Abstract
Objectives
Vaccination of patients with rheumatic disease has been reported to result in lower antibody titres than in healthy individuals. However, studies primarily include patients on immunosuppressive therapy. Here, we investigated the immune response of treatment-naïve patients diagnosed with primary Sjögren’s syndrome (pSS) to an H1N1 influenza vaccine.
Methods
Patients with Sjögren’s syndrome without immunomodulatory treatment and age-matched and gender-matched healthy controls were immunised with an H1N1 influenza vaccine and monitored for serological and cellular immune responses. Clinical symptoms were monitored with a standardised form. IgG class switch and plasma cell differentiation were induced in vitro in purified naïve B cells of untreated and hydroxychloroquine-treated patients and healthy controls. Gene expression was assessed by NanoString technology.
Results
Surprisingly, treatment-naïve patients with Sjögren’s syndrome developed higher H1N1 IgG titres of greater avidity than healthy controls on vaccination. Notably, off-target B cells were also triggered resulting in increased anti-EBV and autoantibody titres. Endosomal toll-like receptor activation of naïve B cells in vitro revealed a greater propensity of patient-derived cells to differentiate into plasmablasts and higher production of class switched IgG. The amplified plasma cell differentiation and class switch could be induced in cells from healthy donors by preincubation with type 1 interferon, but was abolished in hydroxychloroquine-treated patients and after in vitro exposure of naïve B cells to chloroquine.
Conclusions
This comprehensive analysis of the immune response in autoimmune patients to exogenous stimulation identifies a mechanistic basis for the B cell hyperactivity in Sjögren’s syndrome, and suggests that caution is warranted when considering vaccination in non-treated autoimmune patients.
Keywords: Sjögren’s syndrome, B-cells, autoantibodies, vaccination, INFα
Introduction
Infectious diseases are a major cause of morbidity and mortality in patients with systemic rheumatic diseases.1 2 Vaccination is one of the most effective measures to prevent infections; however, safety and efficacy need to be considered in the context of a dysregulated immune system. Studies of individuals with autoimmune rheumatic diseases indicate that patients develop reduced protective antibody titres on immunisation.3–5 However, many patients are treated with immunosuppressive drugs, which likely affect their response to vaccination. In addition, most reports focus on assessing vaccine efficacy by measuring seroconversion rates and production of neutralising antibodies, which leaves other potentially important aspects of the immune response unexplored. The response of an autoimmune-biased immune system to stimuli such as vaccination or infections therefore remains unclear.
Due to early reports of high mortality among younger individuals during the H1N1 influenza pandemic,6 the Swedish government offered protective immunisations to all citizens. We used this opportunity to analyse the immune response to the vaccine of patients with primary Sjögren’s syndrome (pSS) without immunomodulatory treatment.
Here, we report that untreated patients with pSS respond to vaccination with enhanced antibody responses and, importantly, rising autoantibody titres. We find that naïve B cells from untreated patients readily differentiate into class-switched antibody-producing cells on endosomal toll-like receptor (TLR) stimulation. Furthermore, this hyper-reactive state is linked to proinflammatory cytokine exposure and upregulation of several intracellular signalling pathways including those downstream of TLR7 and TLR9 in patients with primary Sjögren’s syndrome.
Methods
Detailed methods and study participant characteristics are provided in the online supplementary materials.
annrheumdis-2016-210509supp001.docx (51.1KB, docx)
Participants and vaccination procedure
Female patients with pSS fulfilling the American-European consensus critera7 and positive for anti-Ro/SSA and/or anti-La/SSB autoantibodies (n=14) and matched healthy controls (n=18) (supplementary table S1) were vaccinated twice with the squalene-adjuvanted inactivated split-virion H1N1 vaccine Pandemrix (GlaxoSmithKline, Brentford, UK). Blood sampling and collection of clinical parameters was performed prior to, and 1 and 3 weeks after each vaccination.
annrheumdis-2016-210509supp002.docx (46.6KB, docx)
In vitro class switch experiments were performed using blood samples from 14 untreated and 11 antimalarial drug-treated patients with Sjögren’s syndrome and 16 matched healthy controls (supplementary table S2). Cytokine stimulation and in vitro chloroquine treatment experiments were performed using cells from buffy coats of healthy blood donors.
The local Ethics Committee Stockholm North approved the study and all participants gave written informed consent.
Statistical analysis
Student’s t-test (normal distribution) or Mann-Whitney U-test (non-normal distribution) was used when comparing two groups, and Wilcoxon paired test when analysing paired data, all using Prism V.7 (GraphPad). Area under the curve (AUC) was calculated and analysed using R. Longitudinal variation of continuous parameters was analysed by quantile regression using Stata (StataCorp, College Station, Texas, USA).
Results
Vaccination induces higher specific and non-specific antibody responses in untreated patients with pSS
To assess the impact of vaccination in autoimmune individuals without interference from immune-targeting therapies, we monitored untreated patients diagnosed with pSS during vaccination with an H1N1 influenza vaccine (Pandemrix) (figure 1A, supplementary table S1).8–10 11 In contrast to previous reports,5 12–14 we observed markedly higher levels of H1N1 influenza-specific IgG antibodies in patients, mainly of the IgG1 subclass, compared with controls. Furthermore, H1N1 antibodies developed by the patients had higher avidity than those of controls (figure 1B-D, supplementary figure S1A). H1N1-specific IgM and IgA titres did not differ between the two groups, and haemagglutinin antibody titres, used as a measure of vaccine-induced protection and previously reported to be lower in patients with rheumatic disease,15 were comparable between the groups (supplementary figure S1B, C).
Figure 1.
H1N1 vaccination induces higher specific IgG response and polyclonal activation of B cells in Sjögren’s syndrome. (A) Untreated patients with primary Sjögren’s syndrome (pSS, n=14) and healthy controls (HC, n=18) were subjected to H1N1 vaccination and boost, and followed by blood sampling five times during 42 days. (B) H1N1-specific IgG levels in pSS and HC measured by ELISA. (C) IgG1 subclass H1N1-specific antibodies in pSS and HC measured by ELISA. (D) Avidity of anti-H1N1-specific IgG in pSS and HC, measured by an ELISA-based 8 M urea competition assay. (E) Anti-EBV-VCA IgG levels in pSS and HC measured by ELISA. (F) Ro52/SSA, Ro60/SSA and La/SSB autoantibody levels in pSS measured by ELISA. (G) Live CD14-CD3-CD19dimCD138+CD27+ plasmablasts in pSS and HC assessed by flow cytometry. (H) IgG producing cells detected by ELISPOT. Representative wells from day 42 are shown in the right panel. Numbers indicate spots/106 peripheral blood mononuclear cell (PBMC). Data are presented as mean±SD. AUC, area under the curve; QR, quantile regression. *p<0.05, **p<0.01 (Mann-Whitney U test, Student’s t-test, Wilcoxon signed-rank test).
annrheumdis-2016-210509supp008.pdf (2.3MB, pdf)
To further explore the impact of vaccine-induced immune activation on humoral responses in non-treated patients with pSS, we analysed the presence of antibodies to other influenza A and B strains. Interestingly, we observed that these antibody titres increased more in patients than in controls on A/H1N1 vaccination (supplementary figure S1D). While this may be due to similarities between the H1N1 vaccine influenza strain and previously encountered viruses, it is however also possible that vaccination reactivated the patients’ memory B cells in an unspecific manner. We therefore investigated antibody levels to the non-influenza pathogen Epstein-Barr virus (EBV), to which immunity is common and which has been implicated in pSS pathogenesis. Notably, antibody titres to EBV increased in patients following vaccination, but not in controls (figure 1E). Next, we analysed whether vaccination had an impact also on autoreactive memory B cells and found that autoantibody titres to the Sjögren’s syndrome-associated autoantigens Ro/SSA (Ro52 and Ro60) and La/SSB16 17 indeed increased significantly during the course of vaccination (figure 1F). No new autoantibody specificities were noted (data not shown), and no expansion or dominance of a specific B cell clonotype was observed in patients compared with controls, as analysed by VH-spectratyping (supplementary figure S2). Consistent with the elevated specific IgG titres and polyclonal B cell activation, higher frequencies of circulating CD19dimCD138+ plasmablasts and IgG-secreting B cells were detected in patients following vaccination (figure 1G and H supplementary figure S3).
Previous studies have indicated that vaccination does not exacerbate disease in patients with autoimmune rheumatic diseases.5 14 Considering a potentially stronger immune reaction in non-treated patients, we monitored common clinical signs of disease activity, such as fever, fatigue, myalgia and arthralgia, by a standardised form filled out by the participants at each visit throughout the study. These parameters are now part of the Sjögren’s syndrome disease activity scores European League Against Rheumatism Sjögren’s Syndrome Patient Reported Index (ESSPRI) and European League Against Rheumatism Sjögren’s Syndrome Disease Activity Index (ESSDAI),18 19 which were not yet established at the time of the study, but confirms their relevance. Notably, fever, fatigue, myalgia and arthralgia also constitute common adverse reactions to vaccination, and the frequencies of patients and controls reporting such symptoms followed similar trends during the vaccination period (figure 2A-D supplementary figure S4).
Figure 2.
Disease activity on vaccination. Information on experienced symptoms of Sjögren’s syndrome during the preceding week was collected. Significant differences were observed after the second vaccination. (A) Fever. (B) Fatigue. (C) Myalgia. (D) Arthralgia. Data are presented as percentage.
In all, our data demonstrate that immunisation with an H1N1 influenza vaccine not only induces higher specific antibody responses in untreated autoimmune patients with pSS, but also drives polyclonal B cell activation, including that of autoreactive cells, leading to an increase in autoantibody titres.
Differential peripheral responses to vaccination between patients with pSS and controls
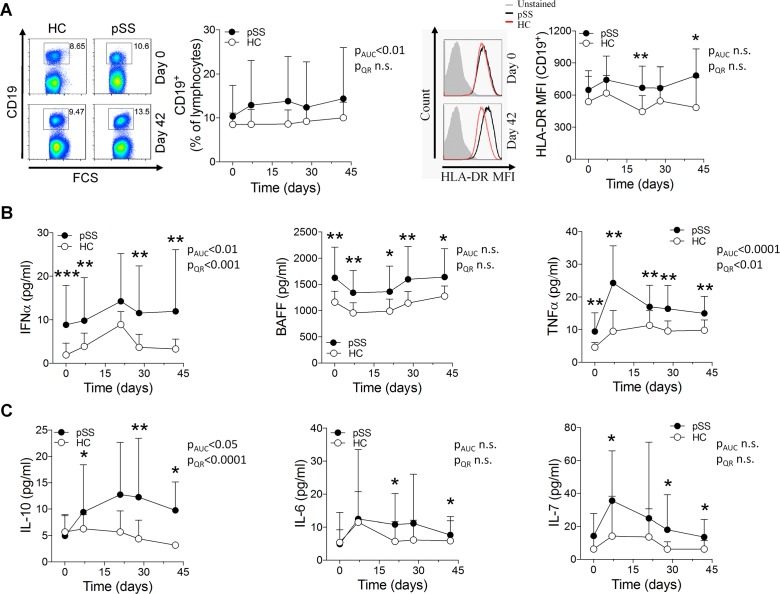
To acquire a more comprehensive understanding of the response triggered by vaccination in untreated autoimmune patients beyond antibody production, we analysed circulating B cell and T cell populations as well as serum levels of cytokines. There were no major differences in lymphocyte populations between patients and controls before vaccination, except for a lower frequency of circulating memory B cells and an increased proportion of naïve B cells in patients, confirming previous studies.20 Vaccination did not induce further differences between patients and controls in either B cell counts or frequencies of B cell subsets. However, B cells from patients expressed significantly more HLA-DR as a sign of activation 3 weeks after both the vaccination and the boost (figure 3A supplementary figure 5). We did not detect any notable difference in either T cell numbers or activation status between groups apart from a decreased CD62L expression on CD4+ T cells after the initial vaccination (supplementary figure S6A-C). None of the hallmark T-helper lineage cytokines interferon-γ (IFN-γ), interleukin (IL)-4 or IL-17 were differently expressed (supplementary figure S6D).
Figure 3.
B cell activation and induction of proinflammatory cytokines on vaccination in patients with pSS. (A) Freshly isolated PBMC from patients with pSS and healthy controls were analysed by flow cytometry at each blood sampling time point during the vaccination protocol. Representative dot or histogram plots (left) and quantification graphs (right) are shown for each analysis. Numbers adjacent to outlined gates indicate percentage positive cells. (B, C) Cytokine concentrations in serum samples analysed by a Luminex assay (tumour necrosis factor-α (TNF-α), interleukin (IL)-10, IL-6, IL-7) or ELISA (interferon-α (IFN-α), B cell activating factor (BAFF)). Data are presented as mean±SD. AUC, area under the curve; QR, quantile regression. *p<0.05, **p<0.01, ***p<0.001 (Mann-Whitney U test).
Analysis of cytokine expression showed high basal serum levels of the proinflammatory cytokines IFN-α, BAFF and TNF-α in untreated patients with pSS (figure 3B), as previously reported.9 21–23 These levels remained significantly elevated in patients throughout the study, or were even further induced. By contrast, the B cell activating cytokines IL-10, IL-6 and IL-7 were detected at comparable serum levels in patients and controls before vaccination, but were significantly more induced in patients on immunisation (figure 3C), suggesting that they might be responsible, at least in part, for the increased antibody production and B cell activation observed in patients after vaccination.24–27
Naïve B cells from patients with pSS more readily differentiate into CD19dimCD138+ plasmablasts after endosomal TLR stimulation
In view of the increase in IgG titres following vaccination, we investigated the possibility that B cells from untreated patients with pSS are more prone to differentiate into antibody-producing cells in response to immune triggering. Therefore, we analysed plasma cell differentiation and immunoglobulin (Ig) class switch in vitro of naïve CD19+IgD+ B cells isolated from untreated patients with pSS and healthy donors (supplementary table S2). Anti-CD40, BAFF as well as TLR7 (imiquimod) and TLR9 (CpG) agonists have previously been described to drive these processes28–35 and were thus selected for in vitro differentiation assays. After an initial screening of IL-4, IL-10 and IL-21 (supplementary figure S7A), supplementation with IL-10 was chosen for all cultures as this cytokine promoted the most efficient plasma cell differentiation and class switch.24 Interestingly, we observed that a higher number of CD19dimCD138+ plasmablasts developed in cultures of pSS patient-derived cells compared with controls on TLR7 and TLR9 stimulation, and higher levels of IgM and IgG were detected in supernatants (figure 4A, supplementary figure S7B). By contrast, anti-CD40 and BAFF stimulation did not differentially affect Ig class switch or plasma cell differentiation between patients and controls.
Figure 4.
Toll-like receptor (TLR)-induced plasma cell differentiation and class switch are enhanced in naïve B cells from patients with primary Sjögren’s syndrome (pSS). (A) fluorescence-activated cell sorter (FACS) sorted naïve CD19+IgD+ B cells from freshly isolated peripheral blood mononuclear cell of untreated patients with pSS (n=14) and healthy controls (HC) (n=16) were cultured for 8 days under plasmablast and class switch promoting conditions with anti-CD40, BAFF, CpG or imiquimod (Imiq). At day 8, the frequency of CD19dimCD138+ plasmablasts and IgM and IgG concentrations in supernatants were assessed by flow cytometry and ELISA. Data represent pooled data from 10 independent experiments. (B) mRNA was extracted from the naïve B cells at day 0 and gene expression analysed by NanoString. Heat map illustrates expression in patients with pSS and HC organised by Ingenuity pathways and Gene ontology analysis using average Z scores. The 14 most differentially regulated gene clusters are displayed in order of mean fold change of the top 10 upregulated genes in each cluster. (C) Network analysis of differentially expressed genes show that pSS-related genes cluster into the pathways type I interferon (IFN) pathway, antigen presentation and BCR signalling and B cell development. Symbols according to Ingenuity. Brighter red indicates higher upregulation in pSS compared with HC. Data are presented as mean±SD. *p<0.05, **p<0.01, ***p<0.001 (Mann-Whitney U test). IL, interleukin.
annrheumdis-2016-210509supp003.docx (58KB, docx)
To understand why endosomal TLR ligands induced plasma cell differentiation and class switch more effectively in naïve B cells isolated from untreated patients with pSS, we investigated gene expression profiles before and after in vitro stimulation, using NanoString nCounter multiplex expression profiling (supplementary figure S8). For the analysis, genes were grouped into functional clusters based on GeneOntology and Ingenuity gene sets (supplementary table S3). Gene expression profiling revealed prominent differences between the naïve, unstimulated B cells derived from patients and controls: immune genes belonging to a broad spectrum of clusters, including the type I IFN pathway, antigen presentation, B cell development and endosomal TLR signalling, were markedly upregulated in B cells from patients (figure 4B,C, supplementary figure S9). After in vitro cell stimulation, the initial gene expression pattern differences diminished, except for gene sets pertaining to plasma cells, reflecting the enhanced differentiation process (supplementary figure S10, table S4).
annrheumdis-2016-210509supp004.xlsx (43.5KB, xlsx)
Taken together, our findings show that naïve B cells from patients with pSS are in a state of hyper-responsiveness, characterised by the overexpression of a variety of immune-related genes, including those downstream of endosomal TLR signalling, and an enhanced capacity to undergo class switch and plasma cell differentiation when triggered by endosomal TLR ligands.
IFN-α sensitises B cells and promotes CD19dimCD138+ plasmablast differentiation on TLR stimulation
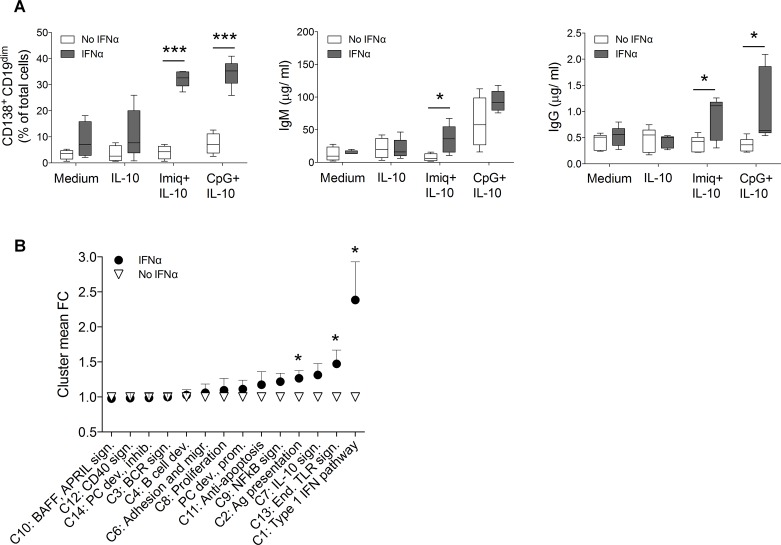
We next set out to understand the mechanisms underlying the hyper-responsiveness of B cells from untreated patients with pSS. Based on our observations that these patients had elevated serum levels of IFN-α (figure 3B) and that genes involved in downstream type I IFN pathways were upregulated in cells isolated from untreated patients (figure 4B), combined with the known role of IFN-α in promoting class switch,36 37 we hypothesised that IFN-α might be responsible for the hyper-reactive state of B cells in untreated patients with pSS. Therefore, to directly assess whether IFN-α could sensitise B cells to subsequent endosomal TLR stimulation, we incubated naïve B cells isolated from healthy donors with IFN-α before culturing them under plasmablast-differentiating conditions. Strikingly, this was enough to recapitulate the enhanced differentiation and class-switch capacity that we had previously observed in B cells isolated from untreated patients with pSS (figure 5A). Gene expression analysis of cells primed with IFN-α revealed upregulation of genes related to type I IFN pathways and endosomal TLR signalling (figure 5B, supplementary table S4), which we had also found to be upregulated in cells from untreated patients with pSS.
Figure 5.
Interferon-α (IFN-α) preactivation recapitulates a Sjögren-like B cell phenotype. (A) fluorescence-activated cell sorter (FACS) sorted CD19+IgD+ cells from healthy donors (n=5) were cultured with IFN-α for 18 hours prior to stimulation with CpG+interleukin (IL)-10, imiquimod (Imiq)+IL-10, IL-10 alone or medium. Frequencies of CD19dimCD138+ plasmablasts, and total IgM and IgG concentrations in supernatants were analysed at day 8. Data represent pooled data from 3 independent experiments. (B) Gene expression of cells was measured at day 0 and 8 by NanoString. Graph depicts values of mean fold change of clusters 1–14 in cells cultured for 8 days and normalised to expression day 0. Data are presented as mean±SD. *p<0.05, **p<0.01, ***p<0.001 (Mann-Whitney U test).
Hydroxychloroquine treatment abolishes B cell hyper-responsiveness to TLR stimulation in patients with pSS
Initially discovered as an antimalarial drug, hydroxycholoroquine (HCQ) is commonly used for treatment of pSS and systemic lupus erythematosus (SLE).38 39 HCQ acts by inhibiting endosomal TLR signalling, and was recently reported to decrease both IFN-α production in SLE40 41 and the IFN signature in patients with pSS.42 Since priming B cells with IFN-α resulted in enhanced B cell differentiation and class switch after TLR stimulation in vitro, we investigated whether HCQ treatment would decrease the B cell hyper-responsiveness in patients with pSS. So far, no direct effect of chloroquine on B cells has been established. We therefore first analysed the effect of chloroquine on B cell class switch. CD19+IgD+ B cells from healthy donors were treated with chloroquine in vitro, simultaneously as class switch was induced by TLR7 and TLR9 stimulation. Addition of chloroquine at pharmacologically relevant concentrations led to significantly less differentiation of CD19dimCD138+ cells, class switch and IgM and IgG production (figure 6A). Strikingly, we further found that naïve B cells isolated from HCQ-treated patients with pSS were significantly less prone to differentiate into CD19dimCD138+ plasmablasts and undergo class switch on TLR9 stimulation (CpG) than cells of untreated patients (figure 6B). A similar trend was observed for TLR7-stimulated cells. Analysis of gene expression revealed that HCQ treatment of patients with pSS abolished the upregulation of immune genes otherwise found in B cells from untreated patients (figure 6C,D, supplementary figure S11, table S4). This suggests that HCQ treatment may act directly on naïve B cells, and reverses the enhanced capacity of B cells from patients with pSS to differentiate into plasma cells on TLR stimulation in vitro by preventing these cells to become hyper-responsive in the first place.
Figure 6.
The B cell hyper-reactivity in patients with Sjögren’s syndrome is abrogated by hydroxychloroquine (HCQ) treatment. (A) Class switch was induced by imiquimod (Imiq)+interleukin (IL)-10 or CpG+IL-10 in freshly prepared CD19+IgD+ B cells from buffy coats (n=5) with or without chloroquine in physiological concentrations (1 μg/mL). At day 8, cells were analysed for CD138+CD19dim expression by flow cytometry, and IgM and IgG concentrations were determined in supernatants. Chloroquine significantly reduced class switch in both Imiq-treated and CpG-treated wells, without affecting cells in IL-10 and untreated wells. Data represent pooled data from 3 independent experiments. (B) FACS sorted CD19+IgD+ B cells from untreated (n=14) and HCQ or chloroquine treated (n=11) patients with pSS and healthy controls (HC) (n=16) were subjected to class switch induction in vitro by Imiq+IL-10 or CpG+IL-10. Data represent pooled data from 18 independent experiments. (C) Heat map of gene expression comparing naïve IgD+ B cells from untreated and HCQ-treated patient with pSS and HC. (D) Mean fold change of clusters 1–14 in cells cultured for 8 days compared with day 0. Gene expression was measured by NanoString and normalised to expression day 0. Data are presented as mean±SD. *p<0.05, **p<0.01, ***p<0.001 (Mann-Whitney U test).
Altogether, our findings demonstrate that B cells from healthy donors can become hyper-responsive to TLR stimulation on exposure to IFN-α and that, conversely, the increased reactive state of B cells from patients with pSS can be reversed by HCQ treatment.
Discussion
Assessing the efficacy of vaccines in patients with autoimmune rheumatic diseases is crucial to provide adequate recommendations for clinical practice. However, these types of studies may miss potential aspects of how a dysregulated immune system reacts to an immune trigger such as a vaccine, especially since they often include patients treated with immunomodulatory drugs. Here, we studied the immune response of treatment-naïve autoimmune patients diagnosed with pSS to an H1N1 influenza vaccine. In contrast to previous studies reporting less vigorous immune responses to vaccination in patients with autoimmune rheumatic disease,5 12 13 we found that untreated patients with pSS developed higher levels of vaccine-specific IgG antibodies than matched controls. Impaired responses to vaccination in autoimmune patients may therefore relate rather to ongoing immunosuppressive therapy than to the immune system being inherently less able to mount protective antibody responses.
Interestingly, we observed that untreated patients with pSS responded to immunisation with higher vaccine-specific antibody titres than matched controls, and with a general increase in non-specific antibody and autoantibody levels, and higher numbers of circulating CD19dimCD138+ plasmablasts. We further found that B cells isolated from untreated patients were more prone to differentiate into class-switched antibody-producing plasma cells than cells from controls when stimulated in vitro with TLR7 and TLR9 agonists, and that they overexpressed a broad panel of immune-related genes compared with controls already prior to stimulation including those in the signalling pathways downstream of TLR7 and TLR9. These findings suggest that B cells from untreated patients with pSS are in a state of hyper-responsiveness, in particular to endosomal TLR stimulation. Interestingly, a recent study showed that TLR7 was required for optimal antibody production after immunisation with the 2009 pandemic split vaccine (Sanofi Pasteur, Swiftwater, Pennsylvania, USA) in mice,43 and that this was likely due to TLR7 recognition of viral RNA present in the split vaccine. It is therefore possible that RNA present in the split-virus Pandemrix vaccine used in our study may contribute to the increased plasma cell differentiation and antibody production detected in patients by stimulating B cell endosomal TLRs. We also observed an increased production of the B cell-promoting cytokine IL-10 following immunisation in patients with pSS compared with controls, suggesting that additional factors besides TLR stimulation of B cells may contribute to enhanced humoral responses in patients with pSS. Altogether, in view of our findings showing that B cells from untreated patients with pSS are especially hyper-responsive to endosomal TLR triggers, caution may be warranted when immunising these patients with vaccines containing TLR agonists—whether added as adjuvants or naturally present in whole or split-virus preparations. Of note, the same patients would presumably respond to vaccination and to natural infections with a broad increase in specific and non-specific antibody responses. An evolutionary advantage of the capacity to respond with high levels of specific antibodies may be the ability to survive infections, and could explain the persistence of autoimmune-associated alleles within our gene pool. The delicate balance is illustrated by the fact that despite the markedly increased B cell responses observed in patients, we noted no significant differences between patients and controls with regard to the recorded clinical parameters fever, fatigue, myalgia and arthralgia. These manifestations represent common symptoms of both Sjögren’s syndrome and adverse reactions to vaccination,8 10 44 and similar frequencies of affected individuals was observed after vaccination.
Further analysis of the mechanism underlying B cell hyper-responsiveness in untreated patients with pSS revealed a major role for type I IFN. Indeed, the phenotype could be recapitulated by priming B cells from healthy donors with IFN-α and was abolished by HCQ treatment in patients with pSS. HCQ has been reported to inhibit IFN-α production by plasmacytoid dendritic cells in SLE,40 as well as decrease the IFN signature in patients with pSS,42 and may therefore exert its therapeutic effect in pSS and SLE at least partly by suppressing TLR-induced IFN-α production and IFN-α-induced B-cell hyper-responsiveness. Such a mechanism may underlie the decrease in both IgM and IgG titres that were previously reported in patients with pSS receiving HCQ treatment.38 Clinical trials of HCQ in pSS on the other hand have not met primary end points.45 46 However, both inclusion criteria and primary outcomes analysed differed from the present study and they are therefore not directly comparable.
In all, our findings reveal that untreated patients with pSS respond to immunisation with increased antibody responses, that this effect is due to a hyper-responsiveness of B cells to endosomal TLR stimulation, and that this phenotype is, in turn, is related to the type I IFN milieu present in these patients.
annrheumdis-2016-210509supp005.xlsx (284.2KB, xlsx)
annrheumdis-2016-210509supp006.xlsx (58.7KB, xlsx)
Acknowledgments
We thank Gull-Britt Almgren, Eva Jemseby, Amina Ossoinak, Lena Jonsson and Rezvan Kiani for excellent technical assistance and Nathalie Pochet for bioinformatic support, Adrián Cortés for AUC analysis, Ulf Hammar for expert guidance on statistical analysis, Marika Rönnholm and David Brodin for excellent technical assistance and computation.
Footnotes
Contributors: SB and MWH conceived the study and designed the experiments with input from VM, CT, LK and VKK; SB, SM, SP, MF-M, KAB and MWH performed the experiments, SB, LF, SP, JM, LW, CT, VM, VKK and MWH analysed the data, MK and GN managed study participant recruitment and clinical data analysis, SB, AA, VKK and MWH wrote the manuscript and all authors participated in revision until its final form.
Funding: This study was supported by grants from the Swedish Research Council, the Heart-Lung Foundation, the Stockholm County Council, the Karolinska Institute, the Swedish Rheumatism Association, King Gustaf the V:th 80-year Foundation, and the Torsten and Ragnar Söderberg Foundation.
Competing interests: None declared.
Patient consent: Detail has been removed from this case description to ensure anonymity. The editors and reviewers have seen the detailed information available and are satisfied that the information backs up the case the authors are making.
Ethics approval: Ethics Committee Stockholm North.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Cervera R, Khamashta MA, Font J, et al. . Morbidity and mortality in systemic lupus Erythematosus during a 5-Year period: a multicenter prospective study of 1,000 patients. Medicine 1999;78:167–75. 10.1097/00005792-199905000-00003 [DOI] [PubMed] [Google Scholar]
- 2.Doran MF, Crowson CS, Pond GR, et al. . Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum 2002;46:2287–93. 10.1002/art.10524 [DOI] [PubMed] [Google Scholar]
- 3.Elkayam O, Paran D, Caspi D, et al. . Immunogenicity and safety of pneumococcal vaccination in patients with rheumatoid arthritis or systemic lupus erythematosus. Clin Infect Dis 2002;34:147–53. 10.1086/338043 [DOI] [PubMed] [Google Scholar]
- 4.Gabay C, Bel M, Combescure C, et al. . Impact of synthetic and biologic disease-modifying antirheumatic drugs on antibody responses to the AS03-adjuvanted pandemic influenza vaccine: a prospective, open-label, parallel-cohort, single-center study. Arthritis Rheum 2011;63:1486–96. 10.1002/art.30325 [DOI] [PubMed] [Google Scholar]
- 5.Saad CG, Borba EF, Aikawa NE, et al. . Immunogenicity and safety of the 2009 non-adjuvanted influenza A/H1N1 vaccine in a large cohort of autoimmune rheumatic diseases. Ann Rheum Dis 2011;70:1068–73. 10.1136/ard.2011.150250 [DOI] [PubMed] [Google Scholar]
- 6.Dawood FS, Jain S, Finelli L, et al. . Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 2009;25:2605–15. [DOI] [PubMed] [Google Scholar]
- 7.Vitali C, Bombardieri S, Jonsson R, et al. . Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 2002;61:554–8. 10.1136/ard.61.6.554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambrosi A, Wahren-Herlenius M. Update on the immunobiology of Sjögren’s syndrome. Curr Opin Rheumatol 2015;27:468–75. 10.1097/BOR.0000000000000195 [DOI] [PubMed] [Google Scholar]
- 9.Hansen A, Lipsky PE, Dörner T. Immunopathogenesis of primary Sjögren’s syndrome: implications for disease management and therapy. Curr Opin Rheumatol 2005;17:558–65. 10.1097/01.bor.0000172801.56744.c3 [DOI] [PubMed] [Google Scholar]
- 10.Wahren-Herlenius M, Dörner T. Immunopathogenic mechanisms of systemic autoimmune disease. Lancet 2013;382:819–31. 10.1016/S0140-6736(13)60954-X [DOI] [PubMed] [Google Scholar]
- 11.Kvarnström M, Ottosson V, Nordmark B, et al. . Incident cases of primary Sjögren’s syndrome during a 5-year period in Stockholm County: a descriptive study of the patients and their characteristics. Scand J Rheumatol 2015;44:135–42. 10.3109/03009742.2014.931457 [DOI] [PubMed] [Google Scholar]
- 12.Del Porto F, Laganà B, Biselli R, et al. . Influenza vaccine administration in patients with systemic lupus erythematosus and rheumatoid arthritis. Safety and immunogenicity. Vaccine 2006;24:3217–23. 10.1016/j.vaccine.2006.01.028 [DOI] [PubMed] [Google Scholar]
- 13.Perdan-Pirkmajer K, Thallinger GG, Snoj N, et al. . Autoimmune response following influenza vaccination in patients with autoimmune inflammatory rheumatic disease. Lupus 2012;21:175–83. 10.1177/0961203311429817 [DOI] [PubMed] [Google Scholar]
- 14.Urowitz MB, Anton A, Ibanez D, et al. . Autoantibody response to adjuvant and nonadjuvant H1N1 vaccination in systemic lupus erythematosus. Arthritis Care Res 2011;63:1517–20. 10.1002/acr.20599 [DOI] [PubMed] [Google Scholar]
- 15.Adler S, Krivine A, Weix J, et al. . Protective effect of A/H1N1 vaccination in immune-mediated disease--a prospectively controlled vaccination study. Rheumatology 2012;51:695–700. 10.1093/rheumatology/ker389 [DOI] [PubMed] [Google Scholar]
- 16.Espinosa A, Dardalhon V, Brauner S, et al. . Loss of the lupus autoantigen Ro52/Trim21 induces tissue inflammation and systemic autoimmunity by disregulating the IL-23-Th17 pathway. J Exp Med 2009;206:1661–71. 10.1084/jem.20090585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wahren-Herlenius M, Muller S, Isenberg D. Analysis of B-cell epitopes of the Ro/SS-A autoantigen. Immunol Today 1999;20:234–40. 10.1016/S0167-5699(99)01458-9 [DOI] [PubMed] [Google Scholar]
- 18.Seror R, Ravaud P, Bowman SJ, et al. . EULAR Sjogren’s syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjogren’s syndrome. Ann Rheum Dis 2010;69:1103–9. 10.1136/ard.2009.110619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seror R, Ravaud P, Mariette X, et al. . EULAR Sjogren’s Syndrome Patient Reported Index (ESSPRI): development of a consensus patient index for primary Sjogren’s syndrome. Ann Rheum Dis 2011;70:968–72. 10.1136/ard.2010.143743 [DOI] [PubMed] [Google Scholar]
- 20.Hansen A, Odendahl M, Reiter K, et al. . Diminished peripheral blood memory B cells and accumulation of memory B cells in the salivary glands of patients with Sjogren’s syndrome. Arthritis Rheum 2002;8:2160–71. [DOI] [PubMed] [Google Scholar]
- 21.Båve U, Nordmark G, Lövgren T, et al. . Activation of the type I interferon system in primary Sjögren’s syndrome: a possible etiopathogenic mechanism. Arthritis Rheum 2005;52:1185–95. 10.1002/art.20998 [DOI] [PubMed] [Google Scholar]
- 22.Groom J, Kalled SL, Cutler AH, et al. . Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjögren’s syndrome. J Clin Invest 2002;109:59–68. 10.1172/JCI14121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emamian ES, Leon JM, Lessard CJ, et al. . Peripheral blood gene expression profiling in Sjögren’s syndrome. Genes Immun 2009;10:285–96. 10.1038/gene.2009.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cerutti A, Zan H, Schaffer A, et al. . CD40 ligand and appropriate cytokines induce switching to IgG, IgA, and IgE and coordinated germinal center and plasmacytoid phenotypic differentiation in a human monoclonal IgM+IgD+ B cell line. J Immunol 1998;5:2145–57. [PMC free article] [PubMed] [Google Scholar]
- 25.Corcoran AE, Riddell A, Krooshoop D, et al. . Impaired immunoglobulin gene rearrangement in mice lacking the IL-7 receptor. Nature 1998;391:904–7. 10.1038/36122 [DOI] [PubMed] [Google Scholar]
- 26.Fujieda S, Saxon A, Zhang K. Direct evidence that gamma 1 and gamma 3 switching in human B cells is interleukin-10 dependent. Mol Immunol 1996;17-18:1335–43. [DOI] [PubMed] [Google Scholar]
- 27.Kikuchi K, Lai AY, Hsu CL, et al. . IL-7 receptor signaling is necessary for stage transition in adult B cell development through up-regulation of EBF. J Exp Med 2005;201:1197–203. 10.1084/jem.20050158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arpin C, Déchanet J, Van Kooten C, et al. . Generation of memory B cells and plasma cells in vitro. Science 1995;268:720–2. 10.1126/science.7537388 [DOI] [PubMed] [Google Scholar]
- 29.Castigli E, Wilson SA, Scott S, et al. . TACI and BAFF-R mediate isotype switching in B cells. J Exp Med 2005;201:35–9. 10.1084/jem.20032000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glaum MC, Narula S, Song D, et al. . Toll-like receptor 7-induced naive human B-cell differentiation and immunoglobulin production. J Allergy Clin Immunol 2009;123:224–30. 10.1016/j.jaci.2008.09.018 [DOI] [PubMed] [Google Scholar]
- 31.He B, Qiao X, Cerutti A. CpG DNA induces IgG class switch DNA recombination by activating human B cells through an innate pathway that requires TLR9 and cooperates with IL-10. J Immunol 2004;173:4479–91. 10.4049/jimmunol.173.7.4479 [DOI] [PubMed] [Google Scholar]
- 32.He B, Santamaria R, Xu W, et al. . The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat Immunol 2010;11:836–45. 10.1038/ni.1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krieg AM, Yi AK, Matson S, et al. . CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 1995;374:546–9. 10.1038/374546a0 [DOI] [PubMed] [Google Scholar]
- 34.Litinskiy MB, Nardelli B, Hilbert DM, et al. . DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol 2002;3:822–9. 10.1038/ni829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruprecht CR, Lanzavecchia A. Toll-like receptor stimulation as a third signal required for activation of human naive B cells. Eur J Immunol 2006;36:810–6. 10.1002/eji.200535744 [DOI] [PubMed] [Google Scholar]
- 36.Kiefer K, Oropallo MA, Cancro MP, et al. . Role of type I interferons in the activation of autoreactive B cells. Immunol Cell Biol 2012;90:498–504. 10.1038/icb.2012.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNab F, Mayer-Barber K, Sher A, et al. . Type I interferons in infectious disease. Nat Rev Immunol 2015;15:87–103. 10.1038/nri3787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuznik A, Bencina M, Svajger U, et al. . Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J Immunol 2011;186:4794–804. 10.4049/jimmunol.1000702 [DOI] [PubMed] [Google Scholar]
- 39.Ramos-Casals M, Tzioufas AG, Stone JH, et al. . Treatment of primary Sjögren syndrome: a systematic review. JAMA 2010;304:452–60. 10.1001/jama.2010.1014 [DOI] [PubMed] [Google Scholar]
- 40.Sacre K, Criswell LA, McCune JM. Hydroxychloroquine is associated with impaired interferon-alpha and tumor necrosis factor-alpha production by plasmacytoid dendritic cells in systemic lupus erythematosus. Arthritis Res Ther 2012;14:R155 10.1186/ar3895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willis R, Seif AM, McGwin G, et al. . Effect of hydroxychloroquine treatment on pro-inflammatory cytokines and disease activity in SLE patients: data from LUMINA (LXXV), a Multiethnic US cohort. Lupus 2012;21:830–5. 10.1177/0961203312437270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feng X, Wu H, Grossman JM, et al. . Association of increased interferon-inducible gene expression with disease activity and lupus nephritis in patients with systemic lupus erythematosus. Arthritis Rheum 2006;54:2951–62. 10.1002/art.22044 [DOI] [PubMed] [Google Scholar]
- 43.Jeisy-Scott V, Kim JH, Davis WG, et al. . TLR7 recognition is dispensable for influenza virus A infection but important for the induction of hemagglutinin-specific antibodies in response to the 2009 pandemic split vaccine in mice. J Virol 2012;86:10988–98. 10.1128/JVI.01064-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gottenberg JE, Cagnard N, Lucchesi C, et al. . Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjögren’s syndrome. Proc Natl Acad Sci U S A 2006;103:2770–5. 10.1073/pnas.0510837103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maria NI, Brkic Z, Waris M, et al. . MxA as a clinically applicable biomarker for identifying systemic interferon type I in primary Sjogren’s syndrome. Ann Rheum Dis 2014;73:1052–9. 10.1136/annrheumdis-2012-202552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gottenberg JE, Ravaud P, Puéchal X, et al. . Effects of hydroxychloroquine on symptomatic improvement in primary Sjögren syndrome: the JOQUER randomized clinical trial. JAMA 2014;312:249–58. 10.1001/jama.2014.7682 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2016-210509supp001.docx (51.1KB, docx)
annrheumdis-2016-210509supp002.docx (46.6KB, docx)
annrheumdis-2016-210509supp008.pdf (2.3MB, pdf)
annrheumdis-2016-210509supp003.docx (58KB, docx)
annrheumdis-2016-210509supp004.xlsx (43.5KB, xlsx)
annrheumdis-2016-210509supp005.xlsx (284.2KB, xlsx)
annrheumdis-2016-210509supp006.xlsx (58.7KB, xlsx)