Summary
Foxp3+ CD4+ regulatory T (Treg) cells are a subset of immune cells that function to regulate tissue inflammation. Skin is one of the largest organs and is home to a large proportion of the body's Treg cells. However, relative to other tissues (such as the spleen and gastrointestinal tract) the function of Treg cells in skin is less well defined. Here, we review our understanding of how Treg cells migrate to skin and the cellular and molecular pathways required for their maintenance in this tissue. In addition, we outline what is known about the specialized functions of Treg cells in skin. Namely, the orchestration of stem cell‐mediated hair follicle regeneration, augmentation of wound healing, and promoting adaptive immune tolerance to skin commensal microbes. A comprehensive understanding of the biology of skin Treg cells may lead to novel therapeutic approaches that preferentially target these cells to treat cutaneous autoimmunity, skin cancers and disorders of skin regeneration.
Keywords: autoimmunity, regulatory T cells, skin
Introduction
As our major barrier to the outside world, the skin performs an array of functions to protect and insulate us from our environment. As such, skin harbours a large community of commensal microbes and is routinely subjected to pathogen invasion. In addition, robust wound‐healing mechanisms have evolved to repair the high frequency of physical trauma inherent to skin, as well as damage associated with exposure to ultraviolet radiation. All of these processes interface with the immune system, and hence the skin is endowed with a complex immune cell repertoire that is unique to this tissue. In addition, there is a finely coordinated system of communication between epithelial cells and immune cells, to both maintain tissue homeostasis and restore normal function after insult. Hence, skin is a highly integrated and immunologically active organ that constantly strives to maintain a delicate balance between pro‐inflammatory and anti‐inflammatory immune responses, in an attempt to protect against invasion and mitigate tissue damage.
Self‐reactive lymphocytes can migrate to tissues and, under specific conditions, initiate autoimmune pathology. Regulatory T (Treg) cells represent a subset of CD4+ T cells that largely act to suppress pathogenic immune responses mediated by self‐reactive cells, thereby establishing and maintaining tissue immune homeostasis. In healthy individuals, the majority of Treg cells arise during thmyic T‐cell maturation upon high avidity recognition of self‐antigen (referred to as thymic Treg cells).1 Alternatively, Treg cells can be generated outside the thymus after naive CD4+ T cells encounter peripheral antigens in the correct immunological context (referred to as peripheral Treg cells).2 An imbalance between pathogenic effector T (Teff) cells and Treg cells results in chronic tissue inflammation and autoimmunity.
Treg cells were initially described based on constitutive expression of the high‐affinity interleukin‐2 (IL‐2) receptor α‐chain, CD25.3 Identification of forkhead box protein 3 (Foxp3) as the lineage defining transcription factor promoting Treg cell development and function, permitted a more comprehensive analysis of their phenotypic and functional diversity.4, 5 Similar to conventional Teff cells, Treg cells represent a heterogeneous population, with multiple subsets defined by their ontogeny, function and tissue residence (reviewed in detail in refs 6, 7, 8, 9). An emerging body of literature suggests that unique populations of Treg cells reside or infiltrate peripheral tissues where they mediate specific functions that depend entirely upon the tissue. In visceral adipose tissue, a population of Treg cells preferentially expresses the peroxisome proliferator‐activated receptor‐γ, which confers highly specialized functions, including expression of genes involved in glucose and lipid metabolism.10 In addition, Treg cells that infiltrate muscle and lung express the epidermal growth factor receptor ligand, amphiregulin, enabling them to directly facilitate tissue repair.11, 12 Although the molecular signature and function of Treg cells in many tissues is well documented, our understanding of the fundamental biology of these cells in skin and how they differ from Treg cells found in other organs is only beginning to be elucidated. Here, we review what is currently known about Treg cells that reside in murine and human skin, with the emphasis on mechanisms of tissue trafficking, in situ migration, maintenance, memory and control of cutaneous autoimmunity.
Treg cell trafficking to skin
The skin and gut are the two largest barrier tissues in mammals. Hence, it is important for these organs to mount strong immune responses in the face of constant threat from the outside environment. Accordingly, these tissues are highly susceptible to ‘collateral damage’ incited by recurrent and robust inflammatory reactions. It is interesting to speculate that the skin and gut house relatively large Treg cell populations in attempts to mitigate tissue damage incited by these responses. Of the CD4+ T cells that reside in the gastrointestinal tract of adult mice, approximately 10–20% are Treg cells.13 In the skin of adult mice, 20–60% of CD4+ T cells are Treg cells.14 In normal human adult skin, approximately 20% of tissue‐resident CD4+ T cells are Treg cells, compared with ~ 5% found in peripheral blood and 5–10% found in human adult colon.15 Interestingly, many of the Treg cells in the gastrointestinal tract are peripheral Treg cells induced by commensal microbes.16 However, the skin appears to be quite different. It has recently been shown that an abrupt wave of T‐cell receptor (TCR) αβ expressing T cells accumulate in murine skin from postnatal day 6 to day 13 of life. Of which, 80% are highly activated Treg cells.14 This marked accumulation during this defined window of postnatal development appeared specific to Treg cells, as other immune and T‐cell subsets were unchanged during this period. An influx of Treg cells was not observed in skin‐draining lymph nodes, nor in the intestinal lamina propria. Inhibition of lymphocyte migration during this window of time resulted in a preferential accumulation of Treg cells in the thymus. Interestingly, both hair follicle development and commensal microbes played a role in Treg cell accumulation in neonatal skin, both of which result in increased expression of the chemokine CCL20 from hair follicle epithelial cells.17 A subset of Treg cells in the neonatal thymus express high levels of CCR6 (the receptor for CCL20) and these Treg cells preferentially migrate to skin during this defined window of postnatal tissue development (Fig. 1a). Hence, it appears that, unlike the gut, Treg cell migration from the thymus is responsible for the initial seeding of this cell population in murine skin early in life. However, similar to the gut, commensal microbes play an active role in this process. Whether a ‘wave’ of Treg cell migration also happens in human skin during a defined developmental window remains to be elucidated. It is also relevant to note that Scurfy mice (which lack functional Treg cells) succumb to a fulminant systemic inflammatory response at a young age. These mice have pronounced skin inflammation and alopecia, perhaps reflecting the necessary and important role of neonatal Treg cells in suppressing skin inflammation early in life, as has been shown in other organs.18
Figure 1.
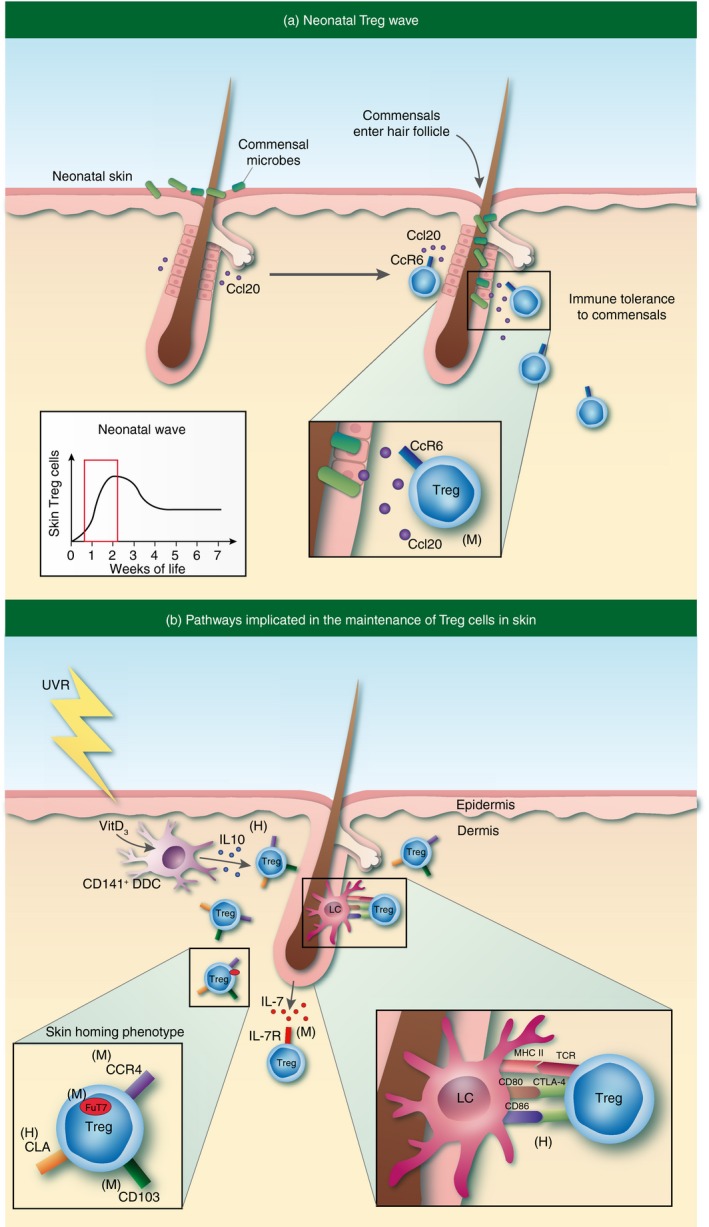
Skin regulatory T (Treg) cell trafficking and maintenance. (a) An abrupt wave of Treg cells accumulate in skin early in neonatal life. Commensal microbe elicitation of the chemokine CCL20 from hair follicle epithelial cells attracts CCR6‐expressing Treg cells to preferentially migrate to skin during this defined window of postnatal tissue development. (b) Treg cells in skin are maintained and/or induced by interactions with both epidermal Langerhans cells (LCs) and CD141+ dermal dendritic cells (DDCs). Skin‐homing Treg cells are defined by expression of CCR4, CD103, cutaneous lymphocyte antigen (CLA), and FuT7. ‘(M)’ or ‘(H)’ denotes pathways that have been identified in mouse or human, respectively.
Investigation of tissue homing receptor expression on CD4+ CD25hi Foxp3+ Treg cells in the peripheral circulation of healthy human beings revealed that the majority (between 68 and 90%) express a carbohydrate modification of P‐selectin glycoprotein ligand‐1 called cutaneous lymphocyte antigen (CLA)19 and between 62 and 84% express the skin‐homing receptor CCR6.20, 21 The functional competency of Treg cell homing receptor expression was demonstrated in a chemotactic response assay to the corresponding chemokines CCL17, CCL20 and CCL22. Similar to human peripheral blood, approximately 60% of Treg cells in murine spleen express P‐selectin glycoprotein ligand‐1,22 which was required for efficient infiltration of CD4+ T cells into inflamed skin.23 In contrast, < 10% of peripheral blood Treg cells were positive for the gut lamina propria homing receptor integrin α 4/β 7. Skin‐homing Treg cells in mice are defined by co‐expression of CCR4 and CD103.24 The ligands for CCR4 are CCL17 and CCL22, which are expressed by endothelial cells in dermal post‐capillary venules, and myeloid cells, respectively.25, 26, 27 The ligand for CD103 is E‐cadherin, which is expressed on epithelial keratinocytes in skin.28 In a study using congenically marked wild‐type and CCR4‐deficient mixed bone marrow chimeras, it was found that the absence of CCR4 expression on Treg cells conferred a competitive disadvantage on these cells to accumulate in skin and lung.24 In contrast, neither CCR6‐deficient nor CCR10‐deficient CD4+ T cells displayed any detectable defects in their ability to migrate to skin.29 These studies support a functional role for CCR4 expression on Treg cells for trafficking to skin. Taken together, several studies suggest that a large proportion of Treg cells in the systemic circulation of mice and humans have the propensity for trafficking to skin.
The carbohydrate determinants of E‐selectin and P‐selectin binding are generated by the enzyme α‐1,3‐fucosyltransferase VII (FuT7).30 FuT7‐deficient Treg cells in the mouse display no overt impairments in Treg cell differentiation or function, as determined by assessment of canonical marker expression and suppression of Teff cells in vitro.31 Interestingly, wild‐type and FuT7‐deficient Treg cells adoptively transferred into neonatal scurfy mice were equal in their ability to accumulate in lymphoid and most non‐lymphoid tissues, including the spleen, and the gastrointestinal tract.31 However, the accumulation of FuT7‐deficient Treg cells in skin was significantly reduced. Consistent with these findings, Treg cells are reduced in non‐inflamed skin of adult mice harbouring a germline mutation in FuT7, as determined by flow cytometric quantification of CD25hi CD4+ T cells. These results indicate that FuT7 expression is required for optimal Treg cell trafficking to skin in the absence of inflammation.
Treg cell localization and migration in skin
Treg cells in both murine and human skin appear to occupy a specialized anatomic niche. In non‐inflamed healthy human skin, CD4+ Foxp3+ Treg cells preferentially reside in close association with hair follicles, with very few cells detected in the interfollicular dermis and epidermis.17, 32, 33, 34 In agreement with these histological findings, flow cytometric quantification of human skin revealed that Treg cells are most abundant in regions with high hair follicle density (i.e. scalp and face).32 Intravital multiphoton imaging of healthy (non‐inflamed) mouse flank skin revealed that intradermal Treg cells are often located in clusters around hair follicles.34 In a separate study, dermal CD4+ T cells and CD11c+ myeloid cells were found in close association around murine hair follicles, which was in part dependent on the T‐cell‐derived chemokine CCL5.35 Taken together, these findings suggest that Treg cells preferentially localize to the hair follicle niche in healthy non‐inflamed skin.
Effector T‐cell‐mediated clearance of pathogens and subsequent resolution of inflammation require finely coordinated migratory behaviour at the inflamed tissue site. Although the molecular mechanisms employed by T cells to extravasate into tissues have been well documented, the pathways that mediate T‐cell trafficking within tissues are less well understood. Intravital monitoring of CD4+ T‐cell migration in inflamed mouse dermis showed that these cells preferentially migrate in directions parallel to local collagen fibres.36 Administration of a blocking antibody directed against integrin β 1, or its pairing subunit partner, integrin αv, blocked T‐cell interactions with the extracellular matrix and led to an arrest of dermal migratory activity. Blockade of other integrin β 1 pairing partners, α 1, α 2, α 4 or α 1 and α 2 in combination failed to impair T‐cell motility, suggesting that α v is uniquely required for CD4+ T‐cell mobility in inflamed dermis.36 This study did not discriminate between Treg and Teff cells in the total CD4+ pool. Hence, it will be important to determine if there is a differential requirement for integrin α v utilization between these different T‐cell lineages in skin during an inflammatory response. This is especially important because dermal Treg and Teff cells have been shown to differ in their interstitial migratory capacity in non‐inflamed mouse skin. In this setting, Treg cells are predominantly stationary, with a small proportion (< 10%) actively migrating or significantly changing cell shape.34 In contrast, Teff cells uniformly demonstrate higher levels of motility (as evidenced by mean displacement rate) relative to skin Treg cells. It is interesting to speculate that the dichotomy in Treg cell motility in non‐inflamed skin may represent distinct spatially organized Treg subsets with unique functions.
Tissue‐resident memory Treg cells
Over the past several years there has been a rapid growth in our understanding of tissue‐resident memory (Trm) cells, with many studies performed in skin (reviewed in ref. 37). These studies have identified the existence of a specialized adaptive immune cell that responds to antigen upon initial encounter and is maintained in tissues after antigen clearance, poised to respond with accelerated kinetics upon subsequent antigen exposure. Although this phenomenon has been well documented in CD8+ T cells and CD4+ Teff cells, memory within the Treg cell lineage is only beginning to be understood (reviewed in detail in ref 38). Several studies support the notion that tissue‐resident memory Treg cells exist and that they may play a major role in mitigating tissue damage upon repeated antigen exposure. To study memory Treg cell responses in skin, transgenic mice were generated in which a defined model antigen (ovalbumin; OVA) could be inducibly expressed in basal epithelial keratinocytes.39 OVA expression in this system largely mimicked the pattern of tissue‐restricted self‐antigen expression, in that it was constitutively expressed in the thymus but tightly regulated in skin through administration of doxycycline. Thymic expression of OVA led to the generation of a large proportion of antigen‐specific Treg cells that populated secondary lymphoid organs. However, these Treg cells were unable to prevent autoimmunity, as induction of OVA expression in skin resulted in pronounced inflammation. This was closely followed by the accumulation of highly activated OVA‐specific Treg cells in skin that resolved the disease, despite continued antigen expression. Interestingly, these Treg cells persisted in skin long after antigen was extinguished, and upon subsequent antigen expression, they were able to respond with accelerated kinetics resulting in an attenuation of skin inflammation. A similar phenomenon was observed in an experimental model of fetal tolerance.40 More recently, a subset of Treg cells induced by inflammation were retained in peripheral tissues that expressed unique gene expression profiles when compared with their counterparts in secondary lymphoid organs.41 Taken together, these studies and others suggest the existence of tissue‐resident Treg cells with ‘memory‐like’ properties. In attempts to determine if these findings translate to humans, Treg cells were extensively phenotyped in adult and fetal human skin. These studies revealed that almost all Treg cells in the skin of healthy adults express the RO isoform of CD45 (CD45RO), indicative of previous or current antigen exposure. In addition, they also expressed T‐cell memory associated markers, including CD27 and BCL‐2. ‘Memory’ Treg cells in skin had a lower proliferative index when compared with a similar population found in peripheral blood and had a reduced capacity to migrate out of skin in a humanized mouse skin transplant model.32 These results suggest that Treg cells in non‐inflamed human skin are a slow cycling tissue‐resident population. Interestingly, Treg cells in human fetal skin (identified as CD45+ CD3+ CD4+ Foxp3+) predominantly lacked CD45RO expression, indicating that most of these cells encounter antigen after or during birth.32 Taken together, an emerging body of literature suggests the existence of Treg Trm cells. How these cells differ from conventional Trm cells and how long they can persist in the absence of antigen in specific tissues remains to be determined.
Control of Treg cell maintenance in skin
Given their critical role in suppressing inflammation, defining the cellular and molecular pathways involved in activating and maintaining Treg cells in tissues is of fundamental importance. It is well established that a large proportion of Treg cells require IL‐2 for their development in the thymus and maintenance in secondary lymphoid organs.42 However, whether IL‐2 plays a central role in the maintenance of Treg cells (or specific Treg subsets) in peripheral tissues remains to be fully elucidated. The Treg cells present in spleen and lymph nodes express low levels of the IL‐7 receptor α chain (CD127),43 but expression of this cytokine receptor is increased on subsets of Treg cells found in peripheral tissues. Antigen‐specific Trm Treg cells in murine skin expressed higher levels of CD127 compared with naive Treg cells found in skin‐draining lymph nodes.33 Using a model of inducible self‐antigen expression, the functional role of the IL‐7 pathway in maintaining this Treg cell subset in skin was tested. Although IL‐2 was necessary for the accumulation of Treg cells in skin during antigen exposure, a subset of Trm Treg cells required IL‐7 for their maintenance in this tissue after cessation of antigen expression.33 The dependence on IL‐7 may represent a dynamic adaptation to survive in a tissue with relatively lower local concentrations of IL‐2 when compared with secondary lymphoid organs. Consistent with this notion, maintenance of both CD4+ and CD8+ tissue‐resident cells in mouse skin has been shown to be dependent on keratinocyte‐derived IL‐7.44 In addition, it has been shown that IL‐2 is necessary for Treg cell maintenance in murine secondary lymphoid organs, but not for subsets of Treg cells that reside in non‐lymphoid tissues such as the liver and intestine. Instead, the maintenance of these cells was dependent on continued signalling through the co‐stimulatory receptor inducible T‐cell co‐stimulator (ICOS).45 Whether a subset of Treg cells in human skin depend on IL‐7 for their maintenance and function remains to be determined. Most tissue‐resident Treg cells in healthy adult skin express low levels of CD127, equivalent to that observed on Treg cells in peripheral blood.32
In addition to growth and survival signals provided by specific cytokines, interactions with other skin‐resident cells have been implicated in Treg cell homeostasis and maintenance in this tissue. Fibroblasts are an abundant accessory cell type found in skin. These cells have been shown to elaborate T‐cell chemoattractant factors and are required for T‐cell emigration from skin explant cultures, as well as maintenance of skin homing marker expression.46 Their role in supporting human skin Treg cell survival was demonstrated using fibroblast monolayer‐skin Treg cell co‐cultures. Fibroblasts were able to preferentially induce proliferation of CFSE‐labelled purified CD25hi CD4+ T cells from skin in a contact‐dependent manner, suggesting that interactions between these cell types may play a role in skin Treg cell biology. Dendritic cells (DCs) resident to tissues have the capacity to readily present antigen to memory T cells in skin, a process that bypasses cellular recruitment from afferent lymphatics or peripheral circulation.47, 48 Langerhans cells (LCs) are an epidermal resident DC population in both mouse and human skin, and are believed to constitute the major first line of defence against pathogenic encounters. Seneschal et al. set out to explore the role of LCs in the function and homeostasis of human skin‐resident Treg cells.49 Relative to dermal DCs, LCs isolated directly from skin preferentially induced the proliferation of Treg cells in a cell‐contact‐dependent manner in vitro. This induction involved interactions with MHC class II and the co‐stimulatory molecules CD80 and CD86, as determined by antibody blockade in the co‐culture system.49 In situ, LCs were found in close association with proliferating Treg cells in the epidermis and follicular epithelium of human skin. It is postulated that the interaction between skin‐resident Treg cells and LCs plays a role in recall or memory responses and so may serve to mediate rapid attenuation of these robust immune reactions. Further evidence supporting a regulatory role for LCs comes from contact hypersensitivity studies in mice, where both constitutive and inducible ablation of LCs result in exaggerated contact hypersensitivity responses.50 Although the cytokine expression or generation of Treg cells in this model was unaltered, a significant expansion of hapten‐specific T cells was observed, that was associated with IL‐10 derived from LCs. Both LC‐derived IL‐10‐mediated suppression and full LC maturation required LC expression of MHC class II, suggesting that cognate CD4–MHC Class II interactions are necessary to inhibit antigen‐specific effector T‐cell responses.50 Overall, these studies ascribe a regulatory role for LCs in both mouse and human skin.
Distinct DC populations may also influence the migratory and retentive signals imparted to skin T cells through the conversion of vitamin metabolites present in tissues. In the gastrointestinal tract, CD103 expressing lamina‐propria‐resident DCs convert dietary vitamin A to retinoic acid, and in doing so, promote expression of the gut homing receptors integrin α 4/β 7 and CCR9.51 Given the direct exposure of skin to ultraviolet radiation, cutaneous DCs uniquely possess the capacity to convert the inactive form of sunlight‐derived vitamin D to its biologically active metabolite, 1,25(OH)2D3 (Fig. 1b). This reaction serves to induce the expression of the chemokine receptor CCR10 on responding T cells, while suppressing gut‐homing receptor expression.52 Although these studies have not specifically discriminated between Teff and Treg cell subsets, vitamin‐D3‐inducible CD141+ dermal DCs resident in human skin have been shown to preferentially expand Treg cells that suppress cutaneous inflammation in vivo.53 The differential metabolic ability of distinct DC populations in skin may serve to appropriately activate or suppress regulatory pathways in specific immunological contexts.
Tissue‐specialized functions of skin Treg cells
Treg cells represent a highly complex and heterogeneous lineage with multiple specialized functions in both lymphoid and non‐lymphoid organs. Independent of their role in immune suppression, these cells are endowed with tissue‐specific functionality (reviewed in ref. 54).We are only beginning to understand the unique functions of Treg cells in skin. In mice, a subset of highly activated Treg cells accumulate in skin early after full thickness wounding.55 Specific deletion of these cells early during the wound‐healing process attenuates wound closure and re‐epithelialization. In this setting, depletion of Treg cells resulted in an interferon‐γ‐dependent accumulation of Ly‐6Chigh pro‐inflammatory macrophages. It was found that Treg cells infiltrating wounds express the epidermal growth factor receptor (EGFR), and that conditional deletion of EGFR on Treg cells resulted in delayed kinetics of wound closure and increased pro‐inflammatory macrophage accumulation (Fig. 2a). These results suggest that Treg cells in skin co‐opt a highly conserved regenerative pathway to mediate this specialized function of tissue repair.
Figure 2.
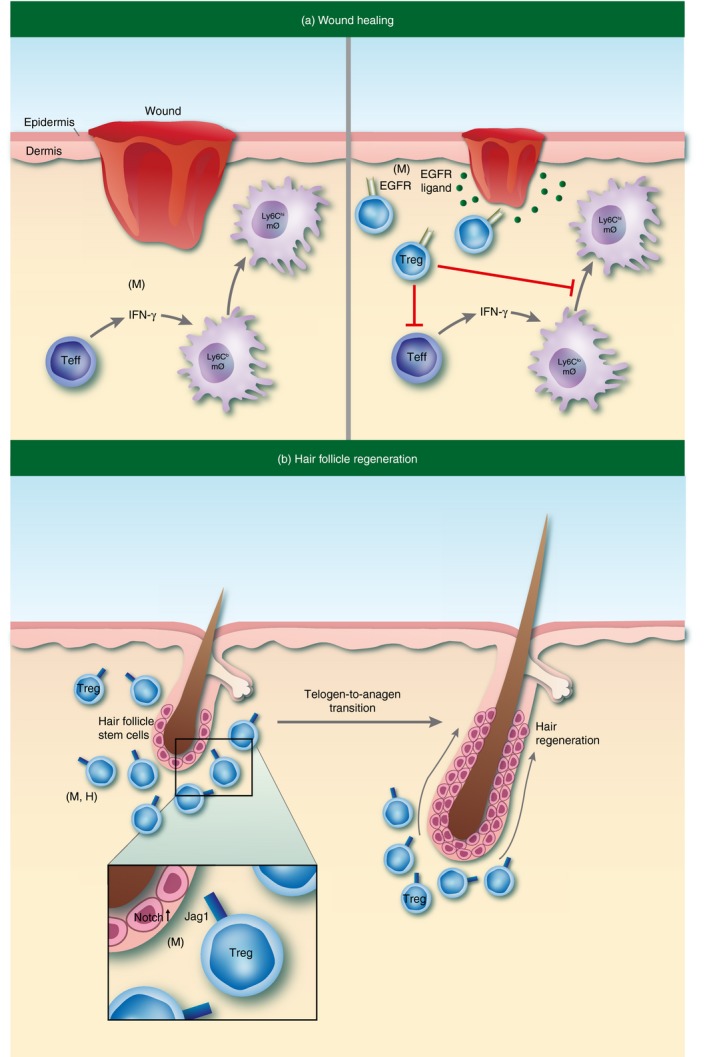
Tissue specialized functions of regulatory T (Treg) cells in skin. (a) Subset of highly activated epidermal growth factor receptor (EGFR) ‐expressing Treg cells accumulate in skin early after full‐thickness wounding. These cells function to promote wound closure and re‐epithelialization through modulation of pro‐inflammatory macrophage induction. (b) A subpopulation of Treg cells in telogen skin localize in close proximity to hair follicle stem cells (HFSCs) in the bulge region of the hair follicle (HF). Treg cells facilitate hair regeneration through expression of the Notch ligand Jagged‐1 (Jag1), which is required to promote HF cycling by enhancing the activation and differentiation of HFSCs. ‘(M)’ or ‘(H)’ denotes pathways that have been identified in mouse or human, respectively.
As discussed previously, tissue‐resident Treg cells predominantly localize around hair follicles (HFs).17, 32, 33, 34 Hair follicles are highly specialized organelles of mammalian skin that undergo perpetual cycles of rest (telogen) and regeneration (anagen), ultimately resulting in the generation of a newly formed hair shaft.56 In addition to immune cells, a major epithelial stem cell (SC) population resides in the bulge region of HFs (HFSCs). Dysregulation of HFSC activation can result in insufficient SC activity and so lead to a failure to regenerate hair.57 Interestingly, several studies link Treg cells with HF biology. Genome‐wide association studies in alopecia areata, an autoimmune disease in humans characterized by aberrant HF cycling, have linked single nucleotide polymorphisms in regions encoding ‘Treg signature’ genes, including the high‐affinity IL‐2 receptor α (IL‐2RA; CD25), the ikaros family member Eos (IKZF4), cytotoxic T‐lymphocyte antigen 4 (CTLA‐4) and Foxp3.58, 59 Consistent with these reports, peri‐follicular Foxp3+ T cells are significantly reduced in alopecia areata skin compared with healthy controls.60 In addition, clinical augmentation of Treg cells with low‐dose IL‐2 administration has shown efficacy in treating individuals with alopecia areata who were resistant to conventional therapies. Notably, the successful hair regeneration observed in 80% of treated patients was associated with an increased accumulation of Treg cells in lesional scalp skin.61 On a functional level, Treg cells have recently been shown to play a role in HF cycling and hair regeneration in murine skin.62, 63 Immunophenotypic profiling by flow cytometry revealed that Treg cell numbers and activation in skin tightly correlate with specific phases of the HF cycle. Treg cells were uniquely more abundant in telogen skin relative to anagen skin, whereas Teff and CD8+ T cells showed an inverse relationship to Treg cells. In addition, the proliferative and activation status of skin Treg cells correlated with phases of the HF cycle. Treg cells displayed a highly activated phenotype in telogen skin compared with skin where HFs were all in the anagen phase of the HF cycle. Interestingly, a subpopulation of Treg cells in telogen skin localized in close proximity to HFSCs in the bulge region of the HF. Lineage‐specific depletion of Treg cells resulted in a marked attenuation of HF regeneration, both after depilation and during the natural hair follicle cycle. Mechanistically, it was found that Treg cell expression of the Notch ligand Jagged‐1 (Jag1) was required to promote HF cycling by enhancing the activation and differentiation of HFSCs (Fig. 2b). These studies reveal that Treg cells in skin play a major role in HF biology and provide a mechanistic link between skin‐resident Treg cells and epithelial stem cells that is critical for tissue function.
Future directions and unanswered questions
Given their robust ability to control inflammatory responses, multiple therapeutic approaches are being developed to augment Treg cell activity in the context of autoimmunity, chronic inflammatory disease and organ transplantation. In contrast, approaches to inhibit Treg cell function are actively being explored for the treatment of cancer. Despite the growing body of work investigating the biology of Treg cells in skin, many fundamental questions remain to be addressed.
Treg cells in human skin may be very different from Treg cells in mouse skin. It is important to appreciate the many anatomical and immunological differences between mouse and human skin. Non‐inflamed mouse epidermis is only two to three cell layers thick, whereas normal human epidermis is much thicker. There are entire populations of immune cells (such as dendritic epidermal T cells and dermal TCR‐gamma delta T cells) that are abundant in mouse skin and present at low numbers if at all in human skin.64, 65, 66
It therefore follows that skin Treg cells analysed from these species may differ with respect to mechanisms required for their establishment, maintenance and function. Treg cells in murine skin are intimately linked with HF morphogenesis early in life,17 and also with adult HF cycling.62 The active growth phase of the HF cycle (anagen) in murine dorsal skin lasts only 2–3 weeks with a high degree of synchronicity between individual HFs. In contrast, human HF cycling is highly mosaic and asynchronous, with an anagen duration that can last for several years.56 Given that Treg cells preferentially localize to HFs and HF biology is quite different between species, it is unknown whether the biology of Treg cells relative to HF function elucidated in mouse skin will translate to humans.
The vast majority of studies analysing mechanisms of Treg‐mediated suppression have focused on Treg cells derived from peripheral blood or secondary lymphoid organs, such as the spleen or lymph nodes; not from peripheral tissues. Of peripheral tissues, the lung and gastrointestinal tract have been the most extensively studied. In these organs, Treg‐derived IL‐10 is essential for maintaining immune homeostasis.70 However, this was not the case for skin, as mice with a specific deletion of IL‐10 in Treg cells showed no signs of de novo cutaneous inflammation. Currently, the mechanisms used by Treg cells to regulate inflammation in skin are largely unknown. There may be a high level of redundancy between well‐accepted mechanisms of immune regulation elucidated in other tissues, such as IL‐10 secretion and CTLA‐4 expression. Alternatively, Treg cells in skin may use unique, previously unidentified pathways. Research focused on elucidating the immune regulatory mechanisms employed by Treg cells in both human and mouse skin is of fundamental importance in attempts to better understand how these cells function in this tissue. With the advent of humanized mice that harbour stably engrafted human skin, in vivo modelling of the human cutaneous immune system and functional aspects of human skin Treg cell biology is possible.32, 53, 71, 72 In addition, because human skin is highly accessible, comprehensive analysis of immune cells in skin biopsy specimens from patients enrolled in clinical trials testing immunomodulatory agents is another important avenue of assessing how Treg cells function in human tissues.
The antigen specificity of skin Treg cells and skin Trm cells in general is poorly understood. What are the antigens that skin Treg cells recognize? Given that thmyic Treg cell selection requires interactions between high‐affinity MHC class II–self peptide complexes, are skin Treg cells specific for self‐antigens expressed in both the thymus and skin, or are the majority restricted to foreign antigens derived from commensal microbes? The thymus may ‘license’ a subset of Treg cells to migrate to skin early in neonatal life in an antigen non‐specific fashion, and those that recognize either self or commensal antigens are retained in the tissue, whereas cells that lack productive TCR engagements are lost over time. Because the skin is an organ that interfaces with the external environment, it is subject to multiple barrier breaches during the lifespan of an individual. Hence, peripheral Treg cells specific for antigens derived from pathogens or from exposure to foreign material may be generated in skin over time. In addition to skin Treg cell antigen specificity, it is currently unknown whether antigen recognition (i.e. TCR engagement) is necessary for the maintenance or function of Treg cells in this tissue. Indeed, a subset of Treg cells that infiltrate the lungs seems to function in a TCR‐independent fashion.12 Elucidating the nature of antigens recognized by skin Treg cells may be critical for the development of both local and systemic antigen‐specific therapies for autoimmune disease and cancer. Single‐cell TCR sequencing of Treg cells from human tissues may uncover α and β chain TCR pairings in individual cells that can be used to re‐construct TCRs to discover the antigens recognized by these cells.
Another fundamental question is how heterogeneous tissue‐resident Treg cells are with respect to ontogeny and function, and how this heterogeneity differs in specific inflammatory contexts when compared with the steady state? Are there distinct skin Treg cell subsets that facilitate HF cycling, skin regeneration and suppression of inflammatory responses? In the lungs of mice, a distinct population of amphiregulin‐expressing Treg cells may preferentially mediate tissue repair when compared with an anti‐inflammatory subset that expresses high levels of IL‐10.12 Interestingly, Treg‐derived amphiregulin and subsequent tissue regeneration were induced in response to innate‐like inflammatory cues from the local environment, independent of TCR signalling. Tissue‐specific cues that activate subsets of Treg cells to perform specialized functions in skin have yet to be defined. In addition, the immune and non‐immune (i.e. parenchymal) cells within skin that Treg cells modulate to mediate these specialized functions are largely unknown. Do Treg cells alter the function of keratinocytes, fibroblasts and/or adipocytes in skin, and if so, how?
Treg cells comprise a large component of tumour‐infiltrating lymphocytes found in most human tumours and in many tumour models in mice. Elevated frequencies of these cells in tumours, for example in ovarian cancer, have been correlated with worse clinical outcomes.73 However, the role of Treg cells in the pathogenesis of human cancer is poorly understood. Do these cells primarily function to suppress anti‐tumour immune responses early during tumorigenesis or do they function to suppress these responses in advanced stage metastatic disease, or both? Do Treg cells in tumours have alternative functions, independent of their roles in suppressing anti‐tumour immune responses? Targeted inhibition of the immune checkpoint pathways programmed death protein‐1 (PD‐1) and CTLA‐4 has revolutionized therapy for advanced cancer, with perhaps the most striking results in melanoma, a tumour that arises in skin and mucous membranes. Interestingly, a flow cytometric‐based analysis of a large cohort of metastatic melanoma patients both before and after anti‐PD‐1 therapy demonstrated an increased activation of CD8+ tumour‐infiltrating lymphocytes, with no observable effect on tumour‐infiltrating Treg cell frequencies or activation status.74 These results indicate that Treg cell modulation plays a minimal role in mediating advanced tumour regression in the context of anti‐PD‐1 monotherapy, and that productive anti‐tumour immune responses can be achieved in the presence of highly activated tumour‐infiltrating Treg cells. Moving forward, it will be important to elucidate whether Treg cells play a functional role in the development or progression of melanoma or non‐melanoma skin cancers such as basal cell carcinoma and squamous cell carcinoma in humans.
In summary, the skin is home to a large proportion of Treg cells and many of these cells in the peripheral circulation have the propensity to migrate to this tissue. Treg cells play a major role in establishing and maintaining immune homeostasis in tissues and are most likely comprised of multiple subsets with unique functions. Some of these functions are most likely unique to skin and we are only beginning to understand the cellular and molecular mechanisms by which Treg cells function in this tissue. Because Treg cells are thought to mediate the majority of their functions in the tissues in which they reside, and optimal therapeutic approaches directed at either augmenting or inhibiting these cells will most likely require strategies that target specific subsets, it is of fundamental importance to further define the function of these cells in tissues such as skin.
Disclosures
The authors have no competing interests to declare.
References
- 1. Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA et al Thymic selection of CD4+ CD25+ regulatory T cells induced by an agonist self‐peptide. Nat Immunol 2001; 2:301–6. [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Jin W, Hardegen N, Lei K‐J, Li L, Marinos N et al Conversion of peripheral CD4+ CD25– naive T cells to CD4+ CD25+ regulatory T cells by TGF‐β induction of transcription factor Foxp3. J Exp Med 2003; 198:1875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self‐tolerance maintained by activated T cells expressing IL‐2 receptor α‐chains (CD25). Breakdown of a single mechanism of self‐tolerance causes various autoimmune diseases. J Immunol 1995; 155:1151–64. [PubMed] [Google Scholar]
- 4. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003; 299:1057–61. [DOI] [PubMed] [Google Scholar]
- 5. Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat Immunol 2003; 4:330–6. [DOI] [PubMed] [Google Scholar]
- 6. Suwanpradid J, Holcomb ZE, MacLeod AS. Emerging skin T‐cell functions in response to environmental insults. J Invest Dermatol 2017; 137:288–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clark RA. Resident memory T cells in human health and disease. Sci Transl Med 2015; 7:269rv1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li Z, Li D, Tsun A, Li B. FOXP3+ regulatory T cells and their functional regulation. Cell Mol Immunol 2015; 12:558–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gratz IK, Campbell DJ. Organ‐specific and memory Treg cells: specificity, development, function, and maintenance. Front Immunol 2014; 5:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE et al PPAR‐γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature 2012; 486:549–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y et al A special population of regulatory T cells potentiates muscle repair. Cell 2013; 155:1282–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S et al A distinct function of regulatory T cells in tissue protection. Cell 2015; 162:1078–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wohlfert EA, Grainger JR, Bouladoux N, Konkel JE, Oldenhove G, Ribeiro CH et al GATA3 controls Foxp3+ regulatory T cell fate during inflammation in mice. J Clin Invest 2011; 121:4503–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scharschmidt TC, Vasquez KS, Truong H‐A, Gearty SV, Pauli ML, Nosbaum A et al A wave of regulatory T cells into neonatal skin mediates tolerance to commensal microbes. Immunity 2015; 43:1011–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wolff MJ, Leung JM, Davenport M, Poles MA, Cho I, Loke P. TH17, TH22 and Treg cells are enriched in the healthy human cecum. PLoS ONE 2012; 7:e41373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio C‐W, Santacruz N et al Peripheral education of the immune system by colonic commensal microbiota. Nature 2011; 478:250–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scharschmidt TC, Vasquez KS, Pauli ML, Leitner EG, Chu K, Truong H‐A et al Commensal microbes and hair follicle morphogenesis coordinately drive Treg migration into neonatal skin. Cell Host Microbe 2017; 21:467–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D. Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self‐tolerance. Science 2015; 348:589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fuhlbrigge RC, Kieffer JD, Armerding D, Kupper TS. Cutaneous lymphocyte antigen is a specialized form of PSGL‐1 expressed on skin‐homing T cells. Nature 1997; 389:978–81. [DOI] [PubMed] [Google Scholar]
- 20. Hirahara K, Liu L, Clark RA, Yamanaka K, Fuhlbrigge RC, Kupper TS. The majority of human peripheral blood CD4+ CD25high Foxp3+ regulatory T cells bear functional skin‐homing receptors. J Immunol 2006; 177:4488–94. [DOI] [PubMed] [Google Scholar]
- 21. Iellem A, Colantonio L, D'Ambrosio D. Skin‐versus gut‐skewed homing receptor expression and intrinsic CCR4 expression on human peripheral blood CD4+ CD25+ suppressor T cells. Eur J Immunol 2003; 33:1488–96. [DOI] [PubMed] [Google Scholar]
- 22. Dioszeghy V, Mondoulet L, Puteaux E, Dhelft V, Ligouis M, Plaquet C et al Differences in phenotype, homing properties and suppressive activities of regulatory T cells induced by epicutaneous, oral or sublingual immunotherapy in mice sensitized to peanut. Cell Mol Immunol 2016; 13:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tietz W, Allemand Y, Borges E, von Laer D, Hallmann R, Vestweber D et al CD4+ T cells migrate into inflamed skin only if they express ligands for E‐ and P‐selectin. J Immunol 1998; 161:963–70. [PubMed] [Google Scholar]
- 24. Sather BD, Treuting P, Perdue N, Miazgowicz M, Fontenot JD, Rudensky AY et al Altering the distribution of Foxp3+ regulatory T cells results in tissue‐specific inflammatory disease. J Exp Med 2007; 204:1335–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Campbell JJ, Haraldsen G, Pan J, Rottman J, Qin S, Ponath P et al The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature 1999; 400:776–80. [DOI] [PubMed] [Google Scholar]
- 26. Alferink J, Lieberam I, Reindl W, Behrens A, Weiss S, Hüser N et al Compartmentalized production of CCL17 in vivo: strong inducibility in peripheral dendritic cells contrasts selective absence from the spleen. J Exp Med 2003; 197:585–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lieberam I, Förster I. The murine β‐chemokine TARC is expressed by subsets of dendritic cells and attracts primed CD4+ T cells. Eur J Immunol 1999; 29:2684–94. [DOI] [PubMed] [Google Scholar]
- 28. Fujita M, Furukawa F, Fujii K, Horiguchi Y, Takeichi M, Imamura S. Expression of cadherin cell adhesion molecules during human skin development: morphogenesis of epidermis, hair follicles and eccrine sweat ducts. Arch Dermatol Res 1992; 284:159–66. [DOI] [PubMed] [Google Scholar]
- 29. Tubo NJ, McLachlan JB, Campbell JJ. Chemokine receptor requirements for epidermal T‐cell trafficking. Am J Pathol 2011; 178:2496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Malý P, Thall A, Petryniak B, Rogers CE, Smith PL, Marks RM et al The α(1,3)fucosyltransferase Fuc‐TVII controls leukocyte trafficking through an essential role in L‐, E‐, and P‐selectin ligand biosynthesis. Cell 1996; 86:643–53. [DOI] [PubMed] [Google Scholar]
- 31. Dudda JC, Perdue N, Bachtanian E, Campbell DJ. Foxp3+ regulatory T cells maintain immune homeostasis in the skin. J Exp Med 2008; 205:1559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sanchez Rodriguez R, Pauli ML, Neuhaus IM, Yu SS, Arron ST, Harris HW et al Memory regulatory T cells reside in human skin. J Clin Invest 2014; 124:1027–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gratz IK, Truong H‐A, Yang SH‐Y, Maurano MM, Lee K, Abbas AK et al Cutting Edge: memory regulatory t cells require IL‐7 and not IL‐2 for their maintenance in peripheral tissues. J Immunol 2013; 190:4483–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chow Z, Mueller SN, Deane JA, Hickey MJ. Dermal regulatory T cells display distinct migratory behavior that is modulated during adaptive and innate inflammation. J Immunol 2013; 191:3049–56. [DOI] [PubMed] [Google Scholar]
- 35. Collins N, Jiang X, Zaid A, Macleod BL, Li J, Park CO et al Skin CD4+ memory T cells exhibit combined cluster‐mediated retention and equilibration with the circulation. Nat Commun 2016; 10:11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Overstreet MG, Gaylo A, Angermann BR, Hughson A, Hyun Y‐M, Lambert K et al Inflammation‐induced interstitial migration of effector CD4+ T cells is dependent on integrin αV. Nat Immunol 2013; 14:949–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Park CO, Kupper TS. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med 2015; 21:688–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rosenblum MD, Way SS, Abbas AK. Regulatory T cell memory. Nat Rev Immunol 2016; 16:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rosenblum MD, Gratz IK, Paw JS, Lee K, Marshak‐Rothstein A, Abbas AK. Response to self antigen imprints regulatory memory in tissues. Nature 2011; 480:538–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature 2012; 490:102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van der Veeken J, Gonzalez AJ, Cho H, Arvey A, Hemmers S, Leslie CS et al Memory of inflammation in regulatory T cells. Cell 2016; 166:977–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sakaguchi S, Vignali DAA, Rudensky AY, Niec RE, Waldmann H. The plasticity and stability of regulatory T cells. Nat Rev Immunol 2013; 13:461–7. [DOI] [PubMed] [Google Scholar]
- 43. Liu W, Putnam AL, Xu‐Yu Z, Szot GL, Lee MR, Zhu S et al CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 2006; 203:1701–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Adachi T, Kobayashi T, Sugihara E, Yamada T, Ikuta K, Pittaluga S et al Hair follicle‐derived IL‐7 and IL‐15 mediate skin‐resident memory T cell homeostasis and lymphoma. Nat Med 2015; 21:1272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Smigiel KS, Richards E, Srivastava S, Thomas KR, Dudda JC, Klonowski KD et al CCR7 provides localized access to IL‐2 and defines homeostatically distinct regulatory T cell subsets. J Exp Med 2014; 211:121–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Clark RA, Kupper TS. IL‐15 and dermal fibroblasts induce proliferation of natural regulatory T cells isolated from human skin. Blood 2007; 109:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell‐induced memory T cell activation in nonlymphoid tissues. Science 2008; 319:198–202. [DOI] [PubMed] [Google Scholar]
- 48. Heath WR, Carbone FR. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat Immunol 2009; 10:1237–44. [DOI] [PubMed] [Google Scholar]
- 49. Seneschal J, Clark RA, Gehad A, Baecher‐Allan CM, Kupper TS. Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity 2012; 36:873–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bobr A, Olvera‐Gomez I, Igyarto BZ, Haley KM, Hogquist KA, Kaplan DH. Acute ablation of Langerhans cells enhances skin immune responses. J Immunol 2010; 185:4724–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song S‐Y. Retinoic acid imprints gut‐homing specificity on T cells. Immunity 2004; 21:527–38. [DOI] [PubMed] [Google Scholar]
- 52. Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D et al DCs metabolize sunlight‐induced vitamin D3 to “program” T cell attraction to the epidermal chemokine CCL27. Nat Immunol 2007; 8:285–93. [DOI] [PubMed] [Google Scholar]
- 53. Chu C‐C, Ali N, Karagiannis P, Di Meglio P, Skowera A, Napolitano L et al Resident CD141 (BDCA3)+ dendritic cells in human skin produce IL‐10 and induce regulatory T cells that suppress skin inflammation. J Exp Med 2012; 209:935–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Panduro M, Benoist C, Mathis D. Tissue Tregs. Annu Rev Immunol 2016; 20:609–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nosbaum A, Prevel N, Truong H‐A, Mehta P, Ettinger M, Scharschmidt TC, et al Cutting edge: regulatory T cells facilitate cutaneous wound healing. J Immunol 2016; 5:2010–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Müller‐Röver S, Handjiski B, van der Veen C, Eichmüller S, Foitzik K, McKay IA et al A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol 2001; 117:3–15. [DOI] [PubMed] [Google Scholar]
- 57. Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol 2009; 10:207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Petukhova L, Duvic M, Hordinsky M, Norris D, Price V, Shimomura Y et al Genome‐wide association study in alopecia areata implicates both innate and adaptive immunity. Nature 2010; 466:113–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Conteduca G, Rossi A, Megiorni F, Parodi A, Ferrera F, Tardito S et al Single nucleotide polymorphisms in the promoter regions of Foxp3 and ICOSLG genes are associated with Alopecia areata. Clin Exp Med 2014; 14:91–7. [DOI] [PubMed] [Google Scholar]
- 60. Han Y‐M, Sheng Y‐Y, Xu F, Qi S‐S, Liu X‐J, Hu R‐M et al Imbalance of T‐helper 17 and regulatory T cells in patients with alopecia areata. J Dermatol 2015; 42:981–8. [DOI] [PubMed] [Google Scholar]
- 61. Castela E, Le Duff F, Butori C, Ticchioni M, Hofman P, Bahadoran P et al Effects of low‐dose recombinant interleukin 2 to promote T‐regulatory cells in alopecia areata. JAMA Dermatol 2014; 150:748–51. [DOI] [PubMed] [Google Scholar]
- 62. Ali N, Zirak B, Rodriguez RS, Pauli ML, Truong H‐A, Lai K et al Regulatory T cells in skin facilitate epithelial stem cell differentiation. Cell 2017; 169:1119–1129.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Horsley V, Naik S. Tregs expand the skin stem cell niche. Dev Cell 2017; 41:455–6. [DOI] [PubMed] [Google Scholar]
- 64. Schlitzer A, Ginhoux F. Organization of the mouse and human DC network. Curr Opin Immunol 2014; 26:90–9. [DOI] [PubMed] [Google Scholar]
- 65. Godfrey DI, Hammond KJ, Poulton LD, Smyth MJ, Baxter AG. NKT cells: facts, functions and fallacies. Immunol Today 2000; 21:573–83. [DOI] [PubMed] [Google Scholar]
- 66. Jameson JM, Sharp LL, Witherden DA, Havran WL. Regulation of skin cell homeostasis by γδ T cells. Front Biosci 2004; 1:2640–51. [DOI] [PubMed] [Google Scholar]
- 67. Gay D, Kwon O, Zhang Z, Spata M, Plikus MV, Holler PD et al Fgf9 from dermal γδ T cells induces hair follicle neogenesis after wounding. Nat Med 2013; 19:916–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. MacLeod AS, Hemmers S, Garijo O, Chabod M, Mowen K, Witherden DA et al Dendritic epidermal T cells regulate skin antimicrobial barrier function. J Clin Invest 2013; 123:4364–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R et al A role for skin γδ T cells in wound repair. Science 2002; 296:747–9. [DOI] [PubMed] [Google Scholar]
- 70. Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X et al Regulatory T cell‐derived interleukin‐10 limits inflammation at environmental interfaces. Immunity 2008; 28:546–58. [DOI] [PubMed] [Google Scholar]
- 71. Kenney LL, Shultz LD, Greiner DL, Brehm MA. Humanized mouse models for transplant immunology. Am J Transplant 2016; 16:389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sagoo P, Ali N, Garg G, Nestle FO, Lechler RI, Lombardi G. Human regulatory T cells with alloantigen specificity are more potent inhibitors of alloimmune skin graft damage than polyclonal regulatory T cells. Sci Transl Med 2011; 3:83ra42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P et al Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004; 10:942–9. [DOI] [PubMed] [Google Scholar]
- 74. Daud AI, Loo K, Pauli ML, Sanchez‐Rodriguez R, Sandoval PM, Taravati K et al Tumor immune profiling predicts response to anti‐PD‐1 therapy in human melanoma. J Clin Invest 2016; 126:3447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]


