Summary
A more complete understanding of immune‐mediated damage to the coronary arteries in children with Kawasaki disease (KD) is required for improvements in patient treatment and outcomes. We recently reported the transcriptional profile of KD coronary arteritis, and in this study sought to determine protein expression of transcriptionally up‐regulated immune genes in KD coronary arteries from the first 2 months after disease onset. We examined the coronary arteries of 12 fatal KD cases and 13 childhood controls for expression of a set of proteins whose genes were highly up‐regulated in the KD coronary artery transcriptome: allograft inflammatory factor 1 (AIF1), interleukin 18 (IL‐18), CD74, CD1c, CD20 (MS4A1), Toll‐like receptor 7 (TLR‐7) and Z‐DNA binding protein 1 (ZBP1). Immunohistochemistry and immunofluorescence studies were performed to evaluate protein expression and co‐localization, respectively. AIF1 was expressed transmurally in KD arteritis and localized to macrophages and myeloid dendritic cells. CD74, which interacts with major histocompatibility complex (MHC) class II on antigen‐presenting cells, localized to the intima‐media. CD1c, a marker of myeloid dendritic cells, was expressed in a transmural pattern, as were IL‐18 and CD20. ZBP1 and TLR‐7 were up‐regulated compared to controls, but less highly compared to the other proteins. These findings provide evidence of antigen presentation and interferon response in KD arteritis. In combination with prior studies demonstrating T lymphocyte activation, these results demonstrate the complexity of the KD arterial immune response.
Keywords: coronary aneurysm, Kawasaki Diseas, AIF1
Introduction
Kawasaki disease (KD) is an acute‐onset systemic inflammatory illness that predominantly affects infants and young children. Inflammation of the coronary arteries in KD can lead to the formation of coronary artery aneurysms, with an accompanying risk for thrombosis leading to myocardial infarction, or stenosis leading to progressive ischaemia 1, 2. KD is now the leading cause of acquired heart disease in children in developed countries 3.
The development and discovery of improved treatments for KD, especially for high‐risk patients, has been hampered by a lack of information regarding the immune response in the target tissues of the disease, the coronary arteries 4. We described recently the transcriptional profile of KD coronary arteritis and demonstrated up‐regulation of genes associated with antigen presentation, dendritic cell (DC) function and type I interferon (IFN) response 5.
Immune protein expression is regulated typically at a transcriptional level 6. In the present study, we selected seven proteins corresponding to highly up‐regulated immune genes in the transcriptome for which externally validated antibodies were available and excluded proteins known to be up‐regulated, such as CD3, CD4 and CD8, and determined their expression in KD coronary artery tissues. The long‐term goal of these studies was to inform our understanding of inflammatory mediators up‐regulated in KD coronary arteritis.
Materials and methods
Subjects
We evaluated the coronary arteries of 12 patients with fatal KD (ages 3·5–11 months, median age 5 months) who died 2–8 weeks after illness onset and 13 control patients who died of non‐KD illness (ages 2 days–4 years, median age 5 months). Demographic and clinical data available in de‐identified autopsy reports for cases and controls are presented in Tables 1 and 2. Cases 1–3, 8, 11 and 12 were included in our recently published transcriptome study 5. Childhood control coronary arteries had normal histology. Coronary artery tissues were formalin‐fixed paraffin‐embedded (FFPE) and sectioned onto glass slides. This study was approved by the Institutional Review Board of the Ann and Robert H. Lurie Children's Hospital of Chicago.
Table 1.
Demographic and clinical data for Kawasaki disease cases
| Case no. | Age (months) | Duration of illness at death (weeks) | Sex (M/F) | Ethnicity | Treatment | Cause of death |
|---|---|---|---|---|---|---|
| 1 | 3·5 | 3·5 | M | C | IVIG, ASA, steroid | Small intestine infarction, mesenteric artery aneurysm |
| 2 | 4 | 5 | M | U | IVIG, ASA, steroid | Myocardial infarction |
| 3 | 4 | 3 | M | C | IVIG, ASA | Myocardial infarction |
| 4 | 4 | 4 | M | H | None | Myocardial infarction |
| 5 | 4 | 4 | M | C | IVIG, steroid, cyclophosphamide, infliximab, anakinra | Myocardial infarction |
| 6 | 4 | 3·5 | M | C | None | Myocardial infarction |
| 7 | 4 | 8 | F | C | IVIG, ASA, steroid, methotrexate | Myocardial infarction |
| 8 | 4·5 | 4 | M | H | IVIG, ASA, infliximab, steroid | Ruptured right common iliac artery aneurysm |
| 9 | 6 | 6·5 | M | A | IVIG, ASA, steroid, cyclophosphamide | Myocardial infarction |
| 10 | 6 | 4·5 | M | C | Steroid | Myocardial infarction |
| 11 | 10 | 4 | F | AA | ASA, dipyridamole | Myocardial infarction |
| 12 | 11 | 2·5 | M | C | None | Ruptured coronary artery aneurysm |
A = Asian; AA = African American; C = Caucasian; H = Hispanic; U = unknown; IVIG = intravenous immunoglobulin; ASA = acetylsalicylic acid.
Table 2.
Demographic and clinical data for childhood controls
| Control no. | Age (months) | Sex (M/F) | Cause of death |
|---|---|---|---|
| 1 | 0 | M | Congenital diaphragmatic hernia |
| 2 | 1 | F | Undetermined |
| 3 | 1 | M | Undetermined |
| 4 | 1 | M | Undetermined |
| 5 | 1 | M | Congenital myopathy, subdural and liver haematomas |
| 6 | 3 | M | Undetermined |
| 7 | 5 | M | Meningitis, disseminated intravascular coagulation |
| 8 | 5 | M | Cholestasis, renal tubular acidosis |
| 9 | 10 | M | Unknown natural causes |
| 10 | 10 | M | Unknown natural causes |
| 11 | 11 | M | Hypoplastic left heart syndrome, respiratory syncytial virus infection |
| 12 | 24 | M | Liver failure, Alpers syndrome |
| 13 | 48 | F | Small bowel obstruction, pneumonia |
Antibodies
Transcriptome results from our published study were used for selection of proteins for analysis 5. We sorted genes from the highest to the lowest degree of up‐regulation. We selected highly up‐regulated genes whose proteins could be examined using antibodies validated by The Human Protein Atlas (www.proteinatlas.org) or Antibodypedia (www.antibodypedia.com) 7. We excluded any proteins that had been demonstrated previously to be up‐regulated at the tissue level in KD coronary arteritis, such as CD3, CD4 and CD8. Polyclonal rabbit anti‐human prestige antibodies (Sigma‐Aldrich, St Louis, MO, USA) directed against allograft inflammatory factor 1 (AIF1), interleukin (IL)‐18, CD74, CD20, Toll‐like receptor 7 (TLR‐7) and Z‐DNA binding protein 1 (ZBP1) were chosen for analysis, as well as a mouse monoclonal anti‐human antibody to CD1c (Novus Biologicals, Littleton, CO, USA). The genes corresponding to these proteins were up‐regulated between 3·4‐ and 14·2‐fold in the KD coronary artery transcriptome 5. Mouse monoclonal antibodies against CD68, CD3 and smooth muscle actin were from Dako (Carpenteria, CA, USA). All antibodies showed excellent staining of control spleen tissue.
Immunohistochemistry
Each antibody was evaluated on 12 cases and 12 controls using immunohistochemistry. Thirteen controls were needed to have sufficient tissue to test each antibody on 12 controls. Immunohistochemical staining was performed using the avidin–biotin complex (ABC) technique.
FFPE coronary artery sections were deparaffinized and rehydrated. Heat‐induced epitope retrieval was performed using a 0·01 M sodium citrate buffer at pH 6.0 in a standard pressure cooker. Background peroxidase was blocked with Pierce stable peroxide substrate (Thermo Fisher Scientific, Waltham, MA, USA) diluted to ×1. Sections were incubated for 30 min with 3% goat blocking‐serum. Sections were then incubated with primary rabbit antibodies: anti‐AIF1 (HPA049234, 1 : 200), anti‐TLR‐7 (HPA059613, 1 : 50), anti‐CD74 (HPA010592, 1 : 250), anti‐CD20 (HPA014341, 1 : 200), anti‐IL‐18 (HPA003980, 1 : 200), anti‐ZBP1 (HPA041256, 1 : 750) and anti‐CD1c (NBP2–46123, 1 : 100). Biotinylated goat anti‐rabbit immunoglobulin (Ig)G antibody (Vector Laboratories, Burlingame, CA, USA) was used for the secondary antibody incubation except for CD1c, in which case biotinylated goat anti‐mouse IgG antibody was used. Immunoperoxidase detection was performed using the Vectastain Elite ABC Kit (Vector Laboratories) and 3,3α‐diaminobenzidine horseradish peroxidase (Vector Laboratories). Counterstaining was accomplished with Hematoxylin QS (Vector Laboratories).
Immunofluorescence
Multiple immunofluorescent labelling was performed following antigen retrieval using the same primary antibodies as above for AIF1 (1 : 20) and CD74 (1 : 10), with goat anti‐rabbit Alexa Fluor 488 as the secondary antibody (Invitrogen A11034, 1 : 300; Invitrogen, Carlsbad, CA, USA). Mouse anti‐human primary antibodies against CD68 (M087629 1 : 10), CD3 (M725429, 1 : 10), CD1c (NBP2–46123, 1 : 10) and smooth muscle actin (M085129, 1 : 50) were used with goat anti‐mouse Alexa Fluor 568 as the secondary antibody (Invitrogen A11031, 1 : 300). ProLong Diamond Antifade Mountant with 4',6‐diamidino‐2‐phenylindole (DAPI) (Thermo Fisher Scientific) was used for mounting and nuclear staining.
Statistical analysis
Two investigators (S.C. and A.R.) reviewed the immunohistochemical staining results independently and discrepant interpretations were resolved by a third investigator (S.W., a pathologist). Blinding was not possible due to the histological differences in case specimens versus controls. Specimens were graded based on the amount of staining present (none, rare, focal or diffuse), with separate grading for the intima‐media and adventitia. All KD cases demonstrated loss of the intima‐media border, precluding individual assessment of intima versus media. Results were compared between the KD case group and the control group for the intima‐media and the adventitial layers individually using Wilcoxon's rank‐sum test and the following data values: no expression = 0, rare = 1, focal = 2 and diffuse = 3.
Results
Results of grading of protein expression and Wilcoxon's rank‐sum test (for each protein in the intima‐media and adventitial layers) are displayed in Table 3 and Supporting information, Table S1.
Table 3.
Immunohistochemistry results for cases and controls
| Intima/media | Adventitia | |||||
|---|---|---|---|---|---|---|
| Case | Control | P | Case | Control | P | |
| AIF1 | <0·002 | < 0·05 | ||||
| None | 0 | 11 | 0 | 0 | ||
| Rare | 0 | 1 | 0 | 7 | ||
| Focal | 0 | 0 | 0 | 1 | ||
| Diffuse | 12 | 0 | 12 | 4 | ||
| IL‐18 | <0·002 | < 0·002 | ||||
| None | 3 | 12 | 1 | 11 | ||
| Rare | 0 | 0 | 0 | 1 | ||
| Focal | 2 | 0 | 2 | 0 | ||
| Diffuse | 7 | 0 | 9 | 0 | ||
| CD20 | <0·002 | < 0·002 | ||||
| None | 0 | 10 | 0 | 10 | ||
| Rare | 2 | 2 | 1 | 0 | ||
| Focal | 6 | 0 | 4 | 2 | ||
| Diffuse | 4 | 0 | 7 | 0 | ||
| TLR‐7 | <0·01 | < 0·002 | ||||
| None | 4 | 12 | 2 | 12 | ||
| Rare | 4 | 0 | 4 | 0 | ||
| Focal | 4 | 0 | 1 | 0 | ||
| Diffuse | 0 | 0 | 5 | 0 | ||
| ZBP1 | <0·01 | < 0·002 | ||||
| None | 4 | 12 | 2 | 8 | ||
| Rare | 3 | 0 | 3 | 3 | ||
| Focal | 2 | 0 | 2 | 0 | ||
| Diffuse | 3 | 0 | 4 | 0 | ||
| CD74 | <0·002 | n.s. | ||||
| None | 0 | 12 | 0 | 0 | ||
| Rare | 0 | 0 | 0 | 2 | ||
| Focal | 0 | 0 | 0 | 2 | ||
| Diffuse | 12 | 0 | 12 | 8 | ||
| CD1c | <0·002 | < 0·01 | ||||
| None | 2 | 11 | 1 | 6 | ||
| Rare | 3 | 1 | 4 | 6 | ||
| Focal | 3 | 0 | 2 | 0 | ||
| Diffuse | 4 | 0 | 5 | 0 | ||
AIF1 = allograft inflammatory factor 1; IL = interleukin; ZBP1 = Z‐DNA binding protein 1; TLR = Toll‐like receptor.
AIF1, an actin‐binding protein induced by IFN, was expressed highly in a transmural distribution in KD coronary arteries in antigen‐presenting cells. AIF1 was up‐regulated 5·5‐fold in the KD coronary artery transcriptome 5. We identified diffuse transmural expression of AIF1 protein in all 12 KD coronary artery specimens (Fig. 1). AIF1‐positive cells co‐localized with macrophage marker CD68 and with myeloid DC marker CD1c (Fig. 2). AIF1‐positive cells did not co‐localize with T lymphocytes (CD3) or smooth muscle actin (data not shown).
Figure 1.
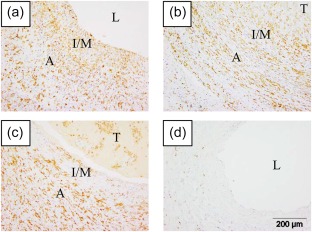
Allograft inflammatory factor 1 (AIF1) is highly expressed in Kawasaki disease (KD) coronary arteries. Three representative KD cases [(a) KD 12; (b) KD 2; (c) KD 3], and a representative control coronary artery [(d) control 12]. Immunohistochemistry, positive cells are brown. A = adventitia; I/M = intima/media; L = lumen; T = thrombus. [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 2.
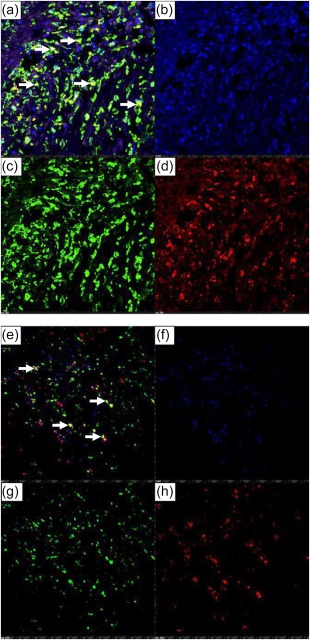
Immunofluorescence demonstrates co‐localization of allograft inflammatory factor 1 (AIF1) with CD68 and CD1c in Kawasaki disease (KD) arteritis. (a–d) AIF1 and CD68. (a) Overlay image, yellow colour (arrows) indicates co‐localization of AIF1 (green)‐ and CD68 (red)‐expressing cells; (b) nuclei stain blue [4,6‐diamidino‐2‐phenylindole (DAPI)]; (c) AIF1‐expressing cells (green); (d) CD68‐expressing cells (red). KD 3, ×10 objective with digital magnification. (e–h) AIF1 and CD1c. (e) Overlay image, yellow colour (arrows) indicates co‐localization of AIF1 (green)‐ and CD1c (red)‐expressing cells; (f) nuclei stain blue (DAPI); (g) AIF1‐expressing cells (green); (h) CD1c‐expressing cells (red). KD 3, ×10 objective. [Colour figure can be viewed at wileyonlinelibrary.com]
CD74, a chaperone that associates with major histocompatibility complex (MHC) class II to regulate antigen presentation for immune response, was up‐regulated significantly in the media of KD coronary arteries compared to controls and localizes to macrophages. CD74 was up‐regulated 7·7‐fold in the KD coronary artery transcriptome 5. CD74 protein was expressed diffusely in both the media and adventitia of all 12 cases, whereas there was no expression of CD74 in the media of any of the 12 control coronary arteries (Fig. 3). CD74 co‐localized with CD68 but not with CD3 or smooth muscle actin by immunofluorescence (Supporting information, Fig. S1).
Figure 3.
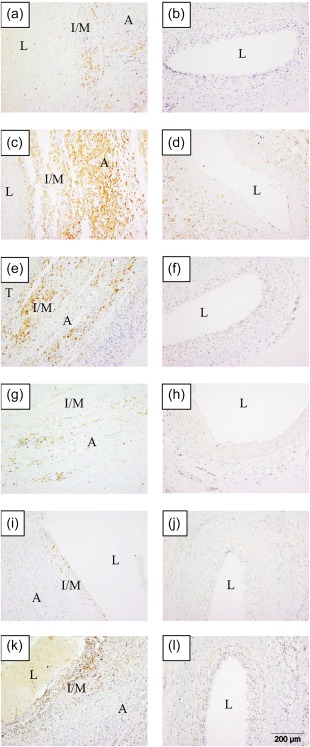
CD20, CD74, interleukin (IL)‐18, Toll‐like receptor (TLR)‐7, Z‐DNA binding protein 1 (ZBP1) and CD1c are expressed in Kawasaki disease (KD) arteritis. Cases in left column; controls in right column. (a,b) CD20 [(a) KD 2, (b) control 2)]; [(c,d) CD74 (c) KD 10, (d) control 13)]; [(e,f) interleukin (IL)‐18, (e) KD 10, (f) control 2)]; [(g,h) TLR‐7, (g) KD 9, (h) control 12)]; [(i,j) ZBP1, (i) KD 12, (j) control 2)]; [(k,l) CD1c, (k) KD 3, (l) control 2). A = adventitia; I/M = intima/media; L = lumen; T = thrombus. [Colour figure can be viewed at wileyonlinelibrary.com]
CD1c, a marker of myeloid DCs, was up‐regulated highly in a transmural pattern in KD coronary arteries. CD1c was up‐regulated 3·9‐fold in the KD coronary artery transcriptome 5. CD1c was expressed highly in a transmural pattern in 10 of 12 cases. CD1c was expressed rarely in the controls and localized to the adventitia when present.
IL‐18, a proinflammatory cytokine produced by macrophages that can stimulate MHC class II molecule expression via IFN‐γ production in T lymphocytes, is expressed highly in a transmural pattern in KD coronary arteries (Fig. 3). IL‐18 was up‐regulated 3·4‐fold in the KD coronary artery transcriptome 5. IL‐18 protein was expressed in a transmural pattern in 9 KD cases and in the adventitia in 2 KD cases. IL‐18 was virtually absent from the control coronary arteries.
CD20, ZBP1 and TLR‐7 proteins were also up‐regulated in KD arteritis. MS4A1 (the gene for CD20), ZBP1 and TLR‐7 were up‐regulated 14·2‐, 5·6‐ and 11·1‐fold, respectively, in the KD coronary artery transcriptome 5. Significantly increased transmural expression of CD20 was observed in all 12 KD cases compared with control coronary arteries (Fig. 3). Although ZBP1 protein was up‐regulated significantly in KD coronary arteries compared to controls, there were few positive cells overall (Fig. 3). ZBP1‐positive cells were located primarily in small clusters near the luminal surface, with rare cells distributed throughout the adventitia. TLR‐7 protein was expressed in a transmural pattern in 8 of 12 KD coronary arteries, but was expressed predominantly in the adventitia (Fig. 3). There was no expression of TLR‐7 in any of the control coronary arteries.
Discussion
Because arterial tissues from fatal KD cases are scarce, there has been little information about immune protein expression in KD coronary arteries. In an early immunological study of a single fatal case of KD, the mononuclear infiltrate in the coronary arteries consisted mainly of macrophages and T lymphocytes with expression of the class II major histocompatibility antigen human leucocyte antigen D‐related (HLA‐DR) 8. We have demonstrated previously the infiltration of CD8‐ and CD4‐positive T lymphocytes and CD68‐positive macrophages in KD coronary artery aneurysms 9. It was demonstrated later that activated myeloid DCs co‐localize with T cells in KD coronary artery tissues 10. Our recent report of the transcriptome of KD arteritis demonstrated marked T lymphocyte activation and also showed transcriptional up‐regulation of genes associated with antigen presentation, DC function and type I IFN response 5. In the present study, we demonstrate the expression of immune protein expression by antigen‐presenting cells (DCs, macrophages and B cells) and proteins related to type I IFN response (TLR‐7, ZBP1).
CD74 is the cell surface form of invariant chain (Ii), which is involved in antigen‐presentation by MHC class II molecules, 11, 12 and was up‐regulated highly in the KD coronary arteries examined. CD74 is expressed in antigen‐presenting cells and production is stimulated by IFN‐γ 13, 14. IL‐18 is a proinflammatory cytokine that stimulates T helper type 1 (Th1) cells and other immune cell types to produce IFN‐γ, which can induce MHC class II expression 15. Furthermore, serum levels of IL‐18 are elevated in KD and have been correlated with the development of coronary artery abnormalities in two prior studies 16, 17. The proinflammatory cytokine IL‐1 was not evaluated in this study, as it was not up‐regulated transcriptionally in KD arterial tissues 5.
TLR‐7 and ZBP1 recognize pathogen‐associated molecular patterns and both are up‐regulated in KD coronary arteritis. TLRs are part of the first line of immune defence against microbes. TLR‐7 activates the production of IFN‐α in response to single‐stranded RNA 18. ZBP1 stimulates type I IFN response, and has been implicated in immune response to viruses such as the herpesviruses 19.
The expression of CD20 is vital for the maturation of B cells to plasma cells, and plasma cells are an important component of the acute KD arterial tissue infiltrate 20, 21. CD20 is expressed by B lymphocytes and follicular DCs. The oligoclonal IgA plasma cell response that we identified previously in KD is consistent with an antigen‐driven response driving an effector IgA plasma cell infiltrate 22.
AIF1, known to be produced by macrophages, was cloned originally from a rat model of chronic cardiac allograft rejection 23. AIF1 expression may promote vascular smooth muscle cell proliferation for the healing of damaged vessels, but also correlates with the development of coronary allograft vasculopathy (CAV) in chronic rejection of transplanted hearts, which is of interest given the pathological similarities between this process and luminal myofibroblastic proliferation in chronic KD 2, 24. We have demonstrated recently the expression of AIF1 in chronic KD arteritis tissues greater than 5 months after illness onset, and also demonstrated that AIF1 is required for antigen‐specific T lymphocyte activation 25. Thus, AIF1 may provide a link between T lymphocyte activation and antigen presentation in KD arteritis.
We suspect that the efficacy of combining steroid therapy with IVIG in high‐risk KD patients in the RAISE trial 26 may relate to the action of steroid therapy in broadly down‐modulating immune responses, at least when administered with IVIG, which is a highly effective therapy for KD 4. The coronary artery protein expression data reported here complement our report of the immune transcriptome of KD arteritis and demonstrate the complexity of the immune response in KD arteritis.
Disclosure
The authors have no financial or commercial conflicts of interest to disclose.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. CD74 co‐localizes with CD68 in Kawasaki disease (KD) arteritis. (a) Overlay image, yellow colour (arrows) indicates co‐localization of CD74 (green) and CD68 (red)‐expressing cells; (b) Nuclei stain blue [4,6‐diamidino‐2‐phenylindole (DAPI)]; (c) CD74‐expressing cells (green); (d) CD68‐expressing cells (red). Immunofluorescence, KD 3; ×10 objective.
Table S1. Individual immunohistochemical staining results for Kawasaki disease (KD) cases by duration of illness and controls.
Acknowledgements
Imaging work was performed at the Northwestern University Center for Advanced Microscopy, generously supported by NCI CCSG P30 CA060553 awarded to the Robert H. Lurie Comprehensive Cancer Center. Research was supported by NIAMS R21AR068041, NIAID R56AI106030, Max Goldenberg Foundation and the Center for Kawasaki Disease at the Ann & Robert H. Lurie Children's Hospital of Chicago.
References
- 1. Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi 1967; 16:178–222. [PubMed] [Google Scholar]
- 2. Orenstein JM, Shulman ST, Fox LM et al Three linked vasculopathic processes characterize Kawasaki disease: a light and transmission electron microscopic study. PLOS ONE 2012; 7:e38998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taubert KA, Rowley AH, Shulman ST. Nationwide survey of Kawasaki disease and acute rheumatic fever. J Pediatr 1991; 119:279–82. [DOI] [PubMed] [Google Scholar]
- 4. McCrindle BW, Rowley AH, Newburger JW et al Diagnosis, treatment, and long‐term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation 2017; 135:e927–99. [DOI] [PubMed] [Google Scholar]
- 5. Rowley AH, Wylie KM, Kim KY et al The transcriptional profile of coronary arteritis in Kawasaki disease. BMC Genomics 2015; 16:1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jovanovic M, Rooney MS, Mertins P et al Immunogenetics. Dynamic profiling of the protein life cycle in response to pathogens. Science 2015; 347:1259038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Uhlen M, Fagerberg L, Hallstrom BM et al Proteomics. Tissue‐based map of the human proteome. Science 2015; 347:1260419. [DOI] [PubMed] [Google Scholar]
- 8. Terai M, Kohno Y, Namba M et al Class II major histocompatibility antigen expression on coronary arterial endothelium in a patient with Kawasaki disease. Hum Pathol 1990; 21:231–4. [DOI] [PubMed] [Google Scholar]
- 9. Brown TJ, Crawford SE, Cornwall ML, Garcia F, Shulman ST, Rowley AH. CD8 T lymphocytes and macrophages infiltrate coronary artery aneurysms in acute Kawasaki disease. J Infect Dis 2001; 184:940–3. [DOI] [PubMed] [Google Scholar]
- 10. Yilmaz A, Rowley A, Schulte DJ et al Activated myeloid dendritic cells accumulate and co‐localize with CD3+ T cells in coronary artery lesions in patients with Kawasaki disease. Exp Mol Pathol 2007; 83:93–103. [DOI] [PubMed] [Google Scholar]
- 11. Stockinger B, Pessara U, Lin RH, Habicht J, Grez M, Koch N. A role of la‐associated invariant chains in antigen processing and presentation. Cell 1989; 56:683–9. [DOI] [PubMed] [Google Scholar]
- 12. Schröder B. The multifaceted roles of the invariant chain CD74 – more than just a chaperone. Biochim Biophys Acta 2016; 1863:1269–81. [DOI] [PubMed] [Google Scholar]
- 13. Momburg F, Koch N, Möller P, Moldenhauer G, Butcher G, Hämmerling G. Differential expression of Ia and Ia‐associated invariant chain in mouse tissues after in vivo treatment with IFN‐gamma. J Immunol 1986; 136:940–8. [PubMed] [Google Scholar]
- 14. Collins T, Korman AJ, Wake CT et al Immune interferon activates multiple class II major histocompatibility complex genes and the associated invariant chain gene in human endothelial cells and dermal fibroblasts. Proc Natl Acad Sci USA 1984; 81:4917–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin‐18 is a unique cytokine that stimulates both Th1 and Th2 responses depending on its cytokine milieu. Cytokine Growth Factor Rev 2001; 12:53–72. [DOI] [PubMed] [Google Scholar]
- 16. Nomura Y, Masuda K, Maeno N, Yoshinaga M, Kawano Y. Serum levels of interleukin‐18 are elevated in the subacute phase of Kawasaki syndrome. Int Arch Allergy Immunol 2004; 135:161–5. [DOI] [PubMed] [Google Scholar]
- 17. Weng KP, Hsieh KS, Huang SH et al Interleukin‐18 and coronary artery lesions in patients with Kawasaki disease. J Chin Med Assoc 2013; 76:438–45. [DOI] [PubMed] [Google Scholar]
- 18. Diebold SS, Kaisho T, Hemmi H, Akira SE, Sousa CR. Innate antiviral responses by means of TLR7‐mediated recognition of single‐stranded RNA. Science 2004; 303:1529–31. [DOI] [PubMed] [Google Scholar]
- 19. Takaoka A, Wang Z, Choi MK et al DAI (DLM‐1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 2007; 448:501–5. [DOI] [PubMed] [Google Scholar]
- 20. Rowley AH, Eckerley CA, Jack HM, Shulman ST, Baker SC. IgA plasma cells in vascular tissue of patients with Kawasaki syndrome. J Immunol 1997; 159:5946–55. [PubMed] [Google Scholar]
- 21. Kuijpers TW, Bende RJ, Baars PA et al CD20 deficiency in humans results in impaired T cell–independent antibody responses. J Clin Invest 2010; 120:214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rowley AH, Shulman ST, Garcia FL et al Cloning the arterial IgA antibody response during acute Kawasaki disease. J Immunol 2005; 175:8386–91. [DOI] [PubMed] [Google Scholar]
- 23. Utans U, Quist WC, McManus BM et al Allograft inflammatory factor‐1 – a cytokine‐responsive macrophage molecule expressed in transplanted human hearts. Transplantation 1996; 61:1387–92. [DOI] [PubMed] [Google Scholar]
- 24. Autieri MV, Kelemen S, Thomas BA, Feller ED, Goldman BI, Eisen HJ. Allograft inflammatory factor‐1 expression correlates with cardiac rejection and development of cardiac allograft vasculopathy. Circulation 2002; 106:2218–23. [DOI] [PubMed] [Google Scholar]
- 25. Rowley AH, Baker SC, Kim KA et al Allograft inflammatory factor‐1 links T‐cell activation, interferon response, and macrophage activation in chronic Kawasaki disease arteritis. J Pediatric Infect Dis Soc 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kobayashi T, Saji T, Otani T et al Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study): a randomised, open‐label, blinded‐endpoints trial. Lancet 2012; 379:1613–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. CD74 co‐localizes with CD68 in Kawasaki disease (KD) arteritis. (a) Overlay image, yellow colour (arrows) indicates co‐localization of CD74 (green) and CD68 (red)‐expressing cells; (b) Nuclei stain blue [4,6‐diamidino‐2‐phenylindole (DAPI)]; (c) CD74‐expressing cells (green); (d) CD68‐expressing cells (red). Immunofluorescence, KD 3; ×10 objective.
Table S1. Individual immunohistochemical staining results for Kawasaki disease (KD) cases by duration of illness and controls.


