Abstract
Background
Carbapenem-resistant (CR) Gram-negative pathogens are recognized as a major health concern. This study examined the prevalence of infections due to 4 CR Gram-negative species (Acinetobacter baumannii, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Escherichia coli) in the United States and assessed their impact on hospital stays and mortality.
Methods
Hospitalized patients with laboratory-confirmed infection due to any of the 4 Gram-negative pathogens were identified from the Premier Healthcare Database. Proportions of CR were calculated by pathogen and infection site (blood, respiratory, urinary, or other) for the United States as whole and by census regions. Crude and adjusted odds ratios for in-hospital mortality were produced using logistic regression.
Results
From 2009 to 2013, 13 262 (4.5%) of 292 742 infections due to these 4 Gram-negative pathogens were CR. Of these CR infections, 82.3% were caused by A. baumannii (22%) or P. aeruginosa (60.3%), while 17.7% were caused by K. pneumoniae or E. coli. CR patients had longer hospital stays than carbapenem-susceptible (CS) patients in all pathogen-infection site cohorts, except in the A. baumannii-respiratory cohort. The crude all cause in-hospital mortality was greater for most pathogen-infection site cohorts of the CR group compared with the CS group, especially for A. baumannii infection in the blood (crude odds ratio [OR], 3.91; 95% confidence interval [CI], 2.69–5.70). This difference for the A. baumannii-blood cohort remained after adjusting for the relevant covariates (adjusted OR, 2.46; 95% CI, 1.43–4.22).
Conclusion
The majority of CR infections and disease burden in the United States was caused by nonfermenters A. baumannii and P. aeruginosa. Patients with CR infections had longer hospital stays and higher crude in-hospital mortality.
Keywords: carbapenem resistance, Gram-negative, nonfermenters, CRE, length of stay, in-hospital mortality
Infections due to carbapenem-resistant (CR) Gram-negative pathogens have been reported from many countries with variable prevalence and associated morbidity and mortality [1–4]. The dramatic increase and spread of CR infections over the past decade are recognized as a major public health concern [2, 5–9].
Recent infection control measures have focused on the identification and spread of CR Enterobacteriaceae (CREs), which is mainly due to mobile carbapenemase enzymes, both serine (KPC, GES, and OXA) and metallo-carbapenemases (NDM, VIM, and IMP) [6, 10]. Resistance mechanisms involving porin channel mutations and efflux pump overproduction may also contribute to the identification of CREs [6, 10, 11]. The frequency of carbapenem resistance among the non-glucose fermenting Pseudomonas aeruginosa and Acinetobacter baumannii has also increased in recent years, but it was not well appreciated as a source of resistance transmission until 2017 [7, 12–16], when the World Health Organization recognized the importance of CR A. baumannii and P. aeruginosa as equal to that of the CREs [17]. These CR nonfermenters are often multidrug resistant and associated with substantial morbidity and mortality [18, 19]. This study was designed to determine the prevalence of CR organisms (CROs) in the United States, specifically the Enterobacteriaceae Escherichia coli and Klebsiella pneumoniae and the nonfermenters Pseudomonas aeruginosa and Acinetobacter baumannii, using an electronic database from a geographically diverse network of US hospitals. Besides determining the prevalence of CR infections in the United States, the study compared similar infections that were carbapenem susceptible (CS) in order to assess the mortality attributed to the CROs.
METHODS
This retrospective study of CR Gram-negative infections between 2009 and 2013 was conducted using microbiology data linked with patient-level in-hospital discharge data from the Premier Healthcare Database [20]. The Premier Healthcare Database is an anonymous census of inpatients and hospital-clinic outpatients from geographically diverse, mixed teaching and nonteaching hospitals with varied bed sizes in the United States. The hospital discharge data contain information on admission, discharge, and a date-stamped log of all billed items, including procedures, medications, laboratory, diagnostic, and therapeutic services at the individual patient level.
Microbiology data, collected from approximately 30% of participating hospitals, include the time the microbiological culture was obtained, type of pathogen identified, and antimicrobial drugs used for susceptibility testing along with the method, result, and interpretation of antibiotic susceptibility. All data were determined by individual hospital laboratories, which were Clinical Laboratory Improvement Amendments (CLIA) compliant using standard laboratory methods, and interpretations of susceptibility results were reported as resistant (R), intermediate (I), susceptible (S), or none (N).
The pathogens were further identified by infection sites (blood, respiratory, urinary, or other) based on the source of the culture sample. Thus, this study had 16 distinct pathogen-infection site cohorts. Within each cohort, pathogens were characterized as CR or CS. Bacteria were defined as CR if they were resistant to at least 1 of the carbapenems tested and as CS if the pathogen was susceptible to or intermediate for the carbapenems tested. If selected patients had multiple hospitalizations during the study period, only data from the first hospitalization were used. If a microbiologic culture identified multiple pathogens, the patient was counted in each pathogen group. If a patient had multiple cultures of the same pathogen, the first culture was used as index date if the pathogen had the same susceptibility test results for all cultures. Otherwise, the first CR culture was used as the index date, and the patient was in the CR group. Only cultured pathogens from patients who were receiving antibiotic treatment were included in the final data set.
The primary outcome variables were the rates of CR in each pathogen-infection site cohort for all hospitals and separately for each US census region. Other outcome variables included in-hospital mortality, total length of stay (LOS) in the hospital, LOS after positive culture (iLOS), total LOS in the intensive care unit (ICU) for the CR group and CS group, and survival status within each pathogen-infection site cohort.
The use of antibiotics with Gram-negative activity was assessed by class (aminoglycoside, carbapenem, cephalosporin, colistin, monobactam, penicillin, polymyxin B, quinolone, tetracycline, and other Gram-negative antibiotics) and the time when the patient received them during the hospitalization. Antibiotics administered in the days prior to the date of the index culture were considered “prior” antibiotics, those received from the date of the index culture through 3 days after the index culture were considered “empiric,” and those received after 3 days from the index culture were considered “definitive” treatment.
Study Population
All hospitalized patients with microbiological cultures identifying A. baumannii, P. aeruginosa, K. pneumoniae, or E. coli from appropriate clinical specimens and with associated interpretations of susceptibility to carbapenem antibiotics (doripenem, ertapenem, imipenem, meropenem) from patients who received at least 1 systemic antibiotic treatment were selected.
Statistical Analyses
Categorical variables were described with the number of patients and percentages and compared between CR and CS in each pathogen-infection site cohort using a chi-square or Fisher’s exact test where appropriate. Continuous variables were described with mean, standard deviation, and median. The differences between CR and CS groups were analyzed using Student’s t test or the Wilcoxon rank-sum test. A P-value <.05 indicated statistically significant differences between groups.
In-hospital mortality was compared between CR and CS for each pathogen infection site cohort with and without adjusting for covariates, such as age, gender, race, ethnicity, mechanical ventilation, renal impairment, geographic region, and each of the Charlson comorbid conditions [21].
All analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, North Carolina).
RESULTS
This study includes data collected from 206 acute care hospitals in the United States between January 1, 2009, and December 31, 2013. The total numbers of pathogen isolates were 173 200 E. coli, 56 552 K. pneumoniae, 56 477 P. aeruginosa, and 6508 A. baumannii.
Carbapenem Resistance Rate
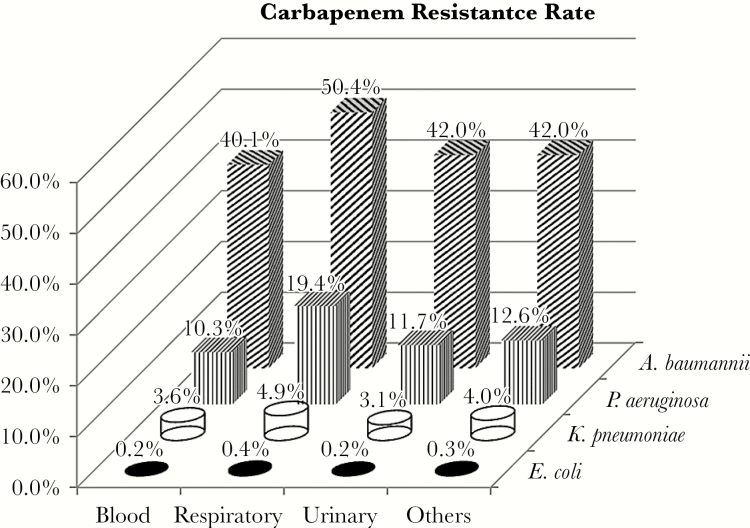
The overall CR rate among the 4 selected pathogens was 4.5% (13 262 of 292 742). A. baumannii had the highest CR rate: blood 40.1%, respiratory 50.4%, urine 42.0%, and other 42%. The CR rate for P. aeruginosa ranked second highest and was more variable by infection site: 10.3% in blood, 19.4% in respiratory, 11.7% in urinary, and 12.6% in other (Figure 1). More than 80% of all CR infections were caused by A. baumannii or P. aeruginosa. This was consistent across 9 US census regions, except for the Middle Atlantic region, where 67% (Table 1) of CR infections were caused by the nonfermenters. For most regions, CR P. aeruginosa outnumbered CR A. baumannii by 3:1, except for the New England region, where 85% of CR infections were due to P. aeruginosa and only 6.4% were from A. baumannii (Table 2).
Figure 1.
Carbapenem resistance rate by pathogen-infection site cohort.
Table 1.
Carbapenem-resistant infections caused by A. baumannii, P. aeruginosa, K. pneumoniae, and E. coli by US census region
| US Census Regions | Number of Hospitals | All 4 Pathogens | A. baumannii + P. aeruginosa | K. pneumoniae + E. coli | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Infections | CR Cases | Total Infections | CR Cases | Total Infections | CR Cases | |||||||
| Total CR Cases | % of Total Infections | CR Cases | % of Total Infections | % of Total CR Cases for 4 Pathogens | CR Cases | % of Total Infections | % of Total CR Cases for 4 Pathogens | |||||
| East North Central | 43 | 47 702 | 3164 | 6.6 | 10 381 | 2542 | 24.5 | 80.3 | 37 321 | 622 | 1.7 | 19.7 |
| East South Central | 9 | 21 440 | 770 | 3.6 | 4293 | 703 | 16.4 | 91.3 | 17 147 | 67 | 0.4 | 8.7 |
| Middle Atlantic | 19 | 37 582 | 2499 | 6.6 | 10 449 | 1672 | 16.0 | 66.9 | 27 133 | 827 | 3.0 | 33.1 |
| Mountain | 15 | 8582 | 765 | 8.9 | 2260 | 710 | 31.4 | 92.8 | 6322 | 55 | 0.9 | 7.2 |
| New England | 9 | 11 964 | 346 | 2.9 | 2417 | 316 | 13.1 | 91.3 | 9547 | 30 | 0.3 | 8.7 |
| Pacific | 27 | 39 045 | 1237 | 3.2 | 6447 | 1070 | 16.6 | 86.5 | 32 598 | 167 | 0.5 | 13.5 |
| South Atlantic | 40 | 62 217 | 2385 | 3.8 | 14 652 | 2017 | 13.8 | 84.6 | 47 565 | 368 | 0.8 | 15.4 |
| West North Central | 18 | 17 854 | 403 | 2.3 | 3130 | 329 | 10.5 | 81.6 | 14 724 | 74 | 0.5 | 18.4 |
| West South Central | 26 | 46 356 | 1693 | 3.7 | 8955 | 1550 | 17.3 | 91.6 | 37 401 | 143 | 0.4 | 8.4 |
| Total | 206 | 292 742 | 13 262 | 4.5 | 62984 | 10 909 | 17.3 | 82.3 | 229 758 | 2353 | 1.0 | 17.7 |
Abbreviations: CR, carbapenem resistant.
Table 2.
A. baumannii and P. aeruginosa CR Infection by Region
| Regions | Number of Hospitals | All 4 Pathogens | Acinetobacter baumannii | Pseudomonas aeruginosa | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Infections | CR Cases | Total Infections | CR Cases | Total Infections | CR Cases | |||||||
| Cases | % of Total Infectionsa | Cases | % of Total Infections | % of Total CR Cases for 4 Pathogens | Cases | % of Total Infections | % of Total CR Cases for 4 Pathogens | |||||
| East North Central | 43 | 47 702 | 3164 | 6.6 | 1360 | 863 | 63.5 | 27.3 | 9021 | 1679 | 18.6 | 53.1 |
| East South Central | 9 | 21 440 | 770 | 3.6 | 498 | 223 | 44.8 | 29.0 | 3795 | 480 | 12.6 | 62.3 |
| Middle Atlantic | 19 | 37 582 | 2499 | 6.6 | 914 | 288 | 31.5 | 11.5 | 9535 | 1384 | 14.5 | 55.4 |
| Mountain | 15 | 8582 | 765 | 8.9 | 326 | 203 | 62.3 | 26.5 | 1934 | 507 | 26.2 | 66.3 |
| New England | 9 | 11 964 | 346 | 2.9 | 183 | 22 | 12.0 | 6.4 | 2234 | 294 | 13.2 | 85.0 |
| Pacific | 27 | 39 045 | 1237 | 3.2 | 739 | 327 | 44.2 | 26.4 | 5708 | 743 | 13.0 | 60.1 |
| South Atlantic | 40 | 62 217 | 2385 | 3.8 | 1322 | 488 | 36.9 | 20.5 | 13 330 | 1529 | 11.5 | 64.1 |
| West North Central | 18 | 17 854 | 403 | 2.3 | 256 | 77 | 30.1 | 19.1 | 2874 | 252 | 8.8 | 62.5 |
| West South Central | 26 | 46 356 | 1693 | 3.7 | 909 | 424 | 46.6 | 25.0 | 8046 | 1126 | 14.0 | 66.5 |
| Total | 206 | 292 742 | 13262 | 4.5 | 6507 | 2915 | 44.8 | 22.0 | 56 477 | 7994 | 14.2 | 60.3 |
Abbreviation: CR, carbapenem resistant.
Demographic and Clinical Characteristics
Compared with patients with CS A. baumannii, patients with CR isolates tended to be older when the source was blood stream infection (mean±SD, 60.5 ± 16.6 years vs 53.6 ± 21.8 years), respiratory infection (mean±SD, 62.5 ± 16.8 vs 55.8 ± 23.5 years), or other infection sites (mean±SD, 60.7 ± 16.5 vs 57.0 ± 20.0 years), but of similar age when the source was urinary tract infection (mean±SD, 61.8 ± 17.5 vs 61.5 ± 19.8 years). More than two-thirds of patients with A. baumannii infections were white; however, the proportions of nonwhite patients in the CR groups were higher than in the CS groups for all infection sites. Slightly more male patients than female patients were identified in both the CR and CS groups. Nearly half of all patients with A. baumannii infections came to the hospitals from their homes, though the proportion was slightly higher in the CS group than in the CR group. Nearly 70% of these patients were admitted via emergency rooms. Patients with CR infections were more likely to be discharged to skilled nursing facilities or rehabilitation or long-term care facilities than patients with CS infections (43% vs 24% for blood, 51% vs 42% for respiratory, 58% vs 33% for urinary, and 51% vs 31% for other). Patients with CR infections also had a higher Charlson comorbidity index score for all infection sites, a longer total LOS for infections in the blood, urinary, and other (median days of CR vs CS, 15 vs 9 for blood, 12 vs 7 for urinary, and 12 vs 8 for other), and longer total ICU stays for blood, urinary, and other (median days of CR vs CS, 8 vs 5, 8 vs 4, and 9 vs 8, respectively). Similarly, a longer LOS after positive culture was seen in the CR group than the CS group among all patients and those who survived for each infection site except respiratory (Table 3). Among nonsurvivors with blood stream infection, the median LOS after positive culture was 2 days after the culture was obtained in the CR group and 4 days in the CS group. Patients with CR infections were slightly more likely to be on dialysis or have renal impairment during their hospital stays.
Table 3.
Median Days Stayed in the Hospital After Positive Culture by Pathogen, Infection Site, and Survival Status
| Pathogen | Length of Stay After Positive Culture | Blood | Respiratory | Urinary | Other | ||||
|---|---|---|---|---|---|---|---|---|---|
| CR | CS | CR | CS | CR | CS | CR | CS | ||
| Acinetobacter baumannii | iLOS | 8 | 6 | 8 | 10 | 8 | 4 | 7 | 5 |
| iLOS among survived | 11 | 6 | 9 | 10 | 8 | 4 | 7 | 5 | |
| iLOS among deceased | 2 | 4 | 6 | 7 | 6 | 6 | 7 | 7 | |
| Pseudomonas aeruginosa | iLOS | 5 | 4 | 20 | 7 | 5 | 3 | 8 | 5 |
| iLOS among survived | 6 | 4 | 20 | 8 | 5 | 3 | 6 | 5 | |
| iLOS among deceased | 3 | 2 | 18.5 | 4 | 7 | 4 | 33.5 | 6 | |
| Klebsiella pneumoniae | iLOS | 10 | 6 | 10 | 7 | 6 | 4 | 9 | 6 |
| iLOS among survived | 10 | 6 | 11 | 8 | 6 | 4 | 9 | 6 | |
| iLOS among deceased | 6 | 3 | 6 | 4 | 8 | 4 | 9 | 6 | |
| Escherichia coli | iLOS | 10 | 6 | 10 | 6 | 7 | 4 | 10 | 5 |
| iLOS among survived | 10 | 7 | 10 | 6 | 6 | 4 | 9 | 5 | |
| iLOS among deceased | 9 | 1 | 11 | 5 | 9 | 5 | 14 | 6 | |
Abbreviations: CR, carbapenem resistant; CS, carbapenem susceptible; iLOS, length of hospital stay after positive culture.
Similar patterns in admission sources, discharge status, hemodialysis, and renal impairment were observed for patients infected with CR P. aeruginosa, CR K. pneumoniae, and CR E. coli compared with their CS counterparts. However, patients with CR P. aeruginosa isolates were younger than patients with CS isolates for all infection sites (mean ± SD, 60.6 ± 17.3 vs 65.2 ± 19.1 for blood, 60.6 ± 19.1 vs 63.2 ± 20.3 for respiratory, 67.1 ± 17.1 vs 71.5 ± 17.1 for urinary, and 60.6 ± 18.5 vs 61.1 ± 20.2 for other), but they had similar Charlson comorbidity index scores. LOS, ICU LOS, and LOS after positive culture were longer for CR infections than CS infections for all infection sites.
The average age of patients with K. pneumoniae was approximately 60 years. There was no significant age difference between patients with CR and CS infections in blood, urine, or respiratory. In other infection sites, CR pathogen–infected patients were approximately 3 years older than those in the CS group.
There were more male patients than female patients in all study cohorts, except for those in the urinary cohort, where significantly higher numbers of female patients were identified. Due to the small number of patients in the CR-urinary cohort, there was no statistical significance in the observed differences in age (average of 60 years), race, or Charlson comorbidity index score.
In-Hospital Mortality
Patients with CR infections consistently had numerically higher crude mortality rates than patients with CS infections for each of the 16 pathogen-infection site cohorts. The crude odds ratio (OR) for CR vs CS ranged from 1.31 for E. coli in respiratory to 3.91 for A. baumannii in blood (Table 4). After adjusting for age, gender, race, ethnicity, Charlson comorbid conditions, mechanical ventilation, renal impairment, and geographic census regions, the odds of dying from CR A. baumannii in blood (adjusted OR, 2.46) and in respiratory (adjusted OR, 1.27) and from P. aeruginosa in other (adjusted OR, 1.20) remained statistically significant (Table 4).
Table 4.
In-Hospital Mortality: Crude and Adjusted Odds Ratio (CR vs CS)
| Pathogen | Site | In-Hospital Mortality by Each Site, No. (%) | Crude | Adjusteda | |||||
|---|---|---|---|---|---|---|---|---|---|
| CR | CS | Odds Ratio | Lower 95% CI | Upper 95% CI | Odds Ratio | Lower 95% CI | Upper 95% CI | ||
| Acinetobacter baumannii | Blood | 103 (38.2) | 55 (13.6) | 3.91 | 2.69 | 5.70 | 2.46 | 1.43 | 4.22 |
| Respiratory | 305 (26.0) | 230 (19.9) | 1.41 | 1.16 | 1.71 | 1.27 | 1.01 | 1.58 | |
| Urinary | 35 (9.6) | 31 (6.2) | 1.62 | 0.98 | 2.69 | 0.62 | 0.32 | 1.19 | |
| Other | 173 (15.6) | 117 (7.7) | 2.24 | 1.74 | 2.87 | 1.19 | 0.88 | 1.61 | |
| Pseudomonas aeruginosa | Blood | 112 (33.3) | 590 (20.1) | 2.02 | 1.58 | 2.58 | 1.19 | 0.88 | 1.63 |
| Respiratory | 661 (20.6) | 1977 (14.8) | 1.50 | 1.36 | 1.65 | 1.11 | 1.00 | 1.24 | |
| Urinary | 208 (9.8) | 1031 (6.3) | 1.58 | 1.35 | 1.85 | 1.05 | 0.88 | 1.26 | |
| Other | 389 (16.8) | 1139 (7.1) | 2.64 | 2.33 | 2.99 | 1.20 | 1.03 | 1.39 | |
| Klebsiella pneumoniae | Blood | 63 (27.3) | 819 (13.2) | 2.46 | 1.83 | 3.32 | 1.10 | 0.75 | 1.61 |
| Respiratory | 98 (25.4) | 1547 (20.6) | 1.32 | 1.04 | 1.67 | 0.97 | 0.76 | 1.25 | |
| Urinary | 90 (9.1) | 1616 (5.2) | 1.81 | 1.45 | 2.27 | 1.07 | 0.83 | 1.37 | |
| Other | 76 (18.1) | 863 (8.7) | 2.33 | 1.80 | 3.01 | 1.02 | 0.76 | 1.37 | |
| Escherichia coli | Blood | 5 (16.1) | 1709 (8.7) | 2.03 | 0.78 | 5.29 | 1.91 | 0.60 | 6.10 |
| Respiratory | 8 (26.7) | 1469 (21.7) | 1.31 | 0.58 | 2.95 | 0.74 | 0.29 | 1.85 | |
| Urinary | 13 (6.9) | 4699 (3.9) | 1.84 | 1.05 | 3.23 | 1.55 | 0.80 | 3.00 | |
| Other | 8 (10.1) | 1466 (5.8) | 1.84 | 0.89 | 3.84 | 0.78 | 0.33 | 1.84 | |
Abbreviations: CI, confidence interval; CR, carbapenem resistant; CS, carbapenem susceptible.
aAdjusted for age, gender, race, ethnicity, various comorbid conditions, mechanical ventilation, renal impairment, and geographic regions.
Patients with CR pathogens were more likely to receive more than 1 systemic antibiotic than patients with CS pathogens. Also, patients with pathogens identified from the blood, respiratory, and other were more likely to receive 2 or more antibiotics than if the source was urinary. The number and types of antibiotics used were highly variable. During the definitive treatment period, more than 100 combinations (defined as different antibiotic classes regardless of the initiation time, dose, or duration) were used for each pathogen, but fewer than 20 of these combinations were used by more than 3% of patients. Fewer different antibiotic combinations were used to treat patients with CS infections than patients with CR infections.
DISCUSSION
This study showed that in the United States the overall disease burden and impact on survival was greatest among the non-glucose fermenting A. baumannii and P. aeruginosa. A. baumannii had the highest rate of CR at each infection site, followed by P. aeruginosa and K. pneumoniae. E. coli consistently had the lowest rate of CR. The combined number of CR A. baumannii and CR P. aeruginosa infections accounted for more than 80% of all CR infections. Though this study included both community-onset and hospital-acquired infections, the pattern of CR from the selected 4 pathogens was comparable with the US Centers for Disease Control and Prevention’s (CDC’s) National Healthcare Safety Network (NHSN) results, which focused on health care–acquired infections via central lines, urinary catheters, and mechanical ventilators [9, 22]. The relative importance of CR A. baumannii has also been recently emphasized by the European Centre for Disease Prevention and Control (ECDC), which reported that CR A. baumannii (resistant through the production of carbapenemases) might be more widely disseminated in Europe than CREs [7, 12].
This study also showed that distribution of CR pathogens differed by infection sites. Respiratory was the most common site of CR infection for A. baumannii (40.3%) and P. aeruginosa (40.1%), while urinary was the most common site of CR infection for K. pneumoniae (48.7%) and E. coli (57.3%). Differences in the frequency of CR infection by infection site have also been reported in other studies [9, 23, 24].
The CR rates varied by the geographic regions. The Middle Atlantic region had the highest proportion of CR infections due to K. pneumoniae and E. coli (33.1%), while the New England region had a higher proportion of CR infections due to P. aeruginosa (85%). Other studies also observed the variations in frequency of CROs by hospitals and by region [22, 24, 25]. Although actual incidence cannot be estimated from this study, the regional differences observed in this study support the understanding that infection control of resistant pathogens needs to be based on local epidemiology.
As shown in this study, a majority of the CR pathogens were treated with multiple antibiotics, especially for A. baumannii and P. aeruginosa or for infections in the blood or respiratory infection sites. Even with combination treatment, patients with CR infections still had longer total hospital (total and after positive culture) and ICU stays, except among nonsurvivors with A. baumannii in blood and respiratory, where CR patients had shorter LOS after positive culture compared with CS patients and the median for CR in blood was 2 days after the culture was obtained. This further highlights the treatment challenges faced by clinicians today and the need for more effective antibiotics and more rapid information on antibiotic susceptibility.
A remarkably high number of different antibiotic combinations were used to treat these infections, especially for CR pathogens. This indicates that there is no generally accepted standard of care regimen for CR infections, which underlies the fact that there are few available antibiotics to treat CR infections, and those that may be available are associated with significant toxicity.
The authors believe that the key strength of this study is the large, geographically diverse data set that includes both detailed microbiologic data as well as patient-level information and outcomes. Unlike the CDC and other data sets that focus on hospital-acquired infections, the Premier Healthcare database includes both hospital-acquired and community-onset infections, many of which are healthcare related, thus better representing the full disease burden related to CR infections in US hospitals.
This study selected subjects based on microbiology results, not based on the discharge diagnosis, avoiding potential biases in the discharge diagnoses due to various reasons, including coding errors or reporting bias. The results of the microbiology and drug susceptibility testing, as well as the documented antibiotic treatments, provide a clear picture of the pattern of infections in acute care hospitals.
However, there are also several limitations in this study. First, case definitions were based on microbiological culture results that may underestimate or overestimate the number of infections. Although some culture results may represent colonization rather than true infection, this study only analyzed patients for whom the cultures had susceptibility testing performed and where their physician prescribed systemic antimicrobial therapy. In addition, focusing only on the 4 most common Gram-negative pathogens underestimates the total disease burden caused by CR infections.
Second, as microbiologic data were not collected from all hospitals in the Premier Network (teaching hospitals were more likely to provide microbiology data), there may be a reporting bias favoring a more complex patient population.
Due to the privacy concern by Premier, participating hospitals can only be identified by US census region. Therefore, it was not possible to estimate CR infection rates in smaller geographic areas, even though infections could vary from city to city and hospital to hospital. This study does identify regional differences in prevalence rates; however, it does not assess whether these findings are driven by individual institutions or episodic outbreaks within institutions.
This study only examined 2 Enterobacteriaceae, E. coli and K. pneumoniae, and 2 nonfermenters, A. baumannii and P. aeruginosa. Other nonfermenters, for example, Stenotrophomas maltophilia, which are highly carbapenem resistant [26, 27], may represent another significant source of disease caused by CR pathogens.
CONCLUSION
This study showed that CR non-glucose fermenting Gram-negative bacteria, such as A. baumannii and P. aeruginosa, contribute to the greater disease and mortality burden when compared with CREs, such as K. pneumoniae and E. coli. This has been observed for the study as whole and for each census region. These nonfermenters contributed more than 80% of CR cases in our analysis of data over a period of 5 years. CR rates differed from region to region, but the predominance of CR nonfermenters over CREs was consistent. The geographic variability was greater among K. pneumoniae and E. coli than the nonfermenters. However, because the authors cannot determine specific population numbers by hospital, the incidence of CR infections cannot be estimated or compared. These data for the United States are in sharp contrast to most reports from Europe and Latin America, where CREs are predominant.
Because patients with CR infections had longer hospital and ICU stays, more antibiotic treatments, and a higher mortality rate, the CR infections likely represent a higher cost burden to the health care system. The development of new antibiotics should not only address CREs, but more importantly, it should address CR non-fermenters such as P. aeruginosa and A. baumannii.
Acknowledgment
Potential conflicts of interest. Dr. Cai, Dr. Morgan, Dr. Arjona Ferreira, Ms. Ariyasu, Ms. Sawada, and Dr. Nagata are full-time employees of Shionogi. Dr. Echols reports personal fees from Shionogi, Inc., during the conduct of the study.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Kim YJ, Jun YH, Kim YR et al. . Risk factors for mortality in patients with Pseudomonas aeruginosa bacteremia; retrospective study of impact of combination antimicrobial therapy. BMC Infect Dis 2014; 14:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Magiorakos AP, Suetens C, Monnet DL et al. . The rise of carbapenem resistance in Europe: just the tip of the iceberg? Antimicrob Resist Infect Control 2013; 2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ny P, Nieberg P, Wong-Beringer A. Impact of carbapenem resistance on epidemiology and outcomes of nonbacteremic Klebsiella pneumoniae infections. Am J Infect Control 2015; 43:1076–80. [DOI] [PubMed] [Google Scholar]
- 4. Xu A, Zheng B, Xu YC et al. . National epidemiology of carbapenem-resistant and extensively drug-resistant Gram-negative bacteria isolated from blood samples in China in 2013. Clin Microbiol Infect 2016; 22(Suppl 1:S1–8. [DOI] [PubMed] [Google Scholar]
- 5. US Department of Health and Human Services, Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013 Available at: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013–508.pdf. Accessed January 10, 2017.
- 6. European Centre for Disease Prevention and Control. Evidence brief: update on the spread of carbapenemase-producing Enterobacteriaceae in Europe – summary of the May 2015 expert assessment Available at: http://ecdc.europa.eu/en/eaad/antibiotics-news/Documents/antimicrobial-resistance-EuSCAPE-Evidence-Brief.pdf. Accessed January 31, 2017.
- 7. European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2015 Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). Stockholm: ECDC; 2017. Available at: http://ecdc.europa.eu/en/publications/Publications/antimicrobial-resistance-europe-2015.pdf. Accessed January 31, 2017. [Google Scholar]
- 8. Sisto A, D’Ancona F, Meledandri M et al. . Carbapenem non-susceptible Klebsiella pneumoniae from Micronet network hospitals, Italy, 2009 to 2012. Euro Surveill 2012; 17: pii: 20247. [PubMed] [Google Scholar]
- 9. Sievert DM, Ricks P, Edwards JR et al. . Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data r eported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009-2010. Infect Control Hosp Epidemiol 2013; 34:1–14. [DOI] [PubMed] [Google Scholar]
- 10. US Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Disease, Division of Healthcare Quality Promotion. Facility guidance for control of carbapenem-resistant Enterobacteriaceae (CRE) – November 2015 update CRE Toolkit Available at: https://www.cdc.gov/hai/pdfs/cre/cre-guidance-508.pdf. Accessed January 10, 2017.
- 11. Livermore DM. Current epidemiology and growing resistance of gram-negative pathogens. Korean J Intern Med 2012; 27:128–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. European Centre for Disease Prevention and Control. Carbapenem-resistant Acinetobacter baumannii in healthcare settings – 8 December 2016. Stockholm: ECDC; 2016. Available at: http://ecdc.europa.eu/en/publications/Publications/8-Dec-2016-RRA-Acinetobacter%20baumannii-Europe.pdf. Accessed January 31, 2017. [Google Scholar]
- 13. Gniadek TJ, Carroll KC, Simner PJ. Carbapenem-resistant non-glucose-fermenting gram-negative bacilli: the missing piece to the puzzle. J Clin Microbiol 2016; 54:1700–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Labarca JA, Salles MJ, Seas C, Guzmán-Blanco M. Carbapenem resistance in Pseudomonas aeruginosa and Acinetobacter baumannii in the nosocomial setting in Latin America. Crit Rev Microbiol 2016; 42:276–92. [DOI] [PubMed] [Google Scholar]
- 15. Lesho E, Chukwuma U, Sparks M et al. . Anatomic, geographic, and taxon-specific relative risks of carbapenem resistance in the health care system of the U.S. Department of Defense. J Clin Microbiol 2016; 54:1546–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zilberberg MD, Kollef MH, Shorr AF. Secular trends in Acinetobacter baumannii resistance in respiratory and blood stream specimens in the United States, 2003 to 2012: a survey study. J Hosp Med 2016; 11:21–6. [DOI] [PubMed] [Google Scholar]
- 17. World Health Organization. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. Published February 27, 2017. Available at: http://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/. Accessed June 16, 2017.
- 18. Vincent JL, Rello J, Marshall J et al. . International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009; 302:2323–9. [DOI] [PubMed] [Google Scholar]
- 19. Micek ST, Wunderink RG, Kollef MH et al. . An international multicenter retrospective study of Pseudomonas aeruginosa nosocomial pneumonia: impact of multidrug resistance. Crit Care 2015; 19:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Premier Inc. Premier Healthcare Database: data that informs and performs Published January 31, 2017. Available at: https://learn.premierinc.com/pharmacy-and-research/premier-healthcare-database-whitepaper. Accessed June 16, 2017.
- 21. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 22. US Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Disease, Division of Healthcare Quality Promotion. Antibiotic Resistance Patient Safety Atlas summary of results. Available at: https://www.cdc.gov/hai/pdfs/patient-safety-atlas/ar-patient-safety-atlas-summary-of-results.pdf. Accessed January 10, 2017.
- 23. Huijskens EG, Rossen JW, Kluytmans JA et al. . Evaluation of yield of currently available diagnostics by sample type to optimize detection of respiratory pathogens in patients with a community-acquired pneumonia. Influenza Other Respir Viruses 2014; 8:243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kleinfauf N, Hausemann A, Kempf VAJ, Gottschalk R, Heudorf U. Burden of carpabenem-resistant organisms in the Frankfurt/Main Metropolitan Area in Germany 2012/2013 – first results and experiences after the introduction of legally mandated reporting. BMC Infect Dis 2014; 12:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Freeman R, Moore LS, Charlett A et al. . Exploring the epidemiology of carbapenem-resistant Gram-negative bacteria in west London and the utility of routinely collected hospital microbiology data. J Antimicrob Chemother 2015; 70:1212–8. [DOI] [PubMed] [Google Scholar]
- 26. Brooke JS. New strategies against Stenotrophomonas maltophilia: a serious worldwide intrinsically drug-resistant opportunistic pathogen. Expert Rev Anti Infect Ther 2014; 12:1–4. [DOI] [PubMed] [Google Scholar]
- 27. Sánchez MB. Antibiotic resistance in the opportunistic pathogen Stenotrophomonas maltophilia. Front Microbiol 2015; 6:658. [DOI] [PMC free article] [PubMed] [Google Scholar]