Summary
Many patients with primary immunodeficiency (PID) who have antibody deficiency develop progressive lung disease due to underlying subclinical infection and inflammation. To understand how these patients are monitored we conducted a retrospective survey based on patient records of 13 PID centres across Europe, regarding the care of 1061 adult and 178 paediatric patients with PID on immunoglobulin (Ig) G replacement. The most common diagnosis was common variable immunodeficiency in adults (75%) and hypogammaglobulinaemia in children (39%). The frequency of clinic visits varied both within and between centres: every 1–12 months for adult patients and every 3–6 months for paediatric patients. Patients diagnosed with lung diseases were more likely to receive pharmaceutical therapies and received a wider range of therapies than patients without lung disease. Variation existed between centres in the frequency with which some clinical and laboratory monitoring tests are performed, including exercise tests, laboratory testing for IgG subclass levels and specific antibodies, and lung function tests such as spirometry. Some tests were carried out more frequently in adults than in children, probably due to difficulties conducting these tests in younger children. The percentage of patients seen regularly by a chest physician, or who had microbiology tests performed following chest and sinus exacerbations, also varied widely between centres. Our survey revealed a great deal of variation across Europe in how frequently patients with PID visit the clinic and how frequently some monitoring tests are carried out. These results highlight the urgent need for consensus guidelines on how to monitor lung complications in PID patients.
Keywords: antibody deficiency, lung disease, monitoring, primary immunodeficiency disease, subclinical infection
Introduction
Approximately 70–75% of patients with primary immunodeficiency disease (PID) suffer from antibody deficiency 1. Many of these patients may develop progressive lung disease as a result of underlying subclinical infection and inflammation, despite apparently adequate levels of replacement immunoglobulin (Ig)G 2, 3, 4, 5, 6. Viral and bacterial pathogens have been detected in secretions from the airways of patients with PID with persistent and/or recurrent infections 7, 8, 9, 10, 11 and from PID patients with no apparent infections at the time of testing 7. Consensus of a meeting of European Union (EU) experts (Paris, June 2013) was that subclinical infection is not monitored adequately, and wide variation may exist between centres in the methods and frequency of monitoring both for evidence of infection and development of chronic lung disease.
Currently, a number of screening measures are used in different centres to diagnose and monitor patients with PID, including lung function tests such as forced expiratory volume at 1 s (FEV1), forced vital capacity (FVC) and transfer factor for carbon monoxide (TLCO); imaging techniques such as high‐resolution computerised tomography (HRCT) and magnetic resonance imaging (MRI); and numerous other tests, including cultures from induced sputum, blood gas analysis and exercise testing. However, the frequency with which these are applied is not standardised, and there is currently a lack of local and national guidelines for screening and treating lung disease in PID.
It is expected that differences may exist between the frequency with which some clinical and laboratory monitoring tests are performed in adult and paediatric patients, due to the challenges of performing some of these tests in infants. For instance, infants can require sedation or a general anaesthetic in order for HRCT or MRI to be carried out 12, 13. In addition, lung function testing, TLCO in particular, can only be performed reliably in children aged more than 6 years 14.
To understand more clearly the current practice in how PID patients with antibody deficiency across Europe are screened and monitored in both adult and paediatric patients with different categories of lung disease, a survey was conducted to identify screening tests and their timing in different centres.
Methods
A survey exploring which screening protocols are used in the assessment of PID was conducted from September to November 2015 (Supporting information). The survey included 12 questions and was e‐mailed to 13 different centres in eight European countries: Belgium, France, Germany, Italy, the Netherlands, Spain, Sweden and the United Kingdom.
Information was requested on the number of paediatric and adult patients with antibody deficiency for six patient groups:
Paediatric/adult PID patients with no apparent lung disease
Paediatric/adult PID patients with bronchiectasis
Paediatric/adult PID patients with other lung disease, e.g. fibrosing lung disease or granulomatous and lymphocytic interstitial lung disease (GLILD) (group referred to as ‘other lung disease’)
Respondents were asked to consider only patients treated with IgG replacement therapy. PIDs were defined according to the European Society for Immunodeficiencies (ESID) criteria. Common variable immunodeficiency (CVID) was diagnosed according to the ESID/Pan‐American Group for Immunodeficiency (PAGID) criteria or revised ESID criteria 15, 16.
For each patient group described above, information was requested on how frequently patients attended the clinic for monitoring and assessment, which screening and monitoring tests were used and how often and which treatments were administered at their centre for patients with PID.
Data on how many patients received different therapies (in addition to IgG replacement therapy), including prophylactic antibiotics, steroids and other therapies such as rituximab, were collected. The proportion of patients treated with each therapy was calculated as a percentage.
Survey respondents were asked to consider how frequently they undertake different types of tests for ongoing monitoring for each group of patients. Tests were divided into five categories: clinical monitoring and assessment, laboratory monitoring, lung function, imaging and other monitoring tests. The frequency of testing was recorded as (1) scheduled regularly or at the clinic visit, (2) as required or (3) never. The proportion of patients included in each frequency group was calculated as a percentage.
A Fisher's exact test was conducted to assess whether there were any statistically significant differences regarding the frequency with which the clinical monitoring and assessment tests were conducted between the different disease groups (without lung disease, with bronchiectasis or with ‘other lung disease’) for either adult or paediatric patients. Information about which guidelines were followed for screening, treating and monitoring patients with PID, the percentage of patients seen regularly by a chest physician at their institute and the frequency of microbiology testing in patients with chest and sinus exacerbations was also collected.
Results
Patient characteristics
A total of 1239 patients with PID presenting with antibody deficiency and receiving IgG treatment were included in this survey (Table 1). The most common underlying PID diagnosis for paediatric patients was hypogammaglobulinaemia (39%), whereas the majority of adult patients had been diagnosed with CVID (75%). Of the patients included in the survey, 24% of children and 36% of adults had been diagnosed with lung disease.
Table 1.
Number of patients treated by participating physicians, with diagnoses
| Paediatric patients | Adult patients | |
|---|---|---|
| Patients and centres | ||
| Number of centres treating patients (n = 13) | 8 | 9 |
| Total number of patients treated (n = 1239) | 178 | 1061 |
| Patients with and without lung disease, number of patients (%) | ||
| Without lung disease | 133 (75) | 539 (51) |
| With bronchiectasis | 38 (21) | 358 (34) |
| With ‘other lung disease’ | 7 (4) | 164 (15) |
| Diagnoses | ||
| Diagnoses for all patients, number of patients (%) | ||
| CVID | 57 (32) | 798 (75) |
| XLA | 33 (19) | 42 (4) |
| SPAD | 13 (7) | 37 (3) |
| ARAG | 6 (3) | 4 (0.4) |
| Hypogammaglobulinaemia | 69 (39) | 180 (17) |
| Diagnoses for patients with progressive lung disease,* number of patients (%) | ||
| CVID | 18 (42) | 307 (80) |
| XLA | 7 (16) | 18 (5) |
| SPAD | 2 (5) | 9 (2) |
| ARAG | 2 (4) | 4 (1) |
| Hypogammaglobulinaemia | 14 (33) | 40 (12) |
ARAG = autosomal recessive agammaglobulinaemia; CVID = common variable immune deficiency; SPAD = selective or partial antibody deficiency; XLA = X‐linked agammaglobulinaemia. *Lung disease that worsens over time, including bronchiectasis and other lung disease.
Patients diagnosed with lung diseases were more likely to receive prophylactic antibiotics, steroids, rituximab or other pharmacological treatments than patients without lung disease. A higher percentage of patients with bronchiectasis or ‘other lung disease’ received prophylactic antibiotics compared to those without lung disease (Fig. 1). A higher percentage of clinicians treating paediatric patients reported prescribing prophylactic antibiotics compared to those treating adult patients, regardless of the presence or type of lung disease. The percentages of patients receiving prophylactic antibiotics varied widely in all categories, as some respondents stated that they treated all patients with antibiotic prophylaxis, while others never recommended this therapy for these patient categories. In addition, a higher percentage of adult patients received inhaled and/or oral steroids than paediatric patients, regardless of the presence or type of lung diseases. Steroids (inhaled and/or oral) were given most commonly to those with ‘other lung disease’ (65% of adult patients and 57% of paediatric patients), followed by patients with bronchiectasis (50% of adult patients and 32% of paediatric patients), and given to 31% of adults and 0% of paediatric patients without lung disease.
Figure 1.
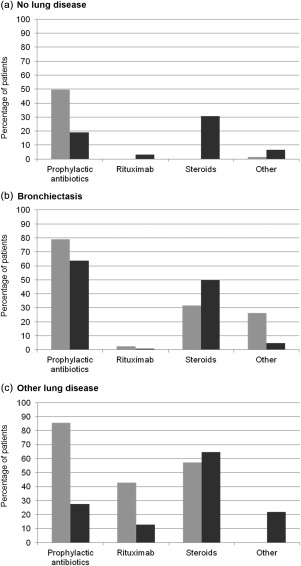
Percentage of primary immunodeficiency (PID) patients receiving prophylactic antibiotics, rituximab, steroids and other treatments, (a) without lung disease, (b) with bronchiectasis, and (c) with ‘other lung disease’.  : Adult patients
: Adult patients  : Paediatric patients.
: Paediatric patients.
Frequency of clinic visits
There was a greater variation between centres in the frequency of clinic visits for adults than paediatric patients. Clinicians saw paediatric patients routinely every 3–6 months, whereas adult patients were seen from once every month to once every 12 months. Variation also existed within centres, with some clinicians seeing patients at fixed intervals (commonly every 3 or 6 months for both adult and paediatric patients) and others seeing patients based on clinical findings; for example, one centre reviewed adult patients every 4–12 months. Clinicians commented that patients were seen more frequently if they were particularly unwell; for example, patients with cancer, chronic lung disease or malabsorption may be seen every month. For paediatric patients, respondents generally reported seeing those with bronchiectasis or ‘other lung disease’ more frequently than those with no lung disease (mean: 3·7, 3·7 and 5·2 months, respectively). For adult patients, respondents generally reported seeing those with ‘other lung disease’ (such as fibrosing lung disease or GLILD) more frequently than those with bronchiectasis and no lung disease (mean: 3·3, 4·7 and 4·9 months, respectively).
Clinical monitoring and assessment
The frequency with which clinical monitoring and assessment was carried out was similar for adult and paediatric patients for most tests, as shown in Fig. 2. In addition, no significant differences were observed between the frequencies of testing for patients without lung disease, with bronchiectasis or with ‘other lung disease’, for either adult or paediatric patients. The majority of clinical monitoring was performed regularly at clinic visits, where all respondents conducted a clinical examination and recorded frequencies of respiratory tract infection and sinusitis since the last visit. For exercise tests, fatigue assessments and quality of life assessments a particularly wide variation was seen in the frequencies of testing at different centres (Fig. 2). Exercise tests were performed less frequently in paediatric patients than adult patients (25 versus 44–63%, respectively), regardless of the presence of lung disease.
Figure 2.
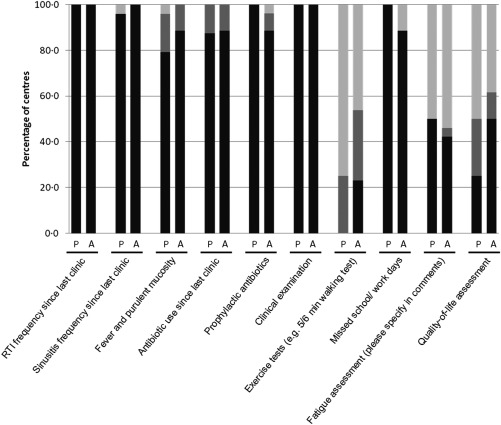
Frequency with which clinical monitoring tests are performed in patients with primary immunodeficiency (PID).  : Performed at clinic visit;
: Performed at clinic visit;  : Performed as required;
: Performed as required;  : Never performed. P = paediatric patients (eight centres); A = adult patients (nine centres).
: Never performed. P = paediatric patients (eight centres); A = adult patients (nine centres).
Laboratory monitoring tests
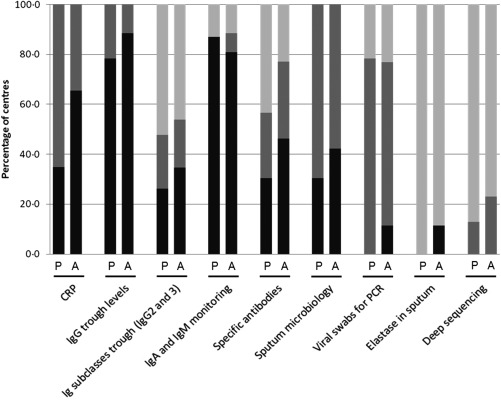
For patients without lung disease, the frequency of laboratory monitoring varied greatly between the different centres, as did the tests used (Fig. 3). However, few differences in the frequency of testing were seen in the monitoring of adult compared to paediatric patients (Fig. 3). In addition, there were no significant differences in the frequency of performing laboratory monitoring, including sputum analysis, viral swabs and deep sequencing, for either adult or paediatric patients with and without lung disease.
Figure 3.
Frequency with which laboratory monitoring tests are performed in patients with primary immunodeficiency (PID).  : Performed at clinic visit;
: Performed at clinic visit;  : Performed as required;
: Performed as required;  : Never performed. P = paediatric patients (eight centres); A = adult patients (nine centres); CRP = C‐reactive protein; PCR = polymerase chain reaction.
: Never performed. P = paediatric patients (eight centres); A = adult patients (nine centres); CRP = C‐reactive protein; PCR = polymerase chain reaction.
Testing for elastase in sputum and deep sequencing for detecting pathogens were carried out by only a minority (0–25%), regardless of the patient's age or the presence of lung disease. The frequency of testing for specific antibodies and IgG2 and IgG3 trough levels varied widely between centres. For all groups of patients at least 71% of clinicians reported that IgG trough levels and IgA and IgM levels were assessed routinely at clinic visits; this was more frequently than for monitoring IgG subclass levels, which were assessed only routinely or as required by approximately 50% of clinicians, regardless of age or the presence of lung disease. Testing for specific antibodies was conducted slightly more frequently by clinicians monitoring adult patients compared to those monitoring paediatric patients; depending on the presence of lung disease, these tests were never performed by 38–50% of centres treating children, compared to 22–25% of centres treating adults. Swabs for viral polymerase chain reaction (PCR) testing were carried out mainly as required, regardless of age or lung disease. One respondent commented that obtaining routine viral swabs for PCR testing was discontinued recently at their institute following an audit, and is now performed only when the patient is symptomatic.
Lung function tests
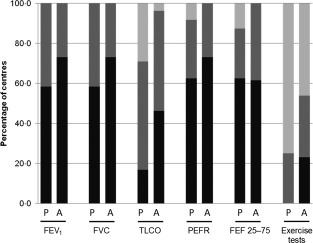
Lung function tests were carried out routinely at the majority of centres. There were generally only small variations in the frequency of testing between adult and paediatric patients (Fig. 4) and no significant differences were observed between different disease groups. However, there was a trend towards more frequent testing in adult versus paediatric patients with ‘other lung disease’. For example, FEV1 and FVC were performed at clinic visits in 50% of paediatric patients with ‘other lung disease’ compared to 78% of adults. Additionally, for both adult and paediatric patients, lung function tests were generally performed more frequently in patients with lung disease than without; for example, FEV1, FVC, peak expiratory flow rate (PEFR) and the forced expiratory flow at 25–75% of FVC (FEF 25–75) were performed routinely at clinic visits by 50–63% of respondents when treating adults with no lung disease, compared to 67–78% of respondents when treating adults with bronchiectasis or ‘other lung disease’. The same was true for the frequency of lung function tests for paediatric patients with no lung disease versus bronchiectasis. There was also no significant difference in the frequency of testing for paediatric patients without lung disease and those with ‘other lung disease’; however, this may be due to the low number of the latter patient category included in this survey (n = 10).
Figure 4.
Frequency with which lung function tests are performed in patients with primary immunodeficiency (PID).  : Performed at clinic visit;
: Performed at clinic visit;  : Performed as required;
: Performed as required;  : Never performed. P = paediatric patients (eight centres); A = adult patients (nine centres). FEV1 = forced expiratory volume at 1 s; FVC = forced vital capacity; TLCO = transfer factor for carbon monoxide; PEFR = peak expiratory flow rate; FEF 25–75 = the forced expiratory flow at 25–75% of FVC.
: Never performed. P = paediatric patients (eight centres); A = adult patients (nine centres). FEV1 = forced expiratory volume at 1 s; FVC = forced vital capacity; TLCO = transfer factor for carbon monoxide; PEFR = peak expiratory flow rate; FEF 25–75 = the forced expiratory flow at 25–75% of FVC.
TLCO was performed routinely by fewer clinicians than the other tests for both adult and paediatric patients. In adults the test was performed at routine clinic visits by 38% of clinicians for patients without lung disease, 44% for patients with bronchiectasis and 56% for patients with ‘other lung disease’. However, TLCO was performed less routinely in paediatric patients; it was performed by only 13% of clinicians treating those with no lung disease or bronchiectasis, and by 25% of clinicians when treating children with ‘other lung disease’. In addition, TLCO was never performed in paediatric patients by 25–38% of clinicians.
Imaging
Although no significant difference between disease groups was observed, there was a trend for all imaging tests to be carried out slightly more frequently in adult than paediatric patients with no lung disease (Fig. 5). Additionally, HRCT imaging was performed at least occasionally by all clinicians for paediatric patients with lung disease, whereas 25% never carried out the test in paediatric patients without lung disease. For adult patients, approximately half of all respondents performed HRCT imaging regularly and the other half performed HRCT imaging as required, regardless of the presence or type of lung disease. When asked which imaging techniques were used to assess which organs, HRCT was the predominant imaging modality used for the lungs.
Figure 5.
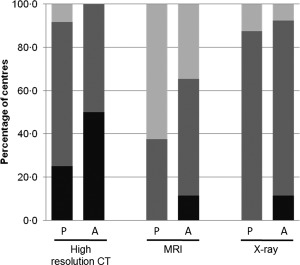
Frequency with which imaging is performed in patients with primary immunodeficiency (PID).  : Performed at clinic visit;
: Performed at clinic visit;  : Performed as required;
: Performed as required;  : Never performed. P = paediatric patients (eight centres); A = adult patients (nine centres). CT = computed tomography; MRI = magnetic resonance imaging.
: Never performed. P = paediatric patients (eight centres); A = adult patients (nine centres). CT = computed tomography; MRI = magnetic resonance imaging.
MRI was used by some respondents for imaging of the brain and central nervous system; however, this was rarely performed. In patients without lung disease, MRI was never performed by 63% of clinicians treating paediatric patients, and was performed as needed by the remainder (Fig. 5). MRI was performed more frequently in adult patients; it was performed routinely by 13% of clinicians and as needed by approximately half of respondents. Only minimal variations were seen in the frequency of MRI for patients with and without lung disease.
X‐rays of the lungs were carried out as needed by the majority of respondents, and this frequency was largely independent of disease state.
Other monitoring tests
The percentage of all PID patients seen regularly by a chest physician varied widely, with answers ranging from 30 to 100%. The percentage of chest and sinus exacerbations which did not have microbiology tests performed also varied; 69% of respondents stated that 50% or more of their patients had no microbiology tests performed.
Guidelines
Centre guidelines were followed when available. Only three of 13 respondents, from centres in Italy and Sweden, stated that they follow national guidelines; respondents from centres in the United Kingdom, Germany, Spain, Belgium and the Netherlands specifically reported a lack of national guidelines. Guidelines followed included those from the Immune Deficiency Foundation 17, Italian Primary Immunodeficiency Network 18, UK Primary Immunodeficiency Network and additional published guidelines 5, 19, 20, 21, 22.
Discussion
Our survey has revealed a great deal of variation across Europe in how frequently PID patients attend clinics and how frequently some monitoring tests are carried out in PID patients with and without lung disease, reflecting the lack of guidelines or standardised routines for respiratory monitoring in patients with PID 3.
The frequency at which patients’ quality of life should be monitored is also not standardised which was reflected in the wide range of frequencies indicated by the survey respondents. However, results from a self‐reported survey of 1526 patients with PID showed that recognising the factors that drive perceived health is key for delivering appropriate treatment to individual patients with PID 23. These findings highlight the importance of selecting the most appropriate methods and standardising quality of life and perceived health assessments in PID patients.
Variation in the frequency with which some monitoring tests were performed may be due to differences in health‐care services provided in different countries. Moreover, international guidelines are not yet available to harmonise disparities in monitoring between different countries. Also, the availability of some laboratory tests, as well as access to physiotherapy and pulmonary rehabilitation, may be influenced by expertise and funding available at different centres; these factors may also influence the frequency of clinic visits, the availability of respiratory physicians and the use of some monitoring protocols/tests.
As mentioned in the Introduction, some differences existed between the frequency with which some clinical and laboratory monitoring tests were performed in adult versus paediatric patients, which may be due to the challenges of performing tests such as MRI, HCRT and TLCO in very young children. Conversely, it should be noted that other variables, such as the rate of growth and weight gain (not investigated in this survey), form important aspects of assessment and monitoring in children but are not an issue in adults.
High‐resolution CT scan is a valuable tool for diagnosing and also monitoring, and can lead to early detection of lung abnormalities. In this study, participants were in agreement that adult patients should have a baseline HRCT, but the exact frequency of CT evaluation for follow‐up is less well defined. Most experts suggest HRCT should be performed every 2–4 years for patients with proven and potentially progressive lung abnormalities and less frequent in patients with normal lung findings, as demonstrated in the literature 5, 20, 24, 25. However, its use must carefully consider a small risk associated with the ionising radiation exposure required for CT scans 26. This consideration is particularly pertinent to children; thus, although HRCT should be considered in paediatric patients to monitor disease progression 1, it should not be used on a regular basis in children without lung disease. The cost of CT scans and whether the results will affect patient management should also be considered. The expectation that imaging studies can be used to monitor patients may be misplaced; if there is accelerated disease progression the achievable interval is too long, and other approaches are required to help optimise the timing of interventions. MRI provides an alternative to HRCT but is not yet used widely for diagnosis and monitoring of lung disorders in PID.
As expected, results from our survey suggested that there is an increase in the perceived prevalence of lung disease in adult PID patients compared to paediatric patients, which appeared to occur despite current monitoring and treatment interventions. Respondents reported that while lung disease is seen less commonly in patients less than 10 years old, this may depend upon the level of screening, and children diagnosed with PID when they were older were more likely to have already established lung disease. Differences in the prevalence of lung disease may reflect the increased duration of infection in the presence of poor antibody function and of exposure to pathogens in adults, and delayed diagnosis and/or under‐treatment. In addition, lung disease may be caused by inflammation secondary to immune dysregulation during an extended period of time 20, and co‐morbidities including sinus disease, airflow obstruction and gastro‐oesophageal reflux disease may influence infective exacerbation rates of bronchiectasis and potentially disease progression. Thus, adults are more likely to be affected than paediatric patients due to their increased duration of exposure. Improvements in the quality of care over the last 20–30 years may also have an impact upon the prevalence of lung disease in adult patients. Children with PID currently receive better care and may be less ill than those treated some time ago, who are now the adults included in this survey. Further investigation is needed to determine whether opportunities exist to improve monitoring and treatment to prevent progression.
The types of PID diagnosed also differed between adult and paediatric patients. This may be due in part to the difficulty in distinguishing between transient hypogammaglobulinaemia and CVID in some young children until the immune system is sufficiently mature, with the opportunity to reassess this over time. For patients with hypogammaglobulinaemia, subclinical infection and inflammation represent an important concern that can lead to persistent immune activation and chronic lung disease 3. Some antibody deficiency syndromes in children may resolve or improve with age and maturation of the immune system, especially transient hypogammaglobulinaemia of infancy (THI) 27; however, only a small proportion of such patients are likely to require immunoglobulin replacement. Large clinical studies are currently under way to assess the impact of microbiology and virology testing, and it is hoped that their results will inform relevant guidelines in the near future.
Our survey has highlighted a lack of local, national and European guidelines for screening and treating lung disease in PID. There is a strong need for evidence‐based consensus guidelines on how to monitor and treat patients both with no lung disease, with different types of lung disease encountered in PID with antibody deficiencies, and in different age groups – especially as emerging therapeutic modalities may be different (for example, IgG replacement therapy and antibiotics compared to corticosteroids, rituximab and other immunosuppressive therapies). Such guidelines will also probably need to accept that monitoring and treatment of any lung disease that is discovered will need to be individualised and provide guidance as to how this should be carried out. Moreover, there are no standardised protocols for the use of biomarkers to predict disease course and complications, nor to record the presence or progression of lung disease 3. In addition, there is currently no formal tool validated or accepted specifically for use in PID patients for the measurement of changes in overall health, in particular the importance of evaluating the patients’ general wellbeing, such as quality of life or fatigue assessment. The development of formal tools or scoring systems, such as the St George's respiratory questionnaire or validated quantitative scales for monitoring sinus and chest infections, could be beneficial for monitoring the progression of symptoms in patients with PID. Tools used to monitor lung disease in other settings, such as the British Thoracic Society quality standard 28, the Bronchiectasis Severity Index 29 or the FACED score 30, may have utility in the setting of PID.
Current guidelines focus mainly on diagnosis over the treatment of lung disease in PID patients. The lack of consensus on assessment, and preventive and therapeutic measures for lung disease in PID patients, may be due to factors such as small patient cohorts, variation in the criteria used for diagnosis, different aetiologies of PID and differing doses of replacement IgG. In addition, although IgG replacement therapy reduces infections in PID patients effectively (particularly severe infections such as pneumonia) 4, the effect of different doses of IgG on chronic lung disease, mucosal infections, bacterial colonisation pathogen persistence, upper airway infections (especially viral infections) and inflammation is less clear.
Some important limitations of this survey should be mentioned. First, this was not a prospective study; our report represents a snapshot of the way patients are treated across different centres at one point in time. As such, we did not assess the use of diagnostic and baseline tests, such as HRCT. Also, there is a possibility that some results could be confounded by conditions such as autoimmune cytopenia, which occur more frequently in patients with lung disease. In patients with these conditions, immunosuppressive therapy is given for reasons not related directly to lung disease, meaning that these patients are more likely to require different therapies and may be required to attend the clinic more often. In addition, co‐morbidities which influence the management of lung disease may differ between adults and children.
In conclusion, our survey has shown differences in how frequently various monitoring methods are carried out at different European centres in PID patients with or without lung disease. Results from this survey define current practice and highlight clearly the need for consensus guidelines on how to monitor and treat lung complications in patients with PID. This would allow the application of agreed standards and the use of key performance indicators to harmonise care and utilise these as outcome measures; an approach that has been used successfully in the clinical accreditation of Immunodeficiency Centres. There is also a need for standardised biomarkers and assessment tools to monitor disease progression, which would allow data to be compared between centres. It is hoped that evidence from well‐planned and controlled clinical studies will allow the development of evidence‐based guidelines for monitoring and treatment of different groups of patients with PID, which would help to ensure that all PID patients receive optimal care.
Disclosure
S. J. has received support from CSL Behring, Baxter, Biotest, Binding Site, BPL, LFB, Shire, Grifols, SOBI, UCB Pharma and Octapharma for projects, advisory boards, meetings and clinical trials. S. S.‐R. has acted on an advisory board with CSL Behring and received speaker fees from Grifols. I. Q. received payment for speaking at European and Italian congresses, funding for research from Kedrion, and grants from Shire and Octapharma for participation in advisory boards. P. S.‐P. has received grants from CSL Behring, and participated as principal investigator in clinical trials by Baxter, Octapharma and CSL Behring. B. F. has acted on advisory boards with CSL Behring and Octapharma and received support to attend scientific meetings from CSL Behring. L.‐J. C. has acted on an advisory board with CSL Behring and received lecture fees and grant support from LVL (an Air Liquide Company). N. B. has acted on advisory boards, as a speaker, or participated in projects, with CSL Behring, Baxter, Octapharma and Meda. A. J. has acted as a speaker for and received funding to attend conferences from CSL Behring and LFB Biopharmaceuticals. HL has received funding to attend conferences or other educational events, as a speaker, donations to departmental fund, financial and other assistance with patient care projects, or has participated in clinical trials, with Biotest, CSL Behring, Grifols, and Shire/Baxalta. K. W. has received grants from CSL Behring, Shire/Baxalta and Bristol‐Myers Squibb, and honoraria/consultation fees from Baxalta, Biotest, CSL Behring, Grifols, LFB, Octapharma, Pfizer, AAAAI and UCB Pharma. F. H. has acted on an advisory board with CSL Behring. AM has acted on advisory boards with CSL Behring and Baxalta. E.d.V. has received unrestricted research grants from CSL Behring, Baxalta and Sanquin.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Survey of Respiratory Monitoring Protocols in European Centres.
Acknowledgements
All authors contributed to designing the study and writing the manuscript. Financial support for the study was provided by CSL Behring. Editorial assistance was provided by Meridian HealthComms Ltd, funded by CSL Behring.
References
- 1. Jesenak M, Banovcin P, Jesenakova B, Babusikova E. Pulmonary manifestations of primary immunodeficiency disorders in children. Front Pediatr 2014; 2:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cunningham‐Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol 1999; 92:34–48. [DOI] [PubMed] [Google Scholar]
- 3. Jolles S. The variable in common variable immunodeficiency: a disease of complex phenotypes. J Allergy Clin Immunol Pract 2013; 1:545–56. [DOI] [PubMed] [Google Scholar]
- 4. Jolles S. Subclinical infection and dosing in primary immunodeficiencies. Clin Exp Immunol 2014; 178:67–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Quinti I, Soresina A, Spadaro G et al Long‐term follow‐up and outcome of a large cohort of patients with common variable immunodeficiency. J Clin Immunol 2007; 27:308–16. [DOI] [PubMed] [Google Scholar]
- 6. Resnick ES, Moshier EL, Godbold JH, Cunningham‐Rundles C. Morbidity and mortality in common variable immune deficiency over 4 decades. Blood 2012; 119:1650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kainulainen L, Nikoskelainen J, Vuorinen T, Tevola K, Liippo K, Ruuskanen O. Viruses and bacteria in bronchial samples from patients with primary hypogammaglobulinemia. Am J Respir Crit Care Med 1999; 159:1199–204. [DOI] [PubMed] [Google Scholar]
- 8. Kainulainen L, Suonpaa J, Nikoskelainen J et al Bacteria and viruses in maxillary sinuses of patients with primary hypogammaglobulinemia. Arch Otolaryngol Head Neck Surg 2007; 133:597–602. [DOI] [PubMed] [Google Scholar]
- 9. Kainulainen L, Vuorinen T, Rantakokko‐Jalava K, Osterback R, Ruuskanen O. Recurrent and persistent respiratory tract viral infections in patients with primary hypogammaglobulinemia. J Allergy Clin Immunol 2010; 126:120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duraisingham SS, Hanson S, Buckland M, Grigoriadou S, Longhurst HJ. Pseudomonas infection in antibody deficient patients. Eur J Microbiol Immunol (Bp) 2014; 4:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Duraisingham SS, Manson A, Grigoriadou S, Buckland M, Tong CY , Longhurst HJ. Immune deficiency: changing spectrum of pathogens. Clin Exp Immunol 2015; 181:267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guillerman RP. Imaging of childhood interstitial lung disease. Pediatr Allergy Immunol Pulmonol 2010; 23:43–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Odegard KC, DiNardo JA, Tsai‐Goodman B, Powell AJ, Geva T, Laussen PC. Anaesthesia considerations for cardiac MRI in infants and small children. Paediatr Anaesth 2004; 14:471–6. [DOI] [PubMed] [Google Scholar]
- 14. Seed L, Wilson D, Coates AL. Children should not be treated like little adults in the PFT lab. Respir Care 2012; 57:61–70. [DOI] [PubMed] [Google Scholar]
- 15. Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan‐American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies). Clin Immunol 1999; 93:190–7. [DOI] [PubMed] [Google Scholar]
- 16. Eijkhout HW, van den Broek PJ, van der Meer JW. Substitution therapy in immunodeficient patients with anti‐IgA antibodies or severe adverse reactions to previous immunoglobulin therapy. Neth J Med 2003; 61:213–7. [PubMed] [Google Scholar]
- 17. Immune Deficiency Foundation (IDF) . Diagnostic and clinical care guidelines for primary immunodeficiency diseases. Towson, MD: IDF, 2009. [Google Scholar]
- 18. Moschese V, Martire B, Soresina A et al Anti‐infective prophylaxis for primary immunodeficiencies: what is done in Italian Primary Immunodeficiency Network centers (IPINet) and review of the literature. J Biol Regul Homeost Agents 2013; 27:935–46. [PubMed] [Google Scholar]
- 19. Italian Primary Immunodeficiencies Strategic Scientific Committee . Common variable immunodeficiency, recommendations for diagnosis and treatment. Bologna, Italy: Centro Operativo AIEOP, 2004. [Google Scholar]
- 20. Cunningham‐Rundles C. How I treat common variable immune deficiency. Blood 2010; 116:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hernandez‐Trujillo HS, Chapel H, Lo Re V III et al Comparison of American and European practices in the management of patients with primary immunodeficiencies. Clin Exp Immunol 2012; 169:57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. O'Shaughnessy D, Hollingsworth R, Kane P, Foster M. Third National Immunoglobulin Database Report (2012). Manchester, UK: Medical Data Services and Solutions, 2013. [Google Scholar]
- 23. Seeborg FO, Seay R, Boyle M, Boyle J, Scalchunes C, Orange JS. Perceived health in patients with primary immune deficiency. J Clin Immunol 2015; 35:638–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Quinti I, Soresina A, Guerra A et al Effectiveness of immunoglobulin replacement therapy on clinical outcome in patients with primary antibody deficiencies: results from a multicenter prospective cohort study. J Clin Immunol 2011; 31:315–22. [DOI] [PubMed] [Google Scholar]
- 25. Maarschalk‐Ellerbroek LJ, de Jong PA, van Montfrans JM et al CT screening for pulmonary pathology in common variable immunodeficiency disorders and the correlation with clinical and immunological parameters. J Clin Immunol 2014; 34:642–54. [DOI] [PubMed] [Google Scholar]
- 26. Kuo W, Ciet P, Tiddens HA, Zhang W, Guillerman RP, van Straten M. Monitoring cystic fibrosis lung disease by computed tomography. Radiation risk in perspective. Am J Respir Crit Care Med 2014; 189:1328–36. [DOI] [PubMed] [Google Scholar]
- 27. Stiehm ER. The four most common pediatric immunodeficiencies. J Immunotoxicol 2008; 5:227–34. [DOI] [PubMed] [Google Scholar]
- 28. Hill AT, Routh C, Welham S. National BTS bronchiectasis audit 2012: is the quality standard being adhered to in adult secondary care? Thorax 2014; 69:292–4. [DOI] [PubMed] [Google Scholar]
- 29. Chalmers JD, Goeminne P, Aliberti S et al The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med 2014; 189:576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martinez‐Garcia MA, de Gracia J, Vendrell Relat M et al Multidimensional approach to non‐cystic fibrosis bronchiectasis: the FACED score. Eur Respir J 2014; 43:1357–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Survey of Respiratory Monitoring Protocols in European Centres.


