Abstract
Introduction
Drug-induced proarrhythmic potential is an important regulatory criterion in safety pharmacology. The application of in silico approaches to predict proarrhythmic potential of new compounds is under consideration as part of future guidelines. Current approaches simulate electrophysiology of a single human adult ventricular cardiomyocyte. However, drug-induced proarrhythmic potential can be different when cardiomyocytes are surrounded by non-muscle cells. Incorporating fibroblasts in models of myocardium is important particularly for predicting a drugs cardiac liability in the aging population – a growing population who take more medications and exhibit increased cardiac fibrosis. In this study, we used computational models to investigate the effects of fibroblast coupling on the electrophysiology and response to drugs of cardiomyocytes.
Methods
A computational model of cardiomyocyte electrophysiology and ion handling (O’Hara et al. 2011) is coupled to a passive model of fibroblast electrophysiology to test the effects of dofetilide block on the rapid delayed rectifier K+ channel. Results are compared to model results without fibroblast coupling to see how fibroblasts affect cardiomyocyte action potential duration at 90% repolarization (APD90) and propensity for early after depolarization (EAD).
Results
Simulation results show an increase in cardiomyocyte APD90 with increasing concentration of three drugs that affect cardiac function: dofetilide, vardenafil and nebivolol, when no fibroblasts are coupled to the cardiomyocyte. Coupling fibroblasts to cardiomyocytes markedly shortens APD90. Moreover, increasing the number of fibroblasts can augment the shortening effect.
Discussion
Coupling cardiomyocytes and fibroblasts are predicted to decrease proarrhythmic susceptibility under dofetilide, vardenafil and nebivolol block. However, this result is sensitive to parameters which define the electrophysiological function of the fibroblast. Fibroblast membrane capacitance and conductance (CFB and GFB) have less of an effect on APD90 than the fibroblast resting membrane potential (EFB). This study suggests that in both theoretical models and experimental tissue constructs that represent cardiac tissue, both cardiomyocytes and nonmuscle cells should be considered when testing cardiac pharmacological agents.
1. Introduction
Cardiomyocytes occupy a major part of the heart muscle by volume, yet >65% of cells in myocardium are non-muscle cells (Bergmann et al. 2015; Nag 1980; Pinto et al. 2016). In vitro experiments have been used to confirm significant effects of the coupling between cardiomyocytes and non-muscle cells on cardiomyocyte electrophysiology (Kohl and Gourdie 2014). While the critical role of electrical coupling between cells in vivo is still under discussions (Kohl and Gourdie 2014), the myocyte-fibroblast coupling has been recorded in healthy hearts and more clearly in those in cardiac remodeling after injury or chronic stress (Ongstad and Kohl 2016).
The aging population (>65 years of age) is the largest group of consumers of pharmaceuticals many of which are taken to treat their high prevalence of chronic heart failure and fibrillation (Vigen, Maddox, and Allen 2012). One distinct feature of the aging heart is its increased number of non-muscle cells including myofibroblasts that contribute to fibrosis (Biernacka and Frangogiannis 2011). This large aging population is expected to grow in accordance with the increasing average life expectancy of the world population and pharmaceuticals targeted to this group must be screened for the possibility of adverse cardiac accounting for the contribution of non-muscle cells to cardiomyocyte electrophysiology.
One of primary objectives of the Comprehensive In vitro Proarrhythmia Assay (CiPA) is to use in silico simulations based on the O’Hara-Rudy (ORd) model (O'Hara et al. 2011) for proarrhythmic potential of compounds applying ion channel inhibition data of more than one channels (e.g., rapid delayed rectifier K+, L-type Ca2+ and/or fast Na+ channels). When using the ORd model to predict cardiomyocyte electrophysiological function in cardiac tissue an understanding of the limitations and scope of the model is important. Like conducting any bench-top experiments, the use of theoretical models must match the question asked. Here, we introduce a simple extension of the ORd model to simulate electrophysiology of an adult cardiomyocyte connected to cardiac fibroblasts and how this computational model responds to the application of cardiac sensitive drugs. The ORd model was chosen as the foundational model to use here because it was developed based on experimental data conducted using human adult ventricular cardiomyocytes isolated from healthy donors and has been adopted as a starting point for the CiPA project aimed at supporting regulatory decisions with respect to cardiac drug proarrhythmic risk (Sager et al. 2014).
Multiple experimental analyses using in vitro models (Xie, Garfinkel, Camelliti, et al. 2009; Vasquez, Benamer, and Morley 2011) and also computational analyses (Nayak et al. 2013; Sridhar, Vandersickel, and Panfilov 2017) support the idea that cardiac fibroblasts affect the arrhythmogenicity of the myocardium including changes in action potential duration and early after depolarization (EAD). However, how the connection between fibroblasts and cardiomyocytes and the current flow through that connection can change the overall electrophysiology of myocardium has not been explored in the context of cardiac sensitive drug response. Specifically, the effects of the magnitude of the gap junctional conductance and the underlying electrophysiology of cardiac fibroblasts has not yet been evaluated quantitatively in the analysis of proarrhythmia risk.
The coupled model, developed in response to these issues, simulates drug-induced changes in electrophysiology not solely in isolated cardiomyocytes but in cardiac tissue which is comprised of both cardiomyocytes and non-muscle cell. In this study, we aim to show how a simple modification of the ORd model can influence the predicted susceptibility of cardiac tissue to the effects of proarrhythmic compounds.
2. Methods
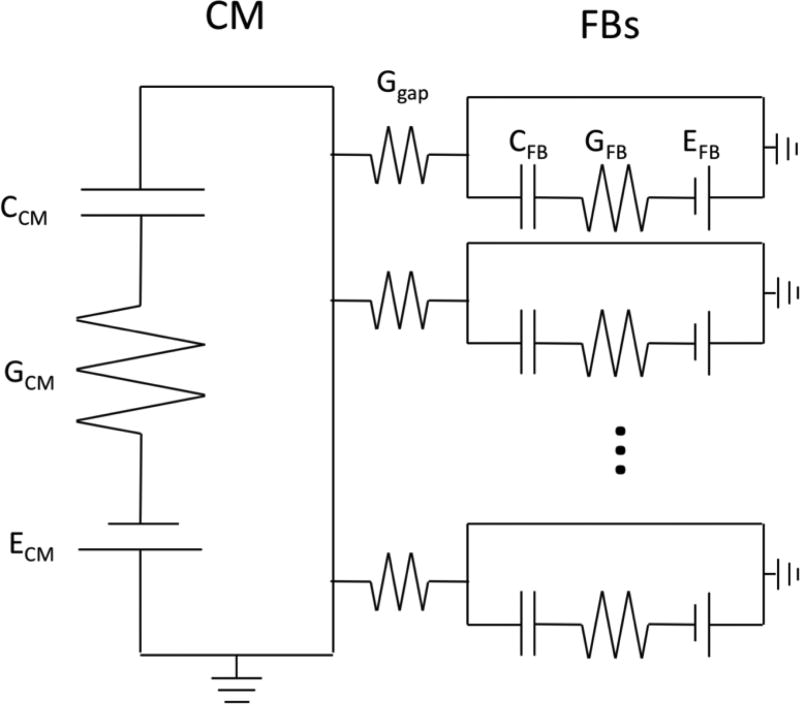
Electrical coupling between cardiomyocytes and fibroblasts were modeled by assuming that a cardiomyocyte is surrounded by N fibroblasts, and the gap junction between cardiomyocyte and fibroblast is an electrical conductor (Figure 1). The fibroblast itself was modeled as an electrically passive cell (Kohl et al. 1994). Thus, the membrane potential of the cardiomyocyte (VCM) and fibroblast (VFB) can be written as:
| (1) |
| (2) |
| (3) |
| (4) |
where CCM and CFB are the membrane capacitances of the cardiomyocyte and fibroblast, ICM and IFB are the membrane currents of the cardiomyocyte and fibroblast, Istim is the stimulus current, GFB and EFB are the electrical conductance and resting potential of the fibroblast, and Igap and Ggap are the current and electrical conductance of gap junction.
Figure 1.
Schematic of cardiomyocyte coupled with one or more fibroblasts. Conductance across the cell membrane in the fibroblast, GFB, is fixed while the conductance across the cardiomyocyte membrane, GCM, is governed by the dynamics of the ORd model. Gap junction conductance (Ggap), fibroblast conductance (GFB), fibroblast membrane capacitance (CFB) and fibroblast membrane Nernst potential (EFB) are chosen from studies by Kohl et al. (Kohl et al. 1994) and Xie et al. (Xie, Garfinkel, Weiss, et al. 2009). Cardiomyocyte membrane capacitance is not explicitly used in the ORd model so the value (185 pF) used in ten Tusscher et al. (ten Tusscher et al. 2004) was used when fibroblasts were coupled to the cardiomyocyte model. Ratio of the number of fibroblast cells to cardiomyocyte cells is typically believed to be between 5 and 12 in normal tissue.
The cardiomyocyte membrane potential and current were simulated with the ORd model which is capable of reproducing experimentally observed human adult ventricular cardiomyocyte action potential shapes with and without the blocking of specific ion channels (O'Hara et al. 2011). The fibroblast membrane conductance (GFB) was chosen as 0.5 nS which is in the range of experimental results (0.1 to 4 nS) (Kohl et al. 1994). The conductance of gap junction (Ggap) was chosen as 1 nS (Kohl et al. 1994). The membrane capacitance of the fibroblast (CFB) was chosen as 25 pF, and the resting potential (EFB) was chosen as −50 mV (Xie, Garfinkel, Weiss, et al. 2009).
The blocking of cardiomyocyte membrane ion channel currents by compounds were modeled by reducing the maximal ion channel conductance as a function of compound concentration. The simplest relationship between the ion channel conductance and the compound concentration is the Hill equation which is written as:
| (5) |
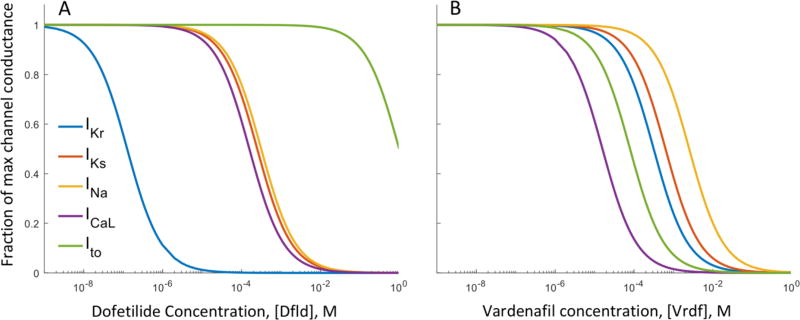
where G and G0 are the electrical conductance of a given ion channel with and without compound, respectively, [IC50] is the half-maximal inhibitory concentration of the cardiac sensitive compound that was determined from the results of IonWorks Quattro screening performed at AstraZeneca (Elkins et al. 2013; Mirams et al. 2014), [C] is the compound concentration, and n is the Hill coefficient which was set to 1 (Mirams et al. 2014). The inhibition of five membrane ion channel currents were modeled in this study: rapid delayed inward rectifying K+ current (IKr), slow delayed inward rectifying K+ current (IKs), fast Na+ current (INa), long-lasting type Ca2+ current (ICaL), and transient outward K+ current (Ito). The effects of dofetilide and vardenafil concentration on the conductance of each channel are shown in Figure 2. Dofetilide and vardenafil were used to illustrate the results of the application of a cardiac sensitive drug to the model. Nebivolol was also tested (see supplement) however, the effects of fibroblasts on the drug-induced proarrhythmia potential of any compound can be analyzed using [IC50]s on each of the five defined ion channel current as described in Mirams et al. study (Mirams et al. 2014).
Figure 2.
Effects of varying concentration of dofetilide ([DFld], A), and vardenafil ([Vdnf], B) on the maximal conductance of five ion channels of the cardiomyocyte. Dofetilide is known to selectively block the rapid delayed rectifier K+ channel current, IKr, however at higher concentrations the slow delayed rectifier K+, fast Na+, L-type Ca2+ and transient outward K+ (IKs, INa, ICaL and Ito, respectively) can be affected. For the range of dofetilide concentrations used in the simulations (0 to 30 μM), dofetilide can be considered specific to block IKr, however the effects on all channels was considered in the model formulation. Vardenafil blocks multiple ion channels at concentrations in the range of ~0.01 to 100 mM and is used to contrast with the specific ion channel blocker dofetilide.
Since cardiomyocytes are outnumbered by fibroblasts in normal cardiac tissue by a ratio of 2 to 3 (Rohr 2012), we simulated the cardiomyocyte action potential by coupling a single cardiomyocyte with 0, 1, or 3 FBs. We chose dofetilide as one reference compound, and the dofetilide concentration was varied from 0 to 1 μM. Vardenafil and nebivolol were also tested in concentrations of 0 to 30 nM and 0 to 100 μM, respectively. For each arrangement of a cardiomyocyte coupled with fibroblasts, the cardiomyocyte was paced at a given frequency without the compound to reach a steady state, and then from that steady state was brought to a new steady state with a given concentration of the cardiac sensitive drug. The steady state was defined as when the relative action potential change is less than 0.1% between pacing cycles (the action potential was evaluated every 100 ms in each pacing cycle to evaluate the relative change). All the simulations were performed with Matlab 2016b (MathWorks Inc., Natick, MA, USA).
3. Results
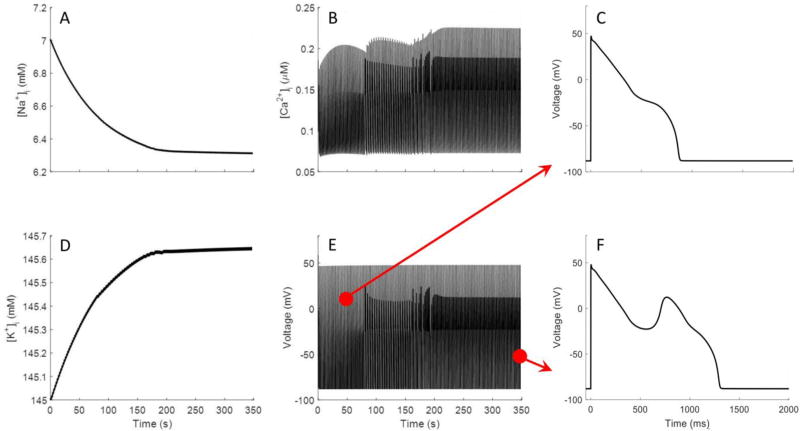
The heart beats continuously and is operating under a relative “steady state” with respect to the ionic concentrations and membrane potential at the start of each beat. However, computational models of cardiac electrophysiology may not include the requisite composition of ion channels to bring the simulation to a steady state. In the context of predicting drug-induced arrhythmogenicity, whether the model is able to run to steady state or not is an important consideration. Even if the formulation of the model supports a steady state many action potential cycles (100s to 1000s) are required to reach their steady state through the balance of cytosolic and extracellular [Na+], [K+], and [Ca2+]. Figure 3 shows the variation of simulated cytosolic concentration of sodium, potassium and calcium ([Na+]i, [K+]i, and [Ca2+]i), and action potential of the cardiomyocyte with respect to the number of cardiac cycles. These results were obtained by pacing the cardiomyocyte at 0.5 Hz without coupling to fibroblasts, and by blocking 90% of IKr. The results demonstrate that it is important to run simulations with enough pacing cycles to reach the steady state. While some models may not reach to a steady state, the ORd model is designed to achieve a physiologically reasonable steady state. In all simulations performed in this study we made sure that the simulations stabilize at physiological levels of [Na+]i, [K+]i, and [Ca2+]i however, the number of cycles to reach steady state varied with different drugs and the number of coupled fibroblasts. Under 90% block of IKr, the ORd model exhibited an EAD, but it requires a reduced pacing frequency, low cytosolic Na+ concentration and appeared only when the model neared the steady state (Figure 3 E, F).
Figure 3.
Simulation results over hundreds of action potentials of the ORd model with an 90% block of IKr used to identify that model reaches steady state. In the ORd model, background currents were tuned to allow cytosolic K+, Na+ and Ca2+ to reach steady-state values when simulated over long time scales. A, B and D shows transition of the cytosolic ion concentrations in the model from initial conditions to steady-state values. E shows the corresponding changes in the action potential while the model goes to steady state. Note that the early after depolarization does not appear until the model nears steady-state as shown in C and F.
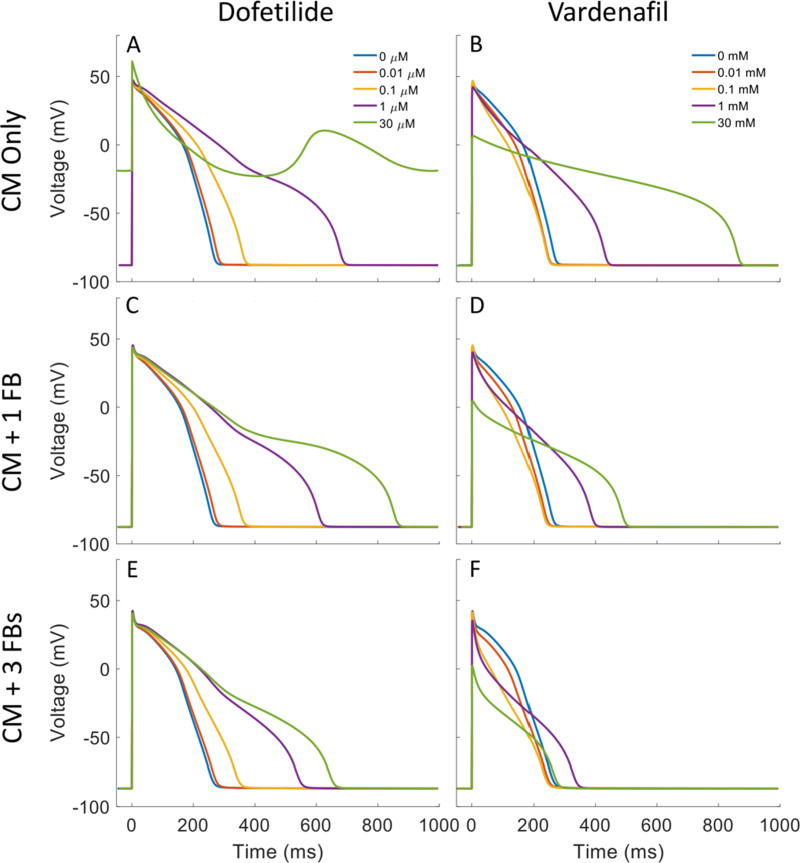
With the simulations at steady state, effects of fibroblasts connected electrically to the ORd model were analyzed for three cardiac sensitive compounds, dofetilide, nebivolol and vardenafil. The results of dofetilide and vardenafil are shown in Figure 4. Figure 4A shows that the action potential of the cardiomyocyte is dramatically prolonged by increasing the concentration of dofetilide (1 nM – 30 μM), and the cardiomyocyte fails to repolarize if the concentration increases to 30 μM. Figures 4C and 4E show that coupling cardiomyocytes with fibroblasts can markedly shorten the prolonged action potential, and the cardiomyocyte can repolarize under the effect of 30 μM dofetilide. Moreover, increasing the number of fibroblasts can augment the shortening effect. Similar results can be seen for nebivolol (see supplement) which primarily blocks INa, IKs and Ito. Figure 4B, D and F show the effects of vardenafil on cardiomyocyte action potential with 0, 1 and 3 coupled fibroblasts respectively. Note that vardenafil causes a shortening of the action potential at concentrations of 0.01 and 0.10 mM however a lengthening of the action potential is observed at higher concentrations of 1 and 30 mM. When coupled with fibroblasts, a shortening of the action potential is observed (Figure 4D and F) for the two higher concentrations of vardenafil however little difference is observed for the 0.01 and 0.1 mM concentrations between coupled and uncoupled simulations.
Figure 4.
Simulation of AP with increasing dofetilide and vardenafil concentration for cardiomyocyte alone (A and B), cardiomyocyte coupled with one fibroblast (C and D) and cardiomyocyte coupled with three fibroblasts (E and F). Concentrations of dofetilide simulated are 0, 0.01, 0.1, 1 and 30 μM while simulated concentrations of vardenafil are 0, 0.01, 0.1, 1 and 30 mM.
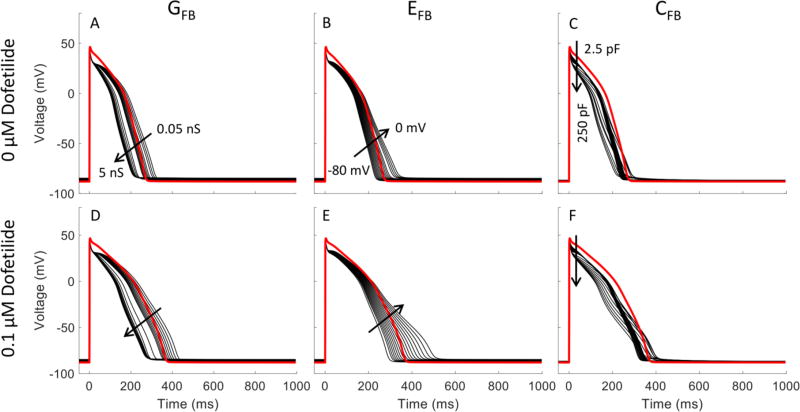
The fibroblast is modeled here as a passive resistive-capacitive element connected in parallel with the cardiomyocyte. The relative ratios of the fibroblast membrane resistance (1/GFB) and total membrane capacitance (CFB) as well as the resting membrane potential of the fibroblast (EFB) will influence how quickly the current flows in and out of the fibroblast as well as its magnitude. By connecting to one or more fibroblasts, electrical current can flow between the cardiomyocyte and the fibroblast in a manner analogous to a capacitor being charged and discharged through a resistor (Figure 1). Therefore, the selection of values for GFB, CFB and EFB influence whether fibroblast coupling shortens or lengthens the cardiomyocyte action potential. We have selected values from the literature that represent the average of the experimentally observed values. However, to see how these three parameters influence the action potential shape, we varied one of three parameters within its experimentally observed range while holding the other two at the average value. Figure 5 shows the change in action potential shape when varying GFB from 0.5 to 5 nS, EFB from −80 to 0 mV and CFB from 2.5 to 25 pF both with and without a 0.1 μM dofetilide block. While an increasing GFB or CFB shortened the action potential duration, an elevating EFB had an opposite effect.
Figure 5.
Effect of fibroblast parameters on myocyte action potential without and with dofetilide. The cardiomyocyte is coupled with 3 fibroblasts. Red lines show the action potential without coupling to fibroblasts. Black lines show the variation of action potential with the change of GFB, EFB, and CFB, respectively. GFB varied from 0.05 to 5 nS; EFB varied from −80 to 0 mV; CFB varied from 2.5 to 250 pF. Arrows indicate increasing of parameter value. In each case, the parameters not varied over a range are fixed at the values used in the simulations of Figure 4.
4. Discussion
In this theoretical study, fibroblasts connected to cardiomyocytes represented by the ORd model demonstrate a potential significant contribution of non-muscle cells on the prediction of cardiac sensitive drug proarrhythmia risk. This brief communication also introduces a theoretical platform for the in silico prediction of the safety of cardiac sensitive drug compounds. While we did not extend our analysis to study all compounds listed in the study by Mirams et al. (Mirams et al. 2014), from this list we selected dofetilide, nebivolol and vardenafil that inhibit different combinations of the ion channel currents IKr, INa, ICaL, IKs and Ito. Any drug with experimental data of its effects on these five channel currents can be simulated in an analogous manner to the three compounds tested here. Comparing the effects of dofetilide and vardenafil on the change in action potential shape we can observe that action potential duration at 90% repolarization (APD90) increases monotonically with increasing dofetilide concentration however with vardenafil APD90 first decreases and then increases with increasing concentration. In all cases where APD90 increases with application of the cardiac sensitive compound coupling with fibroblasts shortens the action potential.
Our group and other (Vasquez et al. 2010) have observed shortening of action potential duration using in vitro co-culture experimental models of cardiac fibroblasts and myocytes. This theoretical model requires further validation and optimization of its parameters against experimental data obtained with human adult ventricular cardiac tissues. However, electrophysiological data obtained using healthy human adult myocardium are rare and difficult to obtain. A recent demonstration of maintaining physiological state of thin slices of human donor hearts for electrophysiological assessments (Kang et al. 2016) is one of promising technologies to determine such parameters.
While our study tested only one set of parameters defining fibroblast conductance, resting membrane potential and capacitance, changing the theoretical model parameters can reverse the results outcome, i.e., action potential elongation or shortening and increased or decreased proarrhythmia drug susceptibility as is shown in Figure 5. A previous study (Nguyen et al. 2012), imposing a virtual fibroblast on isolated cardiomyocytes using a dynamic clamp technique, confirms our theoretical results using our set of average fibroblast parameters by showing shortening of the APD90 in eight different combination of the three fibroblast parameters (CFB, EFB and Ggap).
Theoretical EAD susceptibility was also shown to be affected by the selection of the fibroblast electrophysiological parameters CFB, GFB and EFB in the same study by Nguyen et al. (Nguyen et al. 2012). They used an earlier ventricular cardiomyocyte model developed by Lou and Rudy (Luo and Rudy 1991) formulated to represent a generic mammalian cardiomyocyte and showed a reduced EAD susceptibility to changes in Ggap. Here, we repeated these simulations with the ORd model to represent the effect on an adult human cardiomyocyte. Parameter sensitivity results with the ORd model (Figure 5) show the strongest sensitivity to EFB. APD90 increases with increasing fibroblast resting membrane potential and the effects of dofetilide are accentuated. This confirms that an accurate experimental quantification of a fibroblast’s resting membrane potential would improve the ability of these computational models to predict cardiomyocyte-fibroblast coupling effects on pharmacological proarrhythmia potential.
The ORd model for cardiomyocyte electrophysiology progresses to a steady state solution at a pacing of 0.5 Hz and a block of IKr of 90% as shown in Figure 3. It was also observed in the theoretical model that at a given IKr blockage the APD90 increases with a reduction in pacing frequency. This increased APD90 eventually results in an EAD in the steady state. It was reported by O’Hara et al. that EADs only occur in this model when paced slowly and other models such as those by ten Tusscher et al. (ten Tusscher et al. 2004) and Grandi et al. (Grandi, Pasqualini, and Bers 2010) are not able to replicate this phenomenon. The presence of EADs in the ORd model paced at lower frequencies (~30 beats/min) is accompanied by a reduced cytosolic concentration of sodium, [Na]I, reaching a concentration of 6.31 mM in the steady state. Clamping [Na]i in the theoretical model at the initial condition of 7.0 mM eliminates the development of EADs. Experimental evidence suggests increased cardiomyocyte [Na]i occurs with increased pacing and in disease (Louch et al. 2010; Pieske et al. 2002) however actual experimental measurements of [Na]i seem to be higher (16–32 mM) than that simulated in our computational models (6–9 mM). A further investigation of the effects of pacing on cardiomyocyte [Na]i is required to understand whether observations made computationally are of relevance physiologically.
Limitations
There are a few limitations of the current model. Whereas human adult ventricular cardiac fibroblasts may express ion channels affected by dofetilide (Vasquez, Benamer, and Morley 2011), nebivolol and vardenafil, we assume the electrophysiology of fibroblasts are not affected by the treatment at least acutely. In rat myofibroblasts two dominant K+ channels have been identified: a Shaker-type voltage dependent delayed rectifier channel (Kv1.6) and an inward rectifier channel (KCNA, Kv6.1) (Chilton et al. 2005). The inward rectifier current, IKir, is not thought to be blocked by dofetilide and the effects of the Shaker-type channel current, IShkr, are unknown. However, the rapid delayed rectifier channel current, IKr, known to be affected by dofetilide is a HERG channel and from a different subfamily than the Shaker-type channels. Little is known about the effects of the other drugs simulated here, nebivolol and vardenafil, on fibroblast ion channels. As described previously, proarrhythmic phenomena causing Torsades de pointes (TdP) may be due to action potential heterogeneity in different parts of myocardium, which requires simulations using 2D and 3D special resolutions with different scales (Vandersickel et al. 2016). To validate such computational simulations will require experimental samples by which a composition of fibroblasts and cardiomyocytes can be varied. Engineered heart tissues can serve such purpose (Daily et al. 2015) and models such as those used here can be extended to a spatial domain.
Conclusions
In summary, this study shows the potential importance of the electrophysiological effects of fibroblasts in theoretical models of cardiac tissue response to cardiac pharmacological agents. It also reinforces the concept that experimental tissues developed to test these agents for proarrhythmia potential should also incorporate fibroblasts to more accurately mimic native heart tissue.
Supplementary Material
Acknowledgments
The work was supported by NIH Grants NIA R43AG109735 and NHLBI U01 HL122199-01 for XG, NIA R43AG109735 and NHLBI R01 HL109595 for TE, TW and NIA; R43AG109735 and NHLBI U01 HL122199-01 for BEC.
We thank to our collaborators, Drs. Elliot Elson and Guy Genin at Washington University, St. Louis, constructive discussions about the topics covered in this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, dos Remedios C, Malm T, Andra M, Jashari R, Nyengaard JR, Possnert G, Jovinge S, Druid H, Frisen J. Dynamics of cell generation and turnover in the human heart. Cell. 2015;161:1566–75. doi: 10.1016/j.cell.2015.05.026. [DOI] [PubMed] [Google Scholar]
- Biernacka A, Frangogiannis NG. Aging and cardiac fibrosis. Aging and Disease. 2011;2:158–73. [PMC free article] [PubMed] [Google Scholar]
- Chilton L, Ohya S, Freed D, George E, Drobic V, Shibukawa Y, MacCannell KA, Imaizumi Y, Clark RB, Dixon IMC, Giles WR. K+ currents regulate the resting membrane potential, proliferation, and contractile responses in ventricular fibroblasts and myofibroblasts. American Journal of Physiology-Heart and Circulatory Physiology. 2005;288:H2931–H39. doi: 10.1152/ajpheart.01220.2004. [DOI] [PubMed] [Google Scholar]
- Daily Neil J, Yin Yue, Kemanli Pinar, Wakatsuki Tetsuro. Improving Cardiac Action Potential Measurements: 2D and 3D Cell Culture. Journal of Bioengineering & Biomedical Science. 2015;5 doi: 10.4172/2155-9538.1000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins RC, Davies MR, Brough SJ, Gavaghan DJ, Cui Y, Abi-Gerges N, Mirams GR. Variability in high-throughput ion-channel screening data and consequences for cardiac safety assessment. Journal of Pharmacological and Toxicological Methods. 2013;68:112–22. doi: 10.1016/j.vascn.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandi E, Pasqualini FS, Bers DM. A novel computational model of the human ventricular action potential and Ca transient. Journal of Molecular and Cellular Cardiology. 2010;48:112–21. doi: 10.1016/j.yjmcc.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C, Qiao Y, Li G, Baechle K, Camelliti P, Rentschler S, Efimov IR. Human organotypic cultured cardiac slices: new platform for high throughput preclinical human trials. Scientific Reports. 2016;6 doi: 10.1038/srep28798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl P, Gourdie RG. Fibroblast-myocyte electrotonic coupling: Does it occur in native cardiac tissue? Journal of Molecular and Cellular Cardiology. 2014;70:37–46. doi: 10.1016/j.yjmcc.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl P, Kamkin AG, Kiseleva IS, Noble D. Mechanosensitive fibroblasts in the sino-atrial node region of rat heart: interaction with cardiomyocytes and possible role. Exp Physiol. 1994;79:943–56. doi: 10.1113/expphysiol.1994.sp003819. [DOI] [PubMed] [Google Scholar]
- Louch WE, Hougen K, Mork HK, Swift F, Aronsen JM, Sjaastad I, Reims HM, Roald B, Andersson KB, Christensen G, Sejersted OM. Sodium accumulation promotes diastolic dysfunction in end-stage heart failure following Serca2 knockout. Journal of Physiology. 2010;588:465–78. doi: 10.1113/jphysiol.2009.183517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo CH, Rudy Y. A model of the ventricular cardiac action potential. Depolarization, repolarization, and their interaction. Circulation Research. 1991;68:1501–26. doi: 10.1161/01.res.68.6.1501. [DOI] [PubMed] [Google Scholar]
- Mirams GR, Davies MR, Brough SJ, Bridgland-Taylor MH, Cui Y, Gavaghan DJ, Abi-Gerges N. Prediction of Thorough QT study results using action potential simulations based on ion channel screens. Journal of Pharmacological and Toxicological Methods. 2014;70:246–54. doi: 10.1016/j.vascn.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag AC. Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios. 1980;28:41–61. [PubMed] [Google Scholar]
- Nayak Alok Ranjan, Shajahan TK, Panfilov AV, Pandit Rahul. Spiral-Wave Dynamics in a Mathematical Model of Human Ventricular Tissue with Myocytes and Fibroblasts. PLOS ONE. 2013;8:e72950. doi: 10.1371/journal.pone.0072950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TP, Xie YF, Garfinkel A, Qu ZL, Weiss JN. Arrhythmogenic consequences of myofibroblast-myocyte coupling. Cardiovascular Research. 2012;93:242–51. doi: 10.1093/cvr/cvr292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara T, Virag L, Varro A, Rudy Y. Simulation of the undiseased human cardiac ventricular action potential: model formulation and experimental validation. PLoS Computational Biology. 2011;7:e1002061. doi: 10.1371/journal.pcbi.1002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongstad E, Kohl P. Fibroblast-myocyte coupling in the heart: potential relevance for therapeutic interventions. Journal of Molecular and Cellular Cardiology. 2016;91:238–46. doi: 10.1016/j.yjmcc.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieske B, Maier LS, Piacentino V, Weisser J, Hasenfuss G, Houser S. Rate dependence of [Na+]i and contractility in nonfailing and failing human myocardium. Circulation. 2002;106:447–53. doi: 10.1161/01.cir.0000023042.50192.f4. [DOI] [PubMed] [Google Scholar]
- Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D'Antoni ML, Debuque R, Chandran A, Wang LN, Arora K, Rosenthal NA, Tallquist MD. Revisiting cardiac cellular composition. Circulation Research. 2016;118:400–09. doi: 10.1161/CIRCRESAHA.115.307778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr S. Arrhythmogenic implications of fibroblast-myocyte interactions. Circulation Arrhythmia and Electrophysiology. 2012;5:442–52. doi: 10.1161/CIRCEP.110.957647. [DOI] [PubMed] [Google Scholar]
- Sager PT, Gintant G, Turner JR, Pettit S, Stockbridge N. Rechanneling the cardiac proarrhythmia safety paradigm: a meeting report from the Cardiac Safety Research Consortium. American Heart Journal. 2014;167:292–300. doi: 10.1016/j.ahj.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Sridhar S, Vandersickel Nele, Panfilov Alexander V. Effect of myocyte-fibroblast coupling on the onset of pathological dynamics in a model of ventricular tissue. Scientific Reports. 2017;7:40985. doi: 10.1038/srep40985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Tusscher KHWJ, Noble D, Noble PJ, Panfilov AV. A model for human ventricular tissue. American Journal of Physiology-Heart and Circulatory Physiology. 2004;286:H1573–H89. doi: 10.1152/ajpheart.00794.2003. [DOI] [PubMed] [Google Scholar]
- Vandersickel N, de Boer TP, Vos MA, Panfilov AV. Perpetuation of torsade de pointes in heterogeneous hearts: competing foci or re-entry? Journal of Physiology. 2016;594:6865–78. doi: 10.1113/JP271728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez C, Benamer N, Morley GE. The cardiac fibroblast: functional and electrophysiological considerations in healthy and diseased hearts. Journal of Cardiovascular Pharmacology. 2011;57:380–88. doi: 10.1097/FJC.0b013e31820cda19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez Carolina, Mohandas Poornima, Louie Karen L, Benamer Najate, Bapat Ashwini C, Morley Gregory E. <span hwp:id="article-title-1" class="articletitle">Enhanced Fibroblast–Myocyte Interactions in Response to Cardiac Injury</span><span hwp:id="article-title-47" class="sub-article-title">Novelty and Significance</span>. Circulation Research. 2010;107:1011–20. doi: 10.1161/CIRCRESAHA.110.227421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigen R, Maddox TM, Allen LA. Aging of the United States population: impact on heart failure. Current Heart Failure Reports. 2012;9:369–74. doi: 10.1007/s11897-012-0114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie YF, Garfinkel A, Weiss JN, Qu ZL. Cardiac alternans induced by fibroblast-myocyte coupling: mechanistic insights from computational models. American Journal of Physiology-Heart and Circulatory Physiology. 2009;297:H775–H84. doi: 10.1152/ajpheart.00341.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Yuanfang, Garfinkel Alan, Camelliti Patrizia, Kohl Peter, Weiss James N, Qu Zhilin. Effects of Fibroblast-Myocyte Coupling on Cardiac Conduction and Vulnerability to Reentry: A Computational Study. Heart rhythm : the official journal of the Heart Rhythm Society. 2009;6:1641–49. doi: 10.1016/j.hrthm.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.