Abstract
Emotional stimuli attract attention and lead to increased activity in the visual cortex. The present study investigated the impact of personal relevance on emotion processing by presenting emotional words within sentences that referred to participants’ significant others or to unknown agents. In event-related potentials, personal relevance increased visual cortex activity within 100 ms after stimulus onset and the amplitudes of the Late Positive Complex (LPC). Moreover, personally relevant contexts gave rise to augmented pupillary responses and higher arousal ratings, suggesting a general boost of attention and arousal. Finally, personal relevance increased emotion-related ERP effects starting around 200 ms after word onset; effects for negative words compared to neutral words were prolonged in duration. Source localizations of these interactions revealed activations in prefrontal regions, in the visual cortex and in the fusiform gyrus. Taken together, these results demonstrate the high impact of personal relevance on reading in general and on emotion processing in particular.
Keywords: personal relevance, emotion, language, ERPs, pupillary responses
Introduction
Emotional stimuli–angry faces, the spider in the corner, our favorite food–often have a head start in attracting our attention, most likely due to the high relevance they have in our lives (Lang and Bradley, 2010). By now, electrophysiological and imaging research has established that emotional relevance automatically attracts attention, with effects already measurable in the visual cortex (for review, see Pourtois et al., 2013). However, this line of research has largely ignored the fact that outside of laboratory settings, emotional stimuli seldom appear in isolation: The angry expression we encounter might be the one of our spouse, a letter might be all the more joyful because it was written by our dearest friend. The present study aimed at investigating whether the emotional relevance of a given stimulus is modulated by the personal relevance it holds for the receiver.
Increased cortical activity in response to emotional stimuli has been shown for various stimulus domains, including emotional facial expressions, pictures of emotional objects or scenes, and written language (e.g. Cuthbert et al., 2000; Schupp et al., 2004; Kissler et al., 2007; Bayer and Schacht, 2014). In event-related potentials (ERPs), emotional relevance increases the amplitudes of components generated in extrastriate visual cortex, indexing a boost in attention to enhance sensory processing. In a number of studies, this boost was visible already at around 80 to 100 ms after stimulus onset in the form of increased amplitudes of the P1 component in response to emotional stimuli (Delplanque et al., 2004; Bayer et al., 2012a; Rellecke et al., 2012), although evidence on P1 modulations is mixed (for discussion, see Kissler et al., 2006; Bayer et al., 2012a). In a subsequent time window, emotion effects are more reliably observed in the form of a so-called Early Posterior Negativity (EPN), which occurs at around 150 to 400 ms as an increased relative negativity for emotional stimuli over posterior electrode sites (Junghöfer et al., 2001; Kissler et al., 2007; Bayer et al., 2012b; Bayer and Schacht, 2014). Finally, this boosted sensory processing often results in increased stimulus evaluation as reflected in the late positive complex (LPC, e.g. Cuthbert et al., 2000).
For many theories of emotion, the concept of relevance is a key determinant of emotional reactions. As an example, the motivated attention theory (Lang and Bradley, 2010) assumes that emotional stimuli attract attention because of their high motivational relevance for the observer’s well-being and survival. Thus, the boost in sensory processing described above might reflect the capture and guiding of attention towards these stimuli in the service of prioritized processing (for a review, see Pourtois et al., 2013). Similarly, stimulus relevance plays a paramount role in appraisal theories (e.g. Arnold, 1960; Lazarus, 1966; Scherer, 2001): Assessing a respective stimulus or situation as relevant for the individual can be seen as a prerequisite for appraisal processes ultimately resulting in emotions, including motor expressions of emotions (e.g. smiling), preparations for action (like fight-or-flight responses) and subjective feelings.
Given the theoretically implied importance of stimulus relevance, different sources of relevance need to be considered: As described above, emotional stimuli are assumed to be inherently relevant due to their possible impact on the organism, for example in threatening situations. However, other stimuli obtain increased relevance based on their specific importance for the individual person, either because they directly refer to this person (e.g. to personal traits, self-referential processing), or because they more generally refer to the individual’s personal surroundings (e.g. to personally familiar people), termed personal relevance. Regarding self-referential processing, a number of ERP studies have recently provided evidence for the influence of self-referential context on the processing of emotional words. In a study by Fields and Kuperberg (2012), emotional and neutral words were presented within sentence contexts that either referred to the participants themselves or to an unknown person (e.g. ‘A man knocks on your/Sandra’s hotel room door. You see/she sees that he has a gun in his hand’). Self-referential context increased the amplitudes of ERP components generated in the visual cortex; interactions between emotion and personal relevance occurred within the time window of the LPC (see also Fields and Kuperberg, 2016). Similar results were provided using nouns describing emotional experiences (e.g. ‘my fear/his fear’; Herbert et al., 2011).
Thus, a number of findings suggest that emotional relevance and self-referential context information increase the activity in the visual cortex during reading. However, very little is known about the potential impact of personal relevance: How do we process information that receives its relevance by referring to significant others like a partner or best friend, rather than to ourselves? On the one hand, the presented scenarios do not carry any immediate relevance for the participant and their wellbeing. On the other hand, considering the paramount importance of close personal relationships in our everyday lives, a mechanism that is sensitive to stimulus relevance should also prioritize information that refers to a relevant other. Above that, it remains unclear whether personal relevance would interact with emotional content, for example by augmenting emotion effects in highly relevant contexts.
The present study aimed at investigating the impact of personal relevance and its influence on emotion processing by presenting positive, neutral and negative nouns that were embedded in sentence contexts that either referred to the participant’s significant others, or to an unknown person. Thus, in contrast to other studies cited above, stimuli were not referring to participants themselves, but to their relevant others, expanding the focus to context situations that do not directly involve the participant. During stimulus presentation, we recorded ERPs as a time-sensitive measure of cortical processing. In line with previous research, we expected a boost in early visual processing of the critical word (as indexed by P1 and EPN) reflecting both the emotional relevance of the critical word itself and the personal relevance established by the preceding context. Concerning LPC amplitudes, we expected to replicate previous reports of interactions between emotional content and self-referential processing in the form of increased emotion effects in personally relevant contexts. In addition, we aimed to investigate whether any interactions between emotional content and personal relevance would occur during earlier processing stages, that is, whether emotion effects during visual processing would differ in amplitude and/or duration depending on the relevance of the context. Such interactions would indicate that both factors influence at least one common processing stage (Sternberg, 2001). Importantly, the timing of interactions and their neuronal sources would be indicative of their locus within the reading process.
In addition to ERPs, we recorded changes of pupil size in response to the target words. Previous studies revealed increased pupillary responses to the emotional content of pictures (Bradley et al., 2008) and sounds (Partala and Surakka, 2003). Pupil responses to single words were related to cognitive rather than emotional processing (Bayer et al., 2011); there is, however, little evidence on emotion processing in sentence contexts. More generally, pupillary responses were recently linked to online cognitive and attentional processing (Kang et al., 2014). Accordingly, we expect larger pupil dilations in response to personally relevant stimuli, indexing increased attention allocation as suggested by ERP studies on self-referential processing.
Materials and methods
Participants
The study was approved by the ethics committee of the Department of Psychology at the University of Goettingen, Germany and was conducted in accordance to the Declaration of Helsinki. Data were collected from 25 female participants. The data sets of five participants had to be discarded due to excessive ERP artifacts (2), health problems during data collection (2) and a recording error of pupil data (1). The remaining 20 participants had a mean age of 23.0 years (SD = 2.3 years) and were living in a heterosexual romantic relationship at the time of the investigation (mean duration = 37.8 months, SD = 32.1 months). This sample size allows for the detection of an effect size of ηp2= 0.35 at an alpha level of 0.05 with a power of 0.8 in a repeated-measures ANOVA; comparable effect sizes for EPN effects were reported in previous literature (e.g. Bayer et al., 2012b). All participants were native German speakers, had normal or corrected-to-normal vision and reported the absence of any neurological or psychiatric disorder. Eighteen participants were right-handed and 2 left-handed (according to Oldfield, 1971). Participation was reimbursed with course credit or 8 € per h.
Evaluation and selection of stimulus materials
Prior to the main experiment, a rating study was conducted in order to evaluate and select stimulus materials. Stimuli in the rating study consisted of 120 sentence pairs; the sentences of one pair were identical except for their agent: One sentence contained a personally relevant agent (boyfriend/partner or female best friend), whereas the other referred to a stranger (e.g. customer, pedestrian, guest). Boyfriend and female best friend were chosen since they likely possess the highest and most unambiguous personal relevance for our participant sample. Furthermore, the use of two agents (boyfriend and female best friend) enabled a broader and thus more realistic range of context situations. Finally, the participant sample was limited to female participants because it proved difficult to construct enough sentences that could be referred to both male and female partners/friends.
All sentences contained a positive, neutral or negative critical word at a subsequent sentence position. Materials consisted of 40 sentences per emotion condition. For examples of stimulus sentences, see Table 1.
Table 1.
Example sentences in the high relevance and low relevance condition, including positive, neutral and negative critical words (in bold); English translation in italics
| High personal relevance | Low personal relevance | Sentence ending with positive/neutral/negative critical words (bold) |
|---|---|---|
| Dein Freund Karl | Der Sportler | erwartet eine schnelle Heilung seiner Verletzung. |
| Your boyfriend Karl | The athlethe | expects a fastrecoveryfrom his injury. |
| Deine Freundin Anna | Der Gast | bemerkt, dass noch ein Stuhl frei ist und setzt sich. |
| Your friend Anna | The guest | notices a vacantchairand sits down. |
| Dein Freund Karl | Der Spaziergänger | entdeckt den Schauplatz der Bluttat von letzter Nacht. |
| Your boyfriend Karl | The walker | discovers last night’scrime scene. |
Emotional valence, emotional arousal and expectancy of the 120 critical words within both relevance contexts were rated by 20 participants each. Thus, each participant rated one list containing all sentences (half in each relevance condition) on one dimension (valence, arousal, or expectancy). The complete participant sample consisted of 120 female students (mean age = 23.3, SD = 3.1). First, the initial sentence part was presented, including the (underlined) critical word. Participants rated the critical word in the context of the preceding sentence using a five-point scale displayed underneath the text. After evaluation, the second part of the sentence appeared for the participants to finish reading. An additional set of 30 sentences with unexpected semantic content was included in order to facilitate the utilization of the complete expectancy scale, but was not used further. Based on the rating results, 96 pairs of sentences–32 per emotion condition–were selected for the main experiment (see Table 2).
Table 2.
Descriptive statistics and rating values (Means and Standard Deviations) of critical words
| High personal relevance |
Low personal relevance |
|||||||
|---|---|---|---|---|---|---|---|---|
| Word length | Frequency (Ftot/1mil) | Valence (−2–2) | Arousal (1–5) | Expectancy (1–5) | Valence (−2–2) | Arousal (1–5) | Expectancy (1–5) | |
| Positive | 6.7 (1.8) | 44.6 (51.6) | 1.5 (0.3) | 3.6 (0.6) | 3.0 (0.6) | 1.4 (0.2) | 3.1 (0.5) | 3.1 (0.6) |
| Neutral | 6.2 (1.5) | 50.4 (88.7) | 0.2 (0.4) | 2.5 (0.6) | 2.5 (0.6) | 0.2 (0.3) | 2.1 (0.4) | 3.1 (0.6) |
| Negative | 5.7 (1.5) | 43.2 (64.5) | –1.6 (0.2) | 4.1 (0.4) | 4.1 (0.4) | –1.6 (0.2) | 3.7 (0.5) | 3.4 (0.6) |
Word length is indicated as number of letters, frequency is quantified as occurrence per 1 million words in the CELEX database.
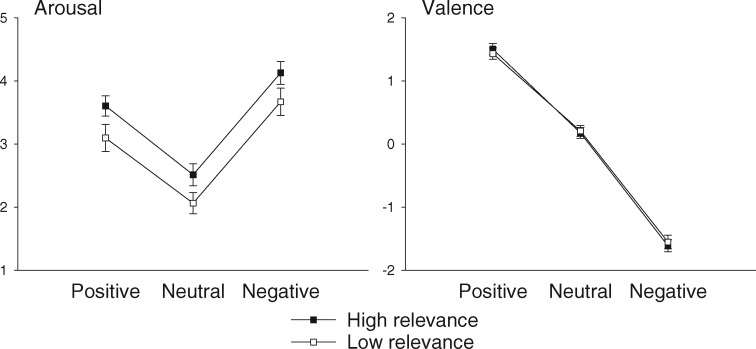
Valence, arousal and expectancy for the selected 96 sentence pairs were analyzed by univariate ANOVAs, including the factors emotion category (3) and personal relevance (2). The results of valence and arousal ratings are depicted in Figure 1. Valence ratings differed significantly between emotion categories as expected (negative < neutral < positive), F(2,89) = 1798.91, P < 0.001; ηp2 = 0.951; posttests: all ps < 0.001. There was no main effect of personal relevance on valence ratings, F(1,90) < 1, and no interaction between personal relevance and emotion category, F(2,89) < 1. Arousal ratings showed a main effect of emotion category, F(2,98) = 181.52, P < 0.001, ηp2 = 0.661, reflecting higher arousal ratings for positive and negative vs neutral words; furthermore, negative words were rated as more arousing than positive words, all ps < 0.001. Additionally, arousal ratings were influenced by the factor personal relevance, F(1,90) = 40.83, P < 0.001, ηp2 = 0.180. Target words presented in high relevance contexts were perceived as more arousing than words in low relevance context; this effect did not interact with emotion category, F(2,89) < 1. Finally, as intended, analyses showed no significant differences in expectancy between emotion categories and relevance conditions, and no interaction, all Fs < 1.97, ps > 0.143. Furthermore, emotion categories did not differ in their frequency of occurrence, F(2,93) < 1, while the number of letter showed a marginal difference between conditions, F(2,93) = 2.761, P = 0.063, with negative words being numerically shorter than positive ones.1
Fig. 1.
Arousal and valence ratings for positive, neutral and negative critical words presented in high relevance and low relevance contexts. Error bars depict 95% confidence intervals.
Experimental design and procedure
Participants were informed about the experimental procedure and signed informed consent. After EEG preparation, participants were seated in a sound-attenuated chamber and placed their chin and forehead on a head rest in order to ensure correct measuring of the pupil. At the start of each sentence, a fixation cross was presented in the middle of the screen for 1000 ms, followed by a blank screen for 300 ms. Sentences were presented word by word in a rapid serial visual presentation (RSVP) design; each word was presented for 300 ms, followed by a blank screen presented for 300 ms. On average, the sentences consisted of 10.5 words (SD = 2.0). The inter-trial interval had a duration of 1000 ms. Words were presented in the center of the screen in black letters on a light grey background with a mean visual angle of 2.3 x 0.6°. Each sentence was presented once in both relevance conditions, resulting in 192 trials. In the high relevance condition, the first name of the participant’s boyfriend/best friend was inserted at the respective position (‘Your boyfriend Karl …) in order to facilitate identification. Sentences of one pair were assigned to two separate lists in order to minimizing the probability of the two versions being presented in direct sequence. The order of lists was counterbalanced; within each list, sentences were presented in randomized order.
Participants were instructed to read the sentences attentively. In order to ensure attention, control questions concerning the content the previous sentence were randomly interspersed after 20% of the trials together with two possible answers; participants chose the correct answer by button press. Sentences were presented in 7 blocks with short breaks between blocks.
After the EEG recording, participants completed the Passionate Love Scale (Hatfield and Sprecher, 1986), an instrument for measuring feelings of passion and infatuation towards one’s partner. In addition, they evaluated the perceived quality of their relationship and friendship using 10-point analogue scales.
Data acquisition and pre-processing
The electroencephalogram was recorded from 64 electrodes mounted in an electrode cap (Biosemi Active Two) according to the International 10–20 system (full montage available under biosemi.com/headcap.htm). During recording, signals were referenced to the CMS-DRL ground, which drives the average potential across all electrodes as close as possible to the amplifier zero. Data were recorded with a sampling rate of 512 Hz and digitally filtered online (low pass filter with 5th order sinc response, –3 dB point at 102.4 Hz); DC offsets were kept within a range of ± 20 μV. Horizontal and vertical electrooculograms were recorded from four additional electrodes. Offline, data were re-referenced to average reference; blinks were corrected using Surrogate Multiple Source Eye Correction (MSEC; Ille et al., 2002) as implemented in BESA (Brain Electric Source Analysis, MEGIS Software GmbH) using default parameters for blink correction. Spontaneous, artifact-free blinks (n > 30) were averaged to obtain individual blink topographies. The procedure uses Principal Component Analyses (PCA) and generic dipole modeling for estimating blink-related activity, which is then subtracted from the continuous EEG signal. Data were filtered from 0.032 to 40 Hz using a 2nd order zero-phase IIR Butterworth filter (12dB/oct); additionally, a Notch filter (50 Hz) was applied. Data were segmented into epochs from 100 ms before to 1000 ms after the onset of the critical word and referred to a 100 ms prestimulus baseline. Channels with poor signals were interpolated by 4th order spherical splines (2.1% of channels on average). Epochs containing artifacts (± 100 μV) were discarded using semi-automatic artifact correction (8,5% of the segments; no significant differences between conditions, all Fs < 2.307, all ps > 0.145).
Pupil diameter was recorded using a desktop-mounted eye tracker (EyeLink 1000, SR Research) with a sampling rate of 500 Hz. Offline analyses of pupil data were performed using Matlab. Pupil diameter was recalculated to absolute values, blinks were corrected by linear interpolation, and a moving average window of 40 ms length was applied. Data were segmented into epochs of 1700 ms, starting 200 ms prior to the onset of the critical word. Segments containing artifacts due to insufficient tracking of the pupil were discarded.
For both EEG and pupil data, segments were averaged per subject and experimental condition (personal relevance x emotion category, resulting in 6 experimental conditions).
Data analyses
The selection of time windows and regions of interest (ROI) were based on previous literature on emotional language processing (e.g. Kissler et al. 2007; Bayer et al., 2012a). For determining the analyses time windows for EPN and LPC, we employed a data-driven approach (corrected for multiple comparisons, see below) to account for the high temporal variability of these components.
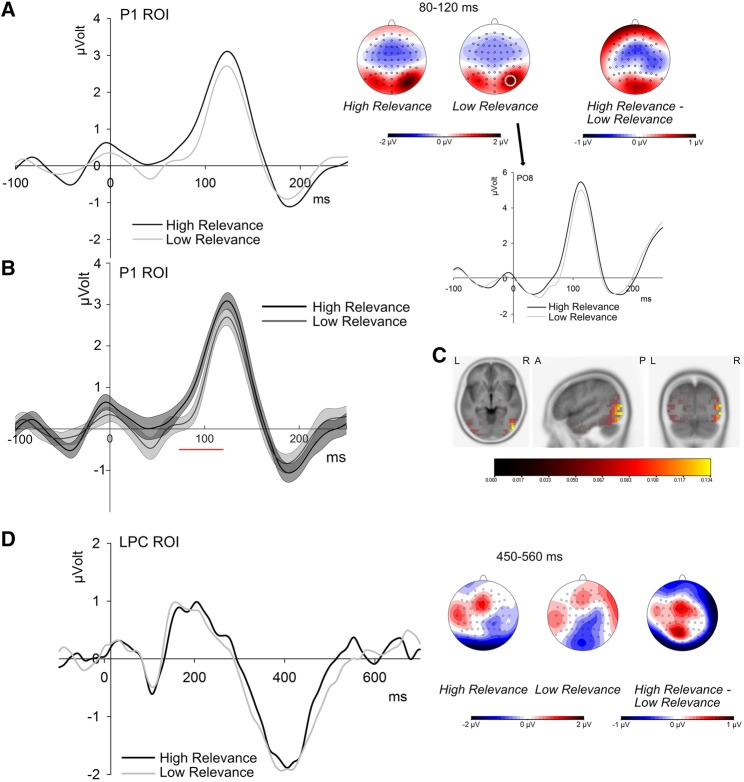
P1 amplitudes were quantified as mean activation at 8 occipital electrodes (O1, Oz, O2, PO7, PO3, POz, PO4, PO8) from 80 to 120 ms after stimulus onset, corresponding to the peak at electrodes PO7/PO8 (see Figure 2) and analyzed by repeated-measures (rm) ANOVAs including the factors personal relevance (2), emotion category (3) and electrode (8).
Fig. 2.
ERP effects of personal relevance. A) Grand mean waveforms for high and low personal relevance averaged over P1-ROI electrodes (left) and for electrode PO8 (right). Scalp distributions show both relevance conditions and their difference in the indicated time window. B) ERPs and 95% confidence intervals for high and low relevance, showing a significant difference from 73 to 120 ms after stimulus onset (red). C) Source localizations across all trials in the P1 time window, showing sources in the occipital and temporal lobes. D) Grand mean waveforms for high and low personal relevance averaged over LPC electrodes and scalp distributions of high and low relevance and their difference between 450 and 560 ms.
The EPN was measured at a group of 9 occipito-parietal electrodes (O1, Oz, O2, Iz, PO3, PO4, POz, PO7, PO8). Since previous EPN effects to emotional words showed a large temporal variation (within the time window of approximately 200 to 500 ms after stimulus onset, e.g. Schacht and Sommer, 2009a; Bayer et al., 2012b), we performed temporal cluster-based permutation tests to determine the time windows of interest. These analyses provide appropriate protection against Type 1 error inflation due to multiple comparisons (Groppe et al., 2011; Winkler et al. 2014). We performed sample-wise General Linear Model (GLM) analyses, including all EPN electrodes, from 200 to 500 ms after stimulus onset. We then applied temporal cluster-based permutation tests (with 5000 permutations), using a sample wise threshold of F = 5.21, ensuring a family-wise error (FWE) rate of P < 0.01. For the LPC, we performed the analyses on a group of centro-parietal electrodes (C1, Cz, C2, CP1, CPz, CP2, P1, Pz, P2) in a time window of 400 to 600 ms after stimulus onset. In order to follow up on the main effects and interactions identified in the GLM analyses, we performed post-tests using rm-ANOVAS. Huynh-Feldt correction was applied to degrees of freedom to correct for violations of sphericity. Results are reported with uncorrected degrees of freedom and corrected P-values. For rm-ANOVAs, we only considered main effects and interactions of experimental factors (emotion and personal relevance), but no interactions with the factor electrode. Within post-hoc comparisons, P-values were Bonferroni adjusted. Within-subject confidence intervals were calculated according to Cousineau (2005).
In order to estimate the neural generators of experimental effects, we performed source localizations using standardized low-resolution brain electromagnetic tomography (sLoreta; Pascual-Marqui, 2002). Computations were performed within a realistic head model computed with a boundary element method (Fuchs et al., 2016) using the MNI152 template (Mazziotta et al., 2001) and scalp electrode coordinates derived from the international 5% system (Jurcak et al., 2007). Standardized current density distribution was estimated for 6239 voxels of 5 mm spatial resolution, each containing an equivalent current dipole. We performed comparisons on log-transformed data using paired-samples t-tests in the time windows corresponding to relevant ERP effects. Only one single t-test per voxel was performed per time window. Statistical analyses were based on a stringent nonparametric randomization (with 5000 permutations) using a t-max procedure, providing a corrected P-value of P < 0.05.
Pupil responses were quantified as mean activations over 1500 ms after stimulus onset and analyzed using repeated-measures ANOVA including the factors personal relevance (2) and emotion category (3). Since pupillary responses to the previous word are still ongoing after onset of the critical word, we performed additional analyses to control for this possible confound. To that aim, we quantified pupillary responses in the time window from 500 to 1500 ms after word onset and referred them to a 0–500 ms baseline, corresponding to the time window of pupillary responses to the previously presented word.
Results
Behavior and self-report
Participants received a mean score of 100.25 points (SD = 12.24) of 135 points on the Passionate Love Scale and were classified as ‘extremely passionate’ (7 participants), ‘passionate’ (11 participants) and ‘average’ (2 participants). As revealed by the analog scales, participants were not only content with their partnership (mean = 7.6, SD = 2.2) but also with their friendship (mean = 8.2, SD = 1.0).
The questions of the 1-back-task were answered accurately in 99.2% of cases, showing that participants read the sentences attentively.
ERPs
P1 amplitudes were modulated by the personal relevance of the sentence context, F(1,19) = 10.40, P < 0.005, ηp2 = 0.354. Target words within high relevance contexts elicited larger P1 amplitudes than target words in low relevance contexts (see Figure 3). P1 amplitudes were not influenced by the factor emotion F(2,38) < 1, and showed no interaction between emotion and personal relevance, F(2,38) < 1. Analyses of 95% confidence intervals showed significant differences between relevance conditions from 73 to 120 ms, thus confirming the time window of analyses (see Figure 2). Source localizations of the difference between relevance conditions did not reveal any significant activation, but analyses of overall neural generators revealed sources in the inferior and middle temporal and occipital gyri and in the fusiform gyrus (MNI coordinates at maximum: x = 50, y = -80, z = -5), see Figure 2.
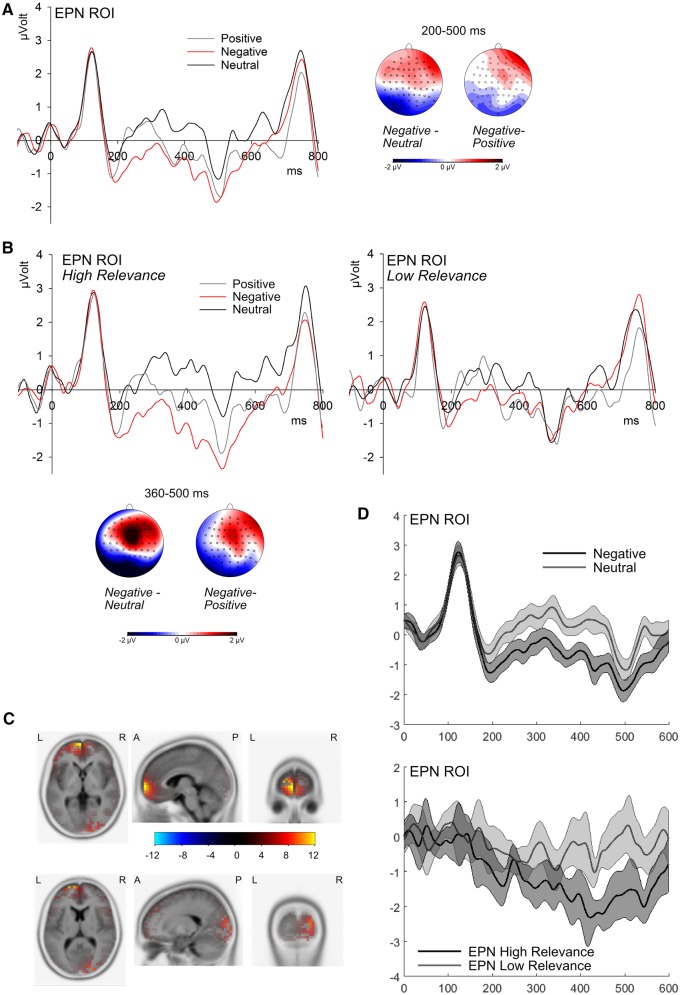
Fig. 3.
ERP effects and source localizations of emotion and personal relevance. A) Grand mean waveforms for emotion categories across relevance conditions. Scalp distributions depict significant emotion effects as differences between indicated emotion categories from 200 to 500 ms. B) Grand mean waveforms for emotion categories, depicted separately for high and low personal relevance. Scalp distributions show difference topographies between indicated emotion categories corresponding to significant emotion effects in the high relevance condition from 360 to 500 ms. C) Results of sLORETA source reconstructions showing maxima of activation in the prefrontal cortex (upper panel, coordinates at max.: x = -5, y = 65, z = 0) and in the visual cortex (lower panel; coordinates at max.: x = 20, y = -95, z = 10); results are corrected for multiple comparisons. D) ERP waveforms and ninety-five percent confidence intervals for negative and neutral critical words (corresponding to the main effect of emotion; upper panel) and for the difference between negative and neutral words in both relevance conditions (depicting the interaction between emotion and personal relevance; lower panel).
Permutation tests within the EPN region of interest revealed significant emotion effects in the time window from 200 to 500 ms after stimulus onset, FWE < 0.001. Rm-ANOVA post-tests showed that negative words elicited more negative amplitudes than both neutral words, F(1,19) = 16.65, P < 0.01, ηp2 = 0.467, and positive words, F(1,19) = 7.28, P = 0.05, ηp2= 0.277. The difference between positive and neutral words did not reach significance, F(1,19) = 5.62, P = 0.075.
Furthermore, interactions between emotion and personal relevance occurred from 290 to 320 ms and from 360 to 500 ms. In the first time window, rm-ANOVAs showed that emotion effects were limited to the high-relevance condition, F(2,38) = 6.332, P < 0.05, ηp2 = 0.250, but post-tests did not show significant differences between emotion conditions. In the later time window between 360 and 500 ms, emotion effects were again limited to target words presented in a high-relevance context, F(2,38) = 14.26, P < 0.001, ηp2 = 0.429, but were absent in low-relevance context, F(2,38) = 1.54, P = 0.454 (see Figure 4). In high-relevant contexts, negative words elicited enhanced EPN amplitudes as compared to neutral, F(1,19) = 19.036, P < 0.05, ηp2 = 0.357, and positive words, F(1,19) = 12.182, P = 0.014, ηp2= 0.391.
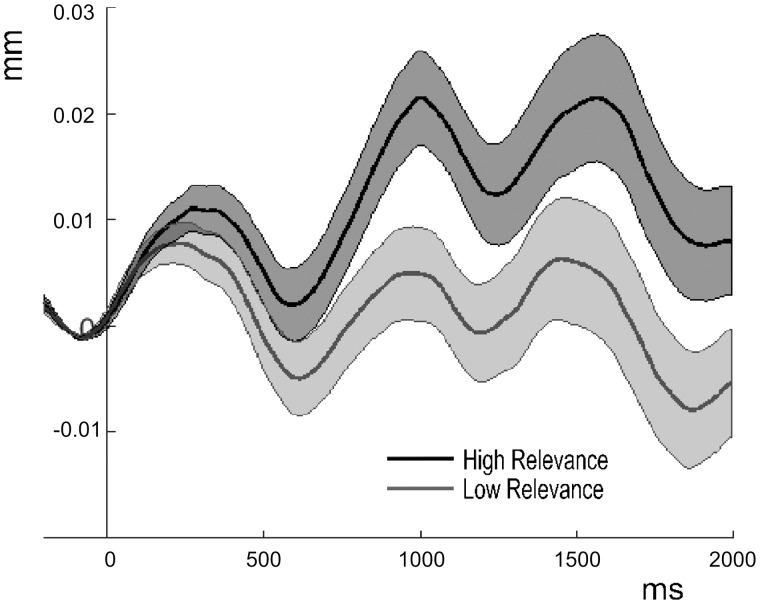
Fig. 4.
Mean pupil dilations and 95% confidence intervals in response to critical words presented in high and low relevance contexts.
Following up on the interaction between personal relevance and emotion in the time window of 360 to 500 ms, we performed source localizations in order to estimate the neural generators of this effect. To that aim, we compared the emotion effect (i.e. the difference between the negative and neutral condition) between both relevance conditions. Results of these estimations show that the emotion effect in the high relevance condition seems to be based on widespread activations in visual areas and the fusiform gyrus (see Figure 3D), with a maximum in the middle occipital gyrus (MNI coordinates at maximum: x = 20, y = –95, z = 10). Furthermore, a large cluster of activation was located in the prefrontal cortex with a maximum in the medial frontal gyrus (x = –5, y = 65, z = 0). A complete list of brain regions and coordinates is provided in Table 3.
Table 3.
Results of source analyses of the interaction between personal relevance and the emotion effect (negative vs neutral)
| Structure | No. of significant Voxels | t-Value at Peak | MNI coordinates at Peak(x, y, z) | ||
|---|---|---|---|---|---|
| Inferior frontal gyrus | 66 | 7.19 | –45 | –50 | 0 |
| Medial frontal gyrus | 94 | 11.99 | –5 | 65 | 0 |
| Middle frontal gyrus | 138 | 8.37 | –25 | 60 | 15 |
| Superior frontal gyrus | 141 | 11.57 | –5 | 65 | –5 |
| Anterior cingulate | 34 | 7.82 | –10 | 50 | 0 |
| Cuneus | 73 | 8.76 | 20 | –90 | 10 |
| Fusiform gyrus | 46 | 6.38 | 20 | –95 | –20 |
| Lingual Gyrus | 38 | 7.72 | 20 | –95 | –5 |
| Middle occipital gyrus | 58 | 9.59 | 20 | –95 | 10 |
| Middle temporal gyrus | 30 | 5.98 | 35 | –85 | 20 |
| Superior temporal gyrus | 36 | 5.66 | –55 | 10 | –15 |
P-values are corrected for multiple comparisons. The list was limited to brain regions showing > 20 significant voxels in order to account for the low resolution of the sLoreta-approach.
Permutation tests of LPC amplitudes showed significant effects of relevance ranging from 450 to 560 ms, FWE < 0.001. In this time window, words presented in high-relevance contexts elicited larger amplitudes than words presented in low-relevance contexts. Furthermore, analyses revealed an interaction between emotion and personal relevance in the time window from 460 to 530 ms, but post-tests did not reveal significant results, Fs(1,19) < 3.00, ps > 0.14.
Pupil data
Analyses of pupil responses to the critical words revealed a significant effect of personal relevance, F(1,19) = 9.22, P < 0.05, ηp2 = 0.327. Critical words presented in high relevance context elicited higher pupil dilations than words presented in low relevance contexts. There was no effect of emotion category and no interaction between emotion and personal relevance, Fs(1,19) < 1. This pattern of results was corroborated by analyses of 95% confidence intervals (see Figure 4) and confirmed by analyses correcting for possible influences of the preceding word, showing a significant effect of personal relevance, F(1,19) = 7.96, P < 0.05, ηp2 = 0.295, and no effect of emotion category or an interaction between emotion and personal relevance, Fs < 1.
Discussion
The present study investigated the impact of personal relevance on the processing of emotional and neutral words embedded in a sentence context. High personal relevance increased the amplitudes of the P1 component, indexing activity in the extrastriate visual cortex, LPC amplitudes, pupillary responses and arousal ratings, showing a general increase of attention and perceived arousal in highly relevant contexts. Crucially, high personal relevance also interacted with emotional content, leading to a prolonged duration of emotion effects in event-related potentials.
Prevailing theories of emotion assume that emotional stimuli capture attention because of their high relevance for human wellbeing. In the present study, emotion effects emerged at around 200 ms after the onset of the critical words in form of an increased posterior negativity (EPN) for negative as compared to neutral critical words. This finding is consistent with previous reports of increased attention allocation at the stage of perceptual processing (Schupp et al., 2004; Kissler et al., 2007; Schacht and Sommer, 2009b; Bayer and Schacht, 2014). In the present study, we were able to show that EPN effects for (identical) emotional words can be modulated by the personal relevance of the sentence context: The EPN had a longer duration when emotional words were presented in highly relevant contexts, that is, referring to the participants’ boyfriend or best friend.
Converging evidence suggests that emotion effects in the visual cortex like the EPN reported here are based on feedback connections from the amygdala (Pourtois et al., 2013), a limbic structure that is known to play an important role in fear learning (Öhman and Mineka, 2001). More generally, it is thought to serve as a ‘relevance detector’ that alarms cognitive and sensory processing systems (Sander et al., 2003; Whalen et al., 2013). Interactions of different forms of stimulus relevance, like emotional and personal relevance in the present study, are in agreement with the assumption that the amygdala might act as a generalized detection mechanism for stimulus relevance. Consistently, previous studies reported interactions between emotional content and bottom-up attention (stimulus size) at the stage of the EPN for both pictures and written words (De Cesarei and Codispoti, 2006; Bayer et al., 2012b).
Source estimations of the interaction between emotional and personal relevance revealed widespread activations in prefrontal regions, in the visual cortex and fusiform gyrus. The extrastriate visual cortex and the fusiform gyrus were previously suggested as generators of the EPN (Kissler et al., 2007; Hofmann et al., 2009); furthermore, communicative contexts were shown to increase fusiform gyrus activation (Schindler et al., 2015). Thus, our results are consistent with previous findings, corroborating our hypothesis that personal relevance has a direct impact on visual and fusiform areas involved in emotional word processing. This influence is most likely mediated by the strong activations in prefrontal regions estimated in our source analyses, which were previously related to social cognition in general (for a review, see Amodio and Frith, 2006), and to the retrieval of emotional contexts in particular (Smith et al., 2004).
Considering increased arousal ratings for personally relevant stimuli, one might be inclined to consider these arousal differences as predominant explanation for our findings, i.e. regardless of personal relevance. We believe this explanation to be unlikely, since increased arousal should have resulted in a generally increased EPN (from the start of the component), instead of a prolonged duration (Bayer et al., 2012a).
The finding of prolonged durations of the EPN caused by personal relevance raises the question about the functional locus of the component within emotional word processing. On the one hand, the EPN was suggested to reflect increased bottom-up attention allocation at the stage of perceptual processing; this assumption is in line with the interaction of emotion and bottom-up attention mentioned above (Bayer et al., 2012b). Furthermore, source localizations in our study confirmed activation differences in the visual cortex even at long latencies, i.e. between 360 and 500 ms after stimulus onset. On the other hand, these extended latencies of the EPN call into question the merely perceptual nature of the EPN, especially considering the pronounced additional sources in the prefrontal cortex. Furthermore, the EPN for written words had previously been located at a lexico-semantic processing stage, i.e. after a word has been accessed in the mental lexicon (Palazova et al., 2011). Taken together, these findings suggest that the EPN might be located at a transitioning stage between perceptual and higher-order, in this case semantic, processing.
Personal relevance increased the amplitudes of the P1 component in response to critical words irrespective of their emotional content. This finding expands previous findings on self-referential processing, where P1 amplitudes in response to critical words were increased in contexts that referred to the participants themselves in contrast to an unknown person (Fields and Kuperberg, 2012). Together, these results suggest that context information (both self-referential and personally relevant) can impact the earliest stages of written word processing. Modulations of early visual potentials by top-down attention are a well-documented phenomenon, which is based on sensory amplification of relevant information in the visual cortex (Hillyard and Anllo-Vento, 1998). Although our source localizations of the relevance effect itself failed to show significant results, overall analyses confirmed sources in the fusiform, temporal and occipital gyri.
The assumption that relevant contexts might lead to a general increase in attention is corroborated by the finding of augmented pupillary responses to critical words presented in personally relevant contexts. As for the P1, this effect was not modulated by the emotion category of the critical word, suggesting that pupillary responses in our study are indexing general top-down attention rather than emotion-specific arousal. In addition, high personal relevance also increased the amplitudes of the LPC, suggesting that personal relevance also evokes sustained attention during higher-order stimulus evaluation. In line with previous literature (Herbert et al., 2011; Fields and Kuperberg, 2016), this effect was based on numerically larger emotion effects in the high-relevance condition, but post-tests did not reveal significant results. Last, and in line with previous literature (Fields and Kuperberg, 2012), high personal relevance increased arousal ratings for critical words irrespective of their emotional valence category, suggesting that relevant context information can also affect subjective measures of emotional arousal.
Finally, two limitations of the present study should be mentioned. First, due to the small sample size, our data did not allow for investigating potential moderating variables of the impact of personal relevance like duration of relationships or level of closeness. Second, our sample included only young, female participants. Future research should aim at uncovering the influence of such moderating variables in order to shed light on the specificity of effects or their generalizability, respectively.
Taken together, our study demonstrated that high personal relevance has an impact on the processing of words embedded into a sentence context: It increased visual cortex activity within 100 ms after stimulus onset, LPC amplitudes, pupillary responses and arousal ratings, suggesting heightened attention allocation to personally relevant contexts. Furthermore, emotion effects were prolonged in relevant contexts, showing an interaction of emotional and personal relevance and suggesting a common detection mechanism for stimulus relevance at the stage of perceptual processing.
Author contributions
All authors contributed to the study design. Data collection was performed by K. Ruthmann; M. Bayer and K. Ruthmann performed data analyses. All authors contributed to data interpretation. M. Bayer drafted the manuscript; A. Schacht and K. Ruthmann provided critical revisions. All authors approved the final version of the manuscript.
Footnotes
During stimulus selection, priority was ultimately given to selecting stimuli that did not differ in expectancy between the relevance (and emotion) conditions, especially since it was not possible to match arousal values between emotion categories in any case. Since we were mainly interested in the modulation of emotion effects by social relevance, it was preferable to avoid confounds in this comparison rather than between emotion categories themselves.
Funding
This research was supported by university grants to MB and by the German Research Foundation (DFG; grant #SCHA1848/1-1 to AS). We acknowledge support by the Open Access Publication Funds of the Göttingen University.
References
- Amodio D.M., Frith C.D. (2006). Meeting of minds: the medial frontal cortex and social cognition. Nature Review in Neuroscience, 7, 268–77. [DOI] [PubMed] [Google Scholar]
- Arnold M.B. (1960). Emotion and Personality. New York: Columbia University Press. [Google Scholar]
- Bayer M., Schacht A. (2014). Event-related brain responses to emotional words, pictures, and faces: a cross-domain comparison. Frontiers in Psychology, 5, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M., Sommer W., Schacht A. (2011). Emotional words impact the mind but not the body: evidence from pupillary responses. Psychophysiology, 48, 1554–62. [DOI] [PubMed] [Google Scholar]
- Bayer M., Sommer W., Schacht A. (2012a). P1 and beyond: functional separation of multiple emotion effects in word recognition. Psychophysiology, 49, 959–69. [DOI] [PubMed] [Google Scholar]
- Bayer M., Sommer W., Schacht A. (2012b). Font size matters-emotion and attention in cortical responses to written words. PLoS One, 7(5), e36042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M.M., Miccoli L., Escrig M.A., et al. (2008). The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology, 45, 602–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cesarei A., Codispoti M. (2006). When does size not matter? Effects of stimulus size on affective modulation. Psychophysiology, 43, 207–15. [DOI] [PubMed] [Google Scholar]
- Cousineau D. (2005). Confidence intervals in within-subject designs: a simpler solution to Loftus and Masson’s method. Tutorial Quantitative Methods for Psychology, 1, 42–5. [Google Scholar]
- Cuthbert B.N., Schupp H.T., Bradley M.M., et al. (2000). Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biological Psychology, 52, 95–111. [DOI] [PubMed] [Google Scholar]
- Delplanque S., Lavoie M.E., Hot P., et al. (2004). Modulation of cognitive processing by emotional valence studied through event-related potentials in humans. Neuroscience Letters, 356, 1–4. [DOI] [PubMed] [Google Scholar]
- Fields E.C., Kuperberg G.R. (2012). It’s All About You: an ERP study of emotion and self-relevance in discourse. Neuroimage, 62, 562–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields E.C., Kuperberg G.R. (2016). Dynamic effects of self-relevance and task on the neural processing of emotional words in context. Frontiers in Psychology, 6. DOI: 10.3389/fpsyg.2015.02003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs M., Kastner J., Wagner M., et al. (2016). A standardized boundary element method volume conductor model. Clinical Neurophysiology, 113, 702–12. [DOI] [PubMed] [Google Scholar]
- Groppe D.M., Urbach T.P., Kutas M. (2011). Mass univariate analysis of event-related brain potentials/fields I: a critical tutorial review. Psychophysiology, 48, 1711–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield E., Sprecher S. (1986). Measuring passionate love in intimate relationships. Journal of Adolescence, 9, 383–410. [DOI] [PubMed] [Google Scholar]
- Herbert C., Herbert B.M., Ethofer T., et al. (2011). His or mine? The time course of self-other discrimination in emotion processing. Social Neuroscience, 6, 277–88. [DOI] [PubMed] [Google Scholar]
- Hillyard S.A., Anllo-Vento L. (1998). Event-related brain potentials in the study of visual selective attention. Proceedings of the National Academy of Sciences of the United States of America, 95, 781–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M.J., Kuchinke L., Tamm S., et al. (2009). Affective processing within 1/10th of a second: High arousal is necessary for early facilitative processing of negative but not positive words. Cognitive Affective Behaviour Neuroscience, 9, 389–97. [DOI] [PubMed] [Google Scholar]
- Ille N., Berg P., Scherg M. (2002). Artifact correction of the ongoing EEG using spatial filters based on artifact and brain signal topographies. Journal of Clinical Neurophysiology, 19, 113–24. [DOI] [PubMed] [Google Scholar]
- Junghöfer M., Bradley M.M., Elbert T.R., et al. (2001). Fleeting images: a new look at early emotion discrimination. Psychophysiology, 38, 175–8. [PubMed] [Google Scholar]
- Jurcak V., Tsuzuki D., Dan I. (2007). 10/20, 10/10, and 10/5 systems revisited: their validity as relative head-surface-based positioning systems. Neuroimage, 34, 1600–11. [DOI] [PubMed] [Google Scholar]
- Kang O.E., Huffer K.E., Wheatley T.P. (2014). Pupil dilation dynamics track attention to high-level information. PLoS One, 9, e102463.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissler J., Assadollahi R., Herbert C. (2006). Chapter 8 Emotional and semantic networks in visual word processing: insights from ERP studies. Progress in Brain Research, 156, 147–83. [DOI] [PubMed] [Google Scholar]
- Kissler J., Herbert C., Peyk P., et al. (2007). Buzzwords–Early cortical responses to emotional words during reading. Psychological Science, 18, 475–80. [DOI] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M. (2010). Emotion and the motivational brain. Biological Psychology, 84, 437–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus R.S. Psychological Stress and the Coping Process. New York, NY: McGraw-Hill, 1966. [Google Scholar]
- Mazziotta J., Toga A., Evans A., et al. (2001). A probabilistic atlas and reference system for the human brain: international consortium for brain mapping (ICBM). Philosophical Transactions of the Royal Society B: Biological Sciences, 356, 1293–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhman A., Mineka S. (2001). Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychological Review, 108, 483–522. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Palazova M., Mantwill K., Sommer W., et al. (2011). Are effects of emotion in single words non-lexical? Evidence from event-related brain potentials. Neuropsychologia, 49, 2766–75. [DOI] [PubMed] [Google Scholar]
- Partala T., Surakka V. (2003). Pupil size variation as an indication of affective processing. International journal of human-computer studies, 59(1), 185–98. [Google Scholar]
- Pascual-Marqui R.D. (2002). Standardized low resolution brain electromagnetic tomography (sLoreta): technical details. Methods and Findings in Experimental and Clinical Pharmacology, 24, 5–12. [PubMed] [Google Scholar]
- Pourtois G., Schettino A., Vuilleumier P. (2013). Brain mechanisms for emotional influences on perception and attention: what is magic and what is not. Biological Psychology, 92, 492–512. [DOI] [PubMed] [Google Scholar]
- Rellecke J., Sommer W., Schacht A. (2012). Does processing of emotional facial expressions depend on intention? Time-resolved evidence from event-related brain potentials. Biological Psychology, 90, 23–32. [DOI] [PubMed] [Google Scholar]
- Sander D., Grafman J., Zalla T. (2003). The human amygdala: an evolved system for relevance detection. Reviews in the Neuroscience, 14, 303–16. [DOI] [PubMed] [Google Scholar]
- Schacht A., Sommer W. (2009a). Time course and task dependence of emotion effects in word processing. Cognitive, Affective, and Behavioral Neurosciences, 9, 28–43. [DOI] [PubMed] [Google Scholar]
- Schacht A., Sommer W. (2009b). Emotions in word and face processing: early and late cortical responses. Brain and Cognition, 69, 538–50. [DOI] [PubMed] [Google Scholar]
- Scherer K.R. (2001). Appraisal considered as a process of multilevel sequential checking In: Scherer K.R., Schorr A., Johnstone T., editors. Appraisal Processes in Emotion: Theory, Methods, Research, 92–120. [Google Scholar]
- Schindler S., Wegrzyn M., Steppacher I., et al. (2015). Perceived communicative context and emotional content amplify visual word processing in the fusiform gyrus. Journal of Neurosciences, 35, 6010–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp H.T., Ohman A., Junghöfer M., et al. (2004). The facilitated processing of threatening faces: an ERP analysis. Emotion, 4, 189–200. [DOI] [PubMed] [Google Scholar]
- Smith A.P.R., Henson R.N.A., Dolan R.J., et al. (2004). fMRI correlates of the episodic retrieval of emotional contexts. Neuroimage, 22, 868–78. [DOI] [PubMed] [Google Scholar]
- Sternberg S. (2001). Separate modifiability, mental modules, and the use of pure and composite measures to reveal them. Acta Psychologica (Amst), 106, 147–246. [DOI] [PubMed] [Google Scholar]
- Whalen P.J., Raila H., Bennett R., et al. (2013). Neuroscience and facial expressions of emotion: the role of amygdala-prefrontal interactions. Emotion Review, 5, 78–83. [Google Scholar]
- Winkler A.M., Ridgway G.R., Webster M.A., et al. (2014). Permutation inference for the general linear model. Neuroimage, 92, 381–97. [DOI] [PMC free article] [PubMed] [Google Scholar]