Abstract
Background and Objectives:
Renal stone disease is a common disorder of the urinary tract and also a significant problem because of incidence, recurrence, and severe consequences. The complex pathogenetic mechanisms of renal stone formation involve both biologic and environmental risk factors. The present study was performed to identify the role of these parameters among renal stone patients and normal individuals from a coastal union territory region in South India.
Methods:
The authors conducted a case–control study of renal stone disease among outpatient department patients more than 30 years of age using systematic random sampling procedure with 100 study participants (50 subjects for each group). A questionnaire to explore some relevant history as well as to note general examination findings was used along with a house visit to collect a sample of water. Analysis was undertaken using appropriate statistical techniques.
Results:
The study showed statistically significant association for renal stones with female sex, illiteracy, body mass index (BMI) (>25 kg/m2), sodium (>50 mg/L), water consumption (<1.5 L/day), water source being borewell, consuming soft drink, sedentary work, and family history of renal stones. The adjusted odds ratios (ORs) were significantly higher for consuming soft drink (OR: 8.19; 95% confidence interval: 1.99–33.69), sedentary work (10.01; 1.27–78.91), and water consumption < 1.5 L/day (7.73; 2.24–26.69).
Interpretation and Conclusions:
We conclude that in this part of India, female gender, illiteracy, high BMI, high sodium in drinking water, inadequate water consumption, borewell drinking water, soft-drink consumption, sedentary work, and family history of renal stones can lead to a significant increase in the risk of renal stone disease.
Keywords: Calcium, drinking water, renal stone, sedentary work, sodium
Introduction
Renal stone disease is a common disorder and a significant problem because of incidence, recurrence, and severe consequences.[1] Calcium oxalate and calcium phosphate make up at least 80% of all kidney stones, infection-induced and uric acid stones occur in 10% and 8%, respectively.[2] Urinary schistosomiasis was the main etiological factor correlated with the occurrence of urolithiasis. Urinary stone disease in association with hypertension, cardiovascular disease, atherosclerosis, obesity, dyslipidemia, diabetes, and other disease states.[3]
Renal stone occurs in all parts of the world. The annual occurrence of urinary tract stones in the industrialized world is estimated to be 0.2%. A lifetime risk of 2%–5% has been noted for in India, 8%–15% for the West, and 20% for Saudi Arabia.[4] In developing countries, calculi in bladder are more common than upper urinary tract stones; the opposite is true in developed countries.[5]
It is estimated that at least 10% of the population in the industrialized part of the world is afflicted by urinary tract stone disease. The prevalence to renal stone is increased in the past 10 years.[6] The renal calculi would lead to the following complications: diminished renal function, urinary fistula formation, ureteral scarring and stenosis, ureteral perforation, extravasation, urosepsis, and renal failure. Acute nephrolithiasis is associated with a unique set of complications during pregnancy.[7]
Eating habits, alcohol consumption and smoking act as risk factors in kidney one stone formation. Some other factors include age, gender, race, diuretic use, low fluid intake, and low urine volume. Regular drinking of tea, urolithiasis history, and brain work are the risk factors of kidney calculi.[8]
Nephrolithiasis is associated with a high cost to society because of high prevalence of the disease and high rates of recurrence. The total annual medical expenditures for renal stones in the US were estimated at $2.1 billion in 2000.[9] There are no exact data available for India. The average outpatient department (OPD) diagnosis in Government Hospital, Karaikal, is 5/day which gives rise to 1800 cases per year. Assuming double the number of cases would happen in Karaikal, 3600 cases receive treatment for symptomatic kidney disease. On an average, 50% of symptomatic cases would be requiring stone removal surgeries and this would amount to 270 lacs, keeping Rs. 15,000 per surgery. As such there are very few epidemiological studies in India, involving all the environmental and other common risk factors responsible for the increasing occurrence of renal stones. This study would enlighten us to know the causative factors mainly prevalent in this region of Puducherry and simple measures to avoid the huge health-care burden on the society as well as the government.
Materials and Methods
The study was carried out among the residents of Karaikal, aged more than 30 years attending the medicine OPD of a tertiary care hospital of Karaikal, Puducherry, between April 2014 and July 2014. This case–control study was done among hospital OPD patients using systematic random sampling procedure till the required sample size was arrived.
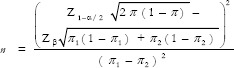
Assuming the proportion of increased mineral content in the drinking water of cases and controls as 50% and 25%, respectively, with alpha error as 10% and power as 80%, the minimum sample size to test the hypothesis was found to be 45 using the above formula and we included 50 subjects in each group.
Newly diagnosed (using ultrasonography [USG] abdomen) renal stone patients more than 30 years of age was the criterion for selecting renal calculi cases. Appropriate hospital-based controls more than 30 years of age were selected after ruling out renal stones using USG abdomen. Cases of carcinoma, chronic infections, endocrine disorder, and nonresidents of Karaikal were excluded from both the cases and controls. Renal stones with complication were excluded from cases. The study protocol was approved by the Institutional Ethical Committee.
After having the informed written consent, freshly diagnosed cases of renal stones were administered a questionnaire and their body composition measurements were made. The controls who were not found to be having renal stones also underwent the same procedure as cases. Apart from this, a house visit was also done to collect water sample from each study participant's house for chemical analysis.
Statistical analysis
Data entry was done in MS Excel. Statistical analysis was done using Epi Info 2002, Centers for Disease Control and Prevention (CDC) in Atlanta, Georgia (USA) and IBM SPSS Statistics 20 (Evaluation copy, AFMC, Pune, India). Continuous data were expressed as mean ± standard deviation and Z-test was used to compare normally distributed variables and Mann–Whitney U-test for nonparametric data. Categorical data were expressed as frequencies and percentages, and wherever applicable, Chi-square test and Fisher's exact test were used to compare. Odds ratio (OR; 95% confidence interval [CI]) was also used to interpret the results. Variables found significantly associated with renal stones were entered into multivariable logistic regression models to control for the potential confounding variables. P <0.05 was considered statistically significant.
Results
The study involved 50 newly diagnosed renal stone patients and 50 controls. The mean age of cases and controls was 47.66 ± 13.87 year and 52.90 ± 13.55 year, respectively. There was no statistically significant difference between case patients and controls with respect to the age (P = 0.059) [Table 1]. Although there was a significant difference in the gender with females having more risk for renal stones, its adjusted OR did not show any significant risk.
Table 1.
Clinical and demographic characteristics of study participants and their drinking water sample parameters
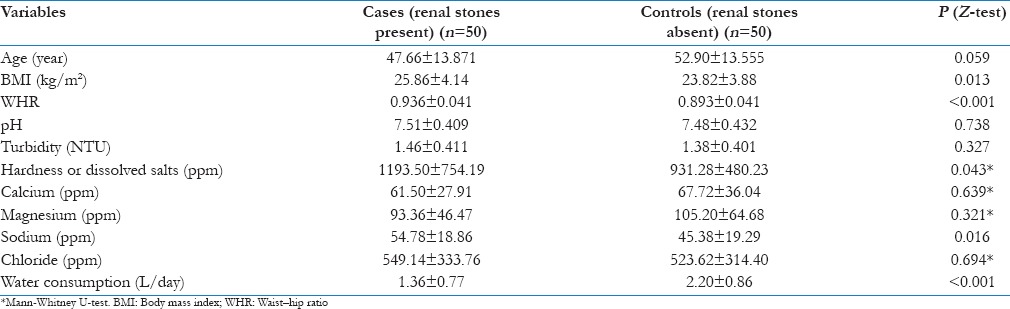
Clinical parameters like BMI and WHR showed statistically significant higher values among renal stone patients than in controls. The renal stone patients had been consuming significantly higher amount of dissolved salts and sodium in their drinking water, although they consumed significantly less water than controls [Table 1].
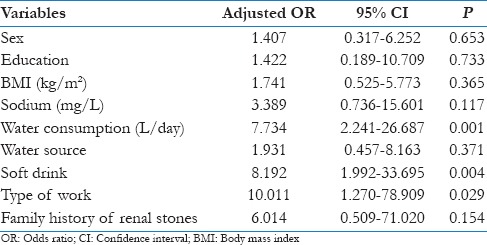
Univariate analysis showed statistically significant for renal stones with female sex, illiterate, BMI (>25 kg/sq.m), sodium (>50 mg/litre), water consumption (<1.5 litre/day), water source being borewell, consuming soft-drink, sedentary work and family history of renal stones [Table 2]. Though Odds ratios were higher for pH>7, TDS>1000mg/litre, magnesium >40mg/litre, chloride >250 mg/litre and turbidity>1NTU, these were not statistically significant (P > 0.05).
Table 2.
Categorical variables associated with renal stones
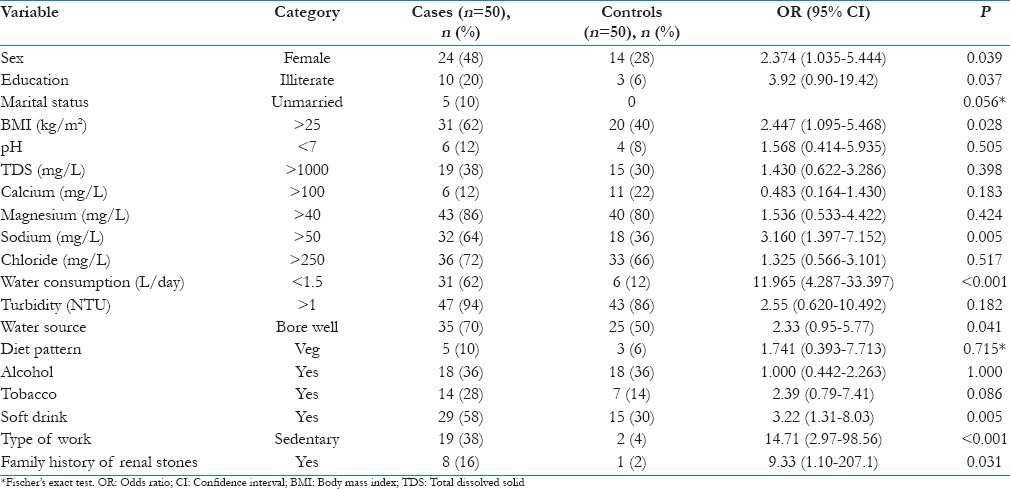
In multivariate analysis, the adjusted ORs were significantly higher for consuming soft-drink (OR: 8.19; 95% CI: 1.99-33.69), sedentary work (10.01; 1.27-78.91) and water consumption< 1.5litre/d (7.73; 2.24-26.69) [Table 3]. Though there was a significant difference in the gender with females having more risk for renal stones, its adjusted odds ratio didn’t show any significant risk [Table 3].
Table 3.
Multivariable logistic regression analysis for possible factors associated with renal stones
Discussion
The predominant age group of study participants (both cases and controls) in this study was around 45–50 years. The maximum incidence was reported in the third and fourth decades in India, by Madhusudan et al.[10] whereas age at peak incidence among Iran, Japan, and the United States was ranging from 40 to 49 years as observed by Romero et al.[11] A study by Chand et al., however, pegged the age of persons at risk for renal stone at a wider range, i.e., 20–60 years.[12] Nevertheless, in the present study, there was no statistically significant difference between case patients and controls with respect to age.
The finding of the present study on the significant association between female gender and renal stones has been corroborated by a study by Roudakova and Monga,[13] albeit with a rider; wherein the actual incidence of renal stones being more in men than among women; however, there is a very significant rise among women and a narrowing of the incidence between the two genders. It is noteworthy that most other studies[14,15] on this particular factor (gender) have come up with different results, where male preponderance in renal stones has been noted. The difference in findings of these studies may lie in the racial and demographic differences between the populations studied. Further, the adjusted OR in our study did not show any significant risk though there was a significant difference in the gender-based incidence, with females having more risk for renal stones.
The present study reported a statistically significant relationship between illiteracy and occurrence of renal stones. This finding is corroborated by a study carried out in Kangra, on the determinants of renal stones as per which a positive association exists between knowledge levels on determinants of renal stones and their incidence.[16]
Taylor et al. in their study[17] have demonstrated that raised BMI correlate well with increased risk of renal stone disease, where the risk tends to be more for women than men. Cupisti et al.[18] also observed a similar finding where incidence of renal stones had up to 75% among overweight and obese patients.[18] The present study also has found that there exists an association between BMI and renal stones.
The present study had arrived at a statistically significant finding with respect to association of renal stones with less water consumption (<1.5 L/day), consumption of soft drinks, and performance of sedentary work. These significant associations were observed both on univariate and on multivariate analyses.
Several studies[17,19,20] have shown that people consuming a quantity < 1.5 L/day of fluids suffered kidney stones. Further, two studies, one observational[21] and one interventional,[22] revealed the favorable effects of water intake in preventing renal stone as well as its recurrences.
Studies[19,20] have reported that the risk of renal stones is lower with an intake of calcium around 1000 mg/day in contrast to an intake < 600 mg/day. Some other studies have also observed that the excretion of calcium is increased with high consumption of cooking salt.[22,23] An increase of about 6 g (100 mmol) of NaCl produces an increase of calciuria of 40 mg (1 mmol) in normal adults and 80 mg (2 mmol) in hypercalciuric patients leading to formation of kidney stones. These findings corroborate those obtained from the present study on the respective determinants (calcium and sodium intake vis-à -vis renal stone formation).
Insofar as our study's finding of a statistically significant association between consumption of soft drinks and occurrence of renal stones is concerned, this is supported by other such studies on the subject. A study on “geography and risk factors for renal stones” by Soucie et al.[24] found a significant relationship between use of any soft drink and occurrence of renal stones (OR = 1.3 for any noncola soft drink and 1.2 for any cola soft drink). Further, a meta-analysis of 5 studies by Vartanian et al.[25] reported that soft-drink consumption was positively associated with urinary or kidney stones.
The same holds true for the relationship between sedentary lifestyle and occurrence of renal stones. Analysis of lifestyle patterns by various studies in affluent countries such as the United States and Northern European countries has shown that how the increased prevalence of stones occurred simultaneously with the increased sedentary lifestyle.[23]
As per our study, those subjects with a family history of renal stones had a greater likelihood of suffering from renal stones than those without a family history. This is similar to the finding of a large, 8-year follow-up study by Curhan et al.,[19] wherein 37,999 men were studied, which showed a strong association between family history and occurrence of renal stones (age-adjusted OR = 3.16, P < 0.05). Further supportive evidence to this finding is available from a study by Jabbar et al.[26] in Quetta, Pakistan. As per the study, 37.5% males and 27.02% females with renal stones had a positive family history.
The limitation of the present study was the wide CI for some variables OR due to smaller sample size which was taken considering the financial constraints due to higher cost for water testing for various mineral contents. Drinking water and soft-drink consumption from study participants were not quantified. Future research can further corroborate these findings using a larger sample.
Conclusions
In this study, female gender, illiteracy, high BMI, high sodium in drinking water, poor water consumption, borewell drinking water, soft-drink consumption, sedentary work, and family history of renal stones were significantly associated with increased risk of stone formation, whereas intakes of water with pH >7, TDS >1000 mg/L, magnesium >40 mg/L, chloride >250 mg/L, and turbidity >1 NTU were related to increased risk. Before definitive recommendations can be given, additional studies are needed to further validate these findings using a larger sample.
Recommendation
Specific dietary considerations for avoiding each type of renal stone formation may be commonly recommended; however, in this coastal region from this study, some of the recommendations would be to reduce obesity, doing exercise or moderate activity, reducing junk foods, especially soft drinks, and to increase water consumption, and the government could increase the supply of providing drinking water after reverse osmosis to reduce the mineral content.
Financial support and sponsorship
The study was supported by the Indian Council of Medical Research.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We acknowledge ICMR for funding the research under the reference ID STS 2014-05063.
References
- 1.Stojimirovic B. Functional evaluation in patients with kidney calculi. Srp Arh Celok Lek. 1998;126:394–8. [PubMed] [Google Scholar]
- 2.Hochreiter W, Knoll T, Hess B. Pathophysiology, diagnosis and conservative therapy of non-calcium kidney calculi. Ther Umsch. 2003;60:89–97. doi: 10.1024/0040-5930.60.2.89. [DOI] [PubMed] [Google Scholar]
- 3.Bagga HS, Chi T, Miller J, Stoller ML. New insights into the pathogenesis of renal calculi. Urol Clin North Am. 2013;40:1–12. doi: 10.1016/j.ucl.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaboré FA, Kambou T, Zango B, Ouattara A, Simporé M, Lougué-Sorgho C, et al. Epidemiology of a cohort of 450 urolithiasis at the Yalgado Ouédraogo University hospital of Ouagadougou (Burkina Faso) Prog Urol. 2013;23:971–6. doi: 10.1016/j.purol.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Trinchieri A. Epidemiology of urolithiasis: An update. Clin Cases Miner Bone Metab. 2008;5:101–6. [PMC free article] [PubMed] [Google Scholar]
- 6.Trinchieri A, Coppi F, Montanari E, Del Nero A, Zanetti G, Pisani E. Increase in the prevalence of symptomatic upper urinary tract stones during the last ten years. Eur Urol. 2000;37:23–5. doi: 10.1159/000020094. [DOI] [PubMed] [Google Scholar]
- 7.Semins MJ, Matlaga BR. Kidney stones and pregnancy. Adv Chronic Kidney Dis. 2013;20:260–4. doi: 10.1053/j.ackd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Luo GT, Niu WJ, Gong MM, Liu L, Zhou J, et al. Risk factors for the kidney stones: A hospital-based case-control study in a distric hospital in Beijing. Beijing Da Xue Xue Bao. 2013;45:971–4. [PubMed] [Google Scholar]
- 9.Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: A 5-year randomized prospective study. J Urol. 1996;155:839–43. [PubMed] [Google Scholar]
- 10.Madhusudan A, Arif M, Hebbar AK, Karthavya SL. Epidemiology and chemical composition of upper urinary tract calculi. SAS J Med. 2015;1:1–6. [Google Scholar]
- 11.Romero V, Akpinar H, Assimos DG. Kidney stones: A global picture of prevalence, incidence, and associated risk factors. Rev Urol. 2010;12:e86–96. [PMC free article] [PubMed] [Google Scholar]
- 12.Chand RB, Shah AK, Pant DK, Paudel S. Common site of urinary calculi in kidney, ureter and bladder region. Nepal Med Coll J. 2013;15:5–7. [PubMed] [Google Scholar]
- 13.Roudakova K, Monga M. The evolving epidemiology of stone disease. Indian J Urol. 2014;30:44–8. doi: 10.4103/0970-1591.124206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abbagani S, Gundimeda SD, Varre S, Ponnala D, Mundluru HP. Kidney stone disease: Etiology and evaluation. Int J Appl Biol Pharm Technol. 2010;I:175–82. [Google Scholar]
- 15.Bangash K, Shigri F, Jamal A, Anwar K. Spectrum of renal stones composition; chemical analysis of renal stones. Int J Pathol. 2011;9:63–6. [Google Scholar]
- 16.Awasthi M. Counseling of Kidney Stone Patients Based on Their Dietary Pattern in the Selected Areas of District Kangra HP. Chaudhary Sarwan Kumar Himachal Pradesh Krishi Vishvavidyalaya. 2013. [Last accessed on 2015 Sep 08]. Available from: https://www.hdl.handle.net/10603/10257 .
- 17.Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293:455–62. doi: 10.1001/jama.293.4.455. [DOI] [PubMed] [Google Scholar]
- 18.Cupisti A, Meola M, D’Alessandro C, Bernabini G, Pasquali E, Carpi A, et al. Insulin resistance and low urinary citrate excretion in calcium stone formers. Biomed Pharmacother. 2007;61:86–90. doi: 10.1016/j.biopha.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med. 1997;126:497–504. doi: 10.7326/0003-4819-126-7-199704010-00001. [DOI] [PubMed] [Google Scholar]
- 20.López M, Hoppe B. History, epidemiology and regional diversities of urolithiasis. Pediatr Nephrol. 2010;25:49–59. doi: 10.1007/s00467-008-0960-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frank M, De Vries A, Tikva P. Prevention of urolithiasis. Arch Environ Health. 1966;13:625–30. doi: 10.1080/00039896.1966.10664630. [DOI] [PubMed] [Google Scholar]
- 22.Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A. Urinary volume, water recurrences in idiopatic calcium nephrolitiasis: A 5 year randomized prospective study. J Urol. 1996;155:839–84. [PubMed] [Google Scholar]
- 23.Dogliotti E, Vezzoli G, Nouvenne A, Meschi T, Terranegra A, Mingione A, et al. Nutrition in calcium nephrolithiasis. J Transl Med. 2013;11:109. doi: 10.1186/1479-5876-11-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soucie JM, Coates RJ, McClellan W, Austin H, Thun M. Relation between geographic variability in kidney stones prevalence and risk factors for stones. Am J Epidemiol. 1996;143:487–95. doi: 10.1093/oxfordjournals.aje.a008769. [DOI] [PubMed] [Google Scholar]
- 25.Vartanian LR, Schwartz MB, Brownell KD. Effects of soft drink consumption on nutrition and health: A systematic review and meta-analysis. Am J Public Health. 2007;97:667–75. doi: 10.2105/AJPH.2005.083782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jabbar F, Asif M, Dutani H, Hussain A, Malik A, Kamal MA, et al. Assessment of the role of general, biochemical and family history characteristics in kidney stone formation. Saudi J Biol Sci. 2015;22:65–8. doi: 10.1016/j.sjbs.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


