Abstract
Background
Implementation of ivermectin-based community treatment for onchocerciasis or lymphatic filariasis elimination has been delayed in Central Africa because of severe adverse events (SAEs), including death, in people with high levels of circulating Loa loa microfilariae (mf). LoaScope, a rapid field-friendly diagnostic tool to quantify L. loa mf in peripheral blood, permits point-of-care identification of individuals “at risk” for SAEs.
Methods
A “Test and not Treat” (TaNT) strategy was used to implement ivermectin treatment in the Okola health district in Cameroon, where ivermectin distribution was halted in 1999 after the occurrence of fatal Loa-related SAEs. The LoaScope was used to identify and exclude individuals with >20,000 mf per milliliter of blood (at-risk for SAEs) from ivermectin treatment. Active surveillance for post-treatment adverse events (AEs) was conducted daily for 7 days.
Results
Between August and October 2015, 16,259 (71.1%) individuals >=5 years of age were tested out of a target population of ~22,800. Among the ivermectin-eligible population, 15,522 (95.5%) received ivermectin; 340 (2.1%) were excluded from ivermectin treatment because of a L. loa density above the risk-threshold and 397 (2.4%) were excluded for pregnancy or illness. No SAEs were observed. Non-severe AEs were recorded in 934 individuals, most (67%) of whom had no detectable L. loa mf.
Conclusions
The LoaScope-based TaNT strategy permitted safe re-implementation of community-wide ivermectin distribution in a heretofore ‘off limits’ health district in Cameroon and is an extremely promising and practical approach for large-scale ivermectin treatment for lymphatic filariasis and onchocerciasis elimination in Loa loa-endemic areas.
Introduction
Mass drug administration (MDA) with ivermectin-containing regimens is the main strategy for elimination of lymphatic filariasis and onchocerciasis. Although generally safe, ivermectin distribution has led to severe adverse events (SAEs) in central African countries. More than 500 cases of characteristic post-ivermectin encephalopathy1 including ~60 fatal case, occurred during MDA and have therefore been reported to the Mectizan Donation Program since 1990. Of note, these neurologic SAEs have occurred exclusively in individuals with peripheral blood Loa loa microfilarial densities >30,000 microfilariae (mf) per milliliter,1 2 and are presumed to be related to eosinophil-mediated inflammation around dying mf and/or micro-embolization with subsequent loss of central nervous system vascular integrity.
Current WHO guidelines allow ivermectin-based MDA to be implemented in areas where onchocerciasis is meso- or hyperendemic because the potential benefits of MDA are felt to outweigh the risk of ivermectin-associated SAEs, although enhanced surveillance for adverse events (AEs) is required. However, areas endemic for loiasis and hypo-endemic for onchocerciasis are spread throughout Central Africa,3 and remain a serious problem. For these areas, a “Test and (not) Treat” (TaNT) strategy has been proposed, wherein individuals with high L. loa mf loads (at risk for SAEs) are excluded from ivermectin treatment and the remaining population (typically >95%) can be treated safely.
Successful implementation of the TaNT strategy requires a rapid, point-of-contact, fieldfriendly and highly accurate method to quantify L. loa mf. To this end, a mobile phone-based videomicroscope – the LoaScope (previously CellScope Loa) – was developed.4 The LoaScope automatically counts L. loa mf in peripheral blood collected in disposable rectangular capillaries without the need for sample processing using a smartphone coupled to a simple optical device (Figure S1 and Movie S1).4
To advance O. volvulus elimination in L. Loa co-endemic countries in Central Africa, we tested the feasibility of this TaNT strategy in the Okola health district in Cameroon, where ivermectin distribution was halted in 1999 after the occurrence of Loa-related encephalopathy. As such, the TaNT strategy allowed the safe reintroduction of ivermectin in all of the communities in this health district without provoking a single SAE.
METHODS
Study site
The Okola health district (Figure S2) includes 11 health areas where, in 1999 MDA was halted by the Ministry of Public Health following 23 cases of encephalopathy that occured during the first treatment campaign. MDA only resumed in 5/11 areas deemed hyper- or mesoendemic (>20% onchocercal nodule prevalence in adult males). In 2013, nodule surveys in the 6 excluded health areas had prevalences of 6% to 40% consistent with hypo- or meso-endemic onchocerciasis.5 The entire Okola health district is known to be highly endemic for L. loa.6 7
Study design
The TaNT strategy was implemented in the 92 villages of the 6 health areas untreated since 1999 (Figure S2). The timeline of the TaNT project is depicted in Figure S3 and the process is described in the Supplementary Methods. All individuals aged >=5 years were invited to participate.
The TaNT process consisted of registration of consenting (or assenting) individuals >=5 years of age, LoaScope quantification of L. loa microfilarial density, treatment of eligible individuals with ivermectin (150μg/kg) and surveillance for AEs. Non-pregnant subjects excluded from ivermectin distribution because of high L. loa mf counts were given albendazole (400 mg) for intestinal deworming. Self-declared pregnant women were not treated with ivermectin or albendazole, but were offered iron and folic acid tablets. Each participant was given a card (Figure S4) with their L. loa mf count, the treatment received and a contact phone number for questions and/or reporting of AEs.
Quantification of L. loa microfilaremia
The use of the LoaScope and its performance have been described previously.4 A threshold of 26,000 mf per milliliter was initially selected for ivermectin treatment, based on the lower 95% confidence interval around the 30,000 mf per milliliter threshold below which no neurologic SAEs were observed in prior studies8-10 and the calculated false negativity rate of 1 in 10 million (<0.00001%).4 Two weeks after the study start, a case of conjunctival hemorrhage, similar to those described previously,11 occurred in a subject with a LoaScope L. loa mf count of 24,599 mf per milliliter. For potential safety reasons, the exclusion threshold of the LoaScope was decreased to 20,000 mf per milliliter for the remainder of the trial.
Calibrated (50 μL) thick smears were performed as a backup for samples unable to be analyzed with the LoaScope, to identify and quantify Mansonella perstans mf, and to corroborate the accuracy of the LoaScope. Smears were read by 2 different microscopists blinded to the LoaScope results. Dried blood spots collected on filter paper were archived and stored at -80°C.
Assessment of exposure to onchocerciasis
Ov16 IgG4 antibodies positivity from eluted single blood spots (10 μl equivalent) was determined the SD Bioline Onchocerciasis IgG4 Rapid Test.13,14
Results were read and recorded at 24 hours.
Monitoring of post-treatment adverse reactions
Monitoring for AEs was performed by two surveillance teams, each composed of a physician and a driver, with the assistance of selected community members (pre-Community Drug Distributors (pre-CDDs)) and local nurses. The surveillance teams visited each village on days 1, 2, 3 and 6 post-treatment, examined all individuals complaining of AEs and provided symptomatic treatment if indicated. In addition, the team toured the entire community by car to identify additional individuals with AEs. All AEs were recorded using a standardized form (Figure S5). A Karnofsky performance score index was assigned to each patient examined. Clinical management was based on reference guidelines.15
Statistical analysis
Medians and interquartile ranges were used as measurements of central tendency. Associations between individual factors (gender, age, L. Loa mf density (assessed by LoaScope), presence of Ov16 IgG4, presence of M. perstans mf (assessed by calibrated blood smear microscopy) and the occurrence of AEs were assessed using multivariable logistic regression.
The logistic regression coefficients were used to calculate population attributable fractions.16 The calibrated thick smear was used as the reference test for assessment of the specificity and negative predictive value of the LoaScope.
Ethical agreement
This study was authorized by the National Ethics Committee of Cameroon (ethical clearance n° 2013/11/370/L/CNERSH/SP) and approved by the Division of Operational Research at the Ministry of Health (Administrative authorization n° D30- 571/L/MINSANTE/SG/DROS/CRSPE/BBM). All volunteers provided written signed consent (or parental consent in the case of minors) before undergoing blood sampling and again before receiving treatment.
Authors’ contributions to the study
Authors’ contributions to this study are as follows: JK, SDP, CDM, ADK, TBN and MB designed the study; JK, SDP, CBC, MHB, MVDA CDM, HCND, RGK, GRN, PN, JBTM, SW, DAF, ADK, TBN and MB gathered the data; SDP and CBC analyzed the data; TNB and MB vouch for the data and the analysis; MHB, MVDA, DAF provided diagnostic technology development and support; JK, SDP, CDM, HCND, WAS, DAF, ADK, TBN and MB wrote the paper; and JK, SDP, CDM, DAF, ADK, TBN and MB decided to publish the paper. SDP wrote the first draft of the manuscript.
RESULTS
Population Characteristics
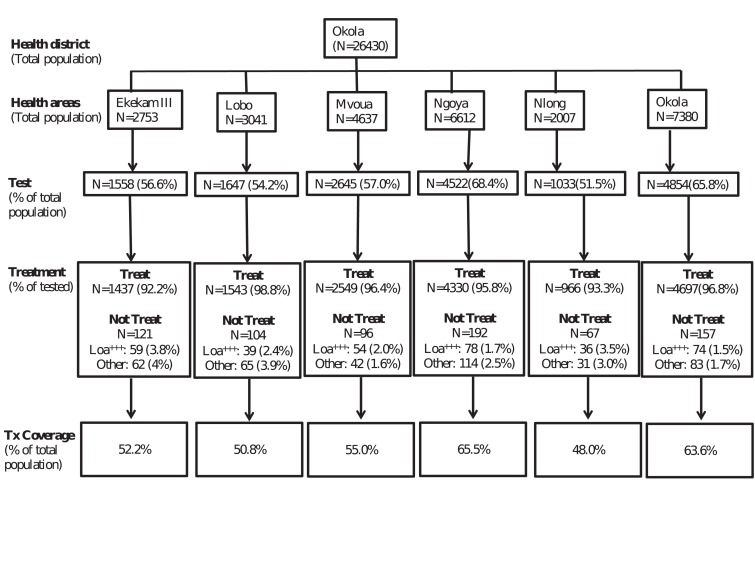
A total of 16,259 individuals were examined during the TaNT process (Figure 1). The median age of the examined populations ranged from 17 to 26 years in the different health areas and the sex distribution was relatively equal (48% male). The prevalence of Ov16 IgG4 antibody in the six health areas varied from 15.3% to 29.9%. The prevalence of L. loa microfilaremia varied from 15.3% to 22.8%, and the proportion of individuals with more than 20,000 Loa mf per milliliter as determined by LoaScope ranged from 1.3% in the Ngoya health area to 2.4% in the Nlong and Ekekam III health areas (Table 1, Table S2).
Figure 1.
Flowchart of the population examined for L. loa microfilaremia and treated with ivermectin in the six health areas of the Okola district. Loa+++ indicates the number of individuals identified as at risk of post-ivermectin severe adverse events and excluded from ivermectin treatment.
Table 1. Demographics, onchocerciasis prevalence and L. loa microfilaremia levels in the population of the six health areas of the Okola district (Cameroon).
| . | Census | Median age (IQR) | M/F | O. volvulus antibody positivity (%) | L. loa microfilaremia (%) | |||
| Prevalence | < 8000 mf per milliliter | 8-20,000 mf per milliliter | > 20,000 mf per milliliter | |||||
| Ekekam III | 2753 | 26 (13-49) | 0.51 | 18.8 | 22.8 | 91.7 | 5.9 | 2.4 |
| Lobo | 3041 | 23 (12-45) | 0.50 | 29.9 | 21.3 | 93.1 | 5.3 | 1.6 |
| Mvoua | 4637 | 17 (10-43) | 0.49 | 20.2 | 18.9 | 94.4 | 3.7 | 1.9 |
| Ngoya | 6612 | 17 (10-38) | 0.47 | 15.3 | 15.3 | 95.4 | 3.3 | 1.3 |
| Nlong | 2007 | 20 (12-50) | 0.51 | 23.8 | 20.3 | 94.5 | 3.1 | 2.4 |
| Okola | 7380 | 18 (11-38) | 0.58 | 27 | 16.1 | 95.1 | 3.5 | 1.4 |
| Total | 26430 | 18 (11-42) | 0.48 | 22.4 | 17.8 | 94.5 | 3.9 | 1.6 |
Table 2. Adverse events recorded during the post-treatment surveillance process.
| Adverse Events | No of adverse events | No. with Loa mf (%) | No. with no Loa mf (%) | P value |
|---|---|---|---|---|
| Pruritus | 564 | 188 (33.3) | 376 (66.7) | <0.001 |
| Asthenia | 389 | 171 (44) | 218 (56) | 0.002 |
| Headache | 326 | 149 (45.7) | 177 (54.3) | 0.14 |
| Rash | 274 | 52 (19) | 222 (81) | <0.001 |
| Back Pain | 257 | 128 (49.8) | 129 (50.2) | 0.97 |
| Arthralgias | 235 | 124 (52.8) | 111 (47.2) | 0.39 |
| Edema | 125 | 21 (16.8) | 104 (83.2) | <0.001 |
| Myalgia | 115 | 51 (44.4) | 64 (55.6) | 0.20 |
| Vertigo | 106 | 48 (45.3) | 58 (54.7) | 0.35 |
| Anorexia | 89 | 42 (47.2) | 47 (52.8) | 0.57 |
| Abdominal pain | 67 | 19 (28.4) | 48 (71.6) | <0.001 |
| Blurred vision | 66 | 27 (40.9) | 39 (59) | 0.15 |
| Difficulty ambulating | 58 | 27 (46.6) | 31 (53.4) | 0.65 |
| Diarrhea | 46 | 18 (39.1) | 28 (60.9) | 0.14 |
| Difficulty in getting upright | 37 | 20 (54) | 17 (46) | 0.63 |
| Lymphadenopathy | 23 | 8 (34.8) | 15 (65.2) | 0.17 |
| Conjunctival hemorrhage | 20 | 14 (68.4) | 6 (31.6) | 0.14 |
| Conjunctival itching | 13 | 7 (53.9) | 6 (46.2) | 0.77 |
| Tinnitus | 6 | 4 (66.7) | 2 (33.3) | Not tested |
| Temporary hearing loss | 2 | 0 (0) | 2 (100) | Not tested |
| Total | 2818 | 1118 (39.7) | 1702 (60.4) | <0.001 |
Test and Not Treat
Between 50 and 100 participants were typically examined per village per day. The mean time from finger prick to LoaScope result was 2-3 minutes. The LoaScope results were immediately available for 16,099/16,259 individuals (99%) and were delayed for 160 individuals (1%) because of technical problems requiring determination of the mf count by calibrated thick smear. Ivermectin was administered to 15,522 individuals (95.5%) with mf levels below the established threshold.
Seven hundred and thirty seven (4.5%) subjects were excluded from ivermectin therapy. Of these, 340 (2.1%) were excluded because of a L. loa density above the risk-threshold, 228 (1.4%) because of poor health (signs or symptoms consistent with a serious acute or chronic concomitant illness) or inebriation, and 169 (1%) because of pregnancy or breastfeeding. The proportion of excluded individuals per village varied from 0% to 15.1% (Figure S6). All excluded individuals (except pregnant women), were treated with albendazole (400 mg). The median treatment coverage in the district was 55% of the total population (interquartile range between villages: 42.9–64.1%), and 64% of the targeted population.
The prevalence of O. volvulus-specific antibody (Ov16 IgG4) was 22.0% in individuals who received ivermectin, 25.4% in those excluded for pregnancy or illness, and 33.5% in those with a L. loa density above the risk-threshold. Thus, individuals who were not treated because of Loa microfilaremia and who were potentially infected with O. volvulus represented only 0.7% of the examined population.
Frequency and types of AEs
Among the 15,522 individuals treated with ivermectin, 934 (6%) had documented AEs. The incidence of AEs decreased slightly from 6.6% (464/7,065) to 5.6% (470/8,457) (p<0.0001) after reducing the exclusion threshold from 26,000 to 20,000 mf per milliliter. Dermatologic manifestations were most common, followed by systemic and rheumatologic manifestations (Table 2). Eight hundred and sixty-nine people (93%) had a Karnofsky score of 90, and 65 (7%) had a score of 80. All AEs resolved within one week without treatment or with basic supportive therapy (anti-histamines, non-steroidal anti-inflammatory drugs, or acetaminophen).
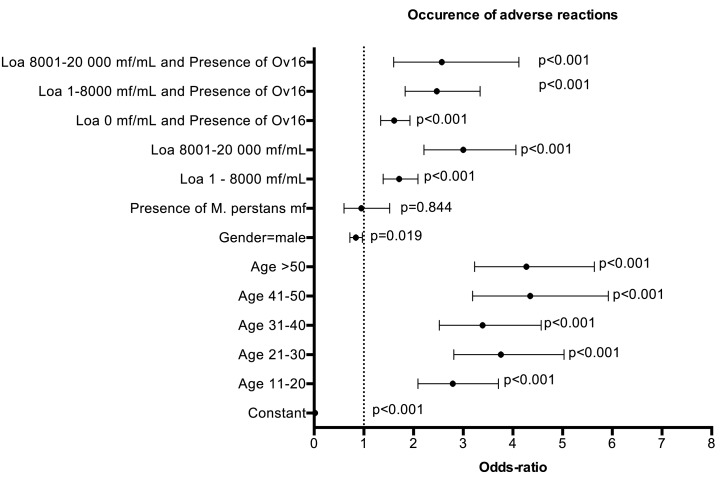
Both L. loa microfilaremia and the presence of Ov16-specific IgG4 were assessed in 888 of the 934 individuals who developed an AE. Among these, 43.2% had neither L. loa mf nor Ov16 IgG4, 22.3% had only L. loa mf, 23.9% had only Ov16 IgG4, and 10.6% had both L. loa mf and Ov16 IgG4. Multivariable regression indicates that AEs were significantly more frequent in older individuals, females, and individuals with either L. loa mf or Ov16 IgG4 (Figure 2). The risk of AEs associated with presence of Ov16 IgG4 was similar to that associated with harboring 1-8000 Loa mf per milliliter (Odds ratio (OR)=1.61 and 1.71, respectively) and was about half the risk associated with harboring 8000-20,000 Loa mf per milliliter (OR=3.00). The risk of AEs associated with both L. loa microfilaremia and O. volvulus IgG4 was similarly increased in persons harboring 1-8000 L. loa mf per milliliter (OR=2.47) and in those harboring 8000-20,000 L. loa mf per milliliter. Population attributable fractions of AEs for L. loa mf density of 1-8000 mf per milliliter, 8000-20,000 mf per milliliter and Ov16 IgG4 were 8.0%, 8.3% and 12.2%, respectively.
Figure 2.
Results of multivariate logistic regression of occurrence of post-ivermectin adverse events in relation to individual factors Dots with error bars represent odds-ratios (OR) and 95% confidence intervals. The dotted line (OR=1) 1 represents an absence of association.
Agreement between LoaScope and calibrated blood smear microscopy
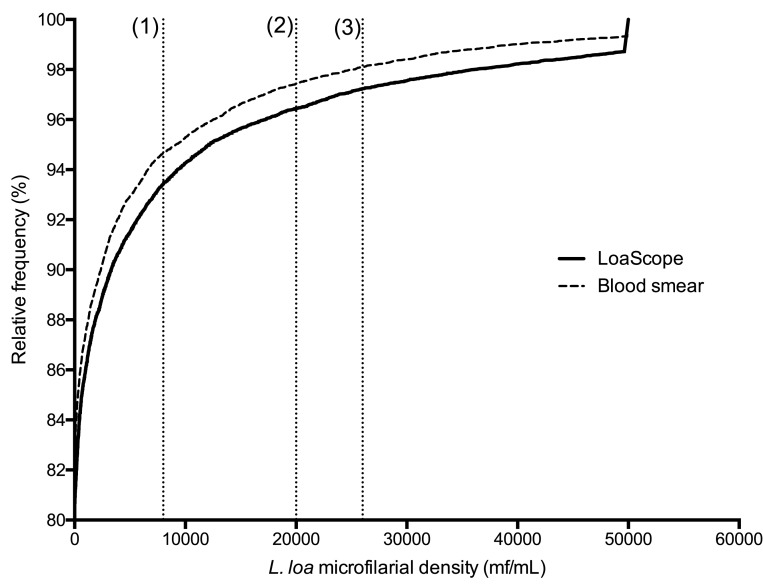
Figure 3 shows that the distributions of L. loa mf density in the population using the LoaScope and thick smear microscopy were similar. The specificity and negative predictive values of the LoaScope to identify individuals with mf counts below 20,000 mf per milliliter (as assessed by microscopy) were 99.7% (95% confidence interval: 99.6 – 99.8) and 99.7% (99.6 – 99.7), respectively.
Figure 3.
Cumulative frequency distribution of L. loa microfilarial density in the population with tails of distribution censored for density above 50,000 mf per milliliter (mL). Dotted vertical lines (1), (2) and (3) correspond to the 8,000 (1), 20, 000 (2) and 26,000 )3) Loa mf/ml cutoffs used to determine treatment exclusion thresholds (2 and 3) and information relevant to the increased likelihood of adverse events (1) provided to each participant.
Discussion
Extension of ivermectin-based MDA to areas hypoendemic for onchocerciasis and coendemic for loiasis remains a significant obstacle to the success of onchocerciasis elimination programs in Africa. In the current study, a LoaScope-based TaNT strategy was used to safely treat more than 15,000 individuals with ivermectin in such an area. Although there was initial reticence to participate in some villages because of the memory of the SAEs (including deaths) that occurred in 1999, 16,259 of the 22,842 individuals aged >= 5 years old recorded during the initial census (71.1%) participated in the TaNT campaign. This suggests that TaNT is an acceptable strategy even in populations with a history of previous ivermectin-related SAEs. Though not formally assessed, it is likely that fear of SAEs was the main reason for non-participation.
During the first MDA campaign conducted in 1999 in Okola, 23 cases of neurological SAEs, including three fatalities, were recorded among the 6,000 individuals who received ivermectin before MDA was stopped.17 The incidences of post-ivermectin neurological SAEs and deaths were therefore 38/10,000 and 5/10,000, respectively. Extrapolating these data to the population enrolled in the present study, a minimum of 62 cases of neurological SAEs and 8 deaths were theoretically prevented by TaNT.
Although some individuals (6%) complained of ivermectin-associated AEs during the TaNT campaign, the proportion was lower than that typically observed after ivermectin MDA for onchocerciasis in areas not endemic for loiasis: 13.1% in south-east Nigeria,18 12% and 20% in northern Cameroon,19 and 21.4% in eastern Sudan.20 It was also much lower than the 26.3% recorded in a neighboring Loa-endemic area of central Cameroon.2 The most likely explanation for the lower frequency of AEs recorded in the Okola district is that onchocerciasis is hypo- and mesoendemic in this area.
The LoaScope operators underwent a 1-hour training session 2 weeks before the field operations. This training was sufficient for the entire study, and the teams noted the ease of use and reliability of the device despite daily use and demanding field conditions. Because L. loa mf are diurnally periodic,21 LoaScope examinations (and treatment) started at 10 am and ended at 4 pm. During this TaNT campaign, up to 162 individuals were examined per village per day.
Whereas the present study clearly shows that the TaNT procedure is safe and feasible at a district level, moving TaNT from the operational research arena to Central African-wide implementation will depend on a number of factors, including greater reliance on local personnel for the census and post-treatment surveillance and smaller teams (a “tester” and a “treater”) for the TaNT process itself. If only a single TaNT round were needed, this would have a major impact on the applicability of this approach at a larger scale. Since ivermectin has marked microfilaricidal and probable embryostatic activity against L. loa, marked and sustained reduction in L. loa mf density is expected for one year after the TaNT campaign. In fact, in a neighboring district, the average reduction in L. loa microfilarial load was >74% one year after a single dose of ivermectin, and no individual with a pre-treatment density <30,000 mf per milliliter had a count above this level after 12 months.22 Thus, it seems likely that a single community-wide round of TaNT will be necessary, with individual testing in subsequent years restricted to previously ivermectin-untreated individuals. This hypothesis, as well as operationality, performance and cost of a TaNT conducted by 3-member teams will be assessed in late 2017.
Given the low percentage (2.4%) of the total population excluded from ivermectin treatment and the proposed implementation of TaNT in areas hypo- and meso-endemic for onchocerciasis, it is unlikely that excluded individuals will be a significant reservoir of O. volvulus microfilariae at the community level. Nevertheless, some excluded individuals are likely to be infected with O. volvulus and, for ethical reasons, should be treated with effective and safe drug regimens, particularly in the setting of clinical manifestations of onchocerciasis. Although a 4-6-week course of doxycycline, a regimen known to be macrofilaricidal for O. volvulus23 but not L. loa24, is impractical at the community level, it could be used safely in this context.
In summary, this TaNT strategy based on a novel and scalable point-of-contact tool that allows rapid identification (and exclusion from ivermectin-based treatment) of individuals at risk of Loa-related SAEs has enabled district-level community treatment of onchocerciasis. Though this TaNT strategy was motivated by the need to tackle hypoendemic onchocerciasis in Central Africa, it could also be considered for other foci co-endemic for onchocerciasis and loiasis. As many (but not all) meso-hyperendemic areas are already covered by CDTI, TaNT would target ivermectinnaïve individuals and systematic non-compliers.
Supplementary Material
Funding and external contributions
The study was funded by the Bill and Melinda Gates Foundation and in part by the Division of Intramural Research, NIAID, NIH and the USAID through the Higher Education Solutions Network partnership with UC Berkeley. Ivermectin was provided by the Mectizan Donation Program through the National Programme for Onchocerciasis Control of Cameroon. KLA-Tencor (Milpitas, CA, USA) supported production of LoaScope devices. The sponsors had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Footnotes
Publisher's Disclaimer: This is an Author Final Manuscript, which is the version after external peer review and before publication in the Journal. The publisher’s version of record, which includes all New England Journal of Medicine editing and enhancements, is available at 10.1056/NEJMoa1612665..
Contributor Information
Joseph Kamgno, Centre for Research on Filariasis and other Tropical Diseases, Yaounde Cameroon; Faculty of Medicine and Biomedical Sciences, University of Yaounde I, Yaounde Cameroon.
Sébastien D. Pion, IRD UMI 233-INSERM U1175-Montpellier University, Montpellier, France.
Cédric B. Chesnais, IRD UMI 233-INSERM U1175-Montpellier University, Montpellier, France.
Matthew H. Bakalar, Department of Bioengineering & Biophysics Program, University of California - Berkeley, Berkeley, CA 94720, US.
Michael V. D'Ambrosio, Department of Bioengineering & Biophysics Program, University of California - Berkeley, Berkeley, CA 94720, USA.
Charles D. Mackenzie, Department of Pathobiology and Diagnostic Investigation, Michigan State University, East Lansing, USA.
Hugues C. Nana-Djeunga, Centre for Research on Filariasis and other Tropical Diseases, Yaounde Cameroon.
Raceline Gounoue-Kamkumo, Centre for Research on Filariasis and other Tropical Diseases, Yaounde Cameroon.
Guy-Roger Njitchouang, Centre for Research on Filariasis and other Tropical Diseases, Yaounde Cameroon.
Philippe Nwane, Centre for Research on Filariasis and other Tropical Diseases, Yaounde Cameroon.
Jules B. Tchatchueng-Mbouga, Centre for Research on Filariasis and other Tropical Diseases, Yaounde Cameroon.
Wilma A. Stolk, Department of Public Health, ErasmusMC, University Medical Center, Rotterdam, the Netherlands.
Daniel A. Fletcher, Department of Bioengineering & Biophysics Program, University of California - Berkeley, Berkeley, CA 94720, USA; Chan Zuckerberg Biohub, San Francisco, CA 94158, USA.
Amy D. Klion, Laboratory of Parasitic Diseases, National Institute of Allergy and Infectious Diseases, Bethesda, MD 20892, USA.
Thomas B. Nutman, Laboratory of Parasitic Diseases, National Institute of Allergy and Infectious Diseases, Bethesda, MD 20892, USA.
Michel Boussinesq, IRD UMI 233-INSERM U1175-Montpellier University, Montpellier, France.
References
- 1.Boussinesq M, Gardon J, Gardon-Wendel N, Chippaux J-P. Clinical picture, epidemiology and outcome of Loa-associated serious adverse events related to mass ivermectin treatment of onchocerciasis in Cameroon. Filaria J 2003;2 Suppl 1:S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gardon J, Gardon-Wendel N, Demanga-Ngangue, Kamgno J, Chippaux JP, Boussinesq M. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet 1997;350(9070):18–22. [DOI] [PubMed] [Google Scholar]
- 3.Kelly-Hope LA, Unnasch TR, Stanton MC, Molyneux DH. Hypo-endemic onchocerciasis hotspots: defining areas of high risk through micro-mapping and environmental delineation. Infect Dis Poverty 2015;1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Ambrosio MV, Bakalar M, Bennuru S, et al. Point-of-care quantification of blood-borne filarial parasites with a mobile phone microscope. Sci Transl Med 2015;7(286):286re4– 286re4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coffeng LE, Pion SDS, O'Hanlon S, et al. Onchocerciasis: The pre-control association between prevalence of palpable nodules and skin microfilariae. PLoS Negl Trop Dis 2013;7(4):e2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boussinesq M, Gardon J, Kamgno J, Pion S, Gardon-Wendel N, Chippaux JP. Relationships between the prevalence and intensity of Loa loa infection in the Central province of Cameroon. Ann Trop Med Parasitol 2001;95(5):495–507. [DOI] [PubMed] [Google Scholar]
- 7.Kouam MK, Tchatchueng-Mbougua JB, Demanou M, Boussinesq M, Pion SDS, Kamgno J. Impact of repeated ivermectin treatments against onchocerciasis on the transmission of loiasis: an entomologic evaluation in central Cameroon. Parasit Vectors 2013;6(1):283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boussinesq M, Gardon J, Gardon-Wendel N, Kamgno J, Ngoumou P, Chippaux JP. Three probable cases of Loa loa encephalopathy following ivermectin treatment for onchocerciasis. Am J Trop Med Hyg 1998;58(4):461–9. [DOI] [PubMed] [Google Scholar]
- 9.Chippaux JP, Boussinesq M, Gardon J, Gardon-Wendel N, Ernould JC. Severe adverse reaction risks during mass treatment with ivermectin in loiasis-endemic areas. Parasitol today 1996;12(11):448–50. [DOI] [PubMed] [Google Scholar]
- 10.Nzenze JR, Kombila M, Boguikouma JB, Belembaogo E, Moussavou-Kombila JB, Nguemby-Mbina C. Encéphalopathie mortelle au cours d’une loase hypermicrofilaremique traitee par ivermectine. Première description au Gabon. Med Afr Noire 2001;48(8/9):375–7. [Google Scholar]
- 11.Fobi G, Gardon J, Santiago M, Ngangue D, Gardon-Wendel N, Boussinesq M. Ocular findings after ivermectin treatment of patients with high Loa loa microfilaremia. Ophthalmic Epidemiol 2000;7(1):27–39. [PubMed] [Google Scholar]
- 12.Weil GJ, Steel C, Liftis F, et al. A rapid-format antibody card test for diagnosis of onchocerciasis. J Infect Dis 2000;182(6):1796–9. [DOI] [PubMed] [Google Scholar]
- 13.Lipner EM, Dembele N, Souleymane S, et al. Field applicability of a rapid-format anti-Ov-16 antibody test for the assessment of onchocerciasis control measures in regions of endemicity. J Infect Dis 2006;194(2):216–21. [DOI] [PubMed] [Google Scholar]
- 14.Gardon J, Kamgno J, Fobi G, et al. Dépistage, identification et prise en charge des effets secondaires graves imputables à la loase et au traitement par ivermectine au cours des campagnes de lutte contre l'onchocercose. Bulletin de liaison et de documentation de l'OCEAC 1999;37–51. [Google Scholar]
- 15.Newson RB. Attributable and unattributable risks and fractions and other scenario comparisons. Stata J 2013;13(4):672–98. [Google Scholar]
- 16.Haselow NJ, Akame J, Evini C, Akongo S. Programmatic and communication issues in relation to serious adverse events following ivermectin treatment in areas co-endemic for onchocerciasis and loiasis. Filaria J 2003;2(Suppl 1):S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chijioke CP, Okonkwo PO. Adverse events following mass ivermectin therapy for onchocerciasis. Trans R Soc Trop Med Hyg 1992;86(3):284–6. [DOI] [PubMed] [Google Scholar]
- 18.Prod'hon J, Boussinesq M, Fobi G, et al. Lutte contre l'onchocercose par ivermectine: résultats d”une campagne de masse au Nord-Cameroun. Bull World Health Org 1991;69(4):443–50. [PMC free article] [PubMed] [Google Scholar]
- 19.Baraka OZ, Khier MM, Ahmed KM, et al. Community based distribution of ivermectin in eastern Sudan: acceptability and early post-treatment reactions. Trans R Soc Trop Med Hyg 1995;89(3):316–8. [DOI] [PubMed] [Google Scholar]
- 20.Hawking F. Periodicity of microfilariae of Loa loa. Trans R Soc Trop Med Hyg 1955;49(2):132–42. [PubMed] [Google Scholar]
- 21.Gardon J, Kamgno J, Folefack G, Gardon-Wendel N, Bouchité B, Boussinesq M. Marked decrease in Loa loa microfilaraemia six and twelve months after a single dose of ivermectin. Trans R Soc Trop Med Hyg 1997;91(5):593–4. [DOI] [PubMed] [Google Scholar]
- 22. Taylor MJ, Bandi C, Hoerauf A. Wolbachia bacterial endosymbionts of filarial nematodes. Adv Parasitol 2005;60:245–84. [DOI] [PubMed] [Google Scholar]
- 23.Dietrich W. Obligatory symbiotic Wolbachia endobacteria are absent from Loa loa. Filaria J 2003;2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.