Abstract
Introduction
Effective chronic disease care is dependent on well-organised quality improvement (QI) strategies that monitor processes of care and outcomes for optimal care delivery. Although healthcare is provincially/territorially structured in Canada, there are national networks such as the Canadian Primary Care Sentinel Surveillance Network (CPCSSN) as important facilitators for national QI-based studies to improve chronic disease care. The goal of our study is to improve the understanding of how patients with chronic kidney disease (CKD) are managed in primary care and the variation across practices and provinces and territories to drive improvements in care delivery.
Methods and analysis
The CPCSSN database contains anonymised health information from the electronic medical records for patients of participating primary care practices (PCPs) across Canada (n=1200). The dataset includes information on patient sociodemographics, medications, laboratory results and comorbidities. Leveraging validated algorithms, case definitions and guidelines will help define CKD and the related processes of care, and these enable us to: (1) determine prevalent CKD burden; (2) ascertain the current practice pattern on risk identification and management of CKD and (3) study variation in care indicators (eg, achievement of blood pressure and proteinuria targets) and referral pattern for specialist kidney care. The process of care outcomes will be stratified across patients’ demographics as well as provider and regional (provincial/territorial) characteristics. The prevalence of CKD stages 3–5 will be presented as age–sex standardised prevalence estimates stratified by province and as weighted averages for population rates with 95% CIs using census data. For each PCP, age–sex standardised prevalence will be calculated and compared with expected standardised prevalence estimates. The process-based outcomes will be defined using established methods.
Ethics and dissemination
The CPCSSN is committed to high ethical standards when dealing with individual data collected, and this work is reviewed and approved by the Network Scientific Committee. The results will be published in peer-reviewed journals and presented at relevant national and international scientific meetings.
Keywords: quality in health care, nephrology, chronic renal failure
Strengths and limitations of this study.
To our knowledge, this is the first nationwide and largest retrospective observational study on the epidemiology and management of chronic kidney disease (CKD) in primary care in Canada with potential to identify opportunities for improving quality of care for CKD at a national level.
It will define the practice patterns on CKD risk identification in primary care by ascertaining whether high-risk groups are appropriately tested, monitored and managed for CKD based on existing guideline recommendations.
It will investigate the variation in CKD care delivery across patient, provider and regional characteristics, relative to established quality indicators.
This study leverages retrospective data collated at point of care, and therefore, limitations include variable data quality and incomplete information in some data domains.
The results of this study will enable the development of strategies and interventions to improve care and outcomes for patients with CKD.
Introduction
There is an absence of effective surveillance mechanisms for chronic kidney disease (CKD) in most countries despite the overwhelming opinion of key stakeholders supporting such developments.1 2 There are a few countries in the world with well-established CKD surveillance systems (Australia, Japan, UK and USA).1 3 As with all other non-communicable diseases (NCDs), planning, development and implementation of effective and efficient care programs require reliable national data systems to monitor the burden of disease, processes of care and clinical outcomes.4–12 Once established, these systems can be used for routine surveillance (including secular trends), quality improvement (QI) and resource allocation (including workforce planning). In Canada, they would also allow for within-country comparisons across provinces and territories, and they would allow for evaluation of how the country compares with similar nations in CKD care. If combined with existing data sources (eg, administrative databases), data on CKD management in primary care might also facilitate efforts to integrate CKD management with care for other major NCDs (eg, diabetes, cardiovascular diseases (CVDs), hypertension, obesity).5
Effective and sustainable chronic disease care is dependent on well-organised QI strategies to monitor processes of care and outcomes and to inform optimal care delivery.1 In the CKD domain, end-stage renal disease (ESRD) care has been the sole focus of national QI activities, often administered in conjunction with national/regional registries (eg, Canadian Organ Replacement Register, US Renal Data System, European Renal Registry).1 5 While there is no surveillance system for non-dialysis-dependent CKD in most countries, including Canada,5 13 a few have developed initiatives in this direction.14 1 5 13 15 A national CKD surveillance system using routinely collected practice data is feasible in nations with well-developed healthcare systems as CKD lends itself particularly well to surveillance because of its laboratory-based diagnosis.16 Although healthcare is provincially/territorially structured and administered in Canada, there are existing national networks and collaborations such as the Canadian Primary Care Sentinel Surveillance Network (CPCSSN)17 that can be important facilitators for national QI-based studies.
We set out to improve the understanding of how patients with CKD are managed and the variation across practitioners, regions and provinces to drive improvements in care delivery. Our study is a multidisciplinary (nephrology, primary care) and cross-jurisdictional effort leveraging data from the CPCSSN to investigate the epidemiology and management of CKD in the Canadian primary care system. The overarching aim is to use data derived from primary care electronic medical records (EMRs) to identify gaps in care and to provide opportunities for interventions to improve care and patient outcomes.
Objectives
Develop and validate a case definition for CKD in primary care and apply this to ascertain the burden of CKD in primary care, obtaining data on unidentified cases using standard criteria.18 19
Determine the current practice patterns on CKD risk identification in primary care by ascertaining whether high-risk groups (eg, individuals with diabetes, hypertension and urological disorders, as well as those with chronic use of nephrotoxic medications) are appropriately screened and managed for CKD.
Determine whether people with CKD are being managed based on established quality indicators and investigate the care variation across patients’ demographics (age, sex, socioeconomic status, rural/urban residence, comorbidity burden), provider characteristics (family physician/nurse practitioner, year of graduation, rural/urban, individual/group, fee for service) and regional characteristics (intraprovincial/territorial and interprovincial/territorial variation, rural versus urban).
Methods and analysis
Setting
The CPCSSN17 20 21 is the first pan-Canadian multidisease surveillance system. The data resource profiles and context within the Canadian health system are detailed elsewhere.22 Canada is considered a high-income country by the World Bank Classification Index with a population of 35 362 905 (2016). It is the second largest country in the world with a land area of 9 984 670 km2. It has a gross domestic product of $C1.634 trillion (2016), and about 10.1% of this is spent on healthcare. In Canada, healthcare is a provincial or territorial mandate under the oversight of the Canada Health Act and with a financial assistance from the federal government ensuring universal, publicly funded health services to the population for medically necessary care including physician visits, hospitalisations and, in some provinces, universal drug coverage. Primary care is the first portal for accessing care particularly for routine chronic disease care.
The structure of CPCSSN comprised a network of 12 practice-based primary care research networks that collect primary care health information from the EMRs of primary care providers (primary care practices (PCPs)) in eight out of the ten provinces and one out of the three territories in the country (figure 1). There are ~1200 sentinels (ie, participating PCPs) contributing data to the CPCSSN, which is updated quarterly. This actively expanding repository currently contains data on approximately 1.5 million Canadians. The initial focus of the CPCSSN was to conduct surveillance on five chronic conditions: diabetes, hypertension, osteoarthritis, chronic obstructive pulmonary disease and depression. Subsequently, it was expanded to included three neurological conditions: dementia, epilepsy and Parkinson's disease. Our project team has been instrumental in working with CPCSSN to further expand on these conditions by including CKD, and this work on CKD will be conducted from January 2017 to December 2018. It will take place under the auspices of the Alberta's Strategy for Patient Oriented Research (SPOR) Primary and Integrated Health Care Innovation Network (PIHCIN).
Figure 1.
Map of Canada showing CPCSSN networks distribution. CPCSSN, Canadian Primary Care Sentinel Surveillance Network.
Population and data sources
Data for the CPCSSN database are extracted from the EMRs of participating sentinels, rendered anonymous, coded and processed using established frameworks described in detail elsewhere.21 23 The data are placed in regional network databases and then merged into the national repository. It is made available for surveillance and research and increasingly for QI projects and for clinical decision support where available, through the implementation of a patient reidentification tool held only within the custodial clinics themselves. With appropriate data sharing agreements with its sentinel PCPs, CPCSSN data may be linked with administrative health data for future work to follow-up on CKD-related outcomes (eg, ascertainment of progression to dialysis requirement using registry data).
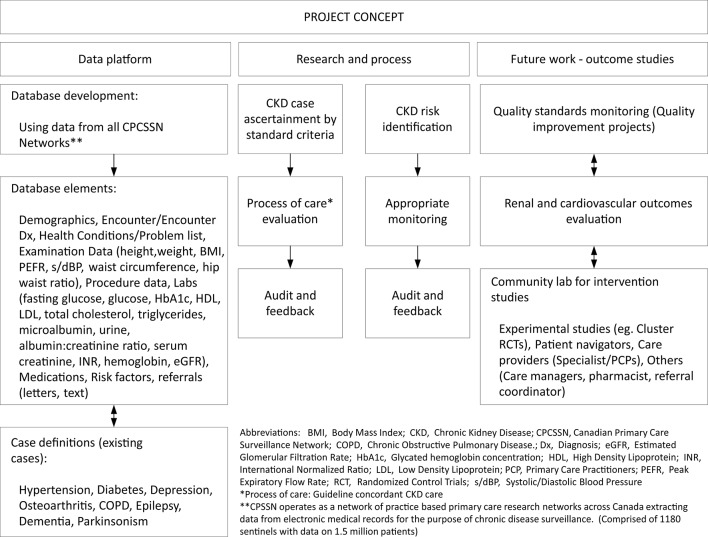
The CPCSSN database will be used to develop a cohort of patients with CKD being managed in primary care between 1 January 2010 and 31 December 2015 (baseline cohort) and from 1 January 2016 onwards (open cohort). The database is updated quarterly, allowing for identification of new patients with CKD who meet the inclusion criteria during the study period. The CPCSSN database contains patient information on sociodemographics (age, sex, socioeconomic status (calculated deprivation category)), treatment (medications data), laboratory results and comorbidities (table 1; figure 2).21 24 Patients under 18 years of age will be excluded from the cohorts, as will those diagnosed with ESRD and on dialysis or having renal transplant. We will leverage validated algorithms, case definitions and guidelines to define CKD, at-risk population and processes of care measures based on established methods and criteria (table 2).25–27
Table 1.
Data variables
| Domain | Variables |
| Patient demographics and clinical data |
|
| Patient–provider pairing |
|
| Provider information |
|
| Billing data |
|
| Clinical encounters |
|
| Encounter diagnoses |
|
| Physical examination data |
|
| Health condition |
|
| Laboratory data |
|
| Medical/surgical procedure |
|
| Medication records |
|
| Referral data |
|
| Risk factor data |
|
BMI, body mass index; BP, blood pressure; COPD, chronic obstructive pulmonary disease; CPCSSN, Canadian Primary Care Sentinel Surveillance Network; eGFR, estimated glomerular filtration rate; EMR, electronic medical records; HbA1C, glycated haemoglobin concentration; HDL, high density lipoprotein; ICD, International Classification of Diseases; LDL, low density lipoprotein.
Figure 2.
The project concept.
Table 2.
Elements of high-quality CKD care as defined by standard national and international CKD guidelines25 44–48
| Domain | Objective | Measures |
| Identification of CKD risk factors | To establish an organised system for identification of people with risk factors and evaluated for the presence of CKD markers | Percentage of patients with risk factors* (present for at least 1 year) tested for CKD within the last 12 months |
| Identification of CKD | To establish an organised system where people with CKD are appropriately identified | Proportion of patients with CKD correctly diagnosed and appropriately coded (validated based on KDIGO definition standard of using Scr measurements to derive eGFR <60 mL/min based on two measures at least 90 days apart based on CKD-EPI equation. |
| Management of CKD: Monitoring of risk factors for progression and CVD | To establish an organised system to ensure patients with CKD are receiving guideline-concordant care appropriate for the stage of CKD. This implies that those with early stages are being monitored appropriately in primary care. | Proportion of patients receiving appropriate testing and monitoring:
|
| Appropriate referral system | To develop a system where patients with CKD that need specialist input to care are appropriately identified and referred. | Proportion of patients appropriately referred for specialist care (defined by any visit to nephrologist or multidisciplinary CKD clinic within the last 12 months, for those patients that meet the KDIGO referral criteria)† |
*Diabetes, hypertension, CVD, nephrotoxic medications (non-steroidal anti-inflammatory drugs, calcineurin inhibitors, lithium), certain urological disease (eg, kidney stones, prostatic hypertrophy), multisystem diseases (eg, lupus) and family history of kidney disease.
†This would include advanced stages of CKD (eGFR<30 mL/min/1.73 m2), significant albuminuria (ACR≥70 mg/mmol), rapid loss of eGFR (>15 mL/min/1.73 m2) refractory hypertension and history of acute kidney injury.
ACEi, ACE inhibitor; ACR, albumin to creatinine ratio; ARB, angiotensin-receptor blocker; BP, blood pressure; CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; EPI, Epidemiology Collaboration; HbA1c, glycated haemoglobin concentration; KDIGO, Kidney Disease Improving Global Outcomes; Scr, serum creatinine.
Identification of CKD
The major focus is to validate a case definition for CKD in primary care using the CPCSSN repository to enable us to identify patients with CKD. We will leverage the existing frameworks and conventions by national and international CKD guidelines, as well as definitions used elsewhere.26 28 Serum creatinine (Scr) measurements will be used to calculate estimated glomerular filtration rate (eGFR) using CKD Epidemiology Collaboration equation.29 Individuals with at least one face-to-face PCP encounter and two calculated eGFR values <60 mL/min per 1.73 m2 more than 90 days apart or having an International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code for CKD used at least twice in an outpatient encounter as of 31 December 2015, will be defined to have CKD. We recognise the significant limitations of the ICD-9 codes when applied for CKD identification in the community; however, its use will allow us to capture those patients with CKD who may or may not have had an abnormal eGFR, for example, cystic kidney diseases and other rare congenital disorders. In a secondary analysis, CKD will be defined by eGFR <45 mL/min/1.73 m2 using two values of eGFR more than 90 days apart. This definition was selected because, although GFR <60 mL/min/1.73 m2 constitutes CKD, individuals with GFR between 45 and 60 mL/min/1.73 m2 usually have favourable prognosis in the absence of proteinuria, and they are substantially fewer in number than those with GFR <45/min/1.73 m2. Patients with eGFR <45 mL/min/1.73 m2 are also a more attractive population for intervention due to higher risk of adverse clinical outcomes including risk of progression to ESRD.2 14 Validation of the primary care CKD case definition algorithm will consist of an expert blinded chart review and identification of cases in a random sample of 1000 CPCSSN charts, containing both original and cleaned text data from the EMRs for patients aged 18 years or older, and comparison with the outcome of an independent search of the same sample using the CPCSSN case finding algorithm. Analysis will consist of calculations of sensitivity, specificity and positive and negative predicted values. The prevalence of CKD stages 3–5 will be presented as unadjusted prevalence estimates and weighted averages for population rates with 95% CIs using the census data and will be stratified by PCP, rural/urban location and province or territory.
Evaluation of processes of care
The process-based outcomes (quality of care metrics) will be defined and evaluated using established methods across key domains of risk identification and case finding for CKD, as well as delivery of appropriate management including monitoring of risk factors for progression and CVD, and referral for specialist nephrology care where necessary (table 2). We are specifically focusing on the key domains of CKD management in primary care that include:
CKD risk identification. This entails the evaluation of the proportion of patients with risk factors (diabetes and/or hypertension) present for at least 1 year that are tested for CKD within the previous 12 months of follow-up.
Identification of CKD. This is to determine the proportion of patients with CKD (defined based on laboratory measures) and/or correctly coded as having CKD in the EMR using defined clinical parameters.
Management of CKD: This examines the current state of practice on the management of the common risk factors (blood pressure (BP), glycaemia, proteinuria, dyslipidaemia) associated with CKD progression and/or risk of CKD. For example, ascertaining the proportion of patients achieving guideline-concordant treatment targets (BP, proteinuria, HbA1c).
Appropriate referral. This is to capture the proportion of patients appropriately referred for specialist kidney care (defined by any visit to nephrologist or multidisciplinary CKD clinic within the last 12 months) based on guideline-concordant criteria for referral.
Data analysis
Covariates definition and evaluation of CKD markers
Sociodemographic, clinical, laboratory and medication data will be classified using standard definitions established by the CPCSSN.17 24 30–34 Other comorbid conditions relevant to the care of patients with CKD but not defined by the CPCSSN (coronary artery disease, stroke, peripheral vascular disease, heart failure) will be identified using standard procedures to search for those diagnoses in patients’ medical records (based on validated ICD-9-CM coding algorithms35). Baseline kidney function (using eGFR) will be calculated using all Scr measurements taken within a 6-month period of the first creatinine measurement, with the index eGFR defined as the mean of the 6-month measurements. Assessment of urine protein excretion will be estimated using the albumin to creatinine ratio based on spot urine measurements using standard conventions.25 All CKD cases will be risk stratified, defined and classified according to current guideline recommendations.25 Demographic information (including age, sex and postal code) of those with and at risk of CKD will be extracted (tables 1 and 2). Normally distributed variables will be summarised as means with SD, and non-normally distributed data will be summarised as medians with IQRs. Dichotomous data will be expressed in percentages. The representativeness of the study population will be tested by comparing their age and sex profiles to the population distribution as reported in the Canadian 2011 Census (adjusting for 2016 census data when available).
Ethics and dissemination
The CPCSSN is committed to high ethical standards when handling individual patient data collected from EMRs of participating PCPs from across Canada. These data are aggregated and stored in CPCSSN's central repository at the Centre for Advanced Computing, Queens University in Kingston, Ontario. There are well-developed organisational, physical and technological safeguards at all levels (clinic, provincial, national) to ensure that the privacy of patients is protected and that all collection, retention, use or disclosure of data complies with applicable privacy legislation and ethical requirements.17 21 All personal identifiers are removed from the data in the national research repository to protect patient confidentiality.21 The protocol has undergone a scientific review and approval by the CPCSSN Surveillance and Research Standing Committee, and ethical approval for the study was granted by the University of Alberta Health Research Ethics Committee.
This project uses an integrated knowledge translation (KT) strategy, where relevant stakeholders (patients, practitioners, policy-makers) have been involved from inception (proposal development) to ensure that the project addresses the needs of patients and practitioners. The activities underpinning the Alberta SPOR PIHCIN centre on the patients are patient oriented, where the voices and perspectives of patients are solicited from research inception (eg, defining research questions) to completion. The patients are collaborators rather than research subjects, and we have a patient representative on the project team to ensure that patients’ voices and perspectives guide the work to completion and that it remains patient centred.
The involvement of the knowledge end-users (practitioners and policy-makers) and the high priority that they place on the project will ensure that we drive it forward to completion and provide an opportunity to meaningfully change the way that care is delivered to patients with CKD managed in primary care. For example, after identifying regions/clusters with especially suboptimal CKD care, we will collaborate with providers, policy-makers and regional health authorities to improve care in those regions. We will apply the KT Canada Knowledge-to-Action Cycle Framework and other established methods (eg, PDSA (Plan, Do, Study, Act) concept) to disseminate our findings to all relevant stakeholders and end-users for action.36 37 We will leverage our collaborations with existing platforms and research networks such as the Canadian Society of Nephrology, CANN-NET (CAnadian KidNey KNowledge TraNslation and GEneration NeTwork), Can-SOLVE CKD (Canadians Seeking Solutions and Innovations to Overcome Chronic Kidney Disease) and the countrywide PIHCIN to ensure the translation of our findings across provinces and territories, as well as internationally through our associations with the International Society of Nephrology and the Kidney Disease Improving Global Outcomes team.5 38 The results will be published in general medicine, nephrology and primary care peer-reviewed journals and presented at relevant national and international scientific meetings. A performance report will be produced against the indicators relevant to each objective, aggregated by practice sentinel, to highlight practice performance and map out areas for improvement, critical for continuing professional development of PCPs and helping to enhance CKD management in primary care. In the future, this database with be linked to the Canadian Organ Replacement Registry and relevant provincial administrative databases to study clinically relevant outcomes of risk and progression toikidney failure (ESRD) and adverse cardiovascular outcomes39 40 (figure 3).
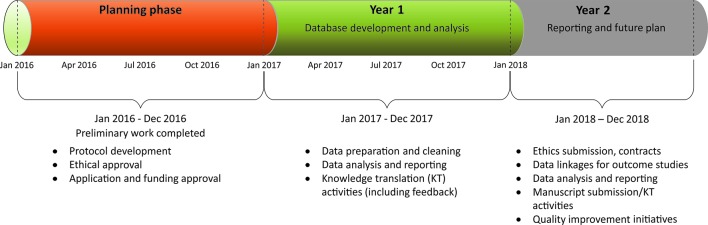
Figure 3.
Project timeline/milestones.
This work will close the information gap between observed and expected burdens and risks of CKD and map out standards of care achieved, providing opportunities for focused and effective population-level QI strategies and other service improvement initiatives. This project will demonstrate proof of concept for an early identification and management of CKD programme in primary care, and this will provide the basis for developing relevant policies and KT strategies to enhance the uptake of findings—for people with CKD and other chronic diseases as well. The work will facilitate identification and appropriate management of patients with CKD at high risk of progression to ESRD. The findings will be reported based on existing reporting frameworks.41
We have anticipated potential threats and have devised strategies to mitigate them. The first is related to feasibility within the defined timeframe (figure 3). This is unlikely to hinder the success of the project as we have completed significant preliminary work for data access, and the team has considerable experience with the use of this kind of data for policy-relevant research.5 19 36 42 43 Second, limitations general to the use of EMR data in research are acknowledged. The collated information is based on clinical encounters and needs and, thus, may be missing for some important key variables, and data quality may vary by region and/or sentinel; specifically, data on patients’ priorities and satisfaction of care are rarely collated in a clinical setting. However, most important demographic, clinical and laboratory information needed for this study are well captured in the existing platform chosen for this work. Third, lack of use of patient-reported outcomes measures such as satisfaction with care, self-management support and so on could not be included as these were not routinely collated in this database.
In summary, planning, development and implementation of nephrology services require reliable information systems and databases to capture information on trends in disease burden, processes of care and related outcomes. In the absence of national/regional health information systems, one way to achieve this is by the creation of surveillance systems using routine practice data, such as the CPCSSN and those established specifically for CKD in other jurisdictions across the world44–48 (table 3). The established conventions and guidelines on CKD can be leveraged for systematic case definition and evaluation of quality of care across settings. This can be facilitated by validation as well as enactment of quality metrics to measure the processes, quality of care and related outcomes and to generate uniformity across databases which may permit analyses across countries and regions. It is important to detect CKD early enough to be able to implement effective interventions. Ongoing primary care management of key risk factors for CKD (eg, hypertension, vascular disease, diabetes) is likely one effective strategy to reduce progression of CKD and to reduce adverse complication rates. Early detection and treatment of CKD and reducing adverse events with appropriate medications also reduces the morbidity and cost of CKD and related complications. The work described in this protocol therefore has a huge potential to address the identified gaps for optimal care delivery of CKD at the primary care level that would impact positively on patients’ outcomes and health system improvement.
Table 3.
Examples of existing CKD surveillance programs
| Programme | Year of inception | Approach/methodology | Data sources | Spread | Funding |
| US CDC* | 2006 | Passive surveillance approach leveraging existing data sources | Administrative surveys (NHANES) | National | Governmental support |
| England QoF | 2006 | An incentive-based system (pay-for-performance system) that required all PCPs to establish a register for adults with CKD stages 3–5 and achieve guideline-concordant treatment targets. | Routine primary care records | National | Governmental support |
| CKD-JAC | 2008 | Routine care data across select CKD clinics across Japan | Practice data | National | Japanese Society of Nephrology and Industry |
| CKD Queensland Registry | 2009 | Routine clinical care data | Practice data | Regional (state of Queensland) | Research organisations and industry |
| CPCSSN-CKD* | 2017 | Passive surveillance using routine practice database | Routine primary care databases | National (multiple regions and territories) | Research organisations and government (PHAC, CIHR) |
*Main database development started in 2005.
CIHR, Canadian Institutes of Health Research; CKD-JAC, Chronic Kidney Disease Japan Cohort; CKD, chronic kidney disease; CPCSSN, Canadian Primary Care Sentinel Surveillance Network; NHANES, National Health and Nutrition Examination Survey; PCP, primary care practice; QoF, Quality and Outcomes Framework; US CDC, US Centres for Disease Control and Prevention CKD Surveillance System.
Supplementary Material
Footnotes
Contributors: AKB and ND had the original idea for this study. AKB and PER wrote the first draft of the manuscript. PER, NT, AS, AG, DN, JAQ, CL, LBS, EF, DT and DM contributed to the development of the idea and the study design and reviewed the manuscript for intellectual content. All authors approved the final submitted version of the manuscript.
Funding: This work is supported by the Canadian Institute of Health Research (CIHR) Operating Grant: SPOR PIHCI Network: Quick Strikes Grant (reference no RN281786) through the Universities of Alberta and Manitoba, and McMaster University.
Disclaimer: The views expressed are those of the authors and not necessarily those of the CIHR,CPCSSN or the various institutions represented.
Competing interests: None declared.
Patient consent: Detail has been removed from this case description/these case descriptions to ensure anonymity. The editors and reviewers have seen the detailed information available and are satisfied that the information backs up the case the authors are making.
Ethics approval: University of Alberta Health Research Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The authors will make available the full statistical analysis of the study results following publication if and when required. The results of the study will be submitted for publication in a leading medical/nephrology peer-reviewed journal.
References
- 1. Radhakrishnan J, Remuzzi G, Saran R, et al. . CDC-CKD Surveillance Team European CKD Burden Consortium CKD.QLD group. Taming the chronic kidney disease epidemic: a global view of surveillance efforts. Kidney Int 2014;86:246–50. 10.1038/ki.2014.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Couser WG, Remuzzi G, Mendis S, et al. . The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int 2011;80:1258–70. 10.1038/ki.2011.368 [DOI] [PubMed] [Google Scholar]
- 3. Bello AK, Levin A, Tonelli M, et al. . Assessment of Global Kidney Health Care Status. JAMA 2017;317:1864 10.1001/jama.2017.4046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. James MT, Hemmelgarn BR, Tonelli M. Early recognition and prevention of chronic kidney disease. Lancet 2010;375:1296–309. 10.1016/S0140-6736(09)62004-3 [DOI] [PubMed] [Google Scholar]
- 5. Bello AK, Levin A, Manns BJ, et al. . Effective CKD care in European countries: challenges and opportunities for health policy. Am J Kidney Dis 2015;65:15–25. 10.1053/j.ajkd.2014.07.033 [DOI] [PubMed] [Google Scholar]
- 6. McBride D, Dohan D, Handley MA, et al. . Developing a CKD registry in primary care: provider attitudes and input. Am J Kidney Dis 2014;63:577–83. 10.1053/j.ajkd.2013.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stevens PE, O'Donoghue DJ, de Lusignan S, et al. . Chronic kidney disease management in the United Kingdom: neoerica project results. Kidney Int 2007;72:92–9. 10.1038/sj.ki.5002273 [DOI] [PubMed] [Google Scholar]
- 8. de Lusignan S, Chan T, Stevens P, et al. . Identifying patients with chronic kidney disease from general practice computer records. Fam Pract 2005;22:234–41. 10.1093/fampra/cmi026 [DOI] [PubMed] [Google Scholar]
- 9. Garg AX, Adhikari NK, McDonald H, et al. . Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA 2005;293:1223–38. 10.1001/jama.293.10.1223 [DOI] [PubMed] [Google Scholar]
- 10. Collins AJ, Foley RN, Gilbertson DT, et al. . United States renal data system public health surveillance of chronic kidney disease and end-stage renal disease. Kidney Int Suppl 2015;5:2–7. (2011) 10.1038/kisup.2015.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Borbolla D, Giunta D, Figar S, et al. . Effectiveness of a chronic disease surveillance systems for blood pressure monitoring. Stud Health Technol Inform 2007;129:223–7. [PubMed] [Google Scholar]
- 12. Indicators for chronic disease surveillance. MMWR Recomm Rep 2004;53:1–6. [PubMed] [Google Scholar]
- 13. Saran R, Hedgeman E, Plantinga L, et al. . Establishing a national chronic kidney disease surveillance system for the United States. Clin J Am Soc Nephrol 2010;5:152–61. 10.2215/CJN.05480809 [DOI] [PubMed] [Google Scholar]
- 14. Mariani L, Stengel B, Combe C, et al. . The CKD Outcomes and Practice patterns study (CKDopps): rationale and methods. Am J Kidney Dis 2016;68:402–13. 10.1053/j.ajkd.2016.03.414 [DOI] [PubMed] [Google Scholar]
- 15. Levey AS, Schoolwerth AC, Burrows NR, et al. . Comprehensive public health strategies for preventing the development, progression, and complications of CKD: report of an expert panel convened by the Centers for Disease Control and Prevention. Am J Kidney Dis 2009;53:522–35. 10.1053/j.ajkd.2008.11.019 [DOI] [PubMed] [Google Scholar]
- 16. Drawz PE, Archdeacon P, McDonald CJ, et al. . CKD as a model for improving chronic disease care through electronic health records. Clin J Am Soc Nephrol 2015;10:1488–99. 10.2215/CJN.00940115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Birtwhistle R, Keshavjee K, Lambert-Lanning A, et al. . Building a pan-Canadian primary care sentinel surveillance network: initial development and moving forward. J Am Board Fam Med 2009;22:412–22. 10.3122/jabfm.2009.04.090081 [DOI] [PubMed] [Google Scholar]
- 18. Bello AK, Hemmelgarn B, Lloyd A, et al. . Associations among estimated glomerular filtration rate, proteinuria, and adverse cardiovascular outcomes. Clin J Am Soc Nephrol 2011;6:1418–26. 10.2215/CJN.09741110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bello AK, Peters J, Wight J, et al. . A population-based screening for microalbuminuria among relatives of CKD patients: the kidney evaluation and awareness program in Sheffield (KEAPS). Am J Kidney Dis 2008;52:434–43. 10.1053/j.ajkd.2007.12.034 [DOI] [PubMed] [Google Scholar]
- 20. Greiver M, Drummond N, Birtwhistle R, et al. . Using EMRs to fuel quality improvement. Can Fam Physician 2015;61:92, e68–9. [PMC free article] [PubMed] [Google Scholar]
- 21. Birtwhistle R, Godwin M, Leggett JA, et al. . Linking health databases for research. Can Fam Physician 2015;61:e223-4:382. [PMC free article] [PubMed] [Google Scholar]
- 22. Garies S, Birtwhistle R, Drummond N, et al. . Data Resource Profile: national electronic medical record data from the canadian primary care Sentinel Surveillance Network (CPCSSN). Int J Epidemiol 2017. 10.1093/ije/dyw248 [DOI] [PubMed] [Google Scholar]
- 23. Williamson T, Green ME, Birtwhistle R, et al. . Validating the 8 CPCSSN case definitions for chronic disease surveillance in a primary care database of electronic health records. Ann Fam Med 2014;12:367–72. 10.1370/afm.1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Godwin M, Williamson T, Khan S, et al. . Prevalence and management of hypertension in primary care practices with electronic medical records: a report from the canadian primary care Sentinel Surveillance Network. CMAJ Open 2015;3:E76–E82. 10.9778/cmajo.20140038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Levin A, Stevens PE. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int 2014;85:49–61. 10.1038/ki.2013.444 [DOI] [PubMed] [Google Scholar]
- 26. Ronksley PE, Tonelli M, Quan H, et al. . Validating a case definition for chronic kidney disease using administrative data. Nephrol Dial Transplant 2012;27:1826–31. 10.1093/ndt/gfr598 [DOI] [PubMed] [Google Scholar]
- 27. Vlasschaert ME, Bejaimal SA, Hackam DG, et al. . Validity of administrative database coding for kidney disease: a systematic review. Am J Kidney Dis 2011;57:29–43. 10.1053/j.ajkd.2010.08.031 [DOI] [PubMed] [Google Scholar]
- 28. Denburg MR, Haynes K, Shults J, et al. . Validation of the Health Improvement Network (THIN) database for epidemiologic studies of chronic kidney disease. Pharmacoepidemiol Drug Saf 2011;20:1138–49. 10.1002/pds.2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Levey AS, Stevens LA, Schmid CH, et al. . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aref-Eshghi E, Leung J, Godwin M, et al. . Low density lipoprotein cholesterol control status among Canadians at risk for cardiovascular disease: findings from the canadian primary care Sentinel Surveillance Network Database. Lipids Health Dis 2015;14:60 10.1186/s12944-015-0056-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Birtwhistle RV. Canadian primary care Sentinel Surveillance Network: a developing resource for family medicine and public health. Can Fam Physician 2011;57:1219–20. [PMC free article] [PubMed] [Google Scholar]
- 32. Green ME, Natajaran N, O'Donnell DE, et al. . Chronic obstructive pulmonary disease in primary care: an epidemiologic cohort study from the canadian primary care Sentinel Surveillance Network. CMAJ Open 2015;3:E15–E22. 10.9778/cmajo.20140040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Greiver M, Williamson T, Barber D, et al. . Prevalence and epidemiology of diabetes in canadian primary care practices: a report from the canadian primary care Sentinel Surveillance Network. Can J Diabetes 2014;38:179–85. 10.1016/j.jcjd.2014.02.030 [DOI] [PubMed] [Google Scholar]
- 34. Kadhim-Saleh A, Green M, Williamson T, et al. . Validation of the diagnostic algorithms for 5 chronic conditions in the canadian primary care Sentinel Surveillance Network (CPCSSN): a Kingston Practice-based Research Network (PBRN) report. J Am Board Fam Med 2013;26:159–67. 10.3122/jabfm.2013.02.120183 [DOI] [PubMed] [Google Scholar]
- 35. Tonelli M, Wiebe N, Guthrie B, et al. . Comorbidity as a driver of adverse outcomes in people with chronic kidney disease. Kidney Int 2015;88:859–66. 10.1038/ki.2015.228 [DOI] [PubMed] [Google Scholar]
- 36. Hemmelgarn BR, Manns BJ, Straus S, et al. . Knowledge translation for nephrologists: strategies for improving the identification of patients with proteinuria. J Nephrol 2012;25:933–43. 10.5301/jn.5000226 [DOI] [PubMed] [Google Scholar]
- 37. Byrne J, Xu G, Carr S. Developing an intervention to prevent acute kidney injury: using the Plan, Do, Study, Act (PDSA) service improvement approach. J Ren Care 2015;41:3–8. 10.1111/jorc.12090 [DOI] [PubMed] [Google Scholar]
- 38. Feehally J, Couser W, Dupuis S, et al. . Nephrology in developing countries: the ISN's story. Lancet 2014;383:1271–2. 10.1016/S0140-6736(13)62711-7 [DOI] [PubMed] [Google Scholar]
- 39. Tangri N, Stevens LA, Griffith J, et al. . A predictive model for progression of chronic kidney disease to kidney failure. JAMA 2011;305:1553–9. 10.1001/jama.2011.451 [DOI] [PubMed] [Google Scholar]
- 40. Hemmelgarn BR, Manns BJ, Lloyd A, et al. . Relation between kidney function, proteinuria, and adverse outcomes. JAMA 2010;303:423–9. 10.1001/jama.2010.39 [DOI] [PubMed] [Google Scholar]
- 41. Motheral B, Brooks J, Clark MA, et al. . A checklist for retrospective database studies—report of the ISPOR Task Force on retrospective databases. Value Health 2003;6:90–7. 10.1046/j.1524-4733.2003.00242.x [DOI] [PubMed] [Google Scholar]
- 42. Bello AK, Thadhani R, Hemmelgarn B, et al. . Design and implementation of the canadian kidney disease cohort study (CKDCS): A prospective observational study of incident hemodialysis patients. BMC Nephrol 2011;12:10 10.1186/1471-2369-12-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bello AK, Nwankwo E, El Nahas AM. Prevention of chronic kidney disease: a global challenge. Kidney Int Suppl 2005:S11–S17. 10.1111/j.1523-1755.2005.09802.x [DOI] [PubMed] [Google Scholar]
- 44. Kilpatrick ES, Verrill H. National Clinical Biochemistry Audit G. A National audit of estimated glomerular filtration rate and proteinuria reporting in the UK. Ann Clin Biochem 2011;48:558–61. [DOI] [PubMed] [Google Scholar]
- 45. Lamb EJ. United Kingdom guidelines for chronic kidney disease. Scand J Clin Lab Invest Suppl 2008;241:16–22. 10.1080/00365510802144920 [DOI] [PubMed] [Google Scholar]
- 46. Johnson DW, Atai E, Chan M, et al. . KHA-CARI guideline: early chronic kidney disease: detection, prevention and management. Nephrology 2013;18:340–50. 10.1111/nep.12052 [DOI] [PubMed] [Google Scholar]
- 47. Akbari A, Clase CM, Acott P, et al. . Canadian Society of Nephrology commentary on the KDIGO clinical practice guideline for CKD evaluation and management. Am J Kidney Dis 2015;65:177–205. 10.1053/j.ajkd.2014.10.013 [DOI] [PubMed] [Google Scholar]
- 48. Crews DC. Chronic Kidney disease: a place for primary care and nephrology to meet. J Gen Intern Med 2016;31:5–6. 10.1007/s11606-015-3506-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.