Abstract
Assessing emotional dynamics in the brain offers insight into the fundamental neural and psychological mechanisms underlying emotion. One such dynamic is emotional inertia—the influence of one’s emotional state at one time point on one’s emotional state at a subsequent time point. Emotion inertia reflects emotional rigidity and poor emotion regulation as evidenced by its relationship to depression and neuroticism. In this study, we assessed changes in cerebral blood flow (CBF) from before to after an emotional task and used these changes to predict stress, positive and negative emotional inertia in daily life events. Cerebral blood flow changes in the lateral prefrontal cortex (lPFC) predicted decreased non-specific emotional inertia, suggesting that the lPFC may feature a general inhibitory mechanism responsible for limiting the impact that an emotional state from one event has on the emotional state of a subsequent event. CBF changes in the ventromedial prefrontal cortex and lateral occipital cortex were associated with positive emotional inertia and negative/stress inertia, respectively. These data advance the blossoming literature on the temporal dynamics of emotion in the brain and on the use of neural indices to predict mental health-relevant behavior in daily life.
Keywords: emotion, emotional inertia, emotion regulation, lpfc, vmpfc, CBF, rumination
Introduction
Emotions change over time (Frijda, 2007; Larsen et al., 2009), often starting as reactions to daily life events (Kuppens et al., 2010) and lasting for seconds to hours even after the initiating event is over (Verduyn et al., 2011). Previous investigators have found that, relative to assessing static emotional experiences, assessing these emotional dynamics in the brain can offer additional insight into the fundamental neural and psychological mechanisms underlying emotion (Waugh et al., 2015). For example, subtle changes in the intensity of an emotional experience is associated more with changes in the duration of the neural activation than with changes in the height of neural activation (Waugh et al., 2010). Evidence suggests that this duration of neural activation to emotional stimuli is also associated with individual differences in negative psychological traits like neuroticism (Schuyler et al., 2014) and depression (Siegle et al., 2002; Heller et al., 2009).
Although these investigations into the neural basis of emotional dynamics are promising, their limitation is that they have yet to capture the natural emotional dynamics that occur in response to real-world events. One such emotional dynamic is emotional inertia—the tendency for emotional states to persist over time (Kuppens et al., 2010). Typically, emotional inertia is assessed as the autocorrelation of daily reports of emotion over time (Kuppens et al., 2010). Individuals with high emotional inertia exhibit a high correlation between successive emotional experiences, suggesting a rigidity in their emotional responding—a relative lack of flexible responding to varying environmental demands (Koval et al., 2012). Supporting this formulation, high emotional inertia has been associated with psychological traits linked with emotional rigidity and poor flexibility such as low self-esteem (Kuppens et al., 2010), neuroticism (Suls et al., 1998) and depression (Koval et al., 2013).
One challenge to this formulation that emotional inertia tracks rigidity in emotional responding is that it is unclear what these emotions are in response to. Typically, the successive mood states being assessed are hours or even days apart with very few attempts by investigators to account for the events that are causing those changes in mood states (see Koval et al., 2013 for an exception). Without knowing whether an emotional event occurred in between assessments of mood states, it is difficult to interpret high emotional inertia as a lack of flexible responding to emotional events when it is equally likely that it could be due to a lack of emotional events occurring. To enable us to interpret emotional inertia as emotional rigidity and a lack of flexible responding in the present study, we assessed emotional inertia in response to daily emotional events. Participants completed a day reconstruction method (DRM; Kahneman et al., 2004) journal every night for up to a week in which they described their day as a series of events (both emotional and non-emotional). They then rated the overall stress level of each event as well as their positive and negative emotional responses throughout the duration of the event and after the event was over.
The overall stress ratings were used to calculate between-event stress inertia, which we hypothesize reflects people’s propensity to allow emotions from one event to carry-over to the next event. The hypothesized mechanisms that underlie this mood carryover include perseverative cognitions such as worry and rumination (Nolen-Hoeksema and Morrow, 1993; Brosschot et al., 2006), and poor mood regulation (Koval et al., 2015). To provide greater emotional specificity than is provided by overall stress responses, we also assessed emotional inertia as the relationship between the negative/positive emotion experienced at the end of one event and the negative/positive emotion experienced at the beginning of the next event. We hypothesized that negative emotional inertia would reflect similar processes as stress inertia (worry, rumination). We also hypothesized that positive emotional inertia may reflect people’s propensity to savor and maintain positive emotional states (Quoidbach et al., 2010), although there is evidence that high positive emotional inertia also reflects emotional rigidity and can be positively related to psychopathology (albeit much less so than is negative emotional inertia; Houben et al., 2015).
Although we are beginning to understand the neural processes that underlay certain temporal dynamics of emotions such as duration and onset (Waugh et al., 2015), no research has yet to explore the neural processes that underlay emotional inertia. Understanding these neural processes may help us more fully understand what emotion and emotional regulatory mechanisms are associated with inertia as well as provide a neural basis for its association with neuroticism and depression. As of now, emotional inertia has been almost exclusively assessed over days in real-life contexts. So the first step in understanding the neural mechanisms involved with it requires adopting the brain-as-predictor approach (Berkman and Falk, 2013). This approach integrates traditional neuroimaging methods with real-world psychosocial and behavioral outcomes by modeling neural activity measured in the laboratory as predictors of measures that are obtained well after the experimental session (Berkman and Falk, 2013). In doing so, the brain-as-predictor approach tests the ecological validity of traditional neuroscience findings, thereby broadening our understanding of the neural processes that underlie daily human thought and behavior. To date, researchers have employed this method to predict, for example, responsivity to depression treatment (Kito et al., 2012), diurnal patterns of cortisol release (Urry et al., 2006), relationship success (Eisenberger et al., 2011), and self-control (Berkman et al., 2011). One particularly relevant study found using this method that neural responses to emotional events predicted the duration of emotional responses to events in real-life (Heller et al., 2015).
In this study, the neural predictor of emotional inertia that we assess is brain cerebral blood flow (CBF) perfusion change from before an emotional task to after that task. Notably, we designed the emotional task to test hypotheses that are not related to inertia and will not be presented here. Therefore, we will be unable to assess specifically how responses to this emotional task related to changes in perfusion. However, assessing participants’ neural activity before and after an emotional task is broadly consistent with how we are assessing emotional inertia as responses to emotional events. In addition, the duration of the emotional task allowed us to maintain a timescale (several minutes) between CBF assessments that is more consistent with the timescale of emotional events that participants report in their daily lives (Verduyn et al., 2009) than is the emotional events typically used in event-related studies, which only last several seconds. Although we were unable to assess ‘neural inertia’ with only two time points, matching the neural and behavioral timescales is important given that emotion dynamics on different time scales are only weakly correlated due to different underlying mechanisms (Koval et al., 2013).
We assessed CBF perfusion change as our neural predictor of emotional inertia. In arterial spin labeling (ASL) perfusion, arterial water is magnetically tagged before the blood flows through neural tissue. After a specified time passes, images are acquired that represent differences in magnetic tagging of blood and tissue water and are indicative of CBF in that brain region (Wong, 1999). Because ASL is less prone to low frequency drift noise than is blood–oxygen level-dependent (BOLD) activation (Wang et al., 2003), it can track changes in slowly varying states better than can BOLD in some instances (Borogovac and Asllani, 2012). For example, previous studies have utilized pre and post measures of resting state ASL perfusion to investigate the neural correlates of long-term memory encoding (Groen et al., 2011) and of meditation-induced pain relief (Zeidan et al., 2015). Perfusion changes are also reliable—within-session changes are highly comparable to changes across sessions (Borogovac et al., 2010).
Because we will not be able to precisely determine the mechanisms underlying the perfusion changes in the scanner, we do not have strong predictions about which brain regions might be involved. Preliminarily, we hypothesized that emotional inertia is in part due to an inability to successfully regulate emotion, so we might expect there to be an association with brain regions responsible for emotion regulation such as the vmPFC, dmPFC/daCC, lPFC, striatum and/or amygdala (Wager et al., 2008b; McRae et al., 2010; Ochsner et al., 2012; Waugh et al., 2014). Many of these regions are also associated with depression (Fitzgerald et al., 2008; Kupfer et al., 2012), a hallmark predictor of emotional inertia (Koval et al., 2013). In addition, the vmPFC, dmPFC, lPFC and amygdala have also been shown to exhibit longer durations of activation to emotional than to non-emotional stimuli (Siegle et al., 2002; Herry et al., 2007; Waugh et al., 2014).
In sum, we measured CBF levels before and after an emotional task, then used these changes in CBF to predict emotional inertia in participants' subjective ratings of stress between life events and positive and negative emotions during those events. We hypothesized that CBF changes in brain regions associated with emotion regulation, depression, and/or the duration of emotional experiences such as the vmPFC, dmPFC, lPFC, striatum and amygdala would be associated with emotional inertia in daily life. We also assessed personality traits that have been previously hypothesized to be associated with emotional inertia such as rumination and neuroticism, as well as ego-resilience and extraversion to explore whether they might be related to positive emotional inertia given their association with positive emotionality (Costa and McCrae, 1980; Fredrickson et al., 2003).
Materials and methods
Participants
Participants included 38 individuals (17 females) aged 21–65 years (M = 40.93, SD = 13.69) from the greater Winston-Salem area. Participants were recruited using ads on Craigslist.com and the Winston-Salem Journal. Individuals who had a history of head injuries, and individuals for whom magnetic resonance imaging may not be safe (e.g. individuals with ferrous metal in their bodies, pregnant women) were excluded from the study. All participants signed informed consent, and all aspects of this study complied with standards set forth by the Wake Forest University Institutional Review Board. Five participants were excluded from perfusion data analysis (N = 33) because of equipment malfunction (response box did not work, so could not complete the emotional processing task; n = 2), excessive artifacts in the ASL data (n = 2), and failure to comply with task instructions for the emotional processing task (n = 1). An additional four participants were excluded from the DRM analyses (N = 29) because of failing to complete more than 1 daily diary.
Tasks and materials
Pseudo-continuous arterial spin labeling (PCASL) perfusion data acquisition. At two times in the scan session, we collected CBF perfusion data. Images were acquired using a 3 Tesla Siemens Skyra MRI scanner with a high-resolution 32 channel human head/neck coil (Siemens Medical, Malvern, PA). High-resolution T1 anatomic images were obtained using a 3D volumetric MPRAGE sequence (192 slices, 1 mm3 resolution, TE = 2.99, flip angle = 9°, FOV = 25cm). Arterial spin labeling to acquire whole-brain resting CBF was performed with a whole brain pseudo-continuous ASL (PCASL) sequence (Dai et al., 2008). The PCASL sequence uses radiofrequency (RF) pulse trains and gradient fields to induce flow-driven adiabatic inversion of the magnetization of arterial blood. PCASL offers higher SNR and a well-controlled temporal bolus leading to robust CBF quantification compared to pulsed ASL. Scan parameters for the present study included: tagging duration = 1.8 s, TI = 3 s, TR = 4 s, repetitions = 64, FOV = 22 × 22 cm, matrix size = 64 × 64, 20 5 mm axial slices with a single shot EPI acquisition, and acquisition time = 4:16 minutes. Quantitative CBF maps (ml/100 gm tissue/min) for each voxel were computed as previously described (Wong et al., 1998).
Emotional task . Between the two perfusion scans, participants completed an emotional processing task while BOLD was collected. In this task, participants viewed 120 statements—60 with negative adjectives and 60 with positive adjectives. The adjectives were selected from the Affective Norms for English Words set (ANEW; Bradley and Lang, 1999) and were previously normed (from 1 [highly negative] to 9 [highly positive]) as being either quite positive (M = 7. 48) or quite negative (M = 2.70).
The statements that participants saw varied in whether they were about the global self (i.e. ‘You are [adjective]’), about another (i.e. ‘He/She is [adjective]’), or about 1, 2 or 3 of their social identities, which they had chosen before entering the scanner (e.g., ‘You are a [adjective] [social identity]’). Participants were instructed to think about how each statement made them feel and were asked to rate the intensity of the emotion elicited by each statement on a scale of 1–5 (1 = low intensity, 5 = high intensity). Participants rated the statements on average as being middle to middle-high intensity (positive M = 3.54, SE = 0.10; negative M = 3.21, SE = 0.16). Each of the five runs lasted 5:36 for a total run time of 28 min. Although this task was not designed specifically to induce state changes in mood, viewing emotional self-relevant statements has been shown in previous research to induce state changes in mood (Velten, 1968). BOLD and additional self-report data from this task will be presented elsewhere.
Modified day reconstruction method (DRM ). In the week following the scan, participants completed daily DRM assessments online using Qualtrics (Qualtrics, Provo, UT). The DRM (Kahneman et al., 2004) is a daily diary in which participants provide detailed accounts of the events that occurred during that day and then provide information about each event including their emotional responses. An advantage to using the DRM is that it is designed to help minimize recall bias (Kahneman et al., 2004), but can be used less frequently and with less technological support than computerized, interval-contingent or signal-contingent experience sampling techniques (Kahneman et al., 2004). Participants were asked to complete the modified DRM at the end of each day to minimize forgetting of events and associated emotions. For each daily DRM, they were first asked to ‘Think of your day as a continuous series of scenes or episodes in a film…’ and to ‘Try to remember each episode in detail… try to remember how you felt, and what your mood was like during each episode’.
After listing the events from the previous night (if any occurred after completing the DRM for the previous day) as well as from that morning, afternoon and evening, participants were asked to provide contextual information for each event (e.g. nature of the event, location, whether and with whom they interacted). Participants were then told to think about how they felt during the event, and then rated that event on a scale of 0–10 (0 = Not at All, 10 = Very) for stressfulness, pleasantness (positive emotion) and unpleasantness (negative emotion). To capture positive and negative emotional inertia, participants provided ratings of pleasantness/unpleasantness for the beginning, middle, end, right after the end (up to 30 min afterwards), and long after the end (30 min to several hours afterwards) of each documented episode. Ratings from the end of the episode were used to predict their ratings of the beginning of the next episode.
Personality questionnaires. Rumination. To assess rumination, we used the 25-item Ruminative Responses Inventory (Whitmer and Gotlib, 2011 adapted from Nolen-Hoeksema et al., 1993), from which we extracted two subscales: brooding (‘Thinking about a recent situation, wishing it had gone better’) and adaptive reflection (‘Go away by yourself and think about why you feel this way’). Brooding has been found to be more associated with maladaptive rumination and depression (Treynor et al., 2003) than has adaptive reflection, which we include as a more adaptive version of rumination.
Neuroticism and Extraversion.We used a shortened version of the NEO five-factor personality inventory (Costa and McCrae, 1992) designed to assess extraversion, neuroticism, and openness to experience (not included here). There were 12 items for each scale, resulting in 36 total items to which participants responded on a scale from 1 (strongly disagree) to 5 (strongly agree).
Resilience. We used an ego-resiliency scale (ER89) to assess trait variation in psychological resilience (Block and Kremen, 1996). Participants were asked to indicate the degree to which they agreed with 14 statements (e.g. ‘I quickly get over and recover from being startled,’ and ‘I enjoy dealing with new and unusual situations.’) on a scale from 1 (does not apply at all) to 4 (applies very strongly). The ER89 has been shown to be a valid measure of trait resilience, but most importantly has been associated with emotional and physiological recovery (Tugade and Fredrickson, 2004) and emotional flexibility (Waugh et al., 2011).
Procedure
Upon arrival, participants provided informed consent and completed an MRI safety screening form. They then completed a variant of the 20 Statements Task (Kuhn and McPartland, 1954) in which they were asked to list twenty social identities by completing the sentence ‘I am a …’ 20 times. After listing 20 identities, participants selected the four social identities that they identified with the most strongly and these social identities were used as targets in the emotional processing task described above.
Next, the experimenter instructed the participants about the emotional task, after which the participants completed six practice trials. Participants were allowed to repeat the practice session until they felt comfortable with the paradigm (maximum number of practice attempts from any participant was three).
While in the scanner, participants first underwent the pre-task cerebral perfusion scan (4:16). They then completed the emotional task (7:00), followed by the post-task cerebral perfusion scan (4:16).
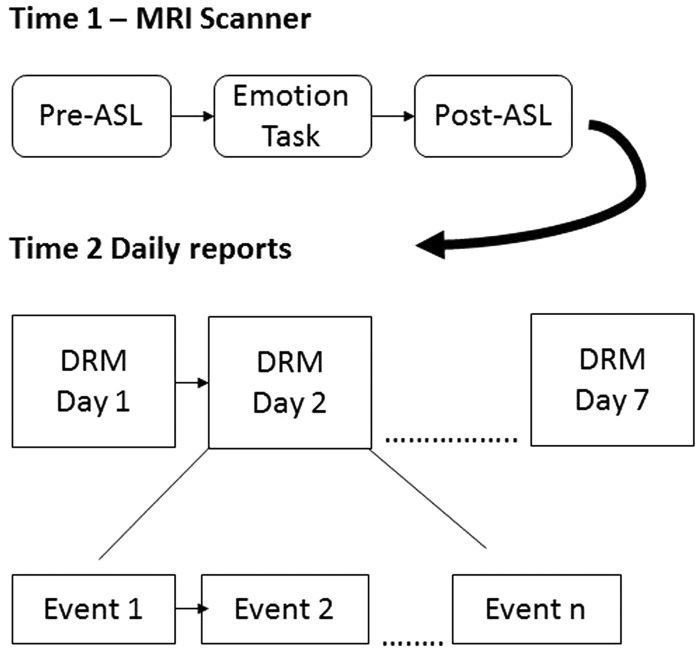
After the MRI scan, a researcher explained the DRM to participants. Participants were given the opportunity to record events for as many days as they wished within 7 days following the scan (see Figure 1 for study schematic). To encourage maximal completion of the DRM, participants were paid $5 for each completed day and an additional $25 for completing all seven days. Daily diaries were counted as complete if participants answered at least 90% of the questions for the day (greater than ∼25 of the 28 questions for each event reported).
Fig. 1.
Schematic of the timing of the study events. Participants began the study with an MRI scan, which was followed up by up to 7 days of completing daily diary reconstructions (DRM), each of which recounted the events of their day.
ASL data pre-processing and analyses
We used SPM (Wellcome Trust Centre for Neuroimaging) through the WFU pipeline to process the ASL data (Maldjian et al., 2009). Quantitative data pre-processing included data cleaning (removal of individual images with noise spikes or severe motion artifact), realignment (separately for label and control images), coregistration to the T1 structural images, normalization to MNI space (resampled to 1.5 mm3), spatial smoothing (8 × 8 × 8 kernel), calculation of mean CBF maps using basic image algebra and signal averaging, and the masking of CBF maps (using an MNI gray matter mask) to eliminate non-gray-matter voxels (Deibler et al., 2008; Tan et al., 2009).
Because participants were engaging in a number of cognitive, motor and emotional processes throughout the task, we sought to increase the probability that the brain regions that showed CBF changes from pre- to post-task were related to emotional processes. To do this, we used Neurosynth.org (Yarkoni, 2011; Yarkoni et al., 2011) to create an emotional-related activation mask. On Neurosynth.org, we conducted an automated meta-analysis with the keyword ‘emotion’, which returned a map of voxels that have shown reliable activation in studies that feature the term ‘emotion’ (number of studies = 790; forward inference). We then masked our CBF maps with this emotion mask. To determine which brain regions exhibited CBF changes from pre- to post- emotion task, we subtracted the pre-task from the post-task quantitative CBF maps and conducted a one-sample robust regression using dummy coded 1s as a predictor, which resembles a one-sample t-test of the difference between pre- and post-task CBF maps but reduces the influence of outliers (Wager et al., 2005). The resulting maps were then thresholded using a Monte Carlo simulation that calculated a cluster size of k = 199 needed for a per-voxel threshold of 0.001 to render a cluster-level corrected p-value of .05 (3dClustSim; estimated autocorrelation function = 0.74, 7.36, 20.74 using 3dFWHMx, one-sided thresholding, nearest neighbor 3 [faces, edges, vertices]; Cox, 1996; Cox et al., 2017). We then averaged the quantitative CBF values across the voxels for each significant cluster (separately for pre- and post-task maps), which then served as regions of interest (ROI) for subsequent data analyses. We also calculated pre- and post-task CBF values across the entire brain (gray matter-masked, but not emotion-masked) and used these values in the analyses to control for overall CBF changes.
Multilevel modeling analyses
We used multilevel linear modeling to account for the nested nature of the DRM data. This analytical method has previously been used in other studies employing the brain-as-predictor approach (Berkman et al., 2011). Several three-level hierarchical linear models were created in HLM version 6.08 (Scientific Software International Inc., Skokie, IL).
We ran three models, one for each of our inertia outcomes. In the ‘Stress inertia’ model, the outcome was participants’ overall level of stress reported about event et and the level 1 predictor was participants’ level of stress reported about the previous event et-1. In the ‘positive emotion inertia’ model, the outcome was participants’ reported level of positive emotion at the beginning of event et, with the level 1 predictor being participants’ reported level of positive emotion at the end of the prior event et – 1. The ‘negative emotion inertia’ model was similar except with negative emotion instead of positive emotion. These models allowed us to estimate both inertia from one event to the next as well as overall levels of stress/emotion controlling for emotion from the previous event.
This level 1 predictor was nested within each episode reported by participants (Level 2), which were themselves nested within each participant (Level 3). There were no additional level 2 predictors. Level 3 predictors for the CBF models included the post-task CBF of a given ROI controlling for pre-task CBF (quantified as the residuals left after regressing post-task values on pre-task values) representing participants’ shift in CBF controlling for their baseline CBF. Separate models were run with each ROI, but all models included pre-post residual changes in whole-brain CBF as a covariate. For example:
- Level 1:
- Level 2:
- Level 3:
The use of ‘z-’ as a prefix in the post-task models connotes that these post-task CBF controlled for pre-task CBF. The γ001 parameters represent post-task CBF in a particular ROI predicting overall stress/negative emotion/positive emotion levels and the γ101 parameters represent CBF predicting stress/negative emotion/positive emotion inertia between one event (et−1) and the next (et). The personality models were similar except they featured each personality variable (e.g., resilience, rumination-brooding) at Level 3 instead of CBF levels. Level 3 predictors were treated as random effects. We report robust standard errors and we used restricted maximum likelihood to estimate the coefficients (Bryk and Raudenbush, 1992).
We also report effect sizes for the main results, using proportional residual variance explained (prv, Peugh, 2010) for the brain-as-predictor MLM findings and semi-partial correlations for the linear regressions (rsp,Aloe and Becker, 2012).
Results
DRM response rates
On average, participants completed 6.4 days of observations (SD = 1.4) with an average of 62.0 total events reported (SD = 22.4) or 9.8 events per day (SD = 2.8).
Relationship between personality and emotional inertia/overall emotion level
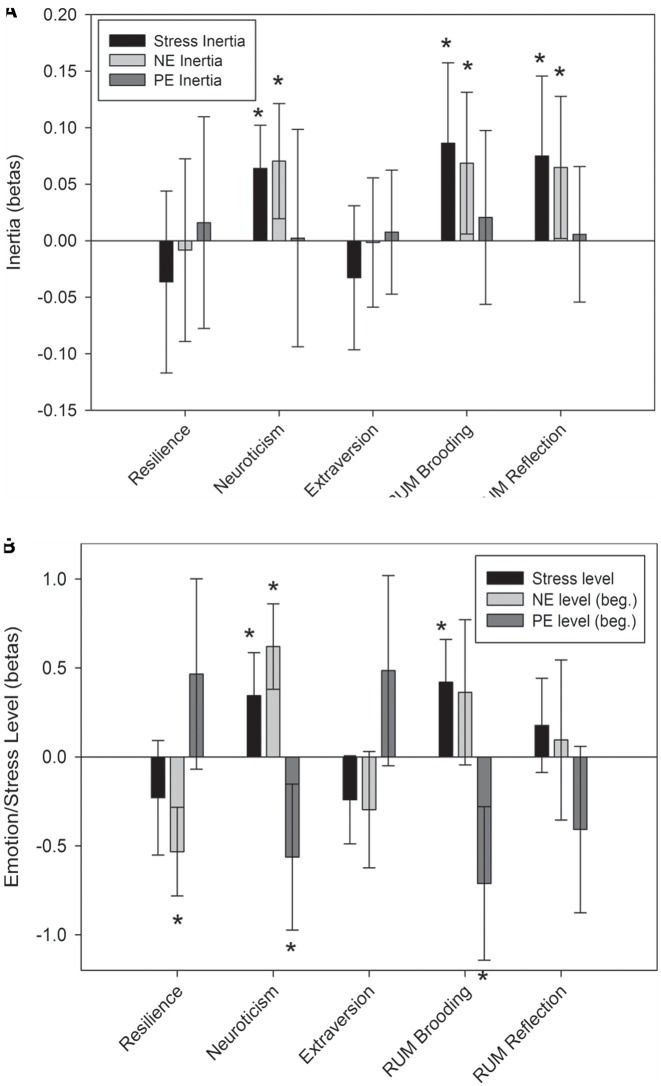
To validate our measure of emotional inertia, we first sought to replicate prior research that personality types like neuroticism and rumination predict increase in negative emotion/stress inertia (Figure 2). Confirming our expectations, neuroticism and rumination (both brooding and reflection) predicted increased stress inertia (neuroticism: γ101 = .064, t(26) = 3.44, P = .002, prv = 0.34; brooding: γ101 = .086, t(26) = 2.49, P = .02, prv = 0.46; reflection: γ101 = 0.075, t(26) = 2.18, P = 0.039, prv = 0.31) and negative emotion inertia (neuroticism: γ101 = 0.07, t(26) = 2.84, P = 0.009, prv = 0.11; brooding: γ101 = 0.069, t(26) = 2.25, P = 0.033, prv = 0.30; reflection: γ101 = 0.065, t(26) = 2.12, P = 0.044; prv = 0.27). Neuroticism and brooding also predicted increases in stress levels (neuroticism: γ001 = 0.34, t(26) = 2.92, P = 0.007, prv = 0.28; brooding: γ001 = 0.42, t(26) = 3.58, P = 0.001, prv = 0.36) and decreases in positive emotion levels (neuroticism: γ001 = −0.563, t(26) = 2.82, P = 0.009, prv = 0.19; brooding: γ001 = −0.711, t(26) = 3.38, P = 0.002, prv = 0.28). Lastly, neuroticism predicted increased negative emotion levels (γ001 = 0.62, t(26) = 5.31, P < 0.001, prv = 0.47) and resilience predicted decreased negative emotion levels (γ001 = −0.53, t(26) = 4.39, P < 0.001, prv = 0.32). These findings replicate past research on emotional inertia to confirm our emotional inertia assessment.
Fig. 2.
The association between personality traits and A. stress inertia from one daily event to the next, negative emotion inertia (NE) and positive emotion inertia (PE) from the end of one daily event to the beginning of the next; and B. levels of stress, negative emotion and positive emotion at the beginning (beg.) of each daily event. RUM = rumination.
Pre- to post-task CBF changes in the brain
Several brain regions exhibited significant increases in pre- to post-task CBF (Table 1; Figure 3), including emotion-generative and emotion-regulatory regions such as the vmPFC, lateral orbitofrontal cortex (lOFC), striatum, dmPFC, right LPFC,1 left dLPFC and precentral gyrus. There were also visuospatial regions including the occipital cortex and the parietal/occipital junction.2 No brain regions exhibited significant decreases in CBF from pre- to post-task.
Table 1.
Brain regions showing significant perfusion changes from Pre- to Post-task
| Region | x | y | z | Voxels | Volume (mm3) | t |
|---|---|---|---|---|---|---|
| Post-task > Pre-task | ||||||
| R OFC | 32 | 27 | −14 | 683 | 2305 | 7.32 |
| vMPFC | 4 | 48 | −16 | 375 | 1266 | 5.26 |
| R Striatum | 18 | 8 | 3 | 660 | 2228 | 6.63 |
| R Mid Temporal G. | 57 | −36 | −3 | 600 | 2025 | 6.73 |
| R Occipital | 50 | −69 | 2 | 452 | 1526 | 5.40 |
| R LPFC | 42 | 15 | 18 | 2869 | 9683 | 7.95 |
| dMPFC | 8 | 36 | 32 | 1790 | 6041 | 6.15 |
| R Parietal/Occipital | 44 | −58 | 34 | 1798 | 6068 | 8.19 |
| L dLPFC | −50 | 20 | 30 | 1240 | 4185 | 5.86 |
| Pre-task > Post-task | ||||||
| No regions survived threshold | ||||||
Note. OFC = orbitofrontal cortex, vMPFC = ventromedial prefrontal cortex, R = right, Mid = middle, G = gyrus, LPFC = lateral prefrontal cortex, dMPFC = dorsomedial prefrontal cortex, dLPFC = dorsolateral prefrontal cortex.
Fig. 3.
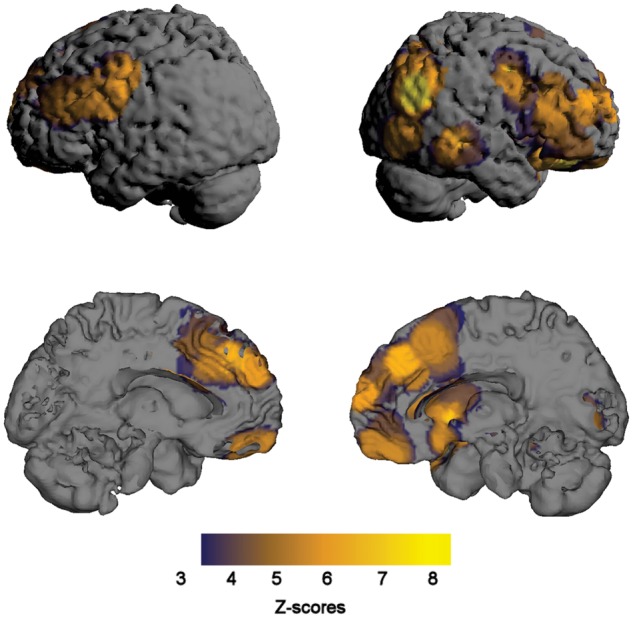
Brain regions that exhibited a significant increase in cerebral blood flow (CBF) as measured by arterial spin labeling (ASL) from before to after an emotional task.
Relationship between post-task CBF (controlling for pre-task CBF) and emotional inertia/overall emotion level
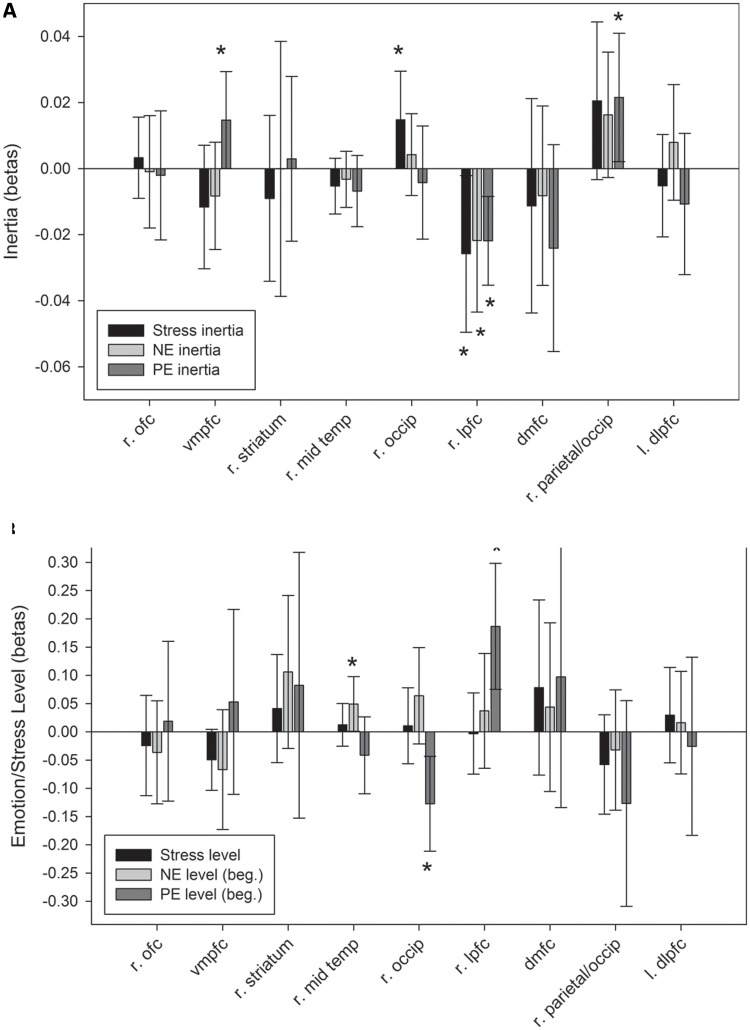
Stress inertia. Overall, there was significant inverse stress inertia (γ100 = −0.099, t(28) = 2.78, P = 0.01)—greater stress about one event tended to predict less stress on the next event. Post-task CBF in the right occipital cortex predicted increased stress inertia (γ101 = 0.015, t(26) = 2.06, P = 0.05, prv = 0.15), whereas CBF in the right lPFC predicted decreased stress inertia (γ101 = −0.026, t(26) = 2.23, P = 0.034, prv = 0.85; Figure 4).
Fig. 4.
The association between CBF changes (assessed as post-task CBF controlling for pre-task CBF) and A. stress inertia from one daily event to the next, negative emotion inertia (NE) and positive emotion inertia (PE) from the end of one daily event to the beginning of the next; and B. levels of stress, negative emotion and positive emotion at the beginning (beg.) of each daily event. The regions examined were those whose CBF changes significantly changes from pre- to post-emotion task and include (r = right, l = left) right orbitofrontal cortex (r. ofc), ventromedial prefrontal cortex (vmpfc), right striatum, right middle temporal gyrus (r. mid temp), right occipital cortex (r. occip), right lateral prefrontal cortex (r. lpfc), dorsomedial frontal cortex (dmfc), right parietal/occipital cortex (r. parietal/occip) and left dorsolateral prefrontal cortex (l. dlpfc).
Stress levels. There were no significant CBF predictors of overall levels of stress.
Positive emotion inertia. On average, there was not significant positive emotional inertia (γ100 = 0.041, t(28) = 1.20, P = 0.241). However, post-task CBF in the vmPFC (γ101 = 0.015, t(26) = 2.05, P = 0.05, prv = 0.52) and right parietal/occipital junction (γ101 = 0.021, t(26) = 2.27, P = 0.031, prv = 0.23) predicted increased positive emotional inertia, whereas, similar to stress inertia, post-task CBF in the right lPFC predicted decreased positive emotional inertia (γ101 = −0.022, t(26) = 3.33, P = 0.003, prv = 89; Figure 4).
Positive emotion levels. Post-task CBF levels in the right lPFC predicted overall increased positive emotion levels at the beginning of events (γ101 = 0.19, t(26) = 3.44, P = 0.002, prv = 0.20), whereas CBF levels in the right occipital cortex predicted overall decreased positive emotion levels (γ101 = −0.13, t(26) = −3.11, P = 0.004, prv = 20).
Negative emotion inertia. As with positive emotional inertia, there was no significant inertia in negative emotions from the end of one event to the beginning of the next (γ100 = −0.029, t(28) = −0.82, P = 0.421). However, as with both stress and positive emotion inertia, post-task CBF in the right lPFC predicted decreased negative emotional inertia between events (γ101 = −0.022, t(26) = 2.05, P = 0.05, prv = 0.54; Figure 4).
Negative emotion levels. Post-task CBF levels in the right middle temporal gyrus predicted increased negative emotion levels at the beginning of events (γ101 = 0.049, t(26) = 2.09, P = 0.046, prv = 0.10).
Correlations between personality and CBF in brain regions predicting inertia
We next ran correlations between the personality variables that showed a relationship to inertia and the post-task CBF of regions that showed a relationship to inertia. Post-task CBF in the occipital cortex, which predicted increased stress inertia and decreased positive emotion levels, was positively correlated with brooding (r = 0.47, P = 0.01) and negatively correlated with resilience (r = −0.49, P = 0.007). There were no other significant correlations between post-task CBF and personality, including in the lPFC, which had consistently predicted all three types of emotional inertia.
Exploring the relationship between post-task CBF in the lPFC and each type of inertia
Because post-task CBF in the lPFC predicted decreased inertia for stress, negative emotion and positive emotion, we next tested whether these effects were unique for each type of emotional inertia or formed a more general ‘inertia’ effect shared by all three emotions. From the HLM model, we extracted each participants’ estimated inertia slopes (e.g., of stress at time 1 on stress at time + 1) for all three types of inertia and correlated these with each other. There was indeed some overlap: stress inertia was positively correlated with negative emotional inertia (r = 0.51, P = 0.005), but not with positive emotional inertia (r = 0.06, P = 0.760). Negative and positive emotional inertia were negatively correlated (r = −0.57, P = 0.05). Because these different types of inertia were somewhat correlated with each other, we next examined whether post-task CBF in the lPFC predicted each type of inertia controlling for the other two types so an example of Level 3 of the model predicting stress inertia would now be:
When controlling for the other types of inertia, post-task CBF in the lPFC no longer predicted stress inertia (γ101 = −0.013, t(24) = −1.147, P = 0.263, prv = 0.13) and negative emotion inertia (γ101 = 0.003, t(24) = 0.293, P = 0.772, prv = −0.13), but still predicted positive emotion inertia (γ101 = −0.018, t(24) = −2.631, P = 0.015, prv = 0.99).
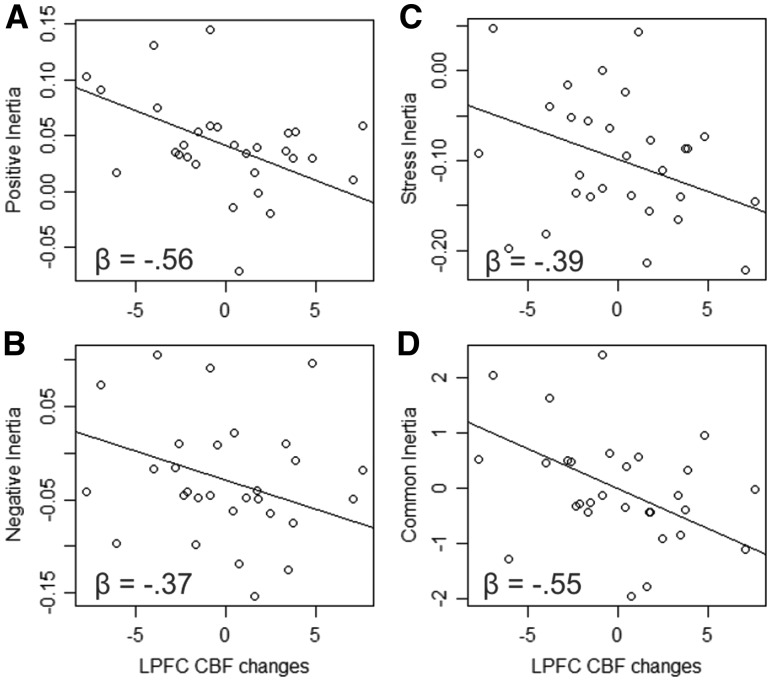
Next, we conducted an exploratory principal components factor analysis on the three types of inertia to examine whether they loaded on a single ‘inertia’ factor. This resulted in three components, only one of which had an eigenvalue above 1 (1.859), which accounted for 62% of the variance, suggesting a single factor solution captured well these types of inertia. All three types of inertia loaded highly on this one factor (stress: 0.712, negative emotion: 0.893, positive emotion: 0.744) suggesting that there may be a common ‘inertia’ mechanism. We next extracted one score for each participant that represented this ‘common inertia’ factor. Post-task CBF in the lPFC (controlling for overall brain CBF) robustly predicted this common inertia factor, β = −0.551, t(26) = −2.78, P = 0.01, rsp = −0.47 (Figure 5). Notably, post-task CBF in the occipital cortex, which had only predicted stress inertia did not predict this common inertia factor, β = 0.199, t(26) = 0.90, P = 0.377, rsp = 0.17, and post-task CBF in the vMPFC, which had only predicted positive emotion inertia, also did not predict this common inertia factor, β = −0.126, t(26) = −0.551, P = 0.586, rsp = −0.11.
Fig. 5.
Plots of the relationships (betas from the hierarchical linear models) between cerebral blood flow (CBF) changes in the lateral prefrontal cortex (LPFC) and each of the three types of inertia (positive emotion, negative emotion and stress) as well as a common factor underlying all three types of inertia (common inertia).
Discussion
This study contributes significantly to the budding literature on the temporal dynamics of emotion in the brain by investigating the neural predictors of emotional inertia. Our primary finding was that increases in CBF in the lPFC from before to after an emotional task predicted decreases in emotional inertia during daily life. We hypothesized that emotional inertia was at least in part due to unsuccessful emotion regulation. Supporting this hypothesis, numerous studies have suggested that the lPFC plays a vital role in emotion regulation (Ochsner and Gross, 2005; Wager et al., 2008b; Otto et al., 2014). Although it has been found to be associated with strategically decreasing (Wager et al., 2008b; Otto et al., 2014), maintaining (Waugh et al., 2014), and increasing emotion (Ochsner et al., 2004), it seems to be most robustly associated with decreasing emotion (Ochsner et al., 2004) through its connections with subcortical regions such as the nucleus accumbens and amygdala (Wager et al., 2008b).
Although the vast majority of emotion regulation studies investigating lPFC activity have shown that it is associated with decreasing negative emotion, the fact that CBF changes in the lPFC in the current study predicted decreased positive and negative emotional inertia suggests that its effect on emotional inertia may reflect a valence-general regulatory mechanism. One such possible mechanism is the inhibition of pre-potent responses, found to be highly associated with and even reliant on the lPFC (Aron et al., 2004). One explanation of the role of the lPFC in emotional inertia might be that its activation increased from before to after the emotional task as a response to the demands of chronically inhibiting emotional stimuli. Those participants who tended to recruit lPFC in response to repeated emotional events in the scanner may have also recruited lPFC in daily life in order to inhibit emotional responses from a prior event and prevent them from spilling over to the next event. However, this explanation is speculative given that we did not assess lPFC activation in daily life, and will need to be tested in future investigations that assess emotional inertia in patients with lesions in this region or with ambulatory neural recordings.
Consistent with prior research, the depressotypic traits of neuroticism and rumination predicted increased stress and negative emotional inertia (Suls et al., 1998; Koval et al., 2013), further supporting the formulation that emotional inertia reflects poor emotional flexibility and emotional regulation. Notably, depression is also associated with functional and structural dysregularities in the lPFC (Fitzgerald et al., 2008; Kupfer et al., 2012). In one study, investigators found that participants with depression engaged the lPFC much less so than did control participants when asked to suppress thoughts—a paradigm meant to simulate rumination (Carew et al., 2013). Therefore, the relationship between neuroticism/rumination and increased negative emotional inertia may be mediated by dysregulation of the lPFC. An important caveat to this formulation, however, is that in the current study, CBF changes in the lPFC were not correlated with any of these personality variables. This may be due to having limited power to detect relationships between broad personality traits and activation in specific brain regions (Yarkoni, 2009), or to lPFC CBF and personality tapping into different mechanisms underlying emotional inertia. This latter formulation is supported in part by the finding that lPFC CBF predicted a valence-general form of emotional inertia, while neuroticism and rumination only predicted negatively valenced emotional inertia. In any case, future investigations should more closely investigate the potential relationships among depression, emotional inertia and lPFC dysregulation.
CBF changes in the lPFC also predicted increased positive emotion at the beginning of daily life events. This finding is consistent with findings from previous studies in which lPFC activation was found to be associated with positive stimuli (Koelsch et al., 2006). In addition, it has been theorized that the lPFC improves emotion regulation not only via inhibiting regions like the amygdala but also through activating regions like the nucleus accumbens (Wager et al., 2008b) a region associated with positive emotional states like reward anticipation (Knutson et al., 2001). In conjunction with the finding from the current study that CBF changes in the lPFC predicted decreased emotional inertia (including positive emotional inertia), it’s clear that the lPFC seems to be involved with improved emotional flexibility—decreasing the emotional carry-over from one event to the next while also improving overall levels of positive emotion (Waugh et al., 2011).
CBF changes in the vmPFC and occipital cortex exhibited more valence-specific associations with emotional inertia. VMPFC CBF predicted increased positive emotion inertia and occipital cortex CBF predicted increased stress inertia and CBF in these regions did not predict the ‘common inertia’ factor that was predicted by lPFC CBF. The relationship between vmPFC and positive emotion inertia is consistent with previous findings that positive emotional stimuli robustly and consistently activate the vmPFC (Wager et al., 2008a; Winecoff et al., 2013). Importantly, however, it is unclear whether the vmPFC is also associated with the up-regulation and/or maintenance of positive emotion, a proposed mechanism underlying positive emotional inertia. Indeed, the few studies examining the neural correlates of increasing or maintaining positive emotions generated from positive emotional stimuli do not show vmPFC to be involved, but rather a slightly more dorsal region in the rostral mPFC (Kim and Hamann, 2007; Waugh et al., 2014). Less clear is why there was a relationship between occipital cortex CBF and increased stress inertia. On one hand, there is robust evidence that occipital cortex’s activation is modulated by the emotional content of visual stimuli (indeed, this is most likely the reason it was included in our meta-analytic ‘emotion mask’; Vuilleumier, 2005; Peelen et al., 2007), especially negative images (Gollan et al., 2015). However, this particular region (lateral occipital cortex or extrastriate body region) seems to respond most selectively to images of human bodies (Downing et al., 2001), and is therefore most consistently modulated when viewing bodies expressing emotion (Peelen et al., 2007). Participants in our emotional task did not view human bodies as stimuli, so it is difficult to discern what element of the emotional task led to increased activation in this region. Furthermore, although there is some evidence that this region is associated with inhibition of emotion (Goldin et al., 2008) and social inhibition (Kret et al., 2011), this region’s role in emotion regulation is still unknown and unsuccessful emotion regulation is our hypothesized mechanism underlying stress inertia. In sum, although occipital cortex CBF in the current study was clearly associated with stress inertia and brooding rumination, its role in these elements of depression will need to be illuminated in future investigations.
There are several open questions from the current study that reflect some if its limitations. Although there is evidence in the current study that top-down emotional regulatory regions such as the lPFC may be involved with stemming emotional inertia, other emotional regulatory regions such as the dmPFC did not exhibit a relationship with emotional inertia, and some emotion-generative regions such as the vmPFC and emotionally modulated regions such as the lateral occipital cortex also exhibited a relationship with emotional inertia. Clarification into these relationships can be attained in future studies that feature emotional tasks that are specifically designed to tease apart emotion generation, regulation and modulation. Another related limitation of the present study is that because we aimed to capture state changes in neural activation (via assessments of CBF) over the course of several minutes from before an emotional task to after it, we cannot infer precisely what was causing the observed changes in CBF. These changes in CBF could have been due to a myriad of emotional processes (as noted above), to non-emotional processes related to the task such as executive control, motor responding, visual stimulation, etc, and/or to processes unrelated to the task such as attention and fatigue. Although we masked our activation patterns with a mask derived from a meta-analysis, this only increases our confidence that activation in these regions may be related to emotion, but does not exclude the possibility that it may be also due to other non-emotion related processes. Related to this uncertainty is the fact that we designed this emotional task to test other hypotheses not presented in this study. Future investigations will need to design more sophisticated and tailored intervening tasks to specify precisely what types of processing these regions were responsible for. Another limitation was our use of DRM to assess emotional inertia. We used DRM so that participants could provide an account of their emotional responses to each episode of the day, however, because these reports are retospective and not moment to moment (as in typical inertia studies; Kuppens et al., 2010), they are subject to memory biases. Although we found similar relationships between neuroticism and rumination and emotional inertia as those studies there remains the possibility that our retrospective measures may be tapping into a different form of inertia than are previous studies.
In conclusion, the present study featured the first investigation into the neural predictors of emotional inertia—a significant correlate of negative psychological traits such as depression and neuroticism. We found that CBF changes in the lPFC predicted decreased valence-general emotional inertia, suggesting that the lPFC may feature a general inhibitory mechanism responsible for limiting the impact that the emotional states from one event have on the emotional states of a subsequent event. We also found that CBF changes in some regions such as the vmPFC and lateral occipital cortex were associated with valence-specific emotional inertia (positive emotional inertia and negative/stress inertia, respectively), although it is not yet clear the mechanisms underlying these relationships. Future investigations can extend these important findings by featuring emotional tasks that better specify the emotional processes hypothesized to underlie emotional inertia.
Acknowledgements
The authors would like to thank Katherine Sams, Cameron Ford and Nathan Hagar for their help in conducting this study.
Funding
This work was supported by internal funds from Wake Forest University awarded to CW and the Louis Argenta Physician-Scientist Fellowship from Wake Forest School of Medicine awarded to ES. We would also like to acknowledge the Translational Imaging Program (TIP) of the Wake Forest CTSI, which is supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR001420.
Conflict of interest. None declared.
Footnotes
1 Using z = 20 as the demarcation between dorsal (z > 20) and ventral (z < 20) regions of the lPFC associated with the cognitive control of emotion (Ochsner and Gross, 2005), the lPFC region in the current study clearly extends to both (∼41% of voxels z < 20, z range −2 to 50).
2 Although there were no significant changes in CBF from pre- to post-emotion task, we tested whether individual differences in CBF changes in the amygdala were related to emotional inertia. We extracted CBF changes (post-task controlling for pre-task) from bilateral amygdala ROIs, which were defined anatomically using the WFU pickatlas (+1 dilation; Maldjian et al., 2003), and then regressed these on each of the emotional inertia indices while controlling for overall CBF changes in the brain. Neither of the amygdala ROIs significantly predicted any of the three emotional inertia indices.
References
- Aloe A.M., Becker B.J. (2012). An effect size for regression predictors in meta-analysis. Journal of Educational and Behavioral Statistics, 37, 278–97. [Google Scholar]
- Aron A.R., Robbins T.W., Poldrack R.A. (2004). Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences, 8, 170–7. [DOI] [PubMed] [Google Scholar]
- Berkman E.T., Falk E.B. (2013). Beyond brain mapping: Using neural measures to predict real-world outcomes. Current Directions in Psychological Science, 22, 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman E.T., Falk E.B., Lieberman M.D. (2011). In the trenches of real-world self-control: Neural correlates of breaking the link between craving and smoking. Psychological Science, 22, 498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block J., Kremen A.M. (1996). IQ and ego-resiliency: conceptual and empirical connections and separateness. Journal of Personality and Social Psychology, 70, 349–61. [DOI] [PubMed] [Google Scholar]
- Borogovac A., Asllani I. (2012). Arterial Spin Labeling (ASL) fMRI: Advantages, theoretical constrains and experimental challenges in neurosciences. International Journal of Biomedical Imaging, 2012, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borogovac A., Habeck C., Small S.A., Asllani I. (2010). Mapping brain function using a 30-day interval between baseline and activation: a novel arterial spin labeling fMRI approach. Journal of Cerebral Blood Flow and Metabolism, 30, 1721–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M.M., Lang P.J. (1999). Affective Norms for English Words (ANEW): Stimuli, Instruction Manual and Affective Ratings. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida. [Google Scholar]
- Brosschot J.F., Gerin W., Thayer J.F. (2006). The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. Journal of Psychosomatic Research, 60, 113–224. [DOI] [PubMed] [Google Scholar]
- Bryk A., Raudenbush S. (1992). Hierarchical Linear Models: Applications and Data Analysis Methods. Newbury Park: Sage Publications. [Google Scholar]
- Carew C.L., Milne A.M., Tatham E.L., MacQueen G.M., Hall G.B. (2013). Neural systems underlying thought suppression in young women with, and at-risk, for depression. Behavioural Brain Research, 257, 13–24. [DOI] [PubMed] [Google Scholar]
- Costa P.T., McCrae R.R. (1980). Influence of extraversion and neuroticism on subjective well-being: Happy and unhappy people. Journal of Personality and Social Psychology, 38, 668–78. [DOI] [PubMed] [Google Scholar]
- Costa P.T., McCrae R.R. (1992). Normal personality assessment in clinical practice: the NEO personality inventory. Psychological Assessment, 4, 5–13. [Google Scholar]
- Cox R.W. (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29, 162–73. [DOI] [PubMed] [Google Scholar]
- Cox R.W., Chen G., Glen D.R., Reynolds R.C., Taylor P.A. (2017). FMRI clustering in AFNI: false positive rates redux. Brain Connectivity, 7, 152–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W., Garcia D., de Bazelaire C., Alsop D.C. (2008). Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magnetic Resonance in Medicine, 60, 1488–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deibler A.R., Pollock J.M., Kraft R.A., Tan H., Burdette J.H., Maldjian J.A. (2008). Arterial spin-labeling in routine clinical practice, part 3: hyperperfusion patterns. American Journal of Neuroradiology, 29, 1428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing P.E., Jiang Y., Shuman M., Kanwisher N. (2001). A cortical area selective for visual processing of the human body. Science, 293, 2470–3. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I., Master S.L., Inagaki T.K., et al. (2011). Attachment figures activate a safety signal-related neural region and reduce pain experience. PNAS Proceedings of the National Academy of Sciences of the United States of America, 108, 11721–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald P.B., Laird A.R., Maller J., Daskalakis Z.J. (2008). A meta-analytic study of changes in brain activation in depression. Human Brain Mapping, 29, 683–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson B.L., Tugade M.M., Waugh C.E., Larkin G.R. (2003). What good are positive emotions in crisis? A prospective study of resilience and emotions following the terrorist attacks on the United States on September 11th, 2001. Journal of Personality and Social Psychology, 84, 365–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijda N.H. (2007). The Laws of Emotion. Hillsdale, NJ: Erlbaum. [Google Scholar]
- Goldin P.R., McRae K., Ramel W., Gross J.J. (2008). The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry, 63, 577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan J.K., Connolly M., Buchanan A., et al. (2015). Neural substrates of negativity bias in women with and without major depression. Biological Psychology, 109, 184–91. [DOI] [PubMed] [Google Scholar]
- Groen G., Sokolov A.N., Jonas C., Roebling R., Spitzer M. (2011). Increased resting-state perfusion after repeated encoding is related to later retrieval of declarative associative memories. PLoS One, 6, e19985.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller A.S., Fox A.S., Wing E.K., McQuisition K.M., Vack N.J., Davidson R.J. (2015). The neurodynamics of affect in the laboratory predicts persistence of real-world emotional responses. Journal of Neuroscience, 35, 10503–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller A.S., Johnstone T., Shackman A.J., et al. (2009). Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proceedings of the National Academy of Sciences, 106, 22445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C., Bach D.R., Esposito F., et al. (2007). Processing of temporal unpredictability in human and animal amygdala. Journal of Neuroscience, 27, 5958–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houben M., Van Den Noortgate W., Kuppens P. (2015). The relation between short-term emotion dynamics and psychological well-being: A meta-analysis. Psychological Bulletin, 141, 901–30. [DOI] [PubMed] [Google Scholar]
- Kahneman D., Krueger A.B., Schkade D.A., Schwarz N., Stone A.A. (2004). A survey method for characterizing daily life experience: the day reconstruction method. Science, 306, 1776–80. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Hamann S. (2007). Neural correlates of positive and negative emotion regulation. Journal of Cognitive Neuroscience, 19, 776–98. [DOI] [PubMed] [Google Scholar]
- Kito S., Hasegawa T., Koga Y. (2012). Cerebral blood flow ratio of the dorsolateral prefrontal cortex to the ventromedial prefrontal cortex as a potential predictor of treatment response to transcranial magnetic stimulation in depression. Brain Stimulation, 5(4), 547–53. [DOI] [PubMed] [Google Scholar]
- Knutson B., Fong G.W., Adams C.M., Varner J.L., Hommer D. (2001). Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport, 12, 3683–7. [DOI] [PubMed] [Google Scholar]
- Koelsch S., Fritz T., Von Cramon D.Y., Muller K., Friederici A.D. (2006). Investigating emotion with music: an fMRI study. Human Brain Mapping, 27, 239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koval P., Kuppens P., Allen N.B., Sheeber L. (2012). Getting stuck in depression: The roles of rumination and emotional inertia. Cognition and Emotion, 26, 1412–27. [DOI] [PubMed] [Google Scholar]
- Koval P., Pe M.L., Meers K., Kuppens P. (2013). Affect dynamics in relation to depressive symptons: Variable, unstable, or inert? Emotion 13, 1132–41. [DOI] [PubMed] [Google Scholar]
- Koval P., Brose A., Pe M.L., et al. (2015). Emotional inertia and external events: The roles of exposure, reactivity, and recovery. Emotion, 15, 625–36. [DOI] [PubMed] [Google Scholar]
- Kret M.E., Denollet J., Grezes J., De Gelder B. (2011). The role of negative affectivity and social inhibition in perceiving social threat: an fMRI study. Neuropsychologia, 49, 1187–93. [DOI] [PubMed] [Google Scholar]
- Kuhn M.H., McPartland T.S. (1954). An empirical investigation of self-attitudes. American Sociological Review, 19, 68–76. [Google Scholar]
- Kupfer D.J., Frank E., Phillips M.L. (2012). Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet, 379, 1045–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppens P., Allen N.B., Sheeber L.B. (2010). Emotional inertia and psychological maladjustment. Psychological Science, 21, 984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen R.J., Augustine A.A., Prizmic Z. (2009). A process approach to emotion and personality: using time as a facet of data. Cognition & Emotion, 23, 1407–26. [Google Scholar]
- Maldjian J.A., Baer A.H., Kraft R.A., Laurienti P.J., Burdette J.H. (2009). Fully automated processing of fMRI data in SPM: from MRI scanner to PACS. Neuroinformatics, 7, 57–72. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fmri data sets. Neuroimage, 19, 1233–9. [DOI] [PubMed] [Google Scholar]
- McRae K., Hughes B., Chopra S., Gabrieli J.D.E., Gross J.J., Ochsner K.N. (2010). The neural bases of distraction and reappraisal. Journal of Cognitive Neuroscience, 22, 248–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S., Morrow J. (1993). Effects of rumination and distraction on naturally occurring depressed mood. Cognition & Emotion, 7, 561–70. [Google Scholar]
- Nolen-Hoeksema S., Morrow J., Fredrickson B.L. (1993). Response styles and the duration of episodes of depressed mood. Journal of Abnormal Psychology, 102, 20–8. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9, 242–9. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Ray R.D., Cooper J.C., et al. (2004). For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage, 23, 483–99. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Silvers J.A., Buhle J.T. (2012). Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Year in Cognitive Neuroscience, 1251, E1–E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto B., Misra S., Prasad A., McRae K. (2014). Functional overlap of top-down emotion regulation and generation: an fMRI study identifying common neural substrates between cognitive reappraisal and cognitively generated emotions. Cognitive Affective and Behavioral Neuroscience, 14, 923–38. [DOI] [PubMed] [Google Scholar]
- Peelen M.V., Atkinson A.P., Andersson F., Vuilleumier P. (2007). Emotional modulation fo body-selective visual areas. Social Cognitive and Affective Neuroscience, 2, 274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peugh J.L. (2010). A practical guide to multilevel modeling. Journal of School Psychology, 48, 85–112. [DOI] [PubMed] [Google Scholar]
- Quoidbach J., Berry E.V., Hansenne M., Mikolajczak M. (2010). Positive emotion regulation and well-being: Comparing the impact of eight savoring and dampening strategies. Personality and Individual Differences, 49, 368–73. [Google Scholar]
- Schuyler B.S., Kral T.R.A., Jacquart J., et al. (2014). Temporal dynamics of emotional responding: amygdala recovery predicts emotional traits. Social Cognitive and Affective Neuroscience, 9, 176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle G.J., Steinhauer S.R., Thase M.E., Stenger V.A., Carter C.S. (2002). Can't shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry, 51, 693–707. [DOI] [PubMed] [Google Scholar]
- Suls J., Green P., Hillis S. (1998). Emotional reactivity to everyday problems, affective inertia, and neuroticism. Personality and Social Psychology Bulletin, 24, 127–36. [Google Scholar]
- Tan H., Maldjian J.A., Pollock J.M., et al. (2009). A fast, effective filtering method for improving clinical pulsed arterial spin labeling MRI. Journal of Magnetic Resonance Imaging, 29, 1134–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treynor W., Gonzalez R., Nolen-Hoeksema S. (2003). Rumination reconsidered: A psychometric analysis. Cognitive Therapy and Research, 27, 247–59. [Google Scholar]
- Tugade M.M., Fredrickson B.L. (2004). Resilient individuals use positive emotions to bounce back from negative emotional experiences. Journal of Personality and Social Psychology, 86, 320–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry H.L., van Reekum C.M., Johnstone T., et al. (2006). Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience, 26, 4415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velten E., Jr. (1968). A laboratory task for induction of mood states. Behaviour Research and Therapy, 6, 473–82. [DOI] [PubMed] [Google Scholar]
- Verduyn P., Delvaux E., Van Coillie H., Tuerlinckx F., Van Mechelen I. (2009). Predicting the duration of emotional experience: Two experience sampling studies. Emotion, 9, 83–91. [DOI] [PubMed] [Google Scholar]
- Verduyn P., Mechelen I.V., Tuerlinckx F. (2011). The relation between event processing and duration of emotional experience. Emotion, 11, 20–8. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. (2005). How brains beware: neural mechanisms of emotional attention. Trends in Cognitive Sciences, 9, 585–93. [DOI] [PubMed] [Google Scholar]
- Wager T.D., Barrett L.F., Bliss-Moreau E., Lindquist K., Duncan S., Kober H. (2008a). The Neuroimaging of Emotion In: Lewis M., editor. Handbook of Emotion, 3rd Edition New York: Guilford Press. [Google Scholar]
- Wager T.D., Davidson M.L., Hughes B.L., Lindquist M.A., Ochsner K.N. (2008b). Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron, 59, 1037–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Keller M.C., Lacey S.C., Jonides J. (2005). Increased sensitivity in neuroimaging analyses using robust regression. Neuroimage, 26, 99–113. [DOI] [PubMed] [Google Scholar]
- Wang J., Aguirre G.K., Kimberg D.Y., Roc A.C., Li L., Detre J.A. (2003). Arterial spin labeling perfusion fMRI with very low task frequency. Magnetic Resonance in Medicine, 49, 796–802. [DOI] [PubMed] [Google Scholar]
- Waugh C.E., Hamilton J.P., Gotlib I.H. (2010). The neural temporal dynamics of the intensity of emotional experience. Neuroimage, 49, 1699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh C.E., Lemus M.G., Gotlib I.H. (2014). The role of the medial frontal cortex in the maintenance of emotional states. Social Cognitive and Affective Neuroscience, 9, 2001–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh C.E., Shing E.Z., Avery B.M. (2015). Temporal dynamics of emotional processing in the brain. Emotion Review, 7, 323–9. [Google Scholar]
- Waugh C.E., Thompson R.J., Gotlib I.H. (2011). Flexible emotional responsiveness in trait resilience. Emotion, 11, 1059–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer A., Gotlib I.H. (2011). Brooding and reflection reconsidered: A factor analytic examination of rumination in currently depressed, formerly depressed, and never depressed individuals. Cognitive Therapy and Research, 35, 99–107. [Google Scholar]
- Winecoff A., Clithero J.A., Carter R.M., Bergman S.R., Wang L., Huettel S.A. (2013). Ventromedial prefrontal cortex encodes emotional value. Journal of Neuroscience, 33, 11032–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E.C. (1999). Potential and pitfalls of arterial spin labeling based perfusion imaging techniques for MRI In: Moone C.T.W., Bandettini P.A., editors. Functional MRI (pp. 63–9). Heidelberg: Springer-Verlag. [Google Scholar]
- Wong E.C., Buxton R.B., Frank L.R. (1998). Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II). Magnetic Resonance in Medicine, 39, 702–8. [DOI] [PubMed] [Google Scholar]
- Yarkoni T. (2009). Big correlations in little studies: Inflated fMRI correlations reflect low statistical power - Commentary on Vul et al., (2009). Perspectives on Psychological Science, 4, 294–8. [DOI] [PubMed] [Google Scholar]
- Yarkoni T. (2011). Neurosynth.org. from neurosynth.org
- Yarkoni T., Poldrack R.A., Nichols T.E., Van Essen D.C., Wager T.D. (2011). Large-scale automated synthesis of human functional neuroimaging data. Nature Methods, 8, 665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan F., Emerson N.M., Farris S.R., et al. (2015). Mindfulness meditation-based pain relief employs different neural mechanisms than placebo and sham mindfulness meditation-induced analgesia. Journal of Neuroscience, 35, 15307–25. [DOI] [PMC free article] [PubMed] [Google Scholar]