Abstract
Objective
Short-term efficacy of induction therapy with intravenous immunoglobulin (Ig) in patients with chronic inflammatory demyelinating polyneuropathy (CIDP) is well established. However, data of previous studies on maintenance therapy were limited up to 24-week treatment period. We aimed to investigate the efficacy and safety of longer-term intravenous Ig therapy for 52 weeks.
Methods
This study was an open-label phase 3 clinical trial conducted in 49 Japanese tertiary centres. 49 patients with CIDP who fulfilled diagnostic criteria were included. After an induction intravenous Ig therapy (0.4 g/kg/day for five consecutive days), maintenance dose intravenous Ig (1.0 g/kg) was given every 3 weeks for up to 52 weeks. The primary outcome measures were the responder rate at week 28 and relapse rate at week 52. The response and relapse were defined with the adjusted Inflammatory Neuropathy Cause and Treatment scale.
Results
At week 28, the responder rate was 77.6% (38/49 patients; 95% CI 63% to 88%), and the 38 responders continued the maintenance therapy. At week 52, 4 of the 38 (10.5%) had a relapse (95% CI 3% to 25%). During 52 weeks, 34 (69.4%) of the 49 enrolled patients had a maintained improvement. Adverse events were reported in 94% of the patients; two patients (66-year-old and 76-year-old men with hypertension or diabetes) developed cerebral infarction (lacunar infarct with good recovery), and the other adverse effects were mild and resolved by the end of the study period.
Conclusions
Maintenance treatment with 1.0 g/kg intravenous Ig every 3 weeks is an efficacious therapy for patients with CIDP, and approximately 70% of them had a sustained remission for 52 weeks. Thrombotic complications should be carefully monitored, particularly in elderly patients with vascular risk factors.
Trial registration number
ClinicalTrials.gov (NCT01824251).
Introduction
Chronic inflammatory demyelinating polyneuropathy (CIDP) is the most common treatable chronic immune-mediated neuropathy.1 2 Randomised controlled trials have shown that intravenous immunoglobulin (Ig), as well as plasma exchange and corticosteroids, is beneficial in the treatment of CIDP.3–7 Currently, intravenous Ig is often considered a first-line treatment for CIDP, largely because its onset of clinical improvement is generally more rapid than that of corticosteroids, and adverse effects of long-term use of corticosteroids are well recognised and can be serious. A recent randomised study has revealed that treatment with monthly intravenous Ig for 6 months was less frequently discontinued because of inefficacy, adverse events or intolerance than was treatment with monthly intravenous methylprednisolone.8 Therefore, intravenous Ig presumably has a better short-term efficacy than corticosteroids.
Thus, the efficacy of induction therapy with intravenous Ig, usually 2.0 g/kg, has been well established, but patients with CIDP who are given a single course of induction intravenous Ig often have a treatment-dependent relapse and require repeated infusion. The longer-term effects of intravenous Ig treatment on the long course of CIDP need to be studied.
A randomised, placebo-controlled study in 117 patients with CIDP treated with intravenous Ig was published in 2008, termed as the ‘ICE’ study (Intravenous Ig in CIDP Efficacy).6 The study consisted of the two periods; after randomisation, enrolled patients were initially given induction intravenous Ig (2.0 g/kg) followed by maintenance intravenous Ig (1.0 g/kg, every 3 weeks) or placebo for the first 24 weeks (first period), and then, only intravenous Ig responders were rerandomised to the maintenance intravenous Ig or placebo group (extension period). The results showed both short-term and long-term improvements induced by intravenous Ig.6 However, according to the study design, the period of maintenance intravenous Ig was variable.
Subsequently, the PRIMA study (Privigen Impact on Mobility and Autonomy) confirmed the findings of the intravenous Ig treatment arm (maintenance intravenous Ig (1.0 g/kg every 3 weeks)) of the ICE study in an open-label study; of the 28 patients enrolled, the overall responder rate was 60.7% at week 24.9 So far, there is no study to evaluate the effects of repeated intravenous Ig for more than 24 weeks. In the present study, we therefore expanded the study period up to 52 weeks and investigated the longer-term efficacy and safety of maintenance intravenous Ig treatment.
Methods
Study design and patients
This study was a multicentre, single-arm, open trial conducted at 49 Japanese tertiary hospitals. The study procedures were in accordance with the Declaration of Helsinki and Japan’s Good Clinical Practice criteria, and approved by the internal review board of each hospital. The design and outcome measures in this trial were based on those of the ICE study to obtain comparable data with those in the placebo arm of the ICE study.6 This study is registered with ClinicalTrials.gov, number NCT01824251.
A total of 49 patients with definite or probable CIDP according to the European Federation of Neurological Societies/Peripheral Nerve Society (EFNS/PNS) clinical diagnostic criteria were enrolled.10 Forty-three of them had typical CIDP, and the remaining six were classified into atypical CIDP by the EFNS/PNS clinical classification. The inclusion criteria were defined as having (1) a progressive or recurrent course, (2) an Inflammatory Neuropathy Cause and Treatment (INCAT) disability score11 of 2 to 9, (3) no additional immunotherapies or, if already treated, not increasing dose of agents for CIDP from 30 days prior to consent and (4) age 20 years or older. The exclusion criteria included suffering (1) a prolonged neurological deficit due to stroke or other central nervous system disorders or other causes of neuropathy; (2) malignancy, other autoimmune disease or POEMS (Polyneuropathy, Organomegaly, Endocrinopathy, Monoclonal gammopathy and Skin changes) syndrome; (3) multifocal motor neuropathy or neuropathy with antimyelin oligodendrocyte associated-glycoprotein antibody; (4) plasma exchange within 3 months prior to consent; (5) rituximab treatment within 6 months prior to consent; (6) high-dose intravenous Ig treatment (1.0 g/kg or greater) within 8 weeks prior to consent and (7) receiving intravenous Ig treatment (any dose) within 3 weeks prior to receiving consent.
Procedures
The study design and trial profile are shown in figure 1. After screening, eligible patients were enrolled in the study. Glovenin-I (freeze-dried polyethylene glycol-treated human Ig; Nihon Pharmaceutical, Tokyo, Japan) was used for intravenous Ig therapy. As the initial induction treatment, intravenous Ig (0.4 g/kg/day for five consecutive days) was administered, followed by maintenance treatment every 3 weeks from week 4 (1.0 g/kg/day for 1 day or 0.5 g/kg/day for two consecutive days). According to the ICE study,6 the primary endpoint measure was done at week 28, and only the responders defined with the INCAT score improvement entered to the further maintenance treatment until week 52.
Figure 1.
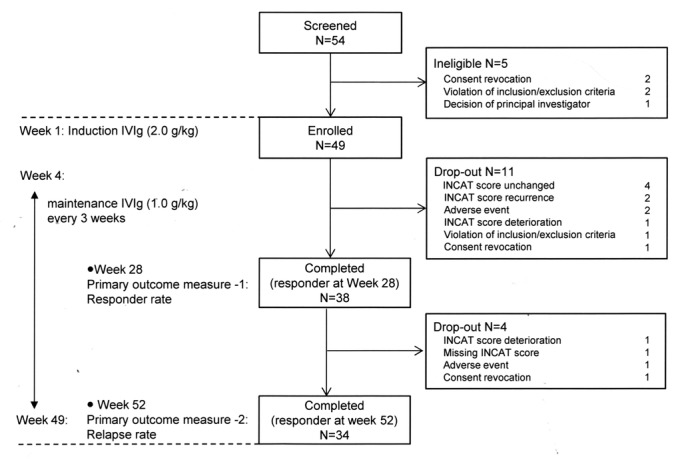
Study design and trial profile. INCAT, Inflammatory Neuropathy Cause and Treatment.
Patients were considered non-responders if the INCAT score remained the same until week 7 or deteriorated by 1 point or more compared with that at week 1 (before administration). Patients who completed the first 28-week treatment were responders and continued the maintenance treatment every 3 weeks from weeks 28 to 49 with observation until week 52. If the INCAT score deteriorated by 1 point or more compared with the score at week 28, the patient was regarded as having a relapse. Discontinuation of intravenous Ig due to reasons other than INCAT score changes was included in relapse according to the predefined statistical analysis plan.
For neurological assessment, the INCAT score, INCAT sensory sum (ISS) score, hand-grip strength and total manual muscle testing score with the Medical Research Council (MRC) score were assessed at each visit. Nerve conduction studies were performed at weeks 1, 4, 28 and 52. Laboratory assessment and urine analysis were conducted at the screening period and at weeks 1, 4, 7, 10, 16, 22, 28, 34, 40, 46 and 52.
Outcome measures
The goal of this trial was the approval by the Pharmaceuticals and Medical Devices Agency (PMDA), Japan. PMDA advised to take comparable data with those of the ICE study,6 and therefore the outcome measures were done at week 28 (the responder rate) and week 52 (the relapse rate), according to the ICE study; the primary outcome measures were (1) the proportion of patients who had sustained improvement of 1 point or more on their adjusted INCAT score at week 28 (the responder rate) and (2) the proportion of patients who had deterioration of the adjusted INCAT score by 1 point or more at week 52 (the relapse rate).
In the present study, the success was predefined as (1) the lower limit of the 95% CI of the responder rate at week 24 in the placebo arm of the ICE study (ie, >20.7%) and (2) the upper limit of the 95% CI of the relapse rate at week 52 of the placebo arm of the ICE study (ie, <42.3%).
The secondary outcome measures were also the same as those in the ICE study: the INCAT score, ISS score, hand-grip strength, total MRC score, compound muscle action potential (CMAP), serum IgG level, the number of days taken to improve the INCAT score and the number of days until recurrence. Safety assessments included any adverse event during the study period.
Statistical analyses
Analyses were performed on the full analysis set, which included all patients who received the drug at least once. Missing data of the INCAT score, ISS score, hand-grip strength and total MRC score were input by last period data (the last observation carried forward method). The coefficients are statistically different from 5% level. The responder rate at week 28 and relapse rate at week 52 with the CI were calculated for the primary outcome measures.
According to the previous studies (ICE6 and PRIMA9), changes in score from both 0 to 1 and 1 to 0 in upper limb INCAT score were not regarded as deterioration or improvement (the adjusted INCAT score). CIs were calculated using the Clopper-Pearson exact method. Survival analysis was performed to apply the number of days taken for the INCAT score to improve by 1 point or more, and on the number of days until relapse; a Kaplan-Meier curve was created.
The target patient number of 49 was calculated based on the predefined criteria described above. All analyses were performed with the statistical software package SAS V.9.2.
Results
Patient disposition
The study lasted from May 2013 and ended in August 2015. A total of 54 patients were screened. Five patients were ineligible, and the remaining 49 were enrolled (figure 1). During the first 28 weeks, 11 patients were discontinued from the study because of no improvement in the adjusted INCAT score, adverse events or consent violation. Also, the remaining 38 patients received continuous maintenance intravenous Ig. Of these, 34 patients completed the study.
Table 1 shows patients’ baseline characteristics. All patients had definite CIDP according to the diagnostic criteria.10 The mean age was 55 years (ranged from 22 to 84 years) and the mean disease duration was 72 months (range, 4–387 months). Ninety-eight per cent of the patients had received intravenous Ig treatment before the study. Forty-three (88%) of the 49 patients had typical CIDP on the EFNS/PNS clinical criteria, and the remaining six had atypical CIDP. The mean baseline serum IgG level was 1210 mg/dL (normal range, 900–1800 mg/dL).
Table 1.
Demographics and baseline disease characteristics
| Category | All patients (n=49) | ||
| Gender (%) | Man | 26 (53.1) | |
| Age (years) | <65 (%) | 27 (55.1) | |
| ≥65 (%) | 22 (44.9) | ||
| Mean (SD) | 55.4 (17.3) | ||
| Range | 22–84 | ||
| Duration of CIDP (months) | Mean (SD) | 72.3 (86.0) | |
| Range | 4–387 | ||
| No of relapses over the 3 years prior to receiving consent | Mean (SD) | 7.0 (7.5) | |
| Range | 0–36 | ||
| CIDP treatment history (%) | Corticosteroid | 28 (57.1) | |
| Intravenous Ig | 48 (98.0) | ||
| Plasma exchange | 3 (6.1) | ||
| INCAT score | Upper limb | Mean (SD) | 2.3 (1.0) |
| Range | 0–5 | ||
| Lower limb | Mean (SD) | 1.8 (1.2) | |
| Range | 0–5 | ||
| Total | Mean (SD) | 4.1 (1.4) | |
| Range | 3–9 | ||
| ISS score on week 1 (before administration) | Pinprick in upper limb | Mean (SD) | 1.3 (1.2) |
| Range | 0–4 | ||
| Pinprick in lower limb | Mean (SD) | 1.2 (1.2) | |
| Range | 0–4 | ||
| Vibratory in upper limb | Mean (SD) | 1.6 (1.5) | |
| Range | 0–4 | ||
| Vibratory in lower limb | Mean (SD) | 2.1 (1.4) | |
| Range | 0–4 | ||
| Two-point discrimination | Mean (SD) | 1.2 (1.1) | |
| Range | 0–4 | ||
| Total | Mean (SD) | 7.4 (4.2) | |
| Range | 0–16 | ||
| Hand-grip strength (kPa) | Dominant | Mean (SD) | 37.1 (25.0) |
| Non-dominant | Mean (SD) | 36.6 (22.5) | |
| MRC score | Upper limb | Mean (SD) | 26.0 (4.8) |
| Range | 7–30 | ||
| Lower limb | Mean value (SD) | 24.7 (4.8) | |
| Range | 6–30 | ||
| Total | Mean value (SD) | 50.7 (7.3) | |
| Range | 28–60 | ||
| Serum IgG concentration (mg/dL) | Mean value (SD) | 1210.29 (357.15) | |
| Range | 537.0–2255.0 | ||
CIDP, chronic inflammatory demyelinating polyneuropathy; INCAT, Inflammatory Neuropathy Cause and Treatment; ISS, INCAT sensory sum; MRC, Medical Research Council.
Efficacy
For the primary outcome measures, the responder rate at week 28 and relapse rate at week 52 are shown in figure 2. At week 28, 77.6% of the patients (95% CI 63.4% to 88.2%) experienced a sustained INCAT score improvement of 1 point or more compared with their score at week 1 (responder). The responder rate and 95% CI were higher than the responder rate in the placebo group of ICE study (20.7%).6 From week 29 to week 52, 10.5% of the patients (95% CI 2.9% to 24.8%) had a relapse (INCAT score deterioration by 1 point or more compared with that at week 28). The relapse rate and 95% CI were lower than the relapse rate in the placebo group of ICE study (42.3%).
Figure 2.
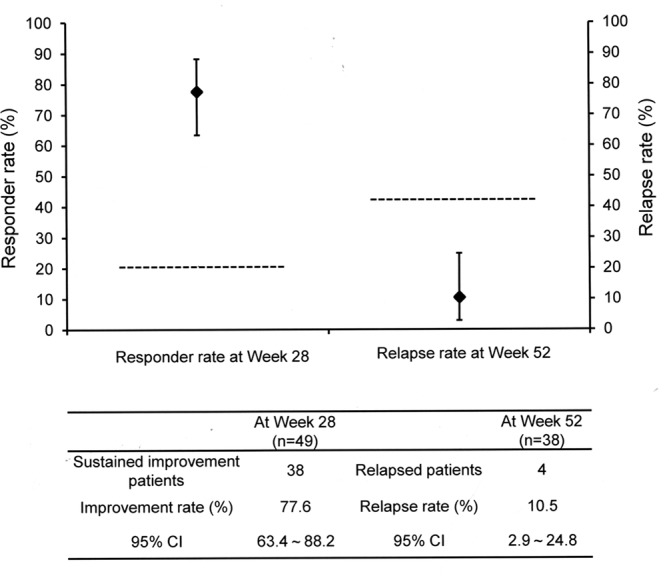
Responder rate and relapse rate. The responder rate is defined as the percentage of patients who had sustained INCAT score improvement of 1 point or more compared with that at week 1 (before administration) at week 28. The relapse rate indicates the percentage of patients whose INCAT score fell by 1 point or more from that of week 28 (before administration) at week 52. Horizontal lines indicate the responder rate (20.7%) for placebo group in the ICE study first period and relapse rate (42.3%) for placebo group in ICE study extension phase.6 9 ICE, Intravenous Ig in CIDP Efficacy; INCAT, Inflammatory Neuropathy Cause and Treatment.
Results of the secondary outcome measures are shown in table 2. The mean value of all the parameters improved from the baseline to week 28, and maintained from weeks 28 to 52, including the INCAT score, ISS score, hand-grip strength and total MRC score. The sequential changes in the total INCAT score and MRC sum score are shown in figure 3. For nerve conduction study data, CMAP amplitude in the most severely affected nerve was increased from the baseline to week 28, and maintained from weeks 28 to 52. Serum IgG levels (trough values) were higher at week 4 than at the baseline, and maintained at approximately 2000 mg/dL up to week 52. The median number of days taken for the INCAT score to improve by 1 point was 45.0 days, and the improvement rate was 97.4% (see online supplementary file 1).
Table 2.
Efficacy of intravenous Ig in patients with CIDP (the secondary measures)
| Week 1–28 (n=49) |
Week 29–52 (n=38)* |
||||
| Week 1 | Week 4 | Week 28 | Week 28* | Week 52 | |
| INCAT score | 4.1 (1.4) | 3.1 (1.8) | 2.8 (1.9)† | 2.2 (1.2) | 1.9 (1.3) |
| ISS score | 7.4 (4.2) | 6.4 (4.5) | 4.9 (3.8)† | 4.3 (3.4) | 4.4 (3.5) |
| Grip strength (kPa) | |||||
| Dominant hand | 37.1 (25.0) | 46.4 (23.9) | 50.1 (27.2)† | 54.2 (24.9) | 57.1 (26.8) |
| Non-dominant hand | 36.6 (22.5) | 46.5 (23.1) | 49.4 (26.1)† | 53.1 (24.0) | 55.8 (25.7) |
| Total MRC score | 50.7±7.3 | 53.8±7.8 | 54.5±8.4 | 57.0±4.5 | 56.9±4.9 |
| CMAP amplitude (mV) (most affected nerve) |
1.1 (1.8)‡ | 1.5 (2.5)§ | 1.8 (2.1)¶ | 1.8 (2.1)¶ | 1.7 (2.2)** |
| Serum IgG (mg/dL) | 1210 (357) | 2058 (369)†† | 1936 (369)‡‡ | 1936 (369)‡‡ | 2012 (341)¶ |
Data are shown as mean (SD).
*Only responders at week 28 were included.
†p<0.01, compared with a baseline value.
‡n=46.
§n=45.
¶n=34.
**n=32.
††n=48.
‡‡n=38.
CMAP, compound muscle action potential; INCAT, Inflammatory Neuropathy Cause and Treatment; ISS, INCAT sensory sum; MRC, Medical Research Council.
Figure 3.
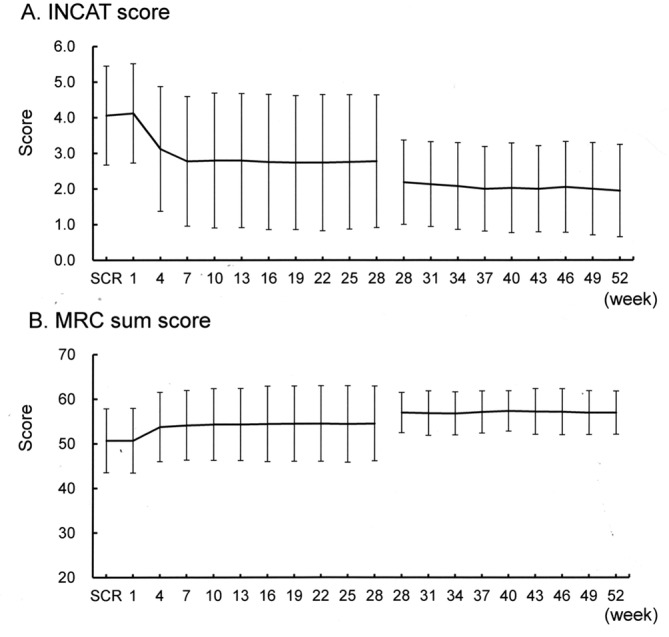
Transition diagram for the total INCAT score (A) and MRC sum score (B). INCAT, Inflammatory Neuropathy Cause and Treatment; MRC, Medical Research Council; SCR, screening.
jnnp-2017-316427supp001.jpg (236.2KB, jpg)
Safety
A total of 46 (93.9%) of the 49 patients experienced adverse events (95% CI 83.1% to 98.7%). Table 3 shows details of adverse events with the incidence of 4% or more. Frequent adverse events were headache (32.7%), nasopharyngitis (28.6%) and skin rash (12.2%). Additionally, adverse drug reactions were observed in 65.3% (32/49 patients, 95% CI 50.4% to 78.3%). No death occurred during the study. Six patients experienced eight serious adverse events, including cerebral infarction (n=2), aggravation of CIDP (n=2), cryptococcal pneumonia (n=1), cholesteatoma (n=1), inguinal hernia (n=1) and anxiety neurosis (n=1).
Table 3.
Adverse events reported in ≥4% of patients
| Total patients | n=49 | |
| Patients developing adverse events | n=46 | |
| Rate of developing adverse events | 93.9% | |
| Total no of developing adverse events | n=230 | |
| Adverse event name (PT) | No of patients | Rate (%) |
| Headache | 16 | 32.7 |
| Nasopharyngitis | 14 | 28.6 |
| Rash | 6 | 12.2 |
| Contusion | 5 | 10.2 |
| Upper respiratory tract inflammation | 4 | 8.2 |
| Diarrhoea | 3 | 6.1 |
| Chronic inflammatory demyelinating polyneuropathy | 3 | 6.1 |
| Erythema | 3 | 6.1 |
| Elevation of aspartate aminotransferase | 3 | 6.1 |
| Sense of fatigue | 3 | 6.1 |
| Pruritus | 2 | 4.1 |
| Abrasion | 2 | 4.1 |
| Influenza | 2 | 4.1 |
| Periodontitis | 2 | 4.1 |
| Pharyngitis | 2 | 4.1 |
| Inguinal hernia | 2 | 4.1 |
| Nausea | 2 | 4.1 |
| Elevation of alanine aminotransferase | 2 | 4.1 |
| Reduction of lymphocyte count | 2 | 4.1 |
| Anthropod bite | 2 | 4.1 |
Medical dictionary for Regulatory Activities (MedDRA), V.18.0.
In the two patients who suffered cerebral infarction, case 1, a 76-year-old man, who had a history of hypertension and steroid-induced hyperlipidaemia, developed mild dysarthria at week 51, 6 days after the last maintenance intravenous Ig (1.0 g/kg for 1 day). T2-weighted and diffusion-weighted brain MRI showed lacunar infraction at the right corona radiate. At stroke onset, the serum IgG level was 2222 mg/dL. Oral aspirin (100 mg/day) was started, and the symptom disappeared 2 months later. He completed the 52-week trial. Case 2, a 66-year-old man, who had a history of type 2 diabetes and hypertension, developed dysarthria and unsteady gait at week 45, 18 days after the last intravenous Ig (0.5 g/kg for two consecutive days). T2-weighted MRI revealed lacunar infarct in the left pons. The serum IgG level was 1946 mg/dL at stroke onset. The trial stopped because of this, and oral cilostazol (200 mg/day) was initiated. The symptoms were resolved 46 days later.
Discussion
Our results showed that after induction therapy with conventional full-dose intravenous Ig (2.0 g/kg), maintenance intravenous Ig treatment (1.0 g/kg) every 3 weeks resulted in sustained clinical remission for 52 weeks in 69.4% of all the enrolled patients with CIDP. During the study at week 28, the responder rate at week 28 was 77.6%, and this was significantly higher than in the placebo group in the ICE study (20.7%)6 and slightly higher than in the intravenous Ig group in the PRIMA study (60.7%)9 using the similar study period of 24 weeks and the same protocol. The clinical remission was maintained for the next 24 weeks, and therefore this study first showed the efficacy of maintenance intravenous Ig therapy for 52 weeks. The results were supported by improvement in the secondary outcome measures including the adjusted INCAT score, ISS score, grip strength and CMAP amplitude.12–14 Thus, this study confirmed the efficacy of maintenance intravenous Ig for 24 weeks in the ICE6 and PRIMA9 studies, and showed the sustained effects during the longer treatment period of 52 weeks.
Among the patients included in this study, 43 had typical CIDP, and the remaining six were classified with atypical CIDP according to the EFNS/PNS clinical criteria.10 Our results therefore showed the long-term efficacy of maintenance intravenous Ig therapy for patients with typical CIDP, whereas effects for atypical CIDP could not be analysed because of the small number of patients.
In this study, the maintenance dose (1.0 g/kg) and interval (3 weeks) of intravenous Ig therapy were the same as those in the previous two studies,6 9 and this treatment regimen appears to be efficacious to prevent relapse of CIDP. However, optimal dose and interval for each patient are still unclear.15 Previous studies have shown different responses among patients with CIDP treated with the same body weight-based dose,16 17 presumably because of different disease activity and Ig metabolism among individual patients. Another issue is the treatment period; two retrospective studies investigating long-term prognosis in patients with CIDP showed that 26% of patients with CIDP reach sustained long-term remission without any treatment.18 19 In the present study, the possibility that CIDP became inactive during the 52-week treatment cannot be excluded. Currently, there is no evidence for predicting the individual dose and interval for each patient. The optimal regimen and algorithm for maintenance intravenous Ig should be studied in future clinical trials.15 19 20
Separately, two patients suffered cerebral infarction (lacunar infarct) during this study; they were aged 76 and 66 years, and had a history of hypertension or diabetes. Intravenous Ig therapy causes an increase in serum viscosity, and this could increase the risk of thromboembolic events.21 The reported incidence of cerebral infarction associated with conventional intravenous Ig treatment in patients with CIDP is not high; a postmarket survey of Glovenin-I shows that two of the total of 5587 patients with CIDP (0.04%) developed cerebral infarction (Nihon Pharmaceutical, unpublished data, 2010), and thromboembolic complications did not occur in the ICE (n=117) and PRIMA (n=28) studies during the 24-week maintenance intravenous Ig treatment. However, because the treatment period of this study was longest for patients with CIDP, the possibility that continuous long-term (52 weeks) hyperviscosity induced lacunar infarction could not be excluded. We suggest that this adverse event should be carefully monitored, particularly in elderly patients with vascular risk factor(s), and that slow infusion rate and occasionally antiplatelet medication may be indicated.
In conclusion, 52-week maintenance intravenous Ig therapy appears to be efficacious to prevent a relapse for typical patients with CIDP. There is a potential risk of thrombotic events, and it should be carefully monitored.
Acknowledgments
We thank our investigators and their patients for their participation. We also acknowledge data management and analysis support from Bell Medical Solutions.
Footnotes
Contributors: SK is the guarantor of the article. SK: study concept and design, and drafting of the manuscript. MM and SM: ethics approval, patient enrolment, data collection and drafting of the manuscript. MS, KN, TM, SD, NK, MK, HY, KA, YN, KO and KS: ethics approval, patient enrolment, data collection and critical revision of the manuscript for important intellectual content. KSe: drafting of the manuscript. SK, GS and RK: study concept and design, critical revision of the manuscript for important intellectual content. Glovenin-I CIDP Study Group members: collected data for this study. All authors approved the final submission.
Funding: This study was funded by Nihon Pharmaceutical (ClinicalTrials.gov number: NCT01824251).
Competing interests: SKuw, SKus, GS, and RK have received consultancy fees, lecture fees and travel expenses on the steering committee from Nihon Pharmaceutical. KSa is employees of Nihon Pharmaceutical. All other authors declare that they have no conflict of interest.
Ethics approval: Institutional review board of each institute participating in this trial.
Provenance and peer review: Not commissioned; externally peer reviewed.
Collaborators: Glovenin-ICIDP Study Group include the following people: K. Shibuya, Y. Iwai (Department of Neurology, Chiba University Hospital, Chiba, Japan); J. Kaneko (Department of Neurology, Kitasato University School of Medicine, Kanagawa, Japan); A. Ueda (Department of Neurology, Fujita Health University School of Medicine, Aichi, Japan); T. Nagashima (Department of Neurology, Dokkyo Medical University, Tochigi, Japan); S. Watanabe (Division of Neurology, Department of Internal Medicine, Hyogo College of Medicine, Hyogo, Japan); K. Abe, K. Deguchi (Department of Neurology, Okayama University School of Medicine, Okayama, Japan): A. Tamaoka, K. Ishii (Department of Neurology, Faculty of Medicine, University of Tsukuba, Ibaraki, Japan); T. Okamoto (Department of Neurology, National Center Hospital of Neurology and Psychiatry, Tokyo, Japan); T. Yokota, T. Okubo (Department of Neurology and Neurological Science, Tokyo Medical and Dental University, Tokyo, Japan); K. Yokoyama, N. Hattori (Department of Neurology, Juntendo University School of Medicine, Tokyo, Japan); K. Nomura, T. Tajima (Department of Neurology, Saitama Medical Center, Saitama Medical University, Saitama, Japan); K. Tanaka, S. Takashima, Y. Nakatsuji (Department of Neurology, Toyama University School of Medicine, Toyama, Japan); M. Morita (Department of Neurology, Fuji City General Hospital, Shizuoka, Japan); M. Iijima, Y. Kawagashira (Department of Neurology, Nagoya University School of Medicine, Aichi, Japan); A. Taniguchi, A. Tamura (Department of Neurology, Mie University School of Medicine, Mie, Japan); T. Ieda (Department of Neurology, Yokkaichi Municipal Hospital, Mie, Japan); M. Kanda (Department of Neurology, Ijinkai Takeda General Hospital, Kyoto, Japan); H. Suzuki (Department of Neurology, Kindai University Faculty of Medicine, Osaka, Japan); T. Okuno (Department of Neurology, Osaka University School of Medicine, Osaka, Japan); T. Toda (Division of Neurology, Kobe University Graduate School of Medicine, Hyogo, Japan); K. Murata: Department of Neurology, Wakayama Medical University, Wakayama, Japan); Y. Takehisa (Department of Neurology, Japanese Red Cross Okayama Hospital, Okayama, Japan); K. Ochi, H. Ueno (Department of Neurology, Hiroshima University School of Medicine, Hiroshima, Japan); T. Kanda, F. Shimizu (Department of Neurology, Yamaguchi University School of Medicine, Yamaguchi, Japan); H. Nodera, N. Matsui (Department of Neurology, Tokushima University School of Medicine, Tokushima, Japan); T. Mitsui (Department of Neurology, National Hospital Organization Tokushima National Hospital, Tokushima, Japan); D. Matsuse (Department of Neurology, Neurological Institute, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan); Y. Misumi, T. Hirahara (Department of Neurology, Kumamoto University School of Medicine, Kumamoto, Japan); H. Takashima, M. Ando (Department of Neurology and Geriatrics Kagoshima University Graduate School of Medical and Dental Sciences, Kagoshima, Japan); I. Yabe (Department of Neurology, Hokkaido University School of Medicine, Hokkaido, Japan); H. Kuroda (Department of Neurology, Tohoku University School of Medicine, Miyagi, Japan); M. Morita (Department of Neurology, Jichi Medical University, Tochigi, Japan); J. Shimizu (Department of Neurology, Tokyo University School of Medicine, Tokyo, Japan); H. Okuma (Department of Neurology, Tokai University Hachioji Hospital, Tokyo, Japan); F. Tanaka (Department of Neurology, Yokohama City University Graduate School of Medicine, Kanagawa, Japan); M. Matsui (Department of Neurology, Kanazawa Medical University, Ishikawa, Japan); K. Fukushima (Department of Medicine (Neurology and Rheumatology), Shinshu University School of Medicine, Nagano, Japan); R. Matsumoto (Department of Neurology, Kyoto University Graduate School of Medicine, Kyoto, Japan); M. Tahara (Department of Neurology, Utano National Hospital, Kyoto, Japan); T. Mizuno (Department of Neurology, Kyoto Prefectural University of Medicine, Kyoto, Japan); T. Hamano (Department of Neurology, Kansai Electric Power Hospital, Osaka, Japan); T. Hazama (Department of Neurology, Osaka General Medical Center, Osaka, Japan); Y. Kita (Neurology Service, Hyogo Brain and Heart Center at Himeji, Hyogo, Japan); K. Deguchi (Department of Gastroenterology & Neurology, Faculty of Medicine, Kagawa University, Kagawa, Japan).
References
- 1. Mathey EK, Park SB, Hughes RA, et al. . Chronic inflammatory demyelinating polyradiculoneuropathy: from pathology to phenotype. J Neurol Neurosurg Psychiatry 2015;86:973–85. 10.1136/jnnp-2014-309697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kuwabara S, Isose S, Mori M, et al. . Different electrophysiological profiles and treatment response in ‘typical’ and ‘atypical’ chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatry 2015;86:1054–9. 10.1136/jnnp-2014-308452 [DOI] [PubMed] [Google Scholar]
- 3. van Doorn PA, Vermeulen M, Brand A, et al. . Intravenous immunoglobulin treatment in patients with chronic inflammatory demyelinating polyneuropathy. Clinical and laboratory characteristics associated with improvement. Arch Neurol 1991;48:217–20. [DOI] [PubMed] [Google Scholar]
- 4. Hahn AF, Bolton CF, Zochodne D, et al. . Intravenous immunoglobulin treatment in chronic inflammatory demyelinating polyneuropathy. A double-blind, placebo-controlled, cross-over study. Brain 1996;119:1067–77. 10.1093/brain/119.4.1067 [DOI] [PubMed] [Google Scholar]
- 5. Mendell JR, Barohn RJ, Freimer ML, et al. . Randomized controlled trial of IVIg in untreated chronic inflammatory demyelinating polyradiculoneuropathy. Neurology 2001;56:445–9. 10.1212/WNL.56.4.445 [DOI] [PubMed] [Google Scholar]
- 6. Hughes RA, Donofrio P, Bril V, et al. . Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): a randomised placebo-controlled trial. Lancet Neurol 2008;7:136–44. 10.1016/S1474-4422(07)70329-0 [DOI] [PubMed] [Google Scholar]
- 7. van Doorn PA, Brand A, Strengers PF, et al. . High-dose intravenous immunoglobulin treatment in chronic inflammatory demyelinating polyneuropathy: a double-blind, placebo-controlled, crossover study. Neurology 1990;40:209–12. 10.1212/WNL.40.2.209 [DOI] [PubMed] [Google Scholar]
- 8. Nobile-Orazio E, Cocito D, Jann S, et al. . Intravenous immunoglobulin versus intravenous methylprednisolone for chronic inflammatory demyelinating polyradiculoneuropathy: a randomised controlled trial. Lancet Neurol 2012;11:493–502. 10.1016/S1474-4422(12)70093-5 [DOI] [PubMed] [Google Scholar]
- 9. Léger JM, De Bleecker JL, Sommer C, et al. . Efficacy and safety of in patients with chronic inflammatory demyelinating polyneuropathy: results of a prospective, single-arm, open-label Phase III study (the PRIMA study). J Peripher Nerv Syst 2013;18:130–40. 10.1111/jns5.12017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Joint Task Force of the EFNS and the PNS. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society—first revision. J Peripher Nerv Syst 2010;15:1–9. 10.1111/j.1529-8027.2010.00245.x [DOI] [PubMed] [Google Scholar]
- 11. Hughes R, Bensa S, Willison H, et al. . Randomized controlled trial of intravenous immunoglobulin versus oral prednisolone in chronic inflammatory demyelinating polyradiculoneuropathy. Ann Neurol 2001;50:195–201. [DOI] [PubMed] [Google Scholar]
- 12. Merkies ISJ, Schmitz PIM, van der Meché FGA, et al. . Psychometric evaluation of a new sensory scale in immune-mediated polyneuropathies. Neurology 2000;54:943–9. [DOI] [PubMed] [Google Scholar]
- 13. Kleyweg RP, van der Meché FG, Schmitz PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barré syndrome. Muscle Nerve 1991;14:1103–9. 10.1002/mus.880141111 [DOI] [PubMed] [Google Scholar]
- 14. Donofrio PD, Bril V, Dalakas MC, et al. . Safety and tolerability of immune globulin intravenous in chronic inflammatory demyelinating polyradiculoneuropathy. Arch Neurol 2010;67:1082–8. 10.1001/archneurol.2010.223 [DOI] [PubMed] [Google Scholar]
- 15. Kuitwaard K, Hahn AF, Vermeulen M, et al. . Intravenous immunoglobulin response in treatment-naïve chronic inflammatory demyelinating polyradiculoneuropathy. J Neurol Neurosurg Psychiatry 2015;86:1331–6. 10.1136/jnnp-2014-309042 [DOI] [PubMed] [Google Scholar]
- 16. Rajabally YA, Wong SL, Kearney DA. Immunoglobulin G level variations in treated chronic inflammatory demyelinating polyneuropathy: clues for future treatment regimens? J Neurol 2013;260:2052–6. 10.1007/s00415-013-6938-7 [DOI] [PubMed] [Google Scholar]
- 17. Dyck PJ, Litchy WJ, Kratz KM, et al. . A plasma exchange versus immune globulin infusion trial in chronic inflammatory demyelinating polyradiculoneuropathy. Ann Neurol 1994;36:838–45. 10.1002/ana.410360607 [DOI] [PubMed] [Google Scholar]
- 18. Kuwabara S, Misawa S, Mori M, et al. . Long term prognosis of chronic inflammatory demyelinating polyneuropathy: a five year follow up of 38 cases. J Neurol Neurosurg Psychiatry 2006;77:66–70. 10.1136/jnnp.2005.065441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Querol L, Rojas-Garcia R, Casasnovas C, et al. . Long-term outcome in chronic inflammatory demyelinating polyneuropathy patients treated with intravenous immunoglobulin: a retrospective study. Muscle Nerve 2013;48:870–6. 10.1002/mus.23843 [DOI] [PubMed] [Google Scholar]
- 20. Adrichem ME, Eftimov F, van Schaik IN. Intravenous immunoglobulin treatment in chronic inflammatory demyelinating polyradiculoneuropathy, a time to start and a time to stop. J Peripher Nerv Syst 2016;21:121–7. 10.1111/jns.12176 [DOI] [PubMed] [Google Scholar]
- 21. Dalakas MC. The use of intravenous immunoglobulin in the treatment of autoimmune neuromuscular diseases: evidence-based indications and safety profile. Pharmacol Ther 2004;102:177–93. 10.1016/j.pharmthera.2004.04.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2017-316427supp001.jpg (236.2KB, jpg)


