Abstract
Purpose
We evaluate the toxicity and plasma toxicokinetic (TK) profile of a biodegradable subconjunctival microrod for sustained prednisolone acetate (PA) release over 12 weeks in a non-human primate model.
Methods
The biodegradable copolymer poly(l-lactide-co-ε-caprolactone) (PLC) and 40-wt% PA microrods were used and fashioned into 8 and 16 mm lengths. Twelve monkeys were divided into two treatment groups of PA-loaded and blank microrods, with six monkeys each receiving either 8- or 16-mm microrods subconjunctively implanted into both eyes. TK and hematology parameters were analyzed. Ophthalmic clinical evaluation, including slit-lamp and ophthalmoscopy examinations, was performed.
Results
Over the study period of 12 weeks, the mean area under the plasma concentration-time curve was 45.7% higher, and the maximum plasma concentration was 17.2% lower for the animals treated with 40-wt% PA 16-mm microrods compared to 8-mm microrods (251.44 versus 172.54 hours × nanograms per milliliter and 8.53 versus 10.30 ng/mL, respectively). The PA release was significantly below the levels of assumed toxicity. There was no significant difference in the time to reach maximum concentration between the 8- and 16-mm microrod groups (7.33 and 8 hours; P = 0.421). Findings from clinical evaluation, hematology, and histopathology showed no ocular side effects and no significant adverse systemic effects.
Conclusion
The PA biodegradable microrods demonstrated safe toxicokinetics even with the larger size implant containing a higher amount of drug. The PA implant may be considered as a safe alternative to the application of topical PA eyedrops.
Translational Relevance
The results provide the evidence of the safety of implanting a steroid delivery system subconjunctively, offering an alternative to topical PA eyedrops.
Keywords: drug delivery, subconjunctival, corticosteroids
Introduction
Topical corticosteroids are among the most frequently prescribed drugs in ophthalmology1 and are used in a broad spectrum of ocular conditions, including ocular anterior segment diseases—for example, viral or allergic conjunctivitis, episcleritis, scleritis, keratitis, and uveitis—and in the management of postoperative conditions, such as inflammation after cataract, glaucoma filtration, corneal transplant, and refractive surgeries. However, patients requiring long-term topical eyedrops have been reported to have noncompliance rates from 5% to 80%.2 The ocular bioavailability for topically applied eyedrops is predicted to range from only 1% to 5% for lipophilic molecules, such as corticosteroids, due to efficient anatomical and physiological barriers and washout by tears, hence effective ocular therapeutic levels may be difficult to reach.1,3,4 To circumvent the drawbacks of topical eyedrops, patients may be given periocular injections, as these provide higher intraocular concentrations of steroids. However, the injected drug is rapidly cleared within 2 weeks of administration.5
A solution to these various drawbacks of eyedrops is a fully biodegradable subconjunctival microrod that offers steady and sustained corticosteroid release. Compared to other corticosteroids, for example, dexamethasone, prednisolone acetate (PA) achieves a high aqueous concentration within 2 hours and maintains higher levels for 24 hours.6 Hence, we developed a subconjunctival 40-wt% PA-loaded poly(l-lactide-co-ε-caprolactone) (PLC) microrod. Implantation of a subconjunctival sustained-release drug-delivery system is a simple and minimally invasive procedure as compared to other anterior chamber drug-delivery device implantations. We have previously shown that the subconjunctival PA-PLC microrods significantly reduced corneal graft rejection after rat corneal transplantation,7,8 reduced postoperative inflammation and prolonged bleb survival following experimental glaucoma filtering surgery,9 and suppressed the severity of uveitis in a rabbit model.10 Once complete PA release has been achieved, the remaining PLC bulk microrod will fully degrade.
While the PA penetration and aqueous concentration profiles from a subconjunctival implant have been elucidated in our previous work and by other groups,6,8 the toxicological profile for PA in plasma after subconjunctival administration is still unknown, with only oral and intravenous values being reported. As a sustained-release microrod for PA is being proposed here, there was a need to understand plasma concentration and time profiles after subconjunctival implantation and to compare this with published data. Proving its toxicological safety would allow further dose refinements and range for maximum efficacy as developments ensue.
This study evaluated the toxicity and plasma toxicokinetic (TK) profile of the PA-PLC microrod following subconjunctival implantation in a non-human primate model.
Methods
Drug-Delivery System
The biodegradable random copolymer used as the matrix material was PLC (Purac Biochem BV, Gorinchem, The Netherlands) with a molar ratio of 70:30, d,l-lactide to ε-caprolactone. The PA drug used was prednisolone 21-acetate (Sigma-Aldrich, Singapore). To fabricate the microrod prototypes, PLC and PA (40 wt%) were dissolved in dichloromethane and cast into films of around 300-μm thick and placed into a 37° C vacuum oven for solvent evaporation/drying for 7 days. Films were then removed, and the microrods were obtained by sectioning into lengths of 8 and 16 mm (PA dose at a 0.1 and 0.2 mg/kg of average body weight, respectively) for two treatment groups. Blank films were produced in the same way, using only PLC without PA addition for the control subgroups. All samples were sterilized by room-temperature ethylene oxide for 24 hours prior to the conduct of this study.
Animals and Experimental Design
This study was approved by the Institutional Animal Care and Use Committee (IACUC) of Maccine, Singapore (no. 308-2013). All animals were treated in accordance with the tenets of the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. Twelve naive female Cynomolgus monkeys (Macaca fascicularis) obtained from Nafovanny, Vietnam, were used. The animals were allocated to four groups (n = six eyes for each): 8-mm blank, 16-mm blank, 8-mm PA-loaded, and 16-mm PA-loaded microrod groups.
Implantation Procedure
For the microrod implantation, the animals were sedated with ketamine and maintained under isoflurane anesthesia. A subconjunctival pocket was created via blunt dissection just above the limbus, and the microrod was inserted into the pocket (Fig. 1). Following insertion, the pocket was closed with 10-0 nylon sutures to ensure secure implantation of the microrod. Following surgery, topical Tobrex (Alcon Laboratories, Ryde, Australia) was applied four times daily for 3 days. The animals were retained for a 12-week follow-up period.
Figure 1.
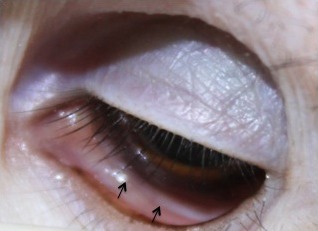
Representative external eye picture showing the microrod implanted in the subconjunctival space (arrows).
Toxicokinetics
Blood samples for the determination of plasma concentrations of PA were collected at the following time points: before implantation; at 1, 2, 4, 6, 8, 24, 72 hours; and 2, 4, and 12 weeks after implantation. Blood samples (2 mL) were collected from the femoral vein and transferred into lithium heparin tubes. The samples were centrifuged, and the resultant plasma samples were frozen before bioanalysis.
Bioanalytical Method
The analyte (PA) was extracted from 100 μL of plasma. Fifty microliters of internal standard, dexamethasone 50 ng/mL, was then added. Ethyl acetate (1 mL) was added, vortexed, and centrifuged. The organic layer was transferred and evaporated. The sample was reconstituted with 50 μL of 50% (0.1% formic acid): 50% methanol. The sample (10 μL) was used for injection into a liquid chromatography tandem-mass spectrometry system for analysis. The mobile phase was a mixture of 70% (0.1% formic acid) and 30% acetonitrile with a flow rate of 0.5 mL/min. The column used was a Zorbax Extend–C18 (Aligent Technologies, Santa Clara, CA) with the column compartment set at 30°C. Identifications of PA and internal standard, dexamethasone, were achieved by comparing the ion sets in multiple reaction monitoring for prednisolone and for dexamethasone in animal plasma with the standard solution, at a similar retention time.
Toxicokinetics Parameter Estimation and Hematology
The TK analysis of plasma concentration-time profiles were conducted using model-independent methods in WinNonlin (version 6.1; WinNonlin: Certara, Princeton, NJ). TK parameters, including mean area under the plasma concentration-time curve (AUC0-t), maximum plasma concentration (Cmax), and time to reach maximum concentration (Tmax). For the hematology analysis, approximately 0.5 mL of the collected blood was transferred into tubes containing ethylenediaminetetraacetic acid and assayed for blood cell morphology and hematology parameters, including hemoglobin, red blood cell count, hematocrit, mean cell volume, mean cell hemoglobin, mean cell hemoglobin concentration, reticulocyte count, platelet count, total white blood cell count, neutrophils, lymphocytes, monocytes, eosinophils, basophils, and large unstained cells. Another 1 mL collected blood was transferred into tubes containing trisodium citrate. The citrated blood samples were centrifuged, and the resultant plasma separated was analyzed for coagulation parameters, including prothrombin time and activated partial thromboplastin time. A further 2 mL blood was collected for blood biochemical analysis, including the analysis for sodium, potassium, chloride, phosphorus, calcium, alkaline phosphatase, alanine amino transferase, aspartate amino transferase, creatine phosphokinase, lactate dehydrogenase, blood urea nitrogen, glucose, total cholesterol, triglycerides, creatinine, protein, albumin, globulin, albumin/globulin ratio, and total bilirubin.
Ophthalmic Clinical Evaluation
All the eyes were examined before the implantation and at day 1, week 1, week 2, and week 12 after the implantation by an experienced ophthalmologist (YCL). All evaluations were performed with animals under sedation with ketamine. Portable slit-lamp biomicroscopy (BA 904; Haag-Streit, Harlow, UK) was used to evaluate the anterior segment of the eyes, and the modified Hackett-McDonald ocular scoring system was used to evaluate the conjunctival congestion (0–3), swelling (0–4), and discharge (0–3) around the implant insertion sites.11 Indirect ophthalmoscopy (12500; Welch Allyn, Singapore) examination after the application of 1% mydriacyl was performed to evaluate the posterior segment of the eyes. It was assumed that there was no drug–drug interaction potential between ketamine and PA in this case.
Histopathology
Ocular tissues from all animals were processed to prepared paraffin wax blocks. Sections were cut 4–6 μm thick, stained with hematoxylin and eosin (H&E), and evaluated for signs of inflammation and toxic reaction.
Statistical Analysis
All data were expressed as mean ± standard deviation. Statistical comparisons among different groups were performed using the Mann-Whitney U test. Statistical analyses were performed using STATA software (version 13; StataCorp, College Station, TX). P values less than 0.05 were considered statistically significant.
Results
Toxicokinetics
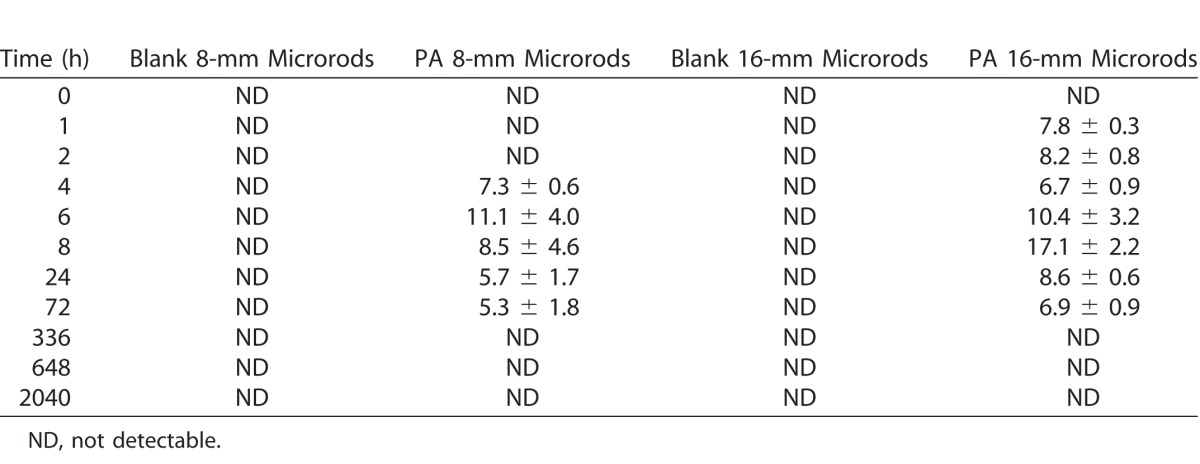
A summary of the plasma toxicokinetics results are presented in Table 1. The exposure (AUC0-t) was 45.7% higher and the maximum measured plasma concentration (Cmax) was 17.2% lower for the animals treated with 40-wt% PA 16-mm microrods than with the 8-mm microrods, respectively. There was no significant difference in time to Tmax for either the 8- or 16-mm microrods (P = 0.421). The plasma concentration-time profiles are illustrated in Figure 2, and it was observed that the Tmax for both PA-loaded groups fell between 6 and 8 hours, indicating similar plasma clearance rates for either a single or double dose of PA. Thereafter, PA plasma concentration maintained at a steady state between 24 and 72 hours, which was due to the sustained-release mechanism for PA from the microrods over an extended period of time. Full PA concentration data for all animals at all time points are presented in Table 2.
Table 1.
Summary of TK Data
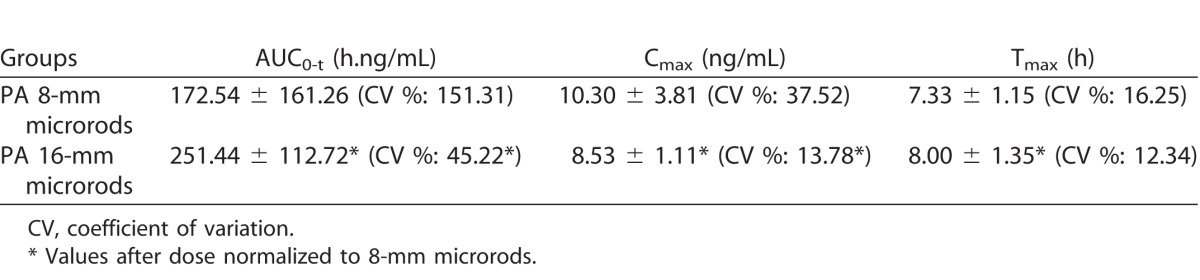
Figure 2.
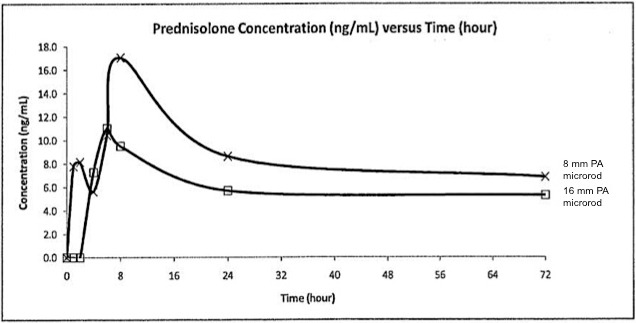
PA plasma concentration-time profiles.
Table 2.
Plasma PA Concentration (ng/mL) at Different Time Points for Different Groups
Hematology
Throughout the study period of 12 weeks, all hematology parameters, coagulation parameters, and blood biochemical parameters were within normal range, and there was no significant difference across the four treatment groups. Data for these results are presented in Supplementary Tables S1–3.
Ophthalmic Clinical Evaluation
Slit-lamp examination revealed a mild degree of conjunctival vessels congestion (score 0–1) and conjunctival swelling (score 0–1) around the margin of both the 40-wt% PA-loaded and blank microrods (8 and 16 mm) at day 1. The mean total Hackett-McDonald ocular scores (0–11) assessing conjunctival congestion, swelling, and discharge were very low throughout the study period of 12 weeks, indicating the blank and PA-loaded microrods elicited only minimal inflammation at the insertion sites. The mean scores at day 1, week 1, week 2, and week 12 was 0.50 ± 0.00, 0.00 ± 0.00, 0.17 ± 0.06, and 0.00 ± 0.00 for the 8-mm blank microrod group; 0.50 ± 0.00, 0.00 ± 0.00, 0.00 ± 0.00, and 0.00 ± 0.00 for the 16-mm blank microrod group; 0.50 ± 0.00, 0.17 ± 0.00, 0.17 ± 0.06, and 0.00 ± 0.00 for the 8-mm 40-wt% PA-loaded microrod group; and 0.50 ± 0.00, 0.00 ± 0.00, 0.33 ± 0.06, and 0.00 ± 0.00 for the 16-mm 40-wt% PA-loaded microrod group (P = 0.67, 0.75, 0.63, and 0.68 among four different groups at day 1, week 1, week 2, and week 12, respectively). All the implanted microrods were visible at 12 weeks, without evidence of protrusion or dislocation. There was no sign of infection, neovascularization, bleeding, or scarring at the site of implantation.
Indirect ophthalmoscopic examination revealed no signs of vitreal or retinal adverse effects, such as vitreous inflammation, retinal hemorrhage or exudate, or macular or optic disc edema, were observed in any of the experimental eyes throughout the study period.
Histopathology
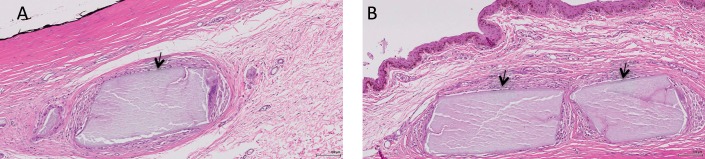
Histologic sections with H&E staining revealed very minimal inflammatory cells within the subconjunctival space. At 12 weeks, there was a mild degree of fibrotic capsule formation, mononuclear cell infiltration, and giant cell infiltration around the microrods in all groups, but the 40-wt% PA microrod groups (both 8- and 16-mm groups) exhibited less fibrosis and fewer inflammatory cell infiltrate as compared to the blank microrods (both 8- and 16-mm groups; Fig. 3). There was no significant foreign body reaction observed in all eyes.
Figure 3.
Representative histologic sections with H&E staining of a 16-mm blank (A) and a 16-mm PA-loaded (B) microrod at 12 weeks at the subconjunctival insertion site. There was a mild degree of fibrotic capsule formation, mononuclear cell infiltration, and giant cell infiltration around the microrods in all groups, but no excessive foreign body reaction was observed. Arrows indicate the degraded fragments of microrods. Original magnification: 200×; scale bar: 100 μm.
Discussion
In the present study, we showed the safe pharmacokinetics (PK) levels and biocompatibility of the PA microrods. Our previous in vitro and in vivo studies on the PLC implants homogeneously impregnated with PA demonstrated sustained drug-release profiles and therapeutic efficacy in different animal models of ocular inflammation and post surgery for up to 12 weeks.9,10,12 We reported that no significant inflammation or vascularization was observed over a period of 6 months by slit-lamp microscopy, histology, immunohistochemistry, and immunofluorescence.12 In addition, PA-PLC implants of various dimensions and PA content were extensively evaluated in rat inflammation and keratoplasty models,7,8 rabbit glaucoma filtration surgery, and anterior uveitis models.9,10 The studies showed that PA-PLC microrods exhibited good biocompatibility, sustained PA-release profiles, and maintained high PA levels in the anterior chamber for up to 12 weeks.7 An estimated PA release of 0.12 mg/day was achieved in the uveitis model, which is comparable to instillation of a commercial 1% Pred Forte eyedrop formulation every 2–3 hours.10
Results from ophthalmic clinical and histopathologic assessments revealed that the PA-loaded microrods were biocompatible and did not appear to cause more localized tissue reactions, such as conjunctival fibrosis or formation of granuloma, compared to the blank microrods. This was similar to the results in our previous rabbit and rat studies.7–10 Taken together with no changes related to treatment for hematology, these results further lend credence to the safety and efficacy aspects of the 40-wt% PA-loaded microrods.
Xu et al. developed a mathematical model for a pharmacokinetic/pharmacodynamic approach to predict total prednisolone concentrations in human plasma based on nine data sets for both intravenous and oral administration from different clinical studies.13 This model was to address the nonlinearity of total prednisolone plasma concentration, and it also showed a good modular fitting between theoretical and empirical data sets for both routes of administration. Peak measured Cmax for oral and intravenous routes were between 100 and 1000 ng/mL and above 1000 ng/mL, respectively, both occurring before 5 hours.13 Our Cmax values of 10.3 and 8.53 ng/mL (normalized) for both PA-loaded 8- and 16-mm microrods, respectively, were far below levels experienced by patients on other regimes, lending further support that the PA implants are safe for clinical use. Also, it was interesting to note that the Tmax was almost similar for both 8- and 16-mm microrods at 7.33 and 8 hours, respectively. This showed that the plasma clearance time difference between a single and double dose of PA administered via such a subconjunctival implant was minimal, and this provides a relatively wide dose range to more accurately target and refine the implant for the future.
To the best of our knowledge, there has been no previously reported plasma TK data that describes a similar drug-delivery system involving a fully biodegradable, steroid-eluting microrod that is subconjunctively implanted. Published reports for plasma Cmax values after 20 mg of oral and intravenous administration were 585 and 1773 ng/mL, respectively, and were 391 and 1055 ng/mL after a 10-mg dose.14 These sets of values agree well with those of Xu et al.13 Kumria et al.15 proposed sustained delivery of prednisolone via buccal films adhered to the cheek. Although not entirely similar to the proposed delivery route described here, increased bioavailability compared to oral suspensions was reported with a relatively high plasma Cmax value of 2700 ng/mL (versus 2290 mg/mL for oral suspensions as a control). All previously reported plasma Cmax values were much higher than our reported values here, thereby attesting to the increased safety of our implants. More recently, extensive results have been gathered for intravitreal implants like Ozurdex (Allergan, Inc.) and Iluvien (Alimera Sciences, Inc.), with dexamethasone concentration levels being reported locally in the vitreous and aqueous humour16 and no quantifiable plasma concentrations of fluocinolone acetonide in rabbits being reported.17
In conclusion, we have evaluated the PK and toxicokinetics of a biodegradable subconjunctival PA implant. The microrods were not associated with any adverse clinical or histologic findings. The AUC0-t was higher with the higher loading 16-mm microrods than with the 8-mm microrods, but there was no significant difference in time to Tmax between the two implant sizes. These reported results, together with efforts to develop this implant into an office-based drug-delivery system for treating anterior segment inflammatory conditions, provide added supporting evidence of the safety of implanting a steroid delivery system subconjunctively, offering an alternative to topical PA eyedrops.
Supplementary Material
Acknowledgments
This study was supported in part by the National Medical Research Council (NMRC) of Singapore under its Translational and Clinical Research Programme Grant (TCR/008-SERI/2013 EyeSITe) administered by the Ministry of Health.
Disclosure: Y.-C. Liu, None; A.H.C. Ng, None; X.W. Ng, None; P. Yan, None; S.S. Venkatraman, None; J.S. Mehta, None; T.T. Wong, None
References
- 1. Gao Y, Sun Y, Ren F, Gao S. . PLGA-PEG-PLGA hydrogel for ocular drug delivery of dexamethasone acetate. Drug Dev Ind Pharm. 2010; 36: 1131– 1138. [DOI] [PubMed] [Google Scholar]
- 2. Olthoff C, Schouten JvdB, Websers CA. Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension: an evidence-based review. Ophthalmology. 2005; 112: 953– 961. [DOI] [PubMed] [Google Scholar]
- 3. Ghate D, Edelhauser HF. . Ocular drug delivery. Expert Opin Drug Deliv. 2006; 3: 275– 287. [DOI] [PubMed] [Google Scholar]
- 4. Barar J, Javadzadeh AR, Omidi Y. . Ocular novel drug delivery: impacts of membranes and barriers. Expert Opin Drug Deliv. 2008; 5: 567– 581. [DOI] [PubMed] [Google Scholar]
- 5. Hyndiuk RA. . Radioactive depot-corticosteroid penetration into monkey ocular tissue. II. Subconjunctival administration. Arch Ophthalmol. 1969; 82: 259– 263. [DOI] [PubMed] [Google Scholar]
- 6. Awan MA, Agarwal PK, Watson DG, McGhee CN, Dutton GN. . Penetration of topical and subconjunctival corticosteroids into human aqueous humour and its therapeutic significance. Br J Ophthalmol. 2009; 93: 708– 713. [DOI] [PubMed] [Google Scholar]
- 7. Liu YC, Peng Y, Lwin NC, Wong TT, Venkatraman SS, Mehta JS. . Optimization of subconjunctival biodegradable microfilms for sustained drug delivery to the anterior segment in a small animal model. Invest Ophthalmol Vis Sci. 2013; 54: 2607– 2615. [DOI] [PubMed] [Google Scholar]
- 8. Liu YC, Peng Y, Lwin NC, Venkatraman SS, Wong TT, Mehta JS. . A biodegradable, sustained-released, prednisolone acetate-loaded microfilm drug delivery system effectively prolongs corneal allograft survival in the rat keratoplasty model. PLoS One. 2013; 8: e70419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ang M, Yan P, Zhen M, Foo S, Venkatraman SS, Wong TT. . Evaluation of sustained release of PLC-loaded prednisolone acetate microfilm on postoperative inflammation in an experimental model of glaucoma filtration surgery. Curr Eye Res. 2011; 36: 1123– 1128. [DOI] [PubMed] [Google Scholar]
- 10. Ang M, Ng X, Wong C,et al. . Evaluation of a prednisolone acetate-loaded subconjunctival implant for the treatment of recurrent uveitis in a rabbit model. PLoS One. 2014; 9: e97555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Munger RJ. . Veterinary ophthalmology in laboratory animal studies. Vet Ophthalmol. 2002; 5: 167– 175. [DOI] [PubMed] [Google Scholar]
- 12. Peng Y, Ang M, Foo S,et al. . Biocompatibility and biodegradation studies of subconjunctival implants in rabbit eyes. PLoS One. 2011; 6: e22507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xu J, Winkler J, Derendorf H. . A pharmacokinetic/pharmacodynamic approach to predict total prednisolone concentrations in human plasma. J Pharmacokinet Pharmacodyn. 2007; 34: 355– 372. [DOI] [PubMed] [Google Scholar]
- 14. Bergrem H, Grottum P, Rugstad HE. . Pharmacokinetics and protein binding of prednisolone after oral and intravenous administration. Eur J Clin Pharmacol. 1983; 24: 415– 419. [DOI] [PubMed] [Google Scholar]
- 15. Kumria R, Nair AB, Goomber G, Gupta S. . Buccal films of prednisolone with enhanced bioavailability. Drug Deliv. 2016; 23: 471– 478. [DOI] [PubMed] [Google Scholar]
- 16. Bhagat R, Zhang J, Farooq S, Li XY. . Comparison of the release profile and pharmacokinetics of intact and fragmented dexamethasone intravitreal implants in rabbit eyes. J Ocul Pharmacol Ther. 2014; 30: 854– 858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kane FE, Green KE. . Ocular pharmacokinetics of fluocinolone acetonide following iluvien implantation in the vitreous humor of rabbits. J Ocul Pharmacol Ther. 2015; 31: 11– 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.