Abstract
Objectives:
The objective of this study is to describe the frequency and type of medication discrepancies (MD) through medication reconciliation and to describe the frequency of potentially inadequate prescription (PIP) medications using screening tool of older persons’ prescriptions criteria.
Design:
Cross-sectional comparison of electronic medical record (EMR) medication lists and patient's self-report of their comprehensive medication histories obtained through telephone interviews.
Inclusion criteria:
Elderly individuals (>65 years old) with more than ten medications recorded in their EMR, who had not been hospitalized in the past year and were not under domiciliary care, affiliated to a private community hospital.
Outcome Measures:
The primary outcomes were the proportion of patients with MD and PIP. Secondary outcomes were the proportion of types of discrepancies and PIP. We analyzed possible associations between these variables and other demographic and clinical variables.
Results:
Out of 214 randomly selected individuals, 150 accepted to participate (70%). The mean number of medications referred to be consumed by patients was 9.1 (95% confidence interval [CI] =8.6–9.6), and the mean number of prescribed medications in their EMR was 13.9 (95% CI = 13.3–14.5). Ninety-nine percent had at least one discrepancy (total 1252 discrepancies); 46% consumed at least one prescription not documented in their EMR and 93% did not consume at least one of the prescriptions documented in their EMR. In 77% of the patients, a PIP was detected (total 186), 87% of them were at least within one of the following categories: Prolonged used of benzodiazepines or proton pump inhibitors and the use of aspirin for the primary prevention of cardiovascular disease.
Conclusions:
There was a high prevalence of MD and PIP within the community of elderly adults affiliated to a Private University Hospital. Future interventions should be aimed at reducing the number of PIP to prevent adverse drug events and improve EMR accuracy by lowering medications discrepancies.
Keywords: Medication discrepancies, medication reconciliation, polypharmacy, potentially inadequate prescriptions, screening tool of older persons’ prescriptions criteria
Introduction
Approximately, one-third of patients over 60-year-old consume daily between 5 and 9 medications, and 12% consume ten or more. The risk of adverse drug events (ADE) increase significantly when the number of consumed medications is 5 or higher.[1] Potentially inadequate prescriptions (PIPs) are those whose benefits are generally outweighed by their potential risks of ADE throughout inadequate dosing or duration of treatment, dangerous interactions or poor clinical effectiveness. PIP can also include the nonprescription of drugs with a significant clinical benefit.[2] Medication discrepancies (MD) are those detected through medication reconciliation (MR). MR is a formal process for creating the most complete and accurate list possible of a patient's current medications and comparing the list to those in the patient record or medication orders. MR was the #8 Patient Safety Goals by the Joint Commission in 2005, and then it was suspended and reformulated within #3 Goal “Improving the safety of using medication.”[3] MD are common in all clinical settings, ranging from 70% to 100%,[4,5] and about one-third are linked to potential harm. It is unclear whether interventions aimed at reducing MD prevents ADE or other harms since most of the research has been focused to inpatient settings and transitions of care.[6] In one outpatient setting study, MR reduced the number of MD from 89% to 66%, though most of them were in minor discrepancies.[7] Another study in our country used screening tool of older persons’ prescriptions (STOPP) and Beers[8] criteria and found that approximately one-third of comorbid elderly adults had PIP.[9]
This study aims to describe the frequency and type of both MD and PIP in a population of elderly adults with polypharmacy in a Private Academic Community Hospital of Buenos Aires, Argentina.
Methods
Study design and population
We performed a cross-sectional study. Random sequence generation was used to identify eligible participants from our hospital database (May 31, 2014) of elderly adults (65 year-old or older) with ten or more active prescriptions in their electronic medical records (EMR). An exclusion criterion was hospital admission or domiciliary care within the last 12 months.
Outcomes
We defined as primary outcomes the proportion of patients with MD and PIP. We defined as secondary outcomes the proportion of types of discrepancies and PIP. We also analyzed the association between the number of MD and PIP and other demographic and clinical variables.
Data collection
A family physician (first author initials, removed for blinding) called patients from the sample and invited them to participate in a telephone interview using a protocolized oral consent. If the patient accepted, then a form [Appendix 1] was used to collect basic demographic characteristics (age, education, marital status) and the complete list of medications currently consumed by the patients (P-LIST). Each patient was called three times at a different time and day before catalogued as “nonrespondent.”
Appendix 1.

Abridged version of the form used in telephone interview (translated from Spanish)
The P-LIST was then compared with the list present in the EMR (EMR LIST), and MD was consigned and classified [Table 1]. PIP was detected using STOPP criteria applied to the P-LIST. Since these criteria require in some cases clinical information when necessary the physician consulted the EMR or asked directly to the patient for information.
Table 1.
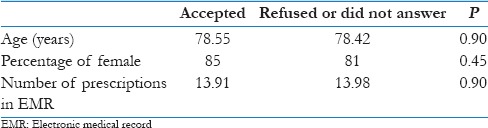
Basic demographic characteristics of those who accepted (n=150) to participate and those who did not (refused or were nonrespondent, n=64)
Sample size and statistical analysis
Sample size calculation was based on an estimated proportion of MD of 75% and a semi-amplitude confidence interval (CI) of 7%. From previous experience in our institution, we estimated a response rate of approximately 50%. Therefore, a randomized sample of 214 patients was needed to achieve 150 individually completed telephone interviews.
We calculated summary statistic measurements using STATA 13 (StataCorp, College Station, Texas, USA) software. We used Chi-square test and two-sample t-test for dichotomous and continuous hypothesis testing respectively. Measures of associations were tested using regression models. We defined an alpha level of P = 0.05.
Ethics
This study protocol and its oral consent form were approved by our Hospital's Research Ethics Committee.
Results
Population characteristics
Out of the 214 randomly selected individual, 150 accepted to participate with a response rate of 70%. Twenty-eight declined the interview, and 36 were nonrespondent. There were no differences in sex, age, and number of prescriptions in the EMR between those who accepted and those who refused or were “nonrespondent” [Table 2].
Table 2.
Other demographic characteristics of those who accepted (n=150) and their 95% confidence intervals
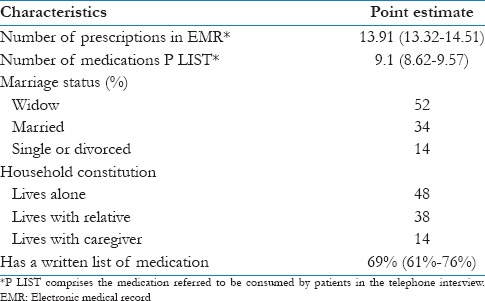
The majority of interviewees were women, and half of them were widows. The mean age was 78 years old. The mean number of medication referred to be consumed by patients was 9.1 (95% CI = 8.6–9.6), and the mean number of prescribed medications in their EMR was 13.9 (95% CI = 13.3–14.5). Table 3 shows the additional characteristics.
Table 3.
Proportions of discrepancies found by type (95% confidence intervals)

Medication discrepancies
When comparing P-LIST with EMR LIST, a total of 1252 discrepancies were found. Ninety-nine percent of patients had at least one discrepancy. The most frequent discrepancy was that in which the patient was not consuming a prescribed medication in the EMR (93%, 95% CI = 88%–97%), and in a minority of patients (5%, 95% CI = 2%–9%) they were consuming a duplicated prescription (e.g. two types of benzodiazepines simultaneously). Other clinically relevant discrepancies are described in Table 1. The mean number of discrepancies per patient was 8.34 (95% CI = 7.65–9.04).
When performing linear regression analysis, we found that the number of prescribed medication was strongly associated with the number of MD even after adjusting by sex, age, household constitution, and marriage status [Figure 1]. For each additional prescription in the EMR, an additional mean of 0.9 MD could be found. No other variables were associated with the number of MD.
Figure 1.
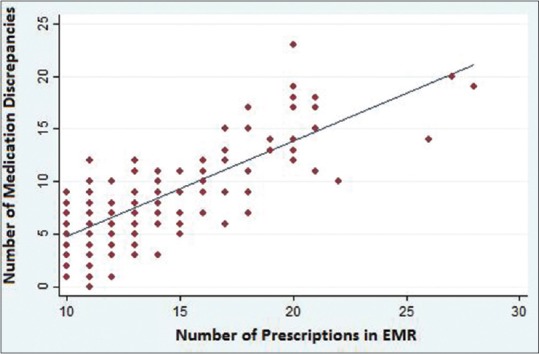
Number of medication discrepancies and number of prescriptions
Potentially inadequate prescriptions
Using STOPP criteria, 186 PIP were detected in 77% of patients (95% CI = 70%–83%). The mean number of PIP per patient was 1.24 (95% CI = 1.09–1.39). The number of PIP per patients is described in Table 4.
Table 4.
Proportion of patients and number of potentially inadequate prescriptions

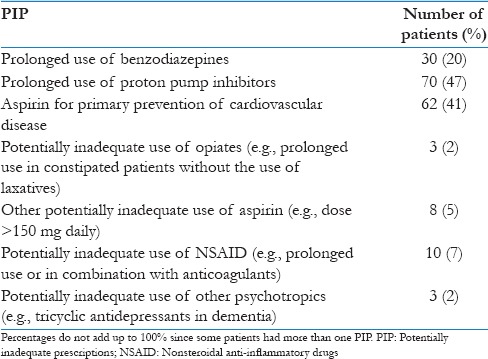
Up to 87% of PIP involved three STOPP criteria: The prolonged use (>1 month) of benzodiazepines, the use of proton pump inhibitors for a period longer than 8 weeks and the use of aspirin for the primary prevention of cardiovascular disease [Table 5].
Table 5.
Description of potentially inadequate prescriptions
Discussion
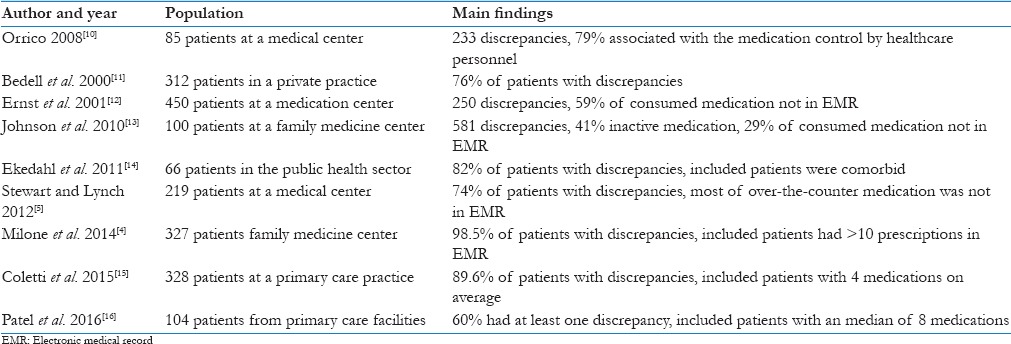
MD was common between their prescriptions in their EMR, and the medication referred to be consumed by patients during their telephone interview. This is consistent with previous findings by Milone et al.[4] where up to 98.5% of patients with 10 or more prescriptions had discrepancies when MR was performed by a pharmacist in a family medicine clinic. In that study, the most frequent source of discrepancies was “patient no longer taking medication” (54.1%) followed by “current medication not on list” with an average of 6.6 discrepancies per patient. In Table 6, other experiences in MR are described. Possible factors associated with the high number of medication no longer taken by the patients are: The inadequate prescription of medication for acute conditions, the inadequate cancellation of old prescriptions when new treatments are indicated, conflicting prescriptions between multiple providers, low adherence, and insufficient stock.
Table 6.
Medication reconciliation and detection of medication discrepancies
A large proportion of patients with PIPs was found in our study sample. Regueiro et al.[9] found a lower proportion in a similar population in our country (21.3%), however, the most frequently found STOPP criteria were similar: Prolonged use of proton pump inhibitors, potentially inadequate use of aspirin and benzodiazepines. In a systematic review of studies using STOPP criteria to detect PIP,[17] a wide range of prevalence of PIP was found (21%–79%), but the most commonly encountered were also the three most frequently found in our study.
Limitations
There are several limitations in our study. The use of telephone interviews could have selected a population of elderly adults, nevertheless there was a high response rate and the demographic characteristics of responders were similar to those who did not. The recall could be a source of bias, especially in patients trying to remember long list of prescriptions or when medication taken by the patient and not registered in EMR could not be recalled. Our data collection method adapted from Stewart and Lynch[5] and Ekedahl et al.[14] was not validated in our population, but was compatible with our current medical practice of comprehensive MR and review.
Conclusions
There is a high prevalence of MD and PIP within the community of elderly adults in ambulatory care affiliated to a Private University Hospital. Future interventions should be aimed at reducing the number of PIP to prevent ADEs and improve EMR accuracy by lowering medications discrepancies. Research in this area should also focus on the effect of these interventions in the incidence of adverse drug reactions.
Financial support and sponsorship
Hospital Italiano de Buenos Aires – Fundación MF.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Johnell K, Klarin I. The relationship between number of drugs and potential drug-drug interactions in the elderly: A study of over 600,000 elderly patients from the Swedish prescribed drug register. Drug Saf. 2007;30:911–8. doi: 10.2165/00002018-200730100-00009. [DOI] [PubMed] [Google Scholar]
- 2.Delgado Silveira E, Muñoz García M, Montero Errasquin B, Sánchez Castellano C, Gallagher PF, Cruz-Jentoft AJ. Inappropriate prescription in older patients: The STOPP/START criteria. Rev Esp Geriatr Gerontol. 2009;44:273–9. doi: 10.1016/j.regg.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 3.Commision TJ. National Patient Safety Goals. [Last accessed on 2015 Oct 10]. Available from: https://www.jointcommission.org/standards_information/npsgs.aspx .
- 4.Milone AS, Philbrick AM, Harris IM, Fallert CJ. Medication reconciliation by clinical pharmacists in an outpatient family medicine clinic. J Am Pharm Assoc. 2014;54:181–7. doi: 10.1331/JAPhA.2014.12230. [DOI] [PubMed] [Google Scholar]
- 5.Stewart AL, Lynch KJ. Identifying discrepancies in electronic medical records through pharmacist medication reconciliation. J Am Pharm Assoc. 2012;52:59–66. doi: 10.1331/JAPhA.2012.10123. [DOI] [PubMed] [Google Scholar]
- 6.Mueller SK, Sponsler KC, Kripalani S, Schnipper JL. Hospital-based medication reconciliation practices: A systematic review. Arch Intern Med. 2012;172:1057–69. doi: 10.1001/archinternmed.2012.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porcelli PJ, Waitman LR, Brown SH. A review of medication reconciliation issues and experiences with clinical staff and information systems. Appl Clin Inform. 2010;1:442–61. doi: 10.4338/ACI-2010-02-R-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Geriatrics Society Beers Criteria Update Expert Panel for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616–31. doi: 10.1111/j.1532-5415.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Regueiro M, Mendy N, Cañás M, Farina HO, Nagel P. Use of medication in non-institutionalized elderly adults. Rev Peru Med Exp Salud Pública. 2011;28:643–7. [PubMed] [Google Scholar]
- 10.Orrico KB. Sources and types of discrepancies between electronic medical records and actual outpatient medication use. J Manag Care Pharm. 2008;14:626–31. doi: 10.18553/jmcp.2008.14.7.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bedell SE, Jabbour S, Goldberg R, Glaser H, Gobble S, Young-Xu Y, et al. Discrepancies in the use of medications: Their extent and predictors in an outpatient practice. Arch Intern Med. 2000;160:2129–34. doi: 10.1001/archinte.160.14.2129. [DOI] [PubMed] [Google Scholar]
- 12.Ernst ME, Brown GL, Klepser TB, Kelly MW. Medication discrepancies in an outpatient electronic medical record. Am J Health Syst Pharm. 2001;58:2072–5. doi: 10.1093/ajhp/58.21.2072. [DOI] [PubMed] [Google Scholar]
- 13.Johnson CM, Marcy TR, Harrison DL, Young RE, Stevens EL, Shadid J. Medication reconciliation in a community pharmacy setting. J Am Pharm Assoc. 2010;50:523–6. doi: 10.1331/JAPhA.2010.09121. [DOI] [PubMed] [Google Scholar]
- 14.Ekedahl A, Brosius H, Jönsson J, Karlsson H, Yngvesson M. Discrepancies between the electronic medical record, the prescriptions in the Swedish national prescription repository and the current medication reported by patients. Pharmacoepidemiol Drug Saf. 2011;20:1177–83. doi: 10.1002/pds.2226. [DOI] [PubMed] [Google Scholar]
- 15.Coletti DJ, Stephanou H, Mazzola N, Conigliaro J, Gottridge J, Kane JM, et al. Patterns and predictors of medication discrepancies in primary care. J Eval Clin Pract. 2015;21:831–9. doi: 10.1111/jep.12387. [DOI] [PubMed] [Google Scholar]
- 16.Patel CH, Zimmerman KM, Fonda JR, Linsky A. Medication complexity, medication number, and their relationships to medication discrepancies. Ann Pharmacother. 2016;50:534–40. doi: 10.1177/1060028016647067. [DOI] [PubMed] [Google Scholar]
- 17.Hill-Taylor B, Sketris I, Hayden J, Byrne S, O’Sullivan D, Christie R. Application of the STOPP/START criteria: A systematic review of the prevalence of potentially inappropriate prescribing in older adults, and evidence of clinical, humanistic and economic impact. J Clin Pharm Ther. 2013;38:360–72. doi: 10.1111/jcpt.12059. [DOI] [PubMed] [Google Scholar]


