Abstract
As a potentially unlimited autologous cell source, patient induced pluripotent stem cells (iPSCs) provide great capability for tissue regeneration, particularly in spinal cord injury (SCI). However, despite significant progress made in translation of iPSC-derived neural progenitor cells (NPCs) to clinical settings, a few hurdles remain. Among them, non-invasive approach to obtain source cells in a timely manner, safer integration-free delivery of reprogramming factors, and purification of NPCs before transplantation are top priorities to overcome. In this study, we developed a safe and cost-effective pipeline to generate clinically relevant NPCs. We first isolated cells from patients’ urine and reprogrammed them into iPSCs by non-integrating Sendai viral vectors, and carried out experiments on neural differentiation. NPCs were purified by A2B5, an antibody specifically recognizing a glycoganglioside on the cell surface of neural lineage cells, via fluorescence activated cell sorting. Upon further in vitro induction, NPCs were able to give rise to neurons, oligodendrocytes and astrocytes. To test the functionality of the A2B5+ NPCs, we grafted them into the contused mouse thoracic spinal cord. Eight weeks after transplantation, the grafted cells survived, integrated into the injured spinal cord, and differentiated into neurons and glia. Our specific focus on cell source, reprogramming, differentiation and purification method purposely addresses timing and safety issues of transplantation to SCI models. It is our belief that this work takes one step closer on using human iPSC derivatives to SCI clinical settings.
Keywords: iPSC, Spinal cord injury, Neural repair, Neuroprotection
1. Introduction
Spinal cord injury (SCI) is one of the most devastating neurological conditions that often causes severe motor and/or sensory deficits in patients. Current managements such as surgeries and physical therapies could only modestly improve patients’ conditions, and leave many patients wheelchair-bound for the rest of their life. Transplantation of neural stem/progenitor cells (NSCs/NPCs) is a novel therapy and has shown promising results in repair and regeneration of lost neural tissues and restoration of neurological deficits (Sahni and Kessler, 2010; Tsuji et al., 2010; Sareen et al., 2014; Salewski et al., 2015). In most reports, human NSCs/NPCs were derived from either fetal brain, spinal cord (Cummings et al., 2005; Salazar et al., 2010; Lu et al., 2012), or human embryonic stem cells (hESCs) (Keirstead et al., 2005; Sharp et al., 2010). These cell sources often have ethical controversies. In addition, they are allogenic, which cause immune rejection and require lifetime immunosuppression.
Patient specific induced pluripotent stem cells (iPSCs) could overcome these hurdles as a potential source for cell-based therapy. Generally, iPSCs are produced from patients’ somatic cells such as dermal fibroblasts, keratinocytes, and blood cells by transient overexpression of four transcription factors, OCT4, SOX2, KLF4 and C-MYC (OSKM) (Takahashi and Yamanaka, 2006; Takahashi et al., 2007; Yu et al., 2007). iPSCs share almost identical properties with hESCs with additional advantages. iPSCs possess unlimited self-renewal capacity and have the potential to manufacture pure and homogenous neural progeny populations in large quantities. In addition, iPSCs offer genetically matched autologous cell source, which might omit the necessity of using immune suppression drugs. These characteristics set the basis for iPSCs to be a major promising candidate for cell-based replacement therapy.
Many reprogramming methods have been rapidly developed to induce a variety of somatic cell types into iPSCs since its invention. The most classical method is infection with retroviruses or lentiviruses. However, both lentivirus and retrovirus integrate into the genome of cells, while effective and sufficient in basic research, neither is suitable for clinical uses due to potential tumorigenicity risks. To avoid the side effects, non-integrating protocols using episomal vectors, Cre-lox system, piggybac vectors, minicircles, recombinant proteins, messenger RNAs, microRNAs, and small molecules, have recently been reported (Chang et al., 2009; Kaji et al., 2009; Kim et al., 2009; Sommer et al., 2009; Woltjen et al., 2009; Yu et al., 2009; Zhou et al., 2009; Jia et al., 2010; Warren et al., 2010; Anokye-Danso et al., 2011; Rao and Malik, 2012; Hou et al., 2013), which have shown variable yields and reproducibility. Recently, Sendai viruses have been established and shown to be able to reprogram dermal fibroblasts, CD34+ hematopoietic cells and urine derived cells (Fusaki et al., 2009; Ye et al., 2013; Afzal and Strande, 2015; Rossbach et al., 2016). As negative sense RNA viruses, Sendai viruses do not integrate into the genome of human cells and are non-pathogenic to humans (Fusaki et al., 2009; Ban et al., 2011; Macarthur et al., 2012a). Most importantly, unlike several other non-integrating reprogramming methods, the reported reprogramming efficiency of Sendai viruses has been high and consistent (Lieu et al., 2013).
Several somatic cell types have been commonly used for iPSC reprogramming such as fibroblasts or keratinocytes from skin biopsies, lymphocytes and CD34+ hematopoietic stem cells harvested from blood (Ye et al., 2009; Mack et al., 2011; Ye et al., 2013). Recently, cells derived from urine were reported to be able to be reprogrammed into iPSCs (Zhou et al., 2012; Wang et al., 2013a; Guan et al., 2014; Afzal and Strande, 2015; Rossbach et al., 2016). Urine can be easily obtained from patients via non-invasive procedures and only 100 mL of urine is sufficient for isolation, culture, and subsequent reprogramming. Therefore, urine cells are a viable somatic cell source for iPSC reprogramming in clinical settings.
Protocols for directed neural induction of human iPSCs have been widely adapted and optimized (Chambers et al., 2009; Yuan et al., 2011; Macarthur et al., 2012b; Reinhardt et al., 2013). However, due to the heterogeneity of NSC/NPC differentiation, it is difficult to obtain pure NSC/NPC populations. Residual undifferentiated iPSCs pose perceived risks of tumorigenicity after transplantation. The contamination of cells skewing from the originally desired lineage during differentiation might interfere with neural differentiation or confound data interpretation. To overcome this obstacle, one strategy is to generate lineage reporters of fluorescent proteins compatible with fluorescence-activated cell sorting (FACS) purification. Although several neural lineage reporters have been developed (Ruby and Zheng, 2009; Xue et al., 2009; Li et al., 2015; Pei et al., 2015; Zhang et al., 2016), these reporters cannot be applied directly to clinical settings. A more straightforward methodology is to identify lineage specific cell surface markers to sort for NPCs from differentiated iPSCs, such as a quadruple sorting strategy of CD184+/CD271−/CD44−/CD24+ (Yuan et al., 2011). While the above approach is effective, a simpler sorting method is desirable to facilitate the clinical application of iPSC-derived neural cells.
A2B5 is a commonly used antibody which recognizes a glycoganglioside that is specifically expressed on the cell surface of neural lineage cells. Previously, A2B5 has been used to identify glial progenitors in mouse and rat embryos (Rao et al., 1998). A2B5 also labels glial progenitors in human fetal brain derived neural cells (Eisenbarth et al., 1979; Mayer-Proschel et al., 1997; Mujtaba et al., 1999; Liu et al., 2002; Liu et al., 2004; Campanelli et al., 2008; Wu et al., 2008).
In the present study, we reported a simple and effective protocol to generate patient specific neural populations for clinical application. We first isolated cells from patients’ urine, and reprogrammed the urine cells into iPSC cells (named as U-iPSCs) with the non-integrating Sendai viral vectors. After neural differentiation and purification with A2B5, a neural progenitor cell population was obtained. The purified A2B5+ progenitor cells were able to proliferate for at least 10 passages and differentiate into both neurons and glial cells in vitro. Importantly, these NPCs survived and integrated well after transplantation into the injured spinal cord. These results suggest that NPCs derived from patients’ U-iPSCs are a valuable source of cell based therapy for SCI.
2. Materials and methods
2.1. Collection and culture of urine cells
Urine samples were collected as previously described (Zhang et al., 2008) with approval from Institutional review board committee. Briefly, about 100 mL of fresh, clean-catch urine samples from each patient or healthy donor (Table 1) was collected, spun down and plated onto one well of a 24-well tissue culture plate. Cells were cultured in urine cell medium consisting of equal volume of keratinocyte serum-free medium and DMEM supplemented with 10% fetal bovine serum with antibiotic antimycotic (all from Life Technologies). The cells from the urine (Table 2) were confirmed to express mesenchymal stem cell (MSC) markers such as CD29, CD73, CD90, CD105, and CD146, but were negative for hematopoietic stem cell markers, such as CD31, CD34 and HLA-DR (Zhang et al., 2008). Hence, we named these cells as urine stem cells (USCs). USCs were passaged in a 1:4 ratio every 4–5 days. It usually took 2–3 weeks from the day of urine collection to obtain sufficient number of USCs at passage 2 or 3 for reprogramming.
Table 1.
Basic information of iPSCs generated from SCI patients and healthy individuals for this study.
| Patient no. | Source | Age | Sex | Race | No. iPSC colonies |
|---|---|---|---|---|---|
| CSCL-27 | Skin fibroblasts | 50+ | F | African | 5 |
| CSCL-13 | Skin fibroblasts | 50+ | M | Caucasian | 10 |
| NR1251 | Skin fibroblasts | 50+ | M | African | 2 |
| CSCL-67 | Skin fibroblasts | 40+ | M | Caucasian | 1 |
| CSCL-14 | Skin fibroblasts | 20+ | M | Caucasian | 30 |
| CSCL-15 | Skin fibroblasts | 50+ | M | Caucasian | 50 |
| B35 | USCs | M | 28 | ||
| B36 | USCs | M | 19 | ||
| K5 | USCs | M | 17 | ||
| K7 | USCs | M | 70 |
Table 2.
Biological features of USCs, U-iPSCs and A2B5+ NPCs in culture.
| USCs | U-iPSCs | A2B5+ NPCs | |
|---|---|---|---|
| Morphology | Rice-grain like | iPSC colony | |
| Cell markers | CD29+, CD73+, CD90+, CD105+, CDI46+, CD31−, CD34− and HLA-DR− | OCT4+, SOX2+, SSEA4+, TRA1-81+, and A2B5− | A2B5+, SOX1+, PAX6+ and TRA1-81− |
2.2. Reprogramming patient USCs into iPSCs using Sendai viruses
To generate iPSCs, USCs were infected with Sendai virus Cytotune Reprogramming Kit (Life Technologies) containing four Yamanaka factors, OCT4, SOX2, KLF4, and c-MYC (OSKM). To evaluate the reprogramming efficiency of USCs, human dermal fibroblasts were also infected in parallel for comparison. Two days before transduction, USCs or fibroblasts were plated onto one well of a 24-well plate at a density of approximately 2 × 104/cm2. On the day of transduction, 25 μL or ¼ of the volume from the original tube of each factor (OSKM) was added onto cells in each well. Twenty-four hours after transduction Sendai viral vector containing medium was replace by fresh medium. On Day 5 post transduction, cells were replated onto irradiated mouse embryonic fibroblast (MEF) feeder layer at a density of 1– 2 × 105 cells/cm2. Culture medium was switched to human iPSC medium containing DMEM-F12, 20% knockout serum replacement, 1% non-essential amino acid, 55 μM 2-mercaptoethanol, 2 mM L-glutamine, supplemented with 12 ng/mL basic FGF. iPSC colonies started to form at day 11 (for USCs) or day 16 (for fibroblasts) post transduction. Colonies were manually picked and transferred to new MEF plates at day 16 or 21 respectively. iPSCs were passaged with Dispase (1 mg/mL, Life Technologies) in a 1:4 ratio every 4– 5 days. Karyotype was examined every 10 passages. iPSCs were transferred to MEF conditioned medium and later adapted to TeSR-E8 medium (Stem Cell Technologies) for feeder free culture and passaged every 4– 5 days in a 1:4– 1:6 ratio using 0.5 mM EDTA. For convenience, iPSCs derived from USCs were named U-iPSCs.
2.3. Monitoring the clearance of Sendai viral components in reprogrammed iPSCs with RT-PCR
Total RNA was extracted from iPSCs using Quick-RNA miniPrep kit (Zymo Research). One microgram of total RNA was reverse transcribed to cDNA using the SuperScript III First-Strand Synthesis System (Life Technologies). Primer sequence for Sendai vector expression according to manufacturer instruction is the following: SeV Forward: 5′ GGATCACTAGGTGATATCGAGC 3′ and Reverse: 5′ ACCAGACAAGA GTTTAAGAGATATGTATC 3′ with expected size of PCR products of 181 bp. GAPDH was used at an internal control. The primers for GAPDH expression were: Forward: 5′ TTTTAACTCTGGTAAAGTGG 3′ and Reverse: 5′ TGTCATACTTCTCATGGTTC 3′. The expected size of the PCR products was 359 bp. PCR was performed using the following conditions: 98 °C for 2 min, 35 cycles of 98 °C for 20 s, 59 °C for 20 s, 72 °C for 30 s, with a final extension at 72 °C for 5 min.
2.4. Differentiation of USC-derived iPSCs into neural progenitor cells
Patient USC-reprogrammed iPSCs were digested into small clumps using 0.5 mM EDTA. Small clumps were then transferred to Corning Petri dishes and suspended as embryoid bodies (EBs) in iPSC medium for 8 days without basic fibroblast growth factor (bFGF) as previously described (Macarthur et al., 2012b). EBs were then seeded onto cell culture plates in neural induction medium containing DMEM/F12 with Glutamax, 1× NEAA, 1× N2, and bFGF (20 ng/mL). After 2– 3 days, neural rosettes were manually isolated and dissociated into single cells. The cells were expanded in Neurobasal medium supplemented with 1× NEAA, L-Glutamine (2 mM), 1× B27, and bFGF (20 ng/mL).
2.5. Purification of A2B5+ cells by fluorescence activated cell sorting (FACS)
Purification of A2B5+ cells was carried out as described previously (Liu et al., 2004). Briefly, upon completion of directed differentiation of iPSCs, cells were harvested using Accutase and resuspended in 1% fetal bovine serum (FBS) in 1× phosphate buffered saline (PBS) at a concentration of 5– 10 × 106 cells/mL. Cells were then stained with anti-A2B5 antibody, followed by a FITC-conjugated secondary antibody, and purified using a FACSAria II cell sorter system (BD) at 4 °C, at a rate of 2500 cells/s. The sorted cells were re-examined by FACS for the percentage of A2B5+ and TRA1-81+ cells to determine purity. All experiments were done in triplicate.
2.6. Immunocytochemistry
A2B5+ cells were characterized by immunocytochemistry (Liu et al., 2004). Briefly, cells grown on glass coverslips were fixed with 2% paraformaldehyde and incubated in blocking buffer (5% goat serum, 1% bovine serum albumin, and 0.1% Triton X-100) for 30 min. Cells were then incubated in primary antibodies diluted in blocking buffer at 4 °C overnight. Appropriate secondary antibodies were used for single and double labeling. All secondary antibodies were tested for cross-reactivity and nonspecific immunoreactivity. The following primary antibodies were used, OCT4 (1:500, Abcam), SOX2 (1:200, R&D Systems), SSEA4 (1:10, Developmental Studies Hybridoma Bank, DSHB), TRA1-81 (1:100, Millipore), A2B5 (1:20, ATCC), β3 tubulin (1:1000, Sigma), HB9 (1:10, DSHB), GABA (1:200, Sigma), NG2 (1:200, Millipore), PDGFRα (1:200, BD), CD44 (1:200, Millipore), S100B (1:200, Sigma), GFAP (1:4000, DAKO), huNA (1:200, Millipore), NFM (1:200, Sigma), MAP2 (1:200, Sigma). Bis-benzamide (DAPI, 1:1000; Sigma) was used to visualize the nuclei. Images were captured using a Zeiss Axiovision microscope with z-stack split view function.
2.7. Classification of A2B5+ cells based on gene expression profiling
To characterize A2B5+ cells based on gene expression, RNA-seq data of several related cell types were retrieved or produced. Data of hESC (WA01 replicates 1, 2 and 3) were retrieved from ENCODE project (Consortium, 2011). Data of neural precursor cells (N2) were from our previous study (Wu et al., 2010), and data of human foreskin fibroblasts were obtained from human body map 2.0 project (Farrell et al., 2014). For USCs collected from two patients (hB35, hK3) the 100 bp paired-end RNA-seq was carried out as previously described (Chen et al., 2013). For A2B5+ cells, Illumina gene expression microarrays using BeadArray technology was performed. Illumina microarray data were first filtered to select probe sets that were called in at least one cell type (with p-value b 0.05 in at least one sample). Probes were then mapped to gene symbols. In the case that multiple probe sets mapped to one gene, the probe set with the greatest standard deviation of expression across cell types was selected. This analysis led to 18,969 unique genes for the subsequent clustering and correlation analysis. In order to compare expression from two platforms, RNA-seq and microarray, expression value was normalized by dividing average expression value from two platforms. Hierarchical cluster analysis for relationship discovery among cell types based on the above gene expression data was performed using the hclust method in R. A correlation matrix as well as a dendrogram indicated their similarities among cell types.
2.8. Transplantation of A2B5+ cells to a mouse model of injured spinal cords
All animal care and surgical interventions were undertaken in strict accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals, Guide for the Care and Use of Laboratory Animals, and with the approval of both Animal Welfare Committee and institutional biosafety committee at the University of Texas Health Science Center at Houston.
Surgical procedures were performed as described previously (Cao et al., 2001; Cao et al., 2002; Cao et al., 2005b). Briefly, after anesthetization with Ketamine (50–80 mg/kg BW, IP) and Xylazine (5–10 mg/kg BW, IP), adult severe combined immunodeficiency (SCID) mice received a dorsal laminectomy at the 9th thoracic vertebral level (T9) to expose the spinal cord and then a moderate contusion at 60 kdyne using Infinite Horizons (IH) impactor. At Day 9 post-injury, mice were re-anesthetized as above and the laminectomy site was re-exposed. Four injections were made at 1 mm cranial to, caudal to, and left and right of the lesion epicenter at a depth of 1.3 mm and 0.6 mm laterally from the midline. At each site, 1 μL of cell suspension was injected through a glass micropipette with an outer diameter 50– 70 μL and the tip sharp-beveled to 30–50° at rate of 0.5 μL/min as described previously (Cao et al., 2001; Cao et al., 2002). Thus, a total of 400,000 cells were grafted into each injured spinal cord. The animals were euthanized 8 weeks after transplantation for analysis.
3. Results
3.1. Successful reprogramming of USCs into iPSCs by integration-free Sendai viral system
USCs and skin fibroblasts collected from patients or healthy individuals were reprogrammed in parallel for comparison. After the initial isolation and culture, USCs were able to proliferate and could be passaged and stocked for further manipulation. These cells were densely-packed with a relatively simple and uniform morphology, resembling an epithelial cell origin (Fig. 1A), in contrast to the spindle shape-like, stromal cell origin of fibroblasts.
Fig. 1.
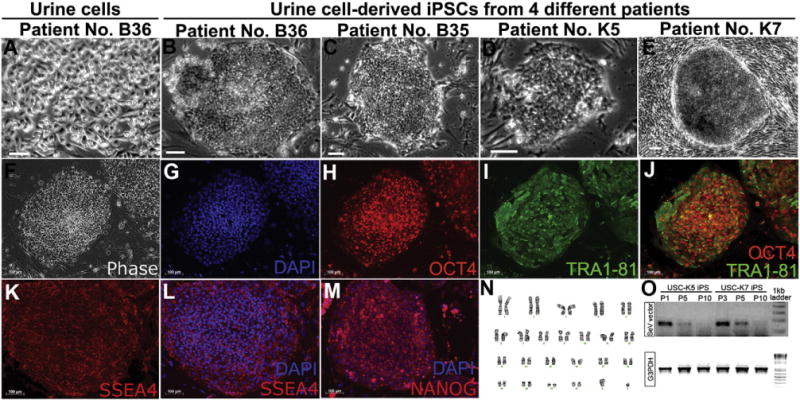
Successful generation of iPSCs using cells derived from patients’ urine. (A) Cells derived from patients’ urine resemble the morphology of epithelial cells. (B–E) Phase images of human iPSCs (U-iPSCs) reprogrammed from urine cells from different patients by Sendai viruses. Typical morphology of human iPSC colonies is observed under phase contrast. The flat cells in panel E are MEF feeders. (F–M) iPSCs expresses pluripotent markers OCT4, SSEA4 and TRA1-81. (N) The human iPSCs also maintain a normal karyotype. (O) RT-PCR results of human iPSCs from two representative clones USC-K5 and K7 show that viral components are depleted after passage 10. Bar, 50 μm.
The reprogramming efficiency of Sendai viral system on USCs ranged from 0.001% to 0.1% (Table 1), comparable with retroviral reprogramming in our labs (Chen et al., 2014). No obvious difference in reprogramming efficiency was noted between USCs and fibroblasts. However, USCs showed better reprogramming consistency compared to fibroblasts, as we only obtained a few iPSC colonies from fibroblasts in some cases. In addition, iPSC colonies appeared earlier from USCs than fibroblasts. By day 11 post transduction, USC derived-iPSC clones (named U-iPSCs) had started to form and appeared to be fully reprogrammed by day 16 as indicated by expression of pluripotency markers OCT4, TRA1-81, SSEA4, and NANOG (Fig. 1F–M), whereas fibroblast-derived iPSCs were obtained at day 21, about 5 days later than USC-iPSCs. iPSCs reprogrammed by Sendai viruses exhibited typical iPSCs morphology that showed compact cells within colonies of distinct margin (Fig. 1B–E). They could also be adapted to feeder-free, serum-free TeSR E8 medium and continued to maintain pluripotency and a normal karyotype (Fig. 1N) for N60 passages during at least two years in culture.
To confirm that our Sendai virus reprogrammed U-iPSCs were integration-free, we monitored the expression of Sendai viral vectors in iPSCs with RT-PCR. Immediately after transduction, all clones showed positive expression of Sendai viral components. However, the expression gradually decreased as cells were passaged, was no longer detectable at passage 10, and remained negative for later passages (Fig. 1O).
3.2. A2B5+ sorted cells resembled characteristics of neural progenitor cells
We induced U-iPSCs along the neural lineage with neural differentiation medium (Fig. 2A). Fourteen days after differentiation, N80% of cells expressed NPC markers SOX1 and PAX6 (Fig. 2G, H). Although the majority of cells expressed NPC markers, a very small percentage of them (<0.1%) still expressed TRA1-81, a pluripotency marker (Fig. 2D, I), indicating the need for further purification to remove undifferentiated iPSCs. We chose A2B5, a cell surface ganglioside epitope, which has been used to label and purify neural lineage specific cells in mouse, rat and human (Eisenbarth et al., 1979; Mayer-Proschel et al., 1997; Mujtaba et al., 1999; Liu et al., 2002; Liu et al., 2004; Wu et al., 2008). Based on flow cytometry data, about 80% of induced neural cells expressed A2B5 at day 15–30 prior to FASC sorting (Fig. 2B). After sorting, 95%–99% of the induced cells were A2B5+, while no cells were TRA1-81+ as assessed by flow cytometry and immunocytochemistry (Fig. 2E, M). Expression of SOX1 and Nestin on A2B5+ cells (Fig. 2L) further confirmed these cells as NPCs. A2B5+ cells continued to proliferate in vitro and gave rise to a variety of neural cell types when cultured in appropriate differentiation medium. Under neuronal differentiation condition, the purified A2B5+ NPCs gave rise to a variety of neuronal subtypes (Fig. 3A–C), including HB9 (MNX1)+ motor neurons and GABA+ neurons, all of which co-expressed the pan-neuronal marker β3 tubulin (Fig. 3A–C). When induced toward the glial lineage, the A2B5+ sorted cells were able to give rise to NG2+ and PDGFRα+ oligodendrocyte progenitors (Fig. 3D–E), and CD44 expressing astro-cyte progenitors and GFAP+/S100B+ astrocytes (Fig. 3F–H). Thus, the A2B5+ sorted cells possessed NPC characteristics.
Fig. 2.
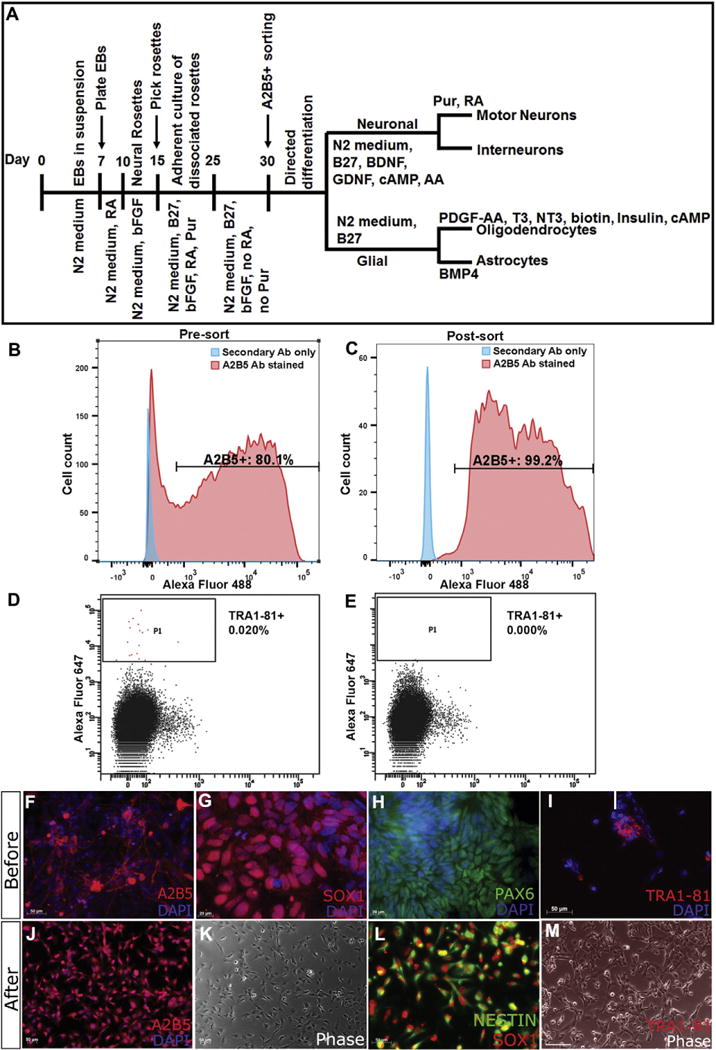
Differentiation and FACS purification of A2B5+ cells from U-iPSCs. (A) Scheme of our differentiation protocol. (B–C) Before sorting, about 80% of differentiated cells have A2B5 immunoreactivity; after A2B5+ sorting, 95–99% of cells are A2B5+. (D–E) On the contrary, the percentage of TRA1-81+ iPSCs is decreased to 0. (F–H) Before sorting, human iPSCs differentiate along neural lineage express A2B5, and NSC markers SOX1 and PAX6. However, a small percentage of cells express pluripotent marker TRA1-81 (I), indicating contamination of undifferentiated cells. (J–L) After A2B5+ sorting, cells maintain A2B5, SOX1 and NESTIN expression. (M) Most importantly, the undifferentiated TRA1-81+ cells have been depleted. Bar, F, I, J–M, 50 μm; G, H, 20 μm.
Fig. 3.
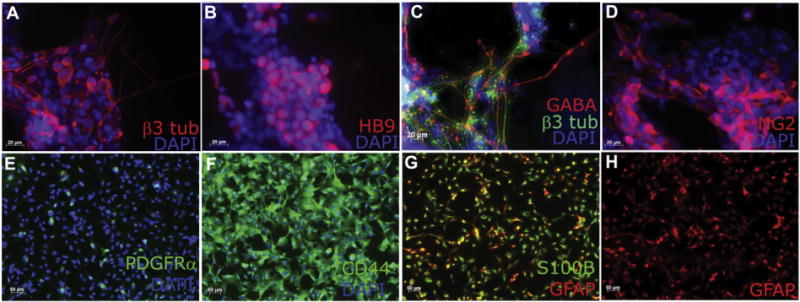
A2B5+ cells derived from patient U-iPSCs give rise to neurons and astrocytes. (A–C) A2B5+ sorted cells continue to proliferate and give rise to a variety of neuronal subtypes including HB9 (MNX1)+ motor neurons and GABA+ neurons, all of which co-express the pan-neuronal marker β3 tubulin. (D–E) A2B5+ sorted cells give rise to NG2+ and PDGFRα+ oligodendrocyte progenitors. (F–H) CD44+ astrocyte progenitors, GFAP+/S100B+ astrocytes are obtained from A2B5+ cells. Bar, A–D, 20 μm; E–H, 50 μm.
3.3. A2B5+ cells shared similar molecular signatures with NSCs/NPCs
To further characterize A2B5+ cells at the molecular level, we performed gene expression microarray analysis and retrieved or produced RNA-seq data on fibroblasts (hFB1, hFB2), USCs (hB35, hK3), NPCs (hN2 NPC), iPSCs (K7 iPS, NR1251 iPS, and UTY1 iPS), and hESCs (WA01 replicates 1, 2 and 3). The hierarchical clustering of the above cell types (Fig. 4A) and the gene expression heatmap (Fig. 4B) revealed most similar cell type groups. Notably, gene expression of A2B5+ cells generated from U-iPSCs was most similar to that of the previously characterized NPCs (hN2 NPC). iPSCs were clustered together regardless of their varied reprogramming methods (retroviruses for UTY1 iPS vs. Sendai viruses for NR1251 iPS). In addition, iPSCs generated from two different types of source cells (USCs for K7 iPS vs. fibroblasts for NR1251 iPS and UTY1 iPS) were also clustered together. The hESCs were clustered together as expected and were closely related to iPSCs. Lastly, as somatic cell types, fibroblasts and USCs were clustered together and were distant to iPSCs and NPCs.
Fig. 4.
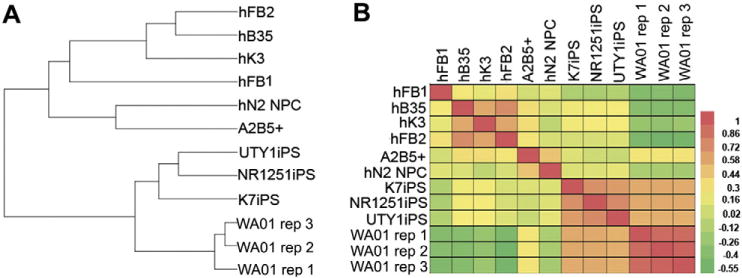
Genome-wide gene expression profiling of U-iPSC derived A2B5 cells and comparison of A2B5 cells to neural progenitor cells. (A) Dendrogram. (B) Heatmap. Global gene expression is compared for human fibroblasts (hFB1, hFB2), patient derived urine cells (USCs, hB35, hK3), iPSCs derived from patient fibroblasts (NR1251 iPS, UTY1 iPS), iPSCs derived from patient urine cells (U-iPSCs, K7 iPS), human embryonic stem cells (WA01 replicates 1, 2, and 3), NPCs derived from hESCs (hN2 NPC), and A2B5+ cells derived from U-iPSCs. Urine cell-derived, Sendai virus reprogrammed U-iPSC K7iPS clusters together with fibroblast-derived, Sendai virus-reprogrammed human iPSC line (NR1251iPS), and fibroblast-derived, retrovirus-reprogrammed human iPSC (UTY1 iPS), as well as previously characterized hESC line WA01 (replicates 1, 2, and 3). iPSC-derived A2B5+ NPCs, patient derived urine cells hB35 and hK3 cluster much closer to the human fibroblasts (hFB1, and hFB2) than to iPSCs or hESCs. Most importantly, A2B5+ NPCs cluster together with previously characterized hESC derived NPCs (hN2 NPC).
3.4. A2B5+ NPCs gave rise to neurons and astrocytes after implanted to a mouse SCI model
To test the in vivo survival and differentiation of A2B5+ NPCs, purified A2B5+ cells were grafted into the contused thoracic spinal cord of adult SCID mice (Fig. 5). Eight weeks after transplantation, a significant number of the grafted A2B5+ cells survived around the injury epicenter as shown by the human nuclei (huNA) staining in the mouse host (Fig. 5A, E, I). The grafted cells were found in the injury cavity as well as the rostral and caudal spared spinal cord. The grafted cells were observed among the spared neurons, astrocytes and axons in the gray and white matter and integrated well into the injured spinal cord. Many grafted cells have started to express neuronal marker MAP2 (Fig. 5K, L), indicating that the grafted A2B5+ cells were able to differentiate into mature neurons in the injured host environment. In addition, a portion of the transplanted cells gave rise to GFAP+ astrocytes (Fig. 5B, D, F, H, J, L), indicating that similar to in vitro differentiation, A2B5+ cells were multipotential in vivo after transplantation. Importantly, no tumor formation was observed in any animals during the 3-month post graft period.
Fig. 5.
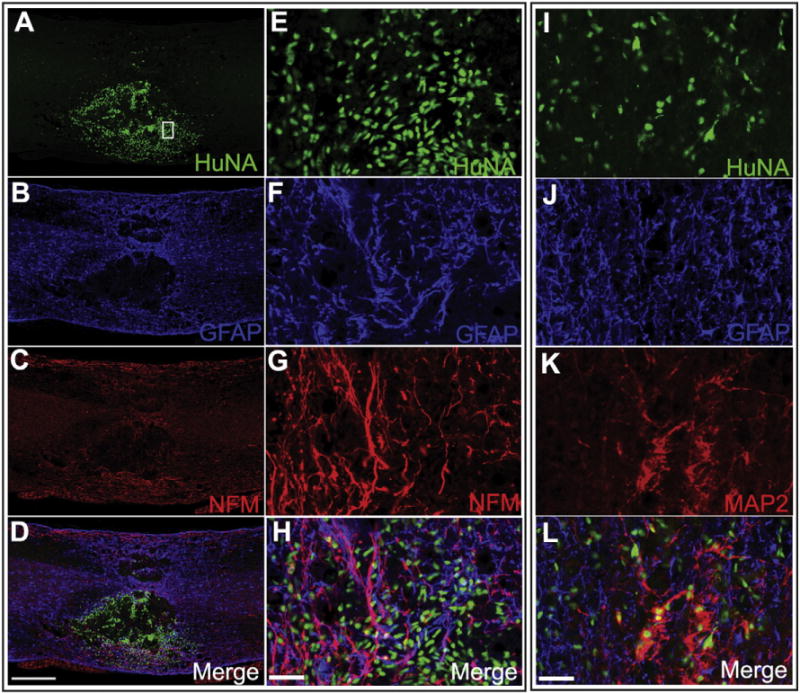
A2B5+ sorted cells derived from patient U-iPSCs give rise to neurons and astrocytes after being grafted to SCI mouse model. FACS purified A2B5+ cells are grafted into the contused thoracic spinal cord of adult mice. Eight weeks after transplantation, grafted human cells survive and integrate into the injured spinal cord as shown by the human nuclei staining (HuNA) (A, E, I). Some have matured to neurons and start to express neuronal marker NFM and MAP2 (C, H, K) some differentiate into GFAP+ astrocytes (B, F, J). Bar, A–D, 500 μm; E–H and I–L, 20 μm. E–H represent higher magnification images of the boxed areas in A–D.
4. Discussion
As a promising source for cell-based regenerative medicine, iPSCs have attracted intensive investigations for their potentials in treating SCI and other neurological conditions. Through meticulously designed protocols, iPSCs can be coaxed into a variety of specific cell types, including NPCs, motor neurons, interneurons, oligodendrocytes and astrocytes (Chambers et al., 2009; Hu and Zhang, 2010; Krencik et al., 2011; Liu et al., 2011; Douvaras et al., 2014). These induced cells have been used to reconstruct in vitro disease models, serving as drug screening and testing platforms, and have been grafted to animal models to test their potential to participate in tissue regeneration or repair (Ruff and Fehlings, 2010; Nakamura et al., 2012; Nutt et al., 2013; Rao, 2013; Lu et al., 2014; Sareen et al., 2014; Baghbaderani et al., 2015; Beers et al., 2015; Liu and Deng, 2015). Importantly, patient specific iPSCs provide autologous cell source, which theoretically omit the need for immune suppression.
To design realistic therapeutic strategies using patient-specific iPSC-derived NSCs/NPCs for the treatment of SCI, several critical issues remain to be addressed. First, iPSCs need to be transgene free and safe to be used in the clinics. Second, somatic cells for reprogramming should be convenient and easy to obtain. Third, it is important to develop a pipeline to differentiate iPSCs into NSCs/NPCs at high efficiency and high purity to avoid contamination of undifferentiated iPSCs and potential for tumorigenesis. Fourth, therapeutic efficacy of iPSC-derived NSCs/NPCs and their long-term safety in vivo after transplantation to SCI patients need to be comprehensively evaluated.
In the current work, we have attempted to address these issues. First, we have successfully generated of iPSCs from USCs, the cells that were derived from patients’ urine, using an integration-free reprogramming protocol. In this protocol, reprogramming factors OSKM were delivered by Sendai viruses, a negative sense, single-stranded RNA virus that is non-pathogenic to humans. Sendai viruses have been safely used to carry vaccines in macaques (Takeda et al., 2008), as well as deliver cystic fibrosis transmembrane conductance regulator (CFTR) gene to restore CFTR levels (Yonemitsu et al., 2000; Ferrari et al., 2007). Notably, consistent with previous efforts on Sendai viral reprogramming (Lieu et al., 2013; Ye et al., 2013), our data showed that although Sendai viral vectors were initially detectable in iPSC clones of earlier passages, they were soon depleted as the cells were passaged (Fig. 1O). Therefore, this protocol could largely eliminate potential long-term adverse effects due to integration of the transgenes or virus delivery.
Although several somatic cell types have been reported to be successfully reprogrammed into iPSCs, USCs display certain advantages. USCs can be easily obtained from a non-invasive, simple and low-cost approach, compared to fibroblasts and keratinocytes taken from skin biopsies, lymphocytes and CD34+ hematopoietic stem cells (HSCs) drew from peripheral blood (Chou et al., 2011), MSCs from bone marrow, or keratinocytes from hair follicles (Petit et al., 2012; Wang et al., 2013b). It seems that a larger number of iPSC colonies could be generated from USCs compared to other somatic cell types (Guan et al., 2014). Furthermore, it takes considerably less time for USCs to be converted to iPSCs than for other cell types. For instance, it usually takes 1– 3 months months to obtain usable dermal fibroblasts after a skin punch, another 2– 3 months to reprogram and bank, and an additional 1– 2 months to get neural lineage specific cells, mounting a total of 4– 8 months. Therefore, it might not be practical to make use of patients’ own isogenic iPSC derivatives to treat SCI at the acute phase, which is a critical window for effective treatment. On the other hand, by simply culturing the cells obtained from about 100 mL clean catch urine sample, one could harvest sufficient number of stem cells or somatic cells for the subsequent iPSC reprogramming experiments within 2– 3 weeks. Compared to dermal fibroblasts, which need at least 21 days to be fully reprogrammed into iPSCs, the USCs can be reprogrammed within 14–16 days. Thus, sufficient iPSCs are usually obtained in 1– 1.5 months after the initial urine sample collection, which greatly reduces the time needed for manufacturing the cells to be grafted. This is particularly important for SCI patients because the best timing for transplantation is probably at the acute or subacute stage. Taken together, iPSCs reprogrammed from USCs with Sendai viral vectors might open a new avenue toward applications in urgent clinical settings such as SCI and stroke. Furthermore, urine cells are a non-invasive resource which is more convenient and easier to access for reprogramming. Thus, urine cells are one of the optimal somatic cell resources to generate iPSCs.
One of the critical issues to apply iPSC-derived NPCs to the treatment of SCI or other neurological conditions is to obtain pure NPCs at high efficiency, which often requires both efficient differentiation protocol and reliable purification approach. In the current work, we successfully induced neural differentiation from human iPSCs with a modified protocol from previous studies (Li and Zhang, 2006; Hu et al., 2010; Zhang and Zhang, 2010; Reinhardt et al., 2013). In addition, we established a method to purify NPCs using FACS with A2B5 antibody. Our results show that the purified NPCs possess characteristics of previously described NPCs from various sources (Fig. 4) and are devoid of undifferentiated iPSCs. This is critical for potential clinical applications since undifferentiated iPSCs could form teratoma after in vivo transplantation.
NPCs/NSCs are commonly isolated by manually dissecting iPSC-derived neural rosettes formed in neural induction medium. Rosettes are dissociated into single cells or smaller clumps which are then cultured as floating neurospheres. Our results show that such an approach can enrich NSCs/NPCs from neural induction, but cannot completely deplete undifferentiated iPSCs (Fig. 2D, I). The contaminated iPSCs in NSCs/NPCs might form teratomas after transplantation following SCI and may even worsen locomotor function of the recipient animals (Tsuji et al., 2010; Nori et al., 2015). The purified A2B5+ NPCs reported in this work are completely devoid of undifferentiated iPSCs (Fig. 2E, M), and importantly, no teratoma was observed after these cells were transplanted into injured mouse spinal cords. Collectively, these results suggest that the purified A2B5+ NPCs are a safe and promising cell source for SCI treatment.
A2B5 is a well-known neural lineage marker. A2B5+ cells isolated from animals improve functional recovery after being grafted into SCI models (Cao et al., 2005a; Lepore et al., 2006; Jin et al., 2011; Haas et al., 2012; Bonner et al., 2013; Haas and Fischer, 2013). Interestingly, although iPSC-derived A2B5+ sorted cells showed neural lineage specific differentiation potential both in vitro and in vivo, they did not completely match the glial progenitor profile to their rodent counterpart. On the contrary, they behaved more like an NPC, as they were able to give rise to all three major lineages of neural populations, including neurons, oligodendrocytes and astrocytes. Indeed, at the molecular level, their genome-wide gene expression profile shared similarities with the previously defined NPCs derived from hESCs (Fig. 4, previously published RNAseq data was retrieved from Gene Expression Omnibus GEO; accession number GSE20301) (Wu et al., 2010), consistent with the conclusion that A2B5+ cells derived from iPSCs have NPC characteristics.
Currently, no consensus on what types of neural cells derived from iPSCs would satisfy the clinical requirement for potential transplantation practices. Multiple types of cells, including NPCs, neuronal progenitors, and glial progenitors have been used individually or in combination (Keirstead et al., 2005; Cao et al., 2010; Tsuji et al., 2010; Bonner et al., 2011; Lepore et al., 2011; Cao and Whittemore, 2012; Sareen et al., 2014). It has been noted that transplantation of a combination of rodent neuronal and glial progenitors exerted optimal effects in survival and functional improvement in SCI animal models (Lepore and Fischer, 2005; Bonner et al., 2011). Usually, compared to postmitotic mature cells, immature cells are more competent to integrate and differentiate after being transplanted, while mature cells have very limited capacity of proliferation, differentiation or integration. On the other hand, cells of very primitive stages might form tumors due to the high capacity of proliferation. In addition, immature cells usually would not respond well to in vivo cues which direct the differentiation and migration of grafts. Proximal neural progenitors, i.e. NPCs, neuronal progenitors, or glial progenitors, which are pre-differentiated, lineage committed, will be able to follow in vivo cues and generate desired neural cells at the injury site. Like NPCs from human fetal brains, iPSC-NPCs are able to survive and differentiate into both neurons and glial cells, promoting functional recovery after SCI (Tsuji et al., 2010; Lu et al., 2014; Sareen et al., 2014). In this work, we have focused on developing a pipeline of generating USCs to iPSCs and then to NPCs. We are currently further transitioning the platform to MEF-free and xeno-free system to better suit for clinical applications.
In summary, we have showed that USCs could be reprogrammed into iPSCs and further induced to NPCs followed by FACS purification using A2B5 antibody. iPSC derived A2B5+ NPCs were able to survive, integrate and differentiate in vitro and in the injured host spinal cord environment without tumor formation. The fact that the A2B5 antibody has been widely tested and used, and that the sorting protocols have been established allows a fast translation of our approach in SCI clinics in the near future.
Acknowledgments
Funding
This work was supported by Department of Neurosurgery, the University of Texas Health Science Center at Houston, Memorial Hermann Foundation-Staman Ogilvie Fund, the Bentsen Stroke Center, the TIRR Foundation through Mission Connect (No. 014-115), Craig H. Neilsen Foundation (No. 338617), NIH (R01 NS061975), NSF (CBET1134449). We also thank the IMM FACS core facility and the Cancer Prevention and Research Institute of Texas (CPRIT) for the supported use of flow cytometry.
Footnotes
Disclosures
None.
Author contributions
YL: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript.
Y Zheng: collection and/or assembly of data, data analysis and interpretation, final approval of manuscript.
SL: collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript.
HX: collection and/or assembly of data, data analysis and interpretation, final approval of manuscript.
KS: provision of study material, collection and/or assembly of data, final approval of manuscript.
GWH: provision of study material, collection and/or assembly of data, final approval of manuscript.
JW: collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript.
Y Zhang: provision of study material, manuscript writing, final approval of manuscript.
DHK: provision of study material, collection and/or assembly of data, final approval of manuscript.
QC: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript.
References
- Afzal MZ, Strande JL. Generation of induced pluripotent stem cells from muscular dystrophy patients: efficient integration-free reprogramming of urine derived cells. J Vis Exp. 2015:52032. doi: 10.3791/52032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokye-Danso F, Trivedi CM, Juhr D, Gupta M, Cui Z, Tian Y, Zhang Y, Yang W, Gruber PJ, Epstein JA, Morrisey EE. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell. 2011;8:376–388. doi: 10.1016/j.stem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghbaderani BA, Tian X, Neo BH, Burkall A, Dimezzo T, Sierra G, Zeng X, Warren K, Kovarcik DP, Fellner T, Rao MS. cGMP-manufactured human induced pluripotent stem cells are available for pre-clinical and clinical applications. Stem Cell Rep. 2015;5:647–659. doi: 10.1016/j.stemcr.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban H, Nishishita N, Fusaki N, Tabata T, Saeki K, Shikamura M, Takada N, Inoue M, Hasegawa M, Kawamata S, Nishikawa S. Efficient generation of transgene-free human induced pluripotent stem cells (iPSCs) by temperature-sensitive Sendai virus vectors. Proc Natl Acad Sci U S A. 2011;108:14234–14239. doi: 10.1073/pnas.1103509108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers J, Linask KL, Chen JA, Siniscalchi LI, Lin Y, Zheng W, Rao M, Chen G. A cost-effective and efficient reprogramming platform for large-scale production of integration-free human induced pluripotent stem cells in chemically defined culture. Sci Rep. 2015;5:11319. doi: 10.1038/srep11319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner JF, Connors TM, Silverman WF, Kowalski DP, Lemay MA, Fischer I. Grafted neural progenitors integrate and restore synaptic connectivity across the injured spinal cord. J Neurosci. 2011;31:4675–4686. doi: 10.1523/JNEUROSCI.4130-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner JF, Haas CJ, Fischer I. Preparation of neural stem cells and progenitors: neuronal production and grafting applications. Methods Mol Biol. 2013;1078:65–88. doi: 10.1007/978-1-62703-640-5_7. [DOI] [PubMed] [Google Scholar]
- Campanelli JT, Sandrock RW, Wheatley W, Xue H, Zheng J, Liang F, Chesnut JD, Zhan M, Rao MS, Liu Y. Expression profiling of human glial precursors. BMC Dev Biol. 2008;8:102. doi: 10.1186/1471-213X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, Whittemore SR. Cell transplantation: stem cells and precursor cells. Handb Clin Neurol. 2012;109:551–561. doi: 10.1016/B978-0-444-52137-8.00034-6. [DOI] [PubMed] [Google Scholar]
- Cao QL, Zhang YP, Howard RM, Walters WM, Tsoulfas P, Whittemore SR. Pluripotent stem cells engrafted into the normal or lesioned adult rat spinal cord are restricted to a glial lineage. Exp Neurol. 2001;167:48–58. doi: 10.1006/exnr.2000.7536. [DOI] [PubMed] [Google Scholar]
- Cao QL, Howard RM, Dennison JB, Whittemore SR. Differentiation of engrafted neuronal-restricted precursor cells is inhibited in the traumatically injured spinal cord. Exp Neurol. 2002;177:349–359. doi: 10.1006/exnr.2002.7981. [DOI] [PubMed] [Google Scholar]
- Cao Q, Xu XM, Devries WH, Enzmann GU, Ping P, Tsoulfas P, Wood PM, Bunge MB, Whittemore SR. Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells. J Neurosci. 2005a;25:6947–6957. doi: 10.1523/JNEUROSCI.1065-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, Zhang YP, Iannotti C, DeVries WH, Xu XM, Shields CB, Whittemore SR. Functional and electrophysiological changes after graded traumatic spinal cord injury in adult rat. Exp Neurol. 2005b;191(Suppl. 1):S3–S16. doi: 10.1016/j.expneurol.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Cao Q, He Q, Wang Y, Cheng X, Howard RM, Zhang Y, DeVries WH, Shields CB, Magnuson DS, Xu XM, Kim DH, Whittemore SR. Transplantation of ciliary neurotrophic factor-expressing adult oligodendrocyte precursor cells promotes remyelination and functional recovery after spinal cord injury. J Neurosci. 2010;30:2989–3001. doi: 10.1523/JNEUROSCI.3174-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CW, Lai YS, Pawlik KM, Liu K, Sun CW, Li C, Schoeb TR, Townes TM. Polycistronic lentiviral vector for “hit and run” reprogramming of adult skin fibroblasts to induced pluripotent stem cells. Stem Cells. 2009;27:1042–1049. doi: 10.1002/stem.39. [DOI] [PubMed] [Google Scholar]
- Chen K, Deng S, Lu H, Zheng Y, Yang G, Kim D, Cao Q, Wu JQ. RNA-seq characterization of spinal cord injury transcriptome in acute/subacute phases: a resource for understanding the pathology at the systems level. PLoS One. 2013;8:e72567. doi: 10.1371/journal.pone.0072567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Jiang P, Xue H, Peterson SE, Tran HT, McCann AE, Parast MM, Li S, Pleasure DE, Laurent LC, Loring JF, Liu Y, Deng W. Role of astroglia in Down’s syndrome revealed by patient-derived human-induced pluripotent stem cells. Nat Commun. 2014;5:4430. doi: 10.1038/ncomms5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou BK, Mali P, Huang X, Ye Z, Dowey SN, Resar LM, Zou C, Zhang YA, Tong J, Cheng L. Efficient human iPS cell derivation by a non-integrating plasmid from blood cells with unique epigenetic and gene expression signatures. Cell Res. 2011;21:518–529. doi: 10.1038/cr.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium, E.P. A user’s guide to the encyclopedia of DNA elements (ENCODE) PLoS Biol. 2011;9:e1001046. doi: 10.1371/journal.pbio.1001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings BJ, Uchida N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, Gage FH, Anderson AJ. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci U S A. 2005;102:14069–14074. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douvaras P, Wang J, Zimmer M, Hanchuk S, O’Bara MA, Sadiq S, Sim FJ, Goldman J, Fossati V. Efficient generation of myelinating oligodendrocytes from primary progressive multiple sclerosis patients by induced pluripotent stem cells. Stem Cell Rep. 2014;3:250–259. doi: 10.1016/j.stemcr.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth GS, Walsh FS, Nirenberg M. Monoclonal antibody to a plasma membrane antigen of neurons. Proc Natl Acad Sci U S A. 1979;76:4913–4917. doi: 10.1073/pnas.76.10.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell CM, O’Leary NA, Harte RA, Loveland JE, Wilming LG, Wallin C, Diekhans M, Barrell D, Searle SM, Aken B, Hiatt SM, Frankish A, Suner MM, Rajput B, Steward CA, Brown GR, Bennett R, Murphy M, Wu W, Kay MP, Hart J, Rajan J, Weber J, Snow C, Riddick LD, Hunt T, Webb D, Thomas M, Tamez P, Rangwala SH, McGarvey KM, Pujar S, Shkeda A, Mudge JM, Gonzalez JM, Gilbert JG, Trevanion SJ, Baertsch R, Harrow JL, Hubbard T, Ostell JM, Haussler D, Pruitt KD. Current status and new features of the Consensus Coding Sequence database. Nucleic Acids Res. 2014;42:D865–D872. doi: 10.1093/nar/gkt1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Griesenbach U, Iida A, Farley R, Wright AM, Zhu J, Munkonge FM, Smith SN, You J, Ban H, Inoue M, Chan M, Singh C, Verdon B, Argent BE, Wainwright B, Jeffery PK, Geddes DM, Porteous DJ, Hyde SC, Gray MA, Hasegawa M, Alton EW. Sendai virus-mediated CFTR gene transfer to the airway epithelium. Gene Ther. 2007;14:1371–1379. doi: 10.1038/sj.gt.3302991. [DOI] [PubMed] [Google Scholar]
- Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85:348–362. doi: 10.2183/pjab.85.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X, Mack DL, Moreno CM, Strande JL, Mathieu J, Shi Y, Markert CD, Wang Z, Liu G, Lawlor MW, Moorefield EC, Jones TN, Fugate JA, Furth ME, Murry CE, Ruohola-Baker H, Zhang Y, Santana LF, Childers MK. Dystrophin-deficient cardiomyocytes derived from human urine: new biologic reagents for drug discovery. Stem Cell Res. 2014;12:467–480. doi: 10.1016/j.scr.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas C, Fischer I. Human astrocytes derived from glial restricted progenitors support regeneration of the injured spinal cord. J Neurotrauma. 2013;30:1035–1052. doi: 10.1089/neu.2013.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas C, Neuhuber B, Yamagami T, Rao M, Fischer I. Phenotypic analysis of astrocytes derived from glial restricted precursors and their impact on axon regeneration. Exp Neurol. 2012;233:717–732. doi: 10.1016/j.expneurol.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou P, Li Y, Zhang X, Liu C, Guan J, Li H, Zhao T, Ye J, Yang W, Liu K, Ge J, Xu J, Zhang Q, Zhao Y, Deng H. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013;341:651–654. doi: 10.1126/science.1239278. [DOI] [PubMed] [Google Scholar]
- Hu BY, Zhang SC. Directed differentiation of neural-stem cells and subtype-specific neurons from hESCs. Methods Mol Biol. 2010;636:123–137. doi: 10.1007/978-1-60761-691-7_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu BY, Weick JP, Yu J, Ma LX, Zhang XQ, Thomson JA, Zhang SC. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci U S A. 2010;107:4335–4340. doi: 10.1073/pnas.0910012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F, Wilson KD, Sun N, Gupta DM, Huang M, Li Z, Panetta NJ, Chen ZY, Robbins RC, Kay MA, Longaker MT, Wu JC. A nonviral minicircle vector for deriving human iPS cells. Nat Methods. 2010;7:197–199. doi: 10.1038/nmeth.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Neuhuber B, Singh A, Bouyer J, Lepore A, Bonner J, Himes T, Campanelli JT, Fischer I. Transplantation of human glial restricted progenitors and derived astrocytes into a contusion model of spinal cord injury. J Neurotrauma. 2011;28:579–594. doi: 10.1089/neu.2010.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–775. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R, Kim KS. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4:472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krencik R, Weick JP, Liu Y, Zhang ZJ, Zhang SC. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat Biotechnol. 2011;29:528–534. doi: 10.1038/nbt.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore AC, Fischer I. Lineage-restricted neural precursors survive, migrate, and differentiate following transplantation into the injured adult spinal cord. Exp Neurol. 2005;194:230–242. doi: 10.1016/j.expneurol.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Lepore AC, Neuhuber B, Connors TM, Han SS, Liu Y, Daniels MP, Rao MS, Fischer I. Long-term fate of neural precursor cells following transplantation into developing and adult CNS. Neuroscience. 2006;142:287–304. doi: 10.1016/j.neuroscience.2005.12.067. [DOI] [PubMed] [Google Scholar]
- Lepore AC, O’Donnell J, Bonner JF, Paul C, Miller ME, Rauck B, Kushner RA, Rothstein JD, Fischer I, Maragakis NJ. Spatial and temporal changes in promoter activity of the astrocyte glutamate transporter GLT1 following traumatic spinal cord injury. J Neurosci Res. 2011;89:1001–1017. doi: 10.1002/jnr.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XJ, Zhang SC. In vitro differentiation of neural precursors from human embryonic stem cells. Methods Mol Biol. 2006;331:169–177. doi: 10.1385/1-59745-046-4:168. [DOI] [PubMed] [Google Scholar]
- Li S, Xue H, Wu J, Rao MS, Kim DH, Deng W, Liu Y. Human induced pluripotent stem cell NEUROG2 dual knockin reporter lines generated by the CRISPR/Cas9 system. Stem Cells Dev. 2015;24:2925–2942. doi: 10.1089/scd.2015.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu PT, Fontes A, Vemuri MC, Macarthur CC. Generation of induced pluripotent stem cells with CytoTune, a non-integrating Sendai virus. Methods Mol Biol. 2013;997:45–56. doi: 10.1007/978-1-62703-348-0_5. [DOI] [PubMed] [Google Scholar]
- Liu Y, Deng W. Reverse engineering human neurodegenerative disease using pluripotent stem cell technology. Brain Res. 2015 doi: 10.1016/j.brainres.2015.09.023. 2015 Sep 27. pii: S0006-8993(15)00721-0. 10.1016/j.brainres.2015.09.023 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wu Y, Lee JC, Xue H, Pevny LH, Kaprielian Z, Rao MS. Oligodendrocyte and astrocyte development in rodents: an in situ and immunohistological analysis during embryonic development. GLIA. 2002;40:25–43. doi: 10.1002/glia.10111. [DOI] [PubMed] [Google Scholar]
- Liu Y, Han SS, Wu Y, Tuohy TM, Xue H, Cai J, Back SA, Sherman LS, Fischer I, Rao MS. CD44 expression identifies astrocyte-restricted precursor cells. Dev Biol. 2004;276:31–46. doi: 10.1016/j.ydbio.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jiang P, Deng W. OLIG gene targeting in human pluripotent stem cells for motor neuron and oligodendrocyte differentiation. Nat Protoc. 2011;6:640–655. doi: 10.1038/nprot.2011.310. [DOI] [PubMed] [Google Scholar]
- Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA, Zheng B, Conner JM, Marsala M, Tuszynski MH. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Woodruff G, Wang Y, Graham L, Hunt M, Wu D, Boehle E, Ahmad R, Poplawski G, Brock J, Goldstein LS, Tuszynski MH. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron. 2014;83:789–796. doi: 10.1016/j.neuron.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macarthur CC, Fontes A, Ravinder N, Kuninger D, Kaur J, Bailey M, Taliana A, Vemuri MC, Lieu PT. Generation of human-induced pluripotent stem cells by a nonintegrating RNA Sendai virus vector in feeder-free or xeno-free conditions. Stem Cells Int. 2012a;2012:564612. doi: 10.1155/2012/564612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macarthur CC, Xue H, Van Hoof D, Lieu PT, Dudas M, Fontes A, Swistowski A, Touboul T, Seerke R, Laurent LC, Loring JF, German MS, Zeng X, Rao MS, Lakshmipathy U, Chesnut JD, Liu Y. Chromatin insulator elements block transgene silencing in engineered human embryonic stem cell lines at a defined chromosome 13 locus. Stem Cells Dev. 2012b;21:191–205. doi: 10.1089/scd.2011.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack AA, Kroboth S, Rajesh D, Wang WB. Generation of induced pluripotent stem cells from CD34+ cells across blood drawn from multiple donors with non-integrating episomal vectors. PLoS One. 2011;6:e27956. doi: 10.1371/journal.pone.0027956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Proschel M, Kalyani AJ, Mujtaba T, Rao MS. Isolation of lineage-restricted neuronal precursors from multipotent neuroepithelial stem cells. Neuron. 1997;19:773–785. doi: 10.1016/s0896-6273(00)80960-5. [DOI] [PubMed] [Google Scholar]
- Mujtaba T, Piper DR, Kalyani A, Groves AK, Lucero MT, Rao MS. Lineage-restricted neural precursors can be isolated from both the mouse neural tube and cultured ES cells. Dev Biol. 1999;214:113–127. doi: 10.1006/dbio.1999.9418. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Tsuji O, Nori S, Toyama Y, Okano H. Cell transplantation for spinal cord injury focusing on iPSCs. Expert Opin Biol Ther. 2012;12:811–821. doi: 10.1517/14712598.2012.681774. [DOI] [PubMed] [Google Scholar]
- Nori S, Okada Y, Nishimura S, Sasaki T, Itakura G, Kobayashi Y, Renault-Mihara F, Shimizu A, Koya I, Yoshida R, Kudoh J, Koike M, Uchiyama Y, Ikeda E, Toyama Y, Nakamura M, Okano H. Long-term safety issues of iPSC-based cell therapy in a spinal cord injury model: oncogenic transformation with epithelial-mesenchymal transition. Stem Cell Rep. 2015;4:360–373. doi: 10.1016/j.stemcr.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt SE, Chang EA, Suhr ST, Schlosser LO, Mondello SE, Moritz CT, Cibelli JB, Horner PJ. Caudalized human iPSC-derived neural progenitor cells produce neurons and glia but fail to restore function in an early chronic spinal cord injury model. Exp Neurol. 2013;248:491–503. doi: 10.1016/j.expneurol.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y, Sierra G, Sivapatham R, Swistowski A, Rao MS, Zeng X. A platform for rapid generation of single and multiplexed reporters in human iPSC lines. Sci Rep. 2015;5:9205. doi: 10.1038/srep09205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit I, Kesner NS, Karry R, Robicsek O, Aberdam E, Muller FJ, Aberdam D, Ben-Shachar D. Induced pluripotent stem cells from hair follicles as a cellular model for neurodevelopmental disorders. Stem Cell Res. 2012;8:134–140. doi: 10.1016/j.scr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Rao M. iPSC-based cell therapy: an important step forward. Stem Cell Rep. 2013;1:281–282. doi: 10.1016/j.stemcr.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS, Malik N. Assessing iPSC reprogramming methods for their suitability in translational medicine. J Cell Biochem. 2012;113:3061–3068. doi: 10.1002/jcb.24183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS, Noble M, Mayer-Proschel M. A tripotential glial precursor cell is present in the developing spinal cord. Proc Natl Acad Sci U S A. 1998;95:3996–4001. doi: 10.1073/pnas.95.7.3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt P, Glatza M, Hemmer K, Tsytsyura Y, Thiel CS, Hoing S, Moritz S, Parga JA, Wagner L, Bruder JM, Wu G, Schmid B, Ropke A, Klingauf J, Schwamborn JC, Gasser T, Scholer HR, Sterneckert J. Derivation and expansion using only small molecules of human neural progenitors for neurodegenerative disease modeling. PLoS One. 2013;8:e59252. doi: 10.1371/journal.pone.0059252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossbach B, Hildebrand L, El-Ahmad L, Stachelscheid H, Reinke P, Kurtz A. Generation of a human induced pluripotent stem cell line from urinary cells of a healthy donor using integration free Sendai technology. Stem Cell Res. 2016;16:133–136. doi: 10.1016/j.scr.2015.12.021. [DOI] [PubMed] [Google Scholar]
- Ruby KM, Zheng B. Gene targeting in a HUES line of human embryonic stem cells via electroporation. Stem Cells. 2009;27:1496–1506. doi: 10.1002/stem.73. [DOI] [PubMed] [Google Scholar]
- Ruff CA, Fehlings MG. Neural stem cells in regenerative medicine: bridging the gap. Panminerva Med. 2010;52:125–147. [PubMed] [Google Scholar]
- Sahni V, Kessler JA. Stem cell therapies for spinal cord injury. Nat Rev Neurol. 2010;6:363–372. doi: 10.1038/nrneurol.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar DL, Uchida N, Hamers FP, Cummings BJ, Anderson AJ. Human neural stem cells differentiate and promote locomotor recovery in an early chronic spinal cord injury NOD-scid mouse model. PLoS One. 2010;5:e12272. doi: 10.1371/journal.pone.0012272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salewski RP, Mitchell RA, Shen C, Fehlings MG. Transplantation of neural stem cells clonally derived from embryonic stem cells promotes recovery after murine spinal cord injury. Stem Cells Dev. 2015;24:36–50. doi: 10.1089/scd.2014.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sareen D, Gowing G, Sahabian A, Staggenborg K, Paradis R, Avalos P, Latter J, Ornelas L, Garcia L, Svendsen CN. Human induced pluripotent stem cells are a novel source of neural progenitor cells (iNPCs) that migrate and integrate in the rodent spinal cord. J Comp Neurol. 2014;522:2707–2728. doi: 10.1002/cne.23578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp J, Frame J, Siegenthaler M, Nistor G, Keirstead HS. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury. Stem Cells. 2010;28:152–163. doi: 10.1002/stem.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer CA, Stadtfeld M, Murphy GJ, Hochedlinger K, Kotton DN, Mostoslavsky G. Induced pluripotent stem cell generation using a single lentiviral stem cell cassette. Stem Cells. 2009;27:543–549. doi: 10.1634/stemcells.2008-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takeda A, Igarashi H, Kawada M, Tsukamoto T, Yamamoto H, Inoue M, Iida A, Shu T, Hasegawa M, Matano T. Evaluation of the immunogenicity of replication-competent V-knocked-out and replication-defective F-deleted Sendai virus vector-based vaccines in macaques. Vaccine. 2008;26:6839–6843. doi: 10.1016/j.vaccine.2008.09.074. [DOI] [PubMed] [Google Scholar]
- Tsuji O, Miura K, Okada Y, Fujiyoshi K, Mukaino M, Nagoshi N, Kitamura K, Kumagai G, Nishino M, Tomisato S, Higashi H, Nagai T, Katoh H, Kohda K, Matsuzaki Y, Yuzaki M, Ikeda E, Toyama Y, Nakamura M, Yamanaka S, Okano H. Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc Natl Acad Sci U S A. 2010;107:12704–12709. doi: 10.1073/pnas.0910106107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wang L, Huang W, Su H, Xue Y, Su Z, Liao B, Wang H, Bao X, Qin D, He J, Wu W, So KF, Pan G, Pei D. Generation of integration-free neural progenitor cells from cells in human urine. Nat Methods. 2013a;10:84–89. doi: 10.1038/nmeth.2283. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu J, Tan X, Li G, Gao Y, Liu X, Zhang L, Li Y. Induced pluripotent stem cells from human hair follicle mesenchymal stem cells. Stem Cell Rev. 2013b;9:451–460. doi: 10.1007/s12015-012-9420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, Daley GQ, Brack AS, Collins JJ, Cowan C, Schlaeger TM, Rossi DJ. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltjen K, Michael IP, Mohseni P, Desai R, Mileikovsky M, Hamalainen R, Cowling R, Wang W, Liu P, Gertsenstein M, Kaji K, Sung HK, Nagy A. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Liu Y, Chesnut JD, Rao MS. Isolation of neural stem and precursor cells from rodent tissue. Methods Mol Biol. 2008;438:39–53. doi: 10.1007/978-1-59745-133-8_5. [DOI] [PubMed] [Google Scholar]
- Wu JQ, Habegger L, Noisa P, Szekely A, Qiu C, Hutchison S, Raha D, Egholm M, Lin H, Weissman S, Cui W, Gerstein M, Snyder M. Dynamic transcriptomes during neural differentiation of human embryonic stem cells revealed by short, long, and paired-end sequencing. Proc Natl Acad Sci U S A. 2010;107:5254–5259. doi: 10.1073/pnas.0914114107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue H, Wu S, Papadeas ST, Spusta S, Swistowska AM, MacArthur CC, Mattson MP, Maragakis NJ, Capecchi MR, Rao MS, Zeng X, Liu Y. A targeted neuroglial reporter line generated by homologous recombination in human embryonic stem cells. Stem Cells. 2009;27:1836–1846. doi: 10.1002/stem.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Zhan H, Mali P, Dowey S, Williams DM, Jang YY, Dang CV, Spivak JL, Moliterno AR, Cheng L. Human-induced pluripotent stem cells from blood cells of healthy donors and patients with acquired blood disorders. Blood. 2009;114:5473–5480. doi: 10.1182/blood-2009-04-217406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Muench MO, Fusaki N, Beyer AI, Wang J, Qi Z, Yu J, Kan YW. Blood cell-derived induced pluripotent stem cells free of reprogramming factors generated by Sendai viral vectors. Stem Cells Transl Med. 2013;2:558–566. doi: 10.5966/sctm.2013-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemitsu Y, Kitson C, Ferrari S, Farley R, Griesenbach U, Judd D, Steel R, Scheid P, Zhu J, Jeffery PK, Kato A, Hasan MK, Nagai Y, Masaki I, Fukumura M, Hasegawa M, Geddes DM, Alton EW. Efficient gene transfer to airway epithelium using recombinant Sendai virus. Nat Biotechnol. 2000;18:970–973. doi: 10.1038/79463. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan SH, Martin J, Elia J, Flippin J, Paramban RI, Hefferan MP, Vidal JG, Mu Y, Killian RL, Israel MA, Emre N, Marsala S, Marsala M, Gage FH, Goldstein LS, Carson CT. Cell-surface marker signatures for the isolation of neural stem cells, glia and neurons derived from human pluripotent stem cells. PLoS One. 2011;6:e17540. doi: 10.1371/journal.pone.0017540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XQ, Zhang SC. Differentiation of neural precursors and dopaminergic neurons from human embryonic stem cells. Methods Mol Biol. 2010;584:355–366. doi: 10.1007/978-1-60761-369-5_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, McNeill E, Tian H, Soker S, Andersson KE, Yoo JJ, Atala A. Urine derived cells are a potential source for urological tissue reconstruction. J Urol. 2008;180:2226–2233. doi: 10.1016/j.juro.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Zhang PW, Haidet-Phillips AM, Pham JT, Lee Y, Huo Y, Tienari PJ, Maragakis NJ, Sattler R, Rothstein JD. Generation of GFAP::GFP astrocyte reporter lines from human adult fibroblast-derived iPS cells using zinc-finger nuclease technology. GLIA. 2016;64:63–75. doi: 10.1002/glia.22903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, Trauger S, Bien G, Yao S, Zhu Y, Siuzdak G, Scholer HR, Duan L, Ding S. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Benda C, Dunzinger S, Huang Y, Ho JC, Yang J, Wang Y, Zhang Y, Zhuang Q, Li Y, Bao X, Tse HF, Grillari J, Grillari-Voglauer R, Pei D, Esteban MA. Generation of human induced pluripotent stem cells from urine samples. Nat Protoc. 2012;7:2080–2089. doi: 10.1038/nprot.2012.115. [DOI] [PubMed] [Google Scholar]


