Summary
Tumor epithelial cells undergo a morphologic shift through the process of EMT with characteristic loss of cell polarity, conferring invasive and metastatic properties during cancer progression. Signaling by transforming growth factor-β mediates EMT programming and its phenotypic reversal to mesenchymal-epithelial transition. The role of EMT in bladder cancer progression to advanced disease is poorly understood. In this study, we conducted a retrospective analysis of the EMT landscape and actin cytoskeleton remodeling in a series of human bladder cancer specimens. Immunoreactivity for E-cadherin, N-cadherin, and vimentin protein expression was performed toward establishing an EMT signature in human bladder cancer. Serial sections were assessed for the primary regulator of the actin cytoskeleton remodeling and transforming growth factor-β signaling effector, cofilin. Our results demonstrate that EMT induction in clinical bladder cancer specimens is significantly associated with bladder cancer progression to high-grade, invasive disease. Evaluation of expression and cellular localization of the cytoskeleton regulator cofilin revealed a significant association between overexpression of nuclear cofilin with bladder cancer progression. This study is of translational significance in defining the value of EMT signature and cytoskeletal cofilin as potential tumor markers and targetable platforms for the treatment of invasive bladder cancer.
Keywords: E-cadherin, N-cadherin, Actin cytoskeleton, Bladder cancer, Phenotypic changes
1. Introduction
It is estimated that 74 000 cases of bladder cancer will be diagnosed in 2016 with nearly 16 000 deaths expected from muscle-invasive disease [1]. At initial presentation, about 75% of bladder cancer patients have disease that does not invade the muscle (carcinoma in situ, Ta, and T1), whereas 25% of patients have invasive cancer extending through the muscularis propria, which is associated with worse prognosis [2,3]. Standard treatment for patients with muscle-invasive tumors includes surgical removal of the bladder (cystectomy) and/or concurrent chemotherapy and radiation in patients with regional lymph node metastasis [4]. Systemic chemotherapy for the treatment for patients with metastatic bladder cancer has resulted in meager clinical benefit with median overall survival of 12 months [5]. Although the TNM staging of bladder cancer guides both treatment and prognosis, there remains substantial heterogeneity among similarly staged patients with respect to treatment response and overall outcomes [4]. Therefore, there is a critical need for identification of biomarkers to diagnose bladder cancer at an early stage, monitor recurrence, predict therapeutic response, and ultimately enable the development of more clinically effective treatment strategies. Emerging biomarkers of disease progression, immunotherapy, and therapy targeted toward specific aberrant signaling pathways that promote malignancy and metastasis have demonstrable value [6,7].
The phenotypic process of EMT is a dysregulation of a homeostatic mechanism vital in tissue differentiation and wound healing [8]. The process of EMT, originally defined as an indispensable developmental program for implantation, embryogenesis, and organogenesis, is now recognized as a critical contributor to cancer progression to metastasis. EMT is characterized by loss of basoapical epithelial polarity, dysregulation and reorganization of the cytoskeletal network, and differential expression of genes conferring a fibroblast-like phenotype while increasing tumor cell invasion and metastatic potential [8]. Perturbation in epithelial cell homeostasis renders EMT a significant venue for epithelial-derived tumors to escape apoptosis, enhance stem cell–like properties, become invasive, and metastasize [9,10]. The complementary process, known as MET, has been implicated in metastatic implant viability [11–13]. The phenomenon of EMT has also been associated with emergence of chemotherapeutic resistance [11,14].
To preserve cellular shape and polarity, the intracellular domains of cadherins connect to the actin cytoskeleton through α-catenin and β-catenin. Loss of epithelial cell markers (E-cadherin, β-catenin) and gain of mesenchymal cell markers (N-cadherin, vimentin) at the leading edge or invasive front of solid tumors are linked to metastatic progression [15]. Upon metastatic colonization, the new microenvironment facilitates paracrine cytokine signaling pathways directing malignant epithelial cells toward MET and survival. During prostate tumorigenesis, “cadherin switching” programs the tumor epithelial cells to undergo EMT mediated by differential expression of N-cadherin (mediator of neural plasticity, cell motility, and invasion) with a simultaneous loss of E-cadherin (regulator of epithelial membrane polarity and integrity through intercellular adherens junctions) [16]. Such a phenotypic switch from E- to N-cadherin is under androgenic regulation and has acquired predictive value in human prostate tumor recurrence and mortality [17]. In transitional carcinoma of the bladder, this cadherin switching is a late event and has been associated with disease progression and poor prognosis [18]. The transcription factors Snail, Slug, Twist, and Zeb1, known molecular drivers of EMT programming via transcriptional repression of E-cadherin, are overexpressed in clinical human bladder cancer, as well as in preclinical models of bladder tumorigenesis [19–22].
Cofilin is a key regulating protein of the actin cytoskeleton organization, directly involved in the actin polymerization and remodeling dynamics in response to extracellular signals such as TGF-β [23]. We recently demonstrated a significant up-regulation of both the native and phosphorylated forms of cofilin in human prostate cancer metastases [23]. The present study evaluated the expression and cellular localization profile of cofilin coupled with the EMT landscape in human bladder cancer specimens in relation to tumor grade and stage. Our results indicate a significant association between an EMT signature and a nuclear localization of cofilin and bladder cancer progression to invasive disease.
2. Materials and methods
2.1. Histopathologic characterization of bladder cancer specimens
Tissue specimens were obtained from patients (n = 48) who underwent radical cystectomy for urothelial carcinoma at the University of Kentucky Medical Center, Department of Urology (2007–2013). Bladder tumors were identified from the University of Kentucky, Department of Pathology database according to the regulatory approval by the institutional review board. The patient cohort evaluated included cases of all tumor stages and pathological grades. Paraffin-embedded tissue specimens were processed and tissue sections (5 µm) were used for immunhistochemical analysis after deidentification. The World Health Organization revised the classification system, which divided bladder tumors into muscle-invasive urothelial carcinoma and non-muscle-invasive urothelial neoplasia. The latter category includes urothelial carcinoma in situ, low- and high-grade non-muscle-invasive papillary urothelial carcinoma, non-muscle-invasive papillary urothelial neoplasm of low malignant potential, and urothelial papilloma [24].
2.2. Quantitative and qualitative analyses of immunostaining
Sections from individual tumors were subjected to immunostaining using specific antibodies against cofilin and E-cadherin (Cell Signaling Technology, Danvers, MA) and N-cadherin (AbCam Cell Signaling, Cambridge, United Kingdom). Immunoreactivity was determined based on “Quick Score” calculated by multiplying staining intensity (on a scale of 1–3) by percentage of tumor cells with positive immunoreactivity [25]. Two blinded reviewers (P. H., D. Z.) independently reviewed 3 nonadjacent fields (×100) per section. Nuclear cofilin accounted for nuclear expression only, whereas total cofilin represented both cytosolic and nuclear staining.
2.3. Genome-wide analysis of messenger RNA transcripts
The publically available Cancer Genome Atlas was interrogated for messenger RNA (mRNA) transcript levels of cofilin in urothelial carcinoma. The results shown are in whole based on data generated by the TCGA Research Network: http://cancergenome.nih.gov/.
2.4. Statistical analysis
The average Quick Score represents an average of the 6 fields (3 from each reviewer). One-way analysis of variance with Tukey correction for multiple comparisons was used to analyze mRNA transcript levels and differences between tumor stage and neoadjuvant therapy. Statistical significance was set at P < .05. Two-tailed t tests with Welch correction for unequal sample size were used to evaluate differences between tumor specimens of different tumor grades and to evaluate differences in bladder cancer–specific survival and metastasis.
3. Results
3.1. Defining EMT signatures in urothelial carcinoma
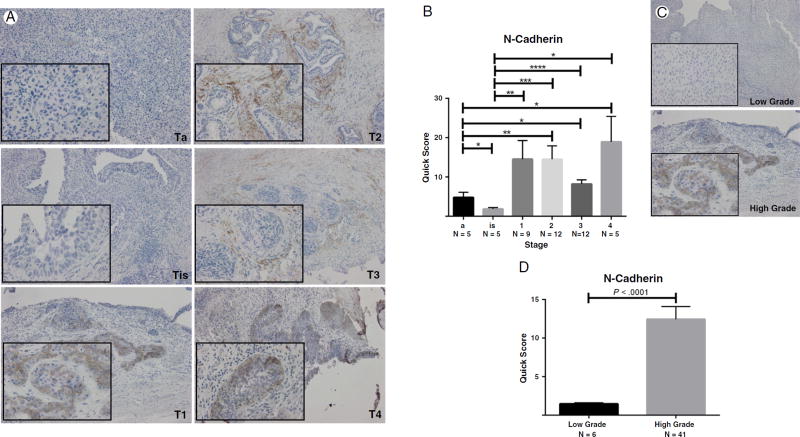
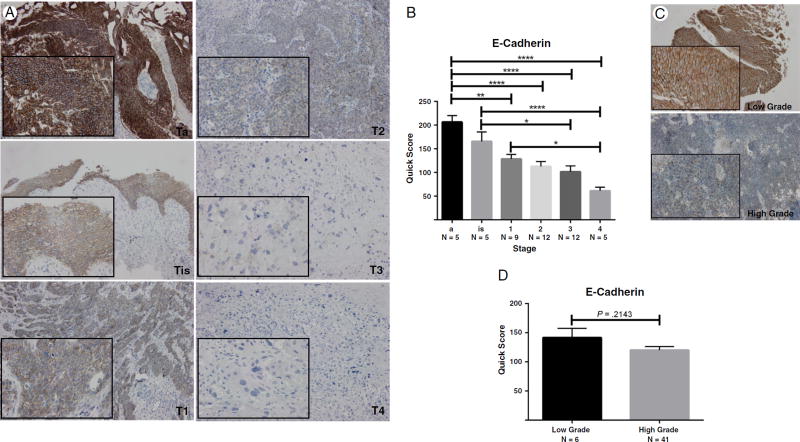
The expression profile of critical protein effectors of the EMT landscape, E-cadherin (marker of epithelial cell membrane polarity), and N-cadherin (a marker of mesenchymal phenotype) was evaluated by immunostaining in serial bladder tumor paraffin-embedded sections. Shown in Fig. 1 are characteristic images of N-cadherin immunoreactivity in bladder cancer specimens of different stages and grades. Quantitative analysis of the staining profile revealed a significantly increased expression of N-cadherin with increased tumor stage and grade (Fig. 1). Immunoreactivity profile of E-cadherin in serial sections of bladder tumors demonstrated a significant decrease in E-cadherin expression with increasing tumor stage and grade (Fig. 2). These changes implicate an association between induction of EMT and invasive potential of urothelial carcinoma.
Fig. 1.
N-cadherin expression in human bladder tumors. A, Representative images of immunostaining of human bladder cancer specimens of increasing pathological stage (×100; inset, ×400). B, Quantitative analysis of N-cadherin immunoreactivity by stage. One-way analysis of variance revealed a P value less than .0256; level of significance was set at P < .05. *Values represent mean ± SEM. C and D, Representative images of N-cadherin staining in human bladder cancer specimens by tumor grade with quantitative analysis, respectively.
Fig. 2.
E-cadherin expression in human bladder tumors. A, Representative images of immunostaining of human bladder cancer tissue specimens of increasing pathological stage (×100; inset, ×400). B, Quantitative analysis of N-cadherin immunoreactivity by stage. One-way analysis of variance revealed a P value less than .0001; level of significance was set at P < .05. *Values represent mean ± SEM. C and D, Representative images of E-cadherin staining in human bladder cancer specimens by tumor grade with quantitative analysis, respectively.
3.2. Cofilin and actin remodeling in bladder cancer
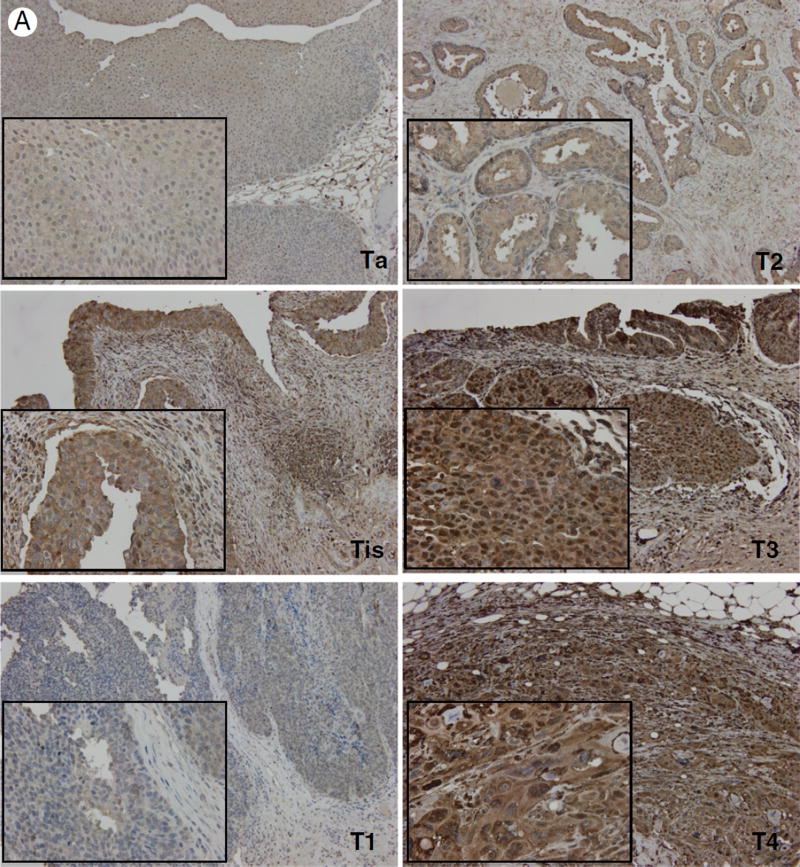
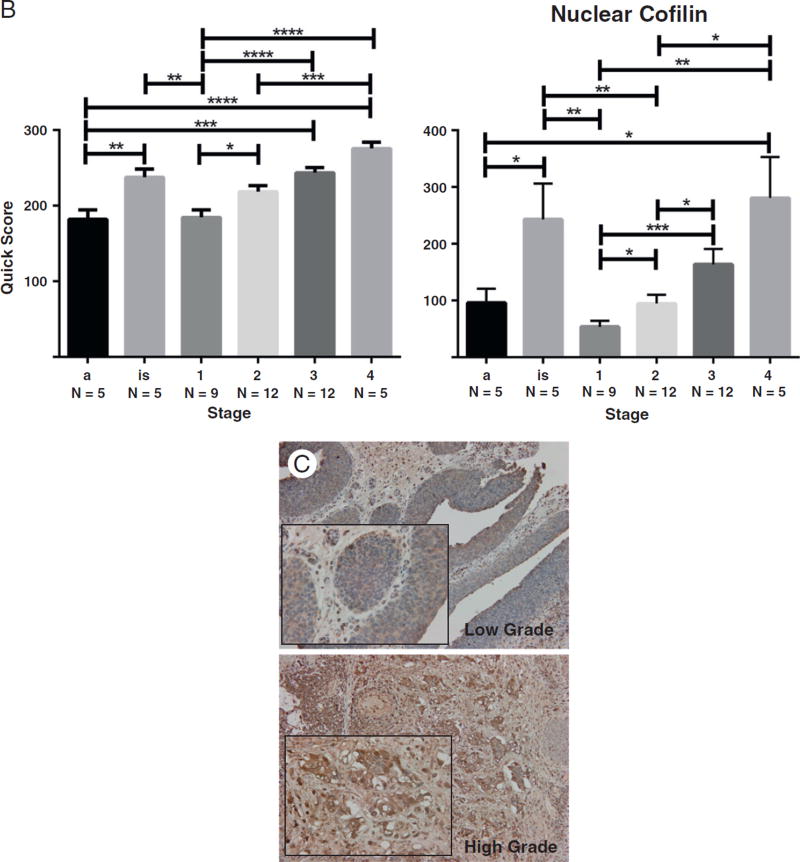
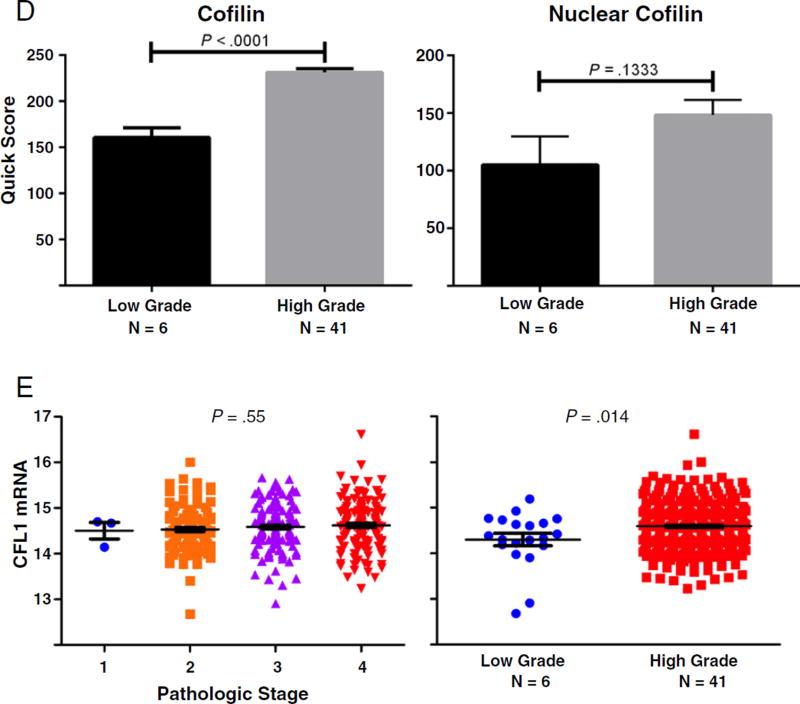
There is a compelling body of work to indicate that during TGF-β–mediated EMT, induction of Snail, Slug, and Twist transcription factors contributes to dissolution of cell junction complexes and consequent functional interference with the actin cytoskeleton organization [13,26–28]. We therefore examined the expression profile and localization of cofilin, a protein required for the actin cytoskeleton organization and a critical effector of TGF-β signaling in prostate cancer [23]. Analysis of cofilin immunoreactivity in bladder cancer specimens demonstrated a significant increase in cofilin expression that correlated with increasing tumor stage and grade (Fig. 3). In addition, we observed increased localization of cofilin to the nucleus with increasing tumor stage and grade (Fig. 3). Over-expression of cytosolic and nuclear cofilin in in situ carcinomas was comparable to that of stage IV bladder tumors (Fig. 4). This implicates cofilin as an early phase EMT mediator relative to regulation of N- and E-cadherin expression, which was similar to that of papillary neoplasms (Figs. 1 and 2, respectively). Genome-wide association analysis depicts higher cofilin (CFL1) mRNA levels in higher-grade bladder tumors (Fig. 3). However, there was no statistically significant association between tumor stage and cofilin transcript levels.
Fig. 3.
Cofilin expression and localization in bladder cancer progression. A, Representative images of cofilin immunoreactivity in human bladder cancer specimens of increasing tumor stage (×100; inset, ×400). B, Quantitative analysis of total and nuclear expression of cofilin by stage. One-way analysis of variance revealed a P value less than .0001 for both total and nuclear cofilin; level of significance was set at P < .05. * denotes level of significance; values ± SEM. C and D, Representative images of cofilin staining in human bladder cancer specimens by tumor grade with quantitative analysis, respectively. A significant increase in total cofilin is found in high-grade bladder tumors (P < .0001). E, Expression of cofilin mRNA transcript levels relative to stage and grade in a data set of 371 urothelial carcinomas indexed in The Cancer Genome Atlas.
Fig. 4.
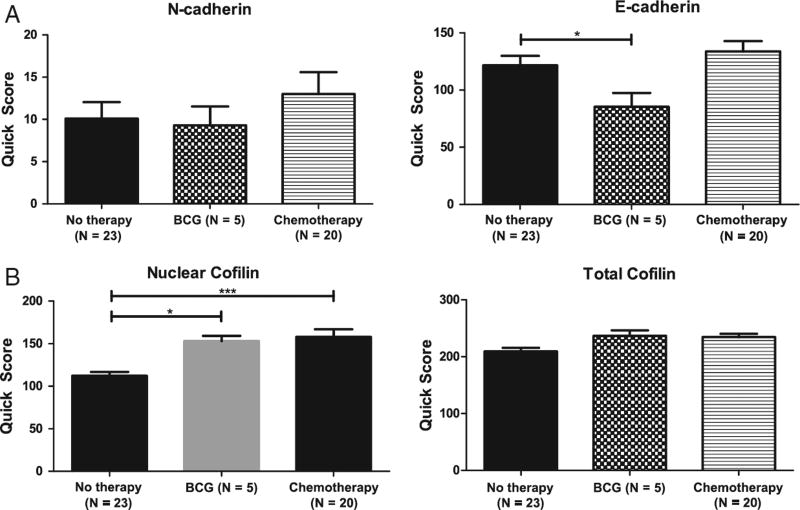
Effect of neoadjuvant therapy on EMT and cofilin expression in human bladder cancer specimens. Subset analysis of patients who underwent neoadjuvant chemotherapy of intravesical bacillus Calmette-Guerin. Correlation of immunoreactivity scoring for the EMT effectors N-cadherin and E-cadherin (A) and nuclear cofilin and total cofilin (B) is shown.
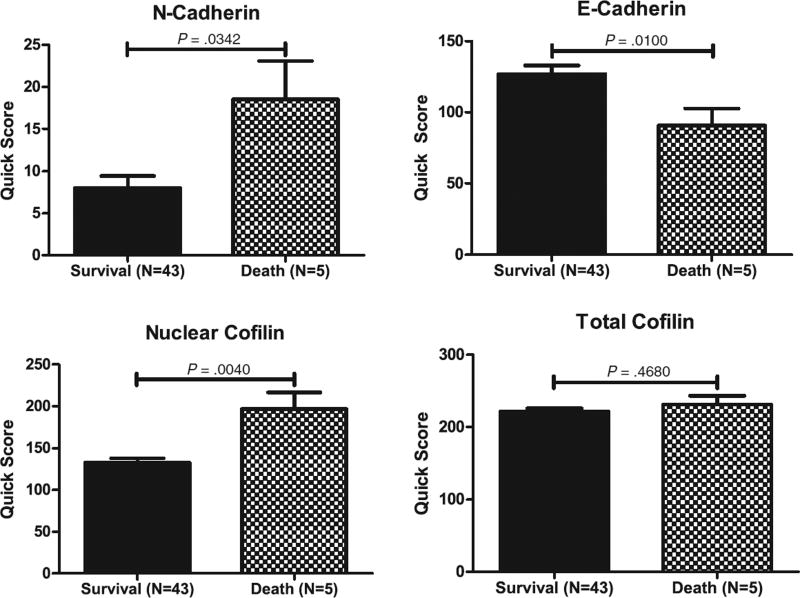
Cofilin and EMT effector expression was analyzed in a subset of patients who underwent neoadjuvant therapy relative to untreated. As shown in Fig. 4, Bacillus Calmette-Guerin treatment (but not chemotherapy) reduced E-cadherin expression, whereas there was no significant effect on N-cadherin. In response to neoadjuvant therapy, there was a significant increase in nuclear cofilin immunoreactivity in bladder cancer specimens (Fig. 4). Analysis of patient clinical outcomes in the context of EMT and cofilin profile revealed a significant correlation between EMT (characterized by reduced E-cadherin and increased N-cadherin levels) and increased nuclear expression of cofilin in patients with bladder cancer– specific mortality among the patient cohort studied (Fig. 5).
Fig. 5.
Association between EMT effectors and cofilin expression in bladder cancer–specific mortality. Immunoreactivity for N-cadherin, E-cadherin, and cofilin was correlated with bladder cancer–specific mortality in the patient cohort analyzed. Increased N-cadherin, reduced E-cadherin, and increased nuclear cofilin were associated with lethal bladder cancer (P = .0342, P = .0100, and P = .0040, respectively).
4. Discussion
Coordinated molecular and genetic events engendering phenotypic alterations in the tumor landscape are associated with the acquisition of mesenchymal traits by the tumor epithelial cells. The phenotypic fluidity that characterizes EMT enables a considerably dynamic transdifferentiation cycle involved in tumorigenesis, with the reversed process, MET, being potentially involved in metastatic urothelial carcinoma cells to invade target tissue to regain epithelial phenotypic traits and become established as secondary tumors [29]. Cadherin switching and profiling of EMT transcription factors Snail, Slug, and Twist in human bladder carcinoma has been reported by other investigators in efforts to characterize EMT in urothelial bladder progression [30]. This study provides the first evidence to define the significance of the EMT landscape in the context of actin cytoskeleton dynamics as controlled by cofilin in human bladder cancer progression to advanced disease. Our findings are of high significance in identifying the biomarker value of these phenotypic conversions in urothelial cancer progression, as well as targeting value for novel chemotherapeutics. It is the signaling activities of mesenchymal cells that facilitate migration and survival in an anchorage-independent anoikis-defying mode [31,32].
A previous study by an independent group has linked phosphorylated cofilin expression to urothelial carcinoma invasive capacity and progression [33]. The present work demonstrates an association of high nuclear localization of cofilin with increasing stage and tumor progression. The expression profile of cofilin in in situ carcinoma specimens paralleled that of advanced-stage and -grade tumors in accord with the existing evidence. Thus, one may argue that cofilin overexpression potentially serves the role of an early phase marker of increased aggressive and invasive properties of bladder tumors. Our findings are consistent with recent evidence suggesting the potential value of cofilin in the context of the actin cytoskeleton in the development of prostate cancer and lymph node metastasis [34]. Phosphorylation of cofilin in colorectal cancer cells caused by migration inhibitory factor accelerated mobility of cancer cells toward an invasive and metastatic phenotype [35]. Moreover, our results demonstrate a significant association between EMT and increased nuclear cofilin levels and bladder cancer–related death. Taken together, this evidence suggests that parallel profiling of the EMT landscape and the actin cytoskeleton in bladder tumors may be of potential prognostic value in urothelial carcinoma progression to lethal disease. Because cofilin protein overexpression in aggressive tumors is not directly mirrored by mRNA transcript levels (Fig. 4), up-regulation of alternative mechanisms involving posttranscriptional modification or translational control may account the role of cofilin in advance disease and provides an investigative platform for further interrogation of therapeutic targeting and biomarker validation.
In summary, this study provides new insights into the significance of 2 tumor microenvironment fronts: actin cytoskeleton reorganization and EMT-MET cycling transitions in invasive bladder urothelial cancer. In advanced stages of tumorigenesis, cellular redifferentiation via MET accounts for similarities between primary tumors and metastatic lesions. EMT reversal to MET includes the reestablishing of epithelial polarity which contributes to the viability of metastatic lesions. We speculate that cofilin-mediated actin cytoskeleton remodeling primes cellular dynamics of urothelial tumor cells toward an enhanced therapeutic response to chemotherapy. Ongoing investigations are pursuing the phenotypic and molecular profiling of a larger patient cohort (after neoadjuvant chemotherapy), including matching primary bladder tumors with metastatic disease.
Acknowledgments
The authors acknowledge the assistance of Lorie Howard in the preparation and submission of the manuscript.
Abbreviations
- EMT
epithelial-mesenchymal transition
- TGF-β
transforming growth factor-β
- MET
mesenchymal-epithelial transition
- ZEB1/2
zinc finger E-box binding homeobox 1 and 2
Footnotes
Competing Interest: The authors declare no potential conflict.
Funding/Support: Supported by the James F. Hardymon Endowment in Urology Research, University of Kentucky College of Medicine.
Dr Natasha Kyprianou is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity and accuracy of the data analysis.
References
- 1.Siegel R, Miller K, Jemal A. Cancer statistics, 2015. Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Ro JY, Staerkel GA, Ayala AG. Cytologic and histologic features of superficial bladder cancer. Urol Clin North Am. 1992;19:435–453. [PubMed] [Google Scholar]
- 3.Holmang S, Hedelin H, Anderstrom C, Johansson SL. The relationship among multiple recurrences, progression and prognosis of patients with stages Ta and T1 transitional cell cancer of the bladder followed for at least 20 years. J Urol. 1995;153:1823–1826. [PubMed] [Google Scholar]
- 4.Morgan TM, Clark PE. Bladder cancer. Curr Opin Oncol. 2010;22:242–249. doi: 10.1097/CCO.0b013e3283378c6b. [DOI] [PubMed] [Google Scholar]
- 5.Abida W, Bajorin DF, Rosenberg JE. First-line treatment and prognostic factors of metastatic bladder cancer for platinum-eligible patients. Hematol Oncol Clin North Am. 2015;29:319–328. doi: 10.1016/j.hoc.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Sonpavde G, Jones BS, Bellmunt J, Choueiri TK, Sternberg CN. Future directions and targeted therapies in bladder cancer. Hematol Oncol Clin North Am. 2015;29:361–376. doi: 10.1016/j.hoc.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Carosella ED, Ploussard G, LeMaoult J, Desgrandchamps F. A systematic review of immunotherapy in urologic cancer: evolving roles for targeting of CTLA-4, PD-1/PD-L1, and HLA-G. Eur Urol. 2015;68:267–279. doi: 10.1016/j.eururo.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 8.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Ouyang G, Wang Z, Fang X, Liu J, Yang CJ. Molecular signaling of the epithelial to mesenchymal transition in generating and maintaining cancer stem cells. Cell Mol Life Sci. 2010;67:2605–2618. doi: 10.1007/s00018-010-0338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mallini P, Lennard T, Kirby J, Meeson A. Epithelial-to-mesenchymal transition: what is the impact on breast cancer stem cells and drug resistance. Cancer Treat Rev. 2014;40:341–348. doi: 10.1016/j.ctrv.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Martin SK, Kamelgarn M, Kyprianou N. Cytoskeleton targeting value in prostate cancer treatment. Am J Clin Exp Urol. 2014;2:15–26. [PMC free article] [PubMed] [Google Scholar]
- 13.Findlay VJ, Wang C, Watson DK, Camp ER. Epithelial-to-mesenchymal transition and the cancer stem cell phenotype: insights from cancer biology with therapeutic implications for colorectal cancer. Cancer Gene Ther. 2014;21:181–187. doi: 10.1038/cgt.2014.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hensley PJ, Desiniotis A, Wang C, Stromberg A, Chen CS, Kyprianou N. Novel pharmacologic targeting of tight junctions and focal adhesions in prostate cancer cells. PLoS One. 2014;9:e86238. doi: 10.1371/journal.pone.0086238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hugo H, Ackland ML, Blick T, et al. Epithelial-mesenchymal transition and mesenchymal-epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 16.Matuszak E, Kyprianou N. Androgen regulation of epithelial-mesenchymal transition in prostate tumorigenesis. Expert Rev J Clin Endocrinol Metab. 2011;6:469–482. doi: 10.1586/eem.11.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu M, Kyprianou N. Role of androgens and the androgen receptor in epithelial-mesenchymal transition and invasion of prostate cancer cells. FASEB J. 2010;24:769–777. doi: 10.1096/fj.09-136994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bryan RT, Atherfold PA, Yeo Y, et al. Cadherin switching dictates the biology of transitional cell carcinoma of the bladder: ex vivo and in vitro studies. J Pathol. 2008;215:184–194. doi: 10.1002/path.2346. [DOI] [PubMed] [Google Scholar]
- 19.Fondrevelle ME, Kantelip B, Reiter RE, et al. The expression of Twist has an impact on survival in human bladder cancer and is influenced by the smoking status. Urol Oncol. 2009;27:268–276. doi: 10.1016/j.urolonc.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Wallerand H, Robert G, Pasticier G, et al. The epithelial-mesenchymal transition-inducing factor TWIST is an attractive target in advanced and/or metastatic bladder and prostate cancers. Urol Oncol. 2010;28:473–479. doi: 10.1016/j.urolonc.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 21.Yu Q, Zhang K, Wang X, Liu X, Zhang Z. Expression of transcription factors Snail, Slug, and Twist in human bladder carcinoma. J Exp Clin Cancer Res. 2010;29:119–121. doi: 10.1186/1756-9966-29-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenney PA, Wszolek MF, Rieger-Christ KM, et al. Novel ZEB1 expression in bladder tumorigenesis. BJU Int. 2011;107:656–663. doi: 10.1111/j.1464-410X.2010.09489.x. [DOI] [PubMed] [Google Scholar]
- 23.Collazo J, Zhu B, Larkin S, et al. Cofilin drives cell-invasive and metastatic responses to TGF-β in prostate cancer. Cancer Res. 2014;74:2362–2373. doi: 10.1158/0008-5472.CAN-13-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyamoto H, Miller JS, Fajardo DA, Lee TK, Netto GJ, Epstein JI. Non-invasive papillary urothelial neoplasms: the 2004 WHO/ISUP classification system. Pathol Int. 2010;60:1–8. doi: 10.1111/j.1440-1827.2009.02477.x. [DOI] [PubMed] [Google Scholar]
- 25.Barnes DM, Dublin EA, Fisher CJ, Levison DA, Millis RR. Immunohistochemical detection of p53 protein in mammary carcinoma: an important new independent indicator of prognosis? Hum Pathol. 1993;24:469–476. doi: 10.1016/0046-8177(93)90158-d. [DOI] [PubMed] [Google Scholar]
- 26.Kajita M, McClinic KN, Wade PA. Aberrant expression of the transcription factors snail and slug alters the response to genotoxic stress. Mol Cell Biol. 2004;24:7559–7566. doi: 10.1128/MCB.24.17.7559-7566.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thuault S, Valcourt U, Kowanetz M, Moustakas A. Transforming Growth Factor-β in Cancer Therapy. I. NJ: Humana Press; 2008. TGF-β and Smad signaling in transcriptome reprogramming during EMT; pp. 259–273. [Google Scholar]
- 28.Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27:6958–6969. doi: 10.1038/onc.2008.346. [DOI] [PubMed] [Google Scholar]
- 29.Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW, Williams ED. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res. 2006;66:11271–11278. doi: 10.1158/0008-5472.CAN-06-2044. [DOI] [PubMed] [Google Scholar]
- 30.Bryan RT, Tselepis C. Cadherin switching and bladder cancer. J Urol. 2010;184:423–431. doi: 10.1016/j.juro.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 31.Gregory PA, Bracken CP, Smith E, et al. An autocrine TGF-Beta/ZEB/ miR-200 signaling network regulates establishment and maintenance of epithelial-mesenchymal transition. Mol Biol Cell. 2011;22:1686–1698. doi: 10.1091/mbc.E11-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheel C, Eaton EN, Hsin-Jung Li S, et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145:826–840. doi: 10.1016/j.cell.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung H, Kim B, Jung SH, et al. Does phosphorylation of cofilin affect the progression of human bladder cancer? BMC Cancer. 2013;13:45. doi: 10.1186/1471-2407-13-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li L, Fu NI, Luo XU, Li XY, Li XP. Overexpression of cofilin 1 in prostate cancer and the corresponding clinical implications. Oncol Lett. 2015;9:2757–2761. doi: 10.3892/ol.2015.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu CT, Guo LL, Feng N, et al. MIF, secreted by human hepatic sinusoidal endothelial cells, promotes chemotaxis and outgrowth of colorectal cancer in liver prometastasis. Oncotarget. 2015;6:22410–22423. doi: 10.18632/oncotarget.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]