Abstract
Monitoring of marine mammal steroid hormone status using matrices alternative to blood is desirable due to the ability to remotely collect samples, which minimizes stress to the animal. However, measurement techniques in alternative matrices such as blubber described to date are limited in the number and types of hormones measured. Therefore, a new method using bead homogenization to QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) extraction, C18 post extraction cleanup and analysis by liquid chromatography tandem mass spectrometry (LC-MS/MS) was developed and applied to the measurement of hormone suites in bottlenose dolphin blubber. Validations were conducted in blubber from fresh dead stranded bottlenose dolphin. The final method consisting of two LC separations and garnet bead homogenization was tested for extraction efficiencies. Steroids were separated using a biphenyl column for reproductive hormones and C18 column for corticosteroids. Three hormones previously noted in blubber, testosterone, progesterone, and cortisol, were quantified in addition to previously unmeasured androstenedione, 17-hydroxyprogesterone, 11-deoxycortisol, 11-deoxycorticosterone, and cortisone in a single sample (0.4 g blubber). Extraction efficiencies of all hormones from blubber ranged from 84% to 112% and all RSDs were comparable to those reported using immunoassay methods (< 15%). The method was successfully applied to remote biopsied blubber samples to measure baseline hormone concentrations. Through this method, increased coverage of steroid hormone pathways from a single remotely collected sample potentially enhances the ability to interpret biological phenomena such as reproduction and stress in wild dolphin populations.
Keywords: Liquid chromatography, Tandem mass spectrometry, Steroids, Dolphin, Blubber
Introduction
Bottlenose dolphins (Tursiops truncatus) are widely distributed in coastal waters of temperate and subtropical regions. Their distribution in near-shore regions and role as apex predators have led to their use as sentinels which provide early warning of threats from zoonotic pathogens, biological toxins, and chemical contaminants including endocrine disruptors [1–3]. However, evaluating endocrinology of wild-ranging dolphin populations is problematic because blood sampling requires capture and release, which is difficult, expensive, and stressful to the animal. Additionally, stress alters the profiles of reproductive and stress hormones, making interpretation of endocrine measurements from stressed animals difficult [4, 5]. Development of techniques to extract and measure steroids in remotely collected alternative matrices that are endocrinologically relevant are needed.
Previous research has demonstrated that dolphin blubber, which can be obtained remotely using dart biopsy technology [6, 7] is a promising alternative matrix for endocrine measurement and has proven informative for reproductive studies. Testosterone concentrations have been used to determine sexual maturity in males and progesterone concentrations have been used to determine pregnancy in wild-ranging dolphins [8–11]. In addition, there is some evidence that blubber cortisol concentrations could be indicative of stress status in dolphins [12, 13].
Currently, methods for the determination of steroid hormones in blubber samples involve a liquid-liquid extraction to enzyme-linked immunosorbent assay (ELISA) detection [6]. Each ELISA is limited to one steroid hormone per analysis, requiring multiple assays, multiple sample aliquots, and potentially multiple extractions to develop endocrine profiles. Additionally, ELISA is an indirect measurement technique relying on antibody binding which is prone to cross-reactivity among the similarly structured hormones and could lead to inaccuracies when comparing across studies that might not adhere to the exact same techniques [14].
Mass spectrometry (MS) methods are advantageous over ELISA techniques in that they are direct detection methods (i.e. MS measures the mass and fragmentation pattern of the hormone rather than measuring a signal generated by a reaction with the hormone) [15]. However, MS methods for the measurement of steroid hormones in marine mammal matrices remain narrow in scope by analyzing only one or two steroids. Gas chromatography (GC) has been used to measure cortisol concentrations in the skin of harbor porpoises [16], but GC-MS methods require derivatization of hydroxyl and amine groups. Cortisol has three hydroxyl groups; therefore, derivatization must be complete to accurately and reproducibly measure cortisol using GC-MS technology. Alternatively, liquid chromatography tandem MS (LC-MS/MS) does not require derivatization to accurately measure steroid hormones. Existing LC-MS methods to measure steroid hormones in alternative cetacean matrices include the analysis of testosterone in bottlenose dolphin saliva and blow [17] and testosterone and progesterone have been measured in North Atlantic right whale blow. [18] To our knowledge, methods have not attempted to analyze hormone suites (androgens, corticosteroids, and progestogens) in alternative marine mammal matrices.
Steroid hormone structures share a cholesterol backbone (many with a log Kow of 3 or higher), thus extraction and measurement of hormones in the lipid-rich blubber matrix presents novel difficulties for LC-MS techniques that can be sensitive to lipid interferences. Despite similar structures of the hormones, the solvent affinities of these compounds vary from hexane for progesterone (polarity index of 0.1) to acetonitrile for cortisol (polarity index of 5.8); consequently, capturing all the hormones in a single extraction while sufficiently removing remaining lipids that can interfere with quantification is difficult.
In this manuscript we describe how we were able to leverage new broad extraction techniques that include lipid removal steps coupled with the greater sensitivity of mass spectrometry methods to achieve steroid hormone profiling of blubber. In this manuscript, we describe a QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) extraction, C18 dispersive solid-phase extraction (dSPE) lipid clean up, to isotope dilution LC-MS/MS method that simultaneously monitors nine hormones in blubber samples. QuEChERS extractions typically have been used for pesticide analysis as it extracts analytes into an organic phase by saturating the aqueous phase using buffered salts (salting-out) whereas C18 dSPE methods aid in the removal of lipids. These extractions allow recovery of a wide range of analytes. Our quality assessment and analysis conducted on stranded bottlenose dolphin blubber successfully quantified eight steroid hormones (five of which were not previously quantified in blubber) in a sample comparable in size to half a typical dart biopsy (approximately 0.4 g) with comparable precision to ELISA techniques (relative standard deviation herein defined as RSD < 15%). Additionally, we applied the developed method to dart biopsies from bottlenose dolphins to demonstrate the ability to measure trace hormone concentrations in relatively unstressed dolphins.
Materials and methods
Sample collection
In order to obtain a large amount of dolphin blubber for method development, samples were collected from dead bottlenose dolphins. Large samples of blubber (approximately 50 g full depth) were collected from two stranded dolphins reported to the Marine Mammal Stranding Network in South Carolina (authorized through a Letter of Authorization from the National Oceanic and Atmospheric Administration’s (NOAA) National Marine Fisheries Service) that had to be euthanized (i.e., code 1). Samples were collected after the dolphins were euthanized. SC1426 was identified as an adult male, possibly senescent, 264.5 cm in length. SC0740 was determined to be a young male 250 cm in length. Blubber sections were stored at −80 °C, minimally thawed for sectioning and stored again at −80 °C until sample processing.
Blubber from stranded animals was used for validation experiments where large homogenous sections of blubber were needed to create replicates. Though there is some concern that development of a method in blubber from a stranded animal might have different matrix qualities compared to a living dolphin (i.e. lipid content reduction due to stress) these changes are most likely minimal. Code 2 (fresh dead) stranded dolphins that have experienced more degradation than code 1 (alive) stranded dolphins were found to have lipid concentrations averaging 44.58%, which is comparable to live wild-ranging dolphins (McFee unpublished). Therefore, we do not expect that conducting validations in stranded dolphin blubber will hinder the interpretation of the experiments in relation to blubber from live wild-ranging dolphins.
In addition, four blubber samples were collected from live, wild-ranging dolphins in the coastal waters near Charleston, South Carolina, via remote biopsy under the Marine Mammal Protection Act Permit numbers 779–1633 and 14,450. Upon collection, samples (approximately 0.8 g) were immediately processed using acetone and hexane rinsed forceps and scalpels. Skin was removed from the biopsy and the blubber was halved to produce two full depth sections. One half, sectioned longitudinally, was collected for this study while the other half was stored for future contaminant analysis. Both halves were placed into individual cryovials and flash frozen in a liquid nitrogen dewar until transported to the National Institute of Standards and Technology Marine Environmental Specimen Bank at the Hollings Marine Laboratory where they were stored in liquid nitrogen freezers until analysis.
After initially testing method reproducibility using blubber from stranded animals, the four remote sampled biopsies from two male and two female adult bottlenose dolphins (sex confirmed by genetic analysis of skin samples) were tested, to explore this method for measurement of baseline concentrations of hormones from non-stressed dolphins.
Internal standards and calibration standards
Internal standards testosterone-13C3, androstenedione-13C3, 17-hydroxyprogesterone-13C3, and cortisol-d4 were purchased from Cerilliant (Round Rock, TX) and were determined to have 99.99% purity by the manufacturer. Progesterone-13C3 was purchased from Cambridge Isotopes (Tewksbury, MA) and was determined to have 98% purity by the manufacturer. Standards for calibration curves were purchased from various vendors. Androstenedione, testosterone, 17-hydroxyprogesterone, progesterone, corticosterone, cortisone, and cortisol were purchased from Sigma Aldrich (St. Louis, MO). 11-Deoxycorticosterone and 11-deoxycortisol were purchased from Steraloids (Newport, RI). The stated purity of all calibration standards was ≥98%.
Homogenization
Previous attempts in our laboratory to homogenize small dart biopsies from Tursiops truncatus using cryohomogenization, bead homogenization, and lyophilization before homogenization have all failed to yield reproducible results for different reasons. Either recovery of the cryohomogenized blubber from the grinding jar was insufficient for mass based calculations on a small biopsy or homogenization was visibly incomplete. Mincing blubber on dry ice before pressurized fluid extraction has been used as a preparatory step in persistent organic pollutant analyses [19], but further homogenization was required before proceeding to the QuEChERS extraction. Therefore, mincing on dry ice followed by bead homogenization was explored.
Blubber from stranded animals was minced in an acetone and hexane rinsed glass beaker on dry ice before bead homogenization using ceramic beads (2.8 mm; Mo Bio, Carlsbad, CA). To examine the homogeneity of steroid measurements following bead homogenization and QuEChERS extraction, minced blubber from SC1426 was mixed by hand and then aliquoted into three replicates of equivalent size to the biopsies (approximately 0.4 g) for analysis.
Isotopic dilution using labeled internal standards was applied to ensure quality of hormone measurements. First, empty tubes for the Precellys 24 bead homogenizer (Bertin Instruments; Montigny-le-Bretonneux, France) were labeled and weighed. Then internal standards (approximately equal to 5 ng of each internal standard; 100 µL total volume) were added to each tube (blanks, calibrants, and samples) and weighed again (see Electronic Supplemental Material (ESM) Table S1 for calibrant and internal standard concentrations). This internal standard mixture was used as a quality normalization factor to account for loss during extraction or differences in injection.
Finally, well mixed minced blubber (approximately 0.4 g) was added to the tube and weighed again to determine the blubber mass extracted. The samples, internal standard blanks (internal standard mixture without added sample), and a steroid hormone calibration curve in methanol (six calibrant concentrations; see ESM Table S1) were processed identically. Deionized water was added to the tube up to the 1.5 mL mark (approximately 800 µL) and samples were immediately homogenized in the bead homogenizer. All tubes were homogenized two times for 30 s each at 6500 rpm with a 30 s rest interval, cooled in a cold tube rack (stored in a – 80 °C freezer until use) for 5 min, and homogenized two additional times for 30 s each.
Different bead materials for homogenization tubes were tested for reproducibility. Ceramic (2.8 mm), metal (2.38 mm) and garnet (0.7 mm) beads from Mo Bio (Carlsbad, CA) were examined. Minced and mixed blubber from SC1426 was gravimetrically weighed into three replicates of approximate equivalent size (≈ 0.4 g) for each bead type, extracted and measured using the technique outlined above. Chi-squared analysis was conducted using JMP Statistical Software (SAS Institute, Cary, NC) to determine differences in quantification of steroids among the different bead types and RSDs were compared to select the most precise method.
Extraction and lipid clean up
Homogenized samples, internal standard blanks and calibration standards were extracted using the QuEChERS method outlined by Agilent (Santa Clara, CA) application note #5990-6589EN (Determination of Hormones in Shrimp) [20] with minor changes. Homogenized samples were poured into a 50 mL Falcon tube and the homogenization tube rinsed twice with 1.5 mL deionized water which was decanted into the 50 mL Falcon tube following each rinse. The volume in the Falcon tube was then brought up to the 5 mL mark with water and vortexed for 10 s. The homogenization tube was then similarly rinsed three times with acetonitrile and added to the 50 mL Falcon tube. The volume was brought up to 15 mL with acetonitrile then shaken vigorously by hand for 30 s. The salt packet from the extraction kit was then added to the tube and the sample was shaken by hand for 1 min. Samples were then centrifuged at 2900 × gn for 5 min at 4 °C using a Beckman Coulter Avanti J-20 XPI centrifuge in a swinging-bucket rotor (Brea, CA). Approximately 9 mL of the top acetonitrile layer (which was clearly delineated from the water by the solid waste layer) was then transferred to the C18 dSPE tube provided by the Agilent Bond Elute QuEChERS dispersive-SPE kit for Drug Residues in Meat (15 mL) for further lipid removal and clean up. C18 dSPE tubes were then vortexed for 1 min and centrifuged at 20000 × gn for 3 min at 4 °C using a fixed-angle rotor and the solvent layer (approximately 8 mL) was then transferred to borosilicate glass tubes and reduced to dryness under nitrogen at 35 °C using a Biotage TurboVap LV (Uppsala, Sweden). Samples were then reconstituted in 2 mL of 80:20 water: acetonitrile (volume fraction), vortexed for one min, then sonicated for 9 min. The solution was then filtered through a 0.22 µm cellulose acetate spin filter at 12000 × gn for 1 min. Filtrate was then transferred to a clean borosilicate glass culture tube, blown down to dryness under nitrogen at 35 °C, then reconstituted in 200 µL of methanol and transferred to a glass insert in an amber autosampler vial.
Instrumental method
The instrumental method is described in Boggs et al. [21] Briefly, a Restek (Bellefonte, PA) Ultra Biphenyl LC column (250 mm × 4.6 mm, 5.0 µm particle size) on an Agilent 1200 Series LC system equipped with a binary pump and autosampler in line with an AB Sciex (Framingham, MA) API4000 QTRAP hybrid triple quadrupole/linear ion trap mass spectrometer was used for measurement. Slight differences in the instrumental method were adjusted for the measurement of steroids in blubber matrices and are as follows. Chromatographic separation was achieved using the gradient outlined in Boggs et al. [21] with increased wash and equilibration time (100% acetonitrile with 0.1% formic acid for 5 min and 20% acetonitrile with 0.1% formic acid for 10 min, respectively). Two transitions were monitored in positive mode ([M + H]+) for each steroid. The largest signal was used for quantitation and the second fragment used for qualitative analyte confirmation (Table 1). Cortisone was included as an additional steroid not previously analyzed by Boggs et al. [21] as it is a metabolite of cortisol typically found in other species, but has never been measured in whales.
Table 1.
Steroid parameters for AB Sciex (Framingham, MA) API4000 QTRAP hybrid triple quadrupole/linear ion trap mass spectrometer tandem mass spectrometry monitoring quantitative (QN) and qualitative (QL) fragmentation patterns
| Steroid Common Name | Fragment Type |
Precursor Ion (m/z) |
Product Ion (m/z) |
Declustering Potential (V) |
Entrance Potential (V) |
Collision Energy (V) |
Cell Exit Potential (V) |
|---|---|---|---|---|---|---|---|
| Testosterone | QN | 289.1 | 97.2 | 100 | 15 | 30 | 12 |
| QL | 289.1 | 109.2 | 100 | 10 | 30 | 5 | |
| Testosterone-13C3 | QN | 292.6 | 112.2 | 75 | 10 | 40 | 5 |
| Androstenedione | QN | 287.2 | 97.2 | 100 | 15 | 30 | 12 |
| QL | 287.2 | 109.2 | 100 | 15 | 30 | 20 | |
| Androstenedione-13C3 | QN | 290.6 | 100.2 | 75 | 10 | 40 | 5 |
| Progesterone | QN | 315.1 | 97.2 | 75 | 15 | 25 | 10 |
| QL | 315.1 | 109.2 | 100 | 15 | 30 | 20 | |
| Progesterone-13C3 | QN | 318.4 | 112.2 | 50 | 10 | 35 | 5 |
| 17-OH-Progesterone | QN | 331.2 | 97.2 | 75 | 10 | 25 | 10 |
| QL | 331.2 | 109.2 | 100 | 15 | 35 | 20 | |
| 17-OH-Progesterone-13C3 | QN | 334.6 | 100.1 | 40 | 5 | 40 | 5 |
| Corticosterone | QN | 347.2 | 135.0 | 25 | 5 | 35 | 5 |
| QL | 347.2 | 121.3 | 40 | 5 | 20 | 15 | |
| 11-Deoxycortisol | QN | 347.3 | 109.2 | 25 | 5 | 35 | 10 |
| QL | 347.3 | 97.1 | 75 | 15 | 30 | 10 | |
| 11-Deoxycorticosterone | QN | 331.7 | 97.1 | 25 | 10 | 25 | 5 |
| QL | 331.7 | 109.3 | 75 | 10 | 35 | 5 | |
| Cortisone | QN | 361.4 | 163.3 | 100 | 5 | 35 | 12 |
| QL | 361.4 | 121.3 | 50 | 15 | 60 | 10 | |
| Cortisol | QN | 363.1 | 121.1 | 75 | 10 | 25 | 10 |
| QL | 363.1 | 267.3 | 25 | 5 | 30 | 30 | |
| Cortisol-d4 | QN | 367.4 | 121.3 | 75 | 10 | 25 | 10 |
After initial experiments, we found that corticosteroid detection was suppressed in this separation, which could be problematic for the measurement of trace baseline concentrations of stress hormones in unstressed animals. Therefore, a second LC method was optimized for the monitoring of corticosteroids in blubber extracts using a separation method similar to Tai et al. [22] A portion of the final extract (50 µL) was transferred to a borosilicate test tube, blown down at 35 °C in the TurboVap and reconstituted with 50 µL of 50:50 methanol: water (volume fraction). An Agilent Eclipse Plus C18 LC column (2.1 mm × 150 mm, 5.0 µm particle size) was used for the separation. Solvent flow was held at 46% methanol for 10 min, increased to 82.5% over 10 min, increased to 83.3% to over 5 min. The column was then washed at 100% methanol for 5 min. And re-equilibrated for 10 min.
Quantitation
Calibration standard mixtures and internal standard blanks were extracted identically to the samples. Internal standard blanks, which contained internal standards in methanol, were used to calculate the limit of detection (LOD). The LOD was calculated as three times the standard deviation plus the mean of the area of internal standard blanks for each analyte. The LOQ was defined as the lowest measured calibration standard unless the LOD was greater than the LOQ, in which case the blank-calculated LOD was defined as the LOQ. This is a conservative method for the calculation of a LOQ as outlined by Ragland et al. [23].
Hormones were quantified using isotopic dilution and by extrapolating from a linear regression of the extracted calibration curve (see ESM Table S2 for regression information). Briefly, analyte peak areas were divided by matched internal standard ratios (cortisol-d4 was used for all corticosteroids). Masses of each analyte were interpolated using a linear regression of at least three calibration standards that bracketed the sample peak area ratios. Concentrations were mass adjusted by dividing the calculated mass of each analyte by the extracted sample mass to yield ng/g concentrations.
Accuracy
Accuracies were determined by spike retrieval experiments. Nine aliquots of approximately 0.4 g of dolphin blubber from a mixture of SC1426 and SC0740 (both male code 1 stranded dolphins) were prepared using the methods described in the biopsy testing section. Five aliquots were measured for endogenous concentrations and four samples were gravimetrically spiked with calibrant B (see ESM Table S3 for spike and calibrant masses). All samples were tracked gravimetrically and spiked with the isotopic internal standard mixture. The samples were prepared using garnet bead homogenization, QuEChERS extraction, C18 dispersive SPE clean-up, and instrument methodologies described above.
Spike retrieval was calculated by comparing the expected total ng (mean endogenous concentrations adjusted by mass plus the known amount of spiked hormone) to the recovered total ng (concentration adjusted by mass from the sample with the spike). Acceptable extraction efficiencies were defined between 70% and 120% with an RSD below 15%.
Results
Chromatography and homogenization quality assessment
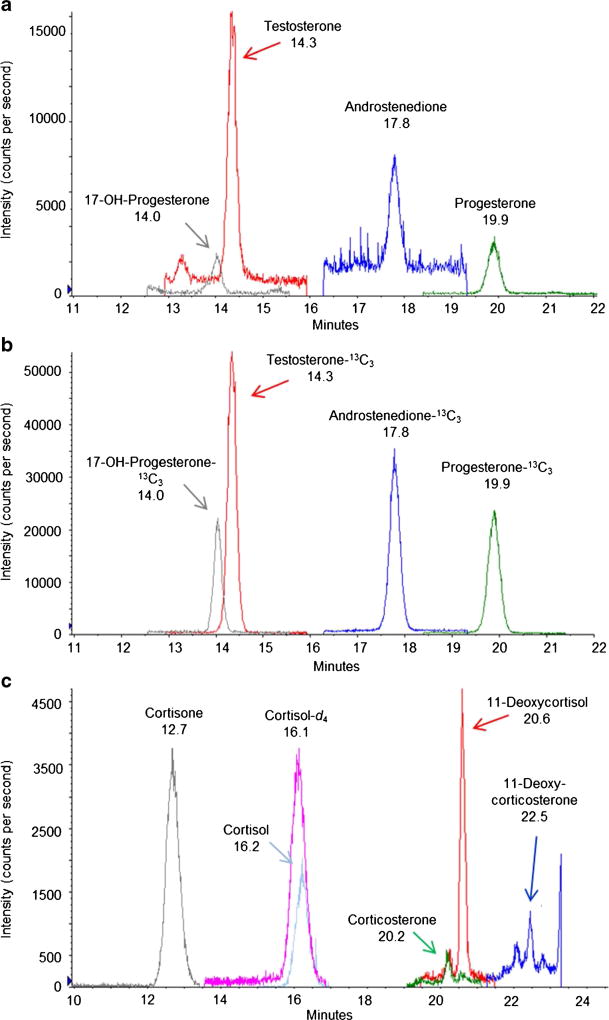
The bead homogenization to QuEChERS extraction followed by biphenyl and C18 LC separation methods provided reliable chromatographic separation and quantification of eight steroid hormones and five internal standards in bottlenose dolphin blubber. Chromatographic separation was achieved using a combination of two different chromatographic separations (Fig. 1). Though two runs increase the amount of instrument time needed, there was no additional extraction necessary and the reproducibility of measurements was increased on different hormone classes by utilizing both separation methods. Retention time RSDs of steroids among samples and calibration standards never exceed 0.5% and all steroids with retention time RSDs above 0.3% had matching internal standards to confirm identity. The biphenyl LC separation produced the lowest RSDs and LODs for the reproductive hormones (testosterone, progesterone, 17-hydroxyprogesterone, and androstenedione), while the C18 LC separation produced the better RSDs and reduced the LODs for the corticosteroids (cortisol, cortisone, 11-deoxycortisol, and corticosterone; Table 2). C18 LC separation increased the signal to noise ratio of corticosteroid analytes compared to the biphenyl LC separation. For example, the peak areas from the same cortisol calibration standard were 91,700 counts on the biphenyl LC separation and 574,000 counts on the C18 LC separation. Noise was reduced by half on the C18 LC separation versus the biphenyl LC separation (data not shown) thereby increasing the ability to detect trace concentrations of cortisol. The combination of improved detection, reduction of noise, and better RSD led to the decision to use a C18 LC separation for the corticosteroids. Using this combination of separations, all RSDs were comparable to ELISA techniques [8, 9, 13] with the added benefit that multiple steroids can be analyzed simultaneously.
Fig. 1.
Chromatographic separation of endogenous hormones in a blubber sample pool from male code 1 stranded Tursiops truncatus. a Reproductive steroids, b reproductive steroid internal standards, and c) corticosteroids and internal standards. Displayed peaks are the quantitative transitions. Reproductive steroids were separated using a biphenyl column and the corticosteroids were separated on a C18 column. Retention times are listed below the steroid name
Table 2.
Analysis of steroid hormones in stranded code-1 Tursiops truncatus (animal SC1426) blubber using two liquid chromatography separation techniques
| Analyte | Biphenyl (n = 3)
|
C18 (n = 3)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LOD (ng/g) | LOQ (ng/g) | Mean (ng/g) | Stdev | RSD (%) | LOD (ng/g) | LOQ (ng/g) | Mean (ng/g) | Stdev | RSD (%) | |
| 11-Deoxycortisol | 0.181 | 0.240 | 0.924 | 0.134 | 14.5 | 0.074 | 0.240 | 0.621 | 0.103 | 16.6 |
| 17-OH-Progesterone | 0.0580 | 0.237 | 1.42 | 0.14 | 10.2 | 0.221 | 0.839 | 1.38 | 0.25 | 18.4 |
| Androstenedione | 0.0667 | 0.238 | 2.00 | 0.23 | 11.5 | 0.216 | 0.464 | 1.96 | 0.22 | 11.2 |
| Corticosterone | 0.128 | 0.252 | 1.40 | 0.16 | 11.5 | 0.0496 | 0.488 | 1.25 | 0.06 | 4.98 |
| Cortisol | 0.357 | 0.400 | 14.1 | 2.00 | 14.4 | 0.263 | 0.400 | 10.2 | 0.60 | 5.84 |
| Cortisone | 0.339 | 0.821 | 9.08 | 0.41 | 4.60 | 0.140 | 0.821 | 5.82 | 0.10 | 1.67 |
| Progesterone | 0.132 | 0.247 | 1.96 | 0.20 | 9.90 | 0.229 | 0.398 | 1.82 | 0.32 | 17.9 |
| Testosterone | 0.0735 | 0.236 | 2.74 | 0.16 | 5.80 | NQ | 0.838 | 2.31 | 0.94 | 40.8 |
NQ: Not quantifiable
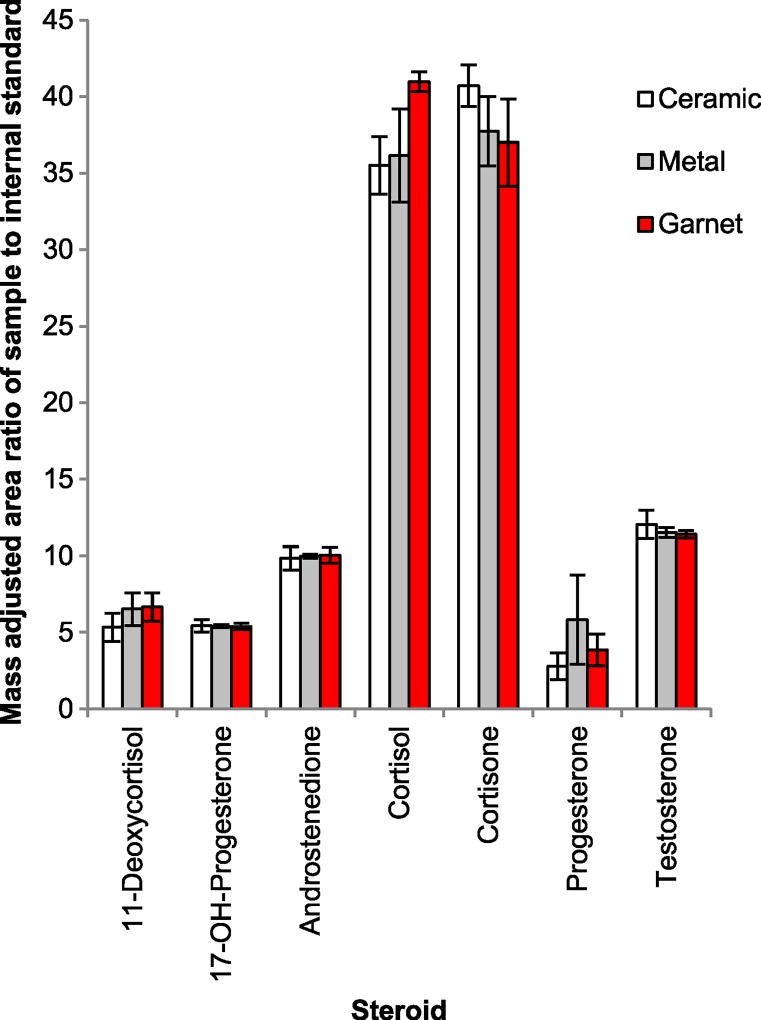
Bead materials were tested to determine differences in homogenization and precision. There was no significant difference (p ≤ 0.05 considered significant for all comparisons) in the mass adjusted peak area ratios among the three tested materials (ceramic, metal, and garnet; Fig. 2). However, upon visual inspection, garnet beads produced a more uniform homogenate. Metal and ceramic beads performed similarly on extraction, but occasionally small visible pieces of blubber could be observed remaining in the homogenate. Additionally, garnet beads yielded smaller standard deviations than the other bead materials for the most hormones analyzed. Therefore, garnet beads were selected for the extraction efficiency testing.
Fig. 2.
Comparison of bead materials used in homogenization of Tursiops truncatus blubber from code 1 stranded male blubber (n = 3). Error bars are ± standard deviations. All p-values were above 0.05, thus, there were no statistically significant differences
Application to remote dart biopsies
Application of this method to dart biopsies allowed the quantification of steroid hormones in the samples (Table 3). Initial screening of the biopsies did not detect 11-deoxycorticosterone or corticosterone, and therefore these analytes were not included in the analysis. Cortisol, progesterone, 17-hydroxyprogesterone, testosterone, and androstenedione were quantifiable in the male blubber samples. Female dart biopsy blubber samples had quantifiable cortisol. Cortisone concentrations were defined as detectable, but not quantifiable because calibration curves for this study were above the LOD but not sufficiently low to bracket the sample concentrations. Additionally, one female had detectable but not quantifiable progesterone also related to calibration curve concentrations above the sample concentration. With further testing with lower concentrations of calibration standards, these samples could be quantifiable. The low (non-quantifiable) concentrations of progesterone suggest that the two females sampled were not pregnant. Quantification in pregnant individuals is feasible based upon the LOD and LOQs here and previous measurements on pregnant individuals in the literature [11].
Table 3.
Concentrations (ng/g) of steroid hormones in remote biopsied blubber from Tursiops truncates
| Analyte | LOD | LOQ | TYP140227–01 | TYP140806–03 | TYP140730–01 | TYP140801–02 |
|---|---|---|---|---|---|---|
| Female | Female | Male | Male | |||
| Feb-14 | Aug-14 | Jul-14 | Aug-14 | |||
| 11-Deoxycortisol | 0.074 | 0.240 | < 0.074 | < 0.074 | 0.084* | < 0.074 |
| 17-OH-Progesterone | 0.0580 | 0.237 | < 0.0580 | < 0.0580 | 3.16 | 1.25 |
| Androstenedione | 0.0670 | 0.238 | < 0.0670 | < 0.0670 | 15.2 | 4.33 |
| Cortisone | 0.140 | 0.821 | 0.160* | 0.214* | 0.483* | 0.217* |
| Cortisol | 0.263 | 0.400 | 0.528 | 0.803 | 1.08 | 0.763 |
| Progesterone | 0.0910 | 0.247 | < 0.0910 | 0.150* | 0.473 | 0.262 |
| Testosterone | 0.0740 | 0.236 | < 0.0740 | < 0.0740 | 31.5 | 10.1 |
Above the LOD but not quantifiable due to concentration below the lowest calibration standard
Accuracy
After improvement of the signal to noise ratios of this method, 11-deoxycorticosterone was added to the analyte list. Previous attempts to measure this hormone were inconsistent or the analyte was not detected and therefore the calibration standards did not include 11-deoxycorticosterone. However, after improving the methods by use of a C18 LC separation, 11-deoxycorticosterone was detectable, and was therefore quantified and tested for reliability during the spike retrieval experiment.
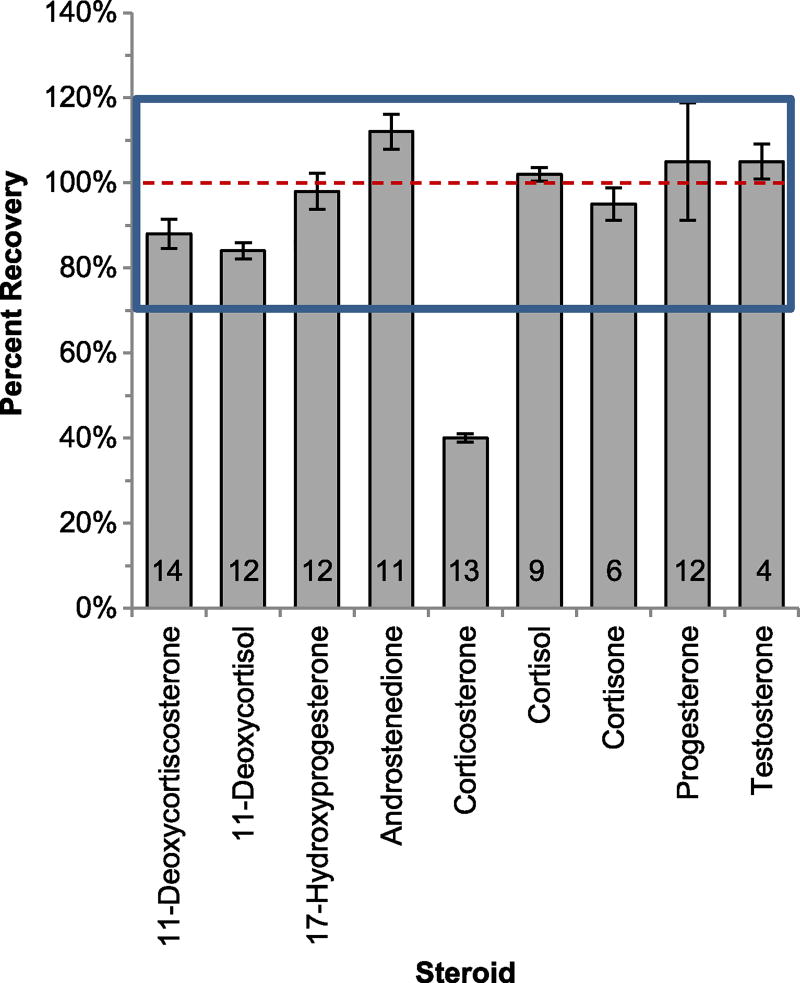
With the exception of corticosterone, all hormones analyzed were within the acceptable limits with the lowest extraction efficiency equal to 84% (11-deoxycortisol) and the highest extraction efficiency equal to 112% (androstenedione; Fig. 3). The fraction of cortisol and cortisone recovered was similar (102% and 95% respectively) which is not surprising as they are similar in structure. Although matrix interferences such as lipids not removed by the C18 dSPE can cause inaccuracies, the spike retrievals in the accuracy experiments were within the acceptable limits defined, and as such, matrix interferences were minimal.
Fig. 3.
Extraction efficiencies of steroids from code-1 stranded dolphin blubber (reproductive steroids n = 3; corticosteroids n = 4). The dotted line delineates 100% extraction efficiency. The box denotes the range of acceptable extraction efficiencies. The number in the bar is the percent relative standard deviation (RSD) of the measurement of endogenous hormones. Error bars are ± standard deviations
Discussion
This method successfully extracts and simultaneously measures all three hormones previously noted in the literature (testosterone, progesterone, and cortisol) and further provides the first information on blubber concentrations of five previously undetected hormones, cortisone, 17-hydroxyprogesterone, 11-deoxycorticosterone, 11-deoxycortisol, and androstenedione, using a single method and relatively small sample mass. Not only is this method advantageous over ELISA for its ability to use mass and structural fragmentation patterns to simultaneously identify and quantify multiple steroids, but there are a number of economic and timesaving advantages of mass spectrometry. There is a reduction in time as there is only one sample processing protocol and automated instrumentation as opposed to steroid specific extractions and manual analysis required by ELISAs. Although cost may be prohibitive for some laboratories due to the need for high tech instrumentation, the supplies and general equipment necessary to perform this extraction are highly cost effective (totaling around $25 per sample). Therefore, if there is access to instrumentation, there is a high return from eight steroid concentrations from one analysis as opposed to purchasing a commercial kit per steroid, in addition to extraction supply costs using ELISA.
Accuracy of this method was within the acceptable limits for all hormones except corticosterone, which could be due to a number of factors. Potentially, matrix interferences may coelute with corticosterone. Different chromatography methods or further sample clean up might improve corticosterone measurements. Additionally, the use of a corticosterone internal standard could improve accuracy as cortisol-d4 was used as a surrogate due to the unavailability of a commercial standard for corticosterone. However, this does not preclude the use of a similarly structured internal standard as the accuracy of the remaining corticosteroids was within the acceptable range using cortisol-d4 as a surrogate. Therefore, when using an internal standard that is not an exact match to the analyte, accuracy testing is necessary to determine applicability.
Some steroids had extraction efficiencies above 100%, likely a result from low concentrations of endogenous hormones, which increased the variability of the measurement. For example, progesterone had an RSD of 12% for the unspiked samples, which could contribute error resulting in the 7% elevation above the 100% spike retrieval. Given this is a solid material that cannot be stripped of hormones and spiked to determine extraction efficiencies, this increase in variability is unavoidable.
Additionally, because blubber is a complex matrix, which cannot be stripped of hormones, we were unable to implement a matrix matched calibration curve. However, by analyzing the accuracy of this method in combination with the use of isotopic dilution, potential matrix interferences that affect the accuracy of this method are quantified. Therefore, a correction factor based on this testing could be employed in future analyses, though the data presented in this manuscript are uncorrected to better interpret the capabilities of the method.
The applications of this method are expansive as demonstrated by the quantification of previously undetected hormones, which all have biological functions in other vertebrates. Investigating these biological endpoints in conjunction with measuring these hormones in blubber could provide more complete health information related to bottlenose dolphins than that gathered by the analysis of cortisol, testosterone, and progesterone alone.
The addition of cortisone and the other corticosteroid metabolites could assist in the modeling of the relationship between blood cortisol and blubber cortisol. Models on stress built by Champagne et al. reported a R2 value of only 57% between blood and blubber cortisol [13]. In other vertebrates, adipose tissue has been shown to convert cortisol to cortisone and it has been suggested that marine mammal blubber could perform this same conversion [24, 25]. Therefore, developing a model that includes cortisone and cortisol concentrations as predictors of blood cortisol concentrations could more accurately reflect blood cortisol and thereby stress status than measuring blubber cortisol alone.
Pregnancy in delphinids has been monitored using progesterone concentrations in blubber. However, using current methods, early pregnancies are not identifiable by blubber measurements alone as the concentrations of progesterone must rise above the model threshold that delineates between non-pregnant females that may be ovulating versus pregnant females. Androstenedione is an androgen, which could be used as a biomarker for pregnancy. Androstenedione is known to increase in the first trimester of pregnancy in other mammals, potentially as a pathway to increase estrone, estriol, and progesterone production in early pregnancy [26–28]. Therefore, increased androstenedione in females could be more sensitive in the detection of early pregnancies than measuring progesterone concentrations alone.
By analyzing a steroid profile rather than one hormone per assay, this method described here provides insight into the steroid production pathways most utilized by dolphins. For example, 17-hydroxyprogesterone and progesterone are in the pathway for production of androgens but also are upstream of production of corticosteroids. Among the dart biopsy blubber samples analyzed in this study, both males had detectable concentrations of 17-hydroxyprogesterone, while females, with comparable concentrations of cortisol and cortisone, did not have detectable 17-hydroxyprogesterone or progesterone concentrations. This suggests that male bottlenose dolphins could utilize 17-hydroxyprogesterone and progesterone for androgen production at baseline stress levels. Further analyses on a larger population and potentially in other matrices such as blood would assist in mapping the steroid synthesis pathways.
The values measured in dart biopsy blubber samples using this method are comparable to previously published values and have the added benefit of being measured by a single method from a single sample. The LOQ achieved with this method is sufficient to measure blubber hormones at concentrations that have been reported from previous research techniques [8–12]. Interestingly, progesterone was quantified only in male biopsy samples, and concentrations in the female samples were low (based upon the LOQ) compared to previously reported values from other delphinids [9, 11]. Previous reports on blubber progesterone in the bottlenose dolphin indicate that they have two-fold (non-pregnant) to six-fold (pregnant) lower concentrations of blubber progesterone as compared to other delphinid species [10]. This pilot application only examined four individual dart biopsies and did not contain calibration standards below the detected sample concentrations for progesterone, but the large increase in progesterone that coincides with pregnancy, in addition to potential increases in androstenedione, would likely allow our method to distinguish pregnant versus non-pregnant individuals. Many prior studies have examined concentrations in stranded or bycaught animals and though bycatch dolphins do experience an extreme stress event, they are the most relevant comparison in the literature for the dart biopsy samples measured here. This is because time to death is short among bycaught individuals compared to stranded individuals and there is a lag time between the increase of steroids in the blood and their transport to the blubber. Cortisol concentrations were approximately three-fold lower in our samples compared to those of bycaught D. delphis [12] and cortisone was not quantifiable in male or female biopsies. This was not surprising as animals from which dart biopsies were collected may experience some brief period of stress due to the ‘chase’ prior to being sampled, but this stress is greatly reduced from that experience by a bycaught animal. Regardless, our method was capable of quantifying cortisol concentrations as low as baseline (non-stressed). Additionally, with the inclusion of lower concentration calibration standards to improve the LOQ of cortisone, we have confidence that cortisone will be quantifiable at baseline concentrations in future studies. Ultimately, this method could provide baseline concentrations in remotely sampled blubber from relatively unstressed delphinids for testosterone, progesterone, and cortisol along with androstenedione, 17-hydroxyprogesterone, and potentially other steroids.
Conclusion
Here we have outlined the use of a QuEChERS extraction to LC-MS/MS analysis of steroid hormones for the reliable measurement of eight steroid hormones in a complex matrix, blubber. The extraction is reliable, general, and efficient for exploration of the endocrinology of bottlenose dolphins and likely other cetaceans. The method is sufficient to have the potential to quantify hormone suites in remote biopsied blubber samples, which could define baseline concentrations of wild populations. Further analyses of dart biopsied blubber samples from living, unstressed animals of different age classes, sex, and reproductive status will be conducted to determine the utility of this method for endocrine analyses of wild living dolphins. Through this methodology, the breadth of information collected from a single sample can be increased to explore pathway alterations in hormone physiology and provide more in depth endocrine health information, which is valuable for the monitoring and health of these protected species.
Supplementary Material
Acknowledgments
We would like to thank Louis J. Guillette Jr. for being the uniting force that made this collaborative project possible. Also, we would like to thank those who contributed their hard work in the coordination and collection of these samples including the South Carolina Marine Mammal Stranding Network, Brian Balmer, and Eric Zolman. We thank Patricia Rosel from the National Marine Fisheries Service, Southeast Fisheries Science Center for genetic analysis of the dart biopsies for sex determination. This research was made possible through a grant from the Office of Naval Research Marine Mammals and Biology Program and additional funding was provided by the National Institute of Standards and Technology, the National Oceanic and Atmospheric Administration, Marine Mammal Health and Stranding Response Program, and the National Academies National Research Council Research Associateship Program for a postdoctoral fellowship from 2013 to 2015.
This research was partially funded by the Office of Naval Research (ONR) under grant award numbers N0001412IP20053, N0001411IP20085, and N000141110542. Commercial equipment, instruments, or materials are identified to specify adequately the experimental procedure. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology nor the National Oceanographic and Atmospheric Administration, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00216-017-0446-z) contains supplementary material, which is available to authorized users.
Compliance with ethical standards All research protocols used were approved by a NOAA Institutional Animal Care and Use Committee. Collections were conducted in an ethical manner in concordance with ethical standard guidelines provided by the Office of Protected Resources, Marine Mammal Health and Stranding Response Program and Animal Welfare Act and under the NOAA authorization 109(h) of the Marine Mammal Protection Act.
The authors declare that they have no conflict of interest in the publication of this manuscript.
References
- 1.Tanabe S. Contamination and toxic effects of persistent endocrine disrupters in marine mammals and birds. Mar Pollut Bull. 2002;45(1–12):69–77. doi: 10.1016/s0025-326x(02)00175-3. [DOI] [PubMed] [Google Scholar]
- 2.Hoekstra PF, Dehn LA, George JC, Solomon KR, Muir DCG, O'Hara TM. Trophic ecology of bowhead whales (Balaena mysticetus) compared with that of other arctic marine biota as interpreted from carbon-, nitrogen-, and sulfur-isotope signatures. Can J Zool-Rev Can Zool. 2002;80(2):223–31. doi: 10.1139/z01-229. [DOI] [Google Scholar]
- 3.Schwacke LH, Gulland FM, White S. Sentinel species in oceans and human health. In: Laws EA, editor. Environmental toxicology: selected entries from the encyclopedia of sustainability Science and technology. New York: Springer; 2013. pp. 503–28. [Google Scholar]
- 4.Stephens MAC, Mahon PB, McCaul ME, Wand GS. Hypothalamic-pituitary-adrenal axis response to acute psychosocial stress: effects of biological sex and circulating sex hormones. Psychoneuroendocrinology. 2016;66:47–55. doi: 10.1016/j.psyneuen.2015.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mastorakos G, Pavlatou MG, Mizamtsidi M. The hypothalamic-pituitary-adrenal and the hypothalamic-pituitary-gonadal axes interplay. Pediatr Endocrinol Rev. 2006;3(Suppl 1):172–81. [PubMed] [Google Scholar]
- 6.Atkinson S, Crocker D, Houser D, Mashburn K. Stress physiology in marine mammals: how well do they fit the terrestrial model? J Comp Physiol B: Biocem Syst Environ. 2015;185(5):463–86. doi: 10.1007/s00360-015-0901-0. [DOI] [PubMed] [Google Scholar]
- 7.Krutzen M, Barre LM, Moller LM, Heithaus MR, Simms C, Sherwin WB. A biopsy system for small cetaceans: darting success and wound healing in Tursiops SPP. Mar Mamm Sci. 2002;18(4):863–78. doi: 10.1111/j.1748-7692.2002.tb01078.x. [DOI] [Google Scholar]
- 8.Kellar NM, Trego ML, Marks CI, Chivers SJ, Danil K, Archer FI. Blubber testosterone: a potential marker of male reproductive status in short-beaked common dolphins. Mar Mamm Sci. 2009;25(3):507–22. doi: 10.1111/j.1748-7692.2009.00291.x. [DOI] [Google Scholar]
- 9.Kellar NM, Trego ML, Marks CI, Dizon AE. Determining pregnancy from blubber in three species of delphinids. Mar Mamm Sci. 2006;22(1):1–16. [Google Scholar]
- 10.Perez S, Garcia-Lopez A, De Stephanis R, Gimenez J, Garcia-Tiscar S, Verborgh P, et al. Use of blubber levels of progesterone to determine pregnancy in free-ranging live cetaceans. Mar Biol. 2011;158(7):1677–80. doi: 10.1007/s00227-011-1676-9. [DOI] [Google Scholar]
- 11.Trego ML, Kellar NM, Danil K. Validation of blubber progesterone concentrations for pregnancy determination in three dolphin species and a porpoise. PLoS One. 2013;8(7):1–9. doi: 10.1371/journal.pone.0069709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kellar NM, Catelani KN, Robbins MN, Trego ML, Allen CD, Danil K, et al. Blubber cortisol: a potential tool for assessing stress response in free-ranging dolphins without effects due to sampling. PLoS One. 2015;10(2):16. doi: 10.1371/journal.pone.0115257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Champagne CD, Kellar NM, Crocker DE, Wasser SK, Booth RK, Trego ML, et al. Blubber cortisol qualitatively reflects circulating cortisol concentrations in bottlenose dolphins. Mar Mamm Sci. 2016;33(1):134–53. doi: 10.1111/mms.12352. [DOI] [Google Scholar]
- 14.Fanelli F, Belluomo I, Di Lallo VD, Cuomo G, De Iasio R, Baccini M, et al. Serum steroid profiling by isotopic dilution-liquid chromatography-mass spectrometry: comparison with current immunoassays and reference intervals in healthy adults. Steroids. 2011;76(3):244–53. doi: 10.1016/j.steroids.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Stanczyk FZ, Clarke NJ. Advantages and challenges of mass spectrometry assays for steroid hormones. J Steroid Biochem Mol Biol. 2010;121(3–5):491–5. doi: 10.1016/j.jsbmb.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Bechshoft T, Wright AJ, Weisser JJ, Teilmann J, Dietz R, Hansen M, et al. Developing a new research tool for use in free-ranging cetaceans: recovering cortisol from harbour porpoise skin. Conserv Physiol. 2015;3:9. doi: 10.1093/conphys/cov016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hogg CJ, Vickers ER, Rogers TL. Determination of testosterone in saliva and blow of bottlenose dolphins (Tursiops truncatus) using liquid chromatography–mass spectrometry. J Chromatogr B. 2005;814(2):339–46. doi: 10.1016/j.jchromb.2004.10.058. [DOI] [PubMed] [Google Scholar]
- 18.Hogg CJ, Rogers TL, Shorter A, Barton K, Miller PJO, Nowacek D. Determination of steroid hormones in whale blow: it is possible. Mar Mamm Sci. 2009;25(3):605–18. doi: 10.1111/j.1748-7692.2008.00277.x. [DOI] [Google Scholar]
- 19.Litz JA, Garrison LP, Fieber LA, Martinez A, Contillo JP, Kucklick JR. Fine-scale spatial variation of persistent organic pollutants in bottlenose dolphins (Tursiops truncatus) in Biscayne Bay. Florida Environ Sci Technol. 2007;41(21):7222–8. doi: 10.1021/es070440r. [DOI] [PubMed] [Google Scholar]
- 20.Fu R, Zhai A. Determination of Hormones in Shrimp by Agilent 1290 Infinity LC with Agilent Poroshell 120 LC Column and Agilent Bond Elut QuEChERS for Sample Preparation. In: Technologies A, editor. Agilent Technologies; 2012. Application note. http://www.chem.agilent.com/Library/applications/5990-6589EN.pdf. [Google Scholar]
- 21.Boggs ASP, Bowden JA, Galligan TM, Guillette LJ, Kucklick JR. Development of a multi-class steroid hormone screening method using liquid chromatography/tandem mass spectrometry (LC-MS/MS) Anal Bioanal Chem. 2016;408(15):4179–90. doi: 10.1007/s00216-016-9512-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tai SS, Welch MJ. Development and evaluation of a candidate reference method for the determination of total cortisol in human serum using isotope dilution liquid chromatography/mass spectrometry and liquid chromatography/tandem mass spectrometry. Anal Chem. 2004;76(4):1008–14. doi: 10.1021/ac034966f. [DOI] [PubMed] [Google Scholar]
- 23.Ragland JM, Liebert D, Wirth E. Using procedural blanks to generate analyte-specific limits of detection for persistent organic pollutants based on GC-MS analysis. Anal Chem. 2014;86(15):7696–704. doi: 10.1021/ac501615n. [DOI] [PubMed] [Google Scholar]
- 24.Kershaw JL, Hall AJ. Seasonal variation in harbour seal (Phoca vitulina) blubber cortisol - a novel indicator of physiological state? Sci Rep. 2016;6:21889. doi: 10.1038/srep21889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Papadopoulos V, Vihma V. Steroid biosynthesis in adipose tissue. Steroids. 2015;103:89–104. doi: 10.1016/j.steroids.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 26.Begumhasan J, Murphy BEP. Invitro stimulation of placental progesterone production by 19-nortestostereone and C-19 steroids in early human-pregnancy. J Clin Endocrinol Metab. 1992;75(3):838–45. doi: 10.1210/jc.75.3.838. [DOI] [PubMed] [Google Scholar]
- 27.Legacki EL, Scholtz EL, Ball BA, Stanley SD, Berger T, Conley AJ. The dynamic steroid landscape of equine pregnancy mapped by mass spectrometry. Reproduction. 2016;151(4):421–30. doi: 10.1530/rep-15-0547. [DOI] [PubMed] [Google Scholar]
- 28.Castracane VD, Asch RH. Testosterone and androstenedione in premature ovarian failure pregnancies: evidence for an ovarian source of androgens in early-pregnancy. Hum Reprod. 1995;10(3):677–80. doi: 10.1093/oxfordjournals.humrep.a136010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.