Abstract
Patient: Male, 58
Final Diagnosis: Bacterial meningitis
Symptoms: Altered mental status • headache • neck stiffness • vomiting
Medication: —
Clinical Procedure: —
Specialty: Infectious Diseases
Objective:
Rare disease
Background:
Gradenigo’s syndrome includes the triad of suppurative otitis media, ipsilateral sixth (abducens) cranial nerve palsy and facial pain in the distribution of the fifth (trigeminal) cranial nerve. Gradenigo’s syndrome is rare, and the diagnosis is easily overlooked. This case is the first to report Gradenigo’s syndrome presenting with meningitis on a background of chronic suppurative otitis media (CSOM) and petrous apicitis (apical petrositis).
Case Report:
A 58-year-old male African American presented with headaches and confusion. Magnetic resonance imaging (MRI) of the head showed petrous apicitis with mastoiditis and abscess formation in the cerebellomedullary cistern (cisterna magna). The case was complicated by the development of palsy of the fourth (trochlear) cranial nerve, fifth (trigeminal) cranial nerve, and sixth (abducens) cranial nerve, with radiological changes indicating infection involving the seventh (facial) cranial nerve, and eighth (vestibulocochlear) cranial nerve. Cerebrospinal fluid (CSF) culture results were positive for Klebsiella pneumoniae, sensitive to ceftriaxone. The patient improved with surgery that included a left mastoidectomy and debridement of the petrous apex, followed by a ten-week course of antibiotics. Follow-up MRI showed resolution of the infection.
Conclusions:
This report is of an atypical case of Gradenigo’s syndrome. It is important to recognize that the classical triad of Gradenigo’s syndrome, suppurative otitis media, ipsilateral sixth (abducens) cranial nerve palsy and facial pain in the distribution of the fifth (trigeminal) cranial nerve, may also involve chronic suppurative otitis media (CSOM), which may lead to involvement of other cranial nerves, petrous apicitis (apical petrositis), and bacterial meningitis.
MeSH Keywords: Klebsiella; Meningitis, Bacterial; Otitis Media; Petrositis
Background
Gradenigo’s syndrome was first described in 1904 by Guiseppe Gradenigo [1]. This syndrome involves a triad of symptoms consisting of diplopia due to palsy of the sixth (abducens) cranial nerve, unilateral periorbital pain due to involvement of the fifth (trigeminal) cranial nerve, and persistent otorrhea due to bacterial otitis media with involvement of the apex of the petrous part of the temporal bone, petrous apicitis (apical petrositis). [1,2].
Gradenigo’s syndrome has become very rare since the availability of antibiotics to treat bacterial otitis media, but cases have been recently documented with chronic suppurative otitis media (CSOM) and cholesteatoma [3,4]. Inflammation of the petrous part of temporal bone, petrous apicitis (apical petrositis), is usually present [5,6].
This case report describes a case of bacterial meningitis in Gradenigo’s syndrome associated with CSOM and petrous apicitis (apical petrositis).
Case Report
We report a case of a male 58-year old African American who presented to the emergency room with a three-day history of confusion, headaches, neck stiffness, and vomiting. The patient had a one-year history of chronic suppurative otitis media (CSOM) and was taking daily amoxicillin with occasional improvement in symptoms. Two months prior to this hospital admission, he had a myringotomy to relieve pressure on the eardrum.
On this hospital admission, the patient’s blood pressure was 138/93, pulse rate 91 per minute, and he was febrile with a temperature of 102°F (38.9°C). He was alert but not oriented to time, place, or person. An urgent computed tomography (CT) image of the head was performed with limited views from the foramen magnum to the vertex, and was negative for any significant pathology. Lumbar puncture showed cloudy, pale yellow cerebrospinal fluid (CSF) with a red blood cell (RBC) count of 3,100/mm3 and white blood cell count (WBC) of 8,880/mm3 (89% polymorphonuclear leucocytes). CSF protein was 711 mg/dl, and CSF glucose was 3 mg/dl.
Although an initial Gram stain of the CSF showed no organisms, culture was positive for Klebsiella pneumoniae, which was sensitive to ceftriaxone.
In the emergency department, an initial diagnosis of meningitis was made and an intravenous normal saline infusion was begun with empiric intravenous vancomycin, ceftriaxone, and ampicillin was administered. On the second day following admission, the patient began to complain of vertical diplopia, described as an upper true image, and a false lower image. The diplopia resolved by closing one eye.
On examination on the second day of hospital admission, his pupils were equal and reactive to light. The left eye was deviated upward and medially with limited abduction and depression of eye movements. Left-beating nystagmus was noticed on left lateral gaze and there was also impairment of auditory acuity on the left. Left superior quadrantanopia was also present. His left ear showed an effusion. The remainder of the neurological examination was unremarkable. Palsy of the fourth (trochlear) cranial nerve, and the sixth (abducens) cranial nerve were diagnosed.
Magnetic resonance imaging (MRI) showed a left mastoiditis and a left petrous apicitis (apical petrositis) with abscess formation in the left petrous apex (Figure 1A, 1B). The infection extended into the left posterior fossa with an abscess formation in the left cerebellomedullary cistern (cisterna magna), the petrosal aspect of the left cerebellar hemisphere, and left foramen of Luschka (lateral aperture of the fourth ventricle) (Figures 2 and 3).
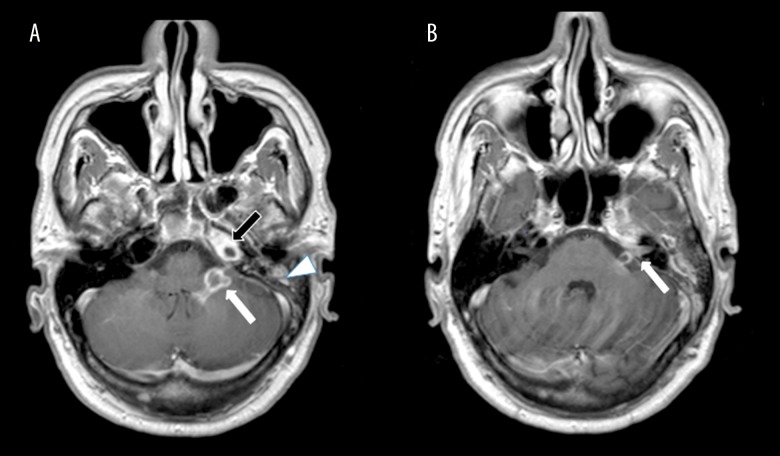
Figure 1.
Magnetic resonance imaging (MRI): Axial T1-weighted images of the left cerebellomedullary cistern (cisterna magna), left petrous apex and mastoid air cells. (A, B) The image shows a rim-enhancing lesion extending into the left cerebellomedullary cistern (cisterna magna) (white arrow, A). There is also abnormal enhancement involving the left seventh and eighth cranial nerves extending into the internal auditory canal (white arrow, B) and enhancement in the left petrous apex and mastoid air cells, consistent with petrous apicitis (apical petrositis) (black arrow, A) and mastoiditis (white arrowhead, A).
Figure 2.
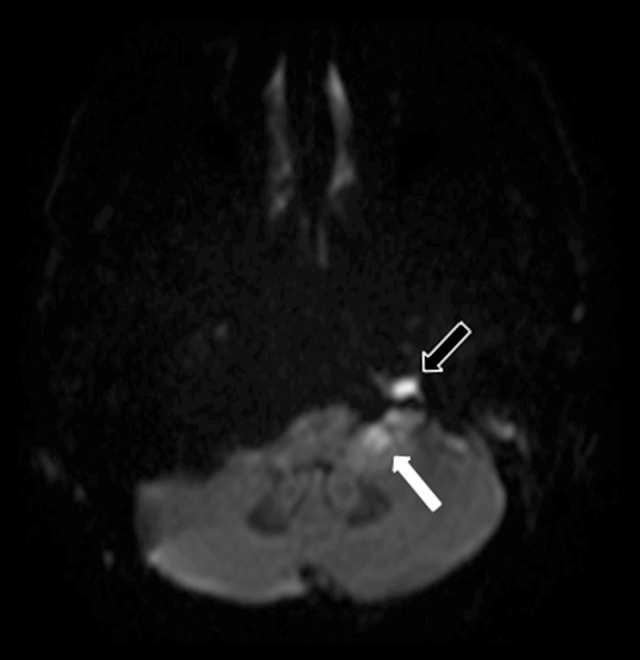
Magnetic resonance imaging (MRI): Axial diffusion-weighted imaging (DWI) of the left cerebellomedullary cistern (cisterna magna) and left petrous apex. The image shows restricted diffusion in the left cerebellomedullary cistern (cisterna magna), consistent with an abscess (white arrow). Restricted diffusion is also seen in the left petrous apex due to purulent material (black outlined arrow).
Figure 3.
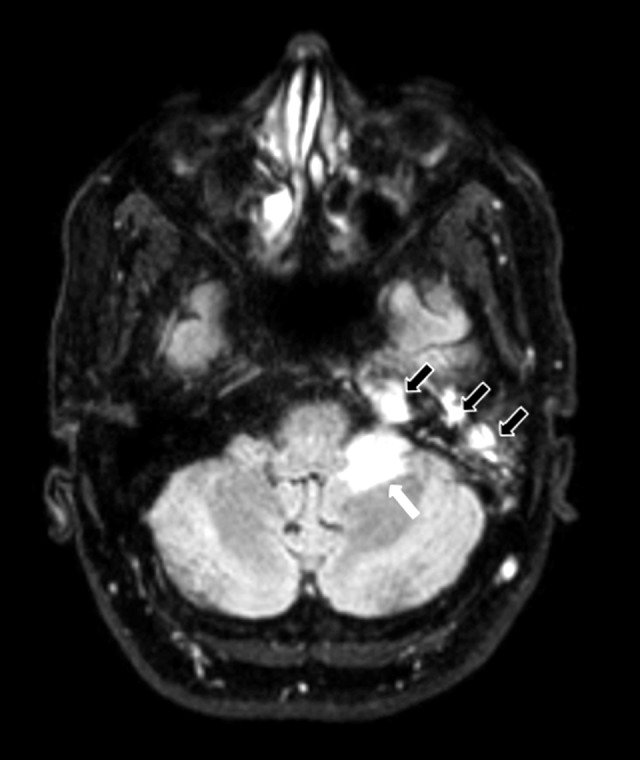
Magnetic resonance imaging (MRI): Axial fluid-attenuated inversion recovery (FLAIR) image of the left cerebral hemisphere, petrous apex, and mastoid air cells. The image shows edema in the left cerebellar hemisphere (white arrow) and fluid in the left petrous apex, and mastoid air cells (black outlined arrows).
The patient was diagnosed with Gradenigo’s syndrome based on his clinical presentation, the involvement of the sixth (abducens) cranial nerve, and petrous apicitis (apical petrositis) due to extension of otitis media with effusion (OME) into the temporal bone. Although the patient did not report symptoms of facial pain, he did have numbness and decreased sensation to light touch and pinprick in the distribution of the left fifth (trigeminal) cranial nerve.
The patient’s antibiotic regimen was adjusted to include metronidazole 500 mg orally, and on the fifth day of hospital admission, the patient underwent a left mastoidectomy with petrous apex debridement.
During the remainder of the hospital stay, the sixth (abducens) cranial nerve palsy resolved by the tenth day, and he was discharged. His WBC levels decreased and microbial culture from the petrous apex culture results showed no growth after 48 hrs. There was also a negative tests for HIV infection and a negative IgG antibody screen for Strongyloides.
A prolonged eight-week course of antibiotics was recommended to cover treatment of the left petrous apicitis (apical petrositis), left cerebellomedullary abscess, and Klebsiella pneumoniae meningitis. This initial treatment was followed by a two-week course of ciprofloxacin and metronidazole, for a total of ten weeks. On outpatient follow-up, at three months following hospital discharge, the patient’s sixth (abducens) cranial nerve palsy was fully resolved, but palsies of the fourth (troch-lear) cranial nerve and fifth (trigeminal) cranial nerve remained unchanged. The patient continued to have a vertical deviation of the left eye, left-beating nystagmus on left lateral gaze, and diminished sensation to light touch and pinprick in the distribution of the fifth (trigeminal) cranial nerve. Follow-up brain MRI showed resolution of the left cerebellomedullary abscess with persistent residual inflammatory changes in the left mastoid air cells and temporal bone (Figure 4).
Figure 4.
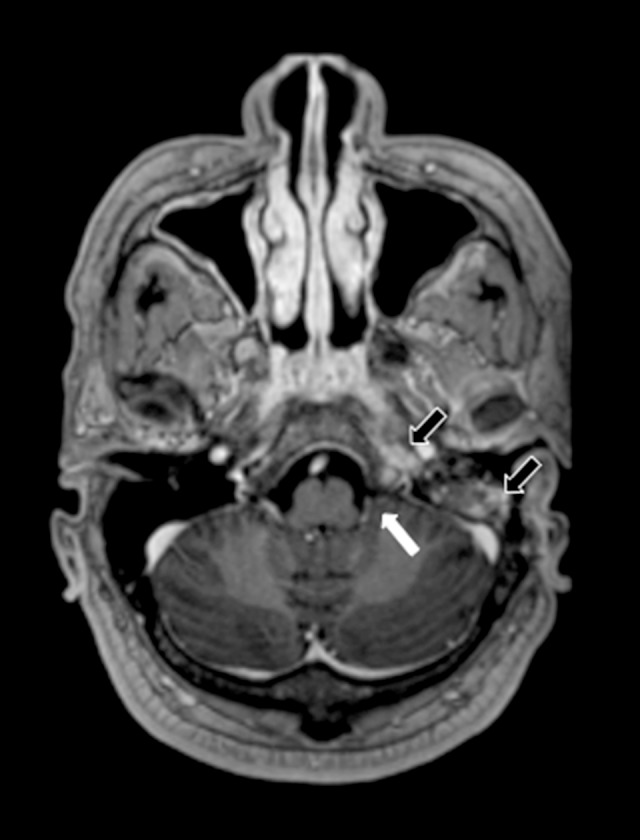
Magnetic resonance imaging (MRI): Axial postcontrast T1-weighted images of the left cerebellomedullary cistern (cisterna magna) and cranial nerve enhancement. The image shows resolution of the abscess of the left cerebellomedullary cistern (cisterna magna) and cranial nerve enhancement following treatment (white arrow). Enhancement of the left petrous apex and mastoid air cells persists but is improved (black arrows).
Discussion
Klebsiella pneumoniae is a common pathogen identified in cases of chronic suppurative otitis media (CSOM) [7]. However, the organisms associated with infection in Gradenigo’s syndrome have not been well studied, but there have been reported in cases of Group A Streptococcus, Pneumococcus, Staphylococcus, Pseudomonas aeruginosa, and Mycobacterium tuberculosis [6].
Although acute otitis media (AOM) is commonly part of Gradenigo’s syndrome, CSOM and cholesteatomas have also been reported [4,8]. Review of previously published case reports of Gradenigo’s syndrome have shown that between one week to three months is the usual time course to develop sixth (abducens) cranial nerve palsy from the onset of AOM [4,8]. However, when CSOM is present in Gradenigo’s syndrome, sixth (abducens) cranial nerve palsy may develop up to three years later [4]. This case report is atypical in that acute meningitis developed as a complication after a one-year history of CSOM in a case of Gradenigo’s syndrome.
Review of the recent published literature has shown that meningitis is the second most common intracranial complication of CSOM, after brain abscess, but no literature currently reports the subsequent development of Gradenigo’s syndrome in these patients [9]. One case report showed the development of Gradenigo’s syndrome in a patient with AOM who presented with acute meningitis, a very similar presentation to our reported case [10]. In this report, the facial pain of trigeminal neuralgia seen in the classical triad of Gradenigo’s syndrome was masked by the symptoms of meningitis, including headache, similar to our case [10]. It may be helpful to note that the classical triad of Gradenigo’s syndrome may not always be present, and symptoms should be taken in the context of all the patient’s presenting symptoms, signs, and investigations. In Gradenigo’s original case series of 57 patients, more than half of the cases did not follow the classical triad [1]. In a case reported in 2004, involvement of the seventh (facial) cranial nerve, and eighth (vestibulocochlear) cranial nerve were reported, which was also found on brain MRI of the patient in our case report (Figure 1B) [11].
The management of Gradenigo’s syndrome typically requires early recognition and timely medical and/or surgical therapy. Intravenous antibiotic therapy is given from between ten and 64 days, as reported in previously published case reports [4]. Duration of therapy is extended, as in this case, to include treatment of osteomyelitis, if the petrous bone becomes involved [4]. Surgical therapy is necessary in very severe cases where the development of abscesses and osteomyelitis warrant surgical debridement [4].
Gradenigo’s syndrome is a very rare condition in current medical practice. Most of the current literature on Gradenigo’s syndrome is from case reports and case series. Timely treatment with antibiotics have led to the containment of otitis infections and prevented them from spreading beyond the middle ear cavity. This syndrome should be suspected with there is the presence of sixth (abducens) cranial nerve palsy in the setting of otitis media, whether chronic or acute [4]. The typical triad of Gradenigo’s syndrome is not always seen, and a low threshold of suspicion should be present to avoid any further complications of chronic suppurative otitis media (CSOM) [5,6,9,10]. This reported case was complicated by meningitis due to CSOM, altering the clinical picture, and resulting in an atypical presentation of Gradenigo’s syndrome.
Conclusions
This report is of an atypical case of Gradenigo’s syndrome. It is important to recognize that the classical triad of Gradenigo’s syndrome, suppurative otitis media, ipsilateral sixth (abducens) cranial nerve palsy and facial pain in the distribution of the fifth (trigeminal) cranial nerve, may also involve chronic suppurative otitis media (CSOM), which may lead to involvement of other cranial nerves, petrous apicitis (apical petrositis), and bacterial meningitis.
Acknowledgments
The authors would like to acknowledge the collaboration from specialist physicians involved in the multi-disciplinary care of this case, from initial presentation to discharge, outpatient follow-up, and rehabilitation. The authors would also like to thank the patient for his co-operation in preparing this case report.
Abbreviations:
- GS
– Gradenigo’s Syndrome;
- CSOM
– chronic suppurative otitis media;
- AOM
– acute otitis media
References:
- 1.Matis GK, de A Silva DO, Chrysou OI, et al. Giuseppe Gradenigo: Much more than a syndrome! Historical vignette. Surg Neurol Int. 2012;3:122. doi: 10.4103/2152-7806.102343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gradenigo G. [Uber die Paralyse des Nervus abducens bei Otitis] Arch Ohrenheilkunde. 1907;774:149–87. [in German] [Google Scholar]
- 3.Zengel P, Wiekström M, Jäger L, Matthias C. Isolated apical petrositis: An atypical case of Gradenigo’s syndrome. HNO. 2007;55:206. doi: 10.1007/s00106-006-1399-2. [DOI] [PubMed] [Google Scholar]
- 4.Jensen PV, Hansen MS, Møller MN, Saunte JP. The forgotten syndrome? Four cases of Gradenigo’s syndrome and a review of the literature. Strabismus. 2016;24(1):21–27. doi: 10.3109/09273972.2015.1130067. [DOI] [PubMed] [Google Scholar]
- 5.Tornabene S, Vilke GM. Gradenigo’s syndrome. J Emerg Med. 2010;38:449–51. doi: 10.1016/j.jemermed.2007.08.074. [DOI] [PubMed] [Google Scholar]
- 6.Lutter SA, Kerschner JE, Chusid MJ. Gradenigo syndrome: A rare but serious complication of otitis media. Pediatr Emerg Care. 2005;21:384–86. doi: 10.1097/01.pec.0000166731.70847.d5. [DOI] [PubMed] [Google Scholar]
- 7.Mittal R, Lisi CV, Gerring R, et al. Current concepts in the pathogenesis and treatment of chronic suppurative otitis media. J Med Microbiol. 2015;64(10):1103–16. doi: 10.1099/jmm.0.000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chole RA, Donald PJ. Petrous apicitis – clinical considerations. Ann Otol Rhinol Laryngol. 1983;92:544–51. doi: 10.1177/000348948309200603. [DOI] [PubMed] [Google Scholar]
- 9.Sharma N, Jaiswal AA, Banerjee PK, Garg AK. Complications of chronic suppurative otitis media and their management: A single institution 12 years experience. Indian J Otolaryngol Head Neck Surg. 2015;67(4):353–60. doi: 10.1007/s12070-015-0836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valles JM, Fekete R. Gradenigo syndrome: Unusual consequence of otitis media. Case Rep Neurol. 2014;6(2):197–201. doi: 10.1159/000365843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sherman SC, Buchanan A. Gradenigo syndrome: A case report and review of a rare complication of otitis media. J Emerg Med. 2004;27:253–56. doi: 10.1016/j.jemermed.2004.03.014. [DOI] [PubMed] [Google Scholar]