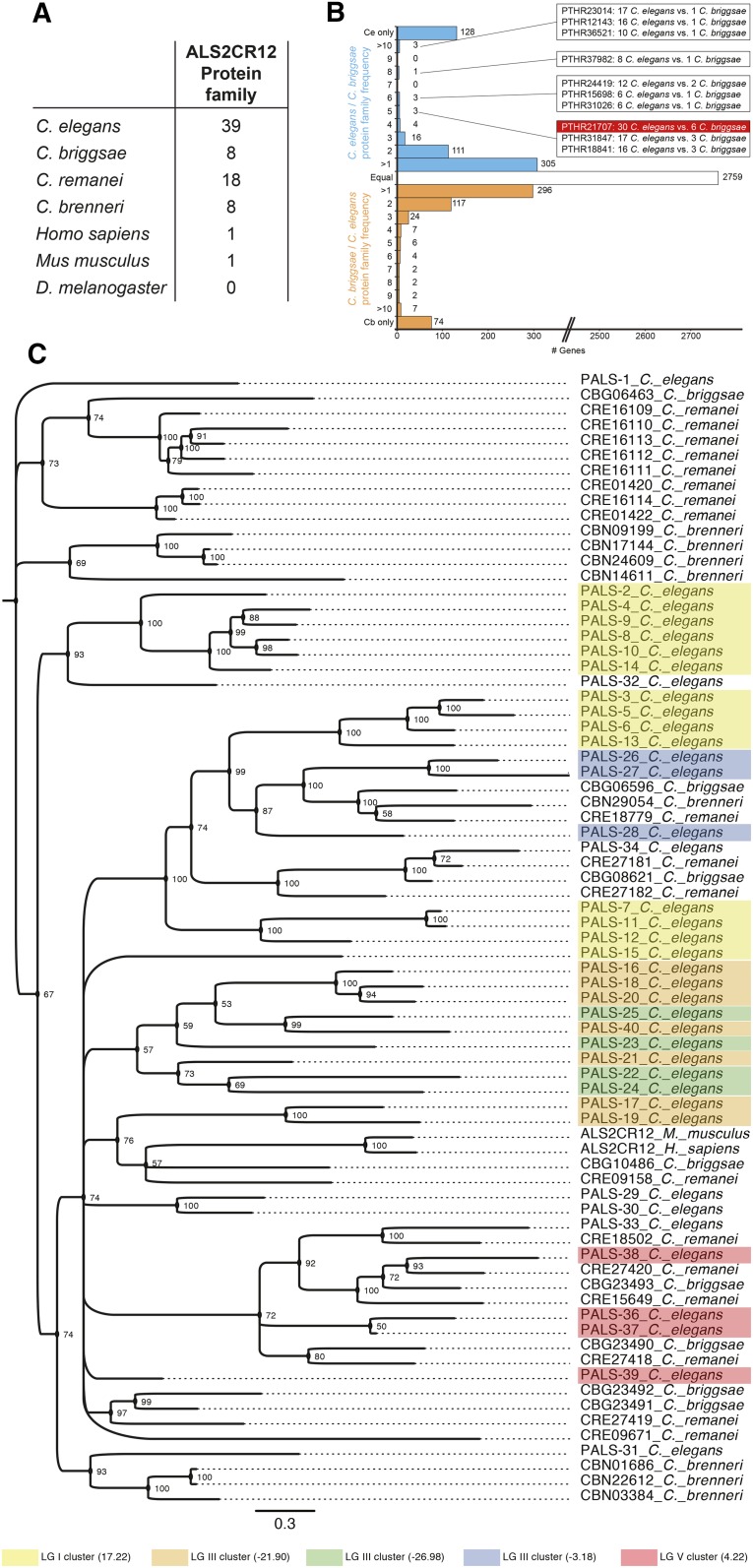
Curiously, while there is only one member of this protein family in mouse and human, the number of ALS2CR12 protein family members is expanded to a total of 39 distinct proteins in C. elegans (Figure 5A and Table 2). In striking contrast, Drosophila seems to be completely devoid of ALS2CR12 family proteins. The 39 C. elegans PALS proteins are very divergent from one another, as reflected in Table 2, with similarity scores of PALS-22 compared to other PALS proteins and reflected in Figure S4B in File S1 with a sequence alignment of PALS-22 with its closest paralogues. Besides poor paralogy, there is also poor orthology. For example, BLAST searches with PALS-22 picks up no sequence ortholog in C. briggsae, and the best homolog from another species (C. brenneri, CBN22612) is less similar to PALS-22 than some of the C. elegans PALS-22 paralogs.
Figure 5.
Sequence and genomic analysis of pals gene family in C. elegans and other species. (A) Number of genes member of the ALS2CR12 protein family as predicted by InterPro (IPR026674) and/or Panther (PTHR21707). (B) Graph representing the C. elegans to C. briggsae Panther protein family frequency for protein families enriched in C. elegans (blue), C. briggsae to C. elegans ratio for protein families enriched in C. briggsae (orange), or white for Panther protein families predicted to occur in the same number of genes in both species. Boxes indicate the gene counts for highly enriched protein families in C. elegans. ALS2CR12 family (Panther protein family PTHR21707) is highlighted in red. The number of Panther protein family hits for the C. elegans and C. briggsae genome were obtained from WormBase. (C) Phylogram of ALS2CR12 family-containing genes, including C. elegans paralogs and orthologs from C. briggsae, C. remanei, C. brenneri, human, and mouse. Node values indicate posterior probabilities for each split as percent. The scale bar indicates average branch length measured in expected substitutions per site. #, number; Cb, C. briggsae; Ce, C. elegans; LG, linkage group.
Table 2. List of all pals genes.
| Linkage Group | Gene | Cosmid-Based Name | WB Gene ID | Location | Protein Size (kDa)a | Similarity to PALS-22b | Upregulated After Microspiridal and/or Viral Infectionc | Expression Enrichment (ModEncode)d |
|---|---|---|---|---|---|---|---|---|
| LGI | pals-1 | F15D3.8 | WBGene00008858 | I:9.26 | 34.9 | Yes | Broad | |
| pals-2 | C17H1.3 | WBGene00007656 | I:17.22 | 45.2 | Yese | Male L4 | ||
| pals-3 | C17H1.4 | WBGene00007657 | I:17.22 | 39.2 | Yese | Male L4 | ||
| pals-4 | C17H1.5 | WBGene00007658 | I:17.22 | 40.5 | Yes | Male L4 | ||
| pals-5 | C17H1.6 | WBGene00007659 | I:17.22 | 35.4 | Yes | Male L4 | ||
| pals-6 | C17H1.7 | WBGene00007660 | I:17.22 | 46.8 | Yes | Male L4 | ||
| pals-7 | C17H1.8 | WBGene00007661 | I:17.22 | 39.3 | Yese | Male L4 | ||
| pals-8 | C17H1.9 | WBGene00007662 | I:17.22 | 41.1 | Yese | Male L4 | ||
| pals-9 | C17H1.10 | WBGene00044237 | I:17.22 | 34.9 | Yes | Male L4 | ||
| pals-10 | C17H1.11 | WBGene00044711 | I:17.22 | 8.4 | Male L4 | |||
| pals-11 | C17H1.13 | WBGene00044708 | I:17.22 | 38.5 | Yes | Male L4 | ||
| pals-12 | C17H1.14 | WBGene00044709 | I:17.22 | 46.4 | Yes | Male L4 | ||
| pals-13 | Y26D4A.8 | WBGene00012505 | I:17.22 | 43.5 | Male L4 | |||
| pals-14 | F22G12.1 | WBGene00009061 | I:17.22 | 41.3 | Yes | Male L4 | ||
| pals-15 | F22G12.7 | WBGene00044707 | I:17.22 | 41.7 | Yes | Male L4 | ||
| LGIII | pals-16 | Y82E9BR.4 | WBGene00022337 | III:−21.90 | 37.7 | Broad | ||
| pals-17 | Y82E9BR.13 | WBGene00022346 | III:−21.90 | 30 | 3e−05 | Yes | Broad | |
| pals-18 | Y82E9BR.21 | WBGene00022353 | III:−21.90 | 29.2 | Broad | |||
| pals-19 | Y82E9BR.23 | WBGene00044761 | III:−21.90 | 30.6 | 3e−05 | Broad | ||
| pals-20 | Y82E9BR.25 | WBGene00194746 | III:−21.90 | 27.5 | Low | |||
| pals-21 | Y82E9BR.32 | WBGene00255597 | III:−21.90 | 20.7 | 3e−12 | Low | ||
| pals-40 | Y82E9BR.11 | WBGene00022344 | III:−21.90 | 24 | 4e−06 | Yes | Low | |
| pals-22 | C29F9.1 | WBGene00016216 | III:−26.98 | 32.7 | 0.0 | Broad | ||
| pals-23 | C29F9.3 | WBGene00016218 | III:−26.98 | 34.7 | 6e−05 | Broad | ||
| pals-24 | C29F9.4 | WBGene00016219 | III:−26.98 | 35.7 | 2e−10 | Broad | ||
| pals-25 | T17A3.2 | WBGene00020540 | III:−26.98 | 34.7 | 2e−06 | Broad | ||
| pals-26 | B0284.1 | WBGene00007131 | III:−3.18 | 49.2 | Yes | Broad | ||
| pals-27 | B0284.2 | WBGene00007132 | III:−3.18 | 48.2 | Yese | Broad | ||
| pals-28 | B0284.4 | WBGene00007134 | III:−3.18 | 48 | Yese | Male L4 | ||
| LGIV | pals-29 | T27E7.6 | WBGene00012091 | IV:12.20 | 48.4 | 7e−05 | Yes | Male L4 + dauer |
| pals-30 | Y57G11B.1 | WBGene00013294 | IV:12.23 | 48.9 | 3e−04 | Yes | Broad | |
| LGV | pals-31 | F48G7.2 | WBGene00018614 | V:−19.97 | 25.6 | Yes | Broad | |
| pals-32 | C31B8.4 | WBGene00016281 | V:−12.86 | 43.7 | 9e−05 | Yese | Male L4 + dauer | |
| pals-33 | W08A12.4 | WBGene00021081 | V:−8.24 | 52 | Yese | Male L4 + dauer | ||
| pals-34 | F26D11.6 | WBGene00017823 | V:0.98 | 19.1 | broad | |||
| pals-36f | C54D10.12 | WBGene00044787 | V:4.22 | 55.4 | 2e−04 | Yes | Male L4 + dauer | |
| pals-37 | C54D10.14 | WBGene00138721 | V:4.22 | 87.3 | Yese | Male L4 + dauer | ||
| pals-38 | C54D10.8 | WBGene00008302 | V:4.22 | 48.1 | Yese | Male L4 + dauer | ||
| pals-39 | C54D10.7 | WBGene00008301 | V:4.22 | 48.5 | 2e−05 | Yes | Male L4 + dauer |
Protein family assignments made by either Interpro (IPR026673) or Panther (PTHR21707), v9.0. A newer release of Panther does not include pals-7, 10, or 11 in the ALS2CR12 family, yet the sequence similarity of these genes to neighboring pals genes is clear. WB Gene ID, WormBase gene identifier.
Protein size of largest predicted isoform.
PSI Blast e-value. Similarities only above e−04 threshold are shown.
Twenty-eight pals genes upregulated in response to microsporidial and/or viral infection according to Bakowski et al. (2014) and Chen et al. (2017) (out of 218 significantly upregulated genes).
According to Celniker et al. (2009).
Ten pals genes upregulated after cadmium exposure according to Cui et al. (2007) (out of 237 significantly upregulated genes).
Possibly a pseudogene.
The expansion of C. elegans ALS2CR12 signature-containing proteins appears to be nematode species-specific, as C. briggsae only contains eight predicted proteins with the ALS2CR12 sequence signature; other nematodes also contain significantly less ALS2CR12 family proteins (Figure 5A). Such C. elegans-specific gene expansion is highly unusual, as shown in Figure 5B. Among 3874 Panther protein families analyzed, 2759 (71.2%) are present in the same number of genes in both C. elegans and C. briggsae, 128 (3.3%) protein families are present only in C. elegans, while 74 (1.9%) are present only in C. briggsae. Most of the remaining families are only slightly enriched in one species or the other. Just 10 protein families (0.3%) are enriched five times or more in C. elegans vs. C. briggsae (Figure 5B). Most of these families contain uncharacterized genes, even though many of them contain human and vertebrate orthologs. Among them, the ALS2CR12 family has not only a noteworthy enrichment, but it also constitutes the family with the largest absolute number of genes (Figure 5B).
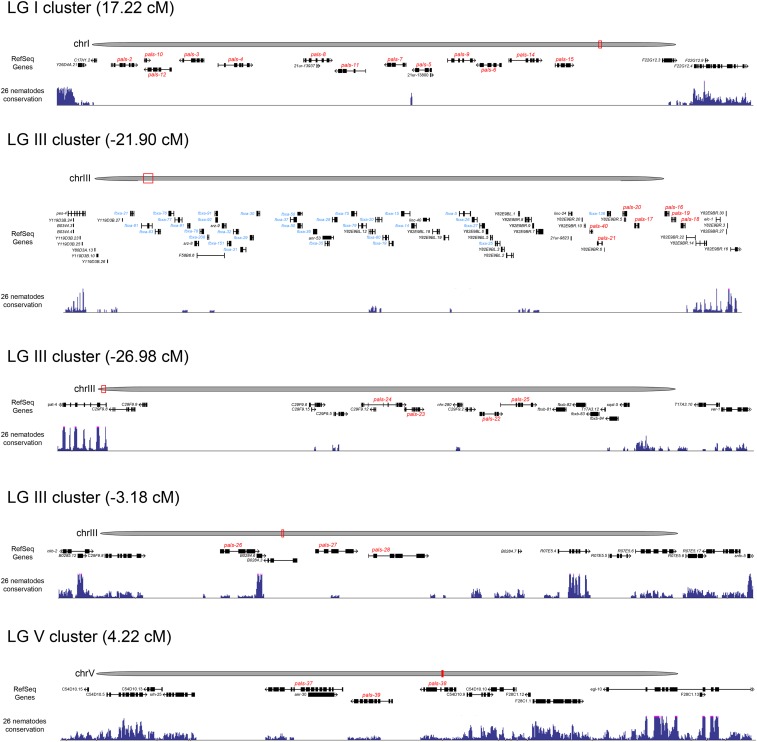
As perhaps expected from a species-specific expansion, the C. elegans pals genes are genomically clustered (Figure 6). Fourteen genes are clustered in chromosome I (position I: 17.22 cM); while three clusters with seven genes (III: −21.90 cM), four genes (III: −26.98 cM), and three genes (III: −3.18 cM) are present in chromosome III; and lastly, four genes are clustered in chromosome V (V: 4.22) (Figure 6 and Table 2). Taking into account the C. elegans-specific family expansion, it is not surprising to find a total lack of conservation in the regions encompassing most of the pals clusters (Figure 6). Only one cluster on chromosome V shows some degree of conservation among other nematode species. Genes surrounding these regions are conserved, suggesting recent gene duplications in the nonconserved areas. The low conservation region in cluster III: −21.90 cM, contains many additional, nonconserved genes that are largely expanded in a nematode-specific manner, namely the previously analyzed fbxa genes (Thomas 2006).
Figure 6.
C. elegans pals genes are clustered and these clusters are poorly conserved. Schematics of different C. elegans genomic regions adapted from the University of California, Santa Cruz Genome Browser (https://genome.ucsc.edu/). One isoform per gene is shown. pals genes are indicated in red and fbxa genes in blue. The following regions are shown: chromosome I, cluster at position 17.22 cM, chrI: 13,099,564–13,160,497 bp; chromosome III, cluster at position −21.90 cM, chrIII: 1,215,665–1,423,550 bp; chromosome III, cluster at position −26.98 cM, chrIII: 89,907–159,962 bp; chromosome III, cluster at position −3.18 cM, chrIII: 4,368,479–4,405,090 bp; and chromosome V, cluster at position 4.22 cM, chrV: 12,427,096–12,462,015 bp. Chr; chromosome; LG, linkage group.
Local gene duplications seem a plausible mechanism for the origin of the expanded C. elegans pals gene family. Consequently, we reasoned that pals genes within the same cluster should be more similar among each other than to other pals genes. To explore this possibility, we built a phylogenetic tree to visualize the phylogenetic relationships between pals genes (Figure 5C). We included all C. elegans pals genes plus orthologs from C. briggsae, C. remanei, C. brenneri, mouse, and human (based on presence of InterPro IPR026674). As expected, pals genes within the same cluster have a closer phylogenetic relationship, suggesting a shared origin.
According to modENCODE expression data (Celniker et al. 2009), most of the genes within each cluster are expressed in the same stage (e.g., all genes clustered in chromosome I are only found in L4 males), suggesting related functions (Table 2). Perhaps most intriguingly, though, out of just a few hundred significantly enriched genes, the majority of pals genes become upregulated upon exposure to specific pathogens, specifically the exposure to intracellular fungal pathogen (microsporidia) or by viral infection (Bakowski et al. 2014; Chen et al. 2017). Induction of pals gene expression is also observed upon various other environmental insults (exposure to toxic compounds) (Cui et al. 2007) (summarized in Table 2). Several fbxa genes present in the low conservation region in cluster III: –21.90 cM, also become upregulated upon exposure to microsporidia or by viral infection (Bakowski et al. 2014; Chen et al. 2017).
Somatic transgene silencing in pals-22 mutants requires rde-4-dependent small RNAs
We examined whether two pals-22 paralogs, pals-19 and pals-25, may also be involved in transgene silencing. Both genes show significant sequence similarity to pals-22 (Table 2), and one (pals-25) is directly adjacent to pals-22 (Figure 6). We tested whether two nonsense alleles generated by the Million Mutant Project, pals-19(gk166606) and pals-25(gk891046) (Thompson et al. 2013), silence the ric-19prom6::NLS::gfp and myo-3::gfp multicopy transgenes. Neither array shows obvious changes in expression in pals-19 or pals-25 mutant backgrounds (data not shown).
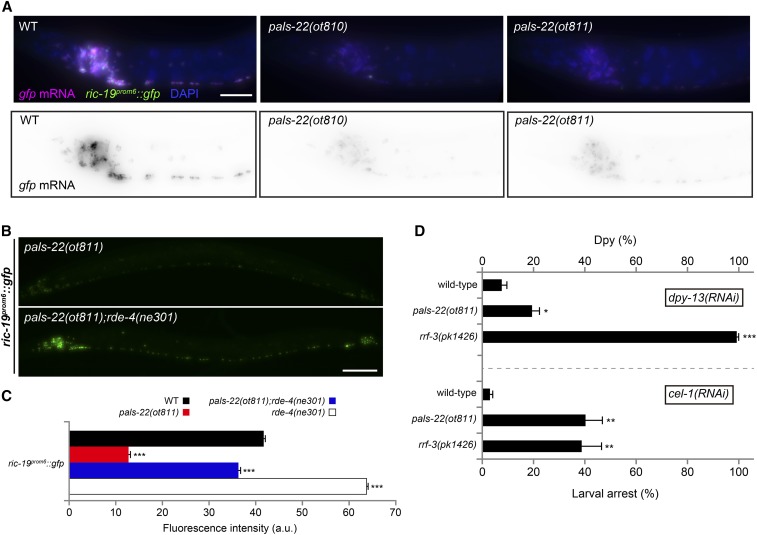
To further pursue the role of pals-22 in transgene silencing, we considered previous reports on transgene silencer mutations. Several genes with transgene-silencing effects are known to be involved in modifying chromatin (Cui and Han 2007). However, the cytoplasmic enrichment of PALS-22 described above argues against a direct role in controlling chromatin architecture (although, a function in the nucleus cannot be ruled out). Nevertheless, we do find that pals-22 affects transgene silencing at the mRNA level. smFISH against gfp mRNA shows that silenced gfp transgenic arrays display a significantly reduced number of transcripts in pals-22 mutants (Figure 7A). Reduced transcript levels can be explained by transcriptional and/or post-transcriptional alterations and we therefore considered the possibility that the effects of cytoplasmically enriched PALS-22 protein on transcript levels may be controlled by intermediary factors. Small interfering RNAs can affect gene expression both at the transcriptional or post-transcriptional level (Zamore et al. 2000; Elbashir et al. 2001; Le Thomas et al. 2013), and through a genetic epistasis test, we asked whether pals-22 requires small RNAs for its function. To this end, we turned to rde-4 mutant animals. The dsRNA-binding protein RDE-4 initiates gene silencing by recruiting an endonuclease to process long dsRNA into short dsRNA and is involved in exogenous as well as endogenous RNAi pathways (Tabara et al. 2002; Parker et al. 2006; Gent et al. 2010; Vasale et al. 2010). We find that loss of rde-4 completely suppresses the pals-22 mutant phenotype (Figure 7, B and C). This result might be in contrast with previous reports showing that rde-4 can also act as a transgene antisilencing factor (Fischer et al. 2013). Interestingly, we find that ric-19prom6::NLS::gfp expression increases in the rde-4(ne301) alone background, in agreement with previous studies suggesting a background level of transgene silencing in wild-type worms (Mello and Fire 1995; Lehner et al. 2006). Although rde-4 could also play other roles in gene expression independently of siRNA production, our result suggests that the gene silencing mediated by pals-22 deficiency requires the production of small dsRNAs.
Figure 7.
Somatic transgene silencing in pals-22 depends on the RNAi pathway. (A) Wild-type (left), pals-22(ot810) (middle), and pals-22(ot811) (right) images of the anterior part of L3 worms showing reduced gfp mRNA levels in mutants compared to control wild-type worms as assessed by single-molecule fluorescence in situ hybridization. Individual transcripts shown as purple dots in top and as black dots in bottom panels. GFP expression is shown in green and DAPI staining is shown in blue. At least 20 animals were examined for each genotype. (B) Silencing of ric-19prom6::NLS::gfp in pals-22(ot811) (top) is suppressed in pals-22(ot811);rde-4(ne301) double mutants (bottom). (C) Quantification of otIs381[ric-19prom6::NLS::gfp] fluorescence intensity in wild-type, pals-22(ot811), pals-22(ot811);rde-4(ne301), and rde-4(ne301) mutants. The data are presented as mean + SEM. Unpaired t-test (comparisons to WT), *** P < 0.001; n ≥ 950 for all genotypes. (D) Animals of the indicated genotype were grown on bacteria expressing dpy-13 or cel-1 dsRNA (feeding RNAi). For dpy-13(RNAi) (top), progeny were scored for the percentage of animals having a dumpy body shape (Dpy). For cel-1(RNAi) (bottom), the percentage of their progeny arresting at the L2 larval stage was determined. Wild-type otIs381[ric-19prom6::NLS::gfp] and rrf-3(pk1426) mutants were used as negative and positive controls. The data are presented as mean + SEM among the data collected from at least four independent experiments. Unpaired t-tests were performed for pals-22(ot811) and rrf-3(pk1426) compared to WT; * P < 0.05, ** P < 0.01, and *** P < 0.001. Bar, 10 µm (A) and 50 µm (B). a.u., arbitrary units; RNAi, RNA interference; WT, wild-type.
Transgene-silencing phenotypes have been observed in mutants that affect multiple distinct small RNA pathways, and exogenous RNAi responses are often enhanced in these mutants (Simmer et al. 2002; Lehner et al. 2006; Fischer et al. 2013). Thus, we tested whether pals-22(ot811) shows an enhanced exogenous RNAi response. Using rrf-3(pk1426) as a positive control (Simmer et al. 2002), we detect enhanced dpy-13 or cel-1 RNAi phenotypes in pals-22(ot811) (dsRNA delivered by feeding; Figure 7D). We conclude that pals-22 physiological function might be related to the regulation of RNAi-dependent silencing, via a mechanism critical to its action as an antisilencing factor.
Locomotory and aging defects of pals-22 mutant animals
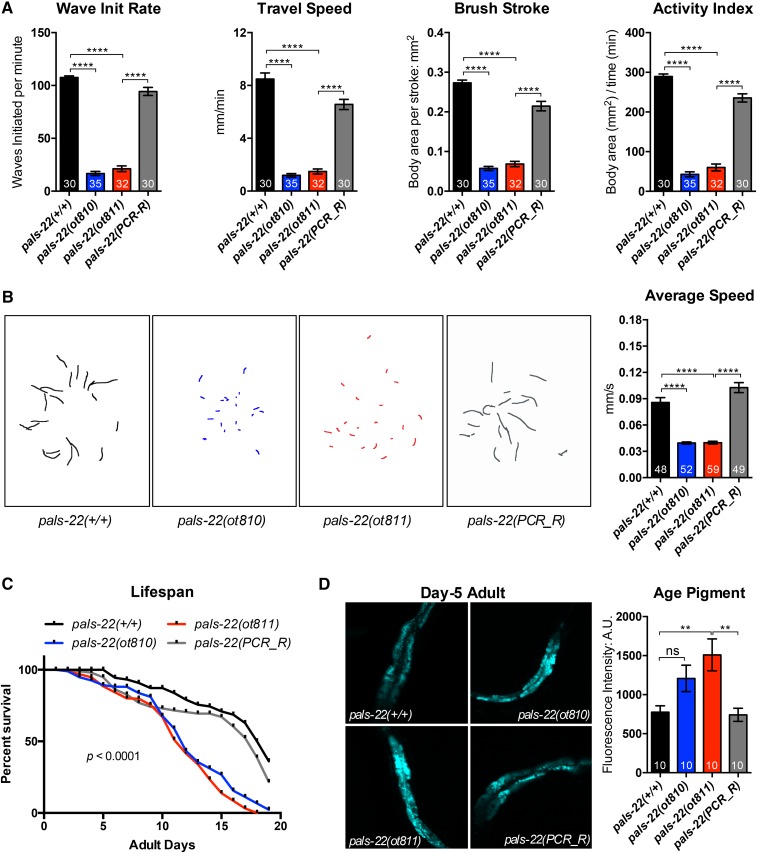
The loss of pals-22 has striking physiological consequences. Since casual observation of pals-22 mutants indicates defects in locomotion, we quantified these defects, by measured swimming behavior (Restif et al. 2014) and crawling activity, comparing wild-type, pals-22(ot810), pals-22(ot811), and pals-22(ot811) carrying a wild-type copy of pals-22 on an extrachromosomal array (Figure 8). Day 1 adult pals-22 mutants show a poor performance in swimming assays as evaluated by multiple parameters, including low wave initiation rate (akin to thrash speed), travel speed (distance moved over time), brush stroke area (area covered in unit time), and activity index (Figure 8A). On agar plates animal movement was also clearly impaired, displaying significantly decreased traveling speed (Figure 8B). All locomotory defects were rescued by the pals-22(+) extrachromosomal array.
Figure 8.
pals-22 mutants show defective locomotion and early onset of aging traits. (A) Day 1 adult pals-22 mutants exhibit defective swimming features, including decreased wave initiation (init) rate, travel speed, brush stroke, and activity index. pals-22(PCR_R) is the pals-22(ot811) mutant carrying a wild-type copy of pals-22 on an extrachromosomal array amplified by PCR. Data shown are mean ± SEM of each parameters, n = 30–35 (number indicated in each bar) from two independent experiments, **** P < 0.0001 (one-way ANOVA) compared to related control. (B) pals-22 mutants crawl slowly on agar plates. The left panel shows the crawling path of each animal in 30 sec. The right panel shows the mean ± SEM of average crawling speed (millimeters per second) for day 1 adults from two independent experiments, n = ∼55 worms for each genotype, **** P < 0.0001 (one-way ANOVA) compared to related control. (C) pals-22 mutants have shorter life spans compared to wild-type or pals-22(PCR_R). Survival study was initiated with 96 worms (for each genotype) at L4 stage (day 0). Data shown is one represented trial of life span; two additional independent trials also show similar changes of life span in pals-22 mutants, P < 0.0001 (Log-rank test), comparing the wild-type to mutants, or comparing pals-22(PCR_R) against pals-22(ot811). (D) pals-22 mutants exhibit early accumulation of age pigment. The left panel shows representative pictures of age pigment (excitation: 364 nm; emission: 380–420 nm). The right panel shows the mean ± SEM of age pigment auto-fluorescence intensity of day 5 adults, n = 10 worms from each genotype, ns (not significant) or ** P < 0.01 (one-way ANOVA) compared to related control. A.U., arbitrary units.
Apart from the locomotory defects, we also noted abnormal survival of pals-22 mutants. Using standard life span assays, we observed premature death of pals-22 mutants, commencing at about day 10 of adulthood (Figure 8C). We also find that 5-day-old adult animals display a signature change in aging, namely increased age pigment in the gut of adult worms (Figure 8D). In light of these premature aging phenotypes, we surmise that the locomotory defects described above may also be an indication of premature aging.
Discussion
We have described here a large, unusual gene family in C. elegans. In the context of whole-animal transcriptome profiling under different conditions, expression of members of the pals gene family has previously been shown to be induced upon various forms of cellular insults, ranging from exposure to intracellular fungal infections, to viral infection, and to toxic compound exposure (Cui et al. 2007; Bakowski et al. 2014; Chen et al. 2017). We define here a function for one of the family members, pals-22, in controlling the silencing of repetitive DNA sequences. Even though the biochemical function of PALS proteins is presently unclear, the upregulation of many pals genes under conditions of cellular stress suggest that this gene family may be part of a host defense mechanism that protects animals from specific insults. The C. elegans-specific expansion of pals genes may relate to their potential function in fending off species-specific stressors and/or encounters with species-specific pathogens.
The mutant phenotype of pals-22 as a transgene silencer, as well as the dependence of this phenotype on small RNA production, indicates that PALS-22 may control gene expression via small RNA molecules. A role of PALS-22 in controlling gene expression is also illustrated in a parallel study in which pals-22 has been found to be required for the proper regulation of a battery of stress- and microsporidial infection-induced genes, including many of the pals genes themselves (Reddy et al. 2017). While the function of pals-22 in the RNAi process is not clear, there are numerous examples of mutants in which somatic transgene silencing is induced as a result of an increase in RNAi sensitivity, including rrf-3, eri-1, lin-35, and others (Simmer et al. 2002; Kennedy et al. 2004; Kim et al. 2005; Wang et al. 2005; Lehner et al. 2006; Fischer et al. 2008, 2011, 2013). A set of genes classically linked to somatic transgene silencing are the class B synMuv genes. synMuv B genes are so-called due to a synthetic phenotype found when mutations are combined with those in synMuv A genes (Ferguson and Horvitz 1989). As pals-22-defective worms, mutants for synMuv B genes show reduced expression from repetitive DNA transgenes. While mutations in some synMuv B genes do not result in transgene silencing (e.g., lin-36) (Hsieh et al. 1999) it has been shown that the silencing of repetitive transgenes in synMuv B mutants results from an enhanced somatic cell RNAi (Wang et al. 2005; Lehner et al. 2006). Interestingly, tam-1 and lex-1 mutants are genetically class B synMuvs; however, some synMuv B mutant characteristics such as enhanced RNAi or defects in P granule localization are not observed in tam-1 mutants, while they still show a transgene-silencing phenotype (Wang et al. 2005; Lehner et al. 2006; Tseng et al. 2007). This suggests that they act through a different mechanism than other synMuv B. While pals-22 animals present some overlapping phenotypes with synMuv B genes, further analysis will reveal in detail the mechanism for somatic transgene silencing in pals-22 mutants. Intriguingly, since the expression levels per copy of repetitive tandem arrays are much lower than for endogenous genes, transgene-targeting siRNAs may be already abundant in wild-type transgenic strains, indicating a background level of transgene silencing in wild-type worms (Mello and Fire 1995). An interesting hypothesis to be tested is that in a mutant, such as pals-22, these transgene-targeted siRNAs may be reduced, perhaps because of a shift in the balance between the loading of transgene siRNAs into a silencing Argonaute (e.g., NRDE-3) vs. an antisilencing Argonaute (e.g., CSR-1) (Shirayama et al. 2012; Fischer et al. 2013).
The precise biochemical function of any PALS protein remains obscure. The only notable sequence relationship that we could find points to potential biochemical function of the PALS proteins in proteostasis. One of the LGIII clusters of pals genes (III: −21.90 cM) also contains a large number of fbxa genes, another large gene family specifically expanded in C. elegans (Thomas 2006) (Figure 6). fbxa genes code for F-box proteins that are involved in protein degradation (Thomas 2006). One of the FBXA proteins in the LGIII cluster, FBXA-138, displays sequence similarities to a number of distinct PALS proteins within and outside the LGIII pals cluster, including PALS-22, PALS-23, PALS-32, PALS-25, and PALS-1. Even though PALS proteins are not predicted to contain a canonical F-box, it is conceivable that their distant sequence relationship to F-box proteins may suggest a role of PALS proteins in protein degradation. How, in the case of PALS-22, such a function may relate to the control of gene expression via small RNA molecules is not clear.
Although it is difficult to unambiguously distinguish accelerated aging from general sickness, young adult pals-22 mutants clearly exhibit multiple features of aged animals: impaired mobility, elevated age pigments/lipofuscin, and shortened life span. Increased expression of repetitive sequences has been documented in aging C. elegans, Drosophila, and humans, and has been suggested to contribute to genomic instability and cell dysfunction (Sedivy et al. 2013); the physiological effect of decreases in the expression of repetitive sequences has not been explored. However, we note that other transgene-silencing mutants (which are also RNAi hypersensitive), like rrf-3 and eri-1, do not display an aging defect (Zhang et al. 2009; Ren et al. 2012). pals-22 may therefore be involved in novel aspects of small RNA-dependent gene silencing that may control the expression of genes involved in animal physiology.
Supplementary Material
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.117.300134/-/DC1.
Acknowledgments
We thank Chi Chen for the generation of transgenic strains, the Caenorhabditis Genetics Center for providing strains (funded by the National Institutes of Health Office of Research Infrastructure Programs, P40 OD010440), Kevin Howe and Gary Williams at WormBase for their help obtaining and analyzing protein family data, Sangeena Salam for initial life span assays, the Million Mutation Project for providing the pals-19(gk166606) and pals-25(gk891046) alleles, Emily Troemel for communicating unpublished results and providing the jySi37 strain, Dylan Rahe for providing the GFP-tagged che-1(ot856) allele, HaoSheng Sun for providing the lin-4prom::yfp MiniMos strain, and members of the Hobert laboratory for comments on this manuscript. This work was supported by grant R01-AG046358 to M.D., and a New Jersey Commission on Cancer Research Postdoctoral Fellowship (DHFS16PPC070) to G.W. and the Howard Hughes Medical Institute (O.H.). E.L.-D. was funded by an European Molecular Biology Organization long-term fellowship.
Footnotes
Communicating editor: D. Greenstein
Literature Cited
- Bakowski M. A., Desjardins C. A., Smelkinson M. G., Dunbar T. L., Lopez-Moyado I. F., et al. , 2014. Ubiquitin-mediated response to microsporidia and virus infection in C. elegans. PLoS Pathog. 10: e1004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celniker S. E., Wheeler D. A., Kronmiller B., Carlson J. W., Halpern A., et al. , 2002. Finishing a whole-genome shotgun: release 3 of the Drosophila melanogaster euchromatic genome sequence. Genome Biol. 3: RESEARCH0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celniker S. E., Dillon L. A., Gerstein M. B., Gunsalus K. C., Henikoff S., et al. , 2009. Unlocking the secrets of the genome. Nature 459: 927–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Franz C. J., Jiang H., Jiang Y., Wang D., 2017. An evolutionarily conserved transcriptional response to viral infection in Caenorhabditis nematodes. BMC Genomics 18: 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong E. B., Elde N. C., Feschotte C., 2017. Regulatory activities of transposable elements: from conflicts to benefits. Nat. Rev. Genet. 18: 71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordaux R., Batzer M. A., 2009. The impact of retrotransposons on human genome evolution. Nat. Rev. Genet. 10: 691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M., Han M., 2007. Roles of chromatin factors in C. elegans development (May 3, 2007), WormBook, ed. The C. elegans Research Community, WormBook /10.1895/wormbook.1.139.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., McBride S. J., Boyd W. A., Alper S., Freedman J. H., 2007. Toxicogenomic analysis of Caenorhabditis elegans reveals novel genes and pathways involved in the resistance to cadmium toxicity. Genome Biol. 8: R122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Koning A. P., Gu W., Castoe T. A., Batzer M. A., Pollock D. D., 2011. Repetitive elements may comprise over two-thirds of the human genome. PLoS Genet. 7: e1002384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doitsidou M., Flames N., Lee A. C., Boyanov A., Hobert O., 2008. Automated screening for mutants affecting dopaminergic-neuron specification in C. elegans. Nat. Methods 5: 869–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doitsidou M., Poole R. J., Sarin S., Bigelow H., Hobert O., 2010. C. elegans mutant identification with a one-step whole-genome-sequencing and SNP mapping strategy. PLoS One 5: e15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle W. F., Sapienza C., 1980. Selfish genes, the phenotype paradigm and genome evolution. Nature 284: 601–603. [DOI] [PubMed] [Google Scholar]
- Edelstein A., Amodaj N., Hoover K., Vale R., Stuurman N., 2010. Computer control of microscopes using μManager. Curr. Protoc. Mol. Biol. CHAPTER: Unit14.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbashir S. M., Lendeckel W., Tuschl T., 2001. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 15: 188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson E. L., Horvitz H. R., 1989. The multivulva phenotype of certain Caenorhabditis elegans mutants results from defects in two functionally redundant pathways. Genetics 123: 109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn R. D., Attwood T. K., Babbitt P. C., Bateman A., Bork P., et al. , 2017. InterPro in 2017-beyond protein family and domain annotations. Nucleic Acids Res. 45: D190–D199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S. E., Butler M. D., Pan Q., Ruvkun G., 2008. Trans-splicing in C. elegans generates the negative RNAi regulator ERI-6/7. Nature 455: 491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S. E., Montgomery T. A., Zhang C., Fahlgren N., Breen P. C., et al. , 2011. The ERI-6/7 helicase acts at the first stage of an siRNA amplification pathway that targets recent gene duplications. PLoS Genet. 7: e1002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S. E., Pan Q., Breen P. C., Qi Y., Shi Z., et al. , 2013. Multiple small RNA pathways regulate the silencing of repeated and foreign genes in C. elegans. Genes Dev. 27: 2678–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedli M., Trono D., 2015. The developmental control of transposable elements and the evolution of higher species. Annu. Rev. Cell Dev. Biol. 31: 429–451. [DOI] [PubMed] [Google Scholar]
- Gent J. I., Lamm A. T., Pavelec D. M., Maniar J. M., Parameswaran P., et al. , 2010. Distinct phases of siRNA synthesis in an endogenous RNAi pathway in C. elegans soma. Mol. Cell 37: 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstbrein B., Stamatas G., Kollias N., Driscoll M., 2005. In vivo spectrofluorimetry reveals endogenous biomarkers that report healthspan and dietary restriction in Caenorhabditis elegans. Aging Cell 4: 127–137. [DOI] [PubMed] [Google Scholar]
- Goke J., Ng H. H., 2016. CTRL+INSERT: retrotransposons and their contribution to regulation and innovation of the transcriptome. EMBO Rep. 17: 1131–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodier J. L., Kazazian H. H., 2008. Retrotransposons revisited: the restraint and rehabilitation of parasites. Cell 135: 23–35. [DOI] [PubMed] [Google Scholar]
- Grishok A., Sinskey J. L., Sharp P. A., 2005. Transcriptional silencing of a transgene by RNAi in the soma of C. elegans. Genes Dev. 19: 683–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S., Von Kalle C., Schmidt M., McCormack M. P., Wulffraat N., et al. , 2003. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 302: 415–419. [DOI] [PubMed] [Google Scholar]
- Hadano S., Hand C. K., Osuga H., Yanagisawa Y., Otomo A., et al. , 2001. A gene encoding a putative GTPase regulator is mutated in familial amyotrophic lateral sclerosis 2. Nat. Genet. 29: 166–173. [DOI] [PubMed] [Google Scholar]
- Hobert O., 2002. PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans. Biotechniques 32: 728–730. [DOI] [PubMed] [Google Scholar]
- Hsieh J., Liu J., Kostas S. A., Chang C., Sternberg P. W., et al. , 1999. The RING finger/B-box factor TAM-1 and a retinoblastoma-like protein LIN-35 modulate context-dependent gene silencing in Caenorhabditis elegans. Genes Dev. 13: 2958–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck J. P., Ronquist F., 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- Jacobs F. M. J., Greenberg D., Nguyen N., Haeussler M., Ewing A. D., et al. , 2014. An evolutionary arms race between KRAB zinc-finger genes ZNF91/93 and SVA/L1 retrotransposons. Nature 516: 242–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji N., van Oudenaarden A., 2012. Single molecule fluorescent in situ hybridization (smFISH) of C. elegans worms and embryos (December 13, 2012), WormBook, ed. The C. elegans Research Community, WormBook, /10.1895/wormbook.1.153.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath R. S., Ahringer J., 2003. Genome-wide RNAi screening in Caenorhabditis elegans. Methods 30: 313–321. [DOI] [PubMed] [Google Scholar]
- Kazazian H. H., Jr, 2004. Mobile elements: drivers of genome evolution. Science 303: 1626–1632. [DOI] [PubMed] [Google Scholar]
- Kelly W. G., Xu S., Montgomery M. K., Fire A., 1997. Distinct requirements for somatic and germline expression of a generally expressed Caernorhabditis elegans gene. Genetics 146: 227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S., Wang D., Ruvkun G., 2004. A conserved siRNA-degrading RNase negatively regulates RNA interference in C. elegans. Nature 427: 645–649. [DOI] [PubMed] [Google Scholar]
- Kim J. K., Gabel H. W., Kamath R. S., Tewari M., Pasquinelli A., et al. , 2005. Functional genomic analysis of RNA interference in C. elegans. Science 308: 1164–1167. [DOI] [PubMed] [Google Scholar]
- Lander E. S., Linton L. M., Birren B., Nusbaum C., Zody M. C., et al. , 2001. Initial sequencing and analysis of the human genome. Nature 409: 860–921. [DOI] [PubMed] [Google Scholar]
- Law J. A., Jacobsen S. E., 2010. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet. 11: 204–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Thomas A., Rogers A. K., Webster A., Marinov G. K., Liao S. E., et al. , 2013. Piwi induces piRNA-guided transcriptional silencing and establishment of a repressive chromatin state. Genes Dev. 27: 390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner B., Calixto A., Crombie C., Tischler J., Fortunato A., et al. , 2006. Loss of LIN-35, the Caenorhabditis elegans ortholog of the tumor suppressor p105Rb, results in enhanced RNA interference. Genome Biol. 7: R4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M., Conery J. S., 2003. The origins of genome complexity. Science 302: 1401–1404. [DOI] [PubMed] [Google Scholar]
- Mello C., Fire A., 1995. DNA transformation. Methods Cell Biol. 48: 451–482. [PubMed] [Google Scholar]
- Mi H., Muruganujan A., Casagrande J. T., Thomas P. D., 2013. Large-scale gene function analysis with the PANTHER classification system. Nat. Protoc. 8: 1551–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H., Huang X., Muruganujan A., Tang H., Mills C., et al. , 2017. PANTHER version 11: expanded annotation data from gene ontology and reactome pathways, and data analysis tool enhancements. Nucleic Acids Res. 45: D183–D189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minevich G., Park D. S., Blankenberg D., Poole R. J., Hobert O., 2012. CloudMap: a cloud-based pipeline for analysis of mutant genome sequences. Genetics 192: 1249–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowick K., Hamilton A. T., Zhang H., Stubbs L., 2010. Rapid sequence and expression divergence suggest selection for novel function in primate-specific KRAB-ZNF genes. Mol. Biol. Evol. 27: 2606–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum-Krammer C. I., Neto M. F., Brielmann R. M., Pedersen J. S., Morimoto R. I., 2015. Investigating the spreading and toxicity of prion-like proteins using the metazoan model organism C. elegans. J. Vis. Exp. 95: 52321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgel L. E., Crick F. H., 1980. Selfish DNA: the ultimate parasite. Nature 284: 604–607. [DOI] [PubMed] [Google Scholar]
- Parker G. S., Eckert D. M., Bass B. L., 2006. RDE-4 preferentially binds long dsRNA and its dimerization is necessary for cleavage of dsRNA to siRNA. RNA 12: 807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quenneville S., Turelli P., Bojkowska K., Raclot C., Offner S., et al. , 2012. The KRAB-ZFP/KAP1 system contributes to the early embryonic establishment of site-specific DNA methylation patterns maintained during development. Cell Rep. 2: 766–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy K. C., Dror T., Dowa J., Panek J., Chen K., et al. , 2017. An intracellular pathogen response pathway promotes proteostasis in C. elegans. bioRxiv. DOI https//.org/10.1101/145235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y., Yang S., Tan G., Ye W., Liu D., et al. , 2012. Reduction of mitoferrin results in abnormal development and extended lifespan in Caenorhabditis elegans. PLoS One 7: e29666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restif C., Ibáñez-Ventoso C., Vora M. M., Guo S., Metaxas D., et al. , 2014. CeleST: computer vision software for quantitative analysis of C. elegans swim behavior reveals novel features of locomotion. PLoS Comput. Biol. 10: e1003702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist F., Huelsenbeck J. P., 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- Rowe H. M., Jakobsson J., Mesnard D., Rougemont J., Reynard S., et al. , 2010. KAP1 controls endogenous retroviruses in embryonic stem cells. Nature 463: 237–240. [DOI] [PubMed] [Google Scholar]
- Schlesinger S., Goff S. P., 2015. Retroviral transcriptional regulation and embryonic stem cells: war and peace. Mol. Cell. Biol. 35: 770–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C. A., Rasband W. S., Eliceiri K. W., 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedivy J. M., Kreiling J. A., Neretti N., De Cecco M., Criscione S. W., et al. , 2013. Death by transposition - the enemy within? Bioessays 35: 1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro J. A., von Sternberg R., 2005. Why repetitive DNA is essential to genome function. Biol. Rev. Camb. Philos. Soc. 80: 227–250. [DOI] [PubMed] [Google Scholar]
- Shirayama M., Seth M., Lee H.-C., Gu W., Ishidate T., et al. , 2012. piRNAs initiate an epigenetic memory of nonself RNA in the C. elegans germline. Cell 150: 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmer F., Tijsterman M., Parrish S., Koushika S., Nonet M., et al. , 2002. Loss of the putative RNA-directed RNA polymerase RRF-3 makes C. elegans hypersensitive to RNAi. Curr. Biol. 12: 1317. [DOI] [PubMed] [Google Scholar]
- Stefanakis N., Carrera I., Hobert O., 2015. Regulatory logic of pan-neuronal gene expression in C. elegans. Neuron 87: 733–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein L. D., Bao Z., Blasiar D., Blumenthal T., Brent M. R., et al. , 2003. The genome sequence of Caenorhabditis briggsae: a platform for comparative genomics. PLoS Biol. 1: E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcomb D. T., Shaw J. E., Carr S. H., Hirsh D., 1985. Extrachromosomal DNA transformation of Caenorhabditis elegans. Mol. Cell. Biol. 5: 3484–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabara H., Sarkissian M., Kelly W. G., Fleenor J., Grishok A., et al. , 1999. The rde-1 gene, RNA interference, and transposon silencing in C. elegans. Cell 99: 123–132. [DOI] [PubMed] [Google Scholar]
- Tabara H., Yigit E., Siomi H., Mello C. C., 2002. The dsRNA binding protein RDE-4 interacts with RDE-1, DCR-1, and a DExH-box helicase to direct RNAi in C. elegans. Cell 109: 861–871. [DOI] [PubMed] [Google Scholar]
- Thomas J. H., 2006. Adaptive evolution in two large families of ubiquitin-ligase adapters in nematodes and plants. Genome Res. 16: 1017–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson O., Edgley M., Strasbourger P., Flibotte S., Ewing B., et al. , 2013. The million mutation project: a new approach to genetics in Caenorhabditis elegans. Genome Res. 23: 1749–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth K. F., Pezic D., Stuwe E., Webster A., 2016. The piRNA pathway guards the germline genome against transposable elements. Adv. Exp. Med. Biol. 886: 51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng R. J., Armstrong K. R., Wang X., Chamberlin H. M., 2007. The bromodomain protein LEX-1 acts with TAM-1 to modulate gene expression in C. elegans. Mol. Genet. Genomics 278: 507–518. [DOI] [PubMed] [Google Scholar]
- Vasale J. J., Gu W., Thivierge C., Batista P. J., Claycomb J. M., et al. , 2010. Sequential rounds of RNA-dependent RNA transcription drive endogenous small-RNA biogenesis in the ERGO-1/Argonaute pathway. Proc. Natl. Acad. Sci. USA 107: 3582–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace I. M., O’Sullivan O., Higgins D. G., Notredame C., 2006. M-Coffee: combining multiple sequence alignment methods with T-Coffee. Nucleic Acids Res. 34: 1692–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Kennedy S., Conte D., Jr, Kim J. K., Gabel H. W., et al. , 2005. Somatic misexpression of germline P granules and enhanced RNA interference in retinoblastoma pathway mutants. Nature 436: 593–597. [DOI] [PubMed] [Google Scholar]
- Wheeler T. J., Clements J., Finn R. D., 2014. Skylign: a tool for creating informative, interactive logos representing sequence alignments and profile hidden Markov models. BMC Bioinformatics 15: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D., Goff S. P., 2009. Embryonic stem cells use ZFP809 to silence retroviral DNAs. Nature 458: 1201–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamore P. D., Tuschl T., Sharp P. A., Bartel D. P., 2000. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell 101: 25–33. [DOI] [PubMed] [Google Scholar]
- Zhang M., Poplawski M., Yen K., Cheng H., Bloss E., et al. , 2009. Role of CBP and SATB-1 in aging, dietary restriction, and insulin-like signaling. PLoS Biol. 7: e1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Strains are available upon request. File S1 contains four supplemental figures, supplemental figure legends, and one supplemental table.