Abstract
A lipid and glycoprotein-rich apical extracellular matrix (aECM) or glycocalyx lines exposed membranes in the body, and is particularly important to protect narrow tube integrity. Lipocalins (“fat cups”) are small, secreted, cup-shaped proteins that bind and transport lipophilic cargo and are often found in luminal or aECM compartments such as mammalian plasma, urine, or tear film. Although some lipocalins can bind known aECM lipids and/or matrix metalloproteinases, it is not known if and how lipocalins affect aECM structure due to challenges in visualizing the aECM in most systems. Here we show that two Caenorhabditis elegans lipocalins, LPR-1 and LPR-3, have distinct functions in the precuticular glycocalyx of developing external epithelia. LPR-1 moves freely through luminal compartments, while LPR-3 stably localizes to a central layer of the membrane-anchored glycocalyx, adjacent to the transient zona pellucida domain protein LET-653. Like LET-653 and other C. elegans glycocalyx components, these lipocalins are required to maintain the patency of the narrow excretory duct tube, and also affect multiple aspects of later cuticle organization. lpr-1 mutants cannot maintain a continuous excretory duct apical domain and have misshapen cuticle ridges (alae) and abnormal patterns of cuticular surface lipid staining. lpr-3 mutants cannot maintain a passable excretory duct lumen, properly degrade the eggshell, or shed old cuticle during molting, and they lack cuticle barrier function. Based on these phenotypes, we infer that both LPR-1 and LPR-3 are required to build a properly organized aECM, while LPR-3 additionally is needed for aECM clearance and remodeling. The C. elegans glycocalyx provides a powerful system, amenable to both genetic analysis and live imaging, for investigating how lipocalins and lipids affect aECM structure.
Keywords: C. elegans, apical extracellular matrix, lipocalin, cuticle, excretory system
A lipid and glycoprotein-rich apical extracellular matrix (aECM) lines exposed membranes in the body (including tube lumens), protects them from pathogens and other damaging agents in the environment, and contributes to structural and barrier properties of tissues. Examples of aECM include lung surfactant (Lopez-Rodriguez and Perez-Gil 2014), the vascular glycocalyx (Tarbell and Cancel 2016), the intestinal mucin lining (Johansson et al. 2013), the epidermal stratum corneum (Feingold 2007), the eye tear film (Green-Church et al. 2011), and plant and invertebrate cuticles (Page and Johnstone 2007; Moussian 2010; Fernandez et al. 2016). In multiple model systems and in humans, luminal aECM shapes biological tubes, with the narrowest tubes often collapsing or rupturing after genetic, mechanical, or chemical aECM perturbations (Jazwinska et al. 2003; Mancuso et al. 2012; Salmon and Satchell 2012; Dong et al. 2014; Herzog et al. 2014; Luschnig and Uv 2014; Gill et al. 2016). There is a growing body of evidence that damage to the luminal aECM may contribute to common progressive diseases that involve a loss of narrow tube integrity, such as chronic obstructive pulmonary disease, end-stage renal disease, and microvascular or “small vessel” disease (Nieuwdorp et al. 2006; Rose and Voynow 2006; Decramer et al. 2012; Salmon et al. 2012; Dane et al. 2014; Seneff et al. 2015). Preserving and restoring the protective function of luminal aECM to prevent and treat such diseases will require a better understanding of its contents and multi-layered organization. Unfortunately, the fragile nature of most luminal aECM has hampered its visualization and study.
Lipocalins (from Greek lipos, “fat” and calyx, “cup”) are a large family of small, secreted, cup-shaped proteins (Flower 1996) that are often found in luminal or aECM compartments such as mammalian plasma (Christoffersen et al. 2011; Luo et al. 2015; di Masi et al. 2016), urine (Barasch et al. 2016), or tear film (Dartt 2011), and are clinically useful biomarkers for detecting tissue damage in the renal and vascular systems (Mishra et al. 2005; Cruz et al. 2012; Fischer et al. 2014; Haase-Fielitz et al. 2014). Lipocalins bind lipophilic cargoes including sterols, phospholipids, and sphingolipids (Watanabe et al. 2014; di Masi et al. 2016), and while their proposed functions are quite diverse, they include transport or sequestration of aECM lipids (Gouveia and Tiffany 2005; Christoffersen et al. 2011; Dartt 2011; Yeh et al. 2013) and regulation of matrix metalloproteinase activity (Yan et al. 2001; Leng et al. 2009). Lipocalin mutant studies in the mouse have revealed defects in tissue integrity, pathogen sensitivity, and signaling (Flo et al. 2004; Quadro et al. 2005; Christoffersen et al. 2011; Watanabe et al. 2014), some of which could be caused by defects in aECM, but difficulties in visualizing the mammalian aECM have prevented direct tests of whether lipocalins affect aECM structure.
We are taking advantage of the transparent model organism Caenorhabditis elegans to study aECM and its tube-protecting functions. C. elegans embryogenesis and the development of all external epithelia (e.g., the epidermis and excretory duct/pore, rectum, and vulva tubes) occur in the context of a glycocalyx-like aECM that contains chondroitin sulfate (Hwang et al. 2003; Dierker et al. 2016; Izumikawa et al. 2016), multiple extracellular leucine-rich repeat-only (eLRRon) proteins (SYM-1, LET-4, and EGG-6) (Mancuso et al. 2012), and multiple zona pellucida (ZP)-domain proteins (FBN-1, NOAH-1, NOAH-2, and LET-653) (Kelley et al. 2015; Gill et al. 2016; Vuong-Brender et al. 2017). At the end of morphogenesis, external epithelia develop a more rigid cuticle consisting primarily of collagens, plus various ZP and other insoluble proteins termed cuticlins (Sapio et al. 2005; Page and Johnstone 2007). The cuticle is shed and resynthesized at each of four larval molts (Singh and Sulston 1978; Frand et al. 2005). Some protein components of the glycocalyx, including the ZP and mucin-like protein LET-653, are cleared prior to cuticle maturation, and then reappear cyclically prior to each new round of cuticle synthesis (Gill et al. 2016). Other protein components, as well as carbohydrates and lipids, are thought to remain associated with the mature cuticle (Priess and Hirsh 1986; Costa et al. 1997; Page and Johnstone 2007). This dynamic system is amenable to live imaging of aECM, and it provides a good opportunity to learn about the mechanisms involved in aECM layer assembly and disassembly, as well as to probe functional importance of individual aECM factors.
Several C. elegans glycocalyx components and the lipocalin LPR-1 are required to maintain integrity of the narrow excretory duct and pore tubes. The excretory system is a simple, renal-like osmoregulatory organ composed of three tandemly connected unicellular tubes: the canal, duct, and pore (Nelson et al. 1983; Sundaram and Buechner 2016) (Figure 1A). The excretory duct is the middle tube in the organ, and during morphogenesis, it undergoes a dramatic shape change in which its lumen elongates and narrows (Gill et al. 2016). The glycocalyx is required to protect lumen integrity during this shape transition. In mutants for let-653 (Gill et al. 2016), let-4, or egg-6 (Mancuso et al. 2012), the duct lumen collapses and fragments during morphogenesis, and the duct and pore cells sometimes detach from each other, leading to massive fluid edema and a “rod-like” L1 larval lethal phenotype. lpr-1 mutants have defects in duct lumen integrity that closely resemble those of glycocalyx mutants (Stone et al. 2009; Pu et al. 2017), consistent with the possibility that lipocalins affect glycocalyx structure.
Figure 1.
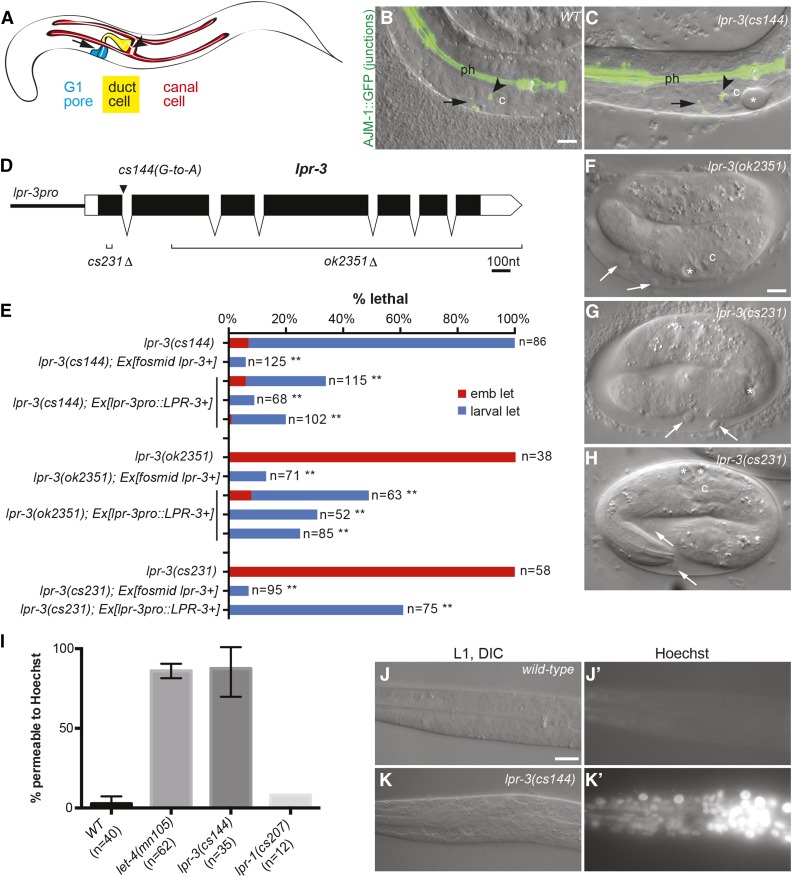
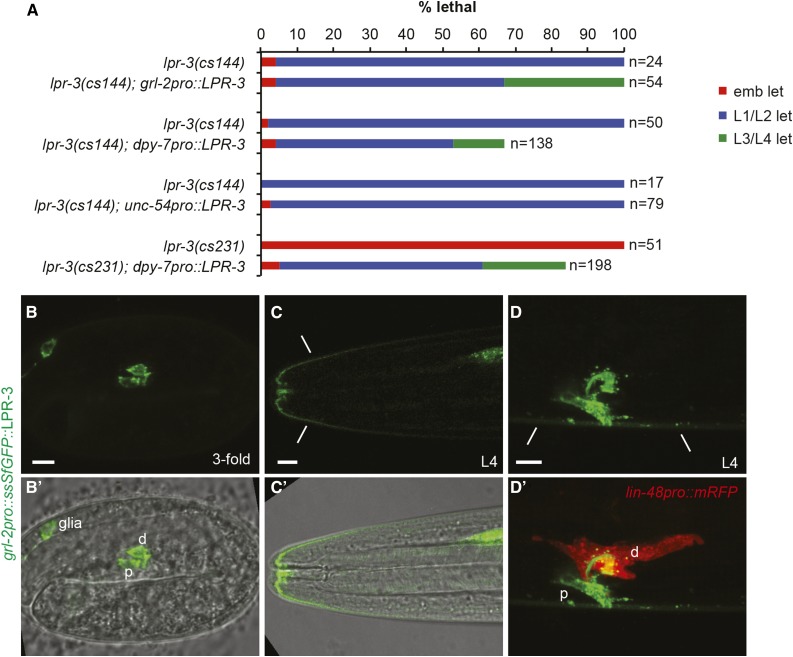
lpr-3 is required for excretory function, epidermal integrity and hatching. (A) Cartoon of the excretory system in an L1 larva. (B and C) Merged DIC and epifluorescence images of L1 larvae expressing junction marker AJM-1::GFP (jcIs1), which marks pore junctions (arrow), the canal-duct junction (arrowhead), and pharyngeal junctions (ph). The duct and canal are seamless and therefore unmarked along their lengths. c, canal nucleus. Bar, 5 μm. (B) Wild type. (C) lpr-3(cs144) mutant with excretory edema (asterisk). (D) lpr-3 mutant alleles. See Materials and Methods and Figure S1 in File S1 for further information. (E) Quantification of lpr-3 embryonic (emb) and larval lethal (let) phenotypes and transgenic rescue. ** P < 0.0001, Fisher’s exact test compared to nontransgenic siblings. The lpr-3 fosmid is WRM0619dE09. lpr-3pro::LPR-3 (pRFR4) contains 0.7 kb of the upstream promoter region driving the lpr-3 cDNA (accession #NM 077448.4) (Materials and Methods); incomplete rescue by this construct likely reflects insufficient promoter activity of this fragment. (F–H) lpr-3(−) mutants arrest as embryos, with shed epidermal fragments (white arrows) accumulating between the embryo and the eggshell. (F and G) embryos are ∼11 hr after egg-lay. H shows an lpr-3(cs231) embryo that is still alive and active 18 hr after egg-lay, ∼5 hr beyond the normal time of hatch. (I–K) lpr-3(cs144) L1 larvae are permeable to Hoechst dye. (I) Quantification of permeability. Wild-type (N2) and let-4(mn105) mutants were included as negative and positive controls, respectively. Data are from 1 to 4 independent experiments per genotype. Error bars show SEM. (J, J' and K, K') Representative wild-type (J, J') and lpr-3(cs144) (K, K') larvae following Hoechst treatment. Bar, 5 μm.
Here we show that LPR-1 and a second lipocalin, LPR-3, are present within distinct compartments of the C. elegans glycocalyx, are required nonredundantly for its narrow tube-protecting function, and also affect multiple aspects of later cuticle structure or molting. These data clearly demonstrate the importance of lipocalins for aECM organization.
Materials and Methods
Worm strains, alleles, and transgenes
All strains were grown at 20° under standard conditions (Brenner 1974). lpr-3 mutants were obtained from mothers rescued with an lpr-3(+) transgene. Failure of some transgenes to rescue was quantified in progeny of mothers carrying both the test transgene and a separate, differently marked, rescuing lpr-3(+) transgene. Transgenes used included: csIs61 I (RDY-2::GFP, lin-48pro::mRFP) (Gill et al. 2016), csIs64 (let-653pro::LET-653::SfGFP, lin-48pro::mRFP, rol-6d) (Gill et al. 2016), jcIs1 IV (AJM-1::GFP, rol-6d) (Koppen et al. 2001), csEx436 (lpr-3+ fosmid WRM0619dE09, myo-2pro::mCherry), csEx573-575 (lpr-3pro::LPR-3+, unc-119pro::GFP), csEx581, csEx591 (lpr-3pro::4xSV40-GFP, grl-2pro::mRFP), and csEx603 (lpr-3pro::ssSfGFP::LPR-3+, lin-48pro::mRFP). See Supplemental Material, Table S1 and Table S2 for a complete list of strains, plasmids, and transgenes used in this study.
lpr-3 mutant isolation and molecular characterization
lpr-3(cs144) was isolated based on its rod-like lethal phenotype after standard ethylmethanesulfonate (EMS) mutagenesis (Brenner 1974) of strain UP2214 (unc-119; jcIs1; qnEx59), as previously described (Gill et al. 2016). cs144 was mapped to the X chromosome based on linkage results with the balancers szT1 and hT2. The lpr-3 lesion was detected after whole genome sequencing followed by bioinformatics analyses with Cloudmap (Minevich et al. 2012), and was determined to be causal based on transgenic rescue experiments (Figure 1E) and analysis of independent alleles. Cloudmap did not detect any other lesions in the area covered by rescuing fosmid WRM0619dE09.
lpr-3 (W04G3.8) is the first in a cluster of four lipocalin-related genes on the X chromosome. This does not appear to be an operon, however (Allen et al. 2011), and indeed by RT-PCR from wild-type N2 animals we could not detect a transcript joining lpr-3 and the next downstream gene, lpr-6 (W04G3.1) (Figure S1 in File S1). Deletion ok2351, provided by the C. elegans knockout consortium (Moerman and Barstead 2008), removes the majority of the lpr-3 coding region and part of the downstream intergenic region; RT-PCR revealed that ok2351 fuses the remainder of lpr-3 to the lpr-6 transcript (Figure S1 in File S1). This does not fuse the coding regions, but presumably leads to misexpression of lpr-6 under control of the lpr-3 promoter. We initially considered the possibility that loss and/or overexpression of lpr-6 explained the more severe embryonic arrest phenotype of ok2351 compared to cs144. However, lpr-6 RNA-mediated interference (RNAi) did not affect cs144 lethality (12% embryonic and 100% overall lethal, n = 115) or ok2351 lethality (100% lethal, n = 70), and an lpr-6 genomic transgene, csEx745, did not affect cs144 lethality (9% embryonic and 100% overall lethal, n = 46) or ok2351 lethality (100% lethal, n = 92), whereas an lpr-3 complementary DNA (cDNA) construct did significantly rescue both alleles (Figure 1E). To resolve this issue definitively, we used CRISPR-Cas9 to generate cs231, a smaller 13 nucleotide deletion/frameshift in the lpr-3 first exon; this allele caused an embryonic lethal phenotype similar to that of ok2351, and was also rescued by both fosmid and lpr-3 cDNA constructs, leading us to conclude that the null phenotype of lpr-3 is embryonic lethal.
The lpr-3(cs231) allele was created in an N2 background by CRISPR-Cas9 using the dpy-10 co-crispr strategy described in Arribere et al. (2014). lpr-3 single-guide RNA (ACGACGTTAGCTGTGGCACT) was inserted into the Cas9 expression plasmid pDD162 (Dickinson et al. 2013). Heterozygous cs231 animals were recognized based on progeny embryonic lethality; the mutant allele was recovered over szT1 and then rescued by lpr-3 transgenes. Lesions in all three alleles were verified by Sanger sequencing of genomic PCR products from mutant worms.
Generation of lpr-3 and lpr-6 transgenes
The lpr-3 promoter was defined as the 0.7-kb region between the next upstream gene and the lpr-3 ATG. This promoter was PCR-amplified from fosmid WRM0619dE09 using primers oRFR3 (GGGCTGCAGGGAAGTGTGAATTGATGCAATC) and oRFR4 (GGGGGATCCCACTGGTGTGCAGAAGCCAATG) and cloned into pPD49.26 (Addgene) as a PstI-BamHI fragment to generate plasmid pRFR3 (lpr-3pro::empty). lpr-3 cDNA (accession #NM 077448.4) was PCR-amplified from cDNA prepared from mixed stage worms with primers oCS66 (GGACCAGCTAGCATGCGTTCGCTTATTTGGATTTTG) and oCS67 (GGGCCCCCATGGTTACTTTCCGAAAACTTCAAAAG) and cloned into pRFR3 as a Nhe1-Nco1 fragment to create pRFR4 (lpr-3pro::LPR-3). To create the transcriptional reporter, lpr-3pro::NLS-GFP (pRFR5), 4xSV40-GFP was PCR-amplified from pPD121.83 (Addgene) and cloned into pRFR3. To create the translational reporter, lpr-3pro::ssSfGFP::LPR-3 (pRFR12), an artificial signal sequence (ss) from pPD95.85 (Addgene) was added to the N-terminus of SfGFP (a kind gift from Max Heiman, Harvard University). ssSfGFP was inserted in place of the lpr-3 signal sequence within the lpr-3 cDNA. A C-terminally tagged translational reporter (pRFR10, lpr-3pro::LPR-3::SfGFP) showed a similar pattern of localization but was completely nonfunctional in cs144 mutant rescue assays.
To test for any influence of lpr-6 on lpr-3 phenotypes, a 3-kb lpr-6+ genomic fragment was PCR-amplified from fosmid WRM0619dE09 using primers oRFR21 (GACCCTGATACTTTCAAGACC) and oCS91 (GCGAAAACACTGGACGGATGAC) and used to generate transgene csEx745.
RT-PCR
Trizol Reagent (Cat# 15596-018; Invitrogen, Carlsbad, CA) was used to isolate RNA from hand-picked lpr-3(cs144) L1 larvae or mixed stage N2, or from lpr-3(ok2351) mutant worms rescued by an lpr-3 cDNA-based extrachromosomal transgene. cDNA was synthesized using SuperScript First-Strand Synthesis System for RT-PCR (11904-018; Invitrogen). Primers for RT-PCR are described in Figure S1 in File S1.
RNAi
lpr-3 and lpr-6 double-stranded RNA (dsRNA) was synthesized using the Ambion MEGAscript RNAi Kit (AM1626; Ambion). Templates used were lpr-3 RNAi bacterial clone W04G3.8 (Ahringer) with primers oRFR33 (TAATACGACTCACTATAGGGTCGCTTATTTGGATTTTGGC) and oRFR34 (TAATACGACTAGGGTTGAGCAGCAGATGGTTGTC) and lpr-6 RNAi bacterial clone W04G3.1 (Ahringer) with primers oRFR35 (TAATACGACTCACTATAGGGTTCAGATGGATTTCCAAGCC) and oRFR36 (TAATACGACTCACTATAGGGTCCAAAGTACGCTTTGGGTC). dsRNA was injected into gravid hermaphrodites at 6–12 ng/μl. Embryos laid 24–48 hr after injection were collected and observed 24 hr later. Nearly all animals treated with lpr-3 RNAi arrested as L1 larvae with molting defects. lpr-6 RNAi had no detected effect.
Staging and microscopy
Embryos were selected at the 1.5-fold stage and then incubated for the indicated number of hours before mounting for imaging. Larvae were staged by hours after egg-lay, with ∼13 hr corresponding to hatch. Young (not yet gravid) adults for alae imaging were selected based on size and vulva morphology. Epifluorescence and Differential Interference Contrast (DIC) images were captured on a compound Zeiss Axioskop fitted with a Leica DFC360 FX camera. Confocal and Dodt gradient contrast (DODT) images were captured with a Leica TCS SP8. Images were processed and merged using ImageJ.
Hoechst staining
Mixed stage worms were washed twice with 1 ml M9 buffer, and then incubated for 15 min in 1 ml of 1 μg/ml Hoechst 33342 (62249; Thermo Fisher), with gentle rocking. Worms were washed 3× with M9 and recovered on plates seeded with OP50. L1 larvae were imaged 10–15 min later.
DiI staining
Lipophilic dye DiI (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine) staining was performed as in (Schultz and Gumienny 2012). Briefly, 20–30 mid- to late-L4-stage larvae were washed once in 1 ml M9 buffer containing 0.5% Triton (X100-100ML; Sigma, St. Louis, MO) and twice in 1 ml M9 buffer. Once all excess buffer had been removed, 400 μl of 30 μg/ml DiI (60010; Biotium) in M9 buffer was added, and larvae were incubated on a rocker for 3 hr at room temperature. After the incubation, worms were pipetted directly from the DiI solution onto NGM-agar plates heavily seeded with OP50. The plates were kept in the dark for 30–45 min while worms swam from DiI solution onto OP50. Worms that had successfully moved onto OP50 were chosen for imaging. Epifluorescence microscopy images were taken using 60-msec exposures, to maximize fluorescence intensity dynamic range. This resulted in nearly undetectable levels of fluorescence in wild-type animals. Images were scored for intensity by a researcher blinded to genotypes.
Fluorescence recovery after photobleaching (FRAP)
Specimens were mounted on 10% agarose pads containing 20 mM sodium azide and 10 mM levamisole in M9. FRAP was performed using Leica Application Suite X software FRAP module on a Leica TCS SP8 MP confocal microscope. A 3 × 3 μm bleach region of interest (ROI) was defined within the wizard, mean fluorescence intensity within the ROI was measured at specified intervals, and values were normalized such that the maximum intensity was set to 1. The following experimental time-course was used: 20 prebleach frames every 0.4 sec, 10 bleach frames every 0.4 sec, and 90 postbleach frames every 2.0 sec. Pre- and postbleach laser intensity was set to 0.5–2% on a specimen-by-specimen basis and set to 100% for bleach frames. To correct for additional bleaching during the postbleach phase, bleached intensity was divided by the intensity of an adjacent, nonbleached ROI for every time point. FRAP plots were created and analyzed using Graphpad Prism, where one-phase association curves derived from the model Y = Y0 + (Plateau-Y0)*[1–e^(−Kx)] were fitted to the data. For statistical tests, mobile fractions and recovery half-times were derived from one-phase association curves fitted to individual experiments. Mobile fraction = Plateau-Y0; t1/2 = ln(2)/K, where K is the recovery rate constant.
Data availability
Strains and plasmids are available upon request. Table S1 contains complete genotypes of all strains. Table S2 contains a list of all plasmids and transgenes.
Results
LPR-3 is required for excretory function, epidermal integrity, and hatching
In genetic screens for rod-like L1 lethal mutants with excretory edema, we previously identified multiple loss-of-function alleles in the lipocalin-related gene lpr-1 (Stone et al. 2009; Pu et al. 2017). Another locus from this screen was defined by a single X-linked recessive allele, cs144 (Figure 1, B and C; Materials and Methods). Whole genome sequencing, followed by transgenic rescue assays, revealed that cs144 is a splice donor mutation in the first intron of the lipocalin-related gene, lpr-3 (W04G3.8) (Figure 1D). RT-PCR revealed failure to splice out this intron in cs144 mutants, resulting in inclusion of a premature stop codon (Figure S1 in File S1). Although normal transcript was not detected, we presume that cs144 embryos have low levels of correct splicing, because it appears to be a hypomorphic allele. Most lpr-3(cs144) mutants arrest as L1 larvae with excretory edema (Figure 1C), and only a small percentage die earlier as embryos (Figure 1E). However, two independent lpr-3 deletion (−) alleles cause a completely penetrant embryonic lethal phenotype (Figure 1, E–H). The CRISPR-induced deletion cs231 removes 13 nucleotides of exon 1, leading to a frameshift (Figure S1 in File S1). The larger deletion ok2351 removes the majority of the lpr-3 coding region and also disrupts expression of the downstream lipocalin gene lpr-6 (Figure S1 in File S1 and Materials and Methods), possibly explaining its earlier onset defects (see Figure 2). Embryonic and larval lethality of all lpr-3 alleles can be rescued by an lpr-3 + fosmid and partially rescued by transgenes expressing the wild-type lpr-3 cDNA under control of a short, 0.7-kb endogenous promoter (Figure 1E).
Figure 2.
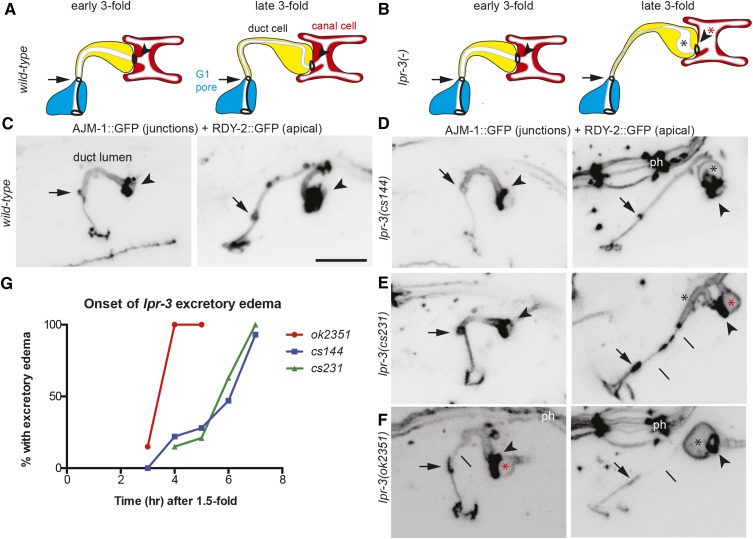
lpr-3 is required to maintain uniform lumen diameter and tube patency during excretory duct tube morphogenesis. (A and B) Cartoons of duct morphogenesis in WT (A) and lpr-3(−) mutants (B). Early and late threefold correspond to 3–4 and 6–7 hr after 1.5-fold, respectively, a time interval during which the lumen elongates and narrows. Arrow points to duct–pore junction and arrowhead points to duct–canal junction. Asterisks indicate lumen dilations in the duct (black) and canal (red). (C–F) Inverted confocal z-projections of early (left panels) and late (right panels) threefold embryos expressing the junction marker AJM-1::GFP (jcIs1) and the apical membrane marker RDY-2::GFP (csIs61). Compare to AJM-1::GFP alone in Figure 1, B and C. The intervening signal between the arrow and arrowhead is RDY-2::GFP marking the apical membrane of the seamless duct tube. Pharyngeal junctions (ph) dorsal to the excretory system are also visible in some panels. Lines indicate abnormally thin but still continuous apical regions. Bar, 5 μm. (G) Timing analysis of excretory edema as assessed by DIC in strains with the AJM-1::GFP marker only. Edema was recognized as a bubble as large, or larger than, the adjacent canal cell nucleolus (Pu et al. 2017). The earlier onset of defects in ok2351 embryos may be caused by abnormal expression of lpr-6 (see Figure S1 in File S1 and Materials and Methods).
lpr-3 mutants exhibit a variety of defects characteristic of glycocalyx or matrix-remodeling mutants. lpr-3(cs144) L1 larvae have defects in cuticle barrier function, as mutants were penetrated by Hoechst dye (Figure 1, I–K); similar barrier defects are observed in let-4 and egg-6 glycocalyx mutants (Mancuso et al. 2012). lpr-3(−) embryos elongate to the threefold stage, but shed epidermal fragments into the extraembryonic space (Figure 1, F and G) and sometimes rupture prior to hatch in a manner similar to that described for let-4, noah-1, and noah-2 mutants (Mancuso et al. 2012; Vuong-Brender et al. 2017). The majority of lpr-3(−) embryos remain intact and active for at least 18 hr after egg-lay, but nevertheless are unable to break through the eggshell and fail to hatch (Figure 1H), suggesting a defect in eggshell dissolution; similar defects have been described for some matrix metalloprotease mutants (Hishida et al. 1996).
LPR-3 is required to maintain excretory duct tube lumen diameter and patency during morphogenesis
To understand the basis for the lpr-3 excretory phenotype, we examined mutants over a time-course of development using markers for the excretory duct and pore tubes’ apical membrane (RDY-2::GFP) and apical junctions (AJM-1::GFP). lpr-3(cs144) and lpr-3(−) embryos appeared normal at early stages of excretory tube development, but then developed excretory edema at the threefold stage, during duct tube elongation (Figure 2). In wild-type animals, in the period between 4 and 6 hr after the 1.5-fold stage, the duct lumen both elongates and narrows (Gill et al. 2016) (Figure 2, A and C). In lpr-3 mutants, distal portions of the duct lumen often became overly narrow and appeared stretched, while other portions failed to narrow or became dilated (Figure 2, B and D–F). The duct and pore cells remained attached, and unlike in lpr-1 mutants, the lumen rarely fragmented until later in larval development. Nevertheless, the upstream canal lumen became progressively more dilated over time (Figure 2), suggesting that excretory fluid was not able to pass through the narrowed part of the duct tubule. We conclude that LPR-3 is required to maintain a uniform duct lumen diameter and tube patency during lumen elongation and narrowing.
The lpr-3 mutant defects somewhat resemble those of glycocalyx mutants and coincide with a known period of glycocalyx remodeling as LET-653 leaves the matrix (Gill et al. 2016). We therefore examined LET-653 dynamics in lpr-3 mutants. A LET-653::SfGFP fusion accumulated robustly in the duct lumen of both wild-type and lpr-3(−) mutant embryos and then was cleared upon cuticle secretion (Figure S2 in File S1), suggesting that LPR-3 is not needed for LET-653 recruitment to, or clearance from, the glycocalyx.
LPR-1 and LPR-3 function nonredundantly
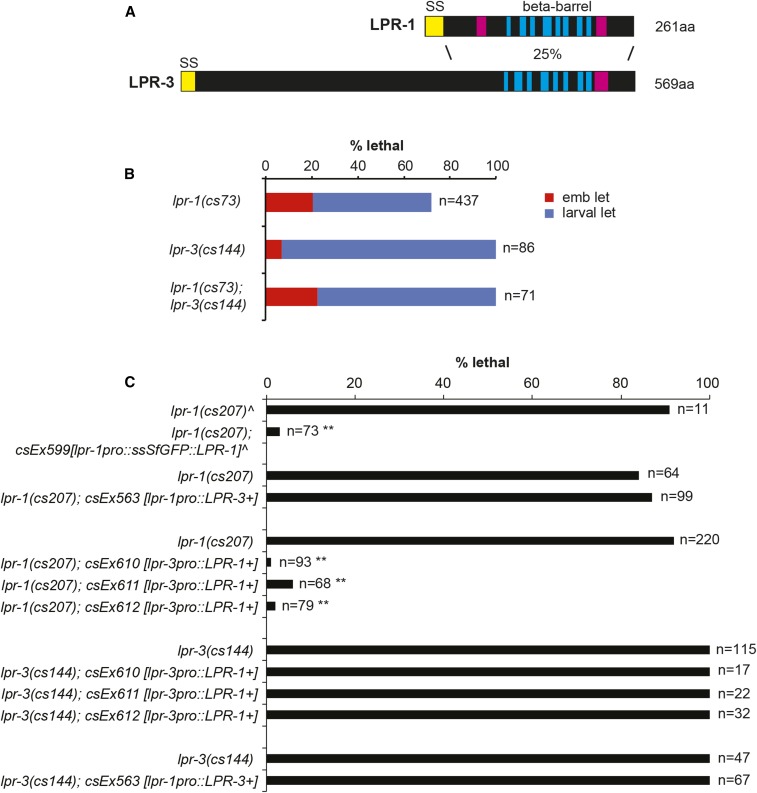
LPR-1 and LPR-3 are 25% identical at the amino acid level (Figure 3A), and are just two of five sequence-related lipocalins encoded by the C. elegans genome (Stone et al. 2009; Pu et al. 2017). Both are predicted to share the signature lipocalin cup structure, consisting of eight antiparallel β-sheets arranged to form a barrel (Flower 1996), but LPR-3 has an additional N-terminal extension not present in LPR-1 (Figure 3A). To ask if lpr-1 and lpr-3 function in a similar or redundant manner, we made lpr-1(cs73); lpr-3(cs144) double mutants and we conducted transgenic cross-rescue experiments by expressing each gene under the other’s promoter. The double mutants resembled the single mutants and did not show increased embryonic lethality (Figure 3B). Overexpression of LPR-3 could not rescue lpr-1 mutant lethality, or vice versa (Figure 3C). These data indicate that LPR-1 and LPR-3 have distinct activities but are consistent with both acting in the same pathway or process in the excretory system. The more penetrant and pleiotropic phenotypes of lpr-3 mutants indicate that it has additional epidermal functions not shared by LPR-1.
Figure 3.
LPR-1 and LPR-3 function nonredundantly. (A) Schematics of LPR-1 and LPR-3 protein structure. Blue and pink rectangles indicate predicted β-sheets and α-helices, respectively (Rost et al. 2004). SS, signal sequence; aa, amino acids. (B) Loss of lpr-1 did not enhance lpr-3(cs144) embryonic lethality. (C) LPR-1 and LPR-3 are not functionally interchangeable. Transgenes with lpr-1pro::LPR-3 (pRFR1) did not rescue lpr-1(cs207) or lpr-3(cs144) lethality; failure to rescue lpr-3 may be due to inappropriate timing of expression, as the lpr-1 promoter comes on later than does the lpr-3 promoter (Pu et al. 2017). Transgenes with lpr-3pro::LPR-1 (pRFR13) did not rescue lpr-3(cs144) lethality (but did rescue lpr-1 lethality). ^Data reproduced from Pu et al. (2017). Lethality of nontransgenic siblings is shown for each experiment. ** P < 0.0001 (Fisher’s exact test) compared to nontransgenic siblings.
The homology between LPR-1 and LPR-3 in the lipocalin cup region raised the possibility that these lipocalins might bind a common cargo. We therefore asked whether the cup region is interchangeable by making a chimeric protein that contains the LPR-3 N-terminus fused to the LPR-1 cup. This chimera did not rescue either lpr-1 or lpr-3 mutants and was toxic in wild-type and lpr-3(cs144) backgrounds, suggesting it functioned as a dominant negative (Figure S3 in File S1).
LPR-3 localizes to an internal layer of the glycocalyx
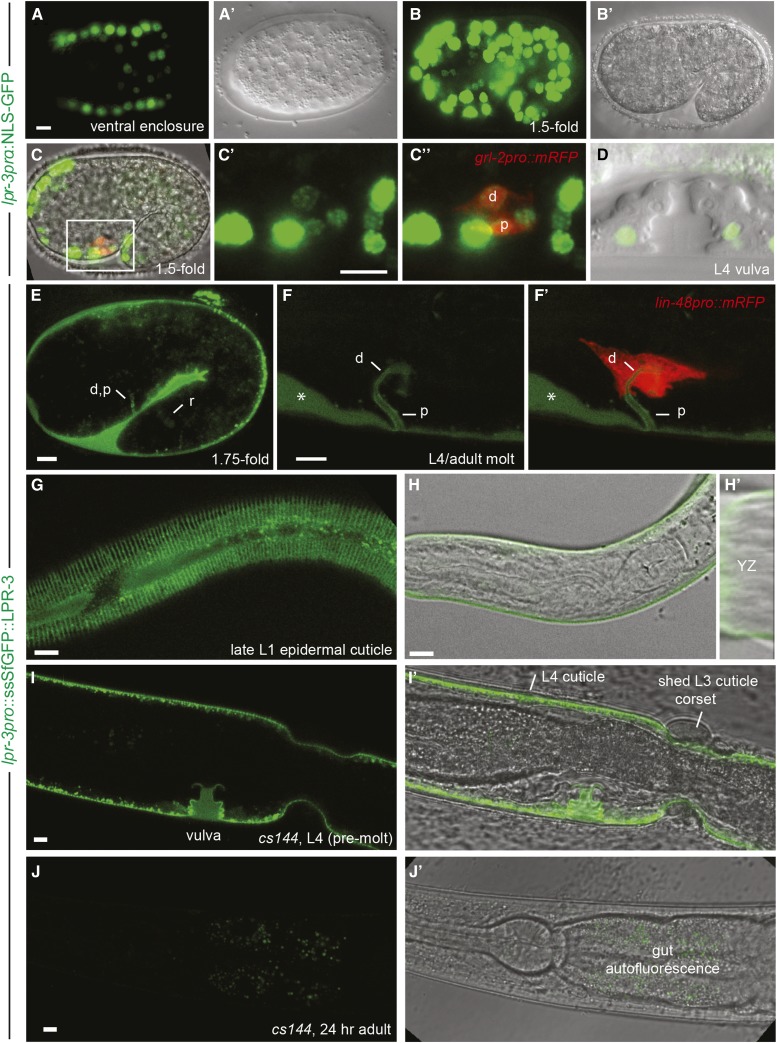
To determine when and where lpr-3 is expressed, we analyzed promoter fusions to nuclear localized Green Fluorescent Protein (GFP) and translational fusions with Superfolder (Sf) GFP (Figure 4 and Figure 5, Figure S3 in File S1). lpr-3 was transcribed in external (cuticle secreting) epithelia, including the epidermis (Figure 4, A and B), excretory duct and pore cells (Figure 4C), and vulva cells (Figure 4D), as well as in the gut, but not in the excretory canal cell. Expression began prior to ventral enclosure (Figure 4A) and persisted throughout larval development, but then disappeared in adults. N- and C-terminally tagged translational fusions showed similar localization patterns, but only the former showed significant rescue activity (Materials and Methods and Figure S3 in File S1). This rescue was weaker than that observed for untagged cDNA constructs (Figure 1E), suggesting that even the N-terminal tag partially interferes with function; nevertheless, ∼25% of transgenic lpr-3(−) mutants survived to be fertile adults (Figure S3 in File S1). ssSfGFP::LPR-3 fusion protein accumulated in apical extracellular compartments, including the extraembryonic space (Figure 4E) and the excretory duct/pore (Figure 4, E and F), rectal (Figure 4E), and vulval (Figure 4I) lumens. ssSfGFP::LPR-3 also associated with the larval cuticle (Figure 4, G–I) and accumulated between the old and new cuticles before and during molting (Figure 4, F and I). However, LPR-3 was not associated with the outer surface of old cuticle or with shed cuticle after molting (Figure 4I), and it completely disappeared in adults (Figure 4J). We infer that LPR-3 is present within a juvenile glycocalyx layer, and that the connection between this layer and the mature cuticle is severed during molting.
Figure 4.
LPR-3 associates with the glycocalyx throughout development. (A–D) lpr-3pro::NLS-GFP (pRFR5) is expressed widely in ventral enclosure (A) and 1.5-fold (B and C) embryos. A’ and B’ show corresponding DIC or DODT images, respectively. (C) Overlay of green (C’ and C”), red [(C”) duct (d) and pore (p) marker grl-2pro::mRFP] and DODT channels. (D) Overlay of green and DIC channels for a late L4 vulva; lpr-3pro drove expression specifically in secondary vulval cells. (E–J) lpr-3pro::ssSfGFP::LPR-3 (pRFR12) is apically secreted from embryos (E) and larvae (F–I) and marks duct (d), pore (p), and rectal (r) luminal glycocalyx (E and F) and juvenile epidermal cuticle (G–I) structures. It is not visible in adults (J). (E–H) Expression in wild-type animals. (F’) Overlay with duct marker lin-48pro::mRFP. Asterisk indicates accumulation between old and new cuticles in molting animal. (G and H) Late L1 (24 hr after egg-lay) and early L1 (13 hr after egg-lay) larvae; LPR-3 is present throughout the molt cycle. (H’) An orthogonal YZ projection, showing LPR-3 at or near the surface of newly synthesized cuticle. (I and J) Expression (or lack thereof) in rescued lpr-3(cs144) animals; I’ and J’ are overlays with corresponding DODT images. Note incomplete rescue of molting defect in (I’) and absence of GFP signal on cuticle surface and in partially shed cuticle corset. See Figure S3 in File S1 for rescue quantification. All bars, 5 μm.
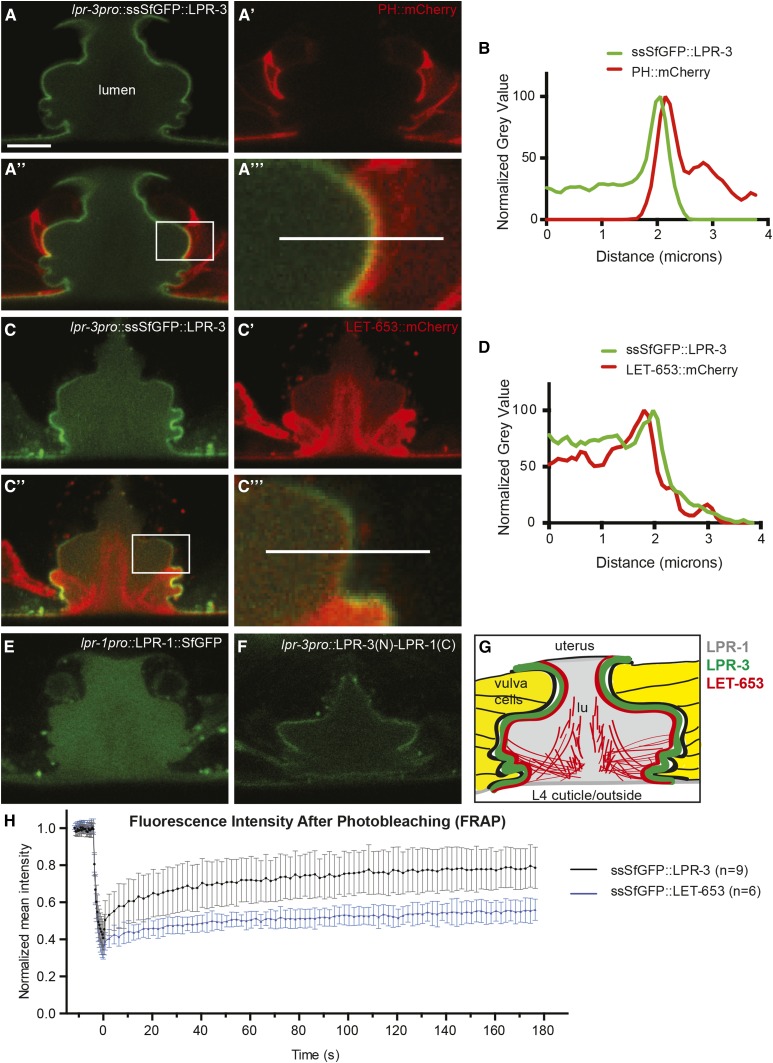
Figure 5.
LPR-3 localizes to an internal layer of the glycocalyx. (A–D) In the L4 vulva, ssSfGFP::LPR-3 localizes apically to PH::mCherry (A and B) and membrane-proximal to LET-653::mCherry (C and D). A and C show lpr-3pro::ssSfGFP::LPR-3 (pRFR12). A’ shows let-653pro::PH::mCherry (pRFR45) alone. C’ shows let-653pro::LET-653::mCherry alone; only a portion is in the membrane-anchored glycocalyx, while the remainder is in a more dynamic central matrix compartment (Gill et al. 2016). Boxed regions in A” and C” (overlays) are enlarged in A”’ and C”’, respectively. Fluorescence intensity along lines in A”’ and C”’ were measured in ImageJ, normalized to the maximum intensity for that specimen in each channel, and plotted in B and D, respectively. Images are representative of at least six animals examined per strain. Bar, 5 μm. (E) LPR-1::SfGFP (pEP96) fills the vulva lumen. (F) The LPR-3 N-terminus confers glycocalyx localization to an LPR-3(N)-LPR-1(C) chimera (pRFR32, see Figure S2 in File S1 for chimera details). (G) Cartoon of the vulva glycocalyx. (H) FRAP fluorescence recovery curves for ssSfGFP::LPR-3 (n = 9) and ssSfGFP::LET-653 (n = 6) showing mean fluorescence and SEM over time. See Figure S4 in File S1 and Materials and Methods for further details.
In the developing vulva tube, where resolution is highest and the glycocalyx is 2–3 μm thick (Gill et al. 2016), ssSfGFP::LPR-3 localized specifically to a narrow region along the apical membrane (Figure 4I and Figure 5, A–D and G). ssSfGFP::LPR-3 localized apically to a PH::mcherry membrane marker (Figure 5, A and B), confirming that it is extracellular. ssSfGFP::LPR-3 localized slightly more membrane-proximal than the ZP protein LET-653 (Figure 5, C and D), indicating that it is present in one of the inner layers of the glycocalyx. FRAP experiments indicated that LPR-3 has a relatively low mobile fraction of 0.36, although it is still higher than the 0.19 mobile fraction observed for LET-653 (Figure 5H and Figure S4 in File S1). The relatively low mobile fraction is consistent with LPR-3 being embedded within a central layer of the membrane-anchored glycocalyx (Figure 5G).
The LPR-3 N-terminus confers glycocalyx localization
We showed previously that LPR-1 is also expressed by all external epithelia and is secreted apically between the embryo and the eggshell (Pu et al. 2017). LPR-1::SfGFP was only variably visible in the embryonic excretory duct/pore lumen (Pu et al. 2017), which is open to the extraembryonic environment, but was more robustly observed in the developing vulval lumen (Figure 5E), which is a closed compartment. LPR-1::SfGFP filled the entire vulva lumen and did not appear to associate specifically with any glycocalyx layer. Thus, both LPR-1 and LPR-3 are found within luminal compartments, but they show distinct localization patterns.
The N-terminal extension of LPR-3 was sufficient to confer a membrane-proximal localization pattern to an LPR-3::LPR-1 chimeric fusion (Figure 5F), suggesting that this LPR-3 domain interacts with some partner in the precuticular glycocalyx.
LPR-3 functions cell nonautonomously but locally
We showed previously that LPR-1 can function tissue nonautonomously, such that lpr-1 mutant duct defects could be rescued by transgenic expression of LPR-1 in the nearby body muscle or pore and hypodermis (Stone et al. 2009; Pu et al. 2017). To test autonomy of LPR-3 action, we performed similar rescue experiments. Expression of lpr-3 cDNA in the duct and pore (as well as in glial cells), under control of the grl-2 promoter, did rescue lpr-3(cs144) excretory defects, but did not restore viability (Figure 6A), instead yielding many larvae that arrested with molting defects (Figure 7A; see below). Expression of lpr-3 cDNA in the hypodermis and excretory pore cells, under control of the dpy-7 promoter, was able to rescue lpr-3(−) mutant excretory defects (Figure 6A), despite the fact that this promoter is not active in the duct (Stone et al. 2009); this construct also rescued embryonic and larval lethality, although many animals still arrested with molting defects, suggesting that the timing or level of expression was not appropriate. Expression of lpr-3 cDNA in body muscle, under control of the unc-54 promoter, did not rescue lpr-3(cs144) excretory duct defects or lethality (Figure 6A). A grl-2pro::ssSfGFP::LPR-3 transgene revealed that LPR-3 could travel a few cell diameters from its site of synthesis, including from sensory glia (Figure 6, B and C) or the excretory duct and pore (Figure 6, C and D) into the nearby epidermal cuticle. We infer that LPR-3 is secreted apically and then can diffuse a limited distance before being captured by unknown partners in the matrix.
Figure 6.
LPR-3 functions cell nonautonomously but locally. (A) Tissue-specific rescue data. Transgenic and nontransgenic siblings were scored in parallel. Arrested L1 animals were examined by DIC for excretory edema. grl-2pro::LPR-3 (pRFR53) and dpy-7pro::LPR-3 (pRFR59) efficiently rescued lpr-3 excretory defects and partially rescued molting defects so that some mutants could survive until late larval stages or adulthood. unc-54pro::LPR-3 (pCS23) did not rescue either defect. (B–D) grl-2pro::ssSfGFP::LPR-3 (pRFR58) expression in the embryo (B) and L4 larvae (C and D). B' and C' show merged DODT and confocal images. The duct is marked by lin-48pro::mRFP (red) in (D’). grl-2pro drives expression in the duct, pore, and amphid glia. Fusion protein also accumulated in epidermal cuticle regions (lines) adjacent to the glial and excretory pore openings. Bar, 5 μm.
Figure 7.
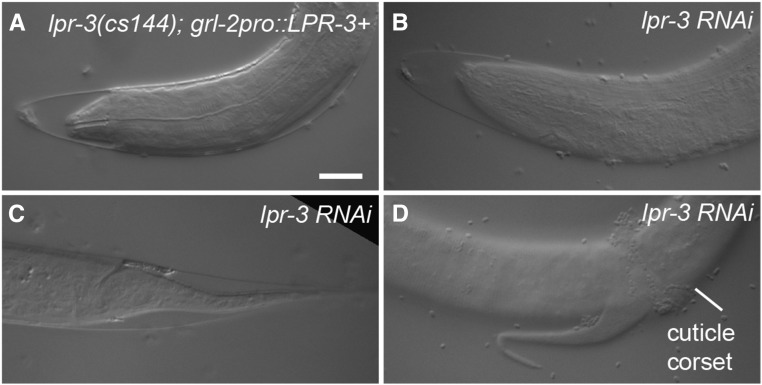
lpr-3 is required for molting. (A–D) Molting defects in lpr-3(cs144); grl-2pro::LPR-3 (A) and lpr-3 RNAi (B–D) animals at the L1–L2 molt. Cuticle partly detached from the nose (A and B) and tail (C and D), but remained attached in the midbody (D). Bar, 5 μm.
LPR-3 is required for molting
The cuticle must be shed and resynthesized at each larval molt (Singh and Sulston 1978; Frand et al. 2005). As mentioned above, lpr-3 mutants rescued for embryonic and excretory defects were molting-defective (Figure 6A and Figure 7A). Similar molting defects were observed in lpr-3(−) mutants incompletely rescued by our lpr-3pro::ssSfGFP::LPR-3 transgene (Figure 4I), or in animals depleted for lpr-3 using RNAi (Figure 7, B–D). In most cases, larvae initiated apolysis (separation of the old and new cuticles) at the nose and tail (Figure 7, A–C), but were unable to fully escape from the old cuticle, and often exhibited a constricted “corset” of incompletely shed cuticle around the midbody (Figure 4G and Figure 7D). In contrast to lpr-3, lpr-1 is not required for molting, since the ∼10% of lpr-1(−) mutants that lack excretory defects develop normally to adulthood (Pu et al. 2017). Similarly, when rescued for excretory defects by duct/pore-specific lin-48- or grl-2-promoter transgenes, mutants for let-653, let-4, or egg-6 all successfully completed the molts and reached adulthood (Gill et al. 2016 and J. D. C. and M. V. S., unpublished data). Thus, among this set of genes, lpr-3 is unique in its molting requirements.
LPR-1 also influences cuticle structure
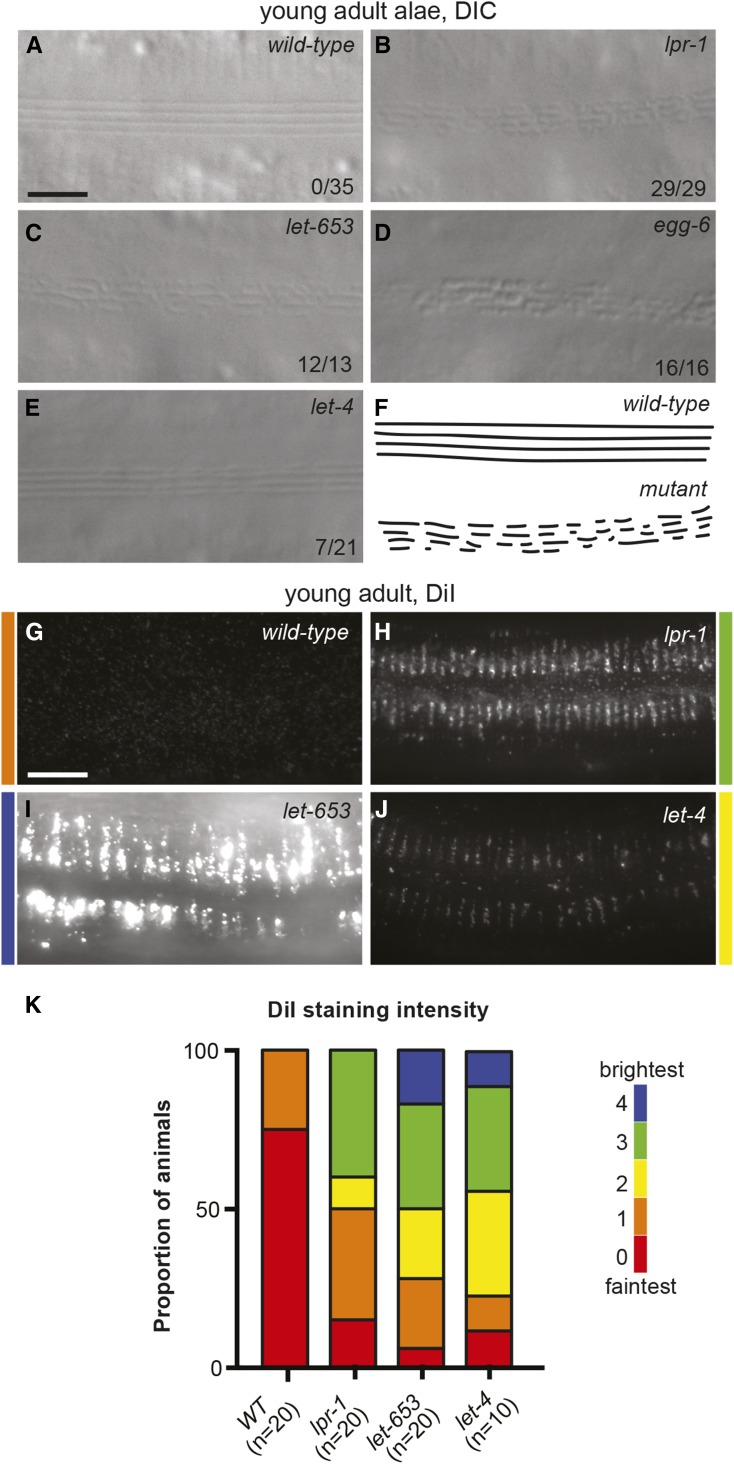
The above observations with lpr-3 led us to explore further roles for lpr-1 in cuticle organization. The cuticle normally serves as an efficient barrier to Hoechst dye penetration (Chisholm and Xu 2012). This barrier function appeared normal in lpr-1(−) mutants (Figure 1I). The lateral cuticle of adult C. elegans contains ridge-like structures termed alae (Figure 8A) (Page and Johnstone 2007). lpr-1(−) mutants all had fragmented adult alae (Figure 8B) similar to those previously described for a variety of secretory pathway mutants (Hyenne et al. 2015) and some mutant collagens (McMahon et al. 2003). Excretory-rescued mutants for let-653, let-4, or egg-6 all showed similar alae phenotypes (Figure 8, C–E). The adult cuticle also has a surface layer of exposed lipids that stain with the lipophilic dye DiI (Schultz and Gumienny 2012) (Figure 8, G–K). lpr-1(−) mutants had dramatically increased levels of DiI staining (Figure 8, H and K), as did excretory-rescued mutants for let-653 and let-4 (Figure 8, I–K), suggesting that either lipid levels are increased or lipids are more exposed in these mutants. We were unable to examine lpr-3 mutant adults due to the molting defect. Since none of the glyocalyx proteins that affected adult alae or DiI staining are present in the adult cuticle, this result indicates that the precuticular glycocalyx is important for building and shaping the adult cuticle. The various cuticle defects in lpr-1 mutants, along with other similarities to glycocalyx mutants, indicate that lpr-1 influences glycocalyx structure and/or function.
Figure 8.
lpr-1 mutants also have cuticle defects. (A–E) DIC images of lateral alae at the L4-adult molt in (A) wild-type, (B) lpr-1(cs207), (C) let-653(cs178); Ex(lin-48pro::LET-653), (D) egg-6(ok1506); Ex(grl-2pro::EGG-6), and (E) let-4(mn105); Ex(grl-2pro::LET-4). The fractions of animals with fragmented alae are listed. The phenotype is incompletely penetrant and variably expressive in the let-4 mutants. Bar, 5 μm. (F) Cartoon interpretation. (G–J) Epifluorescence images of DiI staining in young adults of the indicated genotypes, as above. Colors indicate fluorescence intensity. Bar, 10 μm. (K) Quantification of DiI staining intensity by a researcher blinded to genotypes. Micrographs were scored on a scale of 0–4, from faintest to brightest. Note that all wild-type animals do have some DiI staining, but it was usually not visible with the imaging conditions used here (see Materials and Methods).
Discussion
A lipid and glycoprotein-rich aECM lines and protects the narrowest tubes in our bodies. For example, lung surfactant helps narrow airways remain open (Lopez-Rodriguez and Perez-Gil 2014), and the endothelial glycocalyx protects capillaries in the vascular system (Tarbell and Cancel 2016). Despite the importance of aECM, we have only a rudimentary understanding of its multi-layered organization, how it is assembled and maintained, and how appropriate lipid content is ensured. This study shows that multiple members of the lipocalin family of lipid transport proteins are critical for proper aECM function and organization in C. elegans.
Lipids and lipocalins in the aECM
The C. elegans precuticular glycocalyx has both morphological and molecular similarities to the vascular glycocalyx and other aECMs of mammals. All are multi-layered structures, with inner layers more firmly adherent to the apical plasma membrane, and outer surface layers more loosely or transiently associated (Reitsma et al. 2007; Green-Church et al. 2011; Johansson et al. 2013). Like the C. elegans glycocalyx, most aECMs contain glycoproteins of the eLRRon, ZP, and mucin families, for example, the vascular glycocalyx contains the eLRRons lumican (Friden et al. 2011) and GP1b-GPIX-GPV (Li and Emsley 2013), the ZP proteins betaglycan (Bilandzic and Stenvers 2012) and endoglin (ten Dijke et al. 2008), and the sialomucins CD34 and podocalyxin (Nielsen and McNagny 2008; Strilic et al. 2009). Most aECMs also contain a variety of lipids, often thought to be concentrated in a surface layer between the matrix and the exposed environment (Page and Johnstone 2007; Green-Church et al. 2011; Lopez-Rodriguez and Perez-Gil 2014). Analyses of the C. elegans cuticle surface layer identified the presence of both polar and nonpolar lipids, various complex glycolipids, and free fatty acids (Blaxter 1993); similar lipids are presumed to be present in the early glycocalyx and may comprise the discrete border, visualized by electron microscopy, that separates the membrane-anchored glycocalyx layers from the loose luminal layer(s) (Gill et al. 2016) (Figure 9A).
Figure 9.
Model of lipocalins in the aECM. (A) Cartoon models of C. elegans aECM layers at different stages. LPR-1 does not accumulate in any specific layer, but is seen within cells (yellow) and can pass into the lumen or external environment (blue), where it quickly dissipates unless the compartment is closed. (i) Embryonic glycocalyx. LPR-3 and LET-653 are present in adjacent layers (green) of a membrane-anchored glycocalyx that precedes cuticle secretion. LET-653 also binds to a loose matrix compartment (gray) (Gill et al. 2016). A lipid-rich surface layer (red) may separate the membrane-anchored and loose matrix compartments (Gill et al. 2016). LPR-1, LPR-3, and LET-653 all accumulate between the embryo and the eggshell (Gill et al. 2016; Pu et al. 2017). (ii) Larval cuticle. LET-653 is cleared as the cuticle (black) is secreted, but LPR-3 remains. LPR-3 may be on either or both sides of the cuticle, depending on stage, but is drawn above here based on data from Figure 4H and the assumption that new layers are secreted below preceding layers. (iii) Premolt. (iv) Late molt. In preparation for molting, a new glyocalyx (iii) and then a new cuticle (iv) are synthesized prior to shedding of the old cuticle. The old glycocalyx may be destroyed during the molt, since LPR-3 remains associated with the new cuticle, but not with the older or shed cuticle. Membrane-unattached LET-653 and LPR-3 fill the space between the old and new cuticles during the molt. (v) Adult cuticle. LPR-1, LPR-3, and other glycocalyx components disappear in the adult. The lipid-rich surface layer remains and can be stained by DiI. (B) The molt cycle. A glycocalyx precedes each new round of cuticle synthesis and serves as template for later cuticle synthesis. LPR-1 and LPR-3 are required for proper glycocalyx synthesis and therefore for proper cuticle structure; LPR-3 is also required for old cuticle shedding. (C) Confocal images of the epidermal surface in LPR-1::SfGFP (pEP96) and ssSfGFP::LET-653 (pHG9) animals at the mid-L4 stage. Only faint LPR-1-positive intracellular puncta were observed, while LET-653 accumulated particularly in the seam region, near the future location of cuticle alae. No signal was visible for LPR-1, LPR-3, or LET-653 in 24-hr adults (n = 10 or more).
Lipocalins are often found complexed with lipids in luminal or aECM compartments; for example, circulating blood contains at least five different lipocalins: ApoD, ApoM, orosomucoid (also known as α-1-acid glycoprotein), α-1-microglobulin, and retinol binding protein (Christoffersen et al. 2011; Luo et al. 2015; di Masi et al. 2016). However, it is not clear if any of these lipocalins localize within the vascular glycocalyx or if and how they affect its structure. We showed here that two related C. elegans lipocalins have very different localization properties, but that both are important for aECM structure. LPR-1 can travel between tissues and accumulates to detectable levels only in closed extracellular spaces, while it quickly dissipates from exposed extracellular regions (Stone et al. 2009; Pu et al. 2017); thus LPR-1 appears freely mobile in the extracellular environment (Figure 9A). On the other hand, LPR-3 acts more locally and is embedded in a central layer of the membrane-anchored glycocalyx (Figure 9A). Absence of LPR-1 or LPR-3 results in loss of narrow tube patency and discrete cuticle defects. lpr-1 mutants cannot maintain a continuous excretory duct apical domain or properly shape cuticle ridges (alae), and their adult cuticle has an abnormal lipid-rich surface layer. lpr-3 mutants cannot maintain a passable excretory duct lumen, properly degrade the eggshell, or shed old cuticle during molting, and their L1 cuticle lacks barrier function. Based on these phenotypes, we infer that both LPR-1 and LPR-3 are required to build a properly organized glycocalyx, which then serves as a template for a properly organized cuticle (Figure 9B). LPR-3 is additionally needed for aECM clearance and remodeling during hatching and molting (Figure 9B).
Potential roles of lipocalins in the aECM
The literature suggests a variety of mechanisms through which lipocalins might act to affect aECM structure. Lipocalins are known lipid transport proteins (Flower 1996), and therefore might transport specific aECM structural lipids and help to maintain their proper levels. Bacterial lipocalins are found only in species with an outer membrane, so lipocalins have been hypothesized to transport lipids across the periplasmic space to affect its lipid content (Bishop 2000). Two proposed functions of tear lipocalin are to deliver or remove lipids from different tear film layers to maintain its structure and prevent damage to the eye surface (Gouveia and Tiffany 2005; Dartt 2011; Yeh et al. 2013); however, those models have proven difficult to test because the identity of relevant lipids remains highly debated, and the tear film matrix is difficult to visualize (Green-Church et al. 2011; Butovich 2013). The C. elegans glycocalyx should be a useful system for further testing such models. Since LPR-1 can function tissue-nonautonomously, it is a good candidate for transporting lipids between tissues and delivering them to the excretory duct lumen. The more specific localization pattern of LPR-3 makes it a good candidate for delivering lipids to, or sequestering lipids within, a specific aECM layer.
Another potential function of lipocalins is to regulate matrix metalloproteinases or other matrix components through protein–protein interactions. The mammalian lipocalin NGAL (neutrophil gelatinase-associated lipocalin, also known as Lcn2 or 24p3) was originally discovered in a complex with the matrix metalloproteinase MMP9, and was suggested to protect MMP9 from degradation, thereby promoting its function (Yan et al. 2001; Leng et al. 2009). On the other hand, sphingosine-1-phosphate (S1P), a signaling lipid carried by the lipocalin ApoM (Christoffersen et al. 2011), antagonizes MMP9 function (Zeng et al. 2014). MMP9 and related matrix metalloproteinases have been implicated in glycocalyx proteoglycan shedding (Zeng et al. 2014); however, most of these studies have been carried out in cell culture, and it is still not clear if or how lipocalins and MMPs affect glycocalyx structure in vivo. C. elegans LPR-3 is a good candidate for regulating metalloproteinase activity, since lpr-3 mutants share defects in hatching and molting with a variety of known metalloproteinase mutants (Hishida et al. 1996; Davis et al. 2004; Suzuki et al. 2004). Future studies should address whether LPR-3 is required for proper localization or activity of such metalloproteinases, or of other matrix glycoproteins important for molting (Frand et al. 2005).
A third potential function of lipocalins is to regulate signaling. ApoM transports the signaling lipid S1P in the bloodstream as part of the high-density lipoprotein complex (Christoffersen et al. 2011), and signaling of S1P through G-protein coupled receptors protects vessel integrity (Camerer et al. 2009; Christensen et al. 2016). Retinol binding protein (RBP) transports retinol to promote signaling through both the retinoic acid receptor and the RBP receptor STRA6 (Kawaguchi et al. 2007; Berry and Noy 2012), and the Drosophila lipocalin Swim facilitates long-range Wnt signaling (Mulligan et al. 2012). Lipocalins could also affect signaling indirectly, by affecting matrix structure, which then affects binding of ligands to their receptors. We showed previously that lpr-1 mutant excretory defects can be suppressed by increased signaling through the epidermal growth factor (EGF)–Ras–extracellular signal regulated kinase pathway; however, our genetic analyses suggested that LPR-1 and EGF act in parallel, and that LPR-1 protects duct lumen integrity through an EGF-independent mechanism (Pu et al. 2017). The possibility that LPR-1 and/or LPR-3 regulate some other signaling pathway cannot be ruled out, but the structural aECM defects described here lead us to favor models in which lipocalins exert more direct effects on aECM lipid, metalloproteinase, and/or glycoprotein content. The C. elegans glycocalyx provides a powerful system, amenable to both genetic analysis and live imaging, for further investigating how lipocalins and lipids affect aECM structure.
Supplementary Material
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.117.300207/-/DC1.
Acknowledgments
We thank Jean Parry, Hasreet Gill, Kevin Bickard, and Claudia Lanauze for participation in the genetic screen that identified lpr-3, and Pu Pu for generating localization and Hoechst staining data for lpr-1. We also thank Pu Pu and Fabien Soulavie for helpful discussions throughout the course of this work. Some strains were obtained from the Caenorhabditis Genetics Center (CGC), which is funded by National Institutes of Health (NIH) Office of Research Infrastructure Programs (P40 OD010440). This work was supported by National Science Foundation grant #1257879 and American Heart Association grant #0755524U to M.V.S. NIH training grant T32GM008216 provided support to J.D.C.
Footnotes
Communicating editor: D. Greenstein
Literature Cited
- Allen M. A., Hillier L. W., Waterston R. H., Blumenthal T., 2011. A global analysis of C. elegans trans-splicing. Genome Res. 21: 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arribere J. A., Bell R. T., Fu B. X., Artiles K. L., Hartman P. S., et al. , 2014. Efficient marker-free recovery of custom genetic modifications with CRISPR/Cas9 in Caenorhabditis elegans. Genetics 198: 837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barasch J., Hollmen M., Deng R., Hod E. A., Rupert P. B., et al. , 2016. Disposal of iron by a mutant form of lipocalin 2. Nat. Commun. 7: 12973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry D. C., Noy N., 2012. Signaling by vitamin A and retinol-binding protein in regulation of insulin responses and lipid homeostasis. Biochim. Biophys. Acta 1821: 168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilandzic M., Stenvers K. L., 2012. Betaglycan: a multifunctional accessory. Mol. Cell. Endocrinol. 359: 13–22. [DOI] [PubMed] [Google Scholar]
- Bishop R. E., 2000. The bacterial lipocalins. Biochim. Biophys. Acta 1482: 73–83. [DOI] [PubMed] [Google Scholar]
- Blaxter M. L., 1993. Cuticle surface proteins of wild type and mutant Caenorhabditis elegans. J. Biol. Chem. 268: 6600–6609. [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich I. A., 2013. Tear film lipids. Exp. Eye Res. 117: 4–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerer E., Regard J. B., Cornelissen I., Srinivasan Y., Duong D. N., et al. , 2009. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J. Clin. Invest. 119: 1871–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm A. D., Xu S., 2012. The Caenorhabditis elegans epidermis as a model skin. II: differentiation and physiological roles. Wiley Interdiscip. Rev. Dev. Biol. 1: 879–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen P. M., Liu C. H., Swendeman S. L., Obinata H., Qvortrup K., et al. , 2016. Impaired endothelial barrier function in apolipoprotein M-deficient mice is dependent on sphingosine-1-phosphate receptor 1. FASEB J. 30: 2351–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffersen C., Obinata H., Kumaraswamy S. B., Galvani S., Ahnstrom J., et al. , 2011. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc. Natl. Acad. Sci. USA 108: 9613–9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M., Draper B. W., Priess J. R., 1997. The role of actin filaments in patterning the Caenorhabditis elegans cuticle. Dev. Biol. 184: 373–384. [DOI] [PubMed] [Google Scholar]
- Cruz D. N., Gaiao S., Maisel A., Ronco C., Devarajan P., 2012. Neutrophil gelatinase-associated lipocalin as a biomarker of cardiovascular disease: a systematic review. Clin. Chem. Lab. Med. 50: 1533–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dane M. J., Khairoun M., Lee D. H., van den Berg B. M., Eskens B. J., et al. , 2014. Association of kidney function with changes in the endothelial surface layer. Clin. J. Am. Soc. Nephrol. 9: 698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartt D. A., 2011. Tear lipocalin: structure and function. Ocul. Surf. 9: 126–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. W., Birnie A. J., Chan A. C., Page A. P., Jorgensen E. M., 2004. A conserved metalloprotease mediates ecdysis in Caenorhabditis elegans. Development 131: 6001–6008. [DOI] [PubMed] [Google Scholar]
- Decramer M., Janssens W., Miravitlles M., 2012. Chronic obstructive pulmonary disease. Lancet 379: 1341–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D. J., Ward J. D., Reiner D. J., Goldstein B., 2013. Engineering the Caenorhabditis elegans genome using Cas9-triggered homologous recombination. Nat. Methods 10: 1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierker T., Shao C., Haitina T., Zaia J., Hinas A., et al. , 2016. Nematodes join the family of chondroitin sulfate-synthesizing organisms: identification of an active chondroitin sulfotransferase in Caenorhabditis elegans. Sci. Rep. 6: 34662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Masi A., Trezza V., Leboffe L., Ascenzi P., 2016. Human plasma lipocalins and serum albumin: plasma alternative carriers? J. Control. Release 228: 191–205. [DOI] [PubMed] [Google Scholar]
- Dong B., Hannezo E., Hayashi S., 2014. Balance between apical membrane growth and luminal matrix resistance determines epithelial tubule shape. Cell Rep. 7: 941–950. [DOI] [PubMed] [Google Scholar]
- Feingold K. R., 2007. Thematic review series: skin lipids. The role of epidermal lipids in cutaneous permeability barrier homeostasis. J. Lipid Res. 48: 2531–2546. [DOI] [PubMed] [Google Scholar]
- Fernandez V., Guzman-Delgado P., Graca J., Santos S., Gil L., 2016. Cuticle structure in relation to chemical composition: re-assessing the prevailing model. Front. Plant Sci. 7: 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K., Kettunen J., Wurtz P., Haller T., Havulinna A. S., et al. , 2014. Biomarker profiling by nuclear magnetic resonance spectroscopy for the prediction of all-cause mortality: an observational study of 17,345 persons. PLoS Med. 11: e1001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flo T. H., Smith K. D., Sato S., Rodriguez D. J., Holmes M. A., et al. , 2004. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432: 917–921. [DOI] [PubMed] [Google Scholar]
- Flower D. R., 1996. The lipocalin protein family: structure and function. Biochem. J. 318: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frand A. R., Russel S., Ruvkun G., 2005. Functional genomic analysis of C. elegans molting. PLoS Biol. 3: e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friden V., Oveland E., Tenstad O., Ebefors K., Nystrom J., et al. , 2011. The glomerular endothelial cell coat is essential for glomerular filtration. Kidney Int. 79: 1322–1330. [DOI] [PubMed] [Google Scholar]
- Gill H. K., Cohen J. D., Ayala-Figueroa J., Forman-Rubinsky R., Poggioli C., et al. , 2016. Integrity of narrow epithelial tubes in the C. elegans excretory system requires a transient luminal matrix. PLoS Genet. 12: e1006205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia S. M., Tiffany J. M., 2005. Human tear viscosity: an interactive role for proteins and lipids. Biochim. Biophys. Acta 1753: 155–163. [DOI] [PubMed] [Google Scholar]
- Green-Church K. B., Butovich I., Willcox M., Borchman D., Paulsen F., et al. , 2011. The international workshop on meibomian gland dysfunction: report of the subcommittee on tear film lipids and lipid-protein interactions in health and disease. Invest. Ophthalmol. Vis. Sci. 52: 1979–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase-Fielitz A., Haase M., Devarajan P., 2014. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury: a critical evaluation of current status. Ann. Clin. Biochem. 51: 335–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog B. H., Fu J., Xia L., 2014. Mucin-type O-glycosylation is critical for vascular integrity. Glycobiology 24: 1237–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hishida R., Ishihara T., Kondo K., Katsura I., 1996. hch-1, a gene required for normal hatching and normal migration of a neuroblast in C. elegans, encodes a protein related to TOLLOID and BMP-1. EMBO J. 15: 4111–4122. [PMC free article] [PubMed] [Google Scholar]
- Hwang H. Y., Olson S. K., Esko J. D., Horvitz H. R., 2003. Caenorhabditis elegans early embryogenesis and vulval morphogenesis require chondroitin biosynthesis. Nature 423: 439–443. [DOI] [PubMed] [Google Scholar]
- Hyenne V., Apaydin A., Rodriguez D., Spiegelhalter C., Hoff-Yoessle S., et al. , 2015. RAL-1 controls multivesicular body biogenesis and exosome secretion. J. Cell Biol. 211: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumikawa T., Dejima K., Watamoto Y., Nomura K. H., Kanaki N., et al. , 2016. Chondroitin 4-O-sulfotransferase is indispensable for sulfation of chondroitin and plays an important role in maintaining normal life span and oxidative stress responses in nematodes. J. Biol. Chem. 291: 23294–23304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazwinska A., Ribeiro C., Affolter M., 2003. Epithelial tube morphogenesis during Drosophila tracheal development requires piopio, a luminal ZP protein. Nat. Cell Biol. 5: 895–901. [DOI] [PubMed] [Google Scholar]
- Johansson M. E., Sjovall H., Hansson G. C., 2013. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 10: 352–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi R., Yu J., Honda J., Hu J., Whitelegge J., et al. , 2007. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science 315: 820–825. [DOI] [PubMed] [Google Scholar]
- Kelley M., Yochem J., Krieg M., Calixto A., Heiman M. G., et al. , 2015. FBN-1, a fibrillin-related protein, is required for resistance of the epidermis to mechanical deformation during C. elegans embryogenesis. Elife 4: e06565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppen M., Simske J. S., Sims P. A., Firestein B. L., Hall D. H., et al. , 2001. Cooperative regulation of AJM-1 controls junctional integrity in Caenorhabditis elegans epithelia. Nat. Cell Biol. 3: 983–991. [DOI] [PubMed] [Google Scholar]
- Leng X., Ding T., Lin H., Wang Y., Hu L., et al. , 2009. Inhibition of lipocalin 2 impairs breast tumorigenesis and metastasis. Cancer Res. 69: 8579–8584. [DOI] [PubMed] [Google Scholar]
- Li R., Emsley J., 2013. The organizing principle of the platelet glycoprotein Ib-IX-V complex. J. Thromb. Haemost. 11: 605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rodriguez E., Perez-Gil J., 2014. Structure-function relationships in pulmonary surfactant membranes: from biophysics to therapy. Biochim. Biophys. Acta 1838: 1568–1585. [DOI] [PubMed] [Google Scholar]
- Luo Z., Lei H., Sun Y., Liu X., Su D. F., 2015. Orosomucoid, an acute response protein with multiple modulating activities. J. Physiol. Biochem. 71: 329–340. [DOI] [PubMed] [Google Scholar]
- Luschnig S., Uv A., 2014. Luminal matrices: an inside view on organ morphogenesis. Exp. Cell Res. 321: 64–70. [DOI] [PubMed] [Google Scholar]
- Mancuso V. P., Parry J. M., Storer L., Poggioli C., Nguyen K. C., et al. , 2012. Extracellular leucine-rich repeat proteins are required to organize the apical extracellular matrix and maintain epithelial junction integrity in C. elegans. Development 139: 979–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon L., Muriel J. M., Roberts B., Quinn M., Johnstone I. L., 2003. Two sets of interacting collagens form functionally distinct substructures within a Caenorhabditis elegans extracellular matrix. Mol. Biol. Cell 14: 1366–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minevich G., Park D. S., Blankenberg D., Poole R. J., Hobert O., 2012. CloudMap: a cloud-based pipeline for analysis of mutant genome sequences. Genetics 192: 1249–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra J., Dent C., Tarabishi R., Mitsnefes M. M., Ma Q., et al. , 2005. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365: 1231–1238. [DOI] [PubMed] [Google Scholar]
- Moerman D. G., Barstead R. J., 2008. Towards a mutation in every gene in Caenorhabditis elegans. Brief. Funct. Genomics Proteomics 7: 195–204. [DOI] [PubMed] [Google Scholar]
- Moussian B., 2010. Recent advances in understanding mechanisms of insect cuticle differentiation. Insect Biochem. Mol. Biol. 40: 363–375. [DOI] [PubMed] [Google Scholar]
- Mulligan K. A., Fuerer C., Ching W., Fish M., Willert K., et al. , 2012. Secreted wingless-interacting molecule (swim) promotes long-range signaling by maintaining wingless solubility. Proc. Natl. Acad. Sci. USA 109: 370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson F. K., Albert P. S., Riddle D. L., 1983. Fine structure of the C. elegans secretory-excretory system. J. Ultrastruct. Res. 82: 156–171. [DOI] [PubMed] [Google Scholar]
- Nielsen J. S., McNagny K. M., 2008. Novel functions of the CD34 family. J. Cell Sci. 121: 3683–3692. [DOI] [PubMed] [Google Scholar]
- Nieuwdorp M., van Haeften T. W., Gouverneur M. C., Mooij H. L., van Lieshout M. H., et al. , 2006. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes 55: 480–486. [DOI] [PubMed] [Google Scholar]
- Page A. P., Johnstone I. L., 2007. The cuticle (March 19, 2007). WormBook, ed. The C. elegans Research Community Wormbook, doi/10.1895/wormbook.1.138.1, http://www.wormbook.org. [Google Scholar]
- Priess J. R., Hirsh D. I., 1986. Caenorhabditis elegans morphogenesis: the role of the cytoskeleton in elongation of the embryo. Dev. Biol. 117: 156–173. [DOI] [PubMed] [Google Scholar]
- Pu P., Stone C. E., Burdick J. T., Murray J. I., Sundaram M. V., 2017. The lipocalin LPR-1 cooperates with LIN-3/EGF signaling to maintain narrow tube integrity in Caenorhabditis elegans. Genetics 205: 1247–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadro L., Hamberger L., Gottesman M. E., Wang F., Colantuoni V., et al. , 2005. Pathways of vitamin A delivery to the embryo: insights from a new tunable model of embryonic vitamin A deficiency. Endocrinology 146: 4479–4490. [DOI] [PubMed] [Google Scholar]
- Reitsma S., Slaaf D. W., Vink H., van Zandvoort M. A., oude Egbrink M. G., 2007. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 454: 345–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. C., Voynow J. A., 2006. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol. Rev. 86: 245–278. [DOI] [PubMed] [Google Scholar]
- Rost B., Yachdav G., Liu J., 2004. The PredictProtein server. Nucleic Acids Res. 32: W321–W326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon A. H., Satchell S. C., 2012. Endothelial glycocalyx dysfunction in disease: albuminuria and increased microvascular permeability. J. Pathol. 226: 562–574. [DOI] [PubMed] [Google Scholar]
- Salmon A. H., Ferguson J. K., Burford J. L., Gevorgyan H., Nakano D., et al. , 2012. Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. J. Am. Soc. Nephrol. 23: 1339–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapio M. R., Hilliard M. A., Cermola M., Favre R., Bazzicalupo P., 2005. The zona pellucida domain containing proteins, CUT-1, CUT-3 and CUT-5, play essential roles in the development of the larval alae in Caenorhabditis elegans. Dev. Biol. 282: 231–245. [DOI] [PubMed] [Google Scholar]
- Schultz R. D., Gumienny T. L., 2012. Visualization of Caenorhabditis elegans cuticular structures using the lipophilic vital dye DiI. J. Vis. Exp. 59: e3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneff S., Davidson R. M., Lauritzen A., Samsel A., Wainwright G., 2015. A novel hypothesis for atherosclerosis as a cholesterol sulfate deficiency syndrome. Theor. Biol. Med. Model. 12: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. N., Sulston J. E., 1978. Some observations on molting in C. elegans. Nematologica 24: 63–71. [Google Scholar]
- Stone C. E., Hall D. H., Sundaram M. V., 2009. Lipocalin signaling controls unicellular tube development in the Caenorhabditis elegans excretory system. Dev. Biol. 329: 201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strilic B., Kucera T., Eglinger J., Hughes M. R., McNagny K. M., et al. , 2009. The molecular basis of vascular lumen formation in the developing mouse aorta. Dev. Cell 17: 505–515. [DOI] [PubMed] [Google Scholar]
- Sundaram M. V., Buechner M., 2016. The Caenorhabditis elegans excretory system: a model for tubulogenesis, cell fate specification and plasticity. Genetics 203: 35–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Sagoh N., Iwasaki H., Inoue H., Takahashi K., 2004. Metalloproteases with EGF, CUB, and thrombospondin-1 domains function in molting of Caenorhabditis elegans. Biol. Chem. 385: 565–568. [DOI] [PubMed] [Google Scholar]
- Tarbell J. M., Cancel L. M., 2016. The glycocalyx and its significance in human medicine. J. Intern. Med. 280: 97–113. [DOI] [PubMed] [Google Scholar]
- ten Dijke P., Goumans M. J., Pardali E., 2008. Endoglin in angiogenesis and vascular diseases. Angiogenesis 11: 79–89. [DOI] [PubMed] [Google Scholar]
- Vuong-Brender T. T. K., Suman S. K., Labouesse M., 2017. The apical ECM preserves embryonic integrity and distributes mechanical stress during morphogenesis. Development DOI: 10.1242/dev.150383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H., Takeo T., Tojo H., Sakoh K., Berger T., et al. , 2014. Lipocalin 2 binds to membrane phosphatidylethanolamine to induce lipid raft movement in a PKA-dependent manner and modulates sperm maturation. Development 141: 2157–2164. [DOI] [PubMed] [Google Scholar]
- Yan L., Borregaard N., Kjeldsen L., Moses M. A., 2001. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J. Biol. Chem. 276: 37258–37265. [DOI] [PubMed] [Google Scholar]
- Yeh P. T., Casey R., Glasgow B. J., 2013. A novel fluorescent lipid probe for dry eye: retrieval by tear lipocalin in humans. Invest. Ophthalmol. Vis. Sci. 54: 1398–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y., Adamson R. H., Curry F. R., Tarbell J. M., 2014. Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding. Am. J. Physiol. Heart Circ. Physiol. 306: H363–H372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Strains and plasmids are available upon request. Table S1 contains complete genotypes of all strains. Table S2 contains a list of all plasmids and transgenes.