Abstract
Purpose of Review
Many phenotypes of asthma exist, ranging from mild asthma with onset during childhood to severe asthma with later onset, making asthma a broad disease with different pathologies. A gender disparity exists in asthma prevalence. As adults, women have an increased asthma prevalence compared to men. Further, women are more likely to have severe asthma and a later onset of asthma compared to men. Here, we review clinical and animal studies that have defined the role of sex hormones in airway inflammation, smooth muscle contraction, mucus production, and airway mechanics associated with asthma pathogenesis.
Recent Findings
Clinical evidence shows that increased asthma symptoms occur in females starting at puberty compared to boys. However, after puberty, the role for sex hormones in regulating asthma symptoms during menstruation, pregnancy, and menopause is not as clear. Animal studies have shown that estrogen increases and testosterone decreases Th2-mediated airway inflammation, and that females have increased IL-17A-mediated airway inflammation compared to males. Further, females had increased DC and Mφ function compared to males. However, the mechanisms driving the types of allergic inflammation are not fully elucidated.
Summary
Overall, ovarian hormones increased and testosterone decreased airway inflammation in asthma, but the mechanisms remain unclear. Delineating these pathways using animal models as well as women and men with various phenotypes of asthma will help determine if women with asthma should take (or avoid) hormonal contraceptives as well as predict changes asthma symptoms during life phases, including pregnancy and menopause, when sex hormones are dramatically changing.
Keywords: sex hormones, estrogen, testosterone, type 2 inflammation, IL-17A mediated inflammation
Introduction
Asthma is a chronic disease that causes episodic dyspnea, tightness in the chest, reversible airway obstruction, and wheezing (1). Approximately 25 million people in the United States have asthma, making it a large health care burden (2). In 2007, it was estimated that asthma health care associated costs in the United States were about $3,200 per person or $56 billion total each year (2). Many phenotypes and endotypes of asthma exist, ranging from mild asthma with onset during childhood to severe asthma with later onset (1, 3), making asthma a broad disease with different pathologies. Therefore, it is imperative to understand the pathophysiological mechanisms driving airway inflammation, mucus production, airway hyperreactivity (AHR), and airway remodeling in different phenotypes of asthma.
A gender disparity is well-established in asthma and changes throughout life (4). As children, boys have an increased prevalence of asthma compared to girls (11.9% vs. 7.5%, respectively)(2), and boys are also twice as likely as girls to be hospitalized for an asthma exacerbation (5). However, during adolescence there is a decline in asthma prevalence and morbidity in males concurrent with an increase in females. By adulthood, women have increased asthma prevalence compared to men (9.6% versus 6.3%, respectively)(2, 6), and women are three times more likely than men to be hospitalized for an asthma-related event (7–9). This increase in asthma prevalence in women compared to men is maintained until around the time of menopause, when a decrease in asthma prevalence is noted in women (10). Shifts in asthma prevalence based on gender coincide with changes in sex hormones and suggest that sex hormones modulate pathways associated with asthma pathogenesis. In this review article, we will review the role of sex hormones in asthma pathogenesis using data from epidemiological, clinical, and animal model studies.
Asthma prevalence changes during puberty
Allergic, atopic asthma is associated with onset during childhood, and boys have increased allergic inflammation and serum IgE levels compared to girls (11, 12). Boys also have dysanapsis, smaller airway diameters relative to lung volumes compared to girls, making boys more likely to have asthma symptoms than girls (13). As children age, the switch in asthma prevalence from highest in males to highest in females coincided with the age of puberty onset (5, 14). The Childhood Asthma Management Program (CAMP) study longitudinally tracked the average asthma symptom score as well as progression through puberty, using the Tanner stage metric, in boys and girls ages 4–17 (15•). At approximately age 10, when the Tanner scores start increasing in girls, the average asthma symptom score also increased in girls and declined in boys (15•). Further, asthma symptoms continued to increase in girls as Tanner stages increased (15•). Additional studies have also shown that early aged menarche (≤11 years old) increased the incidence of asthma (16). Collectively these studies present strong epidemiological evidence that the prevalence of asthma in females increased in adolescence and that early menarche further increases the risk of developing asthma.
Pre or peri-menstrual worsening of asthma
Changes in asthma symptoms through the menstrual cycle are well established, and approximately 30–40% of women with asthma report pre or peri-menstrual worsening of asthma. (17–23). Decreased peak expiratory flow rates, increased asthma symptoms, and increased use of rescue mediations were determined during the pre or peri-menstrual phase of the cycle (18–20). Additional studies determined women with pre-menstrual asthma symptoms had increased fractional exhaled nitric oxide (FeNO), a non-invasive measure of epithelial induced nitric oxide that correlates with eosinophilic inflammation (24), and eosinophils in the sputum in the pre-menstrual phase compared to the seventh day of their cycle (25). In the Severe Asthma Research Program (SARP) study, peri-menstrual worsening of asthma had increased oral corticosteroid bursts and increased emergency department visits compared to women without peri-menstrual worsening of asthma (23). However, other studies including multiple phenotypes of asthma found no differences in the phase of the menstrual cycle of women requiring emergency department visits for asthma symptoms was reported (21, 26). While pre-menstrual asthma impacts many women with asthma, the molecular mechanisms driving the cyclic increase in symptoms are poorly understood. The pre-menstrual phase of the cycle occurs after the peaks of serum estrogen and progesterone. Therefore, it is unknown how estrogen, progesterone, or potentially other hormones which are differentially regulated through the menstrual cycle affect airway inflammation.
To delineate if ovarian hormones increased airway inflammation, studies tracking asthma symptoms in women taking hormonal oral contraceptives versus women not taking contraceptives were conducted with discordant findings. Cross-sectional surveys in menstruating women determined that women using oral contraceptives had increased asthma risk compared to women not on oral contraceptives (27, 28). However, Macsali and colleagues found the association between increased asthma risk and oral contraceptive use was not seen in lean (underweight) women (27). Oral contraceptives were associated with increased wheezing in women with asthma in some studies (27, 29), but decreased wheezing and/or asthma symptoms in others (30, 31•). In another study, 28 women with asthma were followed for 12 weeks (2–4 menstrual cycles) and no differences were determined in asthma symptoms in women taking oral contraceptives versus women not taking oral contraceptives (32). These discordant findings from various studies may be due to small sample sizes (for some studies) or the many different forms of birth control medications, including monophasic, multiphasic, progesterone-only, hormonal vaginal or interuterine devices, or extended-cycle pills, used by women. Additional studies with increased sample size and women on similar types of birth control medications should be conducted over several cycles and seasons to determine if hormonal contraceptives affect asthma symptoms.
Pregnancy and asthma
During pregnancy women with asthma are known to have decreased asthma symptoms, increased asthma symptoms, or maintain similar asthma symptoms as prior to pregnancy (33, 34). Women with more severe phenotypes of asthma are more likely to have asthma worsening during pregnancy (35). However, for mild and moderate asthma patients, it is difficult to predict if asthma symptoms will increase, decrease, or stay the same during pregnancy. The National Heart, Lung, and Blood Institute and the Global Initiative for Asthma (GINA) guidelines indicate that pregnant women should maintain their current regiment of asthma medications, including inhaled corticosteroids, long-acting beta agonist, leukotriene modifiers, theophylline, and oral corticosteroids (36, 37). Maintaining asthma control during pregnancy is important as severe asthma, poorly controlled asthma, and asthma exacerbations during pregnancy are associated with increased risk for development of pre-eclampsia and gestational diabetes in the mother and pre-term birth, low birth weight, and perinatal mortality for the baby (36, 38, 39). Future longitudinal studies are needed to track women with asthma prior to pregnancy and throughout the pregnancy to determine if any biomarker or clinical lung function test can be used to predict if asthma symptoms will change during pregnancy. This information would be beneficial in educating the patient on the importance of taking or increasing use of asthma medication during pregnancy.
Menopause and asthma
The age-adjusted risk of asthma decreases in post-menopausal women compared to pre-menopausal women (10). However, variable findings are reported in the literature regarding menopause and asthma. No difference in self-reported asthma between premenopausal and postmenopausal women not taking hormone replacement therapies (HRT) were reported by the European Community Respiratory Healthy Surveys (ECRHS I) (40). However, US Nurses’ Health Study determined postmenopausal women not taking HRT had decreased risk of developing asthma compared to premenopausal women (10), and the ECRHSII cross-sectional study reported increased asthma symptoms in women during the menopause transition (amenorrhea for 6+ months) compared to premenopausal women (41). Further, a new phenotype of asthma with onset after menopause has been recently described for a subset of women (42–44), but the mechanisms that initiate and regulate post-menopausal asthma remain largely unknown.
Role of sex hormones in airway inflammation – summary of animal model data
Patients with asthma have airway inflammation meditated by type 2 cells, IL-17A secreting cells, and IFN-γ secreting cells that leads to increased airway reactivity, inflammation, mucus production and clinical symptoms associated with asthma (45–47). Type 2 airway inflammation is characterized by increased CD4+ Th2 cells, group 2 innate lymphoid cells, eosinophils, mast cells, basophils, and other cells in the airway. IL-17A airway inflammation is meditated by increased secretion of IL-17A from CD4+ Th17 cells, γδ T cells, neutrophils, and group 3 innate lymphoid cells in the airways (48). IFN-γ-mediated airway inflammation is induced by CD4+ Th1 cells, cytotoxic killer T cells and natural killer (NK) cells (49). Clinical and epidemiological data have provided insight into the role of sex hormones in driving asthma symptoms, prevalence, and severity, but mouse models of asthma have elucidated some of the mechanism by which sex hormones regulate airway inflammation. As summarized in Table 1 and Figure 1, ovarian hormones, including estrogen and progesterone, enhanced while androgens, including, testosterone and 5-alpha dihydrotestosterone (5α-DHT), suppressed the innate and adaptive immune responses driving airway inflammation in asthma. In the following subsections, we will describe the findings from these animal studies.
Table 1.
Sex hormones in mouse models of airway inflammation associated with asthma
| Protocol for airway inflammation |
Gender effects on endpoints |
Endpoints | References |
|---|---|---|---|
| OVA sensitization and challenge | Females > Males | • BAL or lung eosinophils | (50–52) |
| • serum IgE | (50–52) | ||
| • IL-13 protein expression | (50–52) | ||
| • AHR | (50–52) | ||
| • airway remodeling | (50, 51) | ||
| • Mucus production | (50, 51) | ||
|
| |||
| OVA sensitization and challenge | Females = Males | • Mucus production | (52•) |
|
| |||
| HDM | Females > Males | • HDM-specific IgE levels in serum | (52•) |
| • IL-17A protein expression | |||
| • AHR | |||
|
| |||
| OVA sensitization and challenge | Castrated Males > Intact Male | • BAL eosinophils and lymphocytes | (50) |
|
| |||
| OVA sensitization and challenge | Intact Females > Ovariectomized Females | • Numbers of eosinophils | (53••) |
| • IL-5 protein expression | |||
| • Total IgE serum levels | |||
| • AHR | |||
|
| |||
| OVA sensitization and challenge | ER-α KO mice > WT and ER-β KO mice | • AHR | (54•) |
|
| |||
| OVA sensitization and challenge | Females > Males | • IL-5 and IL-13 protein expression in ILC2 isolated from whole lung homogenates | (60) |
|
| |||
| Transfer of OVA-specific Th17 cells followed by OVA challenge | Females > Males | • OVA specific IL-17A+ T cells | (72) |
| • Neutrophils | |||
|
| |||
| OVA sensitization and challenge | Females > Males | • Myeloid DCs | (83) |
| • Plasmacytoid DCs | |||
| • AAMφ | |||
AHR, airway hyperresponsiveness, AAMφ, alveolar macrophages; DC, dendritic cells
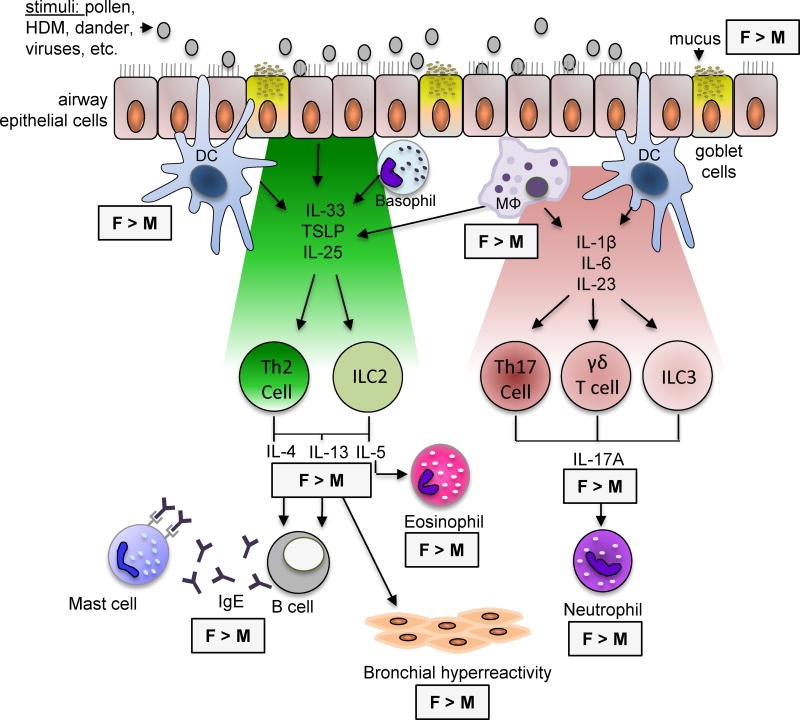
Figure 1.
Gender differences in airway inflammatory pathways associated with asthma. Mouse models of airway inflammation associated with asthma have shown gender differences in both type 2 and IL-17A-mediated airway inflammation. This figure summarizes these differences. DC dendritic cell; F, female; HDM, house dust mice; MΦ, macrophages; M male
Sex hormones and in type 2-mediated allergic airway inflammation
In multiple studies, female mice had increased ovalbumin (OVA)-induced infiltration of eosinophils, serum IgE concentrations, and IL-13 protein expression in the lungs compared to male mice (50–52•). However, OVA-challenged females had increased airway remodeling and mucus production compared to male mice in some reports (50, 51), but no differences were found in others (52•). Presence of ovarian hormones during the sensitization phase is required for maximal Th2-mediated airway inflammation in female mice. In the OVA sensitization and challenge model of allergic airway inflammation, female mice ovariectomized prior to OVA sensitization had decreased IL-5 and eosinophil broncheoalveolar (BAL) levels as well as decreased AHR to methacholine compared to sham-operated female mice (53•). However, if female mice were ovariectomized after sensitization, but before OVA challenge, there was no difference in BAL IL-5 protein expression or eosinophils as well as no effect on the percent increase in AHR (53•). Further, addition of oestradiol benzoate, a synthetic, steroidal estrogen, before OVA sensitization increased eosinophils in the BAL compared to vehicle treated ovariectomized OVA-challenged female mice, but did not restore eosinophil levels to OVA challenged sham-operated female mice (53•). These data show that estrogen signaling is important for OVA-induced allergic airway inflammation prior to sensitization, but that other ovarian hormones, potentially progesterone, are also important in establishing OVA-induced allergic airway inflammation.
Other studies have looked at the direct implication of estrogen or testosterone on allergic airway inflammation. Estrogen can signal through multiple receptors including the nuclear receptors, ER-α and ER-β, as well as the membrane bound G protein-couple estrogen receptor 1 (GPER1). Estrogen signaling through ER-α increased OVA-induced allergic airway inflammation, as mice deficient in ER-α (esr1−/− mice) had decreased AHR compared to WT and ER-β deficient mice (esr2−/− mice) (54•). However, additional studies showed that ER-α signaling increased OVA-induced AHR but had no effect on airway inflammation (54•). Testosterone is also important in type 2 mediated airway inflammation. Castrated male mice had a significant increase in OVA-induced eosinophil and lymphocyte infiltration as well as IL-13 protein expression compared to sham-operated male mice. Further, addition of dehydroepiandrosterone (DHEA), a hormone upstream of testosterone, to the mouse chow decreased house dust mite (HDM)-induced allergic airway inflammation compared to mice on control diet (55). DHEA treated mice undergoing the HDM protocol had decreased serum eosinophils, IL-5, IL-4, and IFN-g levels but no change in serum IgE concentrations compared to HDM stimulated mice on normal chow (55). Combined these data showed sex hormones were important in regulating type 2 inflammation and that many pathways are affected by ovarian hormone and/or testosterone signaling.
Group 2 innate lymphoid cells are also important for the allergic response in the lungs (56–59). Recently, IL-5 and IL-13 were increased in IL-33 stimulated ILC2 from OVA sensitization and challenge female BALB/c mice compared to IL-33 stimulated ILC2 from OVA sensitization and challenge male mice (60). These data suggest that sex hormones may also be important in ILC2-mediated airway inflammation, and future studies need to be conducted to determine the mechanism regulating ILC2-medaited airway inflammation.
Other immune cells, including mast cells, dendritic cells (DCs), and macrophages (Mφ), are also important for type 2 mediated airway inflammation. Allergens increase antigen-specific immunoglobulin E (IgE) production by B cells, and IgE binds to the high affinity IgE receptor, FcεR1, on tissue mast cells and peripheral blood basophils. Antigen crosslinking of the antigen-specific IgE/ FcεR1 complex ignites degranulation of the mast cells and basophils, causing release of soluble mediators, including histamine, cytokines, prostaglandins, and proteases (61, 62). While the role of sex hormones on mast cell degranulation or cytokine expression during airway inflammation is unclear, studies in isolated peritoneal mast cells (PMCs) from female Sprague-Dawley rats stimulated with substance P showed decreased histamine release in PMCs from female rats administered estradiol, progesterone, testosterone, or 5α-DHT. Further, if PMCs from female rats were stimulated with IgE, then histamine release was increased with estradiol treatment and decreased with progesterone, testosterone, or 5α-DHT. PMCs from male rats stimulated with substance P or IgE had no change in histamine release (63). As mentioned above, IgE is increased in the serum of OVA sensitized and challenged female mice compared to male mice (50–52). Therefore, increased activation of mast cells in the lungs of female mice compared to male mice is expected, but has not been fully explored.
DCs and M2 alveolar macrophages also play a central role in allergic airway inflammation, and sex hormones affect cytokine expression and antigen presentation in DCs and alveolar macrophages. Female mice that were OVA sensitized and challenged increased myeloid dendritic DCs and plasmacytoid DC migrating to the lung-draining lymph nodes as well as an increased the percentages of alveolar macrophages (AAMφ) compared to male BALB/c mice (64). Further, 17β-E2 treatment of LPS-stimulated DCs increased T cell activation, proliferation, and protein expression of IL-6, IL-8, and MCP-1 compared to vehicle treated, LPS-stimulated DCs (65). 17β-E2 administration to GM-CSF stimulated bone marrow cells also increased CD11b+, CD11c+ DCs. ER-α deficiency (esr1−/− mice) significantly decreased the number of CD11b+, CD11c+ DCs generated from GM-CSF and IL-4 treated bone marrow cells compared to WT mice (66). Studies on macrophages from other tissues have shown that the administration of E2 to ovariectomized LysM-Cre Erαflox mice significantly reduced numbers of peritoneal macrophages in the peritoneal cavity and increased protein levels of IL-1b and IL-6 as well as mRNA expression of iNOS, compared to ovariectomized LysM-Cre Erαflox mice administered placebo pellets(67). Estrogens are not the only sex hormones that are important for DC and AAMφ function, and future studies need to focus on the role of estrogens, progesterone, and testosterone, on lung DCs and AAMφ differentiation, function, and cytokine expression in airway inflammation associated with asthma.
Sex hormones and IL-17A-mediated airway inflammation
While type 2 immune mediated airway inflammation is found in many patients with asthma, increased IL-17A is associated with more severe phenotypes of asthma (48, 68•). IL-17A is increased in the bronchoalveolar lavage fluid of patients with asthma, leading to increased mucus production and increased neutrophils in the airways (68•–71). In the lungs, IL-17A is secreted by CD4+ T helper 17 (Th17) cells, γδ T cells, and neutrophils and innate lymphoid group 3 (ILC3) cells. Our group showed women with severe asthma had significantly increased IL-17A producing Th17 cells compared to men with severe asthma (72). Using an adoptive transfer mouse model of OVA-specific Th17-mediated inflammation, we also showed that the OVA-specific Th17 cells from female mice had increased IL-17A production and caused increased neutrophilic inflammation in recipient mice compared to OVA-specific Th17 cells from male mice(72). In vivo studies also showed that the combination of estrogen and progesterone increased IL-17A protein expression in Th17 cells by increasing IL-23/IL-23R signaling through a let-7f miRNA expression dependent pathway (72).
IL-17A producing γδ T cells and ILC3 also augment the IL-17A mediated airway inflammatory response and the role of sex hormones on these cell types has also be studied (73, 74). Estradiol (E2) treatment of γδ T cells decreased numbers of IL-17+ T cells in the draining lymph nodes, suggested an estradiol mediated regulation of γδ T cell migration from the lymph nodes to various tissues (75). The role of sex hormones on ILC3 function has not been investigated, but is an important area for future studies. These are the primary findings for sex differences in IL-17A mediated airway inflammation associated with asthma. Additional studies need to be conducted to determine how sex hormones regulate IL-17A-mediated airway inflammation or both type 2 and IL-17A-mediated inflammation, as many patients with asthma have increased eosinophils and neutrophils in the airway (3••).
Role of sex hormones in airway physiology
Non-immunological mechanisms, such as gender differences in AHR, smooth muscle contractility, and mucus production, may also drive the gender differences in asthma. Baseline AHR is increased in male mice compared to female mice for both BALB/c and C57BL/6 strains of mice(76). Increased basal AHR in male mice is potentially caused by fewer numbers of alveoli and decreased alveolar surface area compared to female mice as ovariectomized female mice have similar alveoli structures as male mice (77). Additionally, ER-α- and ER-β signaling is important for alveolar development since ER-α and ER-β deficient mice have decreased alveoli.(78) A gender differences in smooth muscle contractility is also reported with vagal nerve responses increased in male mice compared to female mice in response to methacholine and carabachol challenge (78). However, following gonadectomy male mice has similar levels of vagal nerve responses as intact female mice (78). Restoring androgens to gonadectomized mice increased vagal nerve responses and increased AHR in these mice (78).
Estrogen and progesterone are also important in mucus production and mucociliary clearance. Administration of ethynyl oestradiol to guinea pigs significantly increased mucus cell hyperplasia compared to vehicle treated animals (79). Further, administration of estrogen or progesterone to cultured human airway or nasal epithelial cells the expression of the mucus proteins, Muc5AC and Muc5B, as well as mucus production when compared to vehicle treated cells (80, 81). Progesterone also decreased cilia beat frequency from cultured primary human airway epithelial cells, but cells that were co-administered 17b-E2 with progesterone had cilia beat frequency that was similar to vehicle treated cells (82). In summary, sex hormones regulate baseline airway responsiveness to methacholine, smooth muscle contractility, and mucus production.
Summary and conclusions
Sex hormones regulate asthma pathophysiology via multiple pathways, but some mechanisms remain unclear. Clinical evidence shows that increased asthma symptoms occur in females starting at puberty compared to boys. However, after puberty, the role for sex hormones in regulating asthma symptoms during menstruation, pregnancy, and menopause is not as clear. Animal studies showed that estrogen increased and testosterone decreased Th2-mediated airway inflammation, but how ovarian hormones and testosterone regulate other pathways important in airway inflammation remains to be elucidated. Future research should focus on delineating these pathways using animal models as well as women and men with various phenotypes of asthma. Understanding the pathways will help determine if women with asthma should take (or avoid) hormonal contraceptive use, ways to control perimenstrual asthma, and methods or biomarkers to more accurately predict if asthma symptoms will worsen during pregnancy.
Acknowledgments
Funding: This work was supported by National Institute of Health: R01 HL122554 and R21 AI121420
Abbreviations
- AAMφ
alveolar macrophages
- AHR
airway hyperresponsiveness
- BAL
broncheoalveolar lavage
- DC
dendritic cells
- FeNO
forced nitric oxide
- HDM
house dust mite
- HRT
hormone replacement therapy
- Mφ
macrophages
- OVA
ovalbumin
- SARP
severe asthma research program
References
- 1.Prevention of asthma. Global initiative for asthma (GINA) http://www.ginasthma.org/. 2014 2014. Report No.
- 2.Centers for Disease Control and Prevention. Vital Signs. 2011 May;2013 [Google Scholar]
- 3••.Wu W, Bleecker E, Moore W, Busse WW, Castro M, Chung KF, et al. Unsupervised phenotyping of severe asthma research program participants using expanded lung data. J Allergy Clin Immunol. 2014;133(5):1280–8. doi: 10.1016/j.jaci.2013.11.042. This study was critical in identifying different phenotypes of asthma and determining that women have increased prevalence in the more severe phenotypes of asthma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zein JG, Erzurum SC. Asthma is different in women. Curr Allergy Asthma Rep. 2015;15(6):28. doi: 10.1007/s11882-015-0528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kynyk JA, Mastronarde JG, McCallister JW. Asthma, the sex difference. Curr Opin Pulm Med. 2011;17(1):6–11. doi: 10.1097/MCP.0b013e3283410038. [DOI] [PubMed] [Google Scholar]
- 6.Moorman JE, Zahran H, Truman BI, Molla MT. Current asthma prevalence - United States, 2006–2008. MMWR Suppl. 2011;(60 Suppl):84–6. [PubMed] [Google Scholar]
- 7.Chen Y, Stewart P, Johansen H, McRae L, Taylor G. Sex difference in hospitalization due to asthma in relation to age. J Clin Epidemiol. 2003;56(2):180–7. doi: 10.1016/s0895-4356(02)00593-0. [DOI] [PubMed] [Google Scholar]
- 8.Hyndman SJ, Williams DR, Merrill SL, Lipscombe JM, Palmer CR. Rates of admission to hospital for asthma. BMJ. 1994;308(6944):1596–600. doi: 10.1136/bmj.308.6944.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skobeloff EM, Spivey WH, St Clair SS, Schoffstall JM. The influence of age and sex on asthma admissions. JAMA. 1992;268(24):3437–40. [PubMed] [Google Scholar]
- 10.Troisi RJ, Speizer FE, Willett WC, Trichopoulos D, Rosner B. Menopause, postmenopausal estrogen preparations, and the risk of adult-onset asthma. A prospective cohort study. AmJRespirCrit Care Med. 1995;152(4 Pt 1):1183–8. doi: 10.1164/ajrccm.152.4.7551368. [DOI] [PubMed] [Google Scholar]
- 11.Borish L, Chipps B, Deniz Y, Gujrathi S, Zheng B, Dolan CM, et al. Total serum ige levels in a large cohort of patients with severe or difficult-to-treat asthma. Ann Allergy Asthma Immunol. 2005;95(3):247–53. doi: 10.1016/S1081-1206(10)61221-5. [DOI] [PubMed] [Google Scholar]
- 12.Genuneit J. Sex-specific development of asthma differs between farm and nonfarm children: A cohort study. Am J Respir Crit Care Med. 2014;190(5):588–90. doi: 10.1164/rccm.201403-0428LE. [DOI] [PubMed] [Google Scholar]
- 13.Pagtakhan RD, Bjelland JC, Landau LI, Loughlin G, Kaltenborn W, Seeley G, et al. Sex differences in growth patterns of the airways and lung parenchyma in children. J Appl Physiol Respir Environ Exerc Physiol. 1984;56(5):1204–10. doi: 10.1152/jappl.1984.56.5.1204. [DOI] [PubMed] [Google Scholar]
- 14.Vink NM, Postma DS, Schouten JP, Rosmalen JG, Boezen HM. Gender differences in asthma development and remission during transition through puberty: The tracking adolescents' individual lives survey (trails) study. JAllergy ClinImmunol. 2010;126(3):498–504. doi: 10.1016/j.jaci.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 15•.Fu L, Freishtat RJ, Gordish-Dressman H, Teach SJ, Resca L, Hoffman EP, et al. Natural progression of childhood asthma symptoms and strong influence of sex and puberty. Ann Am Thorac Soc. 2014;11(6):939–44. doi: 10.1513/AnnalsATS.201402-084OC. This study showed that asthma symptoms increased as Tanner stages of puberty increased in girls. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castro-Rodriguez JA. A new childhood asthma phenotype: Obese with early menarche. Paediatric Respiratory Reviews. 2016;18:85–9. doi: 10.1016/j.prrv.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Juniper EF, Kline PA, Roberts RS, Hargreave FE, Daniel EE. Airway responsiveness to methacholine during the natural menstrual cycle and the effect of oral contraceptives. Am Rev Respir Dis. 1987;135(5):1039–42. doi: 10.1164/arrd.1987.135.5.1039. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal AK, Shah A. Menstrual-linked asthma. J Asthma. 1997;34(6):539–45. doi: 10.3109/02770909709055398. [DOI] [PubMed] [Google Scholar]
- 19.Pauli BD, Reid RL, Munt PW, Wigle RD, Forkert L. Influence of the menstrual cycle on airway function in asthmatic and normal subjects. Am Rev Respir Dis. 1989;140(2):358–62. doi: 10.1164/ajrccm/140.2.358. [DOI] [PubMed] [Google Scholar]
- 20.Shames RS, Heilbron DC, Janson SL, Kishiyama JL, Au DS, Adelman DC. Clinical differences among women with and without self-reported perimenstrual asthma. Ann Allergy Asthma Immunol. 1998;81(1):65–72. doi: 10.1016/S1081-1206(10)63111-0. [DOI] [PubMed] [Google Scholar]
- 21.Brenner BE, Holmes TM, Mazal B, Camargo CA., Jr Relation between phase of the menstrual cycle and asthma presentations in the emergency department. Thorax. 2005;60(10):806–9. doi: 10.1136/thx.2004.033928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chandler MH, Schuldheisz S, Phillips BA, Muse KN. Premenstrual asthma: The effect of estrogen on symptoms, pulmonary function, and beta 2-receptors. Pharmacotherapy. 1997;17(2):224–34. [PubMed] [Google Scholar]
- 23.Rao CK, Moore CG, Bleecker E, Busse WW, Calhoun W, Castro M, et al. Characteristics of perimenstrual asthma and its relation to asthma severity and control: Data from the severe asthma research program. Chest. 2013;143(4):984–92. doi: 10.1378/chest.12-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ats clinical practice guideline: Interpretation of exhaled nitric oxide levels (feno) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–15. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oguzulgen IK, Turktas H, Erbas D. Airway inflammation in premenstrual asthma. J Asthma. 2002;39(6):517–22. doi: 10.1081/jas-120004921. [DOI] [PubMed] [Google Scholar]
- 26.Zimmerman JL, Woodruff PG, Clark S, Camargo CA. Relation between phase of menstrual cycle and emergency department visits for acute asthma. Am J Respir Crit Care Med. 2000;162(2 Pt 1):512–5. doi: 10.1164/ajrccm.162.2.9910105. [DOI] [PubMed] [Google Scholar]
- 27.Macsali F, Real FG, Omenaas ER, Bjorge L, Janson C, Franklin K, et al. Oral contraception, body mass index, and asthma: A cross-sectional nordic-baltic population survey. J Allergy Clin Immunol. 2009;123(2):391–7. doi: 10.1016/j.jaci.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 28.Jenkins MA, Dharmage SC, Flander LB, Douglass JA, Ugoni AM, Carlin JB, et al. Parity and decreased use of oral contraceptives as predictors of asthma in young women. Clin Exp Allergy. 2006;36(5):609–13. doi: 10.1111/j.1365-2222.2006.02475.x. [DOI] [PubMed] [Google Scholar]
- 29.Erkocoglu M, Kaya A, Azkur D, Ozyer S, Ozcan C, Besli M, et al. The effect of oral contraceptives on current wheezing in young women. Allergol Immunopathol (Madr) 2013;41(3):169–75. doi: 10.1016/j.aller.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Salam MT, Wenten M, Gilliland FD. Endogenous and exogenous sex steroid hormones and asthma and wheeze in young women. J Allergy Clin Immunol. 2006;117(5):1001–7. doi: 10.1016/j.jaci.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 31••.Dratva J, Schindler C, Curjuric I, Stolz D, Macsali F, Gomez FR, et al. Perimenstrual increase in bronchial hyperreactivity in premenopausal women: Results from the population-based sapaldia 2 cohort. J Allergy Clin Immunol. 2010;125(4):823–9. doi: 10.1016/j.jaci.2009.12.938. This study conducted methacholine challenges on the day of menstruation for over 500 women taking or not taking oral contractives to determine cyclic variations in bronchial hypperactivity. Authers determined systematic variations in BHR durig the menstrual cycle and that oral contraceptives had a protective effect. [DOI] [PubMed] [Google Scholar]
- 32.Murphy VE, Gibson PG. Premenstrual asthma: Prevalence, cycle-to-cycle variability and relationship to oral contraceptive use and menstrual symptoms. J Asthma. 2008;45(8):696–704. doi: 10.1080/02770900802207279. [DOI] [PubMed] [Google Scholar]
- 33.Schatz M, Harden K, Forsythe A, Chilingar L, Hoffman C, Sperling W, et al. The course of asthma during pregnancy, post partum, and with successive pregnancies: A prospective analysis. J Allergy Clin Immunol. 1988;81(3):509–17. [PubMed] [Google Scholar]
- 34.Stenius-Aarniala B, Piirila P, Teramo K. Asthma and pregnancy: A prospective study of 198 pregnancies. Thorax. 1988;43(1):12–8. doi: 10.1136/thx.43.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schatz M. Interrelationships between asthma and pregnancy: A literature review. J Allergy Clin Immunol. 1999;103(2 Pt 2):S330–6. doi: 10.1016/s0091-6749(99)70258-7. [DOI] [PubMed] [Google Scholar]
- 36.National Heart L, Blood I, National Asthma E, Prevention Program A, Pregnancy Working G. Naepp expert panel report. Managing asthma during pregnancy: Recommendations for pharmacologic treatment-2004 update. J Allergy Clin Immunol. 2005;115(1):34–46. doi: 10.1016/j.jaci.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 37.(GINA) GIfA. Global strategy for asthma management and prevention. 2014 [Google Scholar]
- 38.Murphy VE, Clifton VL, Gibson PG. Asthma exacerbations during pregnancy: Incidence and association with adverse pregnancy outcomes. Thorax. 2006;61(2):169–76. doi: 10.1136/thx.2005.049718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim A, Stewart K, Konig K, George J. Systematic review of the safety of regular preventive asthma medications during pregnancy. Ann Pharmacother. 2011;45(7–8):931–45. doi: 10.1345/aph.1P764. [DOI] [PubMed] [Google Scholar]
- 40.Gomez Real F, Svanes C, Bjornsson EH, Franklin KA, Gislason D, Gislason T, et al. Hormone replacement therapy, body mass index and asthma in perimenopausal women: A cross sectional survey. Thorax. 2006;61(1):34–40. doi: 10.1136/thx.2005.040881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Real FG, Svanes C, Omenaas ER, Anto JM, Plana E, Jarvis D, et al. Lung function, respiratory symptoms, and the menopausal transition. J Allergy Clin Immunol. 2008;121(1):72–80. e3. doi: 10.1016/j.jaci.2007.08.057. [DOI] [PubMed] [Google Scholar]
- 42.Balzano G, Fuschillo S, De Angelis E, Gaudiosi C, Mancini A, Caputi M. Persistent airway inflammation and high exacerbation rate in asthma that starts at menopause. Monaldi Arch Chest Dis. 2007;67(3):135–41. doi: 10.4081/monaldi.2007.484. [DOI] [PubMed] [Google Scholar]
- 43.Triebner K, Johannessen A, Puggini L, Benediktsdottir B, Bertelsen RJ, Bifulco E, et al. Menopause as a predictor of new-onset asthma: A longitudinal northern european population study. J Allergy Clin Immunol. 2016;137(1):50–7. e6. doi: 10.1016/j.jaci.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 44.Triebner K, Matulonga B, Johannessen A, Suske S, Benediktsdottir B, Demoly P, et al. Menopause is associated with accelerated lung function decline. Am J Respir Crit Care Med. 2016 doi: 10.1164/rccm.201605-0968OC. [DOI] [PubMed] [Google Scholar]
- 45.Trejo Bittar HE, Yousem SA, Wenzel SE. Pathobiology of severe asthma. Annual Review of Pathology: Mechanisms of Disease. 2015;10:511–45. doi: 10.1146/annurev-pathol-012414-040343. [DOI] [PubMed] [Google Scholar]
- 46.Manni ML, Trudeau JB, Scheller EV, Mandalapu S, Elloso MM, Kolls JK, et al. The complex relationship between inflammation and lung function in severe asthma. Mucosal Immunol. 2014;7(5):1186–98. doi: 10.1038/mi.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fajt ML, Wenzel SE. Asthma phenotypes and the use of biologic medications in asthma and allergic disease: The next steps toward personalized care. Journal of Allergy and Clinical Immunology. 2015;135(2):299–310. doi: 10.1016/j.jaci.2014.12.1871. [DOI] [PubMed] [Google Scholar]
- 48.Fahy JV. Eosinophilic and neutrophilic inflammation in asthma: Insights from clinical studies. Proc Am Thorac Soc. 2009;6(3):256–9. doi: 10.1513/pats.200808-087RM. [DOI] [PubMed] [Google Scholar]
- 49.Ray A, Raundhal M, Oriss TB, Ray P, Wenzel SE. Current concepts of severe asthma. J Clin Invest. 2016;126(7):2394–403. doi: 10.1172/JCI84144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayashi T, Adachi Y, Hasegawa K, Morimoto M. Less sensitivity for late airway inflammation in males than females in balb/c mice. ScandJImmunol. 2003;57(6):562–7. doi: 10.1046/j.1365-3083.2003.01269.x. [DOI] [PubMed] [Google Scholar]
- 51.Takeda M, Tanabe M, Ito W, Ueki S, Konnno Y, Chihara M, et al. Gender difference in allergic airway remodelling and immunoglobulin production in mouse model of asthma. Respirology. 2013;18(5):797–806. doi: 10.1111/resp.12078. [DOI] [PubMed] [Google Scholar]
- 52•.Blacquiere MJ, Hylkema MN, Postma DS, Geerlings M, Timens W, Melgert BN. Airway inflammation and remodeling in two mouse models of asthma: Comparison of males and females. Int Arch Allergy Immunol. 2010;153(2):173–81. doi: 10.1159/000312635. This study showed that female mice had increased OVA or HDM induced airway associated with severe asthma and that airway remodeling is not associated with increased asthma severity in mice. [DOI] [PubMed] [Google Scholar]
- 53••.Riffo-Vasquez Y, Ligeiro de Oliveira AP, Page CP, Spina D, Tavares-de-Lima W. Role of sex hormones in allergic inflammation in mice. ClinExpAllergy. 2007;37(3):459–70. doi: 10.1111/j.1365-2222.2007.02670.x. This study showed that female sex hormones increase OVA induced type 2 airway inflammation by increasing IL-5 and eosinophil numbers in the BAL fluid and airway hyperesponsiveness to methacholine challenges. [DOI] [PubMed] [Google Scholar]
- 54•.Carey MA, Card JW, Bradbury JA, Moorman MP, Haykal-Coates N, Gavett SH, et al. Spontaneous airway hyperresponsiveness in estrogen receptor-alpha-deficient mice. AmJRespirCrit Care Med. 2007;175(2):126–35. doi: 10.1164/rccm.200509-1493OC. Showed that estrogen receptor alpha decreases airway hyperresponsinvess to methacholine challenge and regulates M2 muscarinic receptor expression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu CK, Liu YH, Chen CL. Dehydroepiandrosterone attenuates allergic airway inflammation in dermatophagoides farinae-sensitized mice. J Microbiol Immunol Infect. 2002;35(3):199–202. [PubMed] [Google Scholar]
- 56.Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. Il-33-responsive lineage- cd25+ cd44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012;188(3):1503–13. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Angkasekwinai P, Park H, Wang YH, Wang YH, Chang SH, Corry DB, et al. Interleukin 25 promotes the initiation of proallergic type 2 responses. JExpMed. 2007;204(7):1509–17. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ballantyne SJ, Barlow JL, Jolin HE, Nath P, Williams AS, Chung KF, et al. Blocking il-25 prevents airway hyperresponsiveness in allergic asthma. JAllergy ClinImmunol. 2007;120(6):1324–31. doi: 10.1016/j.jaci.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 59.Barlow JL, Bellosi A, Hardman CS, Drynan LF, Wong SH, Cruickshank JP, et al. Innate il-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J Allergy Clin Immunol. 2012;129(1):191–8. e1–4. doi: 10.1016/j.jaci.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 60.Warren KJ, Sweeter JM, Pavlik JA, Nelson AJ, Devasure JM, Dickinson JD, et al. Sex differences in activation of lung-related type 2 innate lymphoid cells in experimental asthma. Annals of Allergy, Asthma & Immunology. doi: 10.1016/j.anai.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Borish L, Culp JA. Asthma: A syndrome composed of heterogeneous diseases. AnnAllergy Asthma Immunol. 2008;101(1):1–8. doi: 10.1016/S1081-1206(10)60826-5. [DOI] [PubMed] [Google Scholar]
- 62.Busse WW, Lemanske RF., Jr Asthma. NEnglJMed. 2001;344(5):350–62. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 63.Munoz-Cruz S, Mendoza-Rodriquez Y, et al. Gender-related effects of sex steroids on histamine release and FcεR1 expression in rat peritoneal mast cells. Journal of Immunol Res. 2015;2015:10. doi: 10.1155/2015/351829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Melgert BN, Oriss TB, Qi Z, Dixon-McCarthy B, Geerlings M, Hylkema MN, et al. Macrophages: Regulators of sex differences in asthma? Am J Respir Cell Mol Biol. 2010;42(5):595–603. doi: 10.1165/rcmb.2009-0016OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bengtsson AK, Ryan EJ, Giordano D, Magaletti DM, Clark EA. 17beta-estradiol (e2) modulates cytokine and chemokine expression in human monocyte-derived dendritic cells. Blood. 2004;104(5):1404–10. doi: 10.1182/blood-2003-10-3380. [DOI] [PubMed] [Google Scholar]
- 66.Paharkova-Vatchkova V, Maldonado R, Kovats S. Estrogen preferentially promotes the differentiation of CD11c+ CD11b (intermediate) dendritic cells from bone marrow precursors. The J Immunol. 2004;172(3):1426. doi: 10.4049/jimmunol.172.3.1426. [DOI] [PubMed] [Google Scholar]
- 67.Calippe B, Douin-Echinard V, Delpy L, Laffargue M, Lelu K, Krust A, et al. 17beta-estradiol promotes tlr4-triggered proinflammatory mediator production through direct estrogen receptor alpha signaling in macrophages in vivo. J Immunol. 2010;185(2):1169–76. doi: 10.4049/jimmunol.0902383. [DOI] [PubMed] [Google Scholar]
- 68••.Newcomb DC, Peebles RS., Jr Th17-mediated inflammation in asthma. Curr Opin Immunol. 2013;25(6):755–60. doi: 10.1016/j.coi.2013.08.002. First study to show that women with severe asthma have increased numbers of IL-17+ memory Th17 cells compared to men with severe asthma and that transfer of OVA-specific Th17 cells from female mice increased airway neutrophils in OVA challenege recipient mice compared to transfer of OVA-specific Th117 cells from male mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chakir J, Shannon J, Molet S, Fukakusa M, Elias J, Laviolette M, et al. Airway remodeling-associated mediators in moderate to severe asthma: Effect of steroids on tgf-beta, il-11, il-17, and type i and type iii collagen expression. J Allergy Clin Immunol. 2003;111(6):1293–8. doi: 10.1067/mai.2003.1557. [DOI] [PubMed] [Google Scholar]
- 70.Newcomb DC, Boswell MG, Sherrill TP, Polosukhin VV, Boyd KL, Goleniewska K, et al. Il-17a induces signal transducers and activators of transcription-6-independent airway mucous cell metaplasia. Am J Respir Cell Mol Biol. 2013;48(6):711–6. doi: 10.1165/rcmb.2013-0017OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Newcomb DC, Boswell MG, Reiss S, Zhou W, Goleniewska K, Toki S, et al. Il-17a inhibits airway reactivity induced by respiratory syncytial virus infection during allergic airway inflammation. Thorax. 2013;68(8):717–23. doi: 10.1136/thoraxjnl-2012-202404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Newcomb DC, Cephus JY, Boswell MG, Fahrenholz JM, Langley EW, Feldman AS, et al. Estrogen and progesterone decrease let-7f microrna expression and increase il-23/il-23 receptor signaling and il-17a production in patients with severe asthma. J Allergy Clin Immunol. 2015;136(4):1025–34. e11. doi: 10.1016/j.jaci.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ullah MA, Revez JA, Loh Z, Simpson J, Zhang V, Bain L, et al. Allergen-induced il-6 trans-signaling activates γδ t cells to promote type 2 and type 17 airway inflammation. Journal of Allergy and Clinical Immunology. 2015;136(4):1065–73. doi: 10.1016/j.jaci.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 74.Bloodworth MH, Newcomb DC, Dulek DE, Stier MT, Cephus JY, Zhang J, et al. Stat6 signaling attenuates interleukin-17-producing gammadelta t cells during acute klebsiella pneumoniae infection. Infect Immun. 2016;84(5):1548–55. doi: 10.1128/IAI.00646-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Andersson A, Grahnemo L, Engdahl C, Stubelius A, Lagerquist MK, Carlsten H, et al. Il-17-producing γδt cells are regulated by estrogen during development of experimental arthritis. Clinical Immunology. 2015;161(2):324–32. doi: 10.1016/j.clim.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 76.Card JW, Carey MA, Bradbury JA, DeGraff LM, Morgan DL, Moorman MP, et al. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. Journal of immunology (Baltimore, Md : 1950) 2006;177(1):621–30. doi: 10.4049/jimmunol.177.1.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Massaro D, Massaro GD. Estrogen receptor regulation of pulmonary alveolar dimensions: Alveolar sexual dimorphism in mice. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2006;290(5):L866. doi: 10.1152/ajplung.00396.2005. [DOI] [PubMed] [Google Scholar]
- 78.Card JW, Voltz JW, Ferguson CD, Carey MA, DeGraff LM, Peddada SD, et al. Male sex hormones promote vagally mediated reflex airway responsiveness to cholinergic stimulation. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2007;292(4):L908. doi: 10.1152/ajplung.00407.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Helmi AM, El Ghazzawi IF, Mandour MA, Shehata MA. The effect of oestrogen on the nasal respiratory mucosa. An experimental histopathological and histochemical study. J Laryngol Otol. 1975;89(12):1229–41. doi: 10.1017/s0022215100081597. [DOI] [PubMed] [Google Scholar]
- 80.Tam A, Wadsworth S, Dorscheid D, Man SF, Sin DD. Estradiol increases mucus synthesis in bronchial epithelial cells. PLoS One. 2014;9(6):e100633. doi: 10.1371/journal.pone.0100633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Choi HJ, Chung YS, Kim HJ, Moon UY, Choi YH, Van Seuningen I, et al. Signal pathway of 17beta-estradiol-induced muc5b expression in human airway epithelial cells. Am J Respir Cell Mol Biol. 2009;40(2):168–78. doi: 10.1165/rcmb.2007-0377OC. [DOI] [PubMed] [Google Scholar]
- 82••.Jain R, Ray JM, Pan JH, Brody SL. Sex hormone-dependent regulation of cilia beat frequency in airway epithelium. Am J Respir Cell Mol Biol. 2012;46(4):446–53. doi: 10.1165/rcmb.2011-0107OC. Progesterone decreased cilia beat frequency in humany primary epithelial cells, but administration of estrogen in combination with progesterone rescued cilia beat frequency. This study showed that sex hormones affect cilia beat frequency and mucociliary clearance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Melgert BN, Postma DS, Kuipers I, Geerlings M, Luinge MA, van der Strate BW, et al. Female mice are more susceptible to the development of allergic airway inflammation than male mice. Clin Exp Allergy. 2005;35(11):1496–503. doi: 10.1111/j.1365-2222.2005.02362.x. [DOI] [PubMed] [Google Scholar]