Summary
Ikaros is a transcription factor that regulates lymphocyte development from the level of the haematopoietic stem cell. Lack of Ikaros reduces the ability of progenitor cells to commit to the T‐cell lineage, resulting in reduced numbers of early thymic T‐cell progenitors and mature T cells. Mature CD4 T cells that lack Ikaros have defects in proliferation, T helper cell differentiation, cytokine expression and the ability to become anergic. A role for Ikaros in the naive T cell has not yet been identified. The receptors interleukin‐7 receptor α (IL‐7Rα) and l‐selectin are important for ensuring survival and proper homing of naive T cells, respectively. Here we show that lack of Ikaros leads to reduced expression of these receptors in naive T cells, which impacts their ability to home and survive in response to IL‐7. We define the mechanism underlying this phenotype as a requirement for Ikaros in maintenance of expression of Foxo1, a transcriptional regulator that is required for their expression. We also demonstrate that CD4 T cells lacking Ikaros are significantly crippled in their ability to become induced regulatory T cells, a phenotype also linked to reduced Foxo1 expression. Finally, we show that restoring Ikaros function to Ikaros‐deficient CD4 T cells increases levels of Foxo1 message. Together, these studies define, for the first time, a role for Ikaros in naive T cells and establish it as the first transcriptional regulator required for maintaining levels of Foxo1 gene expression in these cells.
Keywords: Foxo1, Ikaros, T cell, transcription
Introduction
CD4 T cells are key players in orchestrating adaptive immunity. Upon activation, they undergo directed programming to become specific subsets of T helper cells, secreting cytokines that dictate the quality of the immune response. Before activation, naive CD4 T cells have homeostatic mechanisms in place that allow them to survive and expand, if needed, under lymphopenic conditions. In addition, they express cell surface receptors that allow them to traffic and be retained in peripheral lymphoid organs. Together, these mechanisms guarantee that there are sufficient numbers of CD4 T cells available to respond quickly upon pathogenic assault.
Survival of naive T cells depends on their ability to respond to homeostatic cytokines and home to peripheral lymphoid organs. Interleukin‐7 (IL‐7) is arguably the most important of the homeostatic cytokines.1 The IL‐7 receptor (IL‐7R), expressed at high levels on the surface of naive T cells, is composed of CD132, or the common γ‐chain, responsible for signalling, and the IL‐7Rα chain, which interacts with IL‐7. Proper homing of naive T cells is dependent on high‐level expression of l‐selectin, or CD62L. The CD62L interacts with its ligands, which are expressed on the surface of high endothelial venules, specialized regions of endothelial cells through which T cells enter lymph nodes from the bloodstream. High levels of cell surface CD62L are required for homing to lymph nodes.2
The Forkhead box O (FoxO) family of transcription factors are regulators of survival, homing and homeostasis in naive T cells. One member of this family, Foxo1, is a direct transcriptional activator for Sell and Il7ra, the genes encoding CD62L and IL‐7Rα, respectively. Studies using conditional knockout mice revealed that Foxo1‐deficient T cells are defective in their ability to survive in response to IL‐7 and to traffic to lymph nodes. In addition, Foxo1 is required for the development of induced regulatory T (iTreg) cells3, 4, 5, 6 and for maintenance of memory T‐cell populations.7, 8 Despite these important roles, transcription factors required for maintenance of Foxo1 gene expression in T cells are unknown.
Ikaros is a DNA‐binding protein expressed at high levels in CD4 T cells.9, 10 It can act as both a transcriptional activator and a transcriptional repressor, and is associated with SWI/SNF and NuRD chromatin remodelling complexes in T cells.11 Much has been revealed about the role of Ikaros in regulating events that take place after T‐cell receptor (TCR) stimulation in CD4 T cells using genetically engineered Ikaros knockout (Ikaros null) mice.12 Lack of Ikaros impacts T helper cell lineage fate decisions, cytokine gene expression, regulation of proliferative responses and the ability of CD4 T cells to become anergic.13, 14, 15, 16, 17, 18 However, whether Ikaros has important functions in naive T cells is not known.
Here, we report that Ikaros regulates the expression of IL‐7Rα and CD62L in naive CD4 T cells. We define the mechanism underlying this role as an inability to express Foxo1 in the absence of Ikaros. We also demonstrate that, in the absence of Ikaros, development of iTreg cells is severely compromised and link this phenotype to decreased Foxo1 expression. This is the first report defining a role for Ikaros in the naive T cell.
Materials and methods
Mice
Ikaros null mice (C57BL/6 × 129 and BALB/c background)12 were generated by intercrossing of Ikaros null heterozygotes. All figures have data contribution from mice of both backgrounds, except for those that use the Foxp3 reporter mice, which are on a BALB/c background. All phenotypes were consistent between backgrounds. Foxp3/GFP reporter mice (BALB/c background)19 were gifted by Dr Vijay Kuchroo at Harvard Medical School. Animals were bred and maintained in a specific pathogen‐free barrier facility at Boston University School of Medicine. Genotypes were determined by PCR analyses. All animal procedures were approved by the Boston University Institutional Animal Care and Use Committee.
Cell purification
CD4+ or CD8+ T cells were purified from pooled spleens of wild‐type or Ikaros null mice using mice using Dynabeads FlowComp CD4 or CD8 kits (Invitrogen, Carlsbad, CA) following the manufacturer's protocol.
RNA isolation and quantitative real‐time PCR
Total RNA was isolated from the cells using the SV Total RNA Isolation System (Promega, Madison, WI) and cDNA was generated with a Superscript III Kit (Invitrogen). Quantitative PCR was performed using iQ SYBR Green (Bio‐Rad, Hercules, CA) and the BioRad MyiQ Real‐Time PCR machine generating results that were analysed using the Pfaffl method. Data are shown as ratios (E target)−CT target : (E reference)−CT reference, where E = efficiency of PCR; target = gene of interest; and reference = HPRT (the gene encoding hypoxanthine‐guanine phosphoribosyltransferase). Primers were synthesized by IDT DNA Technologies. Primer sequences are available upon request.
Western blots
Protein extracts were prepared by whole cell lysis with Lysis Buffer (420 mm NaCl, 20 mm Tris–HCl pH 7·5, 1 mm EDTA, 1% Nonidet P‐40) supplemented with protease inhibitors. Lysates (20 μg) were separated by gel electrophoresis on an 8% SDS–polyacrylamide gel and transferred to a PVDF membrane. Membranes were incubated with anti‐Foxo1 (C29H4, Cell Signaling, Danvers, MA) anti‐Akt (9272, Cell Signaling), anti‐pAkt (92715, Cell Signaling) or anti‐actin (A2066, Sigma, St Louis, MO) followed by horseradish peroxidase‐conjugated secondary antibody (anti‐rabbit or anti‐mouse IgG, Jackson ImmunoResearch, West Grove, PA). Proteins were visualized by incubation with enhanced chemiluminescence reagent and either exposure to film or captured images using the BioRad ChemiDoc MP System. imageJ v1.43u (NIH, Bethesda, MD) was used for densitometry analyses.
Annexin V staining and survival assays
Purified CD4 T cells were plated in 96‐well plates at 6·0 × 105–106 cells/well with or without 10 ng/ml IL‐7 (Peprotech, Rocky Hill, NJ). At 24, 48 and 72 hr post‐plating, 2·0 × 105 cells were stained with Annexin V (Annexin V: PE Apoptosis Detection Kit 1, BD Biosciences, San Jose, CA) and analysed by flow cytometry on a FACSCalibur (BD Biosciences) flow cytometer. Data were analysed using flowjo software.
Flow cytometry and intracellular staining
All antibodies were from eBioscience (San Diego, CA) unless otherwise noted. The following antibodies were used for analyses of cell surface markers: anti‐CD4 (GK1.5), anti‐CD8 (53‐6.7), anti‐H‐2Kk (H100‐27.R55) (Miltenyi Biotech, Auburn, CA), anti‐Thy‐1.1 (HIS51), anti‐IL‐7Rα (SB/199), anti‐CD62L (MEL‐14) and anti‐CD44 (IM7). For intracellular phospho‐Akt analyses, spleens were dissociated directly into 10 ml of 1·6% paraformaldehyde (Electron Microscopy Sciences #15710; Hatfield, PA). Cells were kept at room temperature for 15 min followed by addition of 40 ml 100% ice‐cold methanol to permeabilize cells. Cells were then stained with anti‐phosphoAkt (pS473)‐Alexa Fluor 488 (M89‐61; BD Biosciences). Intracellular staining for Foxo1, Foxp3 and Ki67 was performed using Foxp3/Transcription Factor Fixation/Permeabilization Kit (eBioscience). For Foxo1 staining, cells were stained first with anti‐Foxo1 (C29H4; Cell Signaling) followed by anti‐rabbit IgG FITC (Jackson ImmunoResearch). For Foxp3 and Ki67 staining, cells were stained with anti‐Foxp3‐phycoeryhtrin (150D/E4) or anti‐Ki67‐phycoerythrin (SolA15), respectively. Cells were analysed by flow cytometry on a FACSCalibur (BD Biosciences) flow cytometer. Data were analysed using flowjo software.
T‐cell differentiation cultures
CD4+ T cells purified from pooled spleens from two to four mice were stimulated in 12‐well plates with 2 μg/ml plate‐bound anti‐CD3 (2C11) and 5 μg/ml soluble anti‐CD28 (37.51) in RPMI‐1640 medium supplemented with 10% fetal calf serum, 50 μm 2‐mercaptoethanol, 2 mm l‐glutamine (Hyclone, South Logan, UT) and 100 U/ml of penicillin‐streptomycin (RPMI complete). For iTreg cell differentiations, cells were grown in culture with 2 ng/ml transforming growth factor‐β (TGF‐β) and 50 U/ml IL‐2. Before flow cytometry analyses for the time–course experiment, 5 μl of propidium iodide was added per sample to exclude dead cells. For some iTreg differentiation cultures, 25 nm rapamycin (Sigma) was added. T helper type 17 (Th17) differentiation was induced by adding 5 ng/ml IL‐6, 1 ng/ml TGF‐β, 5 μg/ml anti‐interferon‐γ (XMG1.2) and 5 μg/ml anti‐IL‐4 (11B11). All cytokines were from Peprotech.
Adoptive transfer
CD4+ T cells purified from wild‐type and Ikaros null spleens were differentially labelled with 10 mm Efluor‐450 (eBioscience) or 1 mm CFSE (eBioscience), respectively. Labelled cells were injected retro‐orbitally (2 × 106 cells per genotype) into wild‐type hosts. Inguinal lymph nodes and spleens were harvested after 24 hr, followed by flow cytometric analyses.
Retroviral transduction
MSCV IRES H‐2Kk and MSCV IRES Ik‐1 H‐2Kk constructs were described previously.20 MSCV Foxo1‐A3 Thy‐1.1, MSCV Foxo1 Thy‐1.1 and MSCV Thy‐1.1 constructs were gifts from Dr David Fruman (University of California, Irvine, CA).21 Retroviruses were generated by transfection of retroviral constructs into the Phoenix packaging cell line using a calcium phosphate transfection protocol. Viral supernatants were harvested at 24 hr post‐transfection and used to infect T cells that had been activated overnight with anti‐CD3/anti‐CD28. Supernatants were supplemented with 6 μg/ml polybrene. Plates containing cells and supernatants were centrifuged at 566g for 2 hr at 32° (‘spinfection’). Twenty‐four hours after spinfection, cells were removed from stimulation and plated with 50 U/ml IL‐2. For the ‘resting protocol’, cells were washed 48 hr later, and fresh medium without IL‐2 was added. Seventy‐two hours later, cells were stained, and flow cytometric analyses were performed. Staining with fluorochrome‐conjugated anti‐H‐2Kk (H‐100‐27.R55) or anti‐Thy‐1.1 (H1S51) was used to identify successfully transduced cells. Flow cytometry was performed on a FACSCalibur (BD Biosciences) flow cytometer. Data were analysed using flowjo software.
Statistics
Most statistical analyses were performed using two‐tailed Student's t‐tests. In all graphs, error bars represent standard error of the mean (SEM). A two‐way analysis of varaince with repeated measures analysis was performed for the cell survival assay in Fig. 1(a). All statistics were performed using graphpad prism software (GraphPad, San Diego, CA). P values greater than 0·05 were considered not significant.
Figure 1.
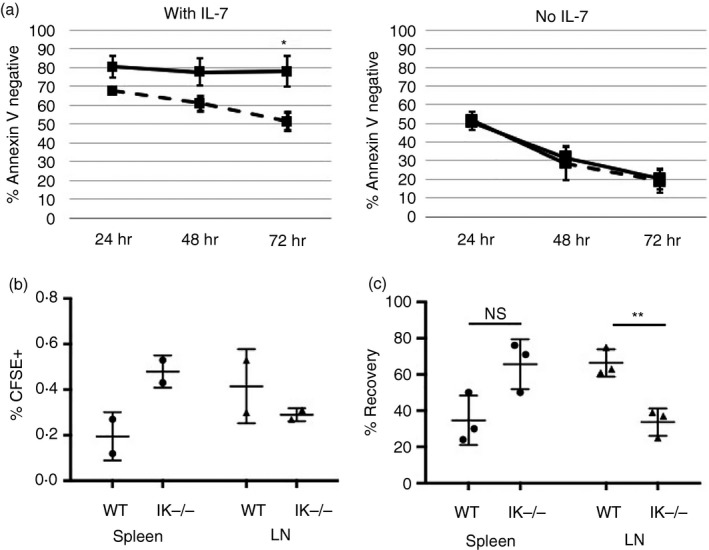
Decreased survival and altered homing of Ikaros null T cells. (a) Cell viability assay for purified wild‐type (solid) and Ikaros null (IK −/−) (dashed) CD4 T cells in culture with or without 10 ng/ml of exogenous interleukin‐7 (IL‐7) measured as per cent of cells that stained negative for Annexin V. Data shown are a compilation from four independent experiments (*P = 0·00684). (b) Purified CD4 T cells from wild‐type and Ikaros null spleens were loaded with CFSE and adoptively transferred into two separate wild‐type mice. Percent (%)CFSE + cells in the spleen and lymph nodes are shown. (c) Differentially labelled wild‐type (Efluor 450) and Ikaros null (CFSE) CD4 T cells were adoptively transferred in a 1 : 1 ratio into the same wild‐type mouse. (NS = Not significant, P = 0·0501; **P = ·0062). % Recovery = Individual percentage of wild‐type (Efluor 450) or Ikaros null (CFSE) cells in the spleen or lymph node/ total percentage of labelled (Efluor 450 + CFSE) cells in the spleen or lymph node. Error bars are representative of ± SEM.
Results
Lack of Ikaros negatively impacts survival and homing of T cells
Ikaros is expressed at high levels in CD4 T cells, yet its role in quiescent CD4 T cells has not been explored. To investigate a potential role, we tested the survival and homing capabilities of peripheral CD4 T cells that lack Ikaros. To measure survival, CD4 T cells purified from spleens of Ikaros null (IK−/−) and wild‐type mice were plated with or without IL‐7, and viability was assessed over time using Annexin V staining. In the absence of IL‐7, viability of Ikaros null and wild‐type CD4 T‐cell populations similarly declined. However, in the presence of IL‐7, wild‐type CD4 T cells were able to fully recover their ability to survive, whereas Ikaros null CD4 T cells could not (Fig. 1a).
To study homing capabilities, adoptive transfer experiments using wild‐type hosts were performed because Ikaros null mice lack lymph nodes.17, 18 In the first set of experiments, Ikaros null and wild‐type T cells were loaded with CFSE and transferred intravenously into separate wild‐type hosts. Ikaros null T cells showed altered homing capabilities, with a reduced ability to traffic to lymph nodes and preferential homing to the spleen (Fig. 1b). To erase the potential confounding factor of injection efficiency discrepancies between hosts, Ikaros null and wild‐type T cells were labelled separately with CFSE and Efluor 450 dyes, respectively, and transferred in a 1 : 1 ratio into the same wild‐type host. Similar results were obtained, where significant reduction in the ability of Ikaros null CD4 T cells to traffic to lymph nodes was observed (Fig. 1c). Taken together, these data suggest a role for Ikaros in regulating survival and homing of peripheral T cells.
Decreased expression of CD62L and IL‐7Rα in Ikaros null CD4 T cells
To begin to define the mechanism underlying the role of Ikaros in survival and homing, we examined the expression of two key molecules required for these processes, IL‐7Rα and CD62L, respectively. Relevant to our data, high‐level expression of CD62L on naive T cells is required for their entry into lymph nodes, but not spleen.22, 23 Expression of both IL‐7Rα and CD62L are significantly reduced on the surface of Ikaros null CD4 T cells (Fig. 2a). In addition, reduced expression of Sell and Il7ra, the genes encoding CD62L and IL‐7Rα, are observed (Fig. 2b). Ikaros null CD8 T cells also display significantly reduced levels of Sell and Il7ra expression (Fig. 2c). These data demonstrate that, in the absence of Ikaros, expression of IL‐7Rα and CD62L are negatively impacted at the level of message.
Figure 2.
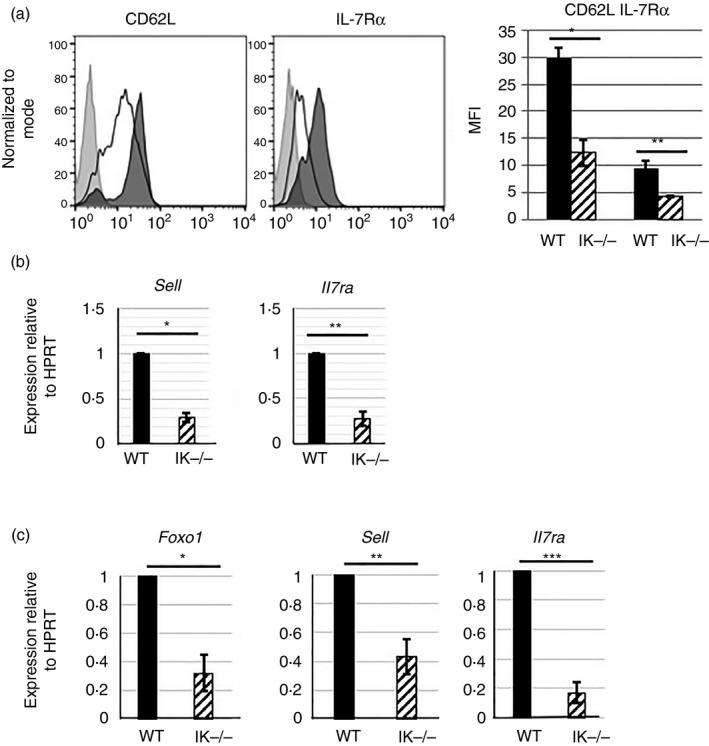
A lack of Ikaros leads to decreased expression of Foxo1 target genes. (a) Flow cytometry analyses measuring expression of CD62L and interleukin‐7 receptor α (IL‐7Rα) on the surface of CD4 splenic T cells from wild‐type (shaded histogram) and Ikaros null (black outlined histogram) mice. Bar graph is representative of mean fluorescence intensities compiled from three independent experiments. (*P = 0·0287, **P = 0·0055). (b) Quantitative RT‐PCR analyses of Sell and Il7ra expression using cDNA from wild‐type and Ikaros null (IK −/−) purified CD4 T cells. Values were normalized to levels of expression of the housekeeping gene, HPRT, for a minimum of six independent experiments. (*P = 2·84 × 10−5, **P = 3·61 × 10−5). (c) Quantitative RT‐PCR analyses of Foxo1, Sell and Il7ra expression using cDNA from wild‐type and Ikaros null purified splenic CD8 T cells. Values were normalized to levels of expression of the housekeeping gene, HPRT, for a minimum of five independent experiments (*P = 0·0016, **P = 0·0094, ***P = 0·00126). Error bars are representative of ± SEM.
Lower levels of CD62L expression are associated with activated and memory T‐cell populations,24 and levels of IL‐7Rα are transiently reduced at early stages of T‐cell activation.25 Ikaros controls thresholds of T‐cell activation in CD4 T cells, such that Ikaros‐deficient T cells are activated by lower levels of TCR stimulation than their wild‐type counterparts.17 Therefore, lower levels of expression of CD62L and IL‐7Rα may be the end product of an expanded activated or memory CD4 T‐cell population in the spleens of Ikaros null mice. To investigate if this were the case, we first analysed expression levels of cell surface CD44 and CD25 on freshly isolated Ikaros null and wild‐type CD4 T cells, as increased expression of these markers occurs on activated and/or previously activated T cells.26 Ikaros null CD4 T cells did not exhibit increased CD44 expression (Fig. 3a). In fact, levels of CD44 were on average lower than those observed on wild‐type cells. CD25 is the α‐chain of the IL‐2R, which is up‐regulated on the surface of activated T cells. CD25 expression was also not increased in Ikaros null CD4 T cells (Fig. 3a). In addition, there was no difference in expression of Ki67, a cell proliferation marker, in wild‐type and Ikaros null CD4 T cells, demonstrating that Ikaros null T cells are quiescent (Fig. 3a). As a positive control, expression levels of CD25, CD44 and Ki67 were assessed on activated Ikaros null and wild‐type CD4 T‐cell populations and were shown to be equivalent (Fig. 3a).
Figure 3.
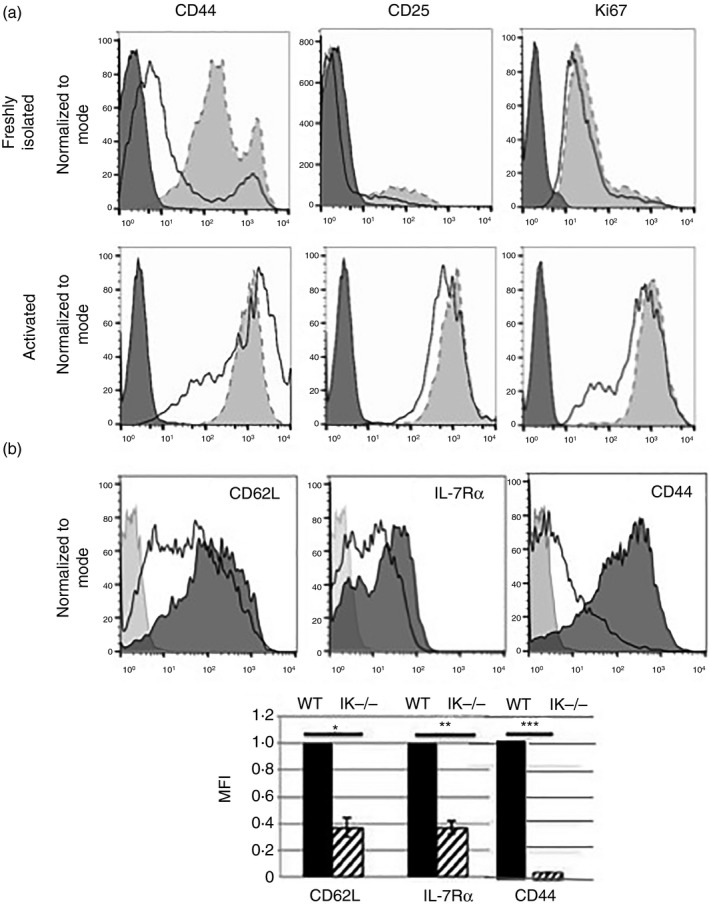
Ikaros null CD4 T cells do not represent an activated or memory cell population. (a) Flow cytometry analyses of CD44, CD25 and Ki67 expression in purified wild‐type (shaded histograms) and Ikaros null (black outlined histograms) freshly isolated and activated CD4 T cells. Data are representative of two independent experiments. (b) Flow cytometric analyses of CD62L, interleukin‐7 receptor α (IL‐7Rα) and CD44 expression in wild‐type (shaded histogram) and Ikaros null (black outlined histogram) CD4 single‐positive T cells in the thymus. Bar graph is representative of mean fluorescence intensities compiled from three independent experiments (*P = 0·00547, **P = 0·0028, ***P = 3·75 × 10−4). Error bars are representative of ± SEM.
Next, levels of CD62L and IL‐7Rα expression were analysed in Ikaros null CD4 single positive thymocytes. These cells were also CD62Llo IL‐7Rlo CD44lo, demonstrating that Ikaros null CD4 T cells acquire this altered phenotype before their exit into the periphery (Fig. 3b). Taken together, these data support a developmental role for Ikaros in the regulation of IL‐7Rα and CD62L expression, rather than an expansion of an activated or memory T‐cell subset in spleens of Ikaros null mice.
Reduced expression of Foxo1 in absence of Ikaros
Foxo1 is a common transcriptional activator of Il7ra and Sell.27, 28, 29 Although it is known that Foxo1 regulates Il7ra and Sell, factors that impact expression of the Foxo1 gene itself in naive T cells are unknown. Foxo1‐deficient CD4 T cells display survival and homing defects similar to those described here for Ikaros null T cells.29 Therefore, we next investigated if Foxo1 expression was altered in Ikaros null CD4 T cells. Western blot and intracellular flow analyses demonstrate a significant reduction in levels of Foxo1 protein in the absence of Ikaros (Fig. 4a,b).
Figure 4.
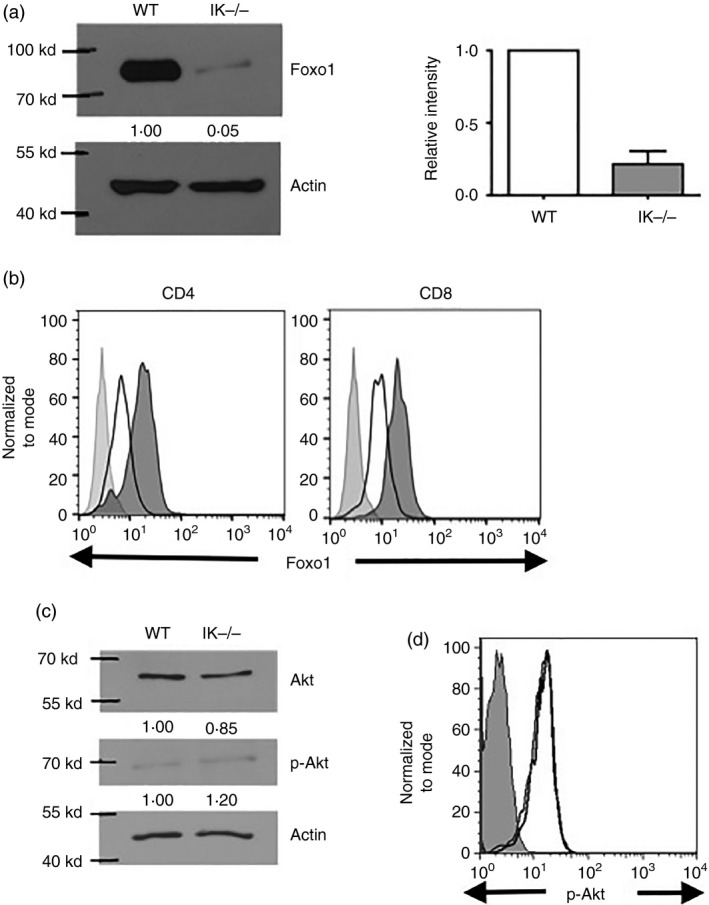
In the absence of Ikaros, decreased levels of Foxo1 protein are observed in T cells. (a) Western blot analysis of Foxo1 expression in purified splenic CD4+ T cells from wild‐type and Ikaros null (IK −/−) mice. Intensity of bands for Foxo1, normalized to actin bands, is shown as fold over wild‐type levels below each band. Average of relative intensity of Foxo1 in Ikaros null compared with wild‐type T cells over three independent experiments are compiled in bar graph. (b) Levels of Foxo1 in CD4 and CD8 T cells in wild‐type (shaded histogram) and Ikaros null (black outlined histogram) spleens as determined by intracellular staining followed by flow cytometric analyses. Data are representative of two independent experiments. (c) Akt and phospho‐Akt (p‐Akt) expression levels were analysed by Western blot using whole‐cell lysates prepared from purified splenic CD4 T cells from wild‐type and Ikaros null (IK −/−) mice. The intensity of the bands for Akt and p‐Akt are normalized to actin and shown as fold over wild‐type levels below each image. Data are representative of two independent experiments. (d) Levels of anti‐phospho‐Akt in CD4 T cells in wild‐type (black line) and Ikaros null (light grey line) spleens as determined by intracellular staining followed by flow cytometric analyses. Error bars are representative of ± SEM. Data are representative of two independent experiments.
Foxo1 protein levels are regulated post‐translationally through phosphorylation by Akt that drives its export from the nucleus and subsequent degradation.30 If Ikaros null T cells have increased basal levels of activated Akt, this could lead to decreased levels of Foxo1 protein. Levels of activated Akt, phosphorylated on serine 473, were compared in Ikaros null and wild‐type CD4 T cells by Western blot and intracellular flow analyses (Fig. 4c,d). By both methods, it was shown that levels of phospho‐Akt were comparable in wild‐type and Ikaros null T cells, demonstrating that decreased Foxo1 is not a consequence of increased pAkt in the absence of Ikaros.
Taken together, these data suggest that the mechanism by which lack of Ikaros results in reduced expression of Il7ra and Sell is through negatively impacting expression of Foxo1.
Lack of Ikaros results in defective initiation of the Foxo1 programme in response to stress
Foxo1 protein is stabilized in the nucleus under conditions of cell stress, when signalling pathways that target it for destruction are silenced. When T cells are cultured under conditions of stress, as induced by cytokine/growth factor withdrawal, increased nuclear Foxo1 leads to up‐regulated expression of CD62L and IL‐7Rα.29 This may be important to promote T‐cell recruitment to lymphoid organs for IL‐7‐mediated survival.31 When Foxo1‐defici ent CD4 T cells are cultured without added growth factors, they are unable to up‐regulate IL‐7Rα and CD62L.29
To test if this pathway is intact in the absence of Ikaros, wild‐type and Ikaros null CD4 T cells were cultured without TCR stimulation or added growth factors. After 24 hr, expression of IL‐7Rα and CD62L were analysed using flow cytometry. Ikaros null CD4 T cells could not up‐regulate the expression of CD62L or IL‐7Rα (Fig. 5a). In fact, levels of IL‐7Rα on stressed Ikaros null cells were significantly decreased relative to those on freshly isolated cells. In addition, stressed Ikaros null CD4 T cells could not restore levels of CD62L or IL‐7Rα to those observed on wild‐type cells (Fig. 5b), providing further support that abnormal activation of signalling pathways that target Foxo1 for degradation in vivo, which would be silenced using this overnight culture protocol, does not underlie their reduced expression.
Figure 5.
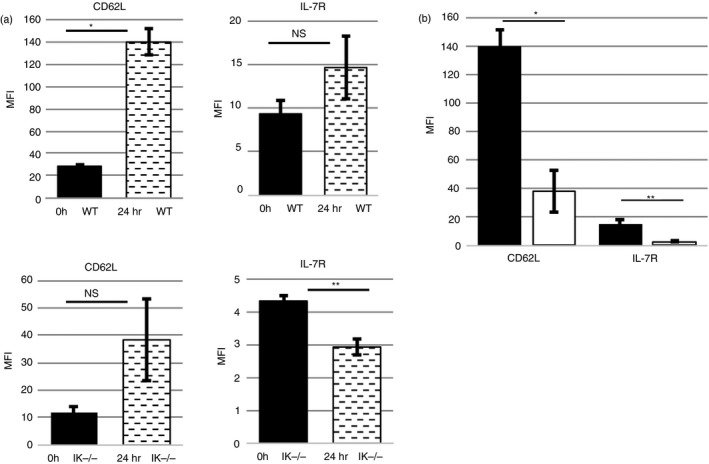
A lack of Ikaros compromises expression of interleukin‐7 receptor α (IL‐7Rα) and CD62L, even under Foxo1‐favouring, cell stress conditions. (a) Compilation of mean fluorescence intensities of three independent experiments measuring cell surface expression levels of CD62L and IL‐7Rα in freshly purified splenic wild‐type and Ikaros null (IK −/−) (0 hr) CD4 T cells as well as cells that have been cultured overnight in the absence of growth factors (24 hr) (NS = Not significant; *P = 0·0007, **P = 0·0092). (b) Compilation of mean fluorescence intensities from three independent experiments measuring cell surface expression levels of CD62L and IL‐7Rα in purified splenic wild‐type (black) and Ikaros null (IK‐) (white) CD4 T cells that have been cultured overnight in the absence of growth factors (*P = 0·0057. **P = 0·0308). Error bars are representative of ± SEM.
Ikaros regulates CD62L and IL‐7Rα expression using two different mechanisms
Next, we asked if restoring Ikaros or Foxo1 expression to Ikaros null T cells could increase expression of CD62L and IL‐7Rα, thereby placing Foxo1 downstream of Ikaros in regulating their expression. To do this, Ikaros null CD4 T cells were transduced with Ikaros (MSCV IRES Ik‐1 H‐2Kk) or Foxo1 (MSCV Foxo1 Thy‐1.1) using retroviral transduction.
Retroviral transduction requires cell proliferation, induced in this case by anti‐CD3/anti‐CD28. This allows for breakdown of the nuclear envelope needed for integration of the viral constructs into the host genome, which is required for their expression. However, induction of TCR signalling pathways results in phosphorylation of Foxo1, which signals its export from the nucleus and degradation.32 To determine if IL‐7Rα or CD62L expression can be increased by transduction of Ikaros or by transduction of Foxo1 itself in Ikaros null CD4 T cells, a ‘resting protocol’ was implemented post‐transduction to drive nuclear localization of Foxo1. For this protocol, CD4 T cells were transduced following overnight activation using anti‐CD3/anti‐CD28. Twenty‐four hours later, they were removed from stimulation and expanded using IL‐2. After 48 hr, cells were removed from IL‐2, and plated with medium that contained no added growth factors for 72 hr to induce a ‘resting’ state before analyses. Successfully transduced cells were identified via staining with anti‐H‐2Kk or anti‐Thy‐1.1.
Transduction of Ikaros into Ikaros null cells increased expression of IL‐7Rα, but not that of CD62L. Conversely, transduction of Foxo1 increased expression levels of CD62L, but not those of IL‐7Rα (Fig. 6a). This dichotomy was unexpected as both Ikaros and Foxo1 are required to maintain high surface levels of CD62L and IL‐7Rα as interfering with the activity of either Ikaros or Foxo1 in wild‐type T cells similarly reduces their expression (see Supplementary material, Fig. S1).
Figure 6.
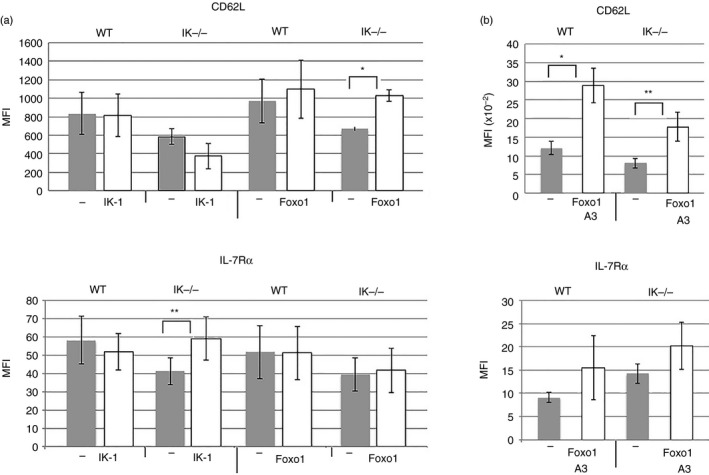
Restoring Ikaros or Foxo1 to Ikaros null T cells differentially impacts the expression of CD62L and interleukin‐7 receptor α (IL‐7Rα). (a) Purified splenic CD4 T cells from wild‐type and Ikaros null (IK −/−) mice were transduced with retroviruses prepared using MSCV IRES H‐2Kk (−), MSCV IRES Ik‐1 H‐2Kk (IK‐1) and MSCV Foxo1 Thy‐1.1 (Foxo1) constructs. Results are presented as a compilation of mean fluorescence intensities for CD62L and IL‐7Rα expression from at least four independent experiments. Differences are not significant unless marked with asterisk(s) (*P = 0·0233, **P = 0·032). Error bars are representative of ± SEM. (b) Purified splenic CD4 T cells from wild‐type and Ikaros null (IK −/−) mice were transduced with retroviruses prepared using MSCV IRES Thy‐1.1 (−) or MSCV Foxo1‐A3 Thy‐1.1 (Foxo1‐A3) constructs. Data are a compilation of results from five independent experiments. Differences are not significant unless marked with asterisk(s) (*P = 0·0152, **P = 0·0353).
Although we used a ‘resting protocol’ in these experiments, Foxo1's nuclear localization would be compromised during the T‐cell activation phase. This may impair its function, leading to the observed inability to increase expression of IL‐7Rα. To neutralize this issue, we asked if transduction with a constitutively active Foxo1 (designated Foxo1‐A3; MSCV Foxo1‐A3 Thy‐1.1)21 could increase expression IL‐7Rα in Ikaros null T cells. Foxo1‐A3 has its three Akt phosphorylation sites mutated to alanine, preventing its transport out of the nucleus. Similar to Foxo1, Foxo1‐A3 was able to increase expression of CD62L but not IL‐7Rα, on the surface of Ikaros null CD4 T cells (Fig. 6b).
Taken together, these data show that lack of Ikaros can be bypassed through transduction of Foxo1 solely for expression of CD62L. Since IL‐7Rα expression was only increased by transduction of Ikaros, which we hypothesize would also increase expression of Foxo1, we suggest that both Ikaros and Foxo1 are needed to co‐regulate expression of the Il7ra gene.
Lack of Ikaros impacts Foxo1 expression at the level of message
It has previously been reported that Ikaros binds to the Foxo1 locus in pre‐B cells and in haematopoietic stem cells, suggesting that Ikaros functions as a direct transcriptional activator of Foxo1.33, 34 In support of this, levels of Foxo1 mRNA are significantly reduced in Ikaros null CD4 T cells (Fig. 7a). Showing the specificity of this result, levels of Foxo3 mRNA are not reduced (Fig. 7b). Not surprisingly, due to the decreased levels of gene expression, levels of acetylated histone H3 at the Foxo1 promoter, a mark associated with open chromatin conformation, are decreased in Ikaros null CD4 T cells (see Supplementary material, Fig. S2).
Figure 7.
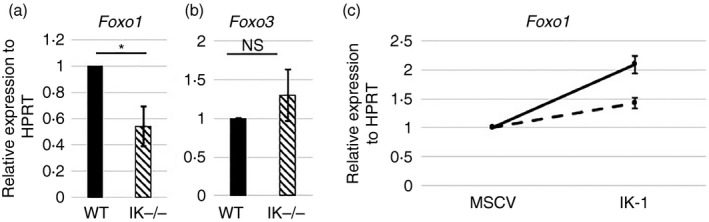
Lack of Ikaros negatively impacts Foxo1 gene expression. (a, b) Quantitative RT‐PCR analyses performed using cDNA from wild‐type and Ikaros null (IK −/−) purified splenic CD4+ T cells. Data are compilation of results from nine independent experiments (NS = Not significant; *P = 0·0121). (c) Purified CD4 T cells from spleens of wild‐type and Ikaros null mice were transduced with retroviruses prepared from the MSCV IRES H‐2Kk (MSCV) or MSCV IRES Ik‐1 H‐2Kk (IK‐1) constructs. Results of quantitative RT‐PCR analyses for Foxo1 expression from two independent experiments are shown. Results have been normalized to expression levels in control (MSCV) transduced cells. Error bars are representative of ± SEM.
If Ikaros controls Foxo1 expression at the level of transcription, increasing levels of Ikaros should increase levels of Foxo1 mRNA. To test if this was the case, Ikaros was transduced into Ikaros null and wild‐type CD4 T cells by retroviral transduction. Transduction of Ikaros significantly increased expression of Foxo1 in both Ikaros null and wild‐type CD4 T cells (Fig. 7c).
This was also translated into an increase in Foxo1 protein in Ikaros‐transduced Ikaros null CD4 T cells (see Supplementary material, Fig. S3).
Lack of Ikaros negatively impacts induced regulatory T‐cell differentiation
Lack of Foxo1 results in a significant reduction in the ability of CD4 T cells to differentiate into iTreg cells.3 To investigate whether a lack of Ikaros leads to decreased iTreg cell differentiation, whole splenocytes or CD4 T cells purified from the spleens of wild‐type and Ikaros null Foxp3/GFP reporter mice were stimulated with anti‐CD3 and anti‐CD28 in the presence of TGF‐β and IL‐2. After 72 hr, flow cytometry was performed to identify the GFP+ iTreg cells. In the absence of Ikaros, CD4 T cells were crippled in their ability to attain the iTreg fate, as determined by decreased GFP and Foxp3 expression (Fig. 8a,b). This was also observed at earlier time‐points (24 and 48 hr), suggesting that this was a defect in iTreg differentiation and not iTreg survival (Fig. 8c). This defect was not the result of decreased expression of the genes encoding TGF‐βR or its two nuclear effectors, Smad2 and Smad3, in Ikaros null CD4 T cells (Fig. 8d).
Figure 8.
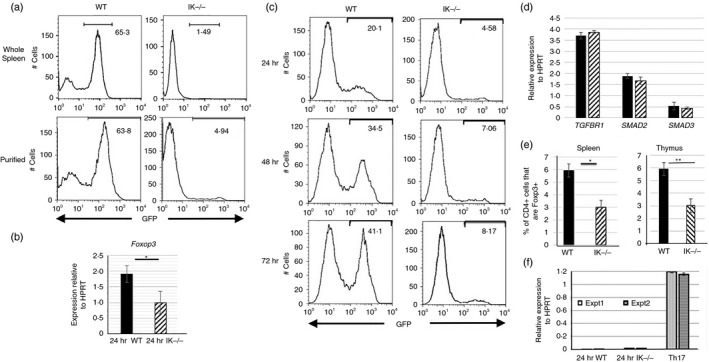
Decreased induced regulatory T (iTreg) cell differentiation in the absence of Ikaros. (a) GFP expression levels in iTreg cell differentiation cultures of wild‐type and Ikaros null (IK −/−) splenocytes (top panel) as well as purified (bottom panel) splenic CD4 T‐cell populations from Foxp3/GFP reporter mice. Cells were grown in culture for 72 hr in the presence of 2 ng/ml transforming growth factor‐β (TGF‐β) and 50 U/ml interleukin‐2 (IL‐2), and were removed from anti‐CD3 and anti‐CD28 stimulation at 24 hr. Data are representative of five independent experiments each. (b) Quantitative RT‐PCR results for Foxp3 expression using cDNA from purified splenic CD4 T cells grown in culture under iTreg cell differentiation conditions for 24 hr (*P = 0·034). Data are compilation of results from three independent experiments. (c) GFP expression levels in iTreg cell differentiation cultures performed with CD4 T cells from wild‐type and Ikaros null (IK −/−) Foxp3/GFP reporter mice. Data shown are from 24, 48 and 72 hr post‐culture. (d) Quantitative RT‐PCR results for TGFBR1,SMAD2 and SMAD3 expression were obtained using cDNA from freshly isolated wild‐type (black bars) and Ikaros null (IK −/−) (patterned bars) CD4 T cells. Data are average of two independent experiments. (e) Percentages of Foxp3+ cells within the CD4+ T‐cell compartment in the spleen (average of five independent experiments) and the CD4 single‐positive compartment in the thymus (average of seven independent experiments) (*P = 0·0126, **P = 0·0046). (f) Quantitative RT‐PCR analyses of Il21 expression using cDNA from wild‐type and Ikaros null purified CD4 T cells cultured under iTreg cell polarization conditions as well as wild‐type cells cultured under T helper type 17 polarization conditions, which served as a positive control. Data are normalized to HPRT and data from two independent experiments are shown. Error bars are representative of ± SEM.
Lack of Ikaros also resulted in decreased peripheral Treg and natural Treg compartments in vivo. This was revealed by reduction in percentages of Foxp3+ cells in spleens and thymuses of Ikaros null mice, respectively (Fig. 8e). Taken together, these data point to an overall defect in differentiation of the Treg lineage in the absence of Ikaros.
CD4 T cells from a genetically engineered Ikaros mutant mouse with deletion of the exon encoding DNA‐binding zinc finger 4 (Ik∆F4), also display defects in iTreg cell differentiation.35 This was attributed to inappropriate expression of large amounts of IL‐21, a Th17 cytokine that blocks Foxp3‐mediated iTreg differentiation, when Ik∆F4 CD4 T cells were plated under iTreg cell differentiation conditions. To determine if this might also underlie the defect in Ikaros null CD4 T cells, levels of Il21 expression were examined in Ikaros null iTreg cultures. In contrast to cells in Ik∆F4 iTreg cultures, which express levels of Il21 similar to those seen in Th17 cultures, cells in Ikaros null iTreg cultures express negligible levels (Fig. 8f). Therefore, the defect underlying lack of iTreg differentiation cannot be attributed to elevated Il21 expression. The difference in phenotype observed in T cells from these two mouse models may be the result of over‐expression of a non‐DNA binding Ikaros protein, which has been shown to result in dominant negative phenotypes, in the Ik∆F4 mice versus a total lack of Ikaros protein in the study presented here.
Rapamycin cannot increase iTreg cell differentiation, but increasing levels of Foxo1 activity can. Next, we asked if decreased levels of Foxo1 in Ikaros null CD4 T cells contributed to the defect in iTreg cell differentiation. First, we attempted to increase levels of nuclear Foxo1 in iTreg differentiation cultures by treating cells with the pharmacological inhibitor rapamycin. Rapamycin enhances iTreg differentiation by preventing activation of Akt by the mammalian target of rapamycin complex 1 (mTORC1) complex.36, 37 Supporting the notion that Foxo1 is the downstream target of Akt responsible for this phenotype, rapamycin does not rescue the defect in iTreg cell differentiation observed with Foxo1‐deficient CD4 T cells.3 In a similar fashion, rapamycin was unable to promote iTreg cell differentiation in Ikaros null cultures (Fig. 9a). This could be because levels of Foxo1 are so low in Ikaros null CD4 T cells that, even in the presence of rapamycin, there is insufficient Foxo1 activity for iTreg cell differentiation to proceed. Alternatively, it could mean that increased Foxo1 activity induced by rapamycin is insufficient to rescue the phenotype in the absence of Ikaros.
Figure 9.
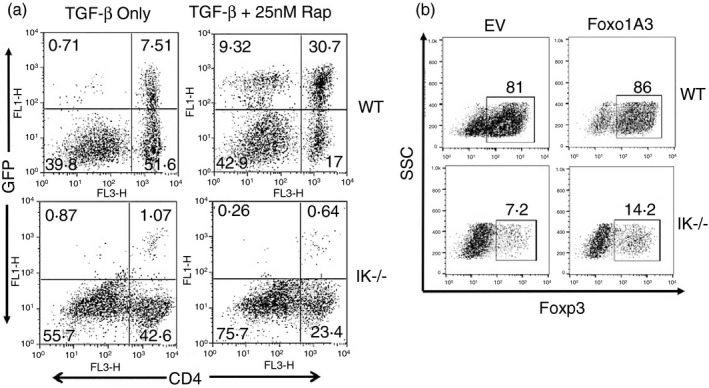
Rapamycin does not increase induced regulatory T (iTreg) cell differentiation in the absence of Ikaros but restoring Foxo1 activity does. (a) GFP expression levels in iTreg cell differentiation cultures performed with wild‐type or Ikaros null (IK −/−) splenocytes from Foxp3/GFP reporter mice either with or without 25 nm rapamycin. Data are representative of two independent experiments. (b) Purified splenic CD4 T cells from Ikaros null (IK−/−) and wild‐type mice were plated under iTreg cell differentiation conditions and transduced with retroviruses prepared from the MSCV Thy‐1.1 construct (EV) or the MSCV Foxo1‐A3 Thy‐1.1 construct (Foxo1‐A3). Data shown are intracellular Foxp3 staining in successfully transduced Thy‐1.1+ cells 4 days after transduction. Data are representative of two independent experiments.
Therefore, next we wanted to directly test if increasing Foxo1 activity by retroviral transduction could increase iTreg differentiation in Ikaros null iTreg cultures. Induction of TCR and cytokine signalling pathways required for iTreg differentiation results in phosphorylation of Foxo1, which leads to its nuclear export and functional inactivation. Therefore, to drive nuclear localization of Foxo1 in the presence of these differentiation signals, the constitutively active form of Foxo1, Foxo1‐A3 was used.21 CD4 T cells were activated in the presence of TGF‐β and IL‐2 for 24 hr, followed by retroviral transduction. Foxo1‐A3 transduction increased iTreg cell differentiation approximately twofold in Ikaros null cultures, demonstrating that lack of Foxo1 contributes to abrogation of iTreg differentiation in these cells (Fig. 9b).
Discussion
Ikaros is a transcription factor expressed at high levels in T cells. It regulates events after TCR signalling that include T‐cell proliferation, T helper cell differentiation and cytokine gene expression.12, 16, 17, 38 Yet, its role in naive T cells was unexplored. Our studies reveal a new role for Ikaros in the expression of receptors that govern homing and survival of naive T cells, and define it as the first identified regulator of Foxo1 expression in quiescent T cells.
The importance of Ikaros in quiescent mature T cells was revealed through the study of CD4 T cells from genetically engineered Ikaros null mice. Ikaros null CD4 T cells show decreased survival in response to IL‐7 and, when adoptively transferred, display defective homing, with a decreased ability to home to lymph nodes. We identified the likely mechanism underlying these defects as significantly reduced expression of IL‐7Rα and CD62L. Reduced expression is not due to expansion of abnormal T‐cell populations in the periphery, because it is observed before Ikaros null CD4 T cells exit the thymus. In addition, peripheral Ikaros null CD4 T cells display no other hallmarks of activation. Therefore, we have demonstrated, for the first time, an important role for Ikaros in quiescent peripheral T cells as a lack of Ikaros impedes their ability to express receptors required for them to survive and home appropriately.
We have demonstrated that the likely mechanism underlying decreased expression of IL‐7Rα and CD62L is severely reduced expression of Foxo1 in Ikaros null T cells. Decreased Foxo1 expression is observed at both the protein and transcript levels, suggesting that Ikaros regulates Foxo1 expression at the level of transcription. Despite its important functions in T cells, little is known about how Foxo1 is regulated at the transcriptional level. To our knowledge, signal transducer and activator of transcription 3 (STAT3) is the only identified transcriptional regulator of the Foxo1 in T cells.39, 40 However, it was described as an activator of Foxo1 in Th17 cells downstream of IL‐6 signalling. STAT3 could not contribute to regulation of Foxo1 in quiescent T cells as it would not be active. Our data demonstrating that restoring expression of Ikaros to Ikaros null CD4 T cells, as well as increasing its expression in wild‐type CD4 T cells, increases levels of Foxo1 mRNA, supports a role for Ikaros as regulator of Foxo1 gene expression. Admittedly, our data do not distinguish between a direct and an indirect role for Ikaros. We undertook chromatin immunoprecipitation experiments using anti‐Ikaros antibodies to demonstrate direct binding of Ikaros to the Foxo1 locus in CD4 T cells. However, numerous attempts with various monoclonal and polyclonal antibodies did not prove successful, as enrichment for Ikaros binding to the il2 promoter, a positive control gene to which Ikaros directly binds,16 was not observed in any experiment. This led us to conclude that the currently available Ikaros antibodies were not appropriate for chromatin immunoprecipitation experiments. However, reports by others showing direct binding of Ikaros to the Foxo1 locus in pre‐B cells33 and haematopoietic stem cells41 support a direct role.
Rescue transduction experiments in Ikaros null CD4 T cells reveal that regulation by Ikaros occurs by two different mechanisms. Transduction of Ikaros or Foxo1/Foxo1‐A3 was only able to increase expression of IL‐7Rα or CD62L, respectively. It is important to note that transduction of Ikaros increases the expression of both Ikaros and Foxo1, whereas transduction of Foxo1 or Foxo1‐A3 increases the expression of only Foxo1, leaving the cells Ikaros‐deficient. Taking this into consideration, our data suggest that both Ikaros and Foxo1 are directly required for regulating expression of Il7ra. This mode of dual regulation is common in pre‐B cells where 56% of Foxo1 target genes are also bound by Ikaros.33 It is puzzling that Ikaros transduction could not increase CD62L expression, especially as expression of dominant negative Ikaros leads to down‐regulation of CD62L expression in mouse T cells (see Supplementary material, Fig. S1), as well as in the CEM T‐cell line and CD34+ cells isolated from human bone marrow.42 This could be the result of an altered Ikaros/Foxo1 ratio as transduction has been linked to over‐expression of the transduced proteins relative to wild‐type levels. Relatively increased Ikaros levels could result in its binding to low‐affinity sites that in turn may interfere with Foxo1's ability to activate the Sell locus either through directly interfering with Foxo1 binding or by interfering with binding of another critical transcription factor.
The importance of Foxo1 in iTreg cell development led us to investigate if Ikaros also plays a role in this process. Lack of Foxo1 not only compromises iTreg cell development but also leads to the appearance of cells with a Th1 phenotype in iTreg cell differentiation cultures.3 This is because Foxo1, the activity of which is reinforced by the iTreg cell differentiation cytokine TGF‐β, is required to maintain Foxp3 expression, which otherwise would be lost as a consequence of the TCR signalling that is required for iTreg cell differentiation.43 Ikaros null CD4 T cells also demonstrate an inability to attain the iTreg cell fate, and instead appear to attain the phenotype of effector T helper cells (unpublished data, P. Agnihotri and S. Winandy). We propose that Ikaros is essential for naive CD4 T cells to maintain the high levels of Foxo1 required to prevent induction of effector lineages and to promote iTreg cell differentiation upon TCR stimulation in the presence of TGF‐β. In the absence of Ikaros, levels of Foxo1 are too low to accomplish this important function. When Foxo1 activity was restored using retroviral transduction, the iTreg cell differentiation potential of Ikaros null CD4 T cells increased about twofold, but was not rescued to wild‐type levels. This relatively modest effect could be due to the fact that Foxo1 activity was not restored until 24 hr after the cells had received TCR stimulation. If, as we propose, levels of Foxo1 must be high in the naive T cell to block TCR‐induced activation of genes that facilitate differentiation of effector lineages, this rescue may be too late. Another possibility is that Ikaros has other roles essential for iTreg differentiation that are not rescued by Foxo1 transduction.
Taken together, these studies contribute to defining a novel Ikaros/Foxo1 axis involved in regulation of important processes in naive CD4 T‐cell development and function. We have demonstrated a lack of Foxo1 and Foxo1 target gene expression in the absence of Ikaros. Transduction experiments led to the creation of a regulatory circuit, placing Ikaros upstream of Foxo1, in expression of the Foxo1 gene itself as well as its target genes important to naive T‐cell survival, homing and iTreg differentiation.
Disclosures
The authors have no financial or commercial conflicts of interest.
Supporting information
Figure S1. Interference with Ikaros or Foxo1 activity in wild‐type T cells decreases expression of Foxo1 targets.
Figure S2. Decreased acetyl‐H3 is observed at the Foxo1 promoter in Ikaros null CD4 T cells.
Figure S3. Transduction of Ikaros into Ikaros null CD4 T cells increases Foxo1 protein levels.
Acknowledgements
We wish to thank Dr. Barbara Slack for the rapamycin, Dr. Hans Dooms for help with the intracellular phospho‐Akt staining, and the Boston University Flow Cytometry Core Facility. This work was supported by Nancy L.R. Bucher Funds awarded to SW. PA was supported by the Immunology Training Program (ITP) training grant funded by the National Institutes of Health (T32 A1007309). SEU was supported by NIH F30 ES015971. SW conceived and coordinated the study. PA and SW wrote the paper. PA, NMR and SEU designed, performed and analysed the experiments. KA contributed to experiments shown in Figs 1(a) and 8(e), and Fig. S3.
References
- 1. Takada K, Jameson SC. Naive T cell homeostasis: from awareness of space to a sense of place. Nat Rev Immunol 2009; 9:823–32. [DOI] [PubMed] [Google Scholar]
- 2. Arbonés ML, Ord DC, Ley K, Ratech H, Maynard‐Curry C, Otten G et al Lymphocyte homing and leukocyte rolling and migration are impaired in l‐selectin‐deficient mice. Immunity 1994; 1:247–60. [DOI] [PubMed] [Google Scholar]
- 3. Kerdiles YM, Stone EL, Beisner DR, Beisner DL, McGargill MA, C'hen IL et al Foxo transcription factors control regulatory T cell development and function. Immunity 2010; 33:890–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ouyang W, Beckett O, Flavell RA, Li MO. An essential role of the Forkhead‐Box transcription factor Foxo1 in control of T cell homeostasis and tolerance. Immunity 2009; 30:358–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ouyang W, Beckett O, Ma Q, Paik J, DePinho RA, Li MO. Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nat Immunol 2010; 11:618–27. [DOI] [PubMed] [Google Scholar]
- 6. Ouyang W, Liao W, Luo CT, Yin N, Huse M, Kim MV et al Novel Foxo1‐dependent transcriptional programs control Treg cell function. Nature 2012; 491:554–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tejera MM, Kim EH, Sullivan JA, Plisch EH, Suresh M. FoxO1 controls effector‐to‐memory transition and maintenance of functional CD8 T cell memory. J Immunol 2013; 191:187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim M, Ouyang W, Liao W, Zhang M, Li M. The transcription factor foxo1 controls central‐memory CD8+ T cell responses to infection. Immunity 2013; 39:286–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Georgopoulos K, Moore DD, Derfler B. Ikaros, an early lymphoid‐specific transcription factor and a putative mediator for T cell commitment. Science 1992; 258:808–12. [DOI] [PubMed] [Google Scholar]
- 10. Molnár A, Wu P, Largespada DA, Vortkamp A, Scherer S, Copeland NG et al The Ikaros gene encodes a family of lymphocyte‐restricted zinc finger DNA binding proteins, highly conserved in human and mouse. J Immunol 1996; 156:585–92. [PubMed] [Google Scholar]
- 11. Kim J, Sif S, Jones B, Jackson A, Koipally J, Heller E et al Ikaros DNA‐binding proteins direct formation of chromatin remodeling complexes in lymphocytes. Immunity 1999; 10:345–55. [DOI] [PubMed] [Google Scholar]
- 12. Wang JH, Nichogiannopoulou A, Wu L, Sun L, Sharpe AH, Bigby M et al Selective defects in the development of the fetal and adult lymphoid system in mice with an Ikaros null mutation. Immunity 1996; 5:537–49. [DOI] [PubMed] [Google Scholar]
- 13. Umetsu SE, Winandy S. Ikaros is a regulator of Il10 expression in CD4+ T cells. J Immunol 2009; 183:5518–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Quirion MR, Gregory GD, Umetsu SE, Winandy S, Brown MA. Cutting edge: Ikaros is a regulator of Th2 cell differentiation. J Immunol 2009; 182:741–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bandyopadhyay S, Duré M, Paroder M, Soto‐Nieves N, Puga I, Macián F. Interleukin 2 gene transcription is regulated by Ikaros‐induced changes in histone acetylation in anergic T cells. Blood 2007; 109:2878–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thomas RM, Chunder N, Chen C, Umetsu SE, Winandy S, Wells AD. Ikaros enforces the costimulatory requirement for IL2 gene expression and is required for anergy induction in CD4+ T lymphocytes. J Immunol 2007; 179:7305–15. [DOI] [PubMed] [Google Scholar]
- 17. Avitahl N, Winandy S, Friedrich C, Jones B, Ge Y, Georgopoulos K. Ikaros sets thresholds for T cell activation and regulates chromosome propagation. Immunity 1999; 10:333–43. [DOI] [PubMed] [Google Scholar]
- 18. Wong LY, Hatfield JK, Brown MA. Ikaros sets the potential for Th17 lineage gene expression through effects on chromatin state in early T Cell Development. J Biol Chem 2013; 288:35170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M et al Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006; 441:235–8. [DOI] [PubMed] [Google Scholar]
- 20. Kathrein KL, Lorenz R, Innes AM, Griffiths E, Winandy S. Ikaros induces quiescence and T‐cell differentiation in a leukemia cell line. Mol Cell Biol 2005; 25:1645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yusuf I, Zhu X, Kharas MG, Chen J, Fruman DA. Optimal B‐cell proliferation requires phosphoinositide 3‐kinase‐dependent inactivation of FOXO transcription factors. Blood 2004; 104:784–7. [DOI] [PubMed] [Google Scholar]
- 22. Gallatin WM, Weissman IL, Butcher EC. A cell‐surface molecule involved in organ‐specific homing of lymphocytes. Nature 1983; 304:30–4. [DOI] [PubMed] [Google Scholar]
- 23. Bradley LM, Watson SR, Swain SL. Entry of naive CD4 T cells into peripheral lymph nodes requires L‐selectin. J Exp Med 1994; 180:2401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jung TM, Gallatin WM, Weissman IL, Dailey MO. Down‐regulation of homing receptors after T cell activation. J Immunol 1988; 141:4110–7. [PubMed] [Google Scholar]
- 25. Park JH, Yu Q, Erman B, Appelbaum JS, Montoya‐Durango D, Grimes HL et al Suppression of IL7Rα transcription by IL‐7 and other prosurvival cytokines: a novel mechanism for maximizing IL‐7‐dependent T cell survival. Immunity 2004; 21:289–302. [DOI] [PubMed] [Google Scholar]
- 26. Puré E, Cuff CA. A crucial role for CD44 in inflammation. Trends Mol Med 2001; 7:213–21. [DOI] [PubMed] [Google Scholar]
- 27. Fabre S, Carrette F, Chen J, Lang V, Semichon M, Denoyelle C et al FOXO1 regulates l‐selectin and a network of human T cell homing molecules downstream of phosphatidylinositol 3‐kinase. J Immunol 2008; 181:2980–9. [DOI] [PubMed] [Google Scholar]
- 28. Dang X, Xiaojiong L, Yuefen L. FOXO1 up‐regulates human L‐selectin expression through binding to a consensus FOXO1 motif. Gene Regul Syst Bio. 2012; 6:139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kerdiles YM, Beisner DR, Tinoco R, Dejean AS, Castrillon DH, DePinho RA et al Foxo1 links homing and survival of naive T cells by regulating l‐selectin, CCR7 and interleukin 7 receptor. Nat Immunol 2009; 10:176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS et al Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999; 96:857–68. [DOI] [PubMed] [Google Scholar]
- 31. Gubbels Bupp MR, Edwards B, Guo C, Wei D, Chen G, Wong B et al T cells require Foxo1 to populate the peripheral lymphoid organs. Eur J Immunol 2009; 39:2991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Van Der Heide LP, Hoekman MFM, Smidt MP. The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J 2004; 380:297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ferreir ´ os‐Vidal I, Carroll T, Taylor B, Terry A, Liang Z, Bruno L et al Genome‐wide identification of Ikaros targets elucidates its contribution to mouse B‐cell lineage specification and pre‐B‐cell differentiation. Blood 2013; 121:1769–82. [DOI] [PubMed] [Google Scholar]
- 34. Ng SYM, Yoshida T, Zhang J, Georgopoulos K. Genome‐wide lineage‐specific transcriptional networks underscore Ikaros‐dependent lymphoid priming in hematopoietic stem cells. Immunity 2009; 30:493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heller JJ, Schjerven H, Li S, Lee A, Qiu J, Chen Z‐ME et al Restriction of IL‐22‐produ cing T cell responses and differential regulation of regulatory T cell compartments by zinc finger transcription factor Ikaros. J Immunol 2014; 193:3934–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M et al T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci USA 2008; 105:7797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Haxhinasto S, Mathis D, Benoist C. The AKT‐mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med 2008; 205:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Georgopoulos K, Bigby M, Wang JH, Molnar A, Wu P, Winandy S et al The Ikaros gene is required for the development of all lymphoid lineages. Cell 1994; 79:143–56. [DOI] [PubMed] [Google Scholar]
- 39. Oh HM, Yu CR, Dambuza I, Marrero B, Egwuagu CE. STAT3 protein interacts with class O forkhead transcription factors in the cytoplasm and regulates nuclear/cytoplasmic localization of FoxO1 and FoxO3a proteins in CD4+ T cells. J Biol Chem 2012; 287:30436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Oh HM, Yu CR, Golestaneh N, Amadi‐Obi A, Lee YS, Eseonu A et al STAT3 protein promotes T‐cell survival and inhibits interleukin‐2 production through up‐regulation of class O forkhead transcription factors. J Biol Chem 2011; 286:30888–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brown KE, Guest SS, Smale ST, Hahm K, Merkenschlager M, Fisher AG. Association of transcriptionally silent genes with Ikaros complexes at centromeric heterochromatin. Cell 1997; 91:845–54. [DOI] [PubMed] [Google Scholar]
- 42. Christopherson I, Piechoki M, Liu G, Ratner S, Galy A. Regulation of L‐selectin expression by a dominant negative Ikaros protein. J Leukoc Biol 2001; 69:675–83. [PubMed] [Google Scholar]
- 43. Bothur E, Raifer H, Haftmann C, Stittrich A‐B, Brüstle A, Brenner D et al Antigen receptor‐mediated depletion of FOXP3 in induced regulatory T‐lymphocytes via PTPN2 and FOXO1. Nat Commun 2015; 6:8576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Interference with Ikaros or Foxo1 activity in wild‐type T cells decreases expression of Foxo1 targets.
Figure S2. Decreased acetyl‐H3 is observed at the Foxo1 promoter in Ikaros null CD4 T cells.
Figure S3. Transduction of Ikaros into Ikaros null CD4 T cells increases Foxo1 protein levels.


