Summary
T helper 9 (Th9) cells and interleukin (IL)‐9 are involved in the pathogenesis of several autoimmune diseases. The exact role of IL‐9 and Th9 cells in patients with systemic sclerosis (SSc) have not yet been studied adequately. IL‐9, IL‐9R, transcription factor PU.1 (PU.1), IL‐4, thymic stromal lymphopoietin (TSLP) and transforming growth factor (TGF)‐β expression were assessed in skin and kidney biopsies of SSc patients and healthy controls (HC) by immunohistochemistry (IHC). The cellular source of IL‐9 was also analysed by confocal microscopy analysis. Peripheral IL‐9‐producing cells were also studied by flow cytometry. The functional relevance of IL‐9 increased expression in SSc was also investigated. Our results demonstrated a strong expression of IL‐9, IL‐9R, IL‐4, TSLP and TGF‐β in skin tissues of patients with both limited and diffuse SSc. IL‐9 expression was observed mainly in the context of skin infiltrating mononuclear cells and keratinizing squamous epithelium. IL‐9 over‐expression was also observed in renal biopsies of patients with SSc. IL‐9 producing cells in the skin were identified as Th9 cells. Similarly, Th9 cells were expanded and were the major source of IL‐9 among SSc peripheral blood mononuclear cells (PBMC), their percentage being correlated directly with the modified Rodnan skin score. Infiltrating mononuclear cells, mast cells and neutrophils expressed IL‐9R. In in‐vitro studies stimulation with rIL‐9 significantly induced NET (neutrophil extracellular traps) release by dying cells (NETosis) in neutrophils, expansion of mast cells and increase of anti‐systemic scleroderma 70 (Scl70) production by B cells. Our findings suggest that Th9 cells and IL‐9 could be implicated in the pathogenesis of SSc.
Keywords: IL‐9, ILC2, systemic sclerosis, Th9
Introduction
Systemic sclerosis (SSc) is a chronic autoimmune disorder in which abnormal immune responses lead to activation and expansion of autoreactive effector immune cells, resulting in fibrosis of multiple organs 1. Accumulating data suggest that infiltrating CD4+ T helper (Th) cells, essentially skewed towards Th2 and Th17 phenotypes, play important roles in essentially mediating inflammation and fibrosis progression 1, 2.
Recently, interleukin (IL)‐9, a pleiotropic cytokine produced mainly by Th9 cells, has been demonstrated to participate actively in the induction of tissue fibrosis and in the pathogenesis of different autoimmune disorders 3, 4. The differentiation of Th9 cells requires a balance of signals, including IL‐4 and transforming growth factor (TGF)‐β 5, and the epithelial cytokine thymic stromal lymphopoietin (TSLP) 6, resulting in the induction of the transcriptional factor PU.1 that, in concert with interferon (IFN) regulatory factor 4 (IRF4), directly binds the IL‐9 promoter and activates gene expression in Th9 cells 7. Once differentiated, Th9 cells require activation of the IL‐25/IL–17RB axis to produce high levels of IL‐9 8, 9
Increased expression of Th9 polarizing cytokines and of IL‐25 has been demonstrated previously in patients with SSc 10, 11, 12, but the expression of IL‐9 and Th9 cells has not yet been studied adequately. In the present study, we analysed the expression of IL‐9 and Th9 cells in SSc. We further examined the relationship of Th9 cells with specific subsets of SSc and the expression of Th9 relevant cytokines, including IL‐17A, TGF‐β1, TSLP and IL‐4 and the role of IL‐9 in modulating the function of IL‐9R‐expressing cells. The aim of the study was to evaluate Th9/IL‐9 expression in different tissues of SSc patients as a possible player in the pathogenesis of SSc, thus representing a potential therapeutic target.
Patients and methods
Skin biopsy specimens of sclerotic cutis and peripheral blood were obtained after informed consent from 20 patients with SSc diagnosed according to the 2013 classification criteria for systemic sclerosis 13 (13 patients with limited and seven with diffuse SSc) (18 female and two male, mean age = 42 ± 12 years, mean disease duration = 34 ± 12 months) and 20 healthy controls (age‐ and sex‐matched) who underwent surgery for non‐cutaneous disease evaluation. Kidney samples were also obtained from an additional eight patients with SSc in whom renal biopsy was performed for hypertensive renal crisis attributable to SSc or persistent renal impairment (serum creatinine value > 2 mg/dl plus a diastolic blood pressure > 110 mmHg), or otherwise inexplicable presence of blood and/or protein in the urine. Renal biopsies were also obtained from five controls with isolated urinary alterations but normal kidney histology. Modified Rodnan skin score was calculated to assess the extension of skin involvement 14. Pulmonary involvement was defined as a forced vital capacity < 70% of predicted, as measured by a dry spirometer, or by the evidence of amorphous or reticulonodular infiltrates in high‐resolution computed tomography scans (HRCT). Pulmonary hypertension (PH) was also evaluated [pulmonary artery pressures (PAPs) > 25 mmHg and peak tricuspid regurgitant jet velocity (TRV) >2·7 m/s] according to Denton et al. 15. Table 1 shows the baseline characteristics of the patients. Age‐ and sex‐matched healthy donors (HD) were enrolled as controls. Collection of samples was approved by the ethical committee and the Institutional Review Board of the University of Palermo and informed consent was obtained from each patient and controls in accordance with the Helsinki Declaration.
Table 1.
Baseline characteristics
| Patients | Limited versus diffuse SSc | Modified Rodnan skin score | Pulmonary involvement | Pulmonary hypertension | Autoantibodies | %Th9 |
|---|---|---|---|---|---|---|
| 1 | Limited | 18 | Yes | Yes | Scl‐70 | 0·72 |
| 2 | Diffuse | 27 | Yes | None | Scl‐70 | 0·89 |
| 3 | Diffuse | 24 | None | None | ANA | 0·92 |
| 4 | Diffuse | 33 | Yes | None | Scl‐70 | 1 |
| 5 | Limited | 15 | None | None | ANA | 0·69 |
| 6 | Diffuse | 23 | None | None | ANA | 0·76 |
| 7 | Limited | 24 | None | Yes | ACA | 0·60 |
| 8 | Limited | 12 | None | Yes | ACA | 0·81 |
| 9 | Limited | 9 | None | Yes | ANA | 0·73 |
| 10 | Limited | 16 | None | Yes | ACA | 1 |
| 11 | Diffuse | 38 | Yes | None | Scl‐70 | 0·87 |
| 12 | Limited | 12 | Yes | None | ANA | 0·60 |
| 13 | Limited | 15 | Yes | None | ANA | 0·79 |
| 14 | Diffuse | 19 | Yes | None | Scl‐70 | 0·88 |
| 15 | Limited | 22 | None | Yes | ANA | 0·62 |
| 16 | Limited | 26 | Yes | None | ANA | 0·67 |
| 17 | Limited | 12 | None | None | ACA | 0·66 |
| 18 | Limited | 14 | Yes | None | ANA | 0·72 |
| 19 | Limited | 28 | Yes | None | ANA | 0·78 |
| 20 | Diffuse | 26 | None | None | ACA | 0·85 |
| Controls (mean ± s.e.m.) | – | – | – | – | – | 0·2040 ± 0·05384 |
| Patients (mean ± s.e.m.) | – | – | – | – | 0·7780 ± 0·02751 |
SSc = systemic sclerosis; Th9 = T helper type 9; s.e.m. = standard error of the mean.
Immunohistochemistry
Tissue samples were fixed immediately with 4% formaldehyde and embedded in paraffin. Immunohistochemistry analysis was performed on 5‐μm‐thick paraffin‐embedded sections. Following rehydration, antigen was unmasked for 45 min at 95°C using Dako Target retrieval solution (pH 6.0) (Dako, Glostrup, Denmark). Endogenous peroxidase was blocked for 10 min with Dako peroxidase blocking reagent, and non‐specific binding was blocked for 20 min with Dako protein block. The primary antibodies were added and incubated for 1 h at room temperature. Isotype‐matched irrelevant antibodies were used as a negative control [mouse immunoglobulin (Ig)G1 monoclonal antibody (mAb) (ab27479) and rabbit IgG polyclonal antibody (ab27472); Abcam, Cambridge, UK)]. Following three washes with Tris‐buffered saline, slides were incubated for 30 min with peroxidase‐conjugated Dako EnVision polymer. After three further washings, peroxidase activity was visualized using diaminobenzidine chromogen (Dako), and slides were counterstained lightly with haematoxylin before dehydration and mounting in DePex (VWR International, Radnor, PA, USA). Sections were analysed by two experienced scientists (A. R., F. C.) who were blinded with regard to subject group. The number of positive cells was determined by counting immunoreactive cells on photomicrographs obtained from three randomly obtained high‐power microscopic fields (×400 magnification) under a DM2000 optical microscope, using a DFC320 digital camera (Leica, Wetzlar, Germany). To address specifically the nature of IL‐9‐producing cells, triple staining for Th17 and Th9 cells were performed on paraffin‐embedded skin sections. Sections were treated with fluorescein isothiocyanate (FITC)‐, Rhodamine Redor Cy‐5‐conjugated anti‐mouse or anti‐rabbit antibodies (Invitrogen, Carlsbad, CA, USA) plus RNasi (200 ng/ml) and counterstained using (4′,6‐diamidino‐2‐phenylindole (DAPI; Life Technologies, Paisley, UK]. Confocal analysis was used to acquire fluorescence staining. A list of the antibodies used is shown in Supporting information, Table S1.
Isolation of peripheral blood mononuclear cells and flow cytometry
Peripheral blood mononuclear cells (PBMC) were obtained by density gradient centrifugation using Ficoll‐Hypaque (Pharmacia Biotech, Uppsala, Sweden) and were cultured in RPMI‐1640 supplemented with 10% fetal calf serum (FCS) and antibiotics from 15 SSc patients (10 with limited SSc and five with diffuse SSc) and 10 healthy controls. Cell viability (trypan blue dye exclusion) was always > 95%. Cells were activated with anti‐CD3/CD28 beads (ratio 1 : 2) for 6 h and then stained with mAb. Flow cytometry analysis was performed using a FACSCanto (BD Biosciences). At least 50 000 cells (events), gated on lymphocyte region, were acquired for each sample. Data were analysed with FlowJo software programs (FloJo, TreeStar Inc., Ashland, OR, USA). A list of the antibodies used is listed in Supporting information Table S1.
Cell cultures
Mast cells and B cells were isolated with microbeads (anti‐CD117 and CD19, respectively) (positive selection) and magnetic‐activated cell sorting techniques (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany), according to the manufacturer's instructions. Neutrophils were isolated from peripheral blood using the density gradient separation method with a mixture of sodium metrizoate and dextran 500 and lysis of residual erythrocytes. Cells were cultured in 24‐well flat‐bottomed plates (Becton Dickinson Labware, Franklin Lakes, NJ, USA) at a density of 1 × 106 cells in 1 ml of RPMI‐1640 medium with 10% FCS, 2 mM L‐glutamine, 20 nM HEPES and 100 U/ml penicillin/streptomycin with or without recombinant IL‐9 (rIL‐9) (10 ng/ml) (R&D Systems, Minneapolis, MN, USA). Cells were incubated at 37°C in a humidified atmosphere containing 5% CO2 for 48 h. After incubation cells were fixed, stained with myeloperoxidase (MPO) and counterstained with DAPI. Confocal analysis was used to acquire fluorescence staining.
Statistical analysis
Student's t‐test or the non‐parametric Mann–Whitney U‐test was used to calculate the statistical significance between groups. P‐values less than 0·05 were considered significant.
Results
IL‐9 is up‐regulated in skin specimens of SSc patients
We first investigated whether IL‐9 was up‐regulated in skin specimens of SSc patients. As shown in Fig. 1, IL‐9 expression was increased in both diffuse and limited SSc patients. In particular, IL‐9 expression was detected mainly between infiltrating cells, endothelial cells and keratinizing squamous epithelial cells of skin of SSc subjects (Fig. 1a–c,l). The cellular source of IL‐9 was represented prevalently by Th9 cells, as demonstrated by the co‐localization of IL‐9 and the transcriptional factor PU.1 (Fig. 1e–h). IL‐9‐producing Th17 cells were also found in the skin specimens of SSc patients (Fig. 1e–h). According to IL‐9 over‐expression, IL‐9R was expressed intensely among infiltrating cells and keratinizing squamous epithelial cells (Fig. 1i–k,m). With regard to the expansion of Th9 cells, we next evaluated the expression of Th9‐inducing cytokines in skin specimens of SSc. As shown in Fig. 2a–i, increased expression of IL‐4, TSLP and TGF‐β was detected in in skin tissues of patients with SSc, especially in those with diffuse disease.
Figure 1.
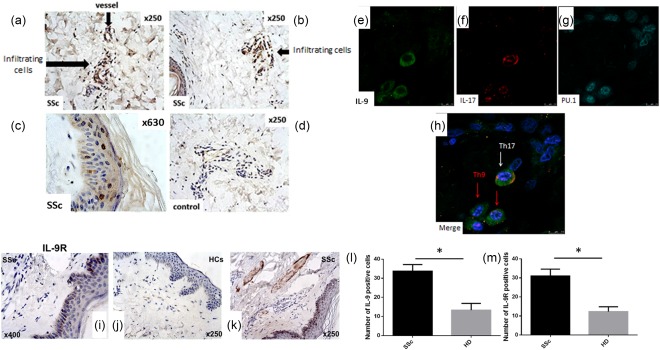
Interleukin (IL)‐9 and IL‐9R expression in the skin of systemic sclerosis (SSc) and HD. IL‐9 expression in the skin of SSc patients (a–c) and controls (d). Single staining for IL‐9 (e), IL‐17 (f) and transcription factor PU.1 (PU.1) (g). (h) Merged triple staining for IL‐9, IL‐17 and PU.1 in the skin of SSc patients. IL‐9R expression in the skin of SSc patients (i,k) and controls (j). Quantification of IL‐9 and IL‐9R‐producing cells from SSc patients and controls (l,m). Original magnification ×400 (i), ×250 (a,b,d,j,k), ×630 (c). Data are expressed as mean ± standard error of the mean (s.e.m.); *P < 0·05. [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 2.
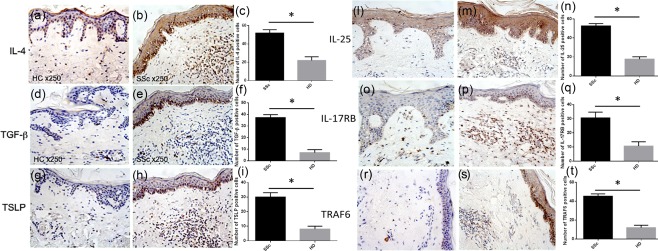
Interleukin (IL)‐4, transforming growth factor (TGF)‐β, thymic stromal lymphopoietin (TSLP), IL‐25, IL‐17RB and tumour necrosis factor (TNF) receptor associated factor 6 (TRAF6) expression in systemic sclerosis (SSc) and healthy donors (HD). IL‐4, TGF‐β, TSLP, IL‐25, IL‐17RB and TRAF6 expression in the skin of SSc patients (b,e,h,m,p,s) and controls (a,d,g,l,o,r). Quantification of IL‐4‐, TGF‐β–, TSLP‐, IL‐25‐, IL‐17RB‐ and TRAF6‐producing cells from SSc patients and controls (c,f,i,n,q,t). Original magnification ×250. Data are expressed as mean ± standard error of the mean (s.e.m.); *P < 0·05. [Colour figure can be viewed at wileyonlinelibrary.com]
The IL‐25–IL‐17RB pathway has been demonstrated to be important in the induction of IL‐9 production by Th9 cells 9. As shown in Fig. 2l–s, we found over‐expression of IL‐25, IL‐17RB and of the specific tumour necrosis factor (TNF) receptor‐associated factor 6 (TRAF6) in the skin of SSc patients compared to controls. IL‐9 over‐expression was also observed in the inflamed glomeruli obtained from SSc patients (Supporting information, Fig. S1). In particular, SSc patients displayed intense staining in the context of mesangial glomerular cells with weak tubular epithelial cells positivity (Supporting information, Fig. S1). Conversely, HC showed intense tubular epithelial cell positivity (Supporting information, Fig. S1). These findings indicate that the Th9 pathway is activated strongly in SSc patients.
Phenotypical analysis of IL‐9‐producing cells among PBMC of SSc patients
The expression of IL‐9 by different subsets of T helper cells was also analysed by FACS analysis on cells isolated from the peripheral blood of patients with SSc and controls. As shown in Fig. 3, IL‐9‐producing CD4+ T cells from patients with SSc were expanded significantly compared to control subjects. Among CD4+ T cells, the percentage of IL‐9‐producing Th9 (0·7 ± 0·02 versus 0·2 ± 0·05) (Fig. 3a,c) and Th17 (1·2 ± 0·3 versus 0·33 ± 0·08) (Fig. 3d) cells was significantly higher in SSc patients respect to controls. Conversely, the percentage of IL‐9‐producing Th2 (0·4 ± 0·09 versus 0·2 ± 0·03) cells was not significantly different in the two groups (Fig. 3b). Interestingly, frequencies of Th9 cells were significantly higher in patients with diffuse SSc and the number of IL‐9‐expressing cells was correlated significantly with the modified Rodnan skin score, but not with pulmonary hypertension (Fig. 3e–g). These findings indicate the presence of a Th9 polarization in the peripheral blood of SSc patients.
Figure 3.
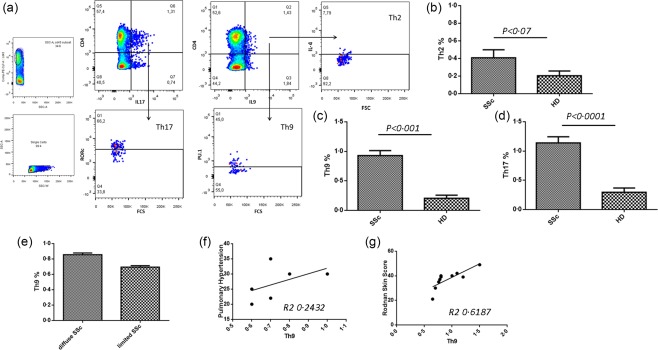
Percentage of T helper type 9 (Th9), Th17 and Th2 in systemic sclerosis (SSc) and healthy donors (HD). Representative dot‐plot analysis and gating strategy in an SSc patient (a). Percentage of Th2 (b), Th9 (c) and Th17 (d) cells in SSc and HD. Mean percentage of Th9 cells in patients with diffuse or limited disease (e). Direct correlation of Th9 percentage in SSc and pulmonary hypertension (f) and Rodnan skin score (g). Data are expressed as mean ± standard error of the mean (s.e.m.). [Colour figure can be viewed at wileyonlinelibrary.com]
Innate lymphoid cells type 2 (ILC2) are expanded in the skin and peripheral blood of SSc
Th9‐derived IL‐9 has been demonstrated to activate ILC2 in a mast cell‐dependent manner 16. Furthermore, ILC2 have been described to be expanded in SSc patients 17. Thus, we next evaluated the frequencies of Th2 polarized ILC2 subsets in the skin and peripheral blood of SSc patients (Fig. 4). According to a previous report, ILC2 were expanded significantly in peripheral blood (Fig. 4a,b), and cells resembling the ILC2 phenotype were also increased in the skin (Fig. 4c) of SSc patients compared to controls. A significant and direct correlation was also found between the percentages of circulating ILC2 and Th9 cells (Fig. 4g).
Figure 4.
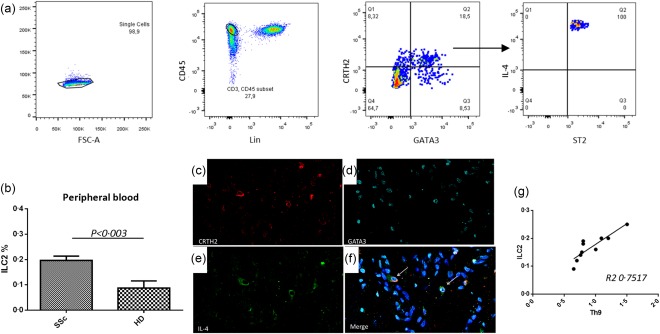
Innate lymphoid cells (ILC) type 2 are expanded in systemic sclerosis (SSc) patients. Representative dot‐plot analysis and gating strategy in a SSc patient (a). Mean percentage of ILC2 cells among peripheral blood mononuclear cells (PBMC) of patients and HD (b). Single staining for chemoattractant receptor Th2 (CRTH2) (c), GATA binding protein 3 (GATA3) (d) and interleukin (IL)‐4 (e). (f) Merged triple staining for CRTH2, GATA3 and IL‐4. ILC2 are directly correlated with Th9 (g). Data are expressed as mean ± standard error of the mean (s.e.m.); *P < 0·05. [Colour figure can be viewed at wileyonlinelibrary.com]
Effect of IL‐9 on B cells, mast cells and neutrophils in SSc
Beyond ILC2, IL‐9 receptor is also expressed on neutrophils and mast cells (MC), which have been involved in the pathogenesis of SSc 18, 19. We next evaluated, in vitro, the role of recombinant IL‐9 in the modulation of IL‐9R‐expressing cells isolated from the peripheral blood of SSc patients. IL‐9 stimulation of neutrophils increased the production of IL‐8 significantly and neutrophil extracellular trap pathogen‐induced cell death (NETosis) strongly, as demonstrated in Fig. 5a where the nuclei of neutrophils lose their shape, and the NETs are released as the cell membrane breaks. Stimulation of MC induces their significant expansion (Fig. 5b) and stimulation of B cells resulted in a significantly increased release of SSc‐related autoantibodies (Fig. 5c).
Figure 5.
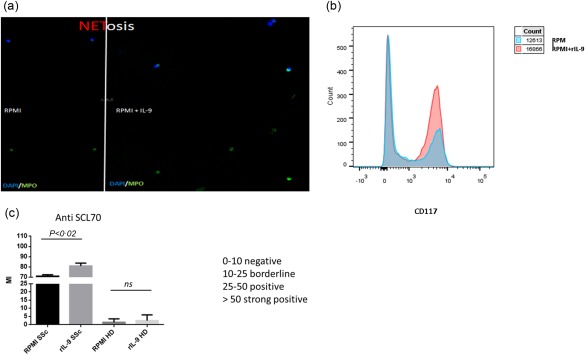
Effect of recombinant interleukin (IL)‐9 on neutrophils, mast cells and B lymphocytes. Immunofluorescences for neutrophil extracellular traps release by dying cells (Netosis): single staining for myeloperoxidase (MPO) in untreated and interleukin (IL)‐9 treated neutrophils (a). Expansion of CD117‐positive mast cells after in‐vitro culture with recombinant IL‐9 (b). Anti‐systemic scleroderma 70 (Scl70) production after in‐vitro stimulation of B lymphocytes with recombinant IL‐9 (c). [Colour figure can be viewed at wileyonlinelibrary.com]
Discussion
In this study we show, for the first time, that the IL‐9/IL‐9R axis is up‐regulated in the inflamed skin of SSc patients and is accompanied by the expansion of pathogenic Th9 cells. We also demonstrated that Th9 polarization occurs in inflamed skin and in the peripheral blood of SSc patients, being correlated directly with the modified Rodnan skin score but not with pulmonary hypertension. Similar to the skin, IL‐9 expression was also found increased in renal biopsy specimens of SSc patients with renal crisis. Finally, in‐vitro treatment with rIL‐9 was associated with a significant induction of NETosis in neutrophils, significantly increased production of SSc‐related autoantibodies by B lymphocytes and the expansion of MC.
IL‐9 is a recently described proinflammatory cytokine because of its capacity to support proliferation of B and T cell infiltration 20. In combination with IL‐17, IL‐9 increases the accumulation of neutrophils and perpetuates chronic inflammation 21, as observed in several autoimmune disorders such as rheumatoid arthritis (RA), psoriatic arthritis and giant cell arteritis (GCA) 22. IL‐9 production was associated first with the Th2 phenotype, even if other T helper subsets appear to have the potential for IL‐9 production such as Th17 cells and regulatory T cells (Treg) 23, 24. More recently, it has been demonstrated that Th0 can differentiate into a specialized subset of IL‐9‐producing T cells, named Th9. Th9 differentiation and IL‐9 expression is enhanced by IL‐4, TGF‐β and TSLP 5, 6, 25 and induced by the activation of the ETS‐family transcription factor PU.1 7, 26. Increased serum levels of IL‐9 have been found recently in SSc, suggesting that the IL‐9 axis might be involved in SSc pathogenesis 10. However, no studies addressing specifically the nature of IL‐9‐producing cells and the functional consequences of IL‐9 over‐expression have yet been published.
In SSc skin, dermal fibroblasts, inflammatory infiltrating cells and endothelial cells produced mainly IL‐9. In particular, Th9 cells and, in a lesser manner, Th17 cells were the major sources of IL‐9 in SSc skin. Th9 cells seem to be involved in the immunopathology of different systemic rheumatic diseases in which this axis is activated and involved potentially in the triggering and/or maintaining inflammation. The differentiation of Th9 cells requires the activation of IL‐2/signal tranducer and activation of transcription‐5 (STAT‐5) and IL‐4/STAT‐6 signalling and TGF‐β that act by inducing redirection of naive T cells from a Th2 to Th9 cell differentiation pathway 5, 27. Recently, it has been demonstrated that TSLP, IL‐25 and IL‐33 may also enhance IL‐9 production by Th9 cells 6, 9. According to the expansion of Th9 cells, TGF‐β, TSLP and IL‐4 were up‐regulated significantly in SSc skin and correlated directly with the expression of IL‐9 and the number of Th9 cells. In addition, the IL‐25/IL‐17RB pathway was also activated in SSc patients, as indicated by over‐expression of IL‐25, IL‐17RB and of the specific transcription factor TRAF6 in the skin of SSc patients. Taken together, these findings seem to point towards a strong activation of IL‐9/Th9 axis in SSc.
A mast cell–ILC2–Th9 pathway has been demonstrated to be functional in human diseases such as cystic fibrosis 28. Although ILC2 expansion has been demonstrated recently in SSc 17, no studies have been performed on the functional pathways involved in their activation. Accordingly, with IL‐9 over‐expression, we observed a significant ILC2 expansion in SSc patients that correlated significantly with the percentages of circulating Th9 cells. Interestingly, ILC2 expansion was also accompanied by the increased frequencies of both circulating and tissue mast cells in SSc patients. Finally, isolated mast cells from the peripheral blood were expanded significantly by the addition of IL‐9 in in‐vitro experiments and produced high levels of IL‐2, indicating that a mast cell–ILC2–Th9 pathway is also functional in SSc patients.
The biological effects of IL‐9 are mediated by IL‐9R, a heterodimeric receptor composed by a α‐chain (IL‐9Rα) and a common γ‐chain receptor shared by other cytokines. According to the increased expression of IL‐9, IL‐9R was also up‐regulated significantly in the inflamed skin of SSc patients, essentially among infiltrating lymphocytes, mast cells and neutrophils. In order to study the functional relevance of the increased frequency of IL‐9R‐expressing cells in SSc, isolated neutrophils and B cells were stimulated with recombinant IL‐9. IL‐9, through interaction with the cognate IL‐9R alpha expressed on human B cells, induces B cell expansion and activation by activating STAT‐3 and STAT‐5 29. In our study, in in‐vitro studies IL‐9 was able to induce the production of SSc‐specific autoantibodies in isolated SSc B lymphocytes, indicating an important role of IL‐9/IL‐9R alpha axis in modulating autoimmunity in SSc patients 24. Stimulation of neutrophils with IL‐9 induces NETosis activation. NETs are chromatin structures loaded with anti‐microbial molecules that represent one of the first lines of defence against pathogens. In vivo, NETs are released during a form of pathogen‐induced cell death, named NETosis. NETosis activation has been demonstrated in autoimmune diseases such as RA and systemic lupus erythematosus but never studied in SSc. Here, we demonstrated that NETosis is also activated in SSc patients and it is possibly induced by IL‐9. The functional consequence of NETosis activation in SSc needs to be addressed specifically in further studies.
Taken together, our results suggest that Th9 cells and IL‐9 could play an important role in the pathogenesis of SSc by modulating adaptive and innate immune responses and the production of autoantibodies and indicate the IL‐9 pathway as a possible therapeutic target.
Disclosure
The authors have declared no conflicts of interest.
Author contributions
G. G.: study conception and design, data interpretation, literature search, figure creation, writing, paper revision and acceptance; F. C.: study conception and design, data interpretation, literature search, writing, paper revision and acceptance; M. L. P.: data collection, data interpretation, literature search, paper revision and acceptance; D. D. L.: data collection, data interpretation, literature search, paper revision and acceptance; P. C.: data collection, literature search, paper revision and acceptance; A. R.: data collection, data interpretation, literature search, paper revision and acceptance; P. R.: data collection, literature search, paper revision and acceptance; G. C.: data collection, literature search, paper revision and acceptance; C. M. G.: data collection, data interpretation, literature search, paper revision and acceptance; G. S.: data collection, data interpretation, literature search, paper revision and acceptance; F. D.: data collection, data interpretation, literature search, paper revision and acceptance; G. T.: data collection, data interpretation, literature search, paper revision and acceptance; and R. G.: study design, data interpretation, paper revision and acceptance. All authors gave final approval for submitting the manuscript for review and agree to be accountable for all aspects of the work.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Table S1. List of antibodies.
Fig. S1. Interleukin (IL)‐9 expression in the kidney of systemic sclerosis (SSc) and healthy donors (HD). IL‐9 expression in the kidney of SSc patients with renal crisis (a) and controls (b). Quantification of IL‐9‐producing cells from kidney tissue from SSc patients and controls (c). Original magnification ×250. Data are expressed as mean ± standard error of the mean (s.e.m.); *P < 0·05.
Acknowledgements
The authors thank Dr Francesca Raiata (Sezione di Anatomia Patologica, Azienda Ospedaliera Ospedali riuniti Villa Sofia Cervello, Palermo, Italy) for her technical support in immunohistochemical experiments. This work was supported by a grant from the Ministero della Università e della Ricerca Scientifica of Italy.
References
- 1. Fuschiotti P. Current perspectives on the immunopathogenesis of systemic sclerosis. Immunotargets Ther 2016; 5:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brembilla NC, Chizzolini C. T cell abnormalities in systemic sclerosis with a focus on Th17 cells. Eur Cytokine Netw 2012; 23:128–39. [DOI] [PubMed] [Google Scholar]
- 3. Jiang S, Wang Z, Ouyang H, Liu Z, Li L, Shi Y. Aberrant expression of cytokine interleukin 9 along with interleukin 4 and interferon gamma in connective tissue disease‐associated interstitial lung disease: association with severity of pulmonary fibrosis. Arch Med Sci 2016; 12:101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Qin SY, Lu DH, Guo XY et al A deleterious role for Th9/IL‐9 in hepatic fibrogenesis. Sci Rep 2016; 6:18694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaplan MH, Hufford MM, Olson MR. The development and in vivo function of T helper 9 cells. Nat Rev Immunol 2015; 15:295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yao W, Zhang Y, Jabeen R et al Interleukin‐9 is required for allergic airway inflammation mediated by the cytokine TSLP. Immunity 2013; 38:360–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ramming A, Druzd D, Leipe J, Schulze‐Koops H, Skapenko A. Maturation‐related histone modifications in the PU.1 promoter regulate Th9‐cell development. Blood 2012; 119:4665–74. [DOI] [PubMed] [Google Scholar]
- 8. Angkasekwinai P, Srimanote P, Wang YH et al Interleukin‐25 (IL‐25) promotes efficient protective immunity against Trichinella spiralis infection by enhancing the antigen‐specific IL‐9 response. Infect Immun 2013; 81:3731–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Angkasekwinai P, Chang SH, Thapa M, Watarai H, Dong C. Regulation of IL‐9 expression by IL‐25 signaling. Nat Immunol 2010; 11:250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yanaba K, Yoshizaki A, Asano Y, Kadono T, Sato S. Serum interleukin 9 levels are increased in patients with systemic sclerosis: association with lower frequency and severity of pulmonary fibrosis. J Rheumatol 2011; 38:2193–7. [DOI] [PubMed] [Google Scholar]
- 11. Lonati PA, Brembilla NC, Montanari E et al High IL‐17E and low IL‐17C dermal expression identifies a fibrosis‐specific motif common to morphea and systemic sclerosis. PLOS ONE 2014; 9:e105008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Christmann RB, Mathes A, Affandi AJ et al Thymic stromal lymphopoietin is up‐regulated in the skin of patients with systemic sclerosis and induces profibrotic genes and intracellular signaling that overlap with those induced by interleukin‐13 and transforming growth factor beta. Arthritis Rheum 2013; 65:1335–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van den Hoogen F, Khanna D, Fransen J et al 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis 2013; 72:1747–55. [DOI] [PubMed] [Google Scholar]
- 14. Furst DE, Clements PJ, Steen VD et al The modified Rodnan skin score is an accurate reflection of skin biopsy thickness in systemic sclerosis. J Rheumatol 1998; 25:84–8. [PubMed] [Google Scholar]
- 15. Denton CP, Cailes JB, Phillips GD, Wells AU, Black CM, Bois RM. Comparison of Doppler echocardiography and right heart catheterization to assess pulmonary hypertension in systemic sclerosis. Br J Rheumatol 1997; 36:239–43. [DOI] [PubMed] [Google Scholar]
- 16. Turner JE, Morrison PJ, Wilhelm C et al IL‐9‐mediated survival of type 2 innate lymphoid cells promotes damage control in helminth‐induced lung inflammation. J Exp Med 2013; 210:2951–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wohlfahrt T, Usherenko S, Englbrecht M et al Type 2 innate lymphoid cell counts are increased in patients with systemic sclerosis and correlate with the extent of fibrosis. Ann Rheum Dis 2016; 75:623–6. [DOI] [PubMed] [Google Scholar]
- 18. Czirjak L, Danko K, Sipka S, Zeher M, Szegedi G. Polymorphonuclear neutrophil function in systemic sclerosis. Ann Rheum Dis 1987; 46:302–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yukawa S, Yamaoka K, Sawamukai N, Shimajiri S, Saito K, Tanaka Y. Involvement of mast cells in systemic sclerosis. Nihon Rinsho Meneki Gakkai Kaishi 2010; 33:81–6. [DOI] [PubMed] [Google Scholar]
- 20. Lv X, Wang X. The role of interleukin‐9 in lymphoma. Leuk Lymphoma 2013; 54:1367–72. [DOI] [PubMed] [Google Scholar]
- 21. Neurath MF, Finotto S. IL‐9 signaling as key driver of chronic inflammation in mucosal immunity. Cytokine Growth Factor Rev 2016; 29:93–9. [DOI] [PubMed] [Google Scholar]
- 22. Ciccia F, Guggino G, Ferrante A, Cipriani P, Giacomelli R, Triolo G. Interleukin‐9 and T helper type 9 cells in rheumatic diseases. Clin Exp Immunol 2016; 185:125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Singh TP, Schön MP, Wallbrecht K, Gruber‐Wackernagel A, Wang XJ, Wolf P. Involvement of IL‐9 in Th17‐associated inflammation and angiogenesis of psoriasis. PLOS ONE 2013; 8:e51752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eller K, Wolf D, Huber JM et al IL‐9 production by regulatory T cells recruits mast cells that are essential for regulatory T cell‐induced immune suppression. J Immunol 2011; 186:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goswami R, Kaplan MH. A brief history of IL‐9. J Immunol 2011; 186:3283–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bauer JH, Liu KD, You Y, Lai SY, Goldsmith MA. Heteromerization of the gammac chain with the interleukin‐9 receptor alpha subunit leads to STAT activation and prevention of apoptosis. J Biol Chem 1998; 273:9255–60. [DOI] [PubMed] [Google Scholar]
- 27. Zhao P, Xiao X, Ghobrial RM, Li XC. IL‐9 and Th9 cells: progress and challenges. Int Immunol 2013; 25:547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moretti S, Renga G, Oikonomou V et al A mast cell‐ILC2‐Th9 pathway promotes lung inflammation in cystic fibrosis. Nat Commun 2017; 8:14017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fawaz LM, Sharif‐Askari E, Hajoui O, Soussi‐Gounni A, Hamid Q, Mazer BD. Expression of IL‐9 receptor alpha chain on human germinal center B cells modulates IgE secretion. J Allergy Clin Immunol 2007; 120:1208–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Table S1. List of antibodies.
Fig. S1. Interleukin (IL)‐9 expression in the kidney of systemic sclerosis (SSc) and healthy donors (HD). IL‐9 expression in the kidney of SSc patients with renal crisis (a) and controls (b). Quantification of IL‐9‐producing cells from kidney tissue from SSc patients and controls (c). Original magnification ×250. Data are expressed as mean ± standard error of the mean (s.e.m.); *P < 0·05.


