Abstract
Background:
Acanthosis nigricans (AN) is one of the signs suggestive of high insulin resistance (IR). IR is one of the mechanisms involved in pathogenesis of diabetes mellitus type-2 (DM Type-2). Thus, early detection of IR in children may allow us time to intervene well before the development of DM Type-2. In this study, 62% of children having AN had high IR. In children having both, AN and high body mass index (BMI), the incidence of IR was about 80%. This suggests that these easily detectable parameters alone can be useful in screening children at high risk of developing DM Type-2 in future. These simple criteria thus hold promise for use in high throughput screening programs for diabetes.
Context:
A pilot study conducted by the authors showed that children with AN have a high incidence of IR. The detection of IR in children may allow us time to intervene well before the development of DM Type-2. Detection of DM Type-2 by hyperglycemia may be too late to prevent the onset of microvascular changes.
Aims:
This study aims to determine whether easily observable presence of AN can be used to screen for increased IR in children, and thereby to detect this important risk factor for DM Type-2.
Settings and Design:
Cross-sectional, observational study. Two schools, one with children belonging to average economic background and the other, a residential school with children of affluent parents. Selection of children was done randomly.
Subjects and Methods:
The study was conducted among 507 children in the age group of 10–18 years. Physical examination for the detection of AN, height and weight measurements, waist circumference, fasting plasma glucose, fasting plasma insulin, and lipid profile was done. Homeostatic model assessment of insulin resistance was calculated.
Statistical Analysis Used:
Data analysis was performed using descriptive statistics and inferential statistical methods. The association between categorical variables was done by Chi-square test.
Results:
The presence of AN positively correlated with high IR, and when combined with increased BMI, the incidence rate of IR is 80%.
Conclusions:
AN can be used as a screening method to identify children at risk of DM Type-2-since those who have high IR have a high possibility of having DM Type-2 in future. Hence, early screening and simple, but effective interventional strategies can be instituted at this age, which may prevent or delay diabetes in the long run.
Keywords: Acanthosis nigricans, body mass index, diabetes mellitus, insulin resistance
Introduction
The American Diabetes Association established acanthosis nigricans (AN) as a risk factor for diabetes in children in the year 2000.[1] We happened to observe that many adolescent children have AN and it is commonly associated with obesity and hyperinsulinemia [Figure 1].[2] AN is thought to be produced because of elevated insulin concentration that results in direct and indirect activation of insulin-like growth factor 1 receptors on keratinocytes and fibroblasts, leading to their proliferation. Clinically, the neck is the most commonly affected area in children.[3] It is established that the genesis of diabetes mellitus type-2 (DM Type-2) is linked to increased IR.[4] The vascular complications of DM may precede the onset of hyperglycemia by several years.[5] Therefore, it will be of tremendous advantage if IR can be detected early so that any possible interventions can be instituted at an early age to prevent or delay the onset of diabetes and its complications.
Figure 1.
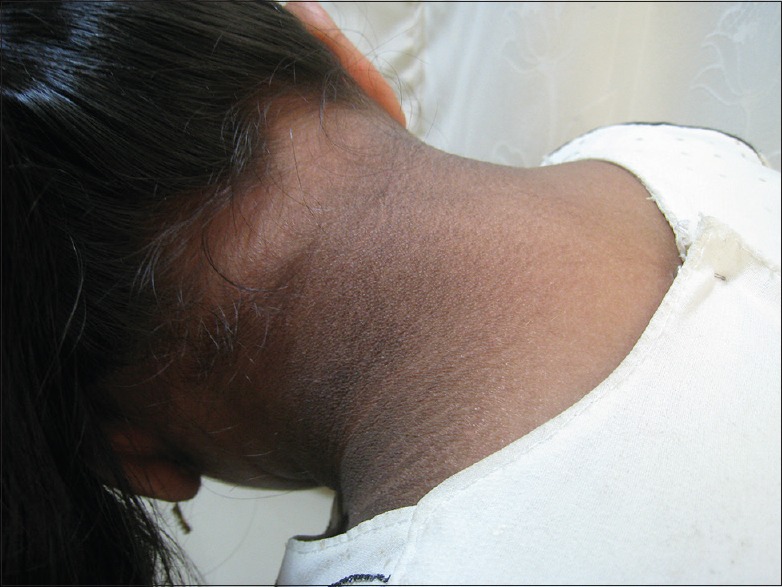
Acanthosis Nigricans over the neck
The observation of the presence of AN could be potentially used as a screening tool to detect IR at an early age. Since increased IR is usually a harbinger of DM Type-2, detection of IR at an early age gives us an opportunity to intervene and institute appropriate interventions.
Subjects and Methods
Two schools, one a residential school with children of affluent parents (School I), and the other, with children belonging to average economic background (School II), were selected.
Children studying from standards 6th–12th, i.e., belonging to age group of 10–18 years, were selected for the study.
The study protocol, the consent, and the assent forms were approved by the Institutional Ethics Committee. Informed consent was taken from a parent; usually, the mother and the assent was taken from the child.
Two hundred and eighty-two children were screened from School I and 225 children from School II.
A prescreening lecture on diabetes and its complications was given to children, teachers and available parents by the investigating team
-
Physical examination was conducted, consisting of the following:
- Presence of AN on and around the neck
- Body mass index (BMI) - Calculated by the formula: Weight in kilograms/(height in meters)2
- Waist measurement in centimeters.
Five milliliter of blood was drawn from each child in the fasting state. Plasma was separated and stored at 2°C–8°C
-
Plasma samples were transported to a tertiary care hospital laboratory, and the following tests were done on it:
- Plasma glucose
- Plasma insulin level-ECLIA (electrochemiluminescence) method was used
- Lipid profile
Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated from the glucose and insulin levels using the equation:
HOMA-IR = {Fasting plasma insulin level (μ units/mL) × Fasting glucose level (mmol/L)}/22.5
Statistical analysis
Statistical analysis was performed using SPSS version 17.0 (Released 2008. SPSS Statistics for Windows, SPSS Inc, Chicago). Descriptive statistics were expressed as frequency (percentage of collected data). Association between AN and IR was calculated using Chi-square test.
HOMA-IR of 2.5 was taken as a cut-off point for determining high values.[6] Fasting plasma glucose >100 mg/dl was taken as evidence of hyperglycemia and BMI above the 85th percentile were taken to be overweight/obese.[7]
Results
The two schools together gave a total number of 507 children. Since the details and values from the 2 individual schools gave similar results, the data are pooled and submitted. There were 325 (64%) boys and 182 (36%) girls.
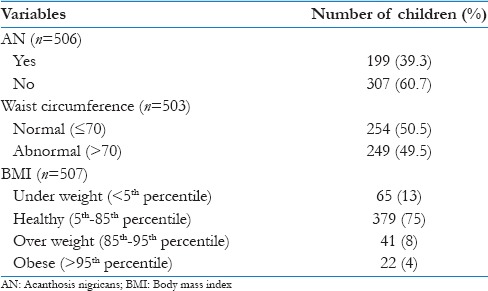
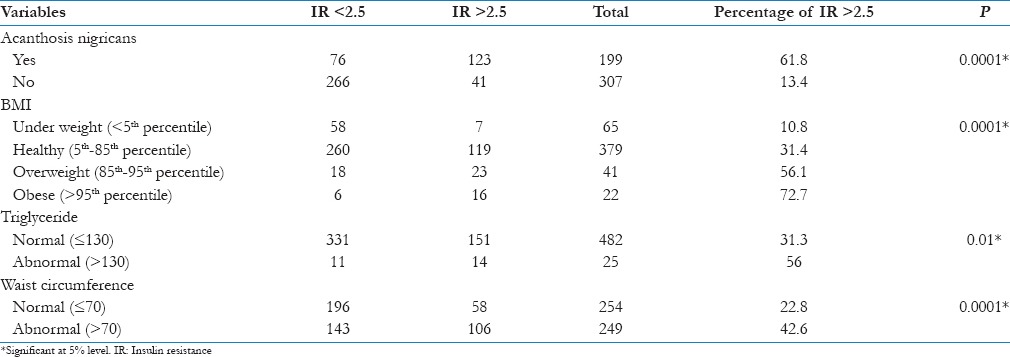
The occurrence of AN, waist circumference and BMI is shown in Table 1.
Table 1.
Occurrence of acanthosis nigricans, waist circumference, and body mass index
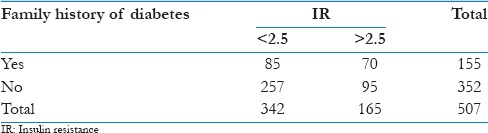
Family history of diabetes as given by the child and or parent is given in Table 2.
Table 2.
Family history of diabetes and insulin resistance
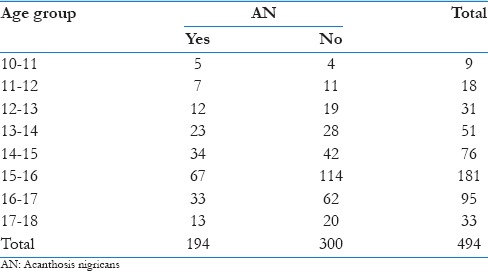
There is no evidence of an association between age and AN (P = 0.792). This shows that occurrence of AN is not age related [Table 3].
Table 3.
Age and acanthosis nigricans
Among the various parameters studied, it was found that total cholesterol levels, HDL and LDL had no association with increased IR [Table 4]. However, positive association was found between triglyceride levels and increased IR. There is a significant association between AN, BMI, and waist circumference with IR [Table 4].
Table 4.
Association of Insulin resistance with various parameters
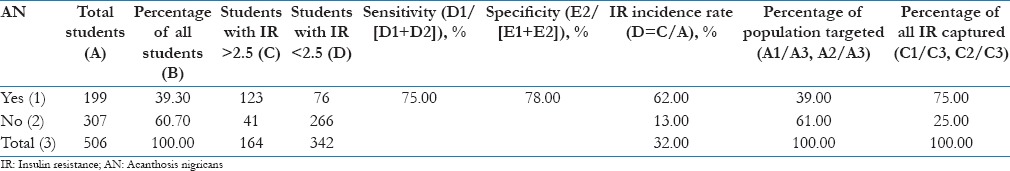
Of the 507 children screened, 199 were found to have AN, which formed 39.3% of the total students screened [Table 5]. One hundred and eighty-two out of the 507 children (approximately, 36%), were girls. Seventy-eight of 182 girls (43%) and 121 of 324 boys (37.3%) were found to have AN. For girls, the high IR incidence with AN was 63%, compared to 19% without AN. The overall high IR incidence rate for girls was 40%. For boys, IR incidence was 63% for AN positive and 9% for AN negative group and an overall high IR incidence of 29%. The overall IR incidence for the entire sample was 32%, but in children with AN, it was 62% [Table 5] and the association between AN and IR is statistically significant (P = 0.0001).
Table 5.
Acanthosis nigricans and insulin resistance incidence
Discussion
AN is known to be associated with obesity, endocrinopathies, and malignancies. It is important to detect AN in day to day clinical practice because it may herald various such disorders.[8] India has one of the highest prevalence of DM Type-2 in the world. The number is expected to be about 57.2 million by the year 2025.[9] Recent studies in India show an increasing trend in DM Type-2 in adolescents and children. The percentage of adult urban subjects affected with Diabetes has more than doubled from 1984 (5.2%) to 2000 (13.9%).[10] Diabetes puts an enormous burden on the patients, their family, and the health-care system. Hence, detection of the disease at an early state with physical markers and instituting preventive measures early on will reduce the economic burden of the society to a great extent.
DM Type-2 is causatively associated with hyperinsulinemia and IR.[11] In the early stages IR results in compensatory hyperinsulinemia, with the result that glucose homeostasis is maintained. At this stage diabetes, as per definition and current understanding as hyperglycemia remains undiagnosed.
It was found in this study that there was a positive relationship between AN and IR. This association was found in both subsets of students and was unrelated to other factors. Another correlating factor is BMI which also showed a positive relationship with IR.
The incidence of AN is equal in men and women.[12] More than half the adults who weigh more than 200% of their ideal body weight have lesions of AN.[5,13] Hud et al. found that 74% of obese population exhibited AN.[14] Some populations have a higher incidence of AN. For example, African-Americans are 25 times more likely to have it as compared to people of American descent.[15] The prevalence of AN in overweight children aged 7–17 years was 23% in Latino patients and 19.4% in African American patients.[16]
A standard method to evaluate AN as a marker for screening is through its “sensitivity” and “specificity.” AN has 75% sensitivity (ratio of true positives to sum of true positives and false negatives) and 78% specificity (ratio of true negatives to sum of true negative and false positives) when we use it as a marker for IR. Another way of viewing AN's performance as a marker is through the “percentage of total population targeted,” “percentage of total IR captured” and the “lift” which is the difference between these two percentages. Using AN, we would screen in 39% of the population, and “capture” 75% of those with high IR. The “lift” (% positive cases captured − % population targeted for screening) of 36% is promising for use in a high throughput screening program.
Along with AN, BMI is also a factor that will add strength to sensitivity in identifying children at risk, the combined high IR incidence rate increasing from 62% with AN alone to 80% when AN is combined with BMI estimation.
Conclusions
Increased fasting Insulin level and IR is prevalent among school children to the extent of 32%
AN is a reliable screening tool to detect Increased IR and can be used to detect children prone to develop DM Type-2. When combined with high BMI, the detection rate of high IR increases to 80%
Early detection of precursor to DM Type-2 may allow the community time to put in practice intervention strategies before onset of diabetes and its complications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kutlubay Z, Engin B, Bairamov O, Tüzün Y. Acanthosis nigricans: A fold (intertriginous) dermatosis. Clin Dermatol. 2015;33:466–70. doi: 10.1016/j.clindermatol.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz RA. Acanthosis nigricans. J Am Acad Dermatol. 1994;31:1–19. doi: 10.1016/s0190-9622(94)70128-8. [DOI] [PubMed] [Google Scholar]
- 3.Stuart CA, Pate CJ, Peters EJ. Prevalence of acanthosis nigricans in an unselected population. Am J Med. 1989;87:269–72. doi: 10.1016/s0002-9343(89)80149-4. [DOI] [PubMed] [Google Scholar]
- 4.Rao G. Insulin resistance syndrome. Am Fam Physician. 2001;63:1159. [PubMed] [Google Scholar]
- 5.Hadi HA, Suwaidi JA. Endothelial dysfunction in diabetes mellitus. Vasc Health Risk Manag. 2007;3:853–76. [PMC free article] [PubMed] [Google Scholar]
- 6.Madeira IR, Carvalho CN, Gazolla FM, de Matos HJ, Borges MA, Bordallo MA. Cut-off point for Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) index established from Receiver Operating Characteristic (ROC) curve in the detection of metabolic syndrome in overweight pre-pubertal children. Arq Bras Endocrinol Metabol. 2008;52:1466–73. doi: 10.1590/s0004-27302008000900010. [DOI] [PubMed] [Google Scholar]
- 7.Vijayan AP, Varma KK, Bhagyanathan M, Dinesh KB, Divia Nath KR, Bijayraj R. Three Physical Markers of Insulin Resistance (Body mass index, waist circumference and acanthosis nigricans): A cross-sectional study among children in South India. Med Pract Rev. 2011;2:37–43. [Google Scholar]
- 8.De Sanctis V, Soliman A, Marsciani A, Timoncini G, Reggiani L, Zucchini A, et al. Acanthosis nigricans in adolescents: A practical approach. Georgian Med News. 2013;222:73–8. [PubMed] [Google Scholar]
- 9.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: Prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–31. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 10.Ramachandran A, Snehalatha C, Vijay V. Temporal changes in prevalence of type 2 diabetes and impaired glucose tolerance in urban southern India. Diabetes Res Clin Pract. 2002;58:55–60. doi: 10.1016/s0168-8227(02)00125-0. [DOI] [PubMed] [Google Scholar]
- 11.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. Hypoadiponectinemia in obesity and type 2 diabetes: Close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–5. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 12.Sinha S, Schwartz RA. Juvenile acanthosis nigricans. J Am Acad Dermatol. 2007;57:502–8. doi: 10.1016/j.jaad.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen TT, Keil MF, Russell DL, Pathomvanich A, Uwaifo GI, Sebring NG, et al. Relation of acanthosis nigricans to hyperinsulinemia and insulin sensitivity in overweight African American and white children. J Pediatr. 2001;138:474–80. doi: 10.1067/mpd.2001.112657. [DOI] [PubMed] [Google Scholar]
- 14.Hud JA, Jr, Cohen JB, Wagner JM, Cruz PD., Jr Prevalence and significance of acanthosis nigricans in an adult obese population. Arch Dermatol. 1992;128:941–4. [PubMed] [Google Scholar]
- 15.Stuart CA, Gilkison CR, Smith MM, Bosma AM, Keenan BS, Nagamani M. Acanthosis nigricans as a risk factor for non-insulin dependent diabetes mellitus. Clin Pediatr (Phila) 1998;37:73–9. doi: 10.1177/000992289803700203. [DOI] [PubMed] [Google Scholar]
- 16.Brickman WJ, Binns HJ, Jovanovic BD, Kolesky S, Mancini AJ, Metzger BE Pediatric Practice Research Group. Acanthosis nigricans: A common finding in overweight youth. Pediatr Dermatol. 2007;24:601–6. doi: 10.1111/j.1525-1470.2007.00547.x. [DOI] [PubMed] [Google Scholar]


