Abstract
Objectives
ABP 501 is a Food and Drug Administration-approved biosimilar to adalimumab; structural, functional and pharmacokinetic evaluations have shown that the two are highly similar. We report results from a phase III study comparing efficacy, safety and immunogenicity between ABP 501 and adalimumab.
Methods
In this randomised, double-blind, active comparator-controlled, 26-week equivalence study, patients with moderate to severe active rheumatoid arthritis (RA) despite methotrexate were randomised (1:1) to ABP 501 or adalimumab (40 mg) every 2 weeks. Primary endpoint was risk ratio (RR) of ACR20 between groups at week 24. Primary hypothesis that the treatments were equivalent would be confirmed if the 90% CI for RR of ACR20 at week 24 fell between 0.738 and 1.355, demonstrating that ABP 501 is similar to adalimumab. Secondary endpoints included Disease Activity Score 28-joint count-C reactive protein (DAS28-CRP). Safety was assessed via adverse events (AEs) and laboratory evaluations. Antidrug antibodies were assessed to determine immunogenicity.
Results
A total of 526 patients were randomised (n=264, ABP 501; n=262 adalimumab) and 494 completed the study. ACR20 response at week 24 was 74.6% (ABP 501) and 72.4% (adalimumab). At week 24, the RR of ACR20 (90% CI) between groups was 1.039 (0.954, 1.133), confirming the primary hypothesis. Changes from baseline in DAS28-CRP, ACR50 and ACR70 were similar. There were no clinically meaningful differences in AEs and laboratory abnormalities. A total of 38.3% (ABP 501) and 38.2% (adalimumab) of patients tested positive for binding antidrug antibodies.
Conclusions
Results from this study demonstrate that ABP 501 is similar to adalimumab in clinical efficacy, safety and immunogenicity in patients with moderate to severe RA.
Trial registration number
NCT01970475; Results.
Keywords: DMARDs (biologic), rheumatoid arthritis, TNF-alpha, anti-TNF, inflammation
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterised by synovial inflammation that results in joint damage. The introduction of biologics in 1998 resulted in improvements in outcomes with RA treatments.1 Tumour necrosis factor (TNF) inhibitors were the first approved biological disease-modifying antirheumatic drugs (bDMARDs) for treatment of RA, followed by additional bDMARDs that had differing mechanisms of action.1 The bDMARD adalimumab (AbbVie, Chicago, Illinois, USA) is a recombinant human IgG1 monoclonal antibody that binds specifically to TNF-α. Adalimumab was approved for the treatment of moderate to severe RA and has been shown to have significant efficacy,2 with improvements in patient’s disease activity, quality of life and prevention of structural damage and disability. Safety concerns have been well delineated and are similar to other biologics, including risk of infections.2 Adalimumab has been approved for other indications, including psoriasis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis, inflammatory bowel disease, hidradenitis suppurativa and non-infectious intermediate and posterior uveitis and panuveitis; it is one of the most frequently prescribed biologics in clinical practice.2–6 Adalimumab has been extensively studied in combination with methotrexate (MTX) and has been shown to improve outcomes versus placebo in patients with RA who demonstrate an incomplete response to MTX.2 7 8
Biosimilars, biological products that are similar to an already licensed reference product (such as adalimumab), are being developed.9 10 Due to complexities involved in developing biological proteins, regulatory agencies have developed guidelines for demonstrating that proposed biosimilars are highly similar to the reference product and that no clinically meaningful differences exist between the proposed biosimilar and reference product in terms of safety, purity and potency.9 11
This pathway differs from innovator biologic product development and requires extensive structural and functional analysis to demonstrate that the biosimilar and originator molecule are highly similar in structure and effector function. Additionally, guidelines on biosimilars indicate that clinical trials should be conducted to compare the biosimilar and reference product in sensitive populations and with appropriate endpoints to enable detection of clinically meaningful differences, if any, between the proposed biosimilar and reference product.12 13 Using this pathway, several biosimilars such as InflectraTM, RemsimaTM, FlixabiTM (infliximab biosimilars) and BenepaliTM (etanercept biosimilar) have received marketing authorisation from the European Medicines Agency (EMA),14–16 and the Food and Drug Administration (FDA) has recently approved biosimilars of filgrastim (ZarxioTM), infliximab (Inflectra), etanercept (ErelziTM) and adalimumab (AMJEVITATM).4 17–20
ABP 501 (AMJEVITA) was approved as the first adalimumab biosimilar by the US FDA.21 Analytical and biofunctional evaluations have demonstrated that ABP 501 and adalimumab are highly similar in their structural and functional properties, as well as biological activity.22 23 A phase I, single-dose study of ABP 501 in healthy adults demonstrated pharmacokinetic equivalence to that of adalimumab.24 To demonstrate similarity in clinical efficacy, safety and immunogenicity of ABP 501 compared with adalimumab, two phase III studies were conducted: one examined effects in patients with moderate to severe plaque psoriasis (NCT01970488) and one in patients with moderate to severe RA (NCT01970475).24 25 Here, we report results from a phase III study designed to assess the clinical efficacy, safety and immunogenicity of ABP 501 compared with adalimumab for the treatment of moderate to severe RA.
Methods
Study design
This was a randomised, double-blind, active comparator-controlled equivalence study designed to show clinical similarity between ABP 501 and adalimumab in adalimumab-naive adult patients with moderate to severe RA who had an inadequate response to MTX. The study was conducted in 12 countries and 100 centres across Europe, North America and Latin America (see online supplementary table 1). Following screening (≤4 weeks), patients were randomised 1:1 to receive either ABP 501 or adalimumab 40 mg subcutaneously on day 1 and then every 2 weeks until week 22. The primary endpoint assessments were conducted at week 24, followed by final safety and immunogenicity assessments at week 26 (see online supplementary methods for additional details on methodology and full protocol).
annrheumdis-2016-210459supp001.docx (112.9KB, docx)
annrheumdis-2016-210459supp002.pdf (435.4KB, pdf)
Study population
Patients ≥18 years to ≤80 years of age were included if they had a diagnosis of moderate to severe RA (per 2010 American College of Rheumatology/European League Against Rheumatism criteria) for ≥3 months. Patients were required to have active RA (≥6 swollen joints and ≥6 tender joints) at screening and baseline. Patients with an erythrocyte sedimentation rate ≥28 mm/hour or serum C reactive protein (CRP) >1.0 mg/dL and positivity for rheumatoid factor or anticyclic citrullinated peptide at screening were included. Patients were also required to have a negative test for tuberculosis at screening, defined as a negative purified protein derivative (<5 mm of induration at 48–72 hours after test) or a negative QuantiFERON (Qiagen, Hilden, Germany) test. Patients were required to have received MTX for ≥12 consecutive weeks and were on a stable oral dose of 7.5–25 mg/week for ≥8 weeks before receiving investigational product (IP). Patients were excluded if they previously used ≥2 biologic therapies for RA or had any previous use of adalimumab or an adalimumab biosimilar.
Concomitant therapies
Patients were required to receive a stable dose of MTX for the study duration, as prescribed by the treating physician. If a patient developed MTX-related side effects, a dose reduction was possible at the investigator’s discretion. Patients were allowed to remain on oral corticosteroids (≤10 mg/day of prednisone or equivalent) if on a stable dose for ≥4 weeks prior to initiation of IP. Prohibited medications included non-biologic DMARDs (other than MTX) and biologic treatment for RA other than those being investigated.
Efficacy endpoints
The primary efficacy endpoint was the risk ratio (RR) of achieving a 20% improvement from baseline in the American College of Rheumatology core set of measurements26 (ACR20) at week 24. Secondary efficacy endpoints included assessments of Disease Activity Score 28-joint count-CRP (DAS28-CRP), the RR for ACR20, ACR50 and ACR70 response (20%, 50%, 70% improvement in ACR core set of measurements) at various time points throughout the study. Additional endpoints included the risk differences (RD) for ACR20, ACR50 and ACR70.
Safety
Key safety endpoints included treatment-emergent adverse events (TEAEs), serious adverse events (SAEs) and incidence of antidrug antibodies (ADAs). Adverse events (AEs) of interest were also assessed based on the Standard Medical Dictionary for Regulatory Activities (MedDRA) queries.
ADAs were assessed at baseline and weeks 4, 12 and 26. In the current study, ADA status was assessed using a highly sensitive and drug tolerant assay based on the Meso Scale Discovery Electrochemiluminescent platform,24 27 followed by a two-tiered test consisting of a screening and specificity assay. Assays were developed for each IP and each serum sample was tested using both assays. Of note, these assays differed from the original ELISA used for immunogenicity assessments of adalimumab.2 Samples positive for binding ADAs were tested in a ligand-binding bioassay for neutralising activity. The sensitivity of the ADA detection assay was the same for both adalimumab and ABP 501. The assay was validated with a tolerance of 25 µg/mL of drug, and the highest observed maximum observed concentration (Cmax) in this study was <6.0 mg/mL. Drug interference was, thus, not expected from the collected samples. The neutralising antibody cell-based bioassay was expected to detect all classes of antibodies that inhibit the biological activity of the drug, including monovalent IgG4 subclass antibodies.24
Statistical analyses
A sample size of approximately 500 patients was chosen to achieve 90% power to demonstrate equivalence between the ABP 501 and adalimumab groups for the primary efficacy endpoint, RR of ACR20 at week 24, with a two-sided significance level of 0.05 and equivalence margin of (0.738, 1.355). It assumed an expected ACR20 response of 63% at week 24 for each group and a 15% dropout by week 24.
All efficacy endpoints were analysed using the full analysis set, which included all randomised patients, based on patients’ randomised treatment. Randomisation, conducted by an independent statistician, was computer-generated and was stratified by geographical region and prior biologic use (with prior biologic use capped at 40% of study population) for RA. The primary hypothesis, that there were no clinically meaningful differences between the ABP 501 and adalimumab groups, was tested by comparing the 2-sided 90% confidence interval (CI) of the RR of ACR20 at week 24 between ABP 501 and adalimumab to the equivalence margin of (0.738, 1.355). The rationale for the equivalence margin was based on considerations in the draft US FDA Non-inferiority Clinical Trials Guidance for Industry.28 The equivalence margin of 0.738, 1/0.738=1.355 for the RR of ACR20 responses was chosen based on a published relevant adequate and well-controlled trial8 and was expected to preserve 50% of the estimated 80% upper confidence bound of the treatment effect of the reference product compared with placebo. The 90% CI for RR was estimated using a generalised linear model adjusted for the stratification factors. All other efficacy endpoints, including RD of ACR20, were analysed descriptively. For DAS28-CRP, treatment differences across time points for change from baseline in DAS28-CRP were evaluated using a mixed model for repeated-measures analysis, with stratification variables, visit, treatment group, treatment-by-visit interactions and baseline DAS28-CRP included in the model. For ACR50 and ACR70, treatment differences were estimated using the same model as described above for ACR20. Analyses of the RDs for ACR20, ACR50 and ACR70 between ABP 501 and adalimumab were descriptive in nature and their corresponding 90% CIs were estimated using the generalised linear model adjusted for stratification factors. For the primary analysis based on the full analysis set, missing values were imputed using the last observation carried forward method. As sensitivity analyses, efficacy endpoints were also analysed for the per-protocol analysis set, which included patients who completed the treatment period and did not have a protocol violation, based on observed cases. In addition, for binary endpoints, such as ACR20, another imputation was performed for sensitivity in which patients with a missing binary response at a certain visit were imputed as non-responders.
All safety data were analysed using the safety analysis set (all randomised patients who received ≥1 dose of IP) based on patients’ actual treatment received. ADA data were analysed using the ADA analysis set, defined as the subset of patients in the safety analysis set who had ≥1 evaluable antibody test result (based on actual treatment received).
Results
Patient disposition
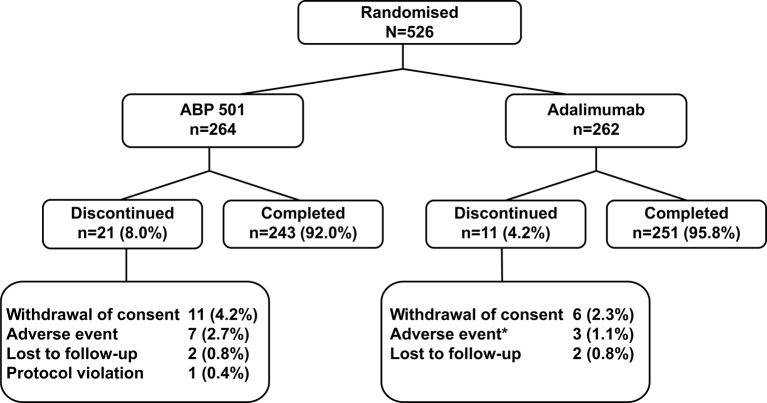
A total of 526 patients were randomised and treated with IP (ABP 501, n=264; adalimumab, n=262) and 494 (93.9%) completed the study (ABP 501, n=243; adalimumab, n=251) (figure 1). The main reason for discontinuation in both groups was withdrawal of consent (ABP 501, 4.2%, n=11; adalimumab, 2.3%, n=6).
Figure 1.
Patient disposition. *n=1 patient took prohibited concomitant medication due to an adverse event and was discontinued from the study. First patient was screened on 15 October 2013 and enrolled on 24 October 2013. Patients screened, n=747; per-protocol analysis set, n=463 (ABP 501, n=230; adalimumab, n=233).
Baseline demographics and clinical characteristics
The majority of patients were female (81.0%) and white (95.1%), with a mean age of 55.9 years (range: 21–80 years) and a mean of 9.39 years and median of 7.09 years since diagnosis. Overall, baseline demographics and clinical characteristics were similar across groups, including mean (standard deviation [SD]) baseline DAS28-CRP scores, which were 5.66 (0.92) and 5.68 (0.91) for the ABP 501 and adalimumab groups, respectively (table 1).
Table 1.
Baseline demographics and clinical characteristics (full analysis set)
| Variable | ABP 501 (n=264) |
Adalimumab (n=262) |
| Age, mean (SD), years | 55.4 (11.9) | 56.3 (11.5) |
| Women, n (%) | 214 (81.1) | 212 (80.9) |
| Race, n (%) | ||
| White | 251 (95.1) | 249 (95.0) |
| Black or African American | 9 (3.4) | 12 (4.6) |
| Asian | 3 (1.1) | 0 (0.0) |
| Other | 1 (0.4) | 1 (0.4) |
| Region, n (%) | ||
| Eastern Europe | 169 (64.0) | 168 (64.1) |
| Western Europe | 22 (8.3) | 20 (7.6) |
| North America | 72 (27.3) | 72 (27.5) |
| Latin America | 1 (0.4) | 2 (0.8) |
| Duration of RA, mean (SD), years | 9.41 (8.08) | 9.37 (8.05) |
| Duration of RA category, n (%) | ||
| <5 years | 101 (38.3) | 90 (34.4) |
| ≥5 years | 163 (61.7) | 172 (65.6) |
| Swollen joint count, mean (SD) | 14.7 (9.1) | 14.1 (8.0) |
| Tender joint count, mean (SD) | 24.3 (14.4) | 23.9 (13.5) |
| Subject Global Health Assessment, mean (SD) | 6.5 (1.9) | 6.6 (1.9) |
| Investigator Global Health Assessment, mean (SD) | 6.8 (1.3) | 6.7 (1.6) |
| HAQ-DI, mean (SD)* | 1.482 (0.617) | 1.498 (0.647) |
| Serum CRP, mean (SD), mg/L | 13.881 (20.687) | 14.678 (19.385) |
| Serum CRP, median, mg/L | 6.140 | 7.630 |
| DAS28-CRP, mean (SD)† | 5.66 (0.92) | 5.68 (0.91) |
| RF status, n (%)‡‡ | ||
| Positive | 243 (92.0) | 240 (91.6) |
| Anti-CCP status, n (%)‡ | ||
| Positive | 212 (80.3) | 230 (87.8) |
| Prior biologic use for RA, n (%) | ||
| Yes | 71 (26.9) | 74 (28.2) |
| MTX dose, mean (SD), mg/week | 16.89 (4.81) | 16.56 (4.93) |
*ABP 501, n=263; adalimumab, n=261; total, n=524.
†ABP 501, n=264; adalimumab, n=261; total, n=525.
‡At screening.
CCP, cyclic citrullinated peptide; CRP, C reactive protein; DAS28, Disease Activity Score 28-joint count; HAQ-DI, Health Assessment Questionnaire-Disability Index; MTX, methotrexate; RA, rheumatoid arthritis; RF, rheumatoid factor.
Concomitant and previous medications
Prior use of biologics for RA and baseline RA medications was balanced across groups; the majority of patients (ABP 501, 73.1%; adalimumab, 71.8%) were treatment-naive for prior use of biologics for RA. Oral corticosteroids were used at baseline by 50.8% and 49.6% of patients in the ABP 501 and adalimumab groups, respectively. Similar percentages of patients used non-steroidal anti-inflammatory drugs in each group (ABP 501, 60.2%; adalimumab, 64.1%). Baseline mean MTX doses were similar across treatment groups (ABP 501, 16.89 mg/week; adalimumab, 16.56 mg/week).
Clinical efficacy
ACR20
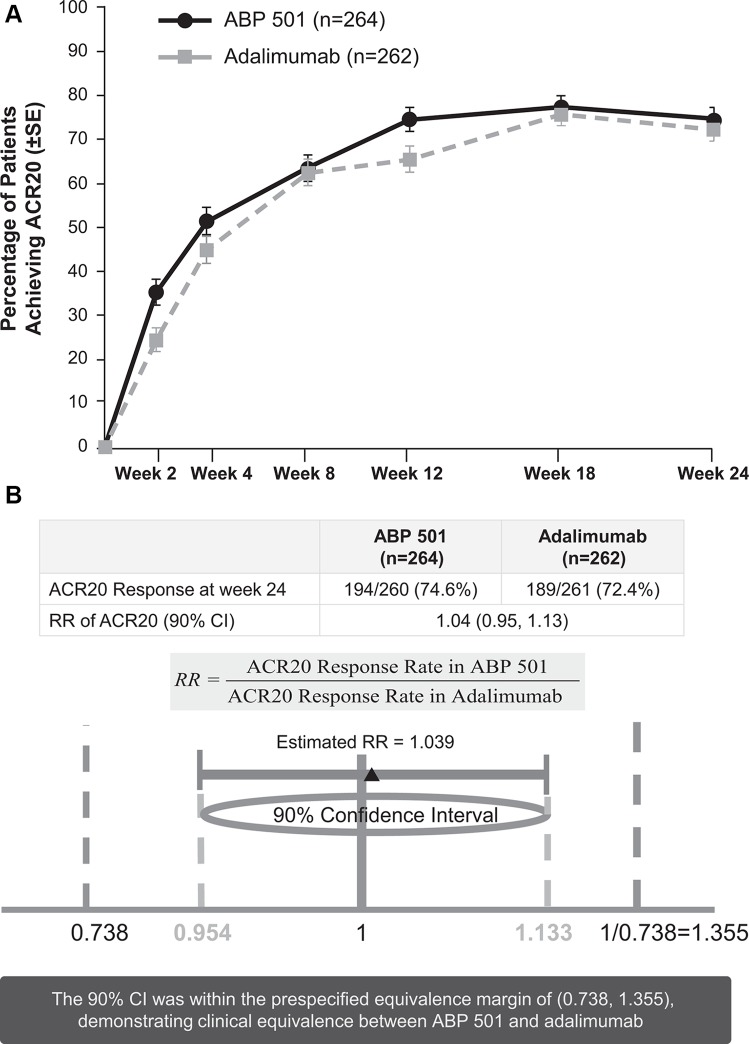
At week 24, 74.6% (194/260) of subjects in the ABP 501 group and 72.4% (189/261) of subjects in the adalimumab group met the ACR20 response criteria (figure 2A, see online supplementary tables 2 and 3). The RR (2-sided 90% CI) of ACR20 at week 24 for ABP 501 versus adalimumab was 1.039 (0.954, 1.133). The 90% CI of (0.954, 1.133) was well within the predefined equivalence margin (0.738, 1.355), demonstrating clinical equivalence between ABP 501 and adalimumab (figure 2B). Additionally, the RD (two-sided 90% CI) between groups for ACR20 at week 24 was 2.604 (−3.728, 8.936).
Figure 2.
(A) Percentage of patients achieving ACR20 by study week (full analysis set). ACR20, 20% improvement from baseline in American College of Rheumatology core set measurements. (B) Ratio of ACR responses at week 24. ACR20, 20% improvement from baseline in American College of Rheumatology core set measurements. RR, risk ratio; 95% CI 0.938, 1.152.
The percentage of patients who achieved ACR20 at weeks 2 and 8 (secondary endpoints) were 35.4% (90/254) of patients who took ABP 501 and 24.5% (63/257) of patients who took adalimumab at week 2 and 63.5% (165/260) versus 62.5% (163/261) of patients, respectively, at week 8 (figure 2A). The percentages of ACR20 responders were comparable across groups at all study time points, supporting clinical similarity between the ABP 501 and adalimumab groups.
ACR50 and ACR70
The percentages of patients who reached ACR50 response criteria at week 24 were 49.2% (120/244) and 52.0% (131/252) for the ABP 501 and adalimumab groups, respectively, with a RR (90% CI) for ABP 501 versus adalimumab of 0.948 (0.819, 1.097) and RD (90% CI) of −2.836% (−10.220%, 4.547%). The proportion of patients who achieved ACR50 was similar across treatment groups throughout the study. A total of 26.0% (64/246) and 22.9% (58/253) of patients reached ACR70 response criteria for ABP 501 and adalimumab at week 24, respectively. The RR (90% CI) for ACR70 at week 24 was 1.130 (0.872, 1.464) and the RD (90% CI) was 3.147% (−3.177%, 9.470%). The percentages of patients who achieved ACR70 were also similar across the ABP 501 and adalimumab groups at all study weeks.
DAS28-CRP
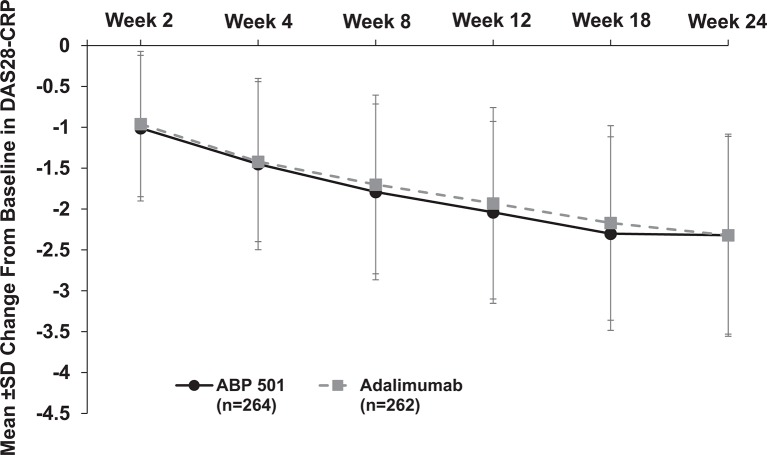
At week 24, the mean change from baseline in DAS28-CRP was −2.32 for both groups, with a difference between treatment groups (two-sided 90% CI) of −0.01 (−0.18, 0.17), further substantiating clinical efficacy equivalence between ABP 501 and adalimumab. Mean change from baseline in DAS28-CRP decreased similarly throughout the study (secondary endpoints) in both groups, indicating similar reduced disease activity (figure 3).
Figure 3.
Mean±SD change from baseline in DAS28-CRP by study week (full analysis set). DAS28-CRP, Disease Activity Score 28-joint count-C reactive protein.
The percentage of patients who achieved DAS28-CRP remission increased over time for both groups from weeks 2 to 18 (range: 6.3%–31.1%, ABP 501; 2.8%–27.1%, adalimumab). At week 24, 30.5% and 35.5% of patients in the ABP 501 and adalimumab groups, respectively, reached DAS28-CRP remission.
The key efficacy data reported here were also analysed for the per-protocol analysis set (patients who completed the treatment period and did not have a protocol violation) as sensitivity analyses of key efficacy endpoints. For these endpoints, the per-protocol analysis set results were similar to that of the full analysis set (see online supplementary figure 1), which further confirms the similarity between ABP 501 and adalimumab.
Safety
Treatment-emergent adverse events
Overall, 52.3% of all patients had ≥1 TEAE during the study and the percentages of patients who reported TEAEs were similar for patients in the ABP 501 and adalimumab groups (50.0% and 54.6%, respectively) (table 2). TEAEs reported by >3% of patients in either group (ABP 501, adalimumab) were nasopharyngitis (6.4%, 7.3%), headache (4.5%, 4.2%), arthralgia (3.0%, 3.4%), cough (2.7%, 3.1%) and upper respiratory tract infection (1.5%, 3.8%). There were no differences between groups that were ≥5% for the percentage of patients who experienced a TEAE by preferred term. Findings from laboratory evaluations and vital sign examinations revealed no clinically significant changes in any outcome.
Table 2.
Overall safety and AEs of interest by treatment (safety population)
| ABP 501 (n=264) |
Adalimumab (n=262) |
|
| Number of patients, n (%) | Number of patients, n (%) | |
| Any TEAE | 132 (50.0) | 143 (54.6) |
| Serious AEs | 10 (3.8) | 13 (5.0) |
| AEs leading to discontinuation of IP | 5 (1.9) | 2 (0.8) |
| AEs leading to study discontinuation | 7 (2.7) | 2 (0.8) |
| AEs of interest | ||
| Any | 80 (30.3) | 94 (35.9) |
| Infections | 61 (23.1) | 68 (26.0) |
| Malignancies | 1 (0.4) | 1 (0.4) |
| Hypersensitivity | 14 (5.3) | 10 (3.8) |
| Haematological reactions | 5 (1.9) | 5 (1.9) |
| Heart failure | 1 (0.4) | 2 (0.8) |
| Liver enzyme elevations | 13 (4.9) | 10 (3.8) |
| Injection-site reactions | 6 (2.3) | 13 (5.0) |
For each category, patients were included only once even if they had multiple events in that category. AEs coded using MedDRA V.17.1.
AE, adverse event; IP, investigational product; TEAE, treatment-emergent adverse event.
Serious adverse events
A total of 23 (4.4%) patients reported having 27 SAEs throughout the study and the percentages of patients were similar between the ABP 501 (n=10; 3.8%) and adalimumab (n=13; 5.0%) groups. The only SAE reported for >1 patient was sepsis (n=2; ABP 501) and both of these events had resolved by study end. Two patients in the ABP 501 group experienced >1 SAE at the same time. One patient had cardiopulmonary failure, pneumonia and sepsis and the other experienced peritoneal abscess, perforated appendicitis and secondary sepsis. No SAEs or treatment-related SAEs occurred in ≥2% of patients for either group by preferred term. No deaths occurred in this study.
Adverse events of interest
Standard searches for AEs of interest identified 80 (30.3%) patients in the ABP 501 group and 94 (35.9%) patients in the adalimumab group who had ≥1 event of interest (table 2). AEs of interest during the study included two malignancies, basal cell carcinoma and squamous cell carcinoma, reported in one subject in the ABP 501 group and squamous cell carcinoma of the skin reported in one subject from the adalimumab group. No cases of active tuberculosis were reported throughout the study. Reported hypersensitivities occurring in >2 patients overall included rash, erythematous rash and allergic dermatitis. Liver enzyme elevations were reported, however, none led to premature study discontinuation and none were associated with increases in bilirubin that would cause concern about drug-induced liver injuries according to Hy’s law.29 Injection-site reactions occurred in 2.3% and 5.0% of patients in the ABP 501 and adalimumab groups, respectively. Most events of interest were grade 1 or 2 in severity. Similar percentages of patients in the ABP 501 (n=3, 1.1%; infection, n=2; hypersensitivity, n=1; heart failure, n=1) and adalimumab (n=4, 1.5%; infections, n=3; heart failure, n=1) groups experienced SAEs of interest.
Immunogenicity
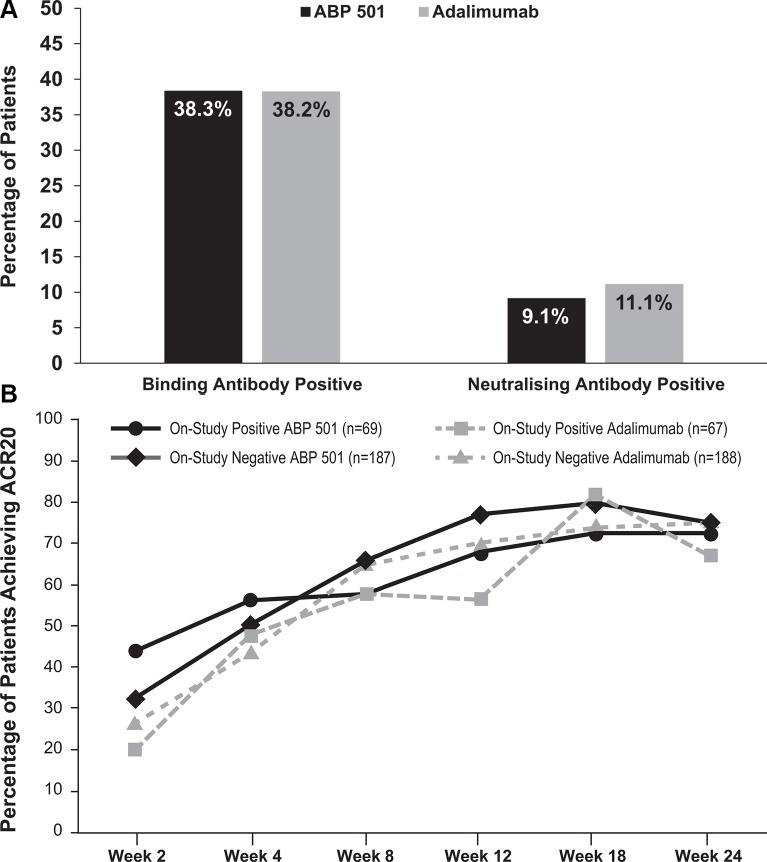
All 526 randomised and treated patients had ≥1 evaluable ADA result and were included in the antibody analysis. For the ABP 501 and adalimumab groups, 5 (1.9%) and 6 (2.3%) patients, respectively, tested positive for pre-existing binding antibodies and no patients tested positive for pre-existing neutralising antibodies. Overall, 201 (38.2%) patients tested positive for binding antibodies at weeks 4, 12, or 26 post-baseline, which was similar to the percentages in each group (ABP 501, n=101, 38.3%; adalimumab, n=100, 38.2%) (figure 4A). A total of 53 (10.1%) patients tested positive for neutralising ADAs at weeks 4, 12, or 26 post-baseline, which was also similar to that for each treatment group (ABP 501, n=24, 9.1%; adalimumab, n=29, 11.1%).
Figure 4.
(A) Immunogenicity at any time point post-baseline throughout the study. (B) ACR20 responders by ADA status throughout the study (full analysis set). ACR20, 20% improvement from baseline in American College of Rheumatology core set measurements.
Descriptive results for ADAs by visit and treatment indicate that the incidence of ADAs were similar in patients across both groups throughout the course of the study (see online supplementary table 4). In addition, the percentage of patients who demonstrated an ACR20 response throughout the study was found to be similar across treatment groups regardless of ADA status (figure 4B).
Pharmacokinetic results
All 526 randomised patients had at least one evaluable result for serum concentration of ABP 501 or adalimumab at any visit and were included in the pharmacokinetic analysis. Pharmacokinetic results revealed that trough serum concentrations were similar between groups across all study weeks, indicating that exposure was similar between treatment groups (see online supplementary table 5). The geometric mean trough serum concentrations at week 24 were 4844.16 ng/mL and 5210.75 ng/mL for ABP 501 and adalimumab, respectively, with similar concentrations across groups at all time points throughout the study (range: ABP 501, 2062.64–4844.16 ng/mL; adalimumab, 1936.11–5210.75 ng/mL).
Discussion
Development of biosimilars is a complex process that requires demonstration of similar efficacy, safety, immunogenicity and pharmacokinetics between the proposed biosimilar and reference product. For regulatory review, demonstration that there are no clinically meaningful differences between the proposed biosimilar and its reference product is necessary. FDA guidance on the development and approval of biosimilars requires that a stepwise, totality-of-evidence-based approach be used to generate data in support of biosimilarity and to evaluate any residual uncertainty.13 Likewise, the EMA requires that biosimilars show similarity to the reference product in pharmacokinetics, pharmacodynamics, clinical efficacy and safety and that a risk management/pharmacovigilance plan is adopted in accordance with EU legislation.12
Analytical comparison has shown that ABP 501 and adalimumab are highly similar molecules with respect to physicochemical properties22 and biological activity.23 Pharmacokinetic equivalence of ABP 501 to adalimumab was also demonstrated in a phase I, single-dose study conducted in healthy adults.24 Results from an additional clinical trial have shown that clinical efficacy and safety profiles for ABP 501 are similar to those of adalimumab in 350 patients with moderate to severe psoriasis (NCT01970488), which adds to the overall demonstration of similarity between the biosimilar and reference product.25
Results from this study indicate that ABP 501 is equivalent in efficacy to the reference product, adalimumab, in patients with RA. Similar efficacy results were observed for ABP 501 and adalimumab for ACR20 (primary endpoint), ACR50 and ACR70 (secondary endpoints). The mean changes from baseline in DAS28-CRP results were also similar between groups at all study time points.
The clinical safety results from this study indicate that ABP 501 and adalimumab have similar safety profiles. No new safety signals were detected in this study compared with other adalimumab clinical trials in patients with RA.2 Hypersensitivity reactions were reported infrequently and occurred at a generally similar frequency in both treatment groups.
Since biosimilars are not identical molecules, the determination of immunogenicity by ADA formation is a major part of the registration programme for approval.2 In the current study, a highly sensitive and drug tolerant electrochemiluminescent assay was used to assess ADA status.27 In pivotal trials of adalimumab in RA, ADA status had been determined by ELISA assays that did not allow for detection of ADAs in the presence of drug and that measured ADA levels that were much lower than those reported in this trial.2 Even though the levels of ADAs detected were higher due to a more sensitive assay than those reported from historical pivotal trials of adalimumab, the percentages of patients who tested positive for binding and neutralising antibodies in this trial were similar between the ABP 501 and adalimumab groups throughout the study. In a phase I study in which the relationship between pharmacokinetics and ADA status was assessed in healthy subjects receiving ABP 501 or adalimumab, results showed that overall exposure (area under the curve (AUC)) was approximately 20%–30% lower in subjects who were ADA positive versus those who were ADA negative.24 Similarly, the elimination half lives (t1/2) for ADA positive subjects were shorter (6–7 days) than patients who were ADA negative (12–15 days). This inverse correlation between ADA levels and serum concentrations of TNFα inhibitors has been previously shown in many studies and has been associated with decreased clinical efficacy.30 Patients who were symptomatic seronegative were not included in this study as they may be misdiagnosed as having RA and would introduce more heterogeneity within the population. Additionally, as biosimilarity is proven via a totality-of-evidence-based approach, the demonstration of similarity between ABP 501 and adalimumab from analytical, functional, pharmacokinetic and clinical perspectives leaves no residual uncertainty that ABP 501 will behave differently in patients who were seronegative. In the present trial, the clinical responses for both ABP 501 and adalimumab were comparable to previously reported results suggesting that the majority of the antibodies detected in these patients may have little or no clinical relevance.2 Further, the percentage of patients who achieved an ACR20 response by ADA status indicates no change in efficacy throughout the study for either treatment group, regardless of ADA status.
Limitations of this study include the 6-month trial design; however, an open-label extension of this study, up to 72 weeks (NCT02114931), is on-going and will provide additional long-term safety and efficacy data for ABP 501 in patients with moderate to severe RA. Moreover, 52-week results from the previously mentioned trial in patients with psoriasis will also provide insights into the long-term immunogenicity and safety profile of ABP 501. Regulatory guidelines for biosimilars do not require monitoring of radiographic progression. While this may be an area of interest, detecting any clinically meaningful differences in radiographic progression would be challenging via studies designed to compare two active treatment groups.
Data from this study indicate that the clinical efficacy, safety and immunogenicity of ABP 501 are similar to that of adalimumab in patients with moderate to severe RA. Additionally, analytical, biofunctional and pharmacokinetic properties of ABP 501 have previously been shown to be highly similar to those of adalimumab.22–24 Taken together, these data contribute to the totality-of-evidence-based requirements to demonstrate that ABP 501 is similar to adalimumab. The FDA has, thus, approved ABP 501 for use as a biosimilar to adalimumab,20 making it a valuable new therapeutic option for the treatment of moderate to severe RA.
annrheumdis-2016-210459supp003.doc (218.5KB, doc)
Acknowledgments
The authors would like to thank all investigators and patients who participated in the study. Medical writing and editorial assistance, funded by Amgen, were provided by Debika Chatterjea, PhD, of MedVal Scientific Information Services, LLC (Princeton, New Jersey) under the guidance of Monica Ramchandani, PhD, Amgen, Inc.
Footnotes
Contributors: Conception and design: PK and NZ. Analysis and interpretation of data: SC, MCG, EC, FP-R, AM, KP, JLP, WR, PH, NZ, WS and PK. Drafting the article and revising it critically for content: All authors. All authors reviewed and revised the manuscript and approved the final version to be published. All authors were involved in the decision to submit the manuscript for publication, and had the right to accept or reject comments or suggestions.
Funding: Amgen Inc funded this study and participated in the design and conduct of the study; collection, management, analysis and interpretation of data; and preparation, review and approval of the manuscript.
Competing interests: SC reports grants and personal fees from Amgen, during the conduct of the study; grants and personal fees from Abbvie, Boehringer Ingelheim, Pfizer and Sandoz, outside the submitted work. MCG reports grants and personal fees from Amgen, AbbVie and Pfizer, and personal fees from Sandoz, FKb, Samsung BioEpis, Merck, Celltrion and Boehringer, during the conduct of the study. EC reports grants and personal fees from Amgen, during the conduct of the study; personal fees from Boehringer Ingelheim, Chelsea Therapeutics, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Hospita, ISIS, Jazz Pharmaceuticals, Janssen, MedImmune, Merrimack Pharmaceutical, Merck, Napp, Novartis, Regeneron, Sanofi-Aventis, Schering Plough, Synovate and Tonix and grants and personal fees from Chugai Pharma, Ferring Pharmaceuticals, Novimmune, Pfizer, Pierre Fabre, Roche and UCB, outside the submitted work. FP-R reports grants from Amgen, during the conduct of the study; and personal fees from Amgen, outside the submitted work. AM reports grants and personal fees from Amgen, during the conduct of the study; grants and personal fees from AbbVie, Pfizer and Bristol-Myers Squibb, grants from Takeda, Janssen, Gilead and UCB and personal fees from GlaxoSmithKline, outside the submitted work. JLP reports lecturing and consultancy fees from Roche, Novartis, Pfizer and Lilly, outside the submitted work. NZ and PK are employees of Amgen Inc.
Patient consent: Written informed consent was obtained from each patient prior to study enrolment.
Ethics approval: This study was conducted in accordance with the current International Conference on Harmonization good clinical practice guidelines and the Declaration of Helsinki. The study protocol was approved by the institutional review board or independent ethics committee at each participating site and adhered to all local regulatory requirements including data protection requirements.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All data from this study, published and unpublished, were made available to all authors. Further inquiry regarding availability of the data can be addressed to PK of Amgen, Inc.
Correction notice: This paper has been amended since it was published Online First. Owing to a scripting error, some of the publisher names in the references were replaced with ’BMJ Publishing Group'. This only affected the full text version, not the PDF. We have since corrected these errors and the correct publishers have been inserted into the references.
References
- 1.McInnes IB, Schett G. The pathogenesis of Rheumatoid Arthritis. N Engl J Med Overseas Ed 2011;365:2205–19. 10.1056/NEJMra1004965 [DOI] [PubMed] [Google Scholar]
- 2.Humira (adalimumab) injection [prescribing information]. North Chicago, IL: AbbVie Inc, 2016. [Google Scholar]
- 3.European Medicines Agency Committee for Medicinal Products for Human Use. Summary of opinion (post authorisation). Humira adalimumab http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion/human/000481/WC500188795.pdf (accessed 6 Jan 2016). [Google Scholar]
- 4.US Food and Drug Administration. FDA approves first biosimilar product Zarxio [press release]. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm436648.htm (accessed 10 Apr 2015).
- 5.Schabert VF, Waston C, Joseph G, et al. Costs of tumor necrosis factor blockers per treated rheumatoid arthritis patient using real-world drug data in a us managed care population [abstract]. Arthritis Rheum 2012;64(suppl 10):S168–S169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atzinger C, Guo JJ. Php38 utilization, price and spending of anti-tumor necrosis factor biologics in the United States medicaid program. Value in Health 2011;14:A18 10.1016/j.jval.2011.02.109 [DOI] [Google Scholar]
- 7.Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 2006;54:26–37. 10.1002/art.21519 [DOI] [PubMed] [Google Scholar]
- 8.Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum 2004;50:1400–11. 10.1002/art.20217 [DOI] [PubMed] [Google Scholar]
- 9.European Medicines Agency. Guideline on similar biological medicinal products. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/10/WC500176768.pdf (accessed 3 Apr 2015). [DOI] [PMC free article] [PubMed]
- 10.US Department of Health and Human Services. Biosimilars: questions and answers regarding implementation of the Biologics Price Competition and Innovation Act of 2009. Guidance for industry. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM444661.pdf (accessed 22 May 2017).
- 11.US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research. Quality considerations in demonstrating biosimilarity of a therapeutic protein product to a reference product. Guidance for industry. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm291134.pdf (accessed 22 May 2017).
- 12.European Medicines Agency Committee for Medicinal Products for Human Use. Guideline on similar biological medicinal products containing monoclonal antibodies - non-clinical and clinical issues http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500128686.pdf (accessed 6 Jan 2016).
- 13.US Department of Health and Human Services. Scientific considerations in demonstrating biosimilarity to a reference product. Guidance for industry http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM291128.pdf (accessed 12 May 2015).
- 14.European Medicines Agency Committee for Medicinal Products for Human Use. Assessment report. Benepali. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/004007/WC500200380.pdf (accessed 22 May 2017).
- 15.European Medicines Agency Committee for Medicinal Products for Human Use. Assessment report. Inflectra. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002778/WC500151490.pdf (accessed 22 May 2017).
- 16.European Medicines Agency Committee for Medicinal Products for Human Use. CHMP assessment report. Flixabi. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/004020/WC500208358.pdf (accessed 22 May 2017).
- 17.US Food and Drug Administration. FDA approves Inflectra, a biosimilar to Remicade. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494227.htm (accessed 22 May 2017).
- 18.INFLECTRA (infliximab-dyyb). Lake Forest, IL: Hospira, 2016. [Google Scholar]
- 19.ERELZI (etanercept-szzs) injection, for subcutaneous use [prescribing information]. Stein, Switzerland: Novartis Pharma AG, 2016. [Google Scholar]
- 20.AMJEVITA (adalimumab-atto) injection for subcutaneous use [prescribing information]. Thousand Oaks, CA: Amgen Inc, 2016. [Google Scholar]
- 21.US Food and Drug Administration. FDA approves Amjevita, a biosimilar to Humira [press release]. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522243.htm (accessed 22 May 2017).
- 22.Liu J, Eris T, Li C, et al. Assessing analytical similarity of proposed amgen biosimilar ABP 501 to adalimumab. BioDrugs 2016;30:321–38. 10.1007/s40259-016-0184-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Velayudhan J, Chen YF, Rohrbach A, et al. Demonstration of functional similarity of proposed biosimilar ABP 501 to Adalimumab. BioDrugs 2016;30:339–51. 10.1007/s40259-016-0185-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaur P, Chow V, Zhang N, et al. A randomised, single-blind, single-dose, three-arm, parallel-group study in healthy subjects to demonstrate pharmacokinetic equivalence of ABP 501 and adalimumab. Ann Rheum Dis 2017;76:526–33. 10.1136/annrheumdis-2015-208914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papp K, Bachelez H, Costanzo A, et al. Clinical similarity of biosimilar ABP 501 to adalimumab in the treatment of patients with moderate to severe plaque psoriasis: a randomized, double-blind, multicenter, phase III study. J Am Acad Dermatol 2017;76:1093–102. 10.1016/j.jaad.2016.12.014 [DOI] [PubMed] [Google Scholar]
- 26.Felson DT, Anderson JJ, Boers M, et al. American College of Rheumatology. preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995;38:727–35. 10.1002/art.1780380602 [DOI] [PubMed] [Google Scholar]
- 27.Colbert A, Umble-Romero A, Prokop S, et al. Bioanalytical strategy used in development of pharmacokinetic (PK) methods that support biosimilar programs. MAbs 2014;6:1178–89. 10.4161/mabs.32114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.US Food and Drug Administration. Guidance for industry: non-inferiority clinical trials. http://www.fda.gov/downloads/Drugs/Guidances/UCM202140.pdf (accessed 10 Apr 2015).
- 29.US Food and Drug Administration. Guidance for industry: drug-induced livery injury: premarketing clinical evaluation. Silver Spring, MD: US Food and Drug Administration, 2009. (accessed 22 May 2017). [Google Scholar]
- 30.Vincent FB, Morand EF, Murphy K, et al. Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspective. Ann Rheum Dis 2013;72:165–78. 10.1136/annrheumdis-2012-202545 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2016-210459supp001.docx (112.9KB, docx)
annrheumdis-2016-210459supp002.pdf (435.4KB, pdf)
annrheumdis-2016-210459supp003.doc (218.5KB, doc)