Abstract
Background
Multiple neuropsychiatric disorders, e.g., depression, are linked to imbalances in excitatory and inhibitory neurotransmission and prefrontal cortical dysfunction, and are concomitant with chronic stress.
Methods
We used electrophysiologic (n = 5–6 animals, 21–25 cells/group), neuroanatomic (n = 6–8/group), and behavioral (n = 12/group) techniques to test the hypothesis that chronic stress increases inhibition of medial prefrontal cortex (mPFC) glutamatergic output neurons.
Results
Using patch clamp recordings from infralimbic mPFC pyramidal neurons, we found that chronic stress selectively increases the frequency of miniature inhibitory postsynaptic currents with no effect on amplitude, which suggests that chronic stress increases presynaptic gamma-aminobutyric acid release. Elevated gamma-aminobutyric acid release under chronic stress is accompanied by increased inhibitory appositions and terminals onto glutamatergic cells, as assessed by both immunohistochemistry and electron microscopy. Furthermore, chronic stress decreases glucocorticoid receptor immunoreactivity specifically in a subset of inhibitory neurons, which suggests that increased inhibitory tone in the mPFC after chronic stress may be caused by loss of a glucocorticoid receptor–mediated brake on interneuron activity. These neuroanatomic and functional changes are associated with impairment of a prefrontal-mediated behavior. During chronic stress, rats initially make significantly more errors in the delayed spatial win-shift task, an mPFC-mediated behavior, which suggests a diminished impact of the mPFC on decision making.
Conclusions
Taken together, the data suggest that chronic stress increases synaptic inhibition onto prefrontal glutamatergic output neurons, limiting the influence of the prefrontal cortex in control of stress reactivity and behavior. Thus, these data provide a mechanistic link among chronic stress, prefrontal cortical hypofunction, and behavioral dysfunction.
Keywords: Chronic variable stress, GABA, Glucocorticoid receptor, mIPSC, Prefrontal cortex, Stress
An imbalance between excitatory and inhibitory neurotransmission is proposed to underlie multiple neuropsychiatric disorders, including major depressive disorder (MDD), schizophrenia, epilepsy, anxiety, Parkinson's disease, and bipolar disorder (1–4). Many of these disorders are comorbid with chronic stress and hypercortisolemia (5–10), which suggests that impaired neurotransmission occurs in the context of altered stress hormone signaling. Importantly, these disorders are accompanied by prefrontal cortical dysfunction (11–14). Moreover, studies in rodents indicate that the ventromedial prefrontal cortex is critical for control of neuroendocrine stress responses, and the infralimbic subdivision is particularly important for chronic stress regulation (15–19). In combination, these lines of evidence suggest that chronic stress may lead to prefrontal cortical dysfunction and stress-related physiologic and emotional pathologies.
Morphologic analysis of the medial prefrontal cortex (mPFC) after chronic stress suggests altered neuronal excitability associated with decreases in the dendritic complexity of pyramidal neurons (20–25) and increases in interneuron dendritic arborization (26). Stress-induced decreases in glutamatergic neuronal complexity in layer V of the mPFC correlate with reduced excitatory neural responses to serotonin, which suggests that chronic stress may affect prefrontal-mediated behaviors (27,28). Morphologic studies are supported by behavioral data suggesting that chronic stress disrupts multiple aspects of mPFC-dependent behaviors, including behavioral flexibility, strategic planning, rule learning, behavioral inhibition, and spatial memory (22,28–36). Collectively, the data suggest that chronic stress may inhibit mPFC output, compromising the functionality of the mPFC.
In this study, we employ an interdisciplinary approach to test the hypothesis that chronic stress shifts the balance between excitatory and inhibitory neurotransmission toward greater inhibition of infralimbic prefrontal glutamatergic output neurons, resulting in impaired prefrontal-mediated behavior. Our data indicate that chronic stress increases inhibition of infralimbic cortex (ilPFC) glutamatergic output neurons via an increase in gamma-aminobutyric acid (GABA) release, likely owing to increased GABAergic innervation of glutamatergic output neurons. Stress-induced inhibition of infralimbic pyramidal cells is accompanied by impaired acquisition of a delayed spatial win-shift (DSWS) contingency, which provides evidence of impaired prefrontal cortical control of behavior.
Methods and Materials
Ex Vivo
Subjects
Male Sprague Dawley rats (Harlan, Indianapolis, IN), aged 54–57 days or 200–225 g upon arrival, were doubly housed throughout the experiment in a temperature- and humidity-controlled room on a 14- and 10-hour light/dark cycle. Food (Teklad, Harlan) and water were available ad libitum. All experimental procedures described subsequently were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Animals and were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Chronic Variable Stress
Half of the animals were unhandled (naive; n = 5–6) or underwent chronic variable stress (CVS) for 14 days (n = 5–6) beginning at about postnatal day 60 (to obviate developmental confounds). The CVS was composed of twice-daily (AM and PM) repeated and unpredictable stressors, including cold swims (10 minutes, 16–18°C), warm swims (20 minutes, 30–32°C), cold room exposure (1 hour, 4°C), shaker stress (1 hour, 100 rpm), open field (5 minutes), and hypoxia (30 minutes, 8% oxygen) (19). The same combination and sequence of stressors were used for all CVS experiments throughout the current study and has consistently led to attenuated body weight gain and adrenal hypertrophy, markers of a successful CVS regimen (19).
Electrophysiology
Patch Clamp
Whole-cell patch clamp recordings were obtained from layer V pyramidal neurons in the ilPFC, which were easily identifiable in the slice on the basis of somal morphology and the presence of a prominent apical dendrite. Miniature excitatory postsynaptic currents (mEPSCs) and miniature inhibitory postsynaptic currents (mIPSCs) were recorded in the presence of tetrodotoxin (500 nmol/L). We recorded mEPSCs and mIPSCs at holding potentials of –70 and 0 mV, respectively, in the same cell (3 minutes each; n = 21–25 cells/group). Bath application of 10 μM gabazine abolished mIPSCs at a holding potential of 0 mV, which showed that the IPSCs observed using this protocol are mediated by GABA type A receptors (Figure 1).
Figure 1.
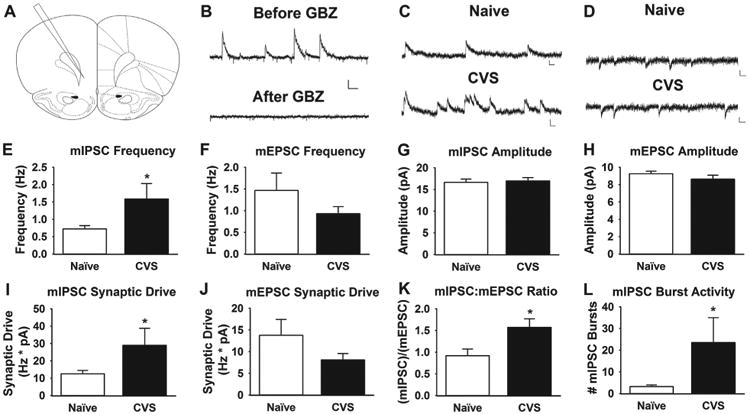
Chronic stress increases inhibitory neurotransmission in the infralimbic cortex. (A) Large pyramidal (.100 picofarad [pF] on average) neurons were recorded from layer V of the infralimbic prefrontal cortex near bregma +3.2 to +2.2 (37). (B) The mIPSCs recorded at a holding potential of 0 mV before and after 10 μmol/L gabazine (GBZ) (gamma-aminobutyric acid type A receptor antagonist) bath application. Scale bars = 20 pA (vertical scaling) and 125 ms (horizontal scaling). (C, D) Representative miniature inhibitory postsynaptic current (mIPSC) and miniature excitatory postsynaptic current (mEPSC) traces, respectively, from naive and chronically stressed animals. Scale bars = 10 pA (vertical scaling) and 25 ms (horizontal scaling). (E, F) The mIPSC and mEPSC frequencies in slices from naive and chronically stressed animals (CVS). Chronic stress significantly increases mIPSC frequency (p < .05), with no effect on mEPSC frequency (p < .05). (G, H) The mIPSC and mEPSC amplitudes in slices from naive and chronically stressed animals. There are no statistical differences in mIPSC (p < .05) or mEPSC (p < .05) amplitudes in chronically stressed vs. naive animals. (I, J) Chronic stress increased mIPSC synaptic drive (p < .05), with no effect of stress on excitatory synaptic drive in slices from chronically stressed animals (p > .05). (K) The ratio of mIPSC to mEPSC frequency in each cell was significantly shifted toward more mIPSCs after chronic stress (p < .05). (L) Bursting, defined as a cluster of three or more mIPSCs separated by less than 150 ms, was also significantly higher in chronically stressed animals (p < .05) (n = 21–25 cells/group, 5 animals/group). *p < .05.
In Vivo Neuroanatomic Studies
Subjects
Male Sprague Dawley rats, 250–275 g upon arrival, were singly housed throughout the experiment on a 12- to 12-hour light/dark cycle.
Chronic Variable Stress
Half of the animals were unhandled (naive; n = 8) or underwent CVS for 14 days (n = 8), as previously described.
Immunohistochemistry
Sections were immunolabeled with primary antibodies against glucocorticoid receptor (GR) (M-20) (1:1,000), glutamic acid decarboxylase (GAD)67 (1:1,000), calcium calmodulin kinase IIα (CaMKIIα) (1:200), GAD65 (1:100), parvalbumin (PV) (1:2,000), cholecystokinin (CCK) (1:1,000), somatostatin (SST) (1:250), calretinin (1:500), and calbindin (1:1,000) using standard immunohistochemical procedures (19) (Supplemental Methods and Supplemental Table S1).
Imaging and Analysis
For analysis of GAD65 appositions onto CaMKIIα-positive cells, three z stacks (at a 0.5-μm interval) from each side of the ilPFC at the rostral (anteroposterior [AP], about +3.2 mm from bregma), middle (AP, about +2.7 mm), and caudal (AP, about +2.2 mm) extent, as defined by the rat stereotaxic brain atlas of Paxinos and Watson (37), were captured at ×63 magnification. All cells within the z stack that met criteria were selected and analyzed. Appositions were defined by immunoreactive boutons with absolutely no visible space between the bouton and the edge of the CaMKIIα-positive cell (Figure 2 and Supplemental Methods).
Figure 2.
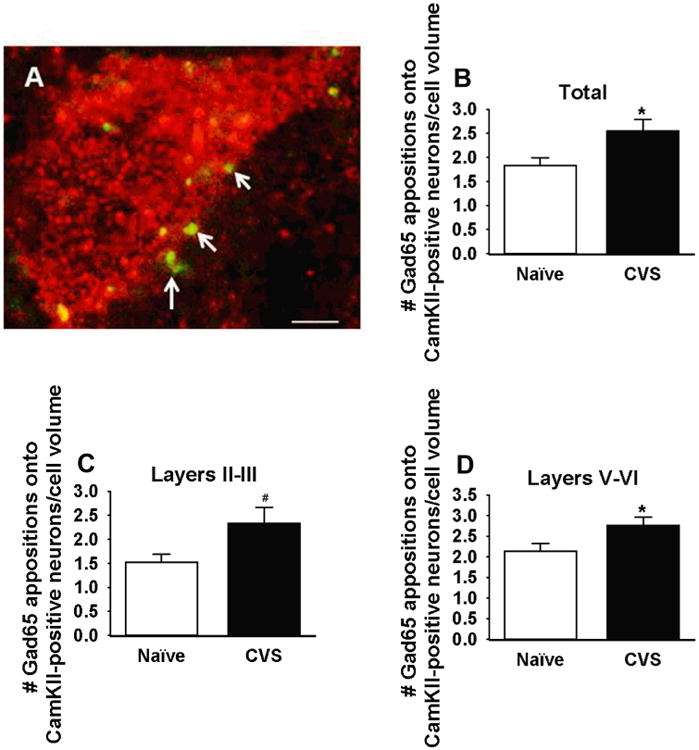
Number of glutamic acid decarboxylase (GAD)65 appositions onto calcium calmodulin kinase (CaM-KII)-positive cells in the infralimbic cortex of naive and chronically stressed animals (CVS). (A) Representative CaMKIIα-immunolabeled cell (red) with GAD65-positive terminals (green). Arrows denote GAD65 terminals that were counted as appositions. (B–D) Chronic stress significantly increases the number of GAD65 appositions onto CaMKII cells in all layers of the infralimbic medial prefrontal cortex (p < .05) and specifically in layers II–III (p < .05) and layers V–VII (p < .05). *p < .05; #p < .05, one-tailed t test (n = 7–8 animals/group, approximately 6 slices/animal; normalized to cell volume [μm3]). Scale bar = 5 μm.
For analysis of GR colocalization with GAD67, calretinin, calbindin, CCK, SST, and PV, z stacks were taken of each side of the rostral, mid, and caudal ilPFC, as noted previously. All image capture and quantification was conducted by an experimenter who was blinded to experimental treatments. After all quantification had taken place, images presented in the article were cropped and contrast and brightness were adjusted uniformly to enhance publication quality without altering the presence or absence of immunolabeling (Supplemental Methods).
Electron Microscopy
Subjects
Male Sprague Dawley rats, 250–275 g upon arrival, were singly housed throughout the experiment on a 12- to 12-hour light/dark cycle.
Chronic Variable Stress
Half of the animals were unhandled (naive; n = 6) or underwent CVS for 14 days (n = 6), as previously described.
Immunohistochemistry
Sections were immunolabeled with primary antibodies against GAD65 (1:500) using standard electron microscopy protocols for tissue preparation, immunohistochemistry, and embedding (Supplemental Methods and Supplemental Table S1).
Ultrastructural Analysis
The GABAergic terminals were counted manually in the two-dimensional plane throughout all layers of the ilPFC at ×8,000 magnification using a Hitachi 7650 transmission electron microscope (Hitachi, Tokoyo, Japan) equipped with an AMT V660 camera (Advanced Microscopy Techniques, Woburn, MA). ImageJ software (National Institutes of Health, Bethesda, MD) was used to measure the area of somata analyzed. Pyramidal cells were identified by their morphologic features (a cell body with a large round nucleus [with homogeneously dispersed karyoplasm] occupying a large portion of the cell and the presence of clear apical and basal dendrites). The GAD65-labeled terminals were identified by black peroxidase label. Terminals were counted by an experimenter who was blinded to the study protocol.
Behavioral Studies
Subjects
Male Sprague Dawley rats, aged 54–57 days upon arrival, were singly–housed throughout the experiment on a 12- to 12-hour reverse light/dark cycle. Animals were allowed to habituate to the vivarium for 1 week before experimentation. Food and water were available ad libitum during habituation to the vivarium and during the initial 14 days of CVS only. Otherwise animals were restricted to 70% of their ab libitum baseline food intake.
Chronic Variable Stress
Half of the animals underwent CVS for 14 days before and throughout the DSWS task (n = 12) during the light phase, as previously described.
Delayed Spatial Win-Shift Task
Animals were pre-exposed to semisweet chocolate morsels in their home cage for 2 nights before testing and were then subjected to 2 days of 10 minutes' habituation in a dimly lit eight-arm radial arm maze (RAM) (the first without chocolate placement and the second with chocolate placed in the center of the maze). Animals began training on the DSWS on the 15th day of CVS. The task consisted of three phases (training, delay, and testing) and was conducted during the dark phase of the light/dark cycle, when animals typically consume most of their calories. During the training phase, animals were placed in the RAM with four blocked arms and four baited arms for a maximum of 5 minutes. After successfully visiting each baited arm, animals were returned to their home cage, with no view of the RAM, for the 5-minute delay phase. During the testing phase, the previously blocked arms were the only four baited arms. Again, the animals had to visit each baited arm to complete the task in a maximum of 5 minutes before being removed from the RAM. Each day, the baited arms for the training and test phases were randomly selected. The RAM was cleaned with 20% ethanol each time an animal was introduced to the RAM, to disrupt olfactory cues, forcing the animal to rely on spatial cues presented around the room. Animals were trained daily on the task until all animals reached the criterion of visiting the four baited arms during the testing phase in five or fewer choices, two consecutive days.
The DSWS requires individual housing to allow for food restriction while monitoring body weight; mild restriction is required to motivate rats to perform the task and is commonly employed in executing this paradigm (38,39). Whereas isolation and food restriction may generate physiologic adaptations, prior work from our laboratory indicates that they do not mimic or interfere with the development of chronic stress phenotypes (19,40,41).
Statistical Analysis
Electrophysiological data were analyzed using Clampfit (Molecular Devices, Sunnyvale, CA) and MiniAnalysis (Synaptosoft, Decatur, GA) software. Data failing normality and/or homogeneity of variance were log-transformed. Properties of excitatory and inhibitory transmission were compared between naive and CVS animals using t test (raw or log-transformed data) or Mann-Whitney when log-transformed data failed normality and/or homogeneity of variance. Immunohistochemical and electromagnetic data were analyzed with t test (raw or log-transformed data) or Mann-Whitney when log-transformed data failed normality and/or homogeneity of variance. Behavioral data were analyzed using two-way repeated measures (RM) analysis of variance (ANOVA) (time [days] × number of errors), with time as the repeated measure or t test. Significance was set at p ≤ .05.
Results
We tested the hypothesis that chronic stress would shift the balance of neurotransmission toward greater synaptic inhibition in the ilPFC. Using whole-cell voltage clamp techniques, we recorded mIPSCs and mEPSCs in layer V pyramidal neurons in the ilPFC (Figure 1). Chronic stress significantly increased mIPSC frequency (t test on log-transformed data: t42 = 2.318, p = .03) with no effect on mIPSC amplitude (t test: t42 = 0.2825, p = .78) or miniature excitatory neurotransmission (t test, amplitude: t43 = 1.217, p = 0.23) or frequency (Mann-Whitney U = 247.0, p = .92) (Figure 1). Furthermore, inhibitory synaptic drive (defined as mIPSC frequency × mIPSC amplitude in an individual neuron) significantly increased after chronic stress (t test on log-transformed data: t43 = 2.133, p = .04), as did the ratio of mIPSC to mEPSC frequency (t test on log-transformed data: t41 = 3.055, p = .004) (Figure 1). Bursting of mIPSCs, defined as a cluster of three or more mIPSCs separated by less than 150 ms, was also significantly higher after chronic stress (Mann-Whitney U = 140.5, p = .012) (Figure 1). Taken together, the data suggest increased inhibitory neurotransmission in the ilPFC after chronic stress.
Because chronic stress increases inhibitory neurotransmission in the ilPFC and arborization of prefrontal interneurons (26), we analyzed the number of GAD65 appositions onto CaMKIIα-positive glutamatergic cells using dual-label immunohistochemistry. Chronic stress increased the number of GAD65 appositions onto glutamatergic soma in all layers of the ilPFC (t test: t10 = 2.27, p = .05), in layers II and III (one-tailed t test: t11 = 2.12, p = .03) as well as V and VI (t test: t10 = 2.297, p = .04), which suggests that chronic stress increases GABAergic innervation of prefrontal output neurons (Figure 2). We corroborated this increase in GABAergic innervation using electron microscopy, which revealed increased growth of GABAergic terminals onto infralimbic pyramidal cells (t test: t118 = 2.042, p = .04) (Figure 3).
Figure 3.
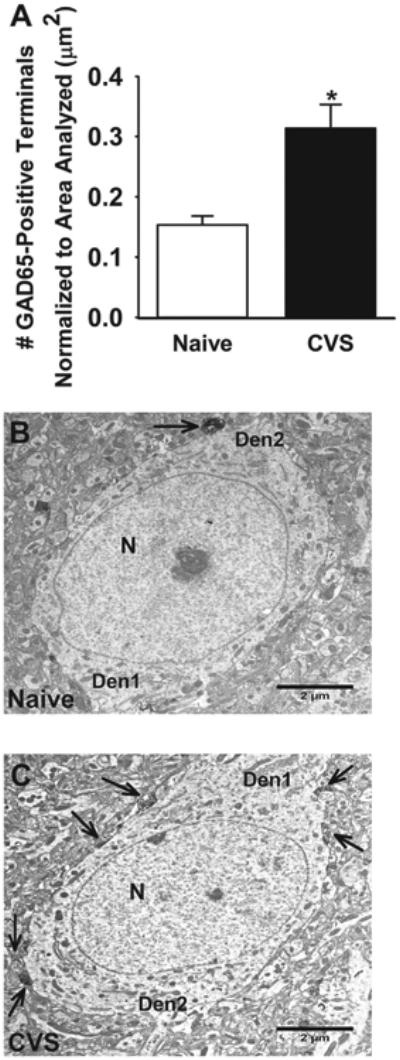
Number of glutamic acid decarboxylase (GAD)65 terminals on glutamatergic cells in the infralimbic cortex of naive and chronically stressed rats (CVS). (A) Chronic stress significantly increased GAD65-immunolabeled terminals onto pyramidal cells (p < .05). (B, C) Representative GAD65-immunolabeled electron micrographs from naive and chronically stressed rats. Images were captured at ×8000 magnification. Arrows indicate GAD65-positive terminals. *p < .05. Scale bar = 2 μm. Den1, apical dendrite; Den2, basal dendrite; N, nucleus.
Chronic stress decreases GR expression in the mPFC (23); however, the cell-type specificity of this downregulation is unknown. Given the specific effects on inhibitory neurotransmission and interneuron plasticity, we used dual-label immunohistochemistry to test the hypothesis that GR down-regulation is interneuron specific. After chronic stress, GR colocalization with GAD67-positive neurons was significantly decreased (t test: t14 = 3.52, p = .003) with no effect on GR and CaMKII colocalization (Mann-Whitney U = 30, p = .88) (Figure 4). Next, we dual-labeled GR with antibodies targeting distinct inhibitory neuronal populations, including PV (Figure 4), SST (not shown), CCK (Supplemental Figure S1), calbindin (Supplemental Figure S1), and calretinin (Supplemental Figure S2). Notably, GR colocalization was significantly decreased only in PV-positive interneurons (t test: t15 = 3.48, p = .003). GR colocalization was absent in calbindin-, SST-, and CCK-positive cells and unaffected in calretinin-positive cells (t test: t10 = 0.52, p = .62), which suggests that GR down-regulation in the ilPFC is directed to specific populations of interneurons.
Figure 4.
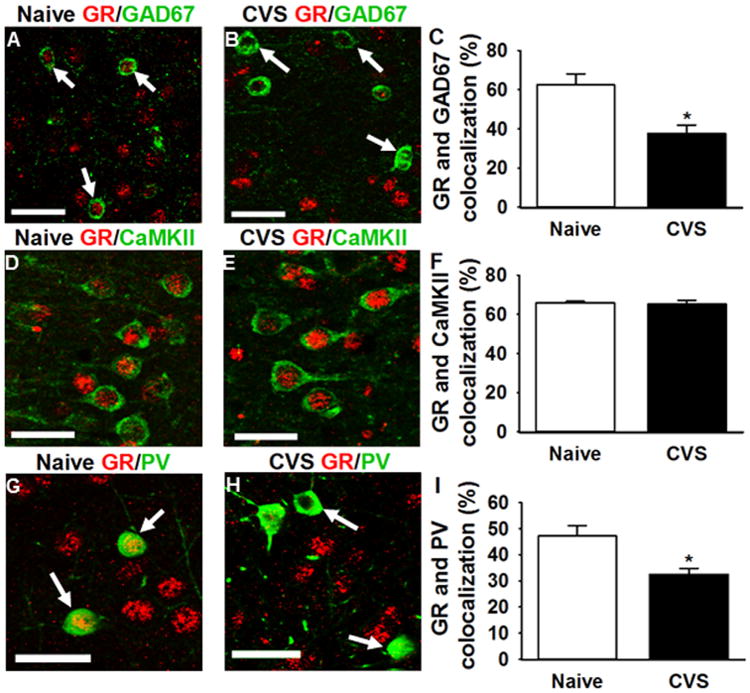
Percentage of glutamic acid decarboxylase (GAD)67, calcium calmodulin kinase II (CaMKII), or parvalbumin (PV) colocalization with glucocorticoid receptor (GR)-positive neurons in infralimbic medial prefrontal cortex in naive and chronically stressed animals (CVS). (A, B) Representative GR immunoreactivity (red) in naive and chronically stressed animals, respectively (×20 magnification). Chronic stress significantly decreases GR immunoreactivity (red) in GAD67-positive neurons (green) (p < .05) (n = 8 animals/group, 6 slices/animal). Arrows indicate GAD67 cells that have GR (A) or lack colocalization with it (B). (D, E) Representative GR (red) immunoreactivity in CaMKII-positive neurons (green), respectively (×20 magnification). Chronic stress does not affect GR expression in CaMKII-positive neurons (p > .05) (n = 8 animals/group, 6 slices/animal). (G, H) Representative GR immunoreactivity (red) in PV-positive neurons (green) (×10 magnification). Arrows indicate PV-positive neurons that have GR (G) or lack colocalization with it (H). Chronic stress significantly decreases GR immunoreactivity in PV-positive neurons (p < .05) (n = 8 animals/group, 6 slices/animal). Arrows indicate PV-positive cells that have or lack colocalization with GR. *p < .05. Scale bar = 50 μm.
The mPFC is critical for behavioral flexibility, a process that requires the individual to update behavioral strategies continually based on the context (30,31,42–44). We used the DSWS paradigm (38,39,45) to test the hypothesis that CVS impairs learning of a prefrontal-mediated task. This task requires animals to use information garnered during the training phase to flexibly plan and alter their behavior prospectively during the testing phase (Figure 5). Chronically stressed animals showed significant impairment in the acquisition of DSWS (higher total errors) (significant stress × time interaction, two-way RM ANOVA: F15,360 = 1.8, p = .03), which was highlighted by greater numbers of errors on days 2 and 3 (Figure 5). Total errors included both within-phase errors (revisiting the same arm during the testing phase) and across-phase errors (visiting un-baited arms during the testing phase that were previously baited during the training phase). Chronically stressed animals made significantly more across-phase errors (on the third day) (main effect of stress, two-way RM ANOVA: F1,25 = 4.13, p = .05) and within-phase errors (on the second and third days) (stress × time interaction, two-way RM ANOVA: F15,350 = 2.02, p = .01) (Supplemental Figure S3). Notably, chronically stressed animals made more within-phase errors, which indicated that these animals had impaired working memory, a prefrontal-mediated function. There was no significant difference in acquisition of the task overall (i.e., number of days to reach criteria) (t test: t22 = 1.07, p = .30) (Supplemental Figure S3).
Figure 5.
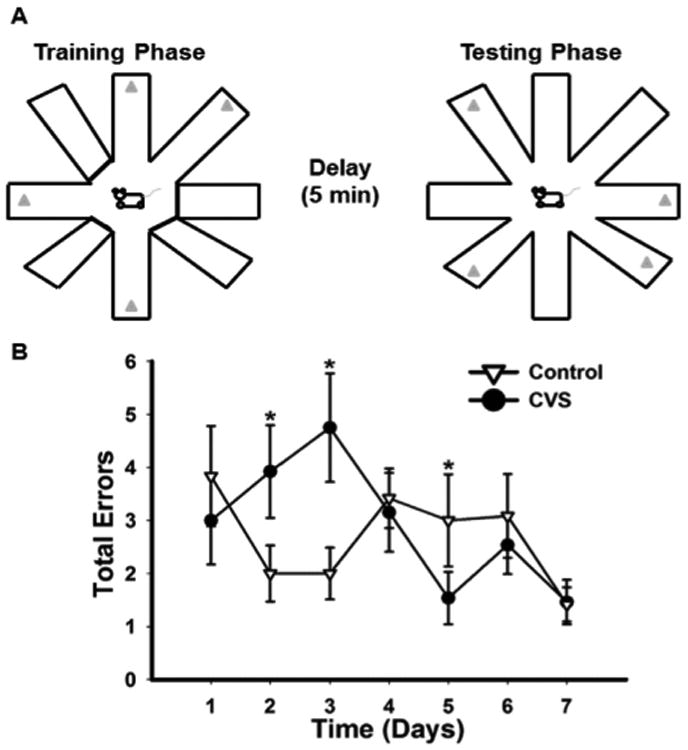
Chronic stress impairs initial behavioral flexibility. (A) Schematic detailing the training, delay, and testing phases of the delayed spatial win-shift (DSWS) task. (B) Total errors made in DSWS task. The graph is truncated to 7 days because there were no significant differences after day 5. Chronically stressed animals (CVS) initially made significantly more errors during acquisition of the DSWS task (p < .05). *p < .05.
Discussion
Our study provides evidence for a profound shift in the balance between excitatory and inhibitory neurotransmission in the ilPFC after chronic stress. Stress biases toward greater inhibition of glutamatergic output neurons manifest as an increased frequency of mIPSCs and enhanced GABAergic innervation of principal output neurons. Morphologic analysis reveals a selective decrease in GR immunoreactivity in PV-expressing inter-neurons, which suggests a possible connection between loss of glucocorticoid signaling in a specific population of interneurons and enhanced inhibition. Morphologic and physiologic plasticity is accompanied by deficits in prefrontal-mediated spatial learning observed in our CVS model, verifying reduced functional activation of prefrontal circuitry.
Increased inhibitory neurotransmission after chronic stress is in stark contrast to the increased excitatory and decreased inhibitory neurotransmission observed after acute stress (46–51). Based on studies from other groups, it appears that acute stress initially allows for the excitation of prefrontal glutamatergic neurons, mediated by GR-dependent (genomic) mobilization of endocannabinoids and subsequent inhibition of interneurons by endocannabinoids. Acute glucocorticoid effects allow for prefrontal glutamatergic regulation of downstream targets to regulate the hypothalamic-pituitary-adrenal (HPA) axis, as well as behavior (46,52). Our data suggest that this brake on interneuron activity is significantly attenuated in chronic stress, perhaps owing to a diminished role of corticosterone, which leads to increased inhibition of glutamatergic output and a diminished influence of the prefrontal cortex (Figure 6). Thus, the data suggest that restoration of inter-neuron GR signaling or an enhancement of endocannabinoid signaling may attenuate the effects of chronic stress on inhibitory neurotransmission. Whether the GR is causally related to or secondary to changes in endocannabinoid signaling (resulting in the enhancement of inhibitory neurotransmission under chronic stress) remains to be determined.
Figure 6.
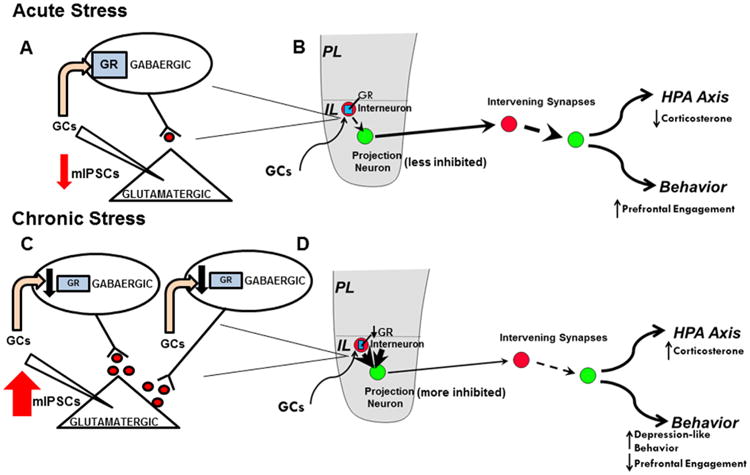
Schematic of effects of acute and chronic stress on prefrontal engagement. (A) Magnified view of inhibitory synapses onto glutamatergic pyramidal cells in the infralimbic (IL) cortex during acute stress. Based on the literature, we propose that acute stress induces genomic glucocorticoid receptor (GR)-dependent inhibition of parvalbumin (PV)-positive interneurons in the IL prefrontal cortex directly and/or via mobilization of endocannabinoids, diminishing inhibitory neurotransmission and allowing the prefrontal cortex to excite downstream targets. (B) How acute stress affects prefrontal engagement on a circuit level. Glucocorticoids (GC) act on the IL GR (represented by blue rectangles) to suppress interneuron (represented by red circles) inhibition of the glutamatergic projection neurons (represented by green circles), allowing the medial prefrontal cortex to become engaged and activating downstream targets such as the basolateral complex of the amygdala. (C) Magnified view of inhibitory synapses onto glutamatergic pyramidal cells in the infralimbic cortex during chronic stress. Chronic stress increases gamma-aminobutyric acidergic (GABAergic) innervation of glutamatergic output neurons and decreases GR in PV-positive neurons. The decrease in GR may directly affect the excitability of PV neurons and/or remove a brake on interneuron axonal growth. Taken together, chronic stress leads to an increase in inhibitory neurotransmission and diminishes the influence of the prefrontal cortex on downstream targets. (D) How chronic stress affects prefrontal engagement on a circuit level. Under chronic stress, GR downregulation diminishes the ability of glucocorticoids to dampen PV-mediated inhibition, resulting in increased inhibition of glutamatergic neurons. In turn, medial prefrontal cortex output is presumably diminished, which offers a potential mechanism for dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis under chronic stress, the depression-like phenotype observed after infralimbic GR knockdown, and general dysfunction of prefrontal-mediated behaviors under chronic stress. mIPSCs, miniature inhibitory postsynaptic currents.
Chronic stress leads to GR downregulation in the prefrontal cortex (23). Our studies indicate that stress-induced down-regulation of GR immunoreactivity is specific to GAD-positive neurons and has no effect on colocalization of GR in glutamatergic projection neurons (identified by CaMKIIα immunolabeling in layer V). Moreover, GR is preferentially depleted in PV-positive neurons, the most predominant interneuron subtype in cortex, making up over half of the interneuron population in layer V (53,54). Thus, a decrease in GR specifically in PV neurons could affect a significant proportion of interneurons and have profound effects on inhibitory neurotransmission. Furthermore, PV inhibits the firing of glutamatergic pyramidal neurons (which is particularly important for directing neural synchrony) and is therefore well-positioned to serve as a target for glucocorticoids (55–59).
In contrast to PV cells, other interneuron subtypes had limited colocalization with the GR (e.g., SST, CCK, and calbindin), which suggests that their activation may not be significantly affected by glucocorticoids. Other populations (e.g., calretinin) showed some degree of colocalization but did not exhibit GR loss upon exposure to chronic stress. Together, the data indicate that chronic stress may preferentially affect the PV-expressing subpopulation of interneurons, which are thought to function at glutamatergic somata to reduce excitability and regulate neuronal synchrony (56). Thus, a possibility for the observed enhancement of inhibitory neurotransmission could be an elevated probability of GABA release and/or increased excitability in PV-expressing interneurons (Figure 6).
Prior studies of chronic stress-induced morphologic plasticity focused on dendritic retraction in glutamatergic pyramidal neurons (20,24,60–63). In general, loss of dendritic complexity is thought to reflect a reduced area for excitatory synaptic connections, which may reflect decreased excitability (28). More recently, Gilabert-Juan and colleagues (26) found increased interneuron arborization using a transgenic mouse line that expressed enhanced green fluorescent protein under the GAD67 promoter. Our data indicate that chronic stress increases the number of inhibitory appositions and terminals onto the glutamatergic output neurons across all layers of the ilPFC, which indicates structural plasticity favoring enhanced inhibition. Together, the morphologic data suggest that chronic stress causes interneuron hypertrophy and terminal sprouting, which may have a role in limiting mPFC excitability. Thus, another possibility for increased inhibitory neurotransmission under chronic stress may be due to an increase in the number of GABA release sites. The GR may have a general role in limiting axonal growth in both interneurons and pyramidal neurons (22,24,25,61). Because chronic stress specifically downregulates GR immunoreactivity in PV-positive neurons, this general brake on axonal growth may be relieved, allowing for interneuron hypertrophy and terminal sprouting. The increase in terminal sprouting would allow for a greater number of GABA release sites during chronic stress (Figure 6). Nonetheless, the exact mechanisms underlying enhanced GABA neurotransmission after chronic stress remain to be determined.
The GR is widely expressed in multiple cell types in the mPFC, including glutamatergic neurons, interneurons, and glia (23,64,65).The neuronal composition of the mPFC is roughly 80%–90% glutamatergic neurons and 10%–20% interneurons (53,66,67). Although it is the most prominent interneuron subtype, PV neurons comprise only half of the interneurons in the mPFC (53). Thus, selective downregulation of GR in PV-positive interneurons suggests specific disruption of glucocorticoid signaling in this cell population after chronic stress. Although it is tempting to speculate that loss of an inhibitory GR signal in PV neurons underlies enhanced GABAergic inhibition of ilPFC output, further analysis is required to provide a mechanistic link between the two observations. Definitive tests of the GR–PV interneuron link await the development of tools to target GR specifically in ilPFC PV neurons in rats.
Loss of GR in ilPFC PV neurons has implications for prefrontal function and numerous stress-related conditions, e.g., schizophrenia and MDD. Gamma oscillations in the mPFC, which are disrupted in both schizophrenia and MDD, are under tight control of the fast-spiking PV neurons (68–71). These oscillations are critical for regulating working memory, attention, and information processing (72–74). Moreover, gamma oscillations are thought to synchronize the output of pyramidal glutamatergic neurons, leading to the enhancement of specific projections to downstream targets to enable information flow out of the mPFC (70,75,76). Hippocampal CA1 pyramidal neurons are similarly under tight perisomatic control of PV neurons, leading to firing synchronization of pyramidal neurons and network oscillations (77). In rat CA1 hippocampal slices, application of bovine serum albumin–conjugated dexamethasone, a nongenomic GR agonist, rapidly increases spontaneous IPSCs. Likewise, acute and chronic stress increased spontaneous IPSC frequency in a bursting pattern, similar to the increased frequency and bursting of mIPSCs observed in the current study. Furthermore, this enhancement of inhibitory neurotransmission in chronically stressed rats was accompanied by impairment in PV rhythmic firing, which may affect network oscillations, and suggests that stress comprises the integrity and functionality of PV neurons in the hippocampus (78). A number of genes that regulate PV function are also associated with susceptibility to schizophrenia and MDD, including disrupted in schizophrenia 1 (DISC1), neuregulin-1 (NRG-1)/ERBB4, and dystrobrevin-binding protein 1 (DTNBP1) (74,79–86). Combined, these findings suggest that stress-induced disruptions in glucocorticoid signaling in PV neurons may have functional consequences for numerous processes controlled by the prefrontal cortex and downstream targets, as well as stress-related neuropsychiatric disorders.
Our finding that chronic stress increased inhibitory neurotransmission in the ilPFC is in contrast to recent data from another group suggesting that chronic restraint and unpredictable stress decreased excitatory neurotransmission with no effect on inhibitory neurotransmission (87). Notably, these studies were conducted in adolescent rats, at a stage when the mPFC is actively undergoing development, including glutamatergic pruning and increasing GABAergic signaling (88,89). For that reason, we ensured that animals were at least aged postnatal day 60 before beginning CVS, to confirm that we were able to study the effects of stress in the absence of potential developmental confounds. Therefore, the current study highlights that chronic stress affects the adult brain much differently from the way it does the adolescent brain (90).
The prefrontal cortex is known to be important for executive functions, including behavioral flexibility, strategic planning, and spatial and working memory. Likewise, GABA is known to regulate excitability and neural circuits, and ultimately it contributes to learning and memory (91). Our data indicate that chronic stress impaired acquisition of a DSWS contingency, which is consistent with reduced behavioral flexibly. Moreover, chronically stressed animals exhibited impaired working memory, which also suggests prefrontal dysfunction. Taken together, these data indicate that chronic stress deleteriously affects prefrontal-mediated behaviors in our experimental model, likely linked to reduced prefrontal cortex output.
The ilPFC is well positioned to regulate both neuroendocrine and behavioral responses to stress. Prior work from our group indicated that knockdown of infralimbic GR enhances HPA axis responses to acute and chronic stress and increases immobility in the forced swim test (19), which indicates the importance of ilPFC stress hormone signaling in stress integration and emotion. Combined with the current study, these findings suggest that stress-induced reductions in infralimbic GR may selectively impair glucocorticoid control of mPFC function, resulting in hyper-inhibition and consequent reduction in prefrontal output. The ilPFC itself provides top-down regulation of areas involved in behavioral and neuroendocrine regulation, including the basolateral amygdala (BLA). Inhibition of infralimbic output neurons thus would be expected to release downstream effectors of prefrontal regulation, resulting in characteristic increases in anxiety-related behaviors, depression-related behaviors, and HPA axis reactivity seen after chronic stress (Figure 6). Thus, diminished prefrontal regulation of the BLA under chronic stress could result from loss of the inhibitory brake (GR) on interneuron activity in the ilPFC. Courtin and colleagues (92) recently demonstrated a similar phenomenon in dorsal mPFC, in which inactivation of PV neurons permits pyramidal cell firing primarily to BLA neurons, enables neural synchrony, and induces freezing in a discriminative fear conditioning task. Whether chronic stress, through loss of a brake on PV activity, diminishes excitability or firing of glutamatergic output neurons in the mPFC and BLA remains to be determined.
Chronic stress increases inhibitory neurotransmission in the ilPFC, which may underlie chronic stress-induced deficits in mPFC function. Thus, restoring the balance between excitatory and inhibitory neurotransmission may attenuate stress-induced effects on mPFC function. Therapeutics designed to achieve this goal may prove useful in treating neuropsychiatric disorders linked to stress and mPFC dysfunction, e.g., MDD or posttraumatic stress disorder.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant Nos. MH097430 (to JMM), MH069860 (to JPH), MH049698 (to JPH), and HL122454 (to BM); and American Heart Association Grant No. 17070152 (to BM).
We thank Liz Kritzer, Dr. Ann Hemerlee, Tara Kyser, Brittany Kopp, and Jenny Shurdack for technical expertise. We also thank Dr. Kim Seroogy for use of equipment and procedural space.
Footnotes
Disclosures: The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at http://dx.doi.org/10.1016/j.biopsych.2016.03.2101.
References
- 1.Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011;16:383–406. doi: 10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2012;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopha-macology for mood disorders. Neuropharmacology. 2012;62:63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skolnick P, Popik P, Trullas R. Glutamate-based antidepressants: 20 years on. Trends Pharmacol Sci. 2009;30:563–569. doi: 10.1016/j.tips.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, Murad H, et al. Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry. 1999;4:163–172. doi: 10.1038/sj.mp.4000473. [DOI] [PubMed] [Google Scholar]
- 6.Altamura AC, Boin F, Maes M. HPA axis and cytokines dysregulation in schizophrenia: potential implications for the antipsychotic treatment. Eur Neuropsychopharmacol. 1999;10:1–4. doi: 10.1016/s0924-977x(99)00017-6. [DOI] [PubMed] [Google Scholar]
- 7.Barden N. Implication of the hypothalamic-pituitary-adrenal axis in the physiopathology of depression. J Psychiatry Neurosci. 2004;29:185–193. [PMC free article] [PubMed] [Google Scholar]
- 8.Corcoran C, Mujica-Parodi L, Yale S, Leitman D, Malaspina D. Could stress cause psychosis in individuals vulnerable to schizophrenia? CNS Spectr. 2002;7:33–38. 41–42. doi: 10.1017/s1092852900022240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan MCM, Sharifi N, Condren R, Thakore JH. Evidence of basal pituitary-adrenal overactivity in first episode, drug naive patients with schizophrenia. Psychoneuroendocrinology. 2004;29:1065–1070. doi: 10.1016/j.psyneuen.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Ventura J, Nuechterlein KH, Lukoff D, Hardesty JP. A prospective study of stressful life events and schizophrenic relapse. J Abnorm Psychol. 1989;98:407–411. doi: 10.1037//0021-843x.98.4.407. [DOI] [PubMed] [Google Scholar]
- 11.Lewis DA, Volk DW, Hashimoto T. Selective alterations in prefrontal cortical GABA neurotransmission in schizophrenia: a novel target for the treatment of working memory dysfunction. Psychopharmacology (Berl) 2004;174:143–150. doi: 10.1007/s00213-003-1673-x. [DOI] [PubMed] [Google Scholar]
- 12.Monchi O, Petrides M, Doyon J, Postuma RB, Worsley K, Dagher A. Neural bases of set-shifting deficits in Parkinson's disease. J Neurosci. 2004;24:702–710. doi: 10.1523/JNEUROSCI.4860-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rogers MA, Kasai K, Koji M, Fukuda R, Iwanami A, Nakagome K, et al. Executive and prefrontal dysfunction in unipolar depression: a review of neuropsychological and imaging evidence. Neurosci Res. 2004;50:1–11. doi: 10.1016/j.neures.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Shad MU, Muddasani S, Prasad K, Sweeney JA, Keshavan MS. Insight and prefrontal cortex in first-episode schizophrenia. Neuroimage. 2004;22:1315–1320. doi: 10.1016/j.neuroimage.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP. The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. Eur J Neurosci. 2003;18:2357–2364. doi: 10.1046/j.1460-9568.2003.02932.x. [DOI] [PubMed] [Google Scholar]
- 17.Jones KR, Myers B, Herman JP. Stimulation of the prelimbic cortex differentially modulates neuroendocrine responses to psychogenic and systemic stressors. Physiol Behav. 2011;104:266–271. doi: 10.1016/j.physbeh.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radley JJ, Arias CM, Sawchenko PE. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J Neurosci. 2006;26:12967–12976. doi: 10.1523/JNEUROSCI.4297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKlveen JM, Myers B, Flak JN, Bundzikova J, Solomon MB, Seroogy KB, et al. Role of prefrontal cortex glucocorticoid receptors in stress and emotion. Biol Psychiatry. 2013;74:672–679. doi: 10.1016/j.biopsych.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- 21.Goldwater DS, Pavlides C, Hunter RG, Bloss EB, Hof PR, McEwen BS, et al. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience. 2009;164:798–808. doi: 10.1016/j.neuroscience.2009.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizoguchi K, Ishige A, Aburada M, Tabira T. Chronic stress attenuates glucocorticoid negative feedback: involvement of the prefrontal cortex and hippocampus. Neuroscience. 2003;119:887–897. doi: 10.1016/s0306-4522(03)00105-2. [DOI] [PubMed] [Google Scholar]
- 24.Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, et al. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol. 2008;507:1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilabert-Juan J, Castillo-Gomez E, Guirado R, Molto MD, Nacher J. Chronic stress alters inhibitory networks in the medial pre-frontal cortex of adult mice. Brain Struct Funct. 2013;218:1591–1605. doi: 10.1007/s00429-012-0479-1. [DOI] [PubMed] [Google Scholar]
- 27.Liu RJ, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci U S A. 2008;105:359–364. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev. 2009;33:773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxietylike behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 2008;33:320–331. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- 30.Cerqueira JJ, Pego JM, Taipa R, Bessa JM, Almeida OFX, Sousa N. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J Neurosci. 2005;25:7792–7800. doi: 10.1523/JNEUROSCI.1598-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cerqueira JJ, Mailliet F, Almeida OFX, Jay TM, Sousa N. The prefrontal cortex as a key target of the maladaptive response to stress. J Neurosci. 2007;27:2781–2787. doi: 10.1523/JNEUROSCI.4372-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delatour B, Gisquet-Verrier P. Functional role of rat prelimbic-infralimbic cortices in spatial memory: evidence for their involvement in attention and behavioural flexibility. Behav Brain Res. 2000;109:113–128. doi: 10.1016/s0166-4328(99)00168-0. [DOI] [PubMed] [Google Scholar]
- 33.Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui DH, Tabira T. Chronic stress induces impairment of spatial working memory because of prefrontal dopaminergic dysfunction. J Neurosci. 2000;20:1568–1574. doi: 10.1523/JNEUROSCI.20-04-01568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy BL, Arnsten AF, Jentsch JD, Roth RH. Dopamine and spatial working memory in rats and monkeys: pharmacological reversal of stress-induced impairment. J Neurosci. 1996;16:7768–7775. doi: 10.1523/JNEUROSCI.16-23-07768.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishimura J, Endo Y, Kimura F. A long-term stress exposure impairs maze learning performance in rats. Neurosci Lett. 1999;273:125–128. doi: 10.1016/s0304-3940(99)00645-x. [DOI] [PubMed] [Google Scholar]
- 36.Ragozzino ME, Wilcox C, Raso M, Kesner RP. Involvement of rodent prefrontal cortex subregions in strategy switching. Behav Neurosci. 1999;113:32–41. doi: 10.1037//0735-7044.113.1.32. [DOI] [PubMed] [Google Scholar]
- 37.Paxinos G, Watson C. The Rat 4th ed In: Brain in Stereotaxic Coordinates. 4th. San Diego, CA: Academic Press Inc; 1998. [Google Scholar]
- 38.Butts KA, Weinberg J, Young AH, Phillips AG. Glucocorticoid receptors in the prefrontal cortex regulate stress-evoked dopamine efflux and aspects of executive function. Proc Natl Acad Sci U S A. 2011;108:18459–18464. doi: 10.1073/pnas.1111746108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flak JN, Jankord R, Solomon MB, Krause EG, Herman JP. Opposing effects of chronic stress and weight restriction on cardiovascular, neuroendocrine and metabolic function. Physiol Behav. 2011;104:228–234. doi: 10.1016/j.physbeh.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flak JN, Solomon MB, Jankord R, Krause EG, Herman JP. Identification of chronic stress-activated regions reveals a potential recruited circuit in rat brain. Eur J Neurosci. 2012;36:2547–2555. doi: 10.1111/j.1460-9568.2012.08161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lapiz MDS, Morilak DA. Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience. 2006;137:1039–1049. doi: 10.1016/j.neuroscience.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 44.Stalnaker TA, Takahashi Y, Roesch MR, Schoenbaum G. Neural substrates of cognitive inflexibility after chronic cocaine exposure. Neuropharmacology. 2009;56(suppl 1):63–72. doi: 10.1016/j.neuropharm.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seamans JK, Floresco SB, Phillips AG. Functional differences between the prelimbic and anterior cingulate regions of the rat prefrontal cortex. Behav Neurosci. 1995;109:1063–1073. doi: 10.1037//0735-7044.109.6.1063. [DOI] [PubMed] [Google Scholar]
- 46.Hill MN, McLaughlin RJ, Pan B, Fitzgerald ML, Roberts CJ, Lee TTY, et al. Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J Neurosci. 2011;31:10506–10515. doi: 10.1523/JNEUROSCI.0496-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Musazzi L, Milanese M, Farisello P, Zappettini S, Tardito D, Barbiero VS, et al. Acute stress increases depolarization-evoked glutamate release in the rat prefrontal/frontal cortex: the dampening action of antidepressants. PLoS One. 2010;5:e8566. doi: 10.1371/journal.pone.0008566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Treccani G, Musazzi L, Perego C, Milanese M, Nava N, Bonifacino T, et al. Stress and corticosterone increase the readily releasable pool of glutamate vesicles in synaptic terminals of prefrontal and frontal cortex. Mol Psychiatry. 2014;19:433–443. doi: 10.1038/mp.2014.5. [DOI] [PubMed] [Google Scholar]
- 49.Treccani G, Musazzi L, Perego C, Milanese M, Nava N, Bonifacino T, et al. Acute stress rapidly increases the readily releasable pool of glutamate vesicles in prefrontal and frontal cortex through non-genomic action of corticosterone. Mol Psychiatry. 2014;19:401. doi: 10.1038/mp.2014.20. [DOI] [PubMed] [Google Scholar]
- 50.Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci U S A. 2009;106:14075–14079. doi: 10.1073/pnas.0906791106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuen EY, Liu W, Karatsoreos IN, Ren Y, Feng J, McEwen BS, Yan Z. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol Psychiatry. 2011;16:156–170. doi: 10.1038/mp.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McLaughlin RJ, Hill MN, Gorzalka BB. A critical role for prefrontocortical endocannabinoid signaling in the regulation of stress and emotional behavior. Neurosci Biobehav Rev. 2014;42:116–131. doi: 10.1016/j.neubiorev.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 53.Kubota Y, Hattori R, Yui Y. Three distinct subpopulations of GABAergic neurons in rat frontal agranular cortex. Brain Res. 1994;649:159–173. doi: 10.1016/0006-8993(94)91060-x. [DOI] [PubMed] [Google Scholar]
- 54.Uematsu M, Hirai Y, Karube F, Ebihara S, Kato M, Abe K, et al. Quantitative chemical composition of cortical GABAergic neurons revealed in transgenic venus-expressing rats. Cereb Cortex. 2008;18:315–330. doi: 10.1093/cercor/bhm056. [DOI] [PubMed] [Google Scholar]
- 55.Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A. Cortical interneurons that specialize in disinhibitory control. Nature. 2013;503:521–524. doi: 10.1038/nature12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Freund TF, Katona I. Perisomatic inhibition. Neuron. 2007;56:33–42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 57.Kvitsiani D, Ranade S, Hangya B, Taniguchi H, Huang JZ, Kepecs A. Distinct behavioural and network correlates of two interneuron types in prefrontal cortex. Nature. 2013;498:363–366. doi: 10.1038/nature12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- 59.Miles R, Toth K, Gulyas AI, Hajos N, Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron. 1996;16:815–823. doi: 10.1016/s0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- 60.Radley JJ, Morrison JH. Repeated stress and structural plasticity in the brain. Ageing Res Rev. 2005;4:271–287. doi: 10.1016/j.arr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 61.Radley JJ, Rocher AB, Miller M, Janssen WGM, Liston C, Hof PR, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- 62.Seib LM, Wellman CL. Daily injections alter spine density in rat medial prefrontal cortex. Neurosci Lett. 2003;337:29–32. doi: 10.1016/s0304-3940(02)01287-9. [DOI] [PubMed] [Google Scholar]
- 63.Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- 64.Vielkind U, Walencewicz A, Levine JM, Bohn MC. Type II glucocorticoid receptors are expressed in oligodendrocytes and astrocytes. J Neurosci Res. 1990;27:360–373. doi: 10.1002/jnr.490270315. [DOI] [PubMed] [Google Scholar]
- 65.Bohn MC, Howard E, Vielkind U, Krozowski Z. Glial cells express both mineralocorticoid and glucocorticoid receptors. J Steroid Biochem Mol Biol. 1991;40:105–111. doi: 10.1016/0960-0760(91)90173-3. [DOI] [PubMed] [Google Scholar]
- 66.McKlveen JM, Myers B, Herman JP. The medial prefrontal cortex: coordinator of autonomic, neuroendocrine and behavioural responses to stress. J Neuroendocrinol. 2015;27:446–456. doi: 10.1111/jne.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gabbott PLA, Warner TA, Jays PRL, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- 68.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Volman V, Behrens MM, Sejnowski TJ. Downregulation of parvalbumin at cortical GABA synapses reduces network gamma oscillatory activity. J Neurosci. 2011;31:18137–18148. doi: 10.1523/JNEUROSCI.3041-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu TY, Hsieh JC, Chen YS, Tu PC, Su TP, Chen LF. Different patterns of abnormal gamma oscillatory activity in unipolar and bipolar disorder patients during an implicit emotion task. Neuropsychologia. 2012;50:1514–1520. doi: 10.1016/j.neuropsychologia.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 72.Jensen O, Kaiser J, Lachaux JP. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 2007;30:317–324. doi: 10.1016/j.tins.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 73.Kim H, Ahrlund-Richter S, Wang X, Deisseroth K, Carlen M. Prefrontal parvalbumin neurons in control of attention. Cell. 2016;164:208–218. doi: 10.1016/j.cell.2015.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murray AJ, Woloszynowska-Fraser MU, Ansel-Bollepalli L, Cole KLH, Foggetti A, Crouch B, et al. Parvalbumin-positive interneurons of the prefrontal cortex support working memory and cognitive flexibility. Sci Rep. 2015;5:16778. doi: 10.1038/srep16778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang X, Carlen M. Optogenetic dissection of cortical information processing-shining light on schizophrenia. Brain Res. 2012;1476:31–37. doi: 10.1016/j.brainres.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 76.Kepecs A, Fishell G. Interneuron cell types are fit to function. Nature. 2014;505:318–326. doi: 10.1038/nature12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hu W, Zhang M, Czeh B, Flugge G, Zhang W. Stress impairs GABAergic network function in the hippocampus by activating non-genomic glucocorticoid receptors and affecting the integrity of the parvalbumin-expressing neuronal network. Neuropsychopharmacol-ogy. 2010;35:1693–1707. doi: 10.1038/npp.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fazzari P, Paternain AV, Valiente M, Pla R, Lujan R, Lloyd K, et al. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 2010;464:1376–1380. doi: 10.1038/nature08928. [DOI] [PubMed] [Google Scholar]
- 80.Hikida T, Jaaro-Peled H, Seshadri S, Oishi K, Hookway C, Kong S, et al. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc Natl Acad Sci U S A. 2007;104:14501–14506. doi: 10.1073/pnas.0704774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marin O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13:107–120. doi: 10.1038/nrn3155. [DOI] [PubMed] [Google Scholar]
- 82.Shen S, Lang B, Nakamoto C, Zhang F, Pu J, Kuan SL, et al. Schizophrenia-related neural and behavioral phenotypes in transgenic mice expressing truncated Disc1. J Neurosci. 2008;28:10893–10904. doi: 10.1523/JNEUROSCI.3299-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wen L, Lu YS, Zhu XH, Li XM, Woo RS, Chen YJ, et al. Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proc Natl Acad Sci U S A. 2010;107:1211–1216. doi: 10.1073/pnas.0910302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ji B, Higa KK, Kim M, Zhou L, Young JW, Geyer MA, et al. Inhibition of protein translation by the DISC1-Boymaw fusion gene from a Scottish family with major psychiatric disorders. Hum Mol Genet. 2014;23:5683–5705. doi: 10.1093/hmg/ddu285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang N, Zhang GF, Liu XY, Sun HL, Wang XM, Qiu LL, et al. Downregulation of neuregulin 1-ErbB4 signaling in parvalbumin interneurons in the rat brain may contribute to the antidepressant properties of ketamine. J Mol Neurosci. 2014;54:211–218. doi: 10.1007/s12031-014-0277-8. [DOI] [PubMed] [Google Scholar]
- 86.Arias B, Serretti A, Mandelli L, Gasto C, Catalan R, Ronchi D De, et al. Dysbindin gene (DTNBP1) in major depression: association with clinical response to selective serotonin reuptake inhibitors. Pharmacogenet Genomics. 2009;19:121–128. doi: 10.1097/FPC.0b013e32831ebb4b. [DOI] [PubMed] [Google Scholar]
- 87.Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012;73:962–977. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cressman VL, Balaban J, Steinfeld S, Shemyakin A, Graham P, Parisot N, et al. Prefrontal cortical inputs to the basal amygdala undergo pruning during late adolescence in the rat. J Comp Neurol. 2010;518:2693–2709. doi: 10.1002/cne.22359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Selemon LD. A role for synaptic plasticity in the adolescent development of executive function. Transl Psychiatry. 2013;3:e238. doi: 10.1038/tp.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jankord R, Solomon MB, Albertz J, Flak JN, Zhang R, Herman JP. Stress vulnerability during adolescent development in rats. Endocrinology. 2011;152:629–638. doi: 10.1210/en.2010-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Castillo PE, Chiu CQ, Carroll RC. Long-term plasticity at inhibitory synapses. Curr Opin Neurobiol. 2011;21:328–338. doi: 10.1016/j.conb.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Courtin J, Chaudun F, Rozeske RR, Karalis N, Gonzalez-Campo C, Wurtz H, et al. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature. 2014;505:92–96. doi: 10.1038/nature12755. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


