Summary
Interleukin 27 (IL‐27) has been identified as a potent cytokine in the differentiation of type 1 regulatory T (Tr1) cells through interactions with several key elements, including transcription factors such as aryl hydrocarbon receptor and IL‐21. Autocrine production of IL‐21 is known to be important for maintaining IL‐10 expression by Tr1 cells. Although previous studies have shown that the phosphoinositide 3‐kinase (PI3K) –Akt axis contributes to the differentiation of helper T‐cell subsets, the role of the PI3K pathway on Tr1 cell differentiation remains to be elucidated. Here, we demonstrate that suppression of the PI3K‐Akt pathway results in impairment of IL‐27‐induced Tr1 (IL‐27–Tr1) cell differentiation in vitro and in vivo. Furthermore, this suppression down‐regulates IL‐21 receptor expression by Tr1 cells, followed by suppression of IL‐10 expression by IL‐27–Tr1 cells. These results suggest that the PI3K pathway enhances IL‐10 expression by IL‐27–Tr1 cells through up‐regulation of IL‐21 receptors.
Keywords: interleukin‐21 receptor, interleukin‐27, phosphoinositide 3‐kinase, type 1 regulatory T cells
Abbreviations
- 4‐HT
4‐hydroxytamoxifen
- AhR
aryl hydrocarbon receptor
- CFSE
5‐(and‐6)‐carboxyfluorescein diacetate succinimidyl ester
- cLP
colonic lamina propria
- GSK3
glycogen synthase kinase 3
- MLN
mesenteric lymph node
- mTORC
mammalian target of rapamycin complex
- PI3K
phosphoinositide 3‐kinase
- PP
Peyer's patch
- Th
helper T cells
- Tr1
type 1 regulatory T cells
- Treg
regulatory T cells
Introduction
The immune response is known to be essential for protecting the host from a wide range of potentially pathogenic microorganisms. Furthermore, an immune‐suppressive response is crucial for preventing reactivity to self‐antigens. Interleukin‐10 (IL‐10) is a regulatory cytokine that plays an essential role in controlling inflammatory processes and autoimmune pathologies. Numerous cell types produce IL‐10, such as activated T cells, mast cells and antigen‐presenting cells, including macrophages and dendritic cells.1, 2, 3 In previous studies, mice deficient in IL‐10 developed a spontaneous inflammation in the colon, and mice with mutations in either IL‐10 or IL‐10 receptor suffered from early‐onset enterocolitis.4, 5 Among helper T (Th) cell subsets, the best understood subsets that express IL‐10 are Foxp3+ CD4+ regulatory T (Treg) cells and Foxp3– IL‐10‐producing type 1 regulatory (Tr1) cells.6
Tr1 cells have received increased attention in recent medical research due to their role in peripheral immune tolerance. Tr1 cells lack expression of Foxp3 but predominantly produce IL‐10.7 Adoptive transfer of Tr1 cells has been shown to suppress tissue inflammation, including colitis and autoimmune diseases.8 Recently, IL‐27, a heterodimeric cytokine that belongs to the IL‐6/IL‐12 family of cytokines, has been identified as a differentiation factor for Tr1 cells. Production of IL‐10 by IL‐27‐induced Tr1 cells was reported to be promoted by signalling pathway through aryl hydrocarbon receptor (AhR), c‐Maf, inducible T‐cell co‐stimulator, and IL‐21 receptor is important for the maintenance of IL‐10 production by IL‐27‐induced Tr1 cells.9, 10 A study using mice deficient for IL‐27 found a lack of Tr1 cells, an increase in Th17 cells, and development of more severe experimental autoimmune encephalitis.11
The phosphoinositide 3‐kinase (PI3K) family controls several biological functions and has emerged as a key molecular regulator in immune responses. Cytokine‐mediated signals in immune cells activate class IA PI3K, consisting of a catalytic subunit (p110α, β or δ) and a regulatory subunit (p85α, p55α, p85β or p55γ). This enzyme is also activated on antigen recognition and co‐stimulatory signalling molecules such as CD28.12, 13
Our previous study showed that deletion of p85α or inhibition of PI3K‐mammalian target of rapamycin complex 1 (mTORC1) impaired Th17 cell differentiation.14 In contrast, another study showed that the inhibition of PI3K and mTORC1 increased inducible regulatory T (iTreg) cell differentiation.15 In the context of IL‐10, we showed that the PI3K‐Akt pathway up‐regulates IL‐10 production by dendritic cells after lipopolysaccharide stimulation.16 However, the role of the PI3K pathway on IL‐10 production by Tr1 cells still remains unclear. Hence, in this study, we analysed the role of the PI3K pathway in the differentiation of Tr1 cells.
Materials and methods
Mice
Female, 8‐ to 12‐week‐old BALB/c mice were purchased from Japan SLC (Hamamatsu, Japan). Foxp3 hCD2 mice on a C57BL/6 background17 were kindly provided by S. Hori (RIKEN RCAI, Yokohama, Japan). Il10 Venus mice on a C57BL/6 background18 were kindly provided by K. Honda (Keio University, Tokyo, Japan). Il10 Venus mice were crossed with Foxp3 hCD2 mice to obtain Il10 Venus Foxp3 hCD2 mice. Akt‐mer tg mice on a C57BL/6 background19 were kindly provided by T. Nakano (Osaka University, Osaka, Japan). All animal experiments were performed in accordance with protocols approved by the Animal Care and Use Committee of Tokyo Medical and Dental University (TMDU; approval number 0170344A) and Kansai Medical University, and 8‐ to 12‐week‐old mice were used for all experiments.
Generation of IL‐10‐producing Tr1 cells in vitro
After single‐cell suspension of splenocytes was isolated, we negatively isolated CD25− CD4+ T cells. After that, CD62L+ cells were purified with anti‐phycoerythrin microbeads by magnetic separation using magnetic‐activated cell sorting (MACS) microbeads (Miltenyi Biotec Inc., San Diego, CA) according to the manufacturer's protocol; the purity of each population was confirmed by FACS to be routinely > 95%. CD62L+ and CD62L− were used as naive and memory CD4+ T cells, respectively. Each population was cultured in triplicate and stimulated for 3 days with plate‐bound αCD3ε (5 μg/ml; 2C11) and αCD28 (2 μg/ml; PV‐1) antibodies in the presence of IL‐10 (10 ng/ml; eBioscience, San Diego, CA) or IL‐27 (10 ng/ml; eBioscience). The pan‐PI3K inhibitor LY294002 (Calbiochem, San Diego, CA) was used at a final concentration of 3 μm, and IC87114 (Symansis, San Diego, CA), a specific inhibitor for class‐IA‐PI3K, was used at a final concentration of 8 μm. 4‐Hydroxytamoxifen (4‐HT) was purchased from Sigma‐Aldrich (St Louis, MO) and used at 2 μm. For the IL‐27‐induced Tr1 cells proliferation assay, naive or memory T cells were labelled with 5‐(and‐6)‐carboxyfluorescein diacetate, succinimidyl ester/CFSE (Molecular Probes, Eugene, OR) or with violet tracker (CellTrace Violet Cell Proliferation Kit; Invitrogen, Carlsbad, CA), respectively, according to the manufacturer's protocol. Both populations were then cultured together at a ratio representing the initial CD4+ CD25− T‐cell population. To investigate the involvement of IL‐21 and IL‐21 receptor, recombinant mouse IL‐21 (80 ng/ml; R&D Systems, Minneapolis, MN) or neutralizing IL‐21 monoclonal antibody (10 μg/ml; clone FFA21, eBioscience) was added when we started to differentiate Tr1 cells by IL‐27.
Antibodies
The following antibodies were used for flow cytometry: monoclonal antibodies against CD3 (145‐2C11), CD4 (RM4‐5), CD44 (IM7), CD62L (MEL‐14), interferon‐γ (XMG1.2), and IL‐10 (JES5‐16E3). All monoclonal antibodies were obtained from Affymetrix (Santa Clara, CA), eBioscience, or BD‐Pharmingen (San Diego, CA). For Western blotting analyses, anti‐pAkt (Ser473, #4058), anti‐pAkt (Thr308, #9275), anti‐Akt (#9272), anti‐pFOXO1 (Ser256, #9461), anti‐pFOXO1/3a (Thr24/32, #9464), anti‐FOXO1 (#2880), anti‐pGSK‐3α/β (Ser21/9, #9331), anti‐GSK‐3β (#9315), and anti‐p‐p70S6K (Thr421/Ser424, #9204) antibodies were purchased from Cell Signaling Technology (Danvers, MA). Anti‐GAPDH (FL‐335) and anti‐S6K1 (C‐18) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Flow cytometry
For intracellular cytokine staining, cells were stimulated for 6 hr with PMA (5 ng/ml) and ionomycin (50 μg/ml) in the presence of brefeldin A (0·5 μg/ml; Sigma‐Aldrich). Stained cells were analysed using FACSVERSE (BD Biosciences, San Jose, CA) with FACsuite software. Data were analysed using flowjo software (Tree Star, Ashland, OR). For intracellular staining for phosphorylated Akt, purified CD4+ CD25− T cells were incubated for 24 hr with IL‐27 in the presence or absence of IC87114. Cells were then fixed with BD Phosflow Lyse/Fix Buffer (BD Biosciences). After fixation, cells were made permeable with BD Phosflow Perm Buffer III (BD Biosciences), and stained for CD4 and phosphorylated Akt (T308) or Akt (S473). Antibodies were purchased from BD Pharmingen.
Western blotting
Western blotting analyses were performed as previously described.14 ECL Prime Western Blotting Detection Kits (GE Healthcare, Piscataway, NJ) were used for detection of chemiluminescence. The LAS‐4010 mini imaging system (Fuji Film, Tokyo, Japan) was used to quantify digital images.
Anti‐CD3 antibody treatment in vivo
Il10 Venus Foxp3 hCD2 mice were treated intraperitoneally with 20 μg of αCD3 (clone 2C11) or isotype‐matched control antibody, in the presence or absence of IC87114 (2 mg/kg). Mice were killed 3 days after injection, and single‐cell suspensions were prepared from spleens, mesenteric lymph nodes (MLNs), Peyer's patches (PPs) and colonic lamina propria (cLP). Single‐cell suspensions of MLN cells from mice were isolated using type I collagenase as described previously. PP and cLP cells were isolated as described previously.20, 21 Briefly, after removal of PPs from the small intestines and separation of the colonic area, the resected tissues were minced and enzymatically digested, followed by density gradient separation. The isolated cells were subjected to flow cytometry analysis. Venus (IL‐10) and hCD2 (Foxp3) expression was analysed using FACSverse.
Quantitative real‐time PCR
Total mRNA was extracted using NucleoSpin RNA II (Macherey‐Nagel, Düren, Germany). cDNA was reverse‐transcribed using Takara PrimeScript RT Master Mix (Takara Bio Inc., Shiga, Japan) according to the manufacturer's instructions. Real‐time PCR was performed using the StepOnePlus Real‐Time PCR System (Applied Biosystems, Foster City, CA) and SYBR Premix Ex Taq II (Takara Bio Inc.). Primers used were as follows: Il10 for, 5′‐GCTGGACAACATACTGCTAA‐3′; rev, 5′‐ATGCTCCTTGATTTCTGG‐3′; il21 for, 5′‐GCACATAGCTAAATGCCCTTCC‐3′; rev, 5′‐TCTCGGATCCTCAGGAATCTTC‐3′; il21r for, 5′‐TACAGTGTGAACATGTAGGGGTG‐3′; rev, 5′‐TCCCAACATGGATGTGCTAA‐3′; ahr for, 5′‐AGCATCATGAGGAACCTTGG‐3′; rev, 5′‐GGATTTCGTCCGTTATGTCG‐3′; cmaf for, 5′‐GTGCAGCAGAGACACGTCCT‐3′; rev, 5′‐CAACTAGCAAGCCCACTC‐3′.
Statistical analysis
Statistical analyses were performed by Mann–Whitney U‐test using prism 6 (GraphPad Software, La Jolla, CA). The significance threshold was set at *P < 0·05 or **P < 0·01.
Results
Generation of Tr1 cells
Although several studies have extensively explored Tr1 cells, the lack of an efficient system to differentiate and maintain Tr1 cells in vitro is a major limitation. Naive CD25− CD62Lhi CD44lo CD4+ T cells have been used to generate Tr1 cells;10, 22 however, recent evidence suggests that CD44hi Foxp3− CD4+ T cells from wild‐type mice rapidly differentiate into Tr1 cells.23 We therefore investigated Tr1 cell differentiation by adding IL‐10 or IL‐27 from different CD4+ T‐cell populations from the spleens of wild‐type mice. We sorted the CD4+ T‐cell populations into CD25−, CD25− CD62Lhi CD44lo (naive), and CD25− CD62Llo CD44hi (memory) CD4+ T cells. Expression of IL‐10 was highly induced with IL‐27 stimulation, especially in CD25− CD4+ T cells. These IL‐10‐producing Th cells did not express Foxp3 marker (data not shown). In contrast, IL‐10 expression was independently induced by IL‐27 from the memory T cells as IL‐10 was highly expressed without any cytokine stimulation and the addition of neither IL‐10 nor IL‐27 induced IL‐10 in this fraction (Fig. 1a).
Figure 1.
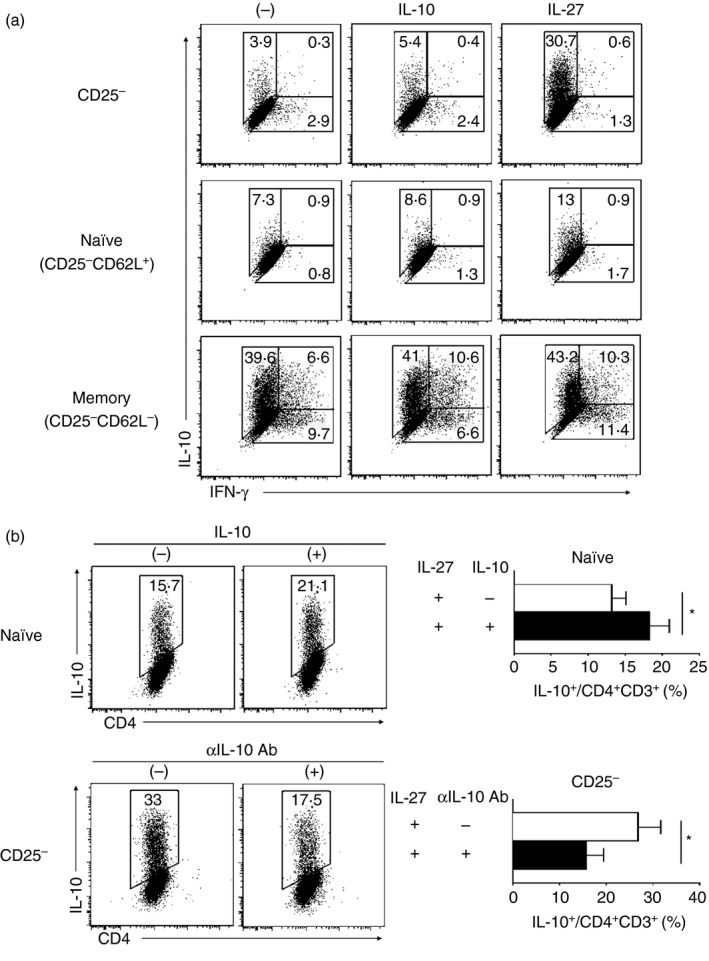
Generation of type 1 regulatory (Tr1) cells. (a) Splenocytes from BALB/c wild‐type mice were phenotypically sorted into CD25−, CD25− CD62Lhi CD44lo (naive), and CD25− CD62Llo CD44hi (memory) CD4+ T cells, and were cultured in the presence of interleukin‐10 (IL‐10) or IL‐27 cytokine. (b) In the presence of IL‐27, naive T cells were cultured with the addition of IL‐10, and CD25− CD4+ T cells were cultured with the addition of α IL‐10 neutralizing antibody. Cells were stimulated with α CD3ε and α CD28 antibody for 3 days. Multicolour fluorescence staining was performed, and the stained cells were analysed by flow cytometry. Data are representative of three independent experiments; the values in the profiles are mean ± standard deviation (SD) from three independent experiments. Significant difference: *P < 0·05.
The fact that IL‐27 augments the expression of IL‐10 in CD25− CD4+ T cells compared with naive T cells leads to the speculation that another factor besides IL‐27 affects IL‐10 expression by Tr1 cells. A previous study suggests that IL‐10 is an essential factor in Tr1 cell differentiation, as the Tr1 cell is generated by a dendritic cell subtype that secrets IL‐10.24 We found that adding IL‐10 to naive T cells enhanced IL‐10 expression by IL‐27‐induced Tr1 (IL‐27–Tr1) cells. In accordance with this result, adding a neutralizing antibody against IL‐10 to the CD25− CD4+ T‐cell population dampened IL‐10 expression by IL‐27–Tr1 cells (Fig. 1b). Therefore, in the CD25− CD4+ T‐cell population, which consists of both CD62Lhi and CD44hi populations, total IL‐10 expression by IL‐27–Tr1 cells might be influenced by IL‐10 cytokine, which is spontaneously expressed by memory T cells (Fig. 1a). Henceforth, the CD25− CD4+ T‐cell population is used for the generation of IL‐27–Tr1 cells.
PI3K activity influences IL‐10 expression by IL‐27‐induced Tr1 cells in vitro
We then examined the involvement of the PI3K pathway in Tr1 cell differentiation in vitro by adding LY294002, a pan‐PI3K inhibitor, or IC87114, a selective inhibitor for class IA subunit p110δ, to the initial culture used for IL‐27–Tr1 cell differentiation. Both inhibitors suppressed IL‐10 expression by IL‐27–Tr1 cells, and the suppressive activity of LY294002 was more effective than that of IC87114 (Fig. 2a). When we generated Tr1 cells using CD25− CD4+ T cells from splenocytes of Akt‐mer transgenic (Akt‐mer tg) mice in which a fusion protein of Akt and mutated estrogen receptor (mer) can be activated with 4‐HT, hyper‐activation of Akt by 4‐HT enhanced IL‐10 expression by Tr1 cells (Fig. 2b). These results suggested that PI3K activity was essential in IL‐27–Tr1 cell differentiation. Furthermore, the involvement of PI3K activity in Tr1 cell differentiation was essential in the early phase of generating IL‐27–Tr1 cells, because the inhibition rate gradually decreased in case of the addition of IC87114 in later phases (day 1 or day 2) (Fig. 2c).
Figure 2.
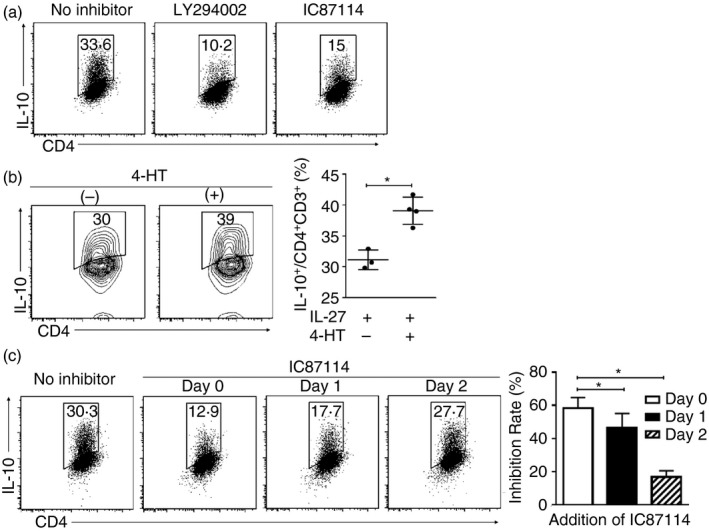
Phosphoinositide 3‐kinase (PI3K) activity influenced interleukin‐10 (IL‐10) expression from interleukin‐27‐induced type 1 regulatory T (IL‐27–Tr1) cells in vitro. (a) Pan PI3K inhibitor (LY294002) or class IA PI3K subunit 110δ inhibitor (IC87114) was added to the initial IL‐27–Tr1 cell culture. (b) Akt‐mer tg CD25− CD4+ T cells differentiated into IL‐27–Tr1 cells for 3 days with the addition of 4‐hydroxytamoxifen (4‐HT). (c) IC87114 was added at three different time‐points: day 0, day 1 or day 2 after initial culture. Data are representative of three independent experiments; the values in the profiles are mean ± standard deviation (SD) from three independent experiments. Significant difference: *P < 0·05.
To investigate whether naive or memory subsets were mainly affected by PI3K inhibition, IL‐27–Tr1 cells were induced in the presence or absence of IC87114 after labelling both subsets with different fluorescent agents and mixing both populations. The suppression of IL‐10 expression was mainly detected by the naive population in CD25− CD4+ T cells (Fig. 3). Furthermore, IL‐10 suppression by IC87114 in IL‐27–Tr1 cells was in a dose‐dependent manner (see Supplementary material, Fig. S1).
Figure 3.
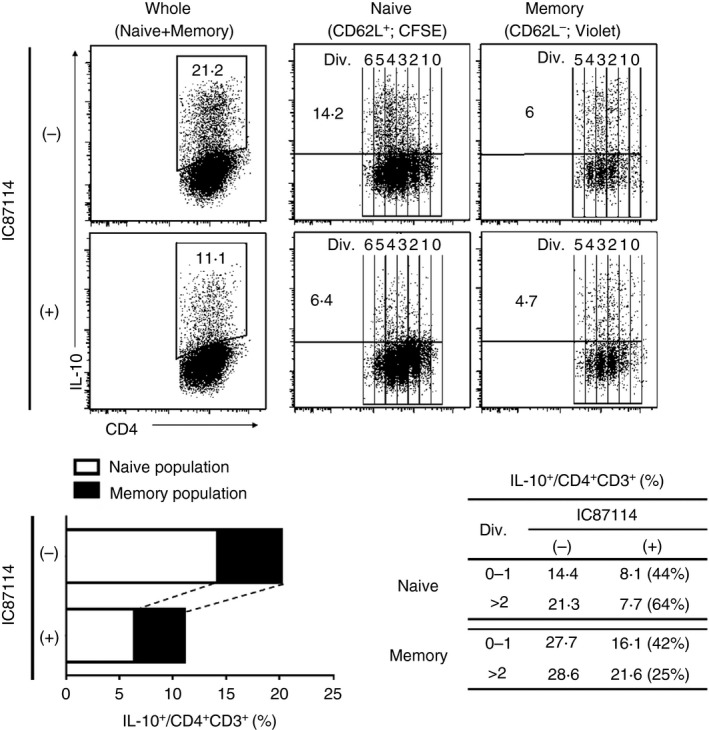
Naive population from CD25− CD4+ T cells was mainly affected by IC87114. CD25− CD4+ T cells were sorted into CD62L+ [labelled with carboxyfluorescein succinimidyl ester (CFSE)] and CD62L− (labelled with violet tracker) fractions. Both populations were then cultured together at a ratio representing the initial CD25− CD4+ T‐cell population, and differentiated into interleukin‐27‐induced type 1 regulatory T (IL‐27–Tr1) cells, with or without addition of IC87114. The table represents IL‐10 expression analysed among divided cells in both fractions, and (%) represents inhibitory rate by IC87114. Results are representative of three independent experiments.
IC87114 suppresses phosphorylation of Akt and FoxO1
T‐cell stimulation via T‐cell receptor (using αCD3 antibody) and co‐stimulation (using αCD28 antibody) induces PI3K activation followed by phosphorylation of Akt at both Ser473 and Thr308.14 We found that adding IC87114 during IL‐27‐induced Tr1 cell differentiation dampened the phosphorylation of Akt at Ser473 at 24 hr, but not Thr308 (Fig. 4a). In line with the result shown in Figure 3 that IC87114 suppression mainly affected the naive population, especially in the highly divided populations, using flow cytometric analysis, the suppression of the phosphorylation of Akt (Ser473) was mainly detected in the FSChi (highly proliferative) population (Fig. 4b).
Figure 4.
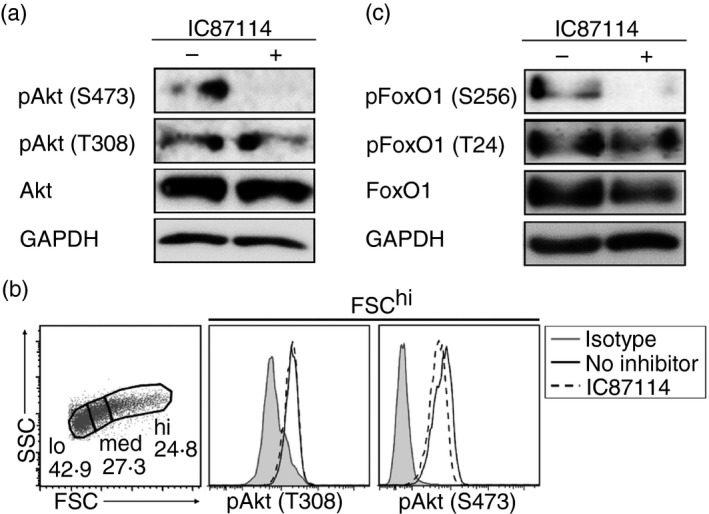
Phosphorylation of Akt and FoxO1 was suppressed by IC87114. Detection of Akt phosphorylation by (a) immunoblotting and (b) flow cytometry. (c) Detection of FoxO1 phosphorylation by immunoblotting. Interleukin‐27‐induced type 1 regulatory T (IL‐27–Tr1) cells were analysed at 24 hr after initial culture. Results are representative of three independent experiments.
To find the key molecule(s) involved in IL‐27–Tr1 cell differentiation, we investigated several molecules downstream of PI3K in the presence of IC87114 at 24 hr after initial culture. To clarify the involvement of molecules downstream of Akt, we examined glycogen synthase kinase 3 (GSK3), but no phosphorylation of GSK3 was detected (see Supplementary material, Fig. S2a). Although phosphorylation of p70S6K, a protein kinase phosphorylated by mTORC1, was detected 24 hr after IL‐27–Tr1 cell differentiation, this phosphorylation was not suppressed even in the presence of IC87114 (Fig. S2b). Our recent study demonstrated that Treg cell differentiation was down‐regulated by phosphorylation of Akt and FoxO transcription factors induced by transforming growth factor‐β.25 Hence, we assessed the involvement of FoxO1 in IL‐27–Tr1 cell differentiation via the PI3K pathway. FoxO1 (Ser256) phosphorylation was suppressed at 24 hr after IC87114 addition during IL‐27–Tr1 cell differentiation (Fig. 4c). These results suggest that the PI3K‐Akt‐FoxO1 pathway might regulate differentiation of IL‐27–Tr1 cells in vitro.
PI3K controls Tr1 cell generation in vivo
Administration of αCD3 antibody to wild‐type mice resulted in significant induction of IL‐10‐producing T cells mediated by IL‐27, as Il27ra −/− mice failed to induce IL‐10+ T cells with this treatment.9 To address the involvement of the PI3K pathway on Tr1 cell differentiation in vivo, we administered αCD3 antibody intraperitoneally to Il10 Venus Foxp3 hCD2 mice and assessed Venus+ IL‐10‐producing T cells in the spleen, MLNs, PPs and cLP at 3 days after injection. In line with our in vitro findings, IL‐10 expression by Foxp3− Tr1 cells in the spleen, MLNs (data not shown), PPs and cLP was decreased after IC87114 injection (Fig. 5a), but not IL‐10 expression by Foxp3+ Treg cells (Fig. 5b). This result suggested that the regulatory effects of the PI3K pathway on IL‐10 expression in Tr1 cells and Treg cells were different in vivo, because inhibition of the PI3K pathway resulted in attenuation of IL‐10 expression by Foxp3− Tr1 cells, but not Foxp3+ Treg cells.
Figure 5.
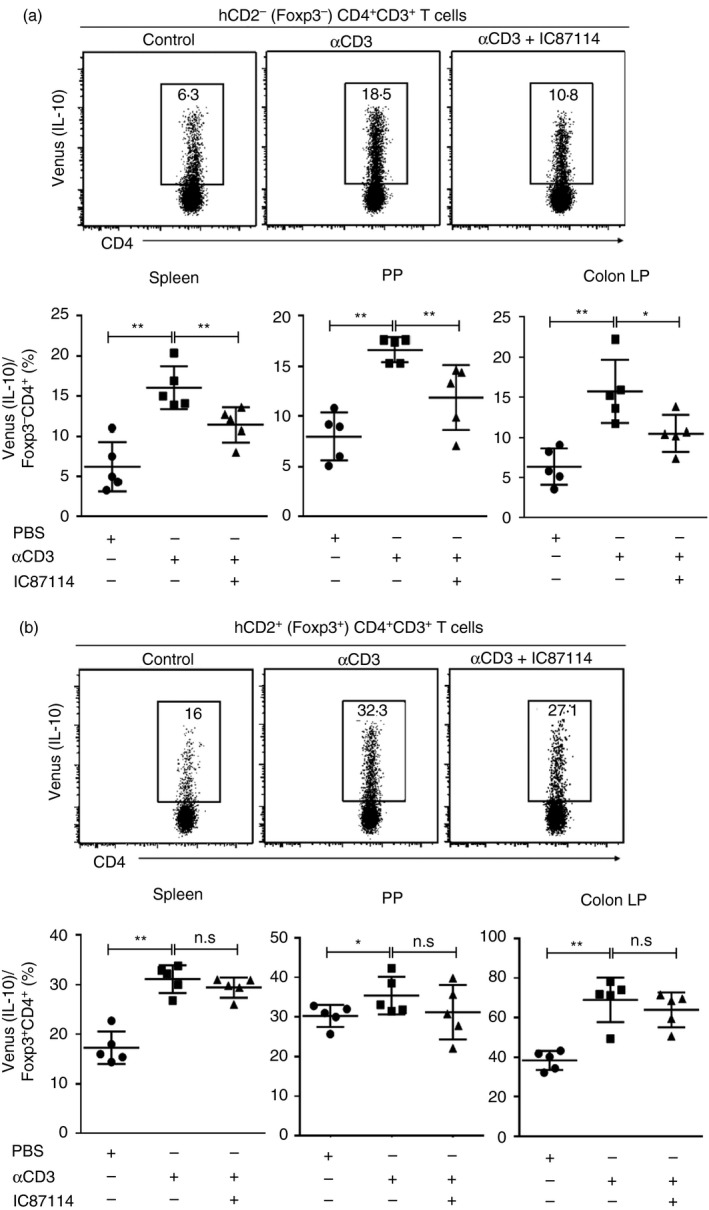
Phosphoinositide 3‐kinase (PI3K) controls type 1 regulatory T (Tr1) cell generation in vivo. Il10 Venus Foxp3 hCD2 mice were treated intraperitoneally with 20 μg of α CD3 alone or with the addition of IC87114. Three days after injection, the mice were killed and spleens, mesenteric lymph nodes (MLNs; data not shown), Peyer's patches (PPs), and colonic lamina propria (cLP) were analysed. (a) Flow cytometry analysis of IL‐10 expression in Foxp3− CD4+ cells and frequency of IL10+ Foxp3− CD4+ from spleen, PP and cLP. (b) Flow cytometry analysis of IL‐10 expression in Foxp3+ CD4+ cells and frequency of IL10+ Foxp3+ CD4+ from spleen, PP and cLP. Values shown are mean ± SD from three independent experiments (five mice per group). Significant difference: *P < 0·05, **P < 0·01.
IC87114 down‐regulates IL‐21 receptor expression
Interleukin‐27 signalling induces transcription factors such as AhR and c‐Maf, both of which together with IL‐21 are associated with Tr1 cell expansion and differentiation.10, 22 Next, we investigated IL‐10 and IL‐21 expression in Tr1 cells by real‐time PCR. Intriguingly, despite suppressing IL‐10 expression, IC87114 enhanced IL‐21 expression in IL‐27‐induced Tr1 cells (Fig. 6a). Activation of AhR and c‐Maf increased IL‐21 production in Tr1 cells,9 in line with the enhancement of IL‐21 expression by IC87114, the addition of IC87114 also enhanced AhR expression and probably c‐Maf expression by IL‐27–Tr1 cells on day 1 (Fig. 6a). In contrast, IL‐21 receptor expression was down‐regulated by IC87114 on day 1 of IL‐27–Tr1 cell differentiation (Fig. 6a). To confirm that IC87114 down‐regulates IL‐21 receptors, recombinant IL‐21 (Fig. 6b) or neutralized IL‐21 antibody (Fig. 6c) were added during IL‐27–Tr1 cell differentiation in the presence or absence of IC87114. Addition of recombinant IL‐21 enhanced IL‐10 expression by IL‐27–Tr1 cells, but not in IC87114‐treated ones (Fig. 6b). Similarly, IL‐10 expression by IL‐27–Tr1 cells was dampened by neutralizing IL‐21 antibody, but not in IC87114‐treated ones (Fig. 6c). RT‐PCR analyses of Il10 expression at day 2 of IL‐27–Tr1 cells treated with recombinant IL‐21 or neutralizing IL‐21 antibody showed a similar result (see Supplementary material, Fig. S3a,b). Together, our results indicate that inhibition of PI3K activity down‐regulates IL‐21 receptor expression in IL‐27–Tr1 cells.
Figure 6.
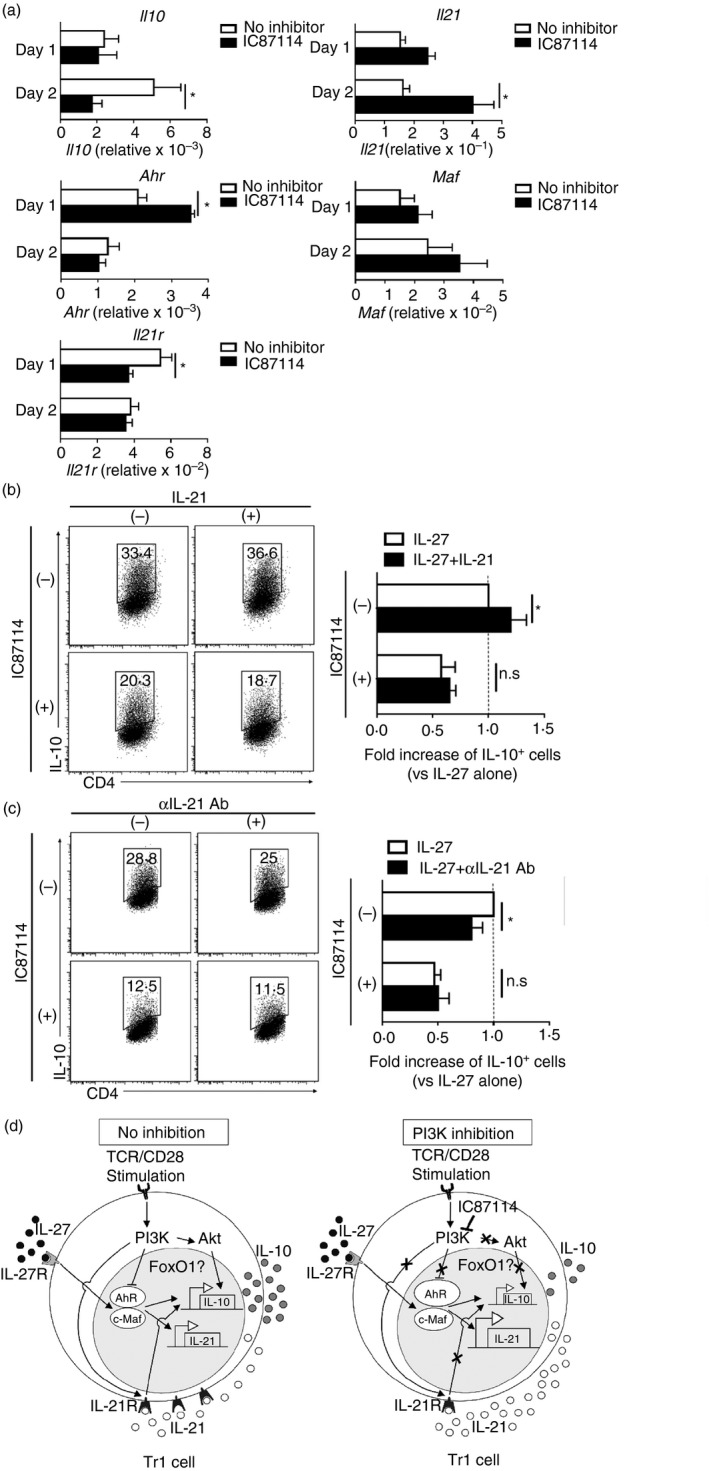
IC87114 down‐regulates interleukin‐21 (IL‐21) receptor expression. (a) Real‐time PCR analyses of Il10, Il21, Ahr, Maf and Il21r on day 1 and day 2 of the interleukin‐27‐induced type 1 regulatory T (IL‐27–Tr1) cell differentiation process. Relative gene expression was calculated by normalization with GAPDH. (b, c) IL‐10 expression in IL‐27–Tr1 cells differentiated with IL‐27 and (b) IL‐21 or (c) α IL‐21 antibody for 3 days. Results are representative of three independent experiments. Values shown are mean ± SD. Significant difference: *P < 0·05. (d) Summary of the regulatory mechanism of IL‐10 expression by the phosphoinositide 3‐kinase (PI3K) pathway in an IL‐27–Tr1 cell. Without any inhibition, T‐cell receptor (TCR) stimulation transduces the PI3K signalling pathway that activates Akt and FoxO1. This PI3K signalling increases IL‐21 receptor expression. IL‐27/IL‐27R signalling induces aryl hydrocarbon receptor (AhR) and c‐Maf expression, which trans‐activates IL‐21 promoter. Autocrine IL‐21 production amplifies Tr1 differentiation. When the PI3K pathway is inhibited, in spite of the up‐regulation of AhR and IL‐21 expression, IL‐21 receptor expression is down‐regulated, which further suppresses IL‐10 expression in Tr1 cells.
Discussion
As one of the suppressive T‐cell subsets, Tr1 cells have been described to regulate inflammation, graft‐versus‐host disease, and autoimmunity by producing high levels of IL‐10.8 Interleukin‐27, a potent inducer of Tr1 cell differentiation, is known to be produced by macrophages and dendritic cells after toll‐like receptor ligation.26 But the precise mechanism has not been clarified. In this study, our results show that the PI3K‐Akt pathway enhances the differentiation of IL‐27–Tr1 cells through up‐regulation of IL‐21 receptors.
Previous studies have tried to overcome the limitation that there is no efficient system for generating Tr1 cells by using CD44hi Foxp3− CD4+ T cells as a major source of IL‐10‐expressing Tr1 cells.23 However, our findings showed that using the CD25− CD4+ T‐cell population that included naive and memory populations was the best source for generating Tr1 cells using IL‐27 stimulation. This might be due to IL‐10 production by the memory population, which further contributes to the differentiation of IL‐27–Tr1 cells, especially of those derived from naive populations. This result emphasizes the importance of IL‐10 for generating Tr1 cells, which has already been shown to be essential for maintaining IL‐10 production in human Tr1 cells.27
The PI3K‐Akt axis generally increases Th cell differentiation.28 Here, we clearly demonstrated that the PI3K‐Akt axis positively regulates IL‐27–Tr1 cell differentiation. Especially in in vivo experiments (Fig. 5), IL‐10 expression from Foxp3− Tr1 but not Foxp3+ Treg cells was affected by PI3K inhibition, inferring several different pathways for IL‐10 expression by Th cells. It has been demonstrated that upon antigen stimulation, protein kinase C θ, which requires mTORC2 for phosphorylation, was essential for inducing IL‐10‐secreting T cells.29, 30 Upon activation, mTORC2 may activate Akt by phosphorylation at Ser473, followed by phosphorylation of FoxO1. This shows that PI3K inhibition in IL‐27–Tr1 cells suppressed phosphorylation of Akt at Ser473, but not at Thr308. In the present study, we found a positive correlation with FoxO1 phosphorylation during IL‐27–Tr1 cell differentiation, whereas PI3K‐Akt‐FoxO1/3a signalling has been known to down‐regulate Treg cell differentiation.5, 31 Therefore, the PI3K‐Akt‐FoxO1 axis might play an essential role in the differentiation of IL‐27–Tr1 cells. However, whether FoxO1 plays a critical role in Tr1 cell differentiation still remains unclear, requiring further studies in the future.
The role of IL‐27 in the generation of IL‐10‐producing Tr1 cells has been clarified in both in vitro and in vivo studies. During IL‐27–Tr1 cell differentiation, cMaf and AhR act synergistically to promote Tr1 cell development through transactivation of Il10 and Il21 promoters.9, 11 In addition, autocrine production of IL‐21 acts as a growth factor for Tr1 cells, which is essential for maintaining Tr1 cell differentiation. In the present study, we demonstrated that inhibition of the PI3K pathway significantly enhanced Ahr expression, followed by IL‐21 expression during the Tr1 cell differentiation process. The mechanism of suppression of Ahr expression by PI3K signalling is also quite interesting but remains to be determined. Enhancement of IL‐21 expression by PI3K inhibition was expected to enhance IL‐10 expression by Tr1 cells, but IL‐10 expression by Tr1 cells was dampened. In fact, we showed that IL‐21 receptor expression, which is important for IL‐21 signalling, was inversely suppressed when the PI3K pathway was inhibited, indicating that the PI3K pathway is involved in the up‐regulation of IL‐21 receptor expression. Hence, these data collectively suggest that the inhibition of the PI3K pathway negatively regulates IL‐21 receptor expression, despite the abundance of IL‐21.
In summary, we demonstrated the importance of the PI3K‐Akt pathway in the differentiation of IL‐27–Tr1 cells through the up‐regulation of IL‐21 receptors. Manipulation of this pathway might offer great opportunities to enhance Tr1 cell function in controlling immune responses, such as in autoimmune disease and inflammatory conditions.
Disclosures
The authors declare no conflict of interest associated with this manuscript.
Supporting information
Figure S1. Interleukin‐10 suppression by IC87114 in the interleukin‐27‐induced type 1 regulatory T (IL‐27–Tr1) cells was in the dose‐dependent manner.
Figure S2. GSK3 and mTORC1 pathway were not involved in the interleukin‐27‐induced type 1 regulatory T (IL‐27–Tr1) cells differentiation.
Figure S3. IC87114 down‐regulates interleukin‐21 receptor expression.
Acknowledgements
We thank Y. Kurebayashi of Keio University School of Medicine for valuable discussions and critical reading of the manuscript. We are grateful to the One‐stop Facility Centre for Future Drug Discoveries, Graduate School of Pharmaceutical Sciences, The University of Tokyo, for image analyses. This work was in part supported by a Grant‐in‐Aid for Scientific Research (B) (26293400 to S.N.), from the Japan Society for the Promotion of Science, Japan.
References
- 1. Hawrylowicz CM, O'Garra A. Potential role of interleukin‐10‐secreting regulatory T cells in allergy and asthma. Nat Rev Immunol 2005; 5:271–83. [DOI] [PubMed] [Google Scholar]
- 2. Saraiva M, O'Garra A. The regulation of IL‐10 production by immune cells. Nat Rev Immunol 2010; 10:170–81. [DOI] [PubMed] [Google Scholar]
- 3. Moore KW, de Waal Malefyt R , Coffman RL, O'Garra A. Interleukin‐10 and the interleukin‐10 receptor. Annu Rev Immunol 2001; 19:683–765. [DOI] [PubMed] [Google Scholar]
- 4. Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin‐10‐deficient mice develop chronic enterocolitis. Cell 1993; 75:263–74. [DOI] [PubMed] [Google Scholar]
- 5. Glocker E‐O, Kotlarz D, Boztug K, Gertz EM, Schäffer AA, Noyan F, et al Inflammatory bowel disease and mutations affecting the interleukin‐10 receptor. N Engl J Med 2009; 361:2033–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jonuleit H, Schmitt E. The regulatory T cell family: distinct subsets and their interrelations. J Immunol 2003; 171:6323. [DOI] [PubMed] [Google Scholar]
- 7. Zeng H, Zhang R, Jin B, Chen L. Type 1 regulatory T cells: a new mechanism of peripheral immune tolerance. Cell Mol Immunol 2015; 12:566–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin‐10‐secreting type 1 regulatory T cells in rodents and humans. Immunol Rev 2006; 212:28–50. [DOI] [PubMed] [Google Scholar]
- 9. Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, et al The aryl hydrocarbon receptor interacts with c‐Maf to promote the differentiation of type 1 regulatory T cells induced by IL‐27. Nat Immunol 2010; 11:854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pot C, Jin H, Awasthi A, Liu SM, Lai CY, Madan R. Cutting edge: IL‐27 induces the transcription factor c‐Maf, cytokine IL‐21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL‐10‐producing Tr1 cells. J Immunol 2009; 183:797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, et al Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17‐producing T cells. Nat Immunol 2006; 7:929–36. [DOI] [PubMed] [Google Scholar]
- 12. Han JM, Patterson SJ, Levings MK. The role of the PI3K signaling pathway in CD4+ T cell differentiation and function. Front Immunol 2012; 3:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koyasu S. The role of PI3K in immune cells. Nat Immunol 2003; 4:313–9. [DOI] [PubMed] [Google Scholar]
- 14. Kurebayashi Y, Nagai S, Ikejiri A, Ohtani M, Ichiyama K, Baba Y, et al PI3K‐Akt‐mTORC1‐S6K1/2 axis controls Th17 differentiation by regulating Gfi1 expression and nuclear translocation of RORγ . Cell Rep 2012; 1:360–73. [DOI] [PubMed] [Google Scholar]
- 15. Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, et al T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci USA 2008; 105:7797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ohtani M, Nagai S, Kondo S, Mizuno S, Nakamura K, Tanabe M, et al Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide‐induced interleukin‐12 production in dendritic cells. Blood 2008; 112:635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Komatsu N, Mariotti‐Ferrandiz ME, Wang Y, Malissen B, Waldmann H, Hori S. Heterogeneity of natural Foxp3+ T cells: a committed regulatory T‐cell lineage and an uncommitted minor population retaining plasticity. Proc Natl Acad Sci USA 2009; 106:1903–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011; 331:337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murayama K, Kimura T, Tarutani M, Tomooka M, Hayashi R, Okabe M, et al Akt activation induces epidermal hyperplasia and proliferation of epidermal progenitors. Oncogene 2007; 26:4882–8. [DOI] [PubMed] [Google Scholar]
- 20. Tezuka H, Abe Y, Iwata M, Takeuchi H, Ishikawa H, Matsushita M, et al Regulation of IgA production by naturally occurring TNF/iNOS‐producing dendritic cells. Nature 2007; 448:929–33. [DOI] [PubMed] [Google Scholar]
- 21. Tezuka H, Abe Y, Asano J, Sato T, Liu J, Iwata M, et al Prominent role for plasmacytoid dendritic cells in mucosal T cell‐independent IgA induction. Immunity 2011; 34:247–57. [DOI] [PubMed] [Google Scholar]
- 22. Mascanfroni ID, Takenaka MC, Yeste A, Patel B, Wu Y, Kenison JE, et al Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1‐α . Nat Med 2015; 21:638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yao Y, Vent‐Schmidt J, McGeough MD, Wong M, Hoffman HM, Steiner TS, et al Tr1 Cells, but Not Foxp3+; regulatory T cells, suppress NLRP3 inflammasome activation via an IL‐10–dependent mechanism. J Immunol 2015; 195:488. [DOI] [PubMed] [Google Scholar]
- 24. McGuirk P, McCann C, Mills KHG. Pathogen‐specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells. J Exp Med 2002; 195:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kurebayashi Y, Baba Y, Minowa A, Nadya NA, Azuma M, Yoshimura A, et al TGF‐β‐induced phosphorylation of Akt and Foxo transcription factors negatively regulates induced regulatory T cell differentiation. Biochem Biophys Res Comm 2016; 480:114–9. [DOI] [PubMed] [Google Scholar]
- 26. Wirtz S, Becker C, Fantini MC, Nieuwenhuis EE, Tubbe I, Galle PR, et al EBV‐induced Gene 3 transcription is induced by TLR signaling in primary dendritic cells via NF‐κB activation. J Immunol 2005; 174:2814. [DOI] [PubMed] [Google Scholar]
- 27. Brockmann L, Gagliani N, Steglich B, Giannou AD, Kempski J, Pelczar P, et al IL‐10 receptor signaling is essential for TR1 cell function in vivo . J Immunol 2017; 198:1130–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arimura Y, Shiroki F, Kuwahara S, Kato H, Dianzani U, Uchiyama T, et al Akt Is a neutral amplifier for Th cell differentiation. J Biol Chem 2004; 279:11408–16. [DOI] [PubMed] [Google Scholar]
- 29. Britton GJ, Mitchell RE, Burton BR, Wraith DC. Protein kinase C θ is required for efficient induction of IL‐10‐secreting T cells. PLoS One 2017; 12:e0171547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang X, Chuang H‐C, Li J‐P, Tan T‐H. Regulation of PKC‐θ function by phosphorylation in T cell receptor signaling. Front Immunol 2012; 3:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Harada Y, Harada Y, Elly C, Ying G, Paik J‐H, DePinho RA, et al Transcription factors Foxo3a and Foxo1 couple the E3 ligase Cbl‐b to the induction of Foxp3 expression in induced regulatory T cells. J Exp Med 2010; 207:1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Interleukin‐10 suppression by IC87114 in the interleukin‐27‐induced type 1 regulatory T (IL‐27–Tr1) cells was in the dose‐dependent manner.
Figure S2. GSK3 and mTORC1 pathway were not involved in the interleukin‐27‐induced type 1 regulatory T (IL‐27–Tr1) cells differentiation.
Figure S3. IC87114 down‐regulates interleukin‐21 receptor expression.


