ABSTRACT
Outer membrane vesicles (OMVs) produced by Gram-negative bacteria are enriched in several outer membrane components, including major and minor outer membrane proteins and lipooligosaccharide. We assessed the functional activity of nontypeable Haemophilus influenzae (NTHi) OMV-specific antisera and the protective ability of NTHi OMVs as vaccine antigens in the chinchilla otitis media model. OMVs were purified from three HMW1/HMW2-expressing NTHi strains, two of which were also engineered to overexpress Hia proteins. OMV-specific antisera raised in guinea pigs were assessed for their ability to mediate killing of representative NTHi in an opsonophagocytic assay. The three OMV-specific antisera mediated killing of 18 of 65, 24 of 65, and 30 of 65 unrelated HMW1/HMW2-expressing NTHi strains. Overall, they mediated killing of 39 of 65 HMW1/HMW2-expressing strains. The two Hia-expressing OMV-specific antisera mediated killing of 17 of 25 and 14 of 25 unrelated Hia-expressing NTHi strains. Overall, they mediated killing of 20 of 25 Hia-expressing strains. OMVs from prototype NTHi strain 12 were used to immunize chinchillas and the course of middle ear infection was monitored following intrabullar challenge with the homologous strain. All control animals developed culture-positive otitis media, as did two of three HMW1/HMW2-immunized animals. All OMV-immunized animals, with or without supplemental HMW1/HMW2 immunization, were completely protected against otitis media. NTHi OMVs are the first immunogens examined in this model that provided complete protection with sterile immunity after NTHi strain 12 challenge. These data suggest that NTHi OMVs hold significant potential as components of protective NTHi vaccines, possibly in combination with HMW1/HMW2 proteins.
KEYWORDS: Haemophilus influenzae, outer membrane vesicles, vaccines
INTRODUCTION
Nontypeable Haemophilus influenzae (NTHi) organisms are small Gram-negative bacteria that colonize the upper respiratory tract of humans beginning at a very early age (1). Although these organisms are normally commensals, when host defenses are compromised by underlying medical conditions such as malnutrition, immunodeficiency, chronic lung disease, or acute viral infection, disease caused by NTHi may develop (2, 3). Among children in the developed world, NTHi infections are currently responsible for an estimated 40 to 50% of the cases of acute otitis media and an even higher percentage of cases of chronic and recurrent disease (4, 5). Among adults, particularly among patients with chronic obstructive pulmonary disease, NTHi infections are a major cause of illness, especially during the acute exacerbations that often characterize this disease (6). A vaccine capable of preventing disease caused by these organisms would offer substantial benefit to the adult and pediatric populations alike.
NTHi vaccine development efforts are ongoing in many laboratories. A large number of protein and lipooligosaccharide antigens have been the subject of detailed investigation as potential vaccine candidates (7, 8). In our early work, we demonstrated that development of bactericidal antibody in the sera of children recovered from acute NTHi otitis media was associated with the appearance of serum antibodies directed against highly immunogenic high molecular weight (HMW) proteins (9). This work led subsequently to the identification and characterization of the HMW1/HMW2 family of proteins (10). The HMW1/HMW2 proteins were later shown to be major adhesins of NTHi (11), as well as targets of human opsonophagocytic antibodies (12, 13) and protective antibodies in the chinchilla otitis model (14). The HMW1/HMW2-like proteins are expressed by approximately 75% of NTHi strains (10, 15). The 25% of NTHi strains that do not express HMW1/HMW2 proteins express other immunogenic high molecular weight proteins that are recognized by human convalescent-phase serum antibodies (16). Almost all HMW1/HMW2-negative strains express a second distinct class of adhesin known as Hia (16). The Hia proteins are members of a large family of bacterial proteins known as autotransporters that are found in many Gram-negative bacteria (17). The Hia proteins have also been demonstrated to serve as targets for antibodies mediating opsonophagocytosis of NTHi (18). Nearly all NTHi strains that lack HMW1/HMW2 proteins contain an hia gene and express an Hia protein, and conversely, strains that express HMW1/HMW2 proteins lack an hia gene (15, 16, 19). Both the HMW1/HMW2 and Hia proteins demonstrate strain heterogeneity (15, 20). However, HMW1/HMW2- and Hia-specific antisera mediate relatively broad-based killing of heterologous NTHi (21), suggesting that the strain heterogeneity present among these proteins would not preclude their potential use as vaccine components.
Both the HMW1/HMW2 and Hia proteins are subject to phase variation in the face of immune pressure, resulting in selection for bacterial variants downregulated in HMW1/HMW2 and Hia protein expression (14, 22). This property could limit the value of the HMW1/HMW2 and Hia proteins as vaccine components. Combining the HMW1/HMW2 and Hia proteins with other NTHi antigens to generate a more robust and broad-based immune response could abrogate the phase variation problem. We have begun investigating the potential of NTHi outer membrane vesicles (OMVs) as immunogens and as supplemental vaccine components. OMVs are membrane blebs naturally produced by all Gram-negative bacteria studied to date, including NTHi (23–25). OMVs are enriched in many outer membrane components, including major and minor outer membrane proteins and lipooligosaccharide (LOS) molecules. OMVs have been investigated as vaccine candidates for prevention of disease caused by a number of Gram-negative pathogens (26), perhaps most notably Neisseria meningitidis, against which several OMV-based vaccines have been or are currently being evaluated in human clinical trials (27). Meningococcal OMVs comprise one component of the 4CMenB vaccine approved for prevention of serogroup B N. meningitidis disease (28, 29).
In the work described here, we demonstrate that antisera raised against prototype NTHi OMVs mediate relatively broad-based opsonophagocytic killing of homologous and heterologous NTHi strains and that immunization with prototype OMVs provides solid protection against homologous bacterial challenge in the chinchilla model of NTHi otitis media. These data suggest that NTHi OMVs hold potential as components of protective NTHi vaccines, possibly in combination with HMW1/HMW2 and Hia proteins.
RESULTS
NTHi OMV preparations with and without Hia protein expression.
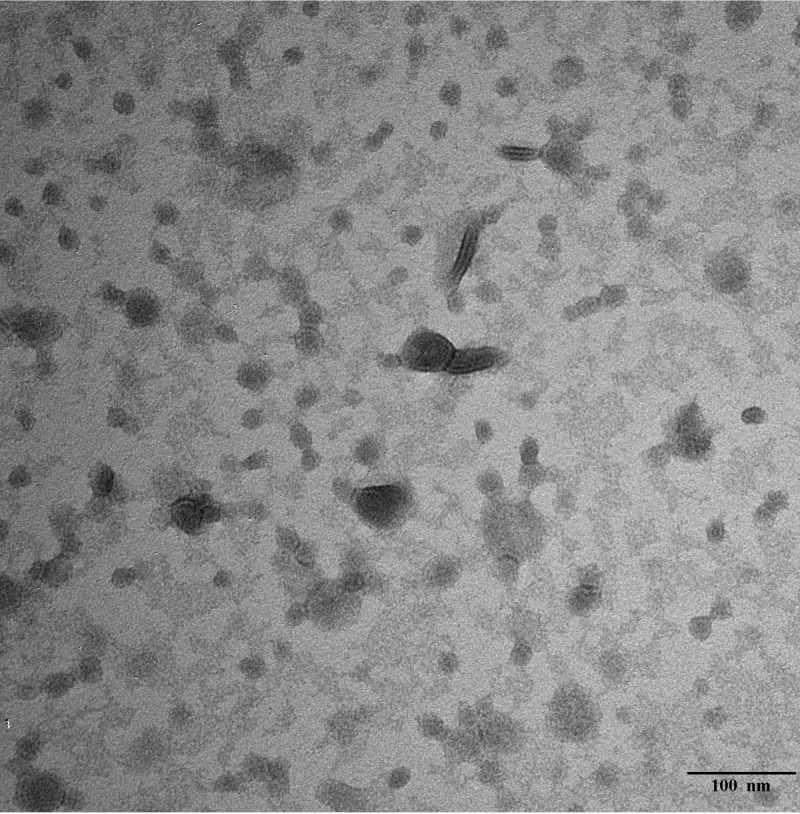
We prepared OMVs from prototype NTHi strains 5, 12, and 15 (12, 18, 21, 30) using methods modified slightly from those described for generation of N. meningitidis OMVs (31). Shown in Fig. 1 is a representative electron micrograph depicting the membrane vesicles generated from prototype NTHi strain 12 when purified as described. Most of the vesicles were 20 to 30 nm in diameter with somewhat larger vesicles occasionally observed. These vesicles are very similar in appearance and physical dimension to naturally released OMVs purified by other groups from other NTHi strains (25, 32, 33).
FIG 1.
Transmission electron micrograph of purified OMV preparation derived from NTHi strain 12 stained with uranyl acetate. Bar, 100 nm.
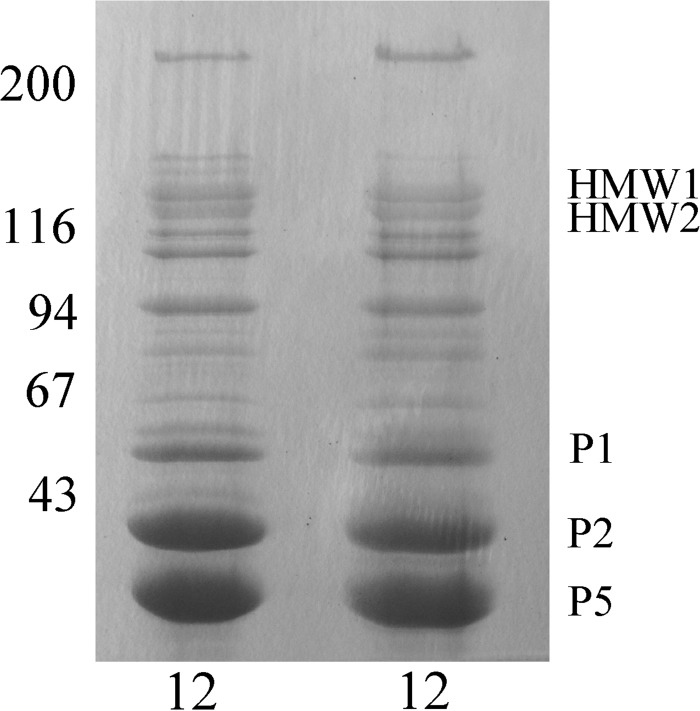
NTHi OMVs have been reported to contain a number of major and minor outer membrane proteins (25, 32, 33). The major outer membrane proteins of Haemophilus influenzae, including proteins P1, P2, P5, and P6, were originally defined based upon their presence in sarcosyl-insoluble membrane preparations and by their migration in SDS-polyacrylamide gels (34). Shown in Fig. 2 is a Coomassie-blue stained 11% SDS-polyacrylamide gel with a sarcosyl-insoluble membrane preparation (lane 1) shown next to an outer membrane vesicle preparation, both prepared from prototype NTHi strain 12. The presence and migration positions of several of the major outer membrane proteins in the two preparations are indicated with arrows. As can be appreciated, the OMV preparation contains significant amounts of proteins P1, P2, and P5 as well as smaller amounts of P6. The HMW1/HMW2 proteins are sarcosyl-soluble and are not present in the sarcosyl-insoluble preparation shown in lane 1, but are present at easily detectable amounts in the OMV preparation. Their specific presence at the position indicated in Fig. 2 was confirmed by their reactivity with several HMW1/HMW2-specific monoclonal antibodies (data not shown). Shown in Fig. 3 is a Coomassie-blue stained 7.5% SDS-polyacrylamide gel demonstrating OMV preparations from NTHi strain 12 purified on different days. One can appreciate the nearly identical protein banding patterns of the two preparations demonstrating the excellent reproducibility of the OMV purification method used.
FIG 2.
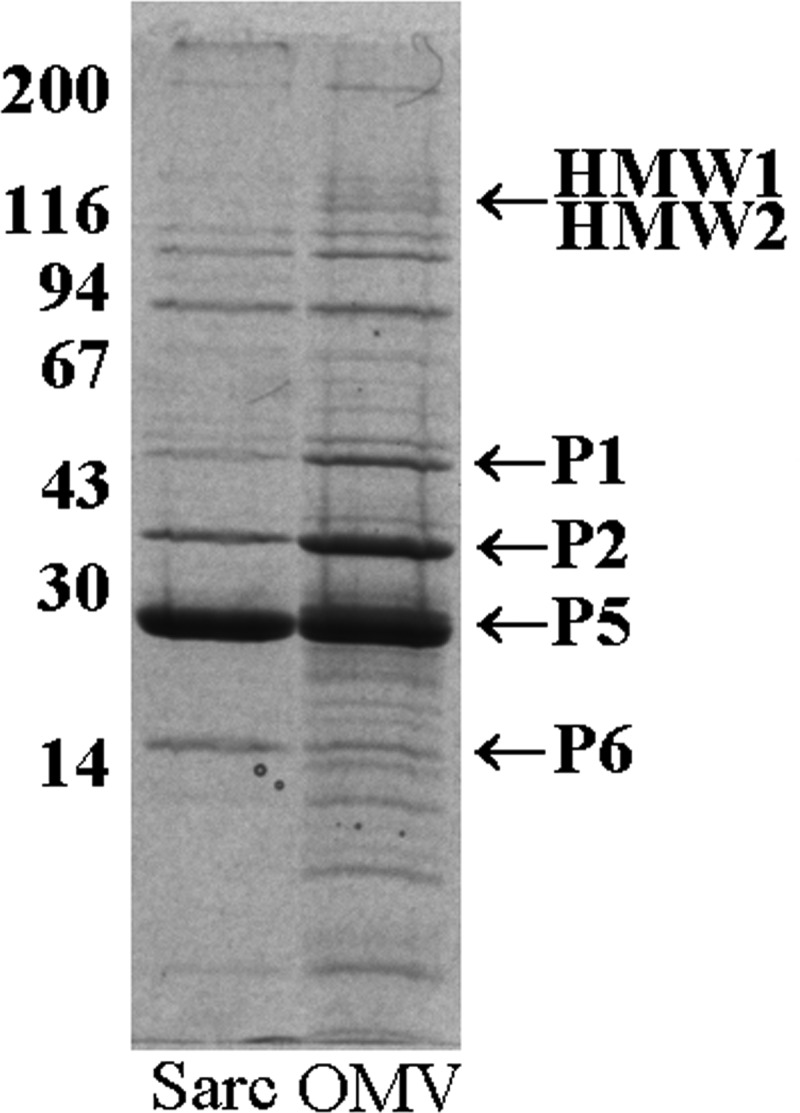
Sarcosyl-insoluble proteins from NTHi strain 12 (lane 1) and an outer membrane vesicle preparation from NTHi strain 12 (lane 2) separated on an 11% SDS-polyacrylamide gel. Values corresponding to molecular weight standards are given on the left and major proteins enriched in the OMV preparations are indicated on the right.
FIG 3.
Two outer membrane vesicle preparations from NTHi strain 12 purified on different days examined together on a 7.5% SDS-polyacrylamide gel. Values corresponding to molecular weight standards are given on the left and major proteins enriched in the OMV preparations are indicated on the right.
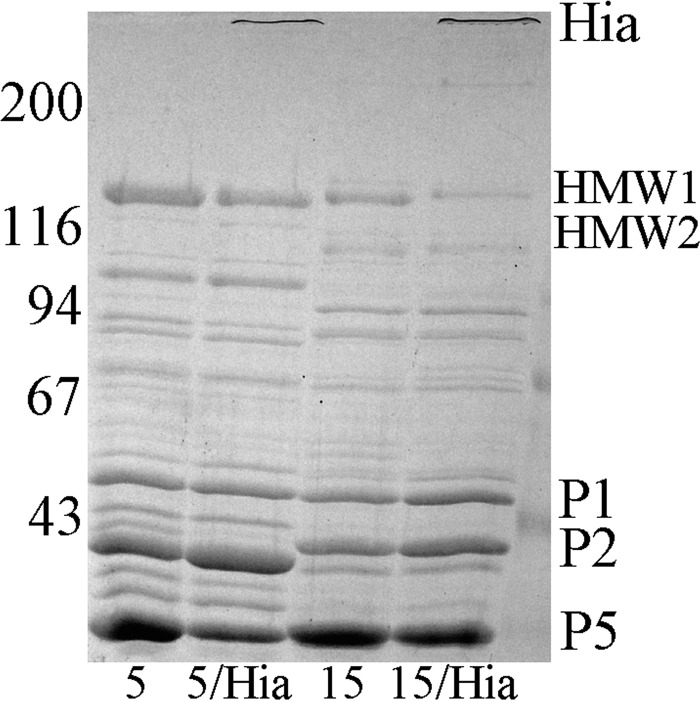
We previously engineered NTHi strains that naturally express HMW1/HMW2 proteins so that they simultaneously expressed high levels of the NTHi Hia protein (18). These strains contain the hia gene on a high copy number plasmid and express the Hia protein at levels at least three to four times the baseline level (18). Shown in Fig. 4 is a Coomassie-blue stained 7.5% SDS-polyacrylamide gel demonstrating OMVs prepared from prototype HMW1/HMW2-expressing strains 5 and 15 and from their respective hia transformants. The OMVs purified from the transformed strains demonstrate high levels of expression of the recombinant Hia protein, its identity confirmed by reaction with an Hia-specific monoclonal antibody (data not shown) (18). The remainder of data determined for the OMV proteins in the respective parent and hia-transformed strains are nearly identical, demonstrating again the excellent reproducibility of the purification method.
FIG 4.
Outer membrane vesicle preparations from NTHi strain 5, strain 5 transformed with the pHia plasmid, strain 15, and strain 15 transformed with the pHia plasmid, each examined on a 7.5% SDS-polyacrylamide gel. Values corresponding to molecular weight standards are given on the left and major proteins enriched in the OMV preparations are indicated on the right.
Opsonophagocytic activity of OMV- and OMV/Hia-specific antisera.
Guinea pig antisera were raised against OMVs prepared from HMW1/HMW2-expressing strain 12 and from the two Hia-coexpressing strains 5/pHia and 15/pHia. These antisera were assessed for their ability to mediate opsonophagocytic killing of a large panel of NTHi strains maintained in our collection (21). Table 1 summarizes the results of screening of 65 HMW1/HMW2-expressing NTHi strains with the three different antisera. As shown, the three antisera mediated killing of 28 to 46% of the heterologous HMW1/HMW2-expressing NTHi, and overall the three antisera mediated killing of 60% of the strains. Of the 39 HMW1/HMW2-expressing strains susceptible to killing, 13 strains were killed by only one of the three antisera, 21 strains were killed by two of the antisera, and 5 strains were killed by all three antisera. When examined in Western immunoblot assays against purified OMVs, the antisera demonstrated antibody responses against an array of different proteins, including the respective HMW1/HMW2-proteins, suggesting that the latter proteins were also very immunogenic even when presented as part of OMVs (data not shown).
TABLE 1.
Opsonophagocytic killing of HMW1/HMW2-expressing NTHi mediated by anti-OMV and OMV/Hia antisera
| Antiserum | No. of strains tested | No. (%) of strains killed |
|---|---|---|
| 5 OMV/Hia | 65 | 18 (28) |
| 12 OMV | 65 | 24 (37) |
| 15 OMV/Hia | 65 | 30 (46) |
| Any of the three antisera | 65 | 39 (60) |
Next, we examined the ability of OMVs purified from the two Hia-coexpressing strains to mediate killing of a collection of 25 Hia-expressing NTHi strains (Table 2). These two OMV/Hia-specific antisera mediated killing of 68% and 56% of the Hia-expressing NTHi, respectively, and overall mediated killing of 80% of these strains. The breadth of this killing activity is comparable to that seen previously when we examined the ability of rHia-specific immune sera to mediate killing of this same set of strains (21). Of the 20 Hia-expressing strains susceptible to killing, 10 strains were killed by both antisera while the remaining 10 were killed by only one of the two antisera. For both the HMW1/HMW2-expressing and the Hia-expressing strains, there was no obvious relationship between susceptibility to opsonophagocytic killing of individual strains by individual antisera and their anatomic site of isolation.
TABLE 2.
Opsonophagocytic killing of Hia-expressing NTHi mediated by anti-OMV/Hia antisera
| Antiserum | No. of strains tested | No. (%) of strains killed |
|---|---|---|
| 5 OMV/Hia | 25 | 17 (68) |
| 15 OMV/Hia | 25 | 14 (56) |
| Either of the two antisera | 25 | 20 (80) |
Chinchilla protection experiments with HMW1/HMW2 and OMVs.
We next examined the ability of the OMV preparations to induce protective immunity in vivo using the chinchilla otitis media model. We previously demonstrated that immunization of chinchillas with purified HMW1/HMW2 proteins provided partial protection against acute otitis media when animals were challenged with homologous NTHi strain 12 (14). In the work described here, NTHi strain 12 HMW1/HMW2 proteins and OMVs purified from strain 12 were used as immunogens in the two experiments summarized in Table 3. Animals received three monthly subcutaneous injections with 100 μg of purified strain 12 HMW1/HMW2 proteins, 250 μg of strain 12 OMVs, or both preparations in combination. Control animals received Freund's adjuvant alone. All animals in both experiments were inoculated on day 0 with 103 CFU of NTHi strain 12 in the left middle ear space. Middle ear otoscopy was performed every 2 to 3 days for the next 10 days and left middle ear aspiration was performed every 4 days, whether or not tympanic membrane inflammation or an effusion was observed by otoscopy. In our hands, middle ear challenge with this dose of NTHi strain 12 uniformly leads to the development of acute otitis media in nonimmune animals. By 2 to 3 days after inoculation, all nonimmune animals will have developed marked middle ear inflammation with culture-positive middle ear effusions that persist for up to 3 weeks (14).
TABLE 3.
Protection against NTHi infection mediated by immunization of chinchillas with strain 12 HMW1/HMW2 and OMV preparations
| Expt no. | Immunogen | No. of animals |
Titerc | |
|---|---|---|---|---|
| Challenged | Infected | |||
| 1 | Freund's | 3 | 3a | <1:10 |
| Strain 12 HMW | 3 | 2 | <1:10 | |
| Strain 12 OMV | 3 | 0a | ≥1:320 | |
| Strain 12 OMV + strain 12 HMW | 3 | 0a | ≥1:320 | |
| 2 | Freund's | 4 | 4b | <1:10 |
| Strain 12 OMV | 4 | 0b | ≥1:320 | |
| Strain 12 OMV + strain 12 HMW | 4 | 0b | ≥1:320 | |
P = 0.014, Fisher exact test.
P = 0.002, Fisher exact test.
Opsonophagocytic titer versus HMW1/HMW2 downregulated strain 12.
As summarized in Table 3, following challenge with NTHi strain 12 all control animals that received Freund's adjuvant alone in experiment 1 developed acute otitis media, as did two of three animals that were immunized with HMW1/HMW2 proteins alone. Middle ear fluid recovered from the middle ear spaces of the four control animals had bacterial concentrations ranging from 106 to 108 CFU per ml on both the day 4 and day 8 aspirations. Middle ear fluid recovered from the two infected HMW1/HMW2 immunized animals had bacterial concentrations ranging from 104 to 106 CFU per ml. In contrast, all six animals immunized with strain 12 OMVs, with or without HMW1/HMW2 proteins, were protected against the development of culture-positive otitis media. Five of the six OMV-immunized animals never developed tympanic membrane inflammation or had any fluid recoverable from the middle ear space with aspiration. A single animal in the OMV-immunized group developed transient tympanic membrane inflammation and had a small amount of middle ear fluid recoverable on aspiration. That fluid was sterile when cultured for the presence of NTHi.
In experiment 2, all control animals immunized with Freund's adjuvant alone developed otoscopic evidence of acute otitis media that was associated with the development of mucopurulent middle ear effusions by 3 days after challenge. Bacterial concentration in the recovered middle ear fluids ranged from 108 to 109 CFU per ml on both day 4 and day 8. In contrast, all eight animals immunized with the strain 12 OMVs, with or without HMW1/HMW2 proteins, were completely protected against the development of acute otitis media. None of the OMV-immunized animals in this experiment had any evidence of tympanic membrane inflammation throughout the course of observation and none developed middle ear fluid recoverable by aspiration.
Characterization of NTHi strains recovered from middle ear aspirates and assessment of the functional activity of chinchilla immune sera.
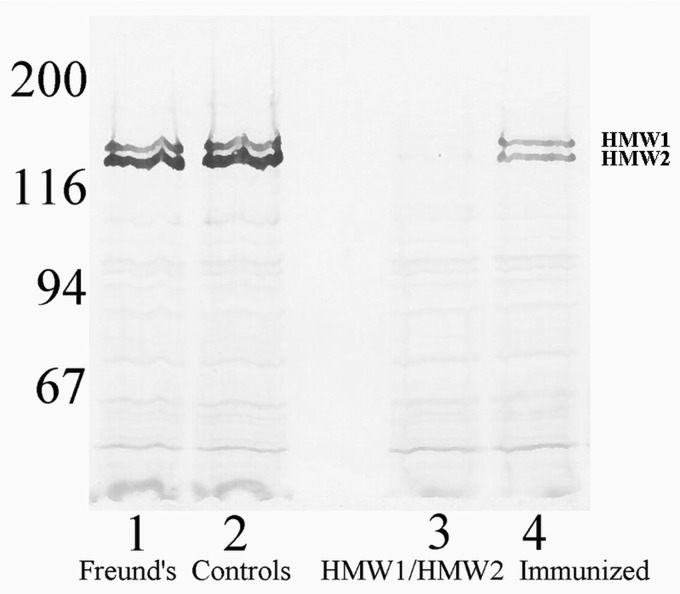
We next characterized the NTHi strains recovered from the culture-positive middle ear fluid specimens of the control animals and from the HMW1/HMW2-immunized animals for HMW1/HMW2 expression. NTHi strains recovered from the middle ear fluid specimens of control animals demonstrated normal levels of HMW1/HMW2 production (Fig. 5, lanes 1 and 2). In contrast, NTHi strains recovered from middle ear fluid specimens of HMW1/HMW2-immunized animals were uniformly downregulated in HMW1/HMW2 protein expression (Fig. 5, lanes 3 and 4). This phenomenon of selection for strains that are downregulated in HMW1/HMW2 protein expression in the face of immune pressure was described earlier in this model (14) and has been repeatedly observed in subsequent experiments in our laboratory.
FIG 5.
Western immunoblot assay with cell suspensions of NTHi recovered from two representative animals from the Freund's control group (lanes 1 and 2) and from the two HMW1/HMW2 immunized animals that developed infection (lanes 3 and 4), all probed with an HMW1/HMW2-specific monoclonal Ab pool. Values corresponding to molecular weight standards are given on the left.
We next examined the ability of sera from animals in the various experimental groups to mediate opsonophagocytic killing of the NTHi strain 12 challenge strain, as well as NTHi strains recovered from Freund's control animals and from HMW1/HMW2-immunized animals. None of the pre-immune sera mediated killing of the challenge strain, the Freund's control middle ear strains, or the HMW1/HMW2-downregulated strains recovered from the HMW1/HMW2-immunized animals. Similarly, none of the immune sera collected immediately prechallenge from the Freund's immunized control groups mediated killing of the challenge strain, the Freund's control strains, or the HMW1/HMW2-downregulated strains. In contrast, prechallenge immune sera from all chinchillas immunized with HMW1/HMW2 alone, OMVs alone, or OMVs in combination with HMW1/HMW2 mediated killing of the challenge strain and the Freund's control middle ear strains at titers ≥1:320. Prechallenge sera from the three animals immunized with HMW1/HMW2 alone, two of whom developed infection, had opsonophagocytic titers of <1:10 against the HMW1/HMW2-downregulated strains (Table 3). Prechallenge sera from the OMV-immunized animals retained their ability to mediate killing of the HMW1/HMW2-downregulated strains at titers ≥1:320.
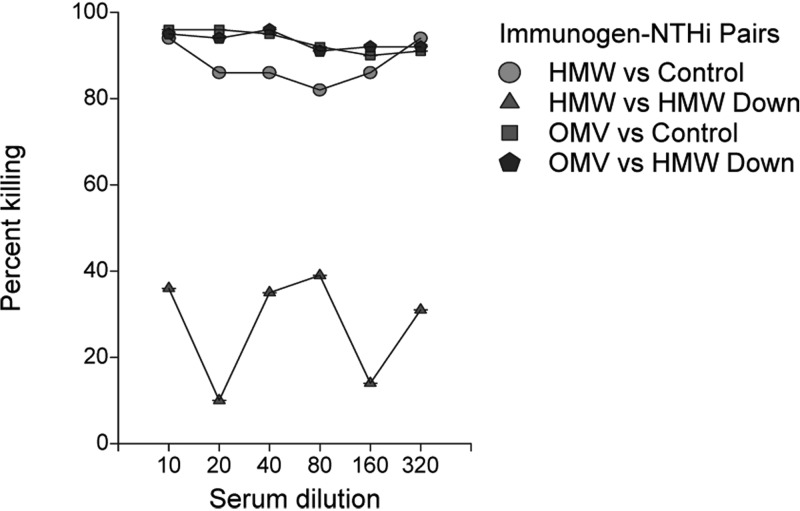
As an example, shown in Fig. 6 are results of an opsonophagocytic assay in which prechallenge sera from an HMW1/HMW2-immunized animal that developed infection and from an OMV-immunized animal that did not develop infection were compared. Each sample was assessed for its ability to mediate killing of a Freund's control middle ear strain and an HMW1/HMW2-downregulated middle ear strain recovered from an HMW1/HMW2-immunized animal. As can be seen, both sera mediated high-titer killing of the Freund's control strain, but only the serum sample from the OMV-immunized animal mediated significant killing of the HMW1/HMW2-downregulated strain. These results mirror those seen with the prechallenge immune sera from the other HMW1/HMW2- and OMV-immunized animals.
FIG 6.
Opsonophagocytic assay with sera from an HMW1/HMW2-immunized (indicated as HMW) and an OMV-immunized (indicated as OMV) chinchilla, each assayed against a NTHi strain recovered from the middle ear fluid of a Freund's immunized animal (indicated as Control) or an HMW1/HMW2-downregulated strain recovered from the middle ear fluid of an HMW1/HMW2-immunized and infected animal (indicated as HMW Down).
DISCUSSION
Two research groups have previously characterized representative naturally released NTHi OMVs in detail (25, 32, 33). These groups defined the protein content of their OMVs using tandem mass spectrometry and found them to be enriched in several of the major outer membrane proteins, including P2 (35), P5 (36), and P6 (37), as well as in the high molecular weight HMW1/HMW2 proteins (14). These other groups also defined the fatty acid and phospholipid composition of their OMVs using gas and thin-layer chromatography. Palmitic acid was found to be the most abundant fatty acid and phosphatidylethanolamine was the major phospholipid. One of these groups also examined the ability of NTHi OMVs to protect against nasal colonization of mice after intranasal immunization followed by intranasal challenge. These investigators reported protection against colonization with the homologous NTHi strain and one heterologous strain (33). No other formal studies of NTHi OMVs as vaccine candidates have been reported to date.
Several different methods have been described in the literature for purification of outer membrane vesicles from Gram-negative bacteria, including those of NTHi (24, 25, 32, 33). When we initiated our project, we attempted to recover so-called naturally released outer membrane vesicles from culture supernatants of our NTHi strains grown in bulk liquid culture, but recovery of vesicles was negligible. Thus, as an alternative, we used a purification method that closely mirrored that described for purification of OMVs from Neisseria meningitidis (31). We have not performed formal biochemical characterizations of our OMV preparations to date, but the proteins demonstrable when the preparations were visualized on standard SDS-polyacrylamide gels included the major outer membrane proteins P1, P2, P5, and P6, as well as the HMW1/HMW2 and Hia proteins (Fig. 2 and 3). As noted above, each of these proteins was also detected in the NTHi OMV preparations previously characterized by others (25, 32, 33). Each of these proteins has been demonstrated capable of inducing functionally active antibodies in vitro and in vivo (14, 35, 37–39). The presence of multiple potentially protective antigens in the NTHi OMV preparations makes the OMVs themselves attractive as possible components of protective NTHi vaccines.
The strain heterogeneity of NTHi is a challenge that must be addressed by any successful NTHi vaccine candidate (40, 41). Efforts to develop effective broad-based NTHi vaccines based upon single purified antigens have met with limited success to date, in part because of this strain heterogeneity (8). We previously demonstrated that antisera raised against representative HMW1/HMW2 proteins and recombinant Hia proteins could provide relatively broad-based opsonophagocytic killing of homologous and heterologous NTHi (21), but even a vaccine based upon these proteins would likely require a number of different representative antigens. OMVs, which contain many different surface-accessible antigens, could conceivably provide more broad-based coverage than the single antigen preparations studied to date, but data in support of this idea are limited. We assessed the ability of the OMVs prepared from representative prototype NTHi to mediate killing of a large panel of NTHi strains. It was notable that OMVs from just three strains, two of which were also engineered to express Hia, could mediate killing of 60% of unrelated HMW1/HMW2-expressing and 80% of unrelated Hia-expressing NTHi strains. As noted earlier, almost all NTHi strains express either HMW1/HMW2 or Hia proteins (15). Thus, these data suggest that a relatively small number of OMVs might be capable of eliciting antibodies capable of mediating killing of the majority of NTHi. Whether a vaccine with less than complete coverage for all NTHi strains might ultimately lead to the emergence of “nonvaccine” strains as predominant causes of disease in the population at large is unknown. However, that theoretic concern should not preclude preclinical studies of OMV-based vaccines, given the current state of the NTHi vaccine development field.
One major reason for our study of the NTHi OMVs was to test the hypothesis that they might serve as supplemental vaccine components for HMW1/HMW2- and/or Hia-based vaccines. We previously reported that HMW1/HMW2 immunization can provide protection against NTHi otitis media in the chinchilla model (14). However, in this model, the development of otitis media in HMW1/HMW2-immunized animals is invariably associated with the appearance of HMW1/HMW2-downregulated strains (14, 42). We reasoned that by combining the HMW1/HMW2 proteins with other NTHi antigens to generate a more robust and broad-based immune response, one might prevent the emergence of such downregulated strains. The results of the chinchilla protection experiments presented above are consistent with this idea. When chinchillas were immunized with HMW1/HMW2 proteins alone, they developed serum antibodies that mediated high-titer killing of the NTHi strain 12 challenge strain, but these same antibodies were unable to mediate killing of the HMW1/HMW2-downregulated strains recovered from the middle ear fluids of infected animals. In contrast, animals immunized with OMV preparations alone or OMV preparations in combination with HMW1/HMW2 proteins were protected against infection and retained the ability to mediate high-titer killing of both the challenge strain and the HMW1/HMW2-downregulated strains (Table 3).
In summary, in this study we have demonstrated that antisera raised against prototype NTHi OMVs mediate relatively broad-based opsonophagocytic killing of homologous and heterologous NTHi strains and that immunization with prototype OMVs provides solid protection against homologous bacterial challenge in the chinchilla model of NTHi otitis media. These data suggest that NTHi OMVs hold potential as components of protective NTHi vaccines, possibly in combination with HMW1/HMW2 and Hia proteins.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The 90 NTHi bacterial strains studied in this work have been described in our previous work (21). These isolates were collected during episodes of clinical disease from children and a few adults between 1975 and 2011. The source patients were drawn from a wide geographic region, with isolates included from Alabama, Michigan, Missouri, New York, Ohio, and Pennsylvania. Of these isolates, 44 were cultured from middle ear effusions collected by tympanocentesis or directly from middle ear drainage, 21 were isolated from blood cultures, 13 were cultured from respiratory tract secretions of patients with acute infection, seven were cultured from eye drainage of patients with acute conjunctivitis, and four were cultured from cerebrospinal fluid. Many of these strains have been described in detail in earlier work from our laboratory and they represent a very heterogeneous population of organisms (15, 18, 20, 21, 30, 43).
NTHi strains were identified as such by standard methods and each was classified as nontypeable by clinical appearance of the bacterial colonies on chocolate agar plates and by failure to agglutinate with a panel of typing antisera for Haemophilus influenzae types a to f (Burroughs Wellcome Co., Research Triangle Park, NC) (44). Each of the 25 strains recovered from blood or cerebrospinal fluid was also examined for the presence of capsular genes by PCR using methods described by Satola and coworkers (45). None of these invasive isolates demonstrated the presence of capsular genes. All NTHi strains were stored at −70°C in skim milk within two or three subpassages of initial clinical isolation. NTHi strains were grown on chocolate agar or in brain heart infusion broth supplemented with hemin and NAD, as previously described (18, 30).
Purification of native HMW1/HMW2 proteins.
HMW1/HMW2 proteins were purified from NTHi strains using previously described methods (30). In brief, a frozen bacterial stock culture was streaked onto chocolate agar and allowed to grow overnight at 37°C. The following day, 5 to 10 colonies were used to inoculate a starter culture that was grown to mid-log phase and then used to inoculate six large flasks containing supplemented brain heart infusion (BHI) broth and were then grown to an optical density of 1.5. Bacteria were pelleted by centrifugation and frozen overnight. The following day, pellets were incubated in a buffered extraction solution for 1 h on ice followed by high-speed centrifugation to remove the cellular debris. The supernatant containing soluble HWW1/HMW2 proteins was ultracentrifuged to remove membrane fragments and then passed over an ion-exchange column followed by a gel filtration column. Fractions containing the purified proteins were pooled and maintained at −70°C.
Purification of NTHi outer membrane vesicles.
NTHi OMV preparations were purified using methods adapted from those used to purify OMVs from Neisseria meningitidis (31). A frozen NTHi bacterial stock culture was streaked onto chocolate agar and allowed to grow overnight at 37°C. The following day, a starter culture was grown to mid-log phase and used to inoculate two large flasks containing supplemented BHI broth. The bacteria were shaken and grown to an optical density of 1.5 before pelleting by centrifugation and then frozen overnight. The next day, the pellets were thawed, resuspended, and maintained in 40 ml of extraction buffer at 56°C for 30 min. The suspension was transferred to an ice bath and sonicated with a fine tip probe for 15 s × 4. The resulting suspension was centrifuged twice at 14,000 × g to remove cellular debris. The supernatant was then ultracentrifuged at 100,000 × g for 60 min to pellet the OMVs and the purified OMVs were maintained at −70°C until needed.
Transmission electron microscopy.
To visualize the NTHi OMVs by transmission electron microscopy, 2 μl of the OMV preparation (0.5 mg/ml [protein equivalent]) was allowed to adsorb onto a Formvar/carbon-coated copper grid for 15 s. After removal of excess liquid by blotting onto filter paper, the samples were negatively stained with 2% uranyl acetate for 15 s and excess uranyl acetate was blotted off. The grids were briefly dipped in distilled water prior to being air dried. Micrographs were recorded using a JEOL 1200EX transmission electron microscope at 80kV.
Generation of HMW1/HMW2 and OMV antisera in guinea pigs.
Guinea pig antisera were prepared using our standard methods (21). In brief, guinea pigs received four or five monthly subcutaneous injections with 100 to 250 μg of total protein administered every 4 weeks. The first dose was mixed with Freund's complete adjuvant and subsequent doses with incomplete Freund's. Serum antibody responses were monitored by enzyme-linked immunosorbent assay (ELISA) and Western immunoblotting using purified HMW1/HMW2, Hia, or OMV proteins as target antigens. Once sufficient antibody titers were achieved, the sera were assessed for their ability to mediate opsonophagocytic killing in the assay described below.
In vitro assessment of opsonophagocytic activity.
Opsonophagocytic activity of guinea pig antisera and chinchilla immune sera was measured as described previously (12, 21, 30). In brief, 10 μl of test serum was added to a capped tube followed by 20 μl of a bacterial suspension containing ∼5 × 103 CFU of the NTHi test strain. Tubes were incubated with shaking at 37°C for 30 min to allow antibodies to opsonize the bacteria. Next 15 μl of complement was added to each tube, followed by 60 μl of a suspension of differentiated HL-60 cells. The HL-60 cells were used at a target cell ratio of 100:1 HL-60 cells to bacteria. Tubes were incubated with shaking at 37°C for 90 min to promote phagocytosis. After incubation, 10 μl from each tube was plated onto a chocolate agar plate and spread for subsequent counting of viable colonies the following day after overnight incubation at 37°C. The immune sera were screened at a 1:10 dilution and any bacterial strain susceptible to killing at that dilution was considered to be susceptible to killing by that antiserum.
Immunogenicity and protective ability of vaccine formulations in chinchillas.
Animals were immunized with three monthly subcutaneous injections of the protein or protein/OMV preparations admixed in Freund's adjuvant (14). Serum antibody responses were quantified by ELISA and by measurement of opsonophagocytic activity (30). Animals were challenged by direct middle ear inoculation via the epitympanic bullae with 103 CFU of freshly grown log-phase bacteria (14). This dose reproducibly causes acute otitis in nonimmune animals. The course of middle ear disease was monitored by otoscopy, aspiration of middle ear fluid, and quantitative culture of the sampled fluid (14). Otoscopy was performed every 2 to 3 days after bacterial challenge and middle ear aspiration was performed every 4 days, whether or not otoscopic examination demonstrated the presence of tympanic membrane inflammation or middle ear effusion. Middle ear fluid specimens were cultured quantitatively overnight on chocolate agar plates and bacterial colonies were counted the following morning. Any recovered NTHi isolates were saved as frozen skim milk stocks for later analyses (see below). Protection was assessed by comparing the number and percentage of animals with culture-positive otitis media in the various experimental groups and by comparing the bacterial densities in middle ear fluid specimens collected from animals in the experimental subgroups. All animal experiments complied with federal and institutional guidelines and were approved by the local Institutional Animal Care and Use Committee under protocol number 2177.
Western immunoblot analyses of HMW1/HMW2 protein expression.
NTHi isolates from individual middle ear fluid specimens were recovered from frozen bacterial stocks by streaking onto chocolate agar and allowing the specimens to grow at 37°C overnight. The following day, cell suspensions of each isolate were prepared in phosphate-buffered saline (PBS) and the optical density of the cell suspensions was adjusted to an absorbance reading of 1.0. Exactly 15 μl of each cell suspension was then solubilized in electrophoresis sample buffer, loaded into an individual lane of a 7.5% acrylamide gels, subjected to SDS-PAGE, and then transferred to nitrocellulose with a Genie electrophoretic blotter (Idea Scientific Company, Corvallis, OR) for 45 min at 24 V. After transfer, the nitrocellulose sheet was blocked and then probed sequentially: first with a pool of murine monoclonal antibodies that recognize epitopes on the HMW1/HMW2 proteins, second by alkaline phosphatase-conjugated goat anti-mouse IgG antibody (Bio-Rad). Bound antibodies were detected by incubation with nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate toluidinium (BCIP) solution.
Statistical analyses.
The Fisher exact test was employed to compare the proportions of animals infected in the control and immunized groups. To compare the middle ear fluid bacterial densities in infected animals in the control and immunized groups, the data were log transformed and then compared with the Mann-Whitney U test.
ACKNOWLEDGMENTS
This work was supported by Public Health Service Grant number AI 81887 from the National Institute of Allergy and Infectious Diseases and grant-in-aid number 17GRNT33630171 from the American Heart Association.
The transmission electron microscopy studies were performed by Barbara Nagel of the Research Microscopy and Histology Core Laboratory, Department of Pathology, St. Louis University School of Medicine.
REFERENCES
- 1.Faden H, Duffy L, Williams A, Krystofik DA, Wolf J. 1995. Epidemiology of nasopharyngeal colonization with nontypeable Haemophilus influenzae in the first 2 years of life. J Infect Dis 172:132–135. doi: 10.1093/infdis/172.1.132. [DOI] [PubMed] [Google Scholar]
- 2.Sethi S, Evans N, Grant BJ, Murphy TF. 2002. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med 347:465–471. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 3.Chonmaitree T, Trujillo R, Jennings K, Alvarez-Fernandez P, Patel JA, Loeffelholz MJ, Nokso-Koivisto J, Matalon R, Pyles RB, Miller AL, McCormick DP. 2016. Acute otitis media and other complications of viral respiratory infection. Pediatrics 137:e20153555. doi: 10.1542/peds.2015-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casey JR, Pichichero ME. 2004. Changes in frequency and pathogens causing acute otitis media in 1995–2003. Pediatr Infect Dis J 23:824–828. doi: 10.1097/01.inf.0000136871.51792.19. [DOI] [PubMed] [Google Scholar]
- 5.Pichichero ME, Casey JR, Hoberman A, Schwartz R. 2008. Pathogens causing recurrent and difficult-to-treat acute otitis media, 2003–2006. Clin Pediatr (Phila) 47:901–906. doi: 10.1177/0009922808319966. [DOI] [PubMed] [Google Scholar]
- 6.Sethi S, Murphy TF. 2008. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 359:2355–2365. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 7.Pelton SI, Pettigrew MM, Barenkamp SJ, Godfroid F, Grijalva CG, Leach A, Patel J, Murphy TF, Selak S, Bakaletz LO. 2013. Panel 6: vaccines. Otolaryngol Head Neck Surg 148:E90–E101. doi: 10.1177/0194599812466535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy TF. 2015. Vaccines for nontypeable Haemophilus influenzae: the future is now. Clin Vaccine Immunol 22:459–466. doi: 10.1128/CVI.00089-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barenkamp SJ, Bodor FF. 1990. Development of serum bactericidal activity following nontypable Haemophilus influenzae acute otitis media. Pediatr Infect Dis J 9:333–339. doi: 10.1097/00006454-199005000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Barenkamp SJ, Leininger E. 1992. Cloning, expression, and DNA sequence analysis of genes encoding nontypeable Haemophilus influenzae high-molecular-weight surface-exposed proteins related to filamentous hemagglutinin of Bordetella pertussis. Infect Immun 60:1302–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.St Geme JW III, Falkow S, Barenkamp SJ. 1993. High-molecular-weight proteins of nontypable Haemophilus influenzae mediate attachment to human epithelial cells. Proc Natl Acad Sci U S A 90:2875–2879. doi: 10.1073/pnas.90.7.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winter LE, Barenkamp SJ. 2003. Human antibodies specific for the high-molecular-weight adhesion proteins of nontypeable Haemophilus influenzae mediate opsonophagocytic activity. Infect Immun 71:6884–6891. doi: 10.1128/IAI.71.12.6884-6891.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winter LE, Barenkamp SJ. 2016. Naturally acquired HMW1- and HMW2-specific serum antibodies in adults and children mediate opsonophagocytic killing of nontypeable Haemophilus influenzae. Clin Vaccine Immunol 23:37–46. doi: 10.1128/CVI.00502-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barenkamp SJ. 1996. Immunization with high-molecular-weight adhesion proteins of nontypeable Haemophilus influenzae modifies experimental otitis media in chinchillas. Infect Immun 64:1246–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.St Geme JW III, Kumar VV, Cutter D, Barenkamp SJ. 1998. Prevalence and distribution of the hmw and hia genes and the HMW and Hia adhesins among genetically diverse strains of nontypeable Haemophilus influenzae. Infect Immun 66:364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barenkamp SJ, St Geme JW III. 1996. Identification of a second family of high-molecular-weight adhesion proteins expressed by non-typable Haemophilus influenzae. Mol Microbiol 19:1215–1223. doi: 10.1111/j.1365-2958.1996.tb02467.x. [DOI] [PubMed] [Google Scholar]
- 17.Cotter SE, Surana NK, Grass S, St Geme JW III. 2006. Trimeric autotransporters require trimerization of the passenger domain for stability and adhesive activity. J Bacteriol 188:5400–5407. doi: 10.1128/JB.00164-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winter LE, Barenkamp SJ. 2009. Antibodies specific for the Hia adhesion proteins of nontypeable Haemophilus influenzae mediate opsonophagocytic activity. Clin Vaccine Immunol 16:1040–1046. doi: 10.1128/CVI.00090-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Chiara M, Hood D, Muzzi A, Pickard DJ, Perkins T, Pizza M, Dougan G, Rappuoli R, Moxon ER, Soriani M, Donati C. 2014. Genome sequencing of disease and carriage isolates of nontypeable Haemophilus influenzae identifies discrete population structure. Proc Natl Acad Sci U S A 111:5439–5444. doi: 10.1073/pnas.1403353111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buscher AZ, Burmeister K, Barenkamp SJ, St Geme JW. 2004. Evolutionary and functional relationships among the nontypeable Haemophilus influenzae HMW family of adhesins. J Bacteriol 186:4209–4217. doi: 10.1128/JB.186.13.4209-4217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winter LE, Barenkamp SJ. 2014. Antibodies to the HMW1/HMW2 and Hia adhesins of nontypeable Haemophilus influenzae mediate broad-based opsonophagocytic killing of homologous and heterologous strains. Clin Vaccine Immunol 21:613–621. doi: 10.1128/CVI.00772-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atack JM, Srikhanta YN, Fox KL, Jurcisek JA, Brockman KL, Clark TA, Boitano M, Power PM, Jen FE, McEwan AG, Grimmond SM, Smith AL, Barenkamp SJ, Korlach J, Bakaletz LO, Jennings MP. 2015. A biphasic epigenetic switch controls immunoevasion, virulence and niche adaptation in non-typeable Haemophilus influenzae. Nat Commun 6:7828. doi: 10.1038/ncomms8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kulp A, Kuehn MJ. 2010. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol 64:163–184. doi: 10.1146/annurev.micro.091208.073413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwechheimer C, Kuehn MJ. 2015. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat Rev Microbiol 13:605–619. doi: 10.1038/nrmicro3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharpe SW, Kuehn MJ, Mason KM. 2011. Elicitation of epithelial cell-derived immune effectors by outer membrane vesicles of nontypeable Haemophilus influenzae. Infect Immun 79:4361–4369. doi: 10.1128/IAI.05332-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaparakis-Liaskos M, Ferrero RL. 2015. Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol 15:375–387. doi: 10.1038/nri3837. [DOI] [PubMed] [Google Scholar]
- 27.Granoff DM. 2010. Review of meningococcal group B vaccines. Clin Infect Dis 50(Suppl 2):S54–S65. doi: 10.1086/648966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JH. 2016. A challenge in vaccine development—Neisseria meningitidis serogroup B. N Engl J Med 375:275–278. doi: 10.1056/NEJMe1606015. [DOI] [PubMed] [Google Scholar]
- 29.Perrett KP, McVernon J, Richmond PC, Marshall H, Nissen M, August A, Percell S, Toneatto D, Nolan T. 2015. Immune responses to a recombinant, four-component, meningococcal serogroup B vaccine (4CMenB) in adolescents: a phase III, randomized, multicentre, lot-to-lot consistency study. Vaccine 33:5217–5224. doi: 10.1016/j.vaccine.2015.06.103. [DOI] [PubMed] [Google Scholar]
- 30.Winter LE, Barenkamp SJ. 2006. Antibodies specific for the high-molecular-weight adhesion proteins of nontypeable Haemophilus influenzae are opsonophagocytic for both homologous and heterologous strains. Clin Vaccine Immunol 13:1333–1342. doi: 10.1128/CVI.00221-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moe GR, Zuno-Mitchell P, Hammond SN, Granoff DM. 2002. Sequential immunization with vesicles prepared from heterologous Neisseria meningitidis strains elicits broadly protective serum antibodies to group B strains. Infect Immun 70:6021–6031. doi: 10.1128/IAI.70.11.6021-6031.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roier S, Blume T, Klug L, Wagner GE, Elhenawy W, Zangger K, Prassl R, Reidl J, Daum G, Feldman MF, Schild S. 2015. A basis for vaccine development: comparative characterization of Haemophilus influenzae outer membrane vesicles. Int J Med Microbiol 305:298–309. doi: 10.1016/j.ijmm.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Roier S, Leitner DR, Iwashkiw J, Schild-Prufert K, Feldman MF, Krohne G, Reidl J, Schild S. 2012. Intranasal immunization with nontypeable Haemophilus influenzae outer membrane vesicles induces cross-protective immunity in mice. PLoS One 7:e42664. doi: 10.1371/journal.pone.0042664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barenkamp SJ, Munson RS Jr, Granoff DM. 1981. Subtyping isolates of Haemophilus influenzae type b by outer-membrane protein profiles. J Infect Dis 143:668–676. doi: 10.1093/infdis/143.5.668. [DOI] [PubMed] [Google Scholar]
- 35.Murphy TF, Bartos LC. 1988. Human bactericidal antibody response to outer membrane protein P2 of nontypeable Haemophilus influenzae. Infect Immun 56:2673–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kyd JM, Cripps AW, Novotny LA, Bakaletz LO. 2003. Efficacy of the 26-kilodalton outer membrane protein and two P5 fimbrin-derived immunogens to induce clearance of nontypeable Haemophilus influenzae from the rat middle ear and lungs as well as from the chinchilla middle ear and nasopharynx. Infect Immun 71:4691–4699. doi: 10.1128/IAI.71.8.4691-4699.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeMaria TF, Murwin DM, Leake ER. 1996. Immunization with outer membrane protein P6 from nontypeable Haemophilus influenzae induces bactericidal antibody and affords protection in the chinchilla model of otitis media. Infect Immun 64:5187–5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bakaletz LO, Kennedy BJ, Novotny LA, Duquesne G, Cohen J, Lobet Y. 1999. Protection against development of otitis media induced by nontypeable Haemophilus influenzae by both active and passive immunization in a chinchilla model of virus-bacterium superinfection. Infect Immun 67:2746–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolduc GR, Bouchet V, Jiang RZ, Geisselsoder J, Truong-Bolduc QC, Rice PA, Pelton SI, Goldstein R. 2000. Variability of outer membrane protein P1 and its evaluation as a vaccine candidate against experimental otitis media due to nontypeable Haemophilus influenzae: an unambiguous, multifaceted approach. Infect Immun 68:4505–4517. doi: 10.1128/IAI.68.8.4505-4517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilsdorf JR. 1998. Antigenic diversity and gene polymorphisms in Haemophilus influenzae. Infect Immun 66:5053–5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gilsdorf JR, Marrs CF, Foxman B. 2004. Haemophilus influenzae: genetic variability and natural selection to identify virulence factors. Infect Immun 72:2457–2461. doi: 10.1128/IAI.72.5.2457-2461.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dawid S, Barenkamp SJ, St Geme JW III. 1999. Variation in expression of the Haemophilus influenzae HMW adhesins: a prokaryotic system reminiscent of eukaryotes. Proc Natl Acad Sci U S A 96:1077–1082. doi: 10.1073/pnas.96.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Musser JM, Barenkamp SJ, Granoff DM, Selander RK. 1986. Genetic relationships of serologically nontypable and serotype b strains of Haemophilus influenzae. Infect Immun 52:183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ledeboer NA, Doern GV. 2012. Haemophilus, p 588–602. In Versalovic R, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW (ed), Manual of clinical microbiology, 10th ed ASM Press, Washington, DC. [Google Scholar]
- 45.Satola SW, Collins JT, Napier R, Farley MM. 2007. Capsule gene analysis of invasive Haemophilus influenzae: accuracy of serotyping and prevalence of IS1016 among nontypeable isolates. J Clin Microbiol 45:3230–3238. doi: 10.1128/JCM.00794-07. [DOI] [PMC free article] [PubMed] [Google Scholar]