Abstract
A long-standing literature implicates activity within the default mode network (DMN) to processes linked to the self. However, contemporary work suggests that other large-scale networks networks might also be involved. For instance, goal-directed autobiographical planning requires positive functional connectivity (FC) between DMN and frontoparietal control (FPCN) networks. The present study examined the inter-relationship between trait self-focus (measured via a self-consciousness scale; SCS), incidental memory in a self-reference paradigm, and resting state FC of large-scale networks. Behaviourally, we found that private SCS was linked to stronger incidental memory for self-relevant information. We also examined how patterns of FC differed according to levels of self-consciousness by using the SCS data to drive multiple regression analyses with seeds from the DMN, the FPCN and the limbic network. High levels of SCS was not linked to differences in the functional behaviour of the DMN, however, it was linked to stronger FC between FPCN and a cluster extending into the hippocampus, which meta analytic decoding using Neurosynth linked to episodic memory retrieval. Subsequent analysis demonstrated that trait variance in this pattern of FC was a moderator for the observed relationship between private SCS and enhanced memory for self-items. Together these findings suggest that interactions between the FPCN and hippocampus may support the memory advantage of self-relevant information associated with SCS and confirm theoretical positions that argue that that self-related processing does not simply depend upon the DMN, but instead relies on complex patterns of interactions between multiple large-scale networks.
Keywords: self-consciousness, resting-state functional connectivity, self-reference memory advantage
Introduction
Human cognition is characterised by the capacity for self-consciousness—the process through which we can become the subject of our own conscious experience. The degree to which individuals engage in self-consciousness appears early in development (Berthental and Fischer, 1978; Lewis and Brooks, 1978; Lipka and Brinthaupt, 1992), and it can have both positive and negative outcomes in daily life. For example, the ability to reflect on our own thoughts and actions is crucial for the development of personal identity (Turner, 1978); however, when taken to extremes, the same process can result in excessive shyness or anxiety (Crozier, 2002). A well established measure of self-consciousness (Carver and Glass, 1976; Scheier and Carver, 1985; Scheier and Carver, 2013) divides the construct into three related, yet independent, dimensions: (1) private self-consciousness, which describes the extent to which people introspect and examine hidden aspects of the self (e.g. their beliefs or values), (2) public self-consciousness, which describes the extent to which people examine how public aspects of the self may be perceived by others (e.g. what impression others might form) and (3) social anxiety, which describes the extent to which people react to perceptions of their public self and evaluations from others.
When people engage in self-conscious thought, schema containing self-relevant information are activated (Nasby, 1985) and this information possesses special mnemonic qualities. For example, people have a robust tendency to remember information more effectively when it is processed with respect to the self, a bias resulting in better memory recall for self-related information (termed ‘The self-reference effect’; Rogers et al., 1977). One possibility is that the self-reference effect is simply an indirect consequence of familiarity: self-relevant information is likely to be highly familiar and familiarity is known to facilitate encoding (Prentice, 1990). However, research has ruled out familiarity as the mechanism underlying the self-reference effect because a self-referent bias has also been observed for neutral shapes (Humphreys and Sui, 2015) and everyday items (Cunningham et al., 2011). Instead, self-reference is thought to improve memory because of the rich network of associations associated with ourselves which in turn allows for the formation of stronger memory traces (Symons and Johnson, 1997). As well as its effect on memory, self-relevant information has strong salient properties which impact on attention (Sui et al., 2015), with studies showing that one’s name (Harris and Pashler, 2004) or face (Brédart et al., 2006) can act like an efficient distractor. Moreover, other studies have shown that self-relevant information has similar properties to salient perceptual stimuli, automatically triggering the reallocation of attentional resources (Sui et al., 2015). These experimental paradigms share similarities with more naturalistic mental processes such as mind-wandering, where salient self-relevant information becomes the focus of conscious attention when we are otherwise engaged in external tasks (Smallwood et al., 2011). Although mind-wandering can often be associated with task errors (Weissman et al., 2006; McVay and Kane, 2009), the reallocation of attention towards the self during a task may serve a broader function because it can facilitate the processing of personally meaningful goals that extend beyond the here-and-now (Medea et al., 2016; for a review see Poerio and Smallwood, 2016).
Recent neuro-imaging work has examined the neural basis of the process of self-reflection, a process important for self-consciousness (Grant et al., 2002). Task-based studies of self-reference often observe activity in the medial prefrontal cortex (mPFC), as well as regions in the posterior cingulate cortex (Kelley et al., 2002, Macrae et al., 2004; Northoff et al., 2006), regions that collectively form what is known as the default mode network (DMN). This large-scale network tends to show a pattern of deactivation during demanding external tasks (Raichle et al., 2001) and shows coherent activity during the resting state (Greicius et al., 2003 In addition, the DMN has also been linked to states of self-generated thought, such as mind-wandering (for recent meta-analyses see Fox et al., 2015; Stawarczyk and D'Argembeau, 2015). Recent work, however, suggests that the DMN often works in tandem with the other networks when internal representation must be manipulated in a goal directed fashion. For example, regions of lateral frontal and parietal cortex (that together form the frontoparietal control network; FPCN), become coupled with the DMN when autobiographical information is organised to form a plan (Spreng et al., 2010) and when identifying perceptual aspects of semantic processing (Krieger-Redwood et al., 2016). Moreover, extensive research has related regions in the FPCN to sustained attention and working memory (Coull et al., 1996; Koechlin et al., 1999; Rottschy et al., 2012), processes that allow conscious manipulation of information. These findings, along with those from Spreng and colleagues (2010), suggest that processing of self-related information present during self-consciousness may recruit the executive system anchored in the FPCN. It is a possibility that differences across individuals in their attentional preferences, i.e., how often one engages in self-conscious thought versus other types of information, is reflected in the FC of the FPCN. Moreover a study conducted by Eisenberger et al. (2005) found a relationship between activity in a cluster in the FPCN, namely in the dorsal anterior cingulate cortex, and self-consciousness during a vigilance task. Similarly, studies have shown that when participants hold social information in mind they use lateral regions of cortex linked to executive control processes (Meyer et al., 2012). In addition to the DMN and the FPCN, the limbic system may also play an important role in self-oriented cognition. Extensive research has shown that negative mood increases self-focussed attention (Sedikides, 1992) as well as mind-wandering (Smallwood et al., 2009; Poerio et al., 2013) and some studies have suggested that the effect of mood on information processing in turn predicts later behaviour (Gendolla, 2000). Moreover, neuroimaging research has shown a distinction within the mPFC between cognitive and affective components of self-oriented cognition (Moran et al., 2006) while the amygdala, a main hub of the limbic system, is important in a range of psychiatric conditions associated with disturbances in the self (Strakowski et al., 1999; Davidson, 2002; Phan et al., 2006).
The current study aimed to determine the functional architecture that underpins different forms of self-consciousness (private, public and social anxiety) and to understand how this is related to the strength of a person’s memory for self-relevant information. We recorded functional imaging data in a large cohort of participants during wakeful rest who later completed the three subscales of the Self-Consciousness Scale (SCS, Scheier and Carver, 2013). Previous research has consistently found a positive relationship between private self-consciousness scores and the magnitude of the self-reference effect (Turner, 1980; Agatstein and Buchanan, 1984; Nasby, 1985; Hull et al., 1988) and so in this experiment we also measured incidental memory for self-relevant information in our participants. We hypothesised that differences in response on the SCS should be reflected in the connectivity patterns of three large-scale networks (Default Mode, Frontoparietal Control and Limbic). To select these large-scale networks we used a parcellation obtained by Yeo and colleagues who applied clustering techniques to resting state data of 1000 individuals (Yeo et al., 2011). We were interested in (a) replicating prior findings linking high levels of private self-consciousness to better memory for the self, (b) identifying patterns of functional connectivity (FC) of the DMN, FPCN and Limbic networks associated with different aspects of self-consciousness and (c) determining whether any neurocognitive patterns linked to different types of self-consciousness explained the hypothesised self-memory advantage.
Materials and methods
Participants
A hundred and forty one healthy right-handed participants were recruited to the study; in exchange for participation they received a monetary reward or course credits. The sample had an average age of 22.50 (SD = 2.93) years. Approval for this project was granted by the York Neuroimaging Centre (YNiC) Ethics Committee and was conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Procedure
Self- consciousness scale
Participants completed a 22-item version of the self-consciousness scale inventory (Scheier and Carver, 2013) from which three subscales can be derived: private self-consciousness, public self-consciousness and social anxiety. Private self-consciousness is a measure of the tendency that an individual has to introspect and study one’s inner self and motives and was assessed with nine statements such as ‘I’m always trying to figure myself out’. Public self-consciousness refers to the tendency of an individual to think about what others think about him/her, and was assessed through seven statements such as ‘ care a lot about how I present myself to others’. Finally social anxiety was measured with six statements such as ‘It takes me time to get over my shyness in new situations’. Participants had to answer to each statement using a scale from 0 (not at all like me) to 3 (a lot like me). Items from each subscale were summed to create an overall score per scale.
Self-reference paradigm
The laboratory task measuring the self-reference memory advantage involved an evaluation and a retrieval phase. The evaluation phase consisted of two social conditions and one syllable count condition. In the social conditions participants were asked to make decisions about the association between adjectives and one of two referents (‘Self’ or ‘Lady Gaga’). In the syllable condition, participants indicated via button press whether the word on screen had three or more syllables or whether it had less. Adjectives were presented sequentially on-screen and participants were required to indicate whether each adjective applied to a particular referent/had three or more syllables by pressing ‘Y’ with the index finger of their right hand for ‘yes’ or ‘N’ with the index finger of their left hand for ‘no’. All words were selected from a pool of normalised personality trait adjectives with meaningfulness and likeability ratings (Anderson, 1968). An equal amount of positive, negative and neutral adjectives (40 adjectives/valence) with the highest meaningfulness rating were selected for this experiment. For each participant, these 120 words were randomly divided into two lists of 60 adjectives. One list contained all the items involved during the encoding phase, the other list contained the items that would be used as foils during the retrieval phase. This first encoding-phase list was divided into three lists of 20 items, each of which was assigned to one of the three conditions (Self, Lady Gaga, Syllables). Finally, these condition-specific lists were subdivided into two 10-item lists, one list per experimental block. During encoding, participants were presented with these lists in separate blocks in an ABCCBA order allowing us to control for order effects within each participant. We also counterbalanced the order in which each category was presented across participants. Each block was preceded by a screen indicating the specific condition and each block started after the participants button press. Stimuli were separated by an inter-stimulus interval of 5000ms during which participants were shown a blank screen with a fixation cross. Following the evaluation phase, participants were presented with a surprise retrieval test in which they were sequentially shown words and asked whether or not that particular item had been presented in the previous phase. This retrieval phase contained all the words from the previous stage of the experiment, plus an equal number of new words contained in the retrieval list. Items were presented in a random order and participants had to either press ‘O’ for old if they thought the word had appeared before or ‘N’ for new if they thought it was a new word. The old/new responses judged as ‘old’ were followed by a source localisation judgement in which participants had to indicate using arrow heads whether they thought the old word had been presented during the self, the Lady Gaga or the syllable-count condition. Confidence ratings ranging from 1 (not confident at all) to 6 (very confident) for each old/new and source localisation judgements were also obtained. At the end of the experiment, familiarity ratings for Lady Gaga were obtained to control for the possible effects familiarity could have on memory. This paradigm allowed for 12 types of response types. Hits are considered old words that were correctly identified as old and correctly localised during the source localisation phase. This results in either ‘Self hits’, ‘Lady Gaga hits’ or ‘syllable hits’. Old words judged as new would be considered misses, again resulting in either a ‘self miss’, a ‘Lady Gaga miss’ or a ‘syllables miss’. New words judged as new were considered ‘correct rejections’, and new words judged as old were considered ‘false alarms’. Based on the incorrect source localisation of these new words, these can further be subdivided into ‘Self false alarms’, ‘Lady Gaga false alarms’ or ‘syllable false alarms’. The false alarms measures specific to each referent allowed us to control for guessing at a referent specific level by subtracting them from the hits. Lastly, if an old word from, for example, the syllable condition was judged as old but then incorrectly source localised as Lady Gaga it was considered a ‘wrong source localisation from syllables to Lady Gaga condition’.
Resting state
Scan acquisition
Functional MRI data was acquired on a 3 Tesla GE scanner. Participants observed a fixation cross for a scan that lasted 9 min. The scan had a repetition time of 2 s, resulting in 210 volumes. We used interleaved slice-timing and isotropic voxel dimensions of 3 mm3 (matrix size of 64 × 64, 192 mm field of view and 32 slices) with a 0.5-mm gap between slices.
Pre-processing
All fMRI preprocessing and analysis was performed using FSL. We extracted the brain from the skull using the BET toolbox for both the flair and the structural T1 weighted images and these scans were registered to standard MNI152 (2mm) space using FLIRT (Jenkinson and Smith, 2001). Prior to conducting the FC analysis, the following pre-statistics processing was applied to the resting state data; motion correction using MCFLIRT (Jenkinson et al., 2002); slice-timing correction using Fourier-space time-series phase-shifting; non-brain removal using BET (Smith, 2002); spatial smoothing using a Gaussian kernel of FWHM 6 mm; grand-mean intensity normalisation of the entire 4D dataset by a single multiplicative factor; highpass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma = 100 s; Gaussian lowpass temporal filtering, with sigma = 2.8s.).
First-level analysis
Following these steps, the time series of three masks of interest (ROI) were extracted. These masks corresponded to (1) the DMN, (2) the FPCN and (3) the limbic system as defined by the 7 network parcellation performed by Yeo and colleagues (Yeo et al., 2011) and can be visualised in Figure 1. The approach of selecting large-scale network masks was based on previous studies using dual-regression, in which networks obtained through ICA group analyses are used as regions of interest in subsequent seed based analyses (Zuo et al., 2010; Rytty et al., 2013; Smith et al., 2014). Instead of using ICA group masks as regions of interest the current study used a reliable parcellation based on 1000 subjects (Yeo et al., 2011).
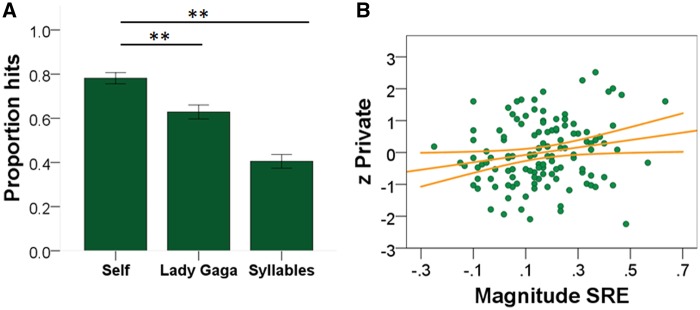
Fig. 1.
(A) ANOVA results between memory scores for the three conditions. (B) Scatterplot reflecting correlation between the standardised residual score for private self-consciousness and the magnitude of the self-reference effect (SRE).
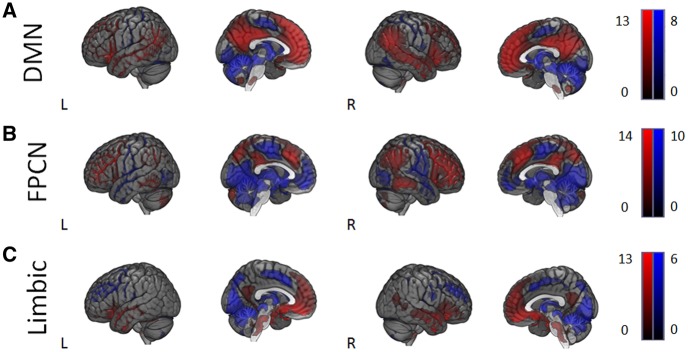
Fig. 2.
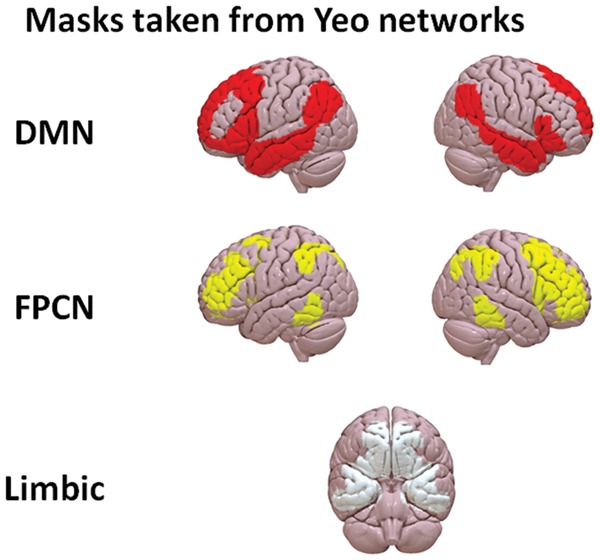
Masks taken from the 7 network parcellation performed by Yeo et al. (2011) used for the current seed-based analyses.
The parcellations in non linear MNI152 volume space were downloaded from https://surfer.nmr.mgh.harvard.edu/fswiki/CorticalParcellation_Yeo2011 and resampled from 1 to 2 mm3. The time series for each voxel within each mask were averaged and used as an explanatory variable in a subject-level FC analysis, which also included the following nuisance regressors: the first five principal time-series components extracted from white matter (WM) and cerebrospinal fluid (CSF) masks in accordance with the CompCor method (Behzadi et al., 2007) and six motion parameters. WM and CSF masks were generated by segmenting each individual’s high-resolution structural image (using FAST in FSL). The default tissue probability maps, referred to as Prior Probability Maps (PPM), were registered to each individual’s high-resolution structural image (T1 space) and the overlap between these PPM and the corresponding CSF and WM maps was identified. Finally, these maps were thresholded (40% for the SCF and 66% for the WM), binarised and combined. The six motion parameters were calculated in the motion-correction step during pre-processing. Linear displacements in each of the three Cartesian directions (x, y, z) and rotations around three axes (pitch, yaw, roll) were included for each individual. No global signal regression was performed (Murphy et al., 2009).
Second-level analysis
To understand whether our psychological measures of self-consciousness varied with either the between or within connectivity of the DMN, limbic and the FPCN, we used FSL to conduct a group-level regression of the connectivity matrices of each mask. In this analysis, we included the residualized mean centred scores for the three self-consciousness subscales as regressors of interest. In order to control for spurious correlations related to subject motion we included framewise displacement as a regressor of no interest, after controlling for four outliers by imputing their data to 2 standard deviations (Power et al., 2012). See Sormaz et al. (2017) for a prior demonstration of this approach. This technique allows us to examine regions within or outside the network mask whose connectivity varies with particular traits (in this case different aspects of self-consciousness). In these analyses the data were processed using FEAT version 5.98 part of FSL (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl) and the analyses were carried out using FMRIB's Local Analysis of Mixed Effects (FLAME). A grey matter mask with a probability threshold of 40% was used as a pre-thresholding mask and the cluster-forming threshold was set as z-score of 2.3. For these analyses we controlled for Type I errors by controlling for the number of voxels in the brain (Worsley, 2001), as well as the number of masks and the two tailed nature of our comparisons yielding an alpha value of P < 0.008 FWE.
Neurosynth meta-analyses
In order to interpret neuro-cognitive patterns of FC predictive of self-consciousness, we performed a meta-analysis using the online Neurosynth database (Yarkoni et al., 2011). We performed a meta-analytic decoding of the unthresholded maps produced in this study by uploading them onto Neurosynth. This allows the identification of psychological terms that are most likely to be associated with the specific spatial pattern that our analysis highlighted providing a quantitative interpretation of our data (for a prior illustration of this technique see de Sormaz et al., 2017). For the purposes of interpretationwe selected the 15 terms most related to the current spatial maps and displayed them in the form of word clouds in which a larger font size indicated a greater probability of association.
Results
Behavioural
Self-consciousness scale
The three subscales (private, public and social anxiety) for the self-consciousness questionnaire were calculated for each individual. The public subscale was correlated with both the private (r = 0.40, P < 0.001) and the social anxiety scale (r = 0.35, P < 0.001). No significant correlations were found between the private and the social anxiety subscales (r = 0.11, P = 0.235). In order to control for these correlations, the standardised residual scores were used in further analyses.
Self-reference paradigm
The first aim of our study was to establish whether in our sample we found a reliable self-relevant memory advantage. A repeated measures one-way analysis of variance (ANOVA) using the proportion of hits for each referent as the within-participants factor indicated a significant effect of referent on incidental memory performance (F (2, 278) = 284, P < 0.001), as measured during the retrieval phase of the self-other reference paradigm. Post-hoc paired-samples t-tests were conducted to compare incidental memory across the three conditions (self, Lady Gaga, syllable count). Participants had significantly better memory in the self (M = 0.78, SD = 0.15) compared to the Lady Gaga (M = 0.62, SD = 0.19) condition; t(139) = 10.85, P < 0.001; they also showed significantly better memory in the self compared to the syllable count(M = 0.4, SD = 0.18) condition; t(139)= 23.56, P < 0.001. Participants also had significantly better memory in the Lady Gaga compared to the syllable condition; t(139) = 12.87, P < 0.001 (Figure 1A). In addition, examination of the confidence intervals obtained from one-sample t-tests suggested memory for the syllable condition was at chance (95% CI [0.37, 0.44], whereas memory for Lady Gaga (95% CI [0.60, 0.66]) and self (95% CI [0.76, 0.81]) were both above chance performance.
Private self-consciousness and magnitude of the self-reference effect
Next, we sought to identify whether in our sample individuals high in private self-consciousness have a stronger memory when referring information to themselves rather than a familiar other (Turner, 1980; Agatstein and Buchanan, 1984; Nasby, 1985; Hull et al., 1988). In order to explore this possibility, we conducted we a repeated measures analysis of covariance (ANCOVA). The within-subject factors included the main effects for the incidental memory for self and Lady Gaga items (corrected for guessing). The different types of self-consciousness scores were included as between participant covariates. This analysis revealed a significant interaction between the incidental memory for the two referents and the private self-consciousness scale (F (1,116) = 5.041, P < 0.05). Post-hoc analyses demonstrated a significant positive correlation between the magnitude of the self-reference effect and private self-consciousness (r = 0.19, P < 0.035) (Figure 1B). Based on previous research revealing that familiarity has a significant influence on memory, familiarity ratings for Lady Gaga were obtained at the end of the experiment. A partial correlation controlling for the Lady Gaga familiarity ratings still showed a positive and significant correlation between the private self-consciousness scores and the magnitude of the self-referent effect both controlling for false alarms (r = 0.18, P = 0.030) and without controlling for false alarms (r = 0.19, P = 0.035). Together these analyses allowed us to establish in our sample that private self-consciousness is linked to a stronger memory for information referred to the self.
Resting state fMRI
Next, we explored whether self-consciousness is reflected in the brain’s intrinsic connectivity by performing a seed based analyses on the DMN, FPCN and limbic networks defined by the 7 network parcellation from Yeo and colleagues (Yeo et al., 2011). We calculated the correlation between the time series for each of these networks and each voxel in the rest of the brain for each individual. The FC group maps obtained for each network can be visualised in Figure 3. Next, we used these spatial maps as the dependent variable in a series of multiple regressions using the standardised residuals of each component of the SCS as explanatory variables. Correction for multiple comparisons included a whole brain correction, correction for two-tailed tests and correction for the number of seeded locations (3), yielding an alpha value of P = 0.008 FWE.
Fig. 3.
Functional connectivity (FC) group maps. (A) Default Mode network (DMN), (B) frontoparietal control network. (C) Limbic Network. Red and blue colors represent positive and negative functional connectivity, respectively.
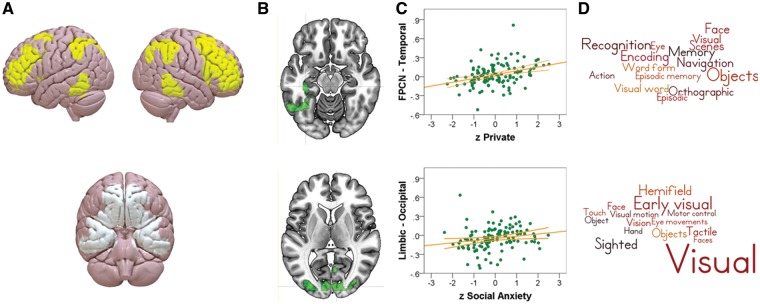
We found two patterns of FC that varied with different types of self-consciousness. The FPCN revealed a pattern of stronger FC between this network and a cluster with a peak in the temporal occipital fusiform cortex that extended into the hippocampus with greater levels of private self-consciousness (Figure 4, top row). The unthresholded map for this contrast can be found at Neurovault at the following link: http://neurovault.org/images/39599/. Possibly due to the fusiform nature of the cluster, meta-analyitic decoding using Neurosynth revealed terms such as ‘objects’, however it also revealed terms such as ‘episodic’, ‘recognition’ and ‘episodic memory’ terms which are consistent with the hypothesised relationship between mnemonic processes and high levels of private self-consciousness. The limbic network revealed a pattern of FC to the occipital cortex predictive of social anxiety. In particular, stronger FC between these regions was predictive of higher social anxiety scores (Figure 4, bottom row). The unthresholded map for this contrast can be found at this link: http://neurovault.org/images/39600/ and meta-analytic decoding revealed this map to be related, among others, to the term ‘face’, which is in line with the social nature of this type of anxiety. Finally, analyses of the DMN did not reveal any patterns of FC predictive of either types of self-consciousness that passed correction for multiple comparisons, although all unthresholded maps are available at the following URL: http://neurovault.org/images/43237/ and http://neurovault.org/collections/2284/. To understand these patterns of data in greater detail we extracted the connectivity with the relevant networks and the region identified through our analyses and plotted these as scatterplots in each figure. The details of these clusters can be found in Table 1.
Fig. 4.
Association between seed regions and clusters predictive of self-consciousness. Top row: results for the Frontoparietal Control Network. Bottom row: Results for the Limbic Network. (A) Seed region. (B) Cluster with functional connectivity (FC) predictive of self-consciousness. (C) Scatterplot reflecting relationship between FC and self-consciousness. (D) Neurosynth’s meta-analytic decoding of cluster in B.
Table 1.
Regions that exhibit FC to seed dependent upon SCS
| Seed | Contrast | Cluster | Regions | Peak | #voxels | P-value |
|---|---|---|---|---|---|---|
| FPCN | Private Up | Temporal | posterior inferior temporal, temporal occipital fusiform, parahippocampal, hippocampus | −48,-52,-28 | 1085 | 0.006 |
| Limbic | Social Anxiety Up | Occipital | inferior lateral occipital, intracalcarine, lingual gyrus | −8,-90,10 | 1324 | 0.001 |
Note: Coordinates are based on the Montreal Neurological Institute coordinate system and regions are based on Harvard-Oxford Cortical Structural Atlas.
Moderation analysis
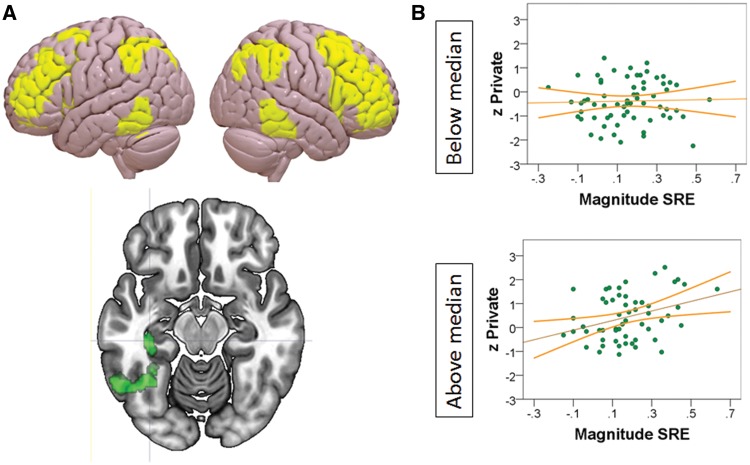
Having identified that private self-consciousness is linked to better memory for information related to the self and that it is also associated with patterns of functional organisation at rest, we next explored whether the expression of better self memory can be related to these patterns of functional organisation. In these analyses we used the correlation coefficients between the FPCN and the cluster in the temporal occipital fusiform cortex as a moderator of the relationship between private self-consciousness and the task outcomes. Moderation analyses using PROCESS (Hayes, 2013) revealed that the FC between the FPCN and the temporal occipital fusiform cluster moderated the relationship between private self-consciousness and the magnitude of the self-reference effect, ΔR2 = 0.037, F(1, 119) = 4.846.08, P < 0.05. This can be visualised in Figure 5 in which the data has been divided using a median split of the FC coefficients. Here it is apparent that the positive relationship found between private SCS and the magnitude of the self-reference effect is present in individuals with a high FC between the FPCN and the temporal occipital fusiform cortex (r = 0.334, P = 0.011) and absent in individuals situated below the median (r = 0.03, P = 0.812) (Figure 5B).
Fig. 5.
Moderation of the relationship between memory for self-items and private self-consciousness by the functional connectivity of the Frontoparietal Control Network (FPCN). (A) FPCN (red) and cluster with functional connectivity (FC) to the network predictive of private self-consciousness (green). (B) Median split of FC between FPCN and cluster. Top: Scatterplot reflecting the lack of relationship between memory for self-items and private self-consciousness in the below median group. Bottom: Scatterplot reflecting the positive correlation between memory for self-items and private self-consciousness in the above median group
Discussion
Our data suggest that the tendency towards private self-consciousness is characterised by a memory bias for self-relevant information that is rooted in the functional organisation of the brain at rest. We replicated prior studies showing that high levels of private self-consciousness are associated with a mnemonic advantage for self-relevant information (Turner, 1980; Agatstein and Buchanan, 1984; Nasby, 1985; Hull et al., 1988). Our FC analyses indicated that private self-consciousness was also associated with strong connectivity between the FPCN and regions of lateral occipital cortex, fusiform cortex and hippocampus, a pattern that meta-analytic decoding suggests is often associated with functions including episodic memory. Critically, our moderation analysis demonstrated that these two effects are related: we found that the relationship between private self-consciousness and a heightened memory for the self was only present in participants who exhibited this episodic neural fingerprint at rest. Taken together, our results suggest that patterns of neural organisation associated with the effective retrieval of episodic details may be central to the ability to consciously reflect on who we are as indexed by private self-consciousness. They also support functional studies linking executive regions to process of self-consciousness (Eisenberger et al., 2005) and when working memory is focused on more personally relevant information (Meyer et al., 2012). Our study also raises the question of whether these patterns exhibited by individuals high on private self-consciousness may also have a relationship to the thoughts experienced during the resting state, a question that could be addressed using experience sampling after resting state scans (Gorgolewski et al., 2014; Smallwood et al., 2016).
Unlike private self-consciousness, we found that social anxiety was related to heightened connectivity between the limbic network and regions of visual cortex. It is not surprising that the FC of the limbic system predicted social anxiety scores, given the well documented links between these regions and emotion (Strakowski et al., 1999; Cardinal et al., 2002; Davidson, 2002; Phelps and LeDoux, 2005; Phan et al., 2006). Moreover, our analysis suggests that social anxiety is linked to heightened connectivity between the limbic system and regions of occipital cortex, a pattern that may explain the hyper vigilance to social cues that are often associated with this form of self-consciousness (Eysenck, 1992; Mogg and Bradley, 1998). Thus unlike private self-consciousness, which was linked to heightened memory, our data is consistent with the view that social anxiety is linked to an attentional bias concerned with external attention, potentially to the reaction of other people to the public self (Bögels and Mansell, 2004; Mueller et al., 2009). It will be important in the future to determine whether the pattern of FC that we show supports social anxiety is a moderator for some of the attentional biases that this trait has been linked to in the past.
Our analysis did not link the DMN to any of the types of self-consciousness measured in our study. Our analytic strategy highlights differences between types of self-consciousness, so it is possible that the absence of any observed associations with the DMN may be because this network plays a role common to all three types of self-consciousness. Given evidence that the DMN is activated by states of self-focus (Northoff et al., 2006; Andrews‐Hanna et al., 2014) perhaps the absence of an association with this system reflects the fact that it is generally important in all states of self-consciousness, rather than in the expression of specific types. On the other hand, our observation that private self-consciousness is described by the connectivity of the FPCN supports accounts of states of self-focus which have linked self-biases to the function of regions of the control networks such as the inter-parietal sulcus (Sui et al., 2015; Humphreys and Sui, 2016). More broadly, our findings are consistent with theoretical positions that advocate a more complex component process architecture for states of higher-order cognition, such that different types of cognition emerge through the interaction of multiple different large scale networks (Smallwood and Schooler, 2015; Moscovitch et al., 2016). For example, an emerging literature has begun to show that the DMN is important in many situations beyond those linked to internal focus, such as working memory (Konishi et al., 2015; Vatansever et al., 2015), social memory (Meyer et al., 2012), or demanding semantic task performance (Krieger-Redwood et al., 2016), observations that are consistent with the notion that the DMN acts to integrate information from across the cortex (Margulies et al., 2016). For example, research exploring autobiographical planning has shown co-activation in the FPCN and medial temporal lobe structures (e.g. the hippocampus) when we consider future goals (Gerlach et al., 2011), particularly those that are high on episodic detail (Spreng et al., 2010). It is possible, for example, that trait levels of private self-consciousness may relate to particular aspects of mental life characterised by simulations of the future that contain high levels of detail, a perspective that is supported by studies that have shown priming self-relevant information increases an individual’s tendency to consider events in the future (Smallwood et al., 2011). This is an important question for future research to address.
One limitation of our study is that we focused on a relatively coarse description of neural function that is characterized by a neural parcellation that divides the cortical landscape into seven large-scale networks. Recent accounts of the DMN suggest that it can be subdivided into different sub-networks (e.g. Andrews-Hanna et al., 2010; Yeo et al., 2011). One of these sub-divisions, known as the medial-temporal subsystem, encompasses regions of posterior parietal cortex, but critically aspects of the medial temporal lobe. It is therefore possible that a more fine-grained analysis of the relationship between DMN sub-networks at rest and different aspects of self-consciousness would have revealed a role for one of these subsystems. Although our coarse analysis revealed patterns of neural activity that described two out of three forms of self-consciousness it remains an open question whether looking at the behaviour of subsystems of the DMN at rest, or during tasks, may reveal a role for aspects of this large scale network in trait differences in self-consciousness. Not withstanding this limitation, our current study suggests that trait variation in private self-consciousness is related to the FC of the FPCN, and in particular to its communication with regions involved in episodic memory retrieval. This pattern of FC moderates the association between private self-consciousness and a heightened memory for self-relevant information, identified by prior investigations. Together these findings suggest that a greater capacity for the retrieval of self relevant information may explain important aspects of the processes through which we become the subject of our own conscious evaluations.
Funding
EJ was supported by a grant from the European Research Council (SEMBIND – 283530) and JS was supported by European Research Council (WANDERINGMINDS – 646927) and from the Volkswagen Foundation (Wandering Minds - 89440 and 89439). This publication was also made possible through the support of a grant from the John Templeton Foundation, ‘Prospective Psychology Stage 2: A Research Competition’ to Martin Seligman. The opinions expressed in this publication are those of the author(s) and do not necessarily reflect the views of the John Templeton Foundation.
Conflict of interest. None declared.
References
- Agatstein F.C., Buchanan D.B. (1984). Public and private self-consciousness and the recall of self-relevant information. Personality and Social Psychology Bulletin, 10,314–25. [Google Scholar]
- Anderson N.H. (1968). Likableness ratings of 555 personality-trait words. Journal of Personality and Social Psychology, 9, 272.. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Reidler J.S., Sepulcre J., Poulin R., Buckner R.L. (2010). Functional-anatomic fractionation of the brain's default network. Neuron, 65(4), 550–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna J.R., Smallwood J., Spreng R.N. (2014). The default network and self‐generated thought: component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences, 1316, 29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage, 37, 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertenthal B.I., Fischer K.W. (1978). Development of self-recognition in the infant. Developmental Psychology, 14, 44. [Google Scholar]
- Bögels S.M., Mansell W. (2004). Attention processes in the maintenance and treatment of social phobia: hypervigilance, avoidance and self-focused attention. Clinical Psychology Review, 24, 827–56. [DOI] [PubMed] [Google Scholar]
- Brédart S., Delchambre M., Laureys S. (2006). Short article one's own face is hard to ignore. Quarterly Journal of Experimental Psychology, 59, 46–52. [DOI] [PubMed] [Google Scholar]
- Cardinal R.N., Parkinson J.A., Hall J., Everitt B.J. (2002). Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neuroscience & Biobehavioral Reviews, 26, 321–52. [DOI] [PubMed] [Google Scholar]
- Carver C.S., Glass D.C. (1976). The self-consciousness scale: A discriminant validity study. Journal of Personality Assessment, 40, 169–72. [DOI] [PubMed] [Google Scholar]
- Coull J.T., Frith C.D., Frackowiak R.S.J., Grasby P.M. (1996). A fronto-parietal network for rapid visual information processing: a PET study of sustained attention and working memory. Neuropsychologia, 34(11), 1085–95. [DOI] [PubMed] [Google Scholar]
- Crozier W. R. (Ed.). (2002). Shyness: Development, Consolidation and Change. Routledge. [Google Scholar]
- Cunningham S.J., Brady-Van den Bos M., Turk D.J. (2011). Exploring the effects of ownership and choice on self-memory biases. Memory, 19, 449–61. [DOI] [PubMed] [Google Scholar]
- Davidson R.J. (2002). Anxiety and affective style: role of prefrontal cortex and amygdala. Biological Psychiatry, 51, 68–80. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I., Lieberman M.D., Satpute A.B. (2005). Personality from a controlled processing perspective: an fMRI study of neuroticism, extraversion, and self-consciousness. Cognitive, Affective, & Behavioral Neuroscience, 5(2), 169–81. [DOI] [PubMed] [Google Scholar]
- Eysenck M.W. (1992). Anxiety: The Cognitive Perspective. Hillsdale, NJ: Lawrence Erlbaum Associates Inc. [Google Scholar]
- Fox K.C., Spreng R.N., Ellamil M., Andrews-Hanna J.R., Christoff K. (2015). The wandering brain: Meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. Neuroimage, 111, 611–21. [DOI] [PubMed] [Google Scholar]
- Gendolla G.H. (2000). On the impact of mood on behavior: An integrative theory and a review. Review of General Psychology, 4, 378. [Google Scholar]
- Gerlach, K.D., Spreng, R.N., Gilmore, A.W., Schacter, D.L. (2011). Solving future problems: default network and executive activity associated with goal-directed mental simulations. Neuroimage, 55(4), 1816–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, A.M., Franklin, J., Langford, P. (2002). The self-reflection and insight scale: A new measure of private self-consciousness. Social Behavior and Personality: an international journal, 30(8), 821–35. [Google Scholar]
- Gorgolewski K.J., Lurie D., Urchs S., et al. (2014). A correspondence between individual differences in the brain's intrinsic functional architecture and the content and form of self-generated thoughts. PloS One, 9, e97176.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Krasnow B., Reiss A.L., Menon V. (2003). Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences, 100, 253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C.R., Pashler H. (2004). Attention and the processing of emotional words and names not so special after all. Psychological Science, 15, 171–8. [DOI] [PubMed] [Google Scholar]
- Hayes A.F. (2013). Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. Guilford Press. [Google Scholar]
- Hull J.G., Van Treuren R.R., Ashford S.J., Propsom P., Andrus B.W. (1988). Self-consciousness and the processing of self-relevant information. Journal of Personality and Social Psychology, 54, 452. [Google Scholar]
- Humphreys G.W., Sui J. (2015). The salient self: Social saliency effects based on self-bias. Journal of Cognitive Psychology, 27, 129–40. [Google Scholar]
- Humphreys, G.W., Sui, J. (2016). Attentional control and the self: the Self-Attention Network (SAN). Cognitive neuroscience, 7(1–4), 5–17. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Smith S. (2001). A global optimisation method for robust affine registration of brain images. Medical Image Analysis, 5, 143–56. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage, 17, 825–41. [DOI] [PubMed] [Google Scholar]
- Kelley W.M., Macrae C.N., Wyland C.L., Caglar S., Inati S., Heatherton T.F. (2002). Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience, 14, 785–94. 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Koechlin, E., Basso, G., Pietrini, P., Panzer, S., Grafman, J. (1999). The role of the anterior prefrontal cortex in human cognition. Nature, 399(6732), 148–51. [DOI] [PubMed] [Google Scholar]
- Konishi M., McLaren D.G., Engen H., Smallwood J. (2015). Shaped by the past: the default mode network supports cognition that is independent of immediate perceptual input. PloS One, 10, e0132209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger-Redwood K., Jefferies E., Karapanagiotidis T., et al. (2016). Down but not out in posterior cingulate cortex: Deactivation yet functional coupling with prefrontal cortex during demanding semantic cognition. Neuroimage, 141, 366–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae C.N., Moran J.M., Heatherton T.F., Banfield J.F., Kelley W.M. (2004). Medial prefrontal activity predicts memory for self. Cerebral Cortex, 14, 647–54. 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- Margulies D.S., Ghosh S.S., Goulas A., et al. (2016). Situating the default-mode network along a principal gradient of macroscale cortical organization. Proceedings of the National Academy of Sciences, 113, 12574–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay J.C., Kane M.J. (2009). Conducting the train of thought: working memory capacity, goal neglect, and mind wandering in an executive-control task. Journal of Experimental Psychology: Learning, Memory, and Cognition, 35, 196.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medea B., Karapanagiotidis T., Konishi M., et al. (2016). How do we decide what to do? Resting-state connectivity patterns and components of self-generated thought linked to the development of more concrete personal goals. Experimental Brain Research, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, M.L., Spunt, R.P., Berkman, E.T., Taylor, S.E., Lieberman, M.D. (2012). Evidence for social working memory from a parametric functional MRI study. Proceedings of the National Academy of Sciences, 109(6), 1883–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg K., Bradley B.P. (1998). A cognitive-motivational analysis of anxiety. Behaviour Research and Therapy, 36, 809–48. [DOI] [PubMed] [Google Scholar]
- Moran J.M., Macrae C.N., Heatherton T.F., Wyland C.L., Kelley W.M. (2006). Neuroanatomical evidence for distinct cognitive and affective components of self. Journal of Cognitive Neuroscience, 18, 1586–94. [DOI] [PubMed] [Google Scholar]
- Moscovitch M., Cabeza R., Winocur G., Nadel L. (2016). Episodic memory and beyond: the hippocampus and neocortex in transformation. Annual Review of Psychology, 67, 105–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller E.M., Hofmann S.G., Santesso D.L., Meuret A.E., Bitran S., Pizzagalli D.A. (2009). Electrophysiological evidence of attentional biases in social anxiety disorder. Psychological Medicine, 39, 1141–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K., Birn R.M., Handwerker D.A., Jones T.B., Bandettini P.A. (2009). The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage, 44, 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasby W. (1985). Private self-consciousness articulation of the self-schema, and recognition memory of trait adjectives. Journal of Personality and Social Psychology, 49, 704. [Google Scholar]
- Northoff G., Heinzel A., De Greck M., Bermpohl F., Dobrowolny H., Panksepp J. (2006). Self-referential processing in our brain—a meta-analysis of imaging studies on the self. Neuroimage, 31, 440–57. [DOI] [PubMed] [Google Scholar]
- Lewis M., Brooks J. (1978). Self-knowledge and emotional development In The Development of Affect (pp. 205–226). Springer; US. [Google Scholar]
- Lipka R. P., Brinthaupt T. M. (Eds.). (1992). Self-Perspectives across the Life Span. (pp 12) SUNY Press. [Google Scholar]
- Phan K.L., Fitzgerald D.A., Nathan P.J., Tancer M.E. (2006). Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biological Psychiatry, 59, 424–9. [DOI] [PubMed] [Google Scholar]
- Phelps E.A., LeDoux J.E. (2005). Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron, 48, 175–87. [DOI] [PubMed] [Google Scholar]
- Poerio G.L., Smallwood J. (2016). Daydreaming to navigate the social world: What we know, what we don't know, and why it matters. Social and Personality Psychology Compass, 10, 605–18. [Google Scholar]
- Poerio G.L., Totterdell P., Miles E. (2013). Mind-wandering and negative mood: Does one thing really lead to another? Consciousness and Cognition, 22, 1412–21. [DOI] [PubMed] [Google Scholar]
- Power, J.D., Barnes, K.A., Snyder, A.Z., Schlaggar, B.L., Petersen, S.E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage, 59(3), 2142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice D.A. (1990). Familiarity and differences in self-and other-representations. Journal of Personality and Social Psychology, 59, 369. [DOI] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98, 676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T.B., Kuiper N.A., Kirker W.S. (1977). Self-reference and the encoding of personal information. Journal of Personality and Social Psychology, 35, 677.. [DOI] [PubMed] [Google Scholar]
- Rottschy C., Langner R., Dogan I.. et al. (2012). Modelling neural correlates of working memory: a coordinate-based meta-analysis. Neuroimage, 60(1), 830–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rytty R., Nikkinen J., Paavola L.. et al. (2013). GroupICA dual regression analysis of resting state networks in a behavioral variant of frontotemporal dementia. Frontiers in Human Neuroscience, 26(7), 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheier M.F., Carver C.S. (1985). The self‐consciousness scale: a revised version for use with general populations. Journal of Applied Social Psychology, 15, 687–99. [Google Scholar]
- Scheier M.F., Carver C.S. (2013). Self-Consciousness Scale–(SCS-R). Measurement Instrument Database for the Social Science.
- Sedikides C. (1992). Mood as a determinant of attentional focus. Cognition & Emotion, 6, 129–48. [Google Scholar]
- Smallwood J., Fitzgerald A., Miles L.K., Phillips L.H. (2009). Shifting moods, wandering minds: negative moods lead the mind to wander. Emotion, 9, 271.. [DOI] [PubMed] [Google Scholar]
- Smallwood J., Schooler J.W. (2015). The science of mind wandering: empirically navigating the stream of consciousness. Annual Review of Psychology, 66, 487–518. [DOI] [PubMed] [Google Scholar]
- Smallwood J., Schooler J.W., Turk D.J. (2011). Self-reflection and the temporal focus of the wandering mind. Consciousness and Cognition, 20(4), 1120–6. [DOI] [PubMed] [Google Scholar]
- Smallwood J., Karapanagiotidis T., Ruby F.. et al. (2016). Representing representation: Integration between the temporal lobe and the posterior cingulate influences the content and form of spontaneous thought. PLoS One, 11(4), e0152272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood, J., Ruby, F.J., Singer, T. (2013). Letting go of the present: mind-wandering is associated with reduced delay discounting. Consciousness and cognition, 22(1), 1–7. [DOI] [PubMed] [Google Scholar]
- Smith S.M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17, 143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.V., Utevsky A.V., Bland A.R.. et al. (2014). Characterizing individual differences in functional connectivity using dual-regression and seed-based approaches. NeuroImage, 95, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormaz M., Jefferies E., Bernhardt B.C.. et al. (2017). Knowing what from where: Hippocampal connectivity with temporoparietal cortex at rest is linked to individual differences in semantic and topographic memory. Neuroimage, 152, 400–10. [DOI] [PubMed] [Google Scholar]
- Spreng R.N., Stevens W.D., Chamberlain J.P., Gilmore A.W., Schacter D.L. (2010). Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage, 53, 303–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawarczyk D., D'Argembeau A. (2015). Neural correlates of personal goal pocessing during episodic future thinking and mind‐wandering: An ALE meta‐analysis. Human Brain Mapping, 36, 2928–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski S.M., DelBello M.P., Sax K.W., et al. (1999). Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Archives of General Psychiatry, 56, 254–60. [DOI] [PubMed] [Google Scholar]
- Sui J., Liu M., Mevorach C., Humphreys G.W. (2015). The salient self: the left intraparietal sulcus responds to social as well as perceptual-salience after self-association. Cerebral Cortex, 25, 1060–8. [DOI] [PubMed] [Google Scholar]
- Symons C.S., Johnson B.T. (1997). The self-reference effect in memory: a meta-analysis. Psychological Bulletin, 121, 371.. [DOI] [PubMed] [Google Scholar]
- Turner R.G. (1978). Effects of differential request procedures and self-consciousness on trait attributions. Journal of Research in Personality, 12, 431–8. [Google Scholar]
- Turner R.G. (1980). Self-consciousness and memory of trait terms. Personality and Social Psychology Bulletin, 6, 273–7. [Google Scholar]
- Vatansever D., Menon D.K., Manktelow A.E., Sahakian B.J., Stamatakis E.A. (2015). Default mode dynamics for global functional integration. Journal of Neuroscience, 35, 15254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman D.H., Roberts K.C., Visscher K.M., Woldorff M.G. (2006). The neural bases of momentary lapses in attention. Nature Neuroscience, 9, 971–8. [DOI] [PubMed] [Google Scholar]
- Worsley K.J. (2001). Functional MRI: An Introduction to Methods 14, 251–70. Statistical analysis of activation images. [Google Scholar]
- Yarkoni, T., Poldrack, R.A., Nichols, T.E., Van Essen, D.C., Wager, T.D. (2011). Large-scale automated synthesis of human functional neuroimaging data. Nature methods, 8(8), 665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo B.T., Krienen F.M., Sepulcre J., et al. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106, 1125–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X.N., Kelly C., Adelstein J.S., Klein D.F., Castellanos F.X., Milham M.P. (2010). Reliable intrinsic connectivity networks: testretest evaluation using ICA and dual regression approach. Neuroimage, 49(3), 2163–77. [DOI] [PMC free article] [PubMed] [Google Scholar]