Abstract
Objective:
This article compares trends in CD4+ T-cell recovery and proportions achieving optimal restoration (≥500 cells/μl) after viral suppression following combination antiretroviral therapy (cART) initiation between rapid and nonrapid progressors.
Methods:
We included HIV-1 seroconverters achieving viral suppression within 6 months of cART. Rapid progressors were individuals experiencing at least one CD4+ less than 200 cells/μl within 12 months of seroconverters before cART. We used piecewise linear mixed models and logistic regression for optimal restoration.
Results:
Of 4024 individuals, 294 (7.3%) were classified as rapid progressors. At the same CD4+ T-cell count at cART start (baseline), rapid progressors experienced faster CD4+ T-cell increases than nonrapid progressors in first month [difference (95% confidence interval) in mean increase/month (square root scale): 1.82 (1.61; 2.04)], which reversed to slightly slower increases in months 1–18 [−0.05 (−0.06; −0.03)] and no significant differences in 18–60 months [−0.003 (−0.01; 0.01)]. Percentage achieving optimal restoration was significantly lower for rapid progressors than nonrapid progressors at months 12 (29.2 vs. 62.5%) and 36 (47.1 vs. 72.4%) but not at month 60 (70.4 vs. 71.8%). These differences disappeared after adjusting for baseline CD4+ T-cell count: odds ratio (95% confidence interval) 0.86 (0.61; 1.20), 0.90 (0.38; 2.17) and 1.56 (0.55; 4.46) at months 12, 36 and 60, respectively.
Conclusion:
Among people on suppressive antiretroviral therapy, rapid progressors experience faster initial increases of CD4+ T-cell counts than nonrapid progressors, but are less likely to achieve optimal restoration during the first 36 months after cART, mainly because of lower CD4+ T-cell counts at cART initiation.
Keywords: CD4 responses, HIV-viral suppression, rapid progression
Introduction
Humans show a remarkable variation in clinical outcomes following HIV-1 infection. Although some individuals are able to control HIV replication for long periods (elite controllers), others experience rapid CD4+ T-cell loss after seroconversion (rapid progressors) in the absence of combination antiretroviral therapy (cART) [1–2]. Over the last few years, many studies have focused on these extreme HIV phenotypes in a search for clues relating to viral pathogenesis. Accumulating evidence suggests that a combination of viral and host factors play a role in HIV disease outcomes [3–7].
Rapid HIV progression is defined by a decay of CD4+ T-cell counts below a threshold ranging between 100 and 350 cells/μl in a time frame from 6 months to 3 years [2,5] after seroconversion. Studies of rapid progressors have been limited by small numbers [7–9] and by the heterogeneity of definitions used for rapid progressors. Both a documented seroconversion date and a narrow seroconversion window are generally required to characterize these uncommon phenotypes. Indeed, Muñoz et al.[10] reported frequency of the rapid progressors to be under 10% in the MACS, and Rotger et al.[5] reported that approximately 8% of seroconverters in the Swiss cohort were rapid progressors. Recently, Olson et al.[2] showed that 2.8, 7.3 and 24.9% of seroconverters in the Concerted Action of Seroconversion to AIDS and Death in Europe (CASCADE) Collaboration experienced at least one CD4+ cell count less than 100, 200 and 350 cells/μl, respectively, within 1 year of seroconverters.
Rapid progression could be clinically relevant for immune restoration after cART initiation as poorer CD4+ T-cell recovery has been associated with low CD4+ T-cell counts at the initiation of therapy in a cross-sectional study [11]. However, the link between rapid progression and immune recovery is unknown.
The CASCADE Collaboration has previously reported that individuals with steeper precART CD4+ T-cell decline are more likely to experience greater CD4+ T-cell increases after cART [12] but did not examine if these responses varied among those who are virologically suppressed. Several studies have revealed a substantial prevalence of immunological nonresponders among patients who are virologically suppressed on cART, with rates ranging from 17 to 40%, depending on the study criteria and the population [13–15]. Compared with concordant responders (i.e. those with a good CD4+ T-cell response while virally suppressed on cART), these nonconcordant responders are at increased risk of clinical progression to AIDS-related and non–AIDS-related illnesses and death [11,13,16–23].
We hypothesized that rapid progression before cART initiation predicts poor CD4+ T-cell recovery in virologically suppressed individuals and hinders optimal CD4+ T-cell restoration. The objective of this analysis was, therefore, to compare trends in CD4+ T-cell recovery after cART initiation and the proportion achieving counts at least 500 cells/μl after 12, 36 and 60 months between rapid and nonrapid progressors achieving virological suppression.
Methods
Ethics statement
All collaborating cohorts received approval from their respective or national ethics review boards. Ethics approval for CASCADE collaborating cohorts within EuroCoord has been granted by the committees detailed in the ‘Acknowledgements’ section.
Study population
We used data from CASCADE, updated in 2013 within EuroCoord (www.EuroCoord.net), which consists of 29 884 individuals with well estimated dates of HIV seroconversion (seroconverters) from 28 cohorts across Europe, Canada, Australia and Sub-Saharan Africa [24]. Individuals followed-up in the two African cohorts were excluded as both CD4+ T-cell count evolution during natural history and treatment guidelines applied to these populations differ from those in high-income countries [25]. We also excluded individuals infected through a route other than injecting drug use or sexual intercourse (i.e. haemophilia, transfusion, other and unknown) to avoid other clinical complications that affect HIV disease progression. Eligible individuals were patients initiating their first cART regimen from naive and who achieved viral suppression (plasma HIV-RNA level ≤200 copies/ml) within the first 6 months of therapy and maintained it thereafter until their censoring date. Patients had to have both CD4+ T-cell counts and HIV-RNA measurements available at start of cART (i.e. within the last 6 months prior to cART initiation). Individuals with a viral load less than 1000 copies/ml at the time of starting cART were excluded as they may have been misclassified as treatment naïve. Furthermore, to be able to classify patients as rapid or nonrapid progressors, we required that seroconversion dates were estimated in one of three ways: as the midpoint between the last negative and first positive HIV antibody test dates with an interval of less than 12 months between tests, as the date of laboratory evidence of acute seroconversion (PCR positivity in the absence of HIV antibodies or antigen positivity with fewer than four bands on Western blot), or as the date of seroconversion illness for individuals with a test interval of 12 months or less. Patients also had to have at least one CD4+ T-cell count within the first 12 months of seroconversion in the absence of antiretroviral therapy (ART).
Individuals were classified as rapid progressors if there was at least one determination of CD4+ T-cell counts less than 200 cells/μl within 12 months from seroconverters before ART initiation, and as nonrapid progressors otherwise. Additional definitions were also tested [2], and more details are provided in the sensitivity analyses section.
Follow-up time started at cART initiation and was censored at the first of the following dates: the first of two consecutive occasions when the plasma HIV-RNA level increased above 200 copies/ml (considered to be treatment failure), the first of two consecutive HIV-RNA measurements separated by more than 12 months (considered to be lost to follow-up) or at the last date when an HIV-RNA measurement was available within 60 months of starting therapy. Patients who modified their cART regimens were not censored at date of modification, provided plasma HIV-RNA levels remained 200 copies/ml or less.
CART was defined as a protease inhibitor-based, nonnucleoside reverse transcriptase inhibitor (NNRTI), or fusion inhibitor-based regimen, in combination with at least two nucleoside or nucleotide reverse transcriptase inhibitors, or a triple nucleotide reverse transcriptase inhibitor regimen including abacavir or tenofovir.
Statistical analysis
Differences in sociodemographic and clinical characteristics between rapid and nonrapid progressors were assessed through the nonparametric Mann–Whitney test for continuous variables and the χ2 test for independence for categorical variables.
Trends in CD4+ T-cell counts after cART initiation were modelled using a piecewise linear mixed-effects model (to take into account the correlation between measurements in the same individual) with three slopes (including random effects for the intercept and the three slopes); the best model (Akaike criterion) obtained allowed for changes of slopes at months 1 and 18. The square root transformation of the CD4+ T-cell counts was used to fulfil the model assumptions.
As an initial approach, multivariable piecewise linear mixed-effects models were initially adjusted for sex, age at cART initiation, risk group (MSM, sex between men and women, IDUs), geographical origin (non-sub-Saharan Africa, migrants from sub-Saharan Africa, unknown) used as a proxy for HIV subtype and log10 HIV-RNA levels at cART initiation. However, as CD4+ T-cell count at cART initiation is known to be a strong predictor of immunological outcome and is significantly lower in rapid progressors, this initial approach was confounded by the CD4+ T-cell counts at cART initiation. As attempting to remove this confounding by including the observed CD4+ T-cell counts at cART initiation as a covariate in a model for later measurements is likely to introduce bias, we used a method based on first following the initial approach and then applying a correction to the parameter estimates to compare rapid and nonrapid progressors with the same underlying CD4+ T-cell counts at start of cART [26].
We calculated the proportion of patients who experienced an optimal CD4+ T-cell restoration, defined as achieving CD4+ T-cell counts of at least 500 cells/μl, at 12, 36 and 60 (±3) months from cART initiation and used logistic regression models to calculate odds ratios (ORs) and 95% confidence intervals (CIs) for association between rapid progressors status and optimal CD4+ T-cell count restoration. Multivariate logistic regression models were initially adjusted for the same factors as in the piecewise linear mixed-effects models, and additionally adjusted for CD4+ T-cell count at cART initiation (<100, 100–199, ≥200).
A set of sensitivity analyses was undertaken. Analyses were repeated using 100 and 350 as CD4+ T-cell thresholds to define rapid progressors; considering the first 6 months from seroconverters to classify patients as rapid and nonrapid progressors; defining an optimal CD4+ T-cell restoration as achieving CD4+ T-cell counts of at least 600 cells/μl; restricting to individuals who started cART with CD4+ T-cell counts less than 200 cells/μl; restricting to patients who started cART after year 2000 when boosted protease inhibitors became widely available and allowing patients to achieve viral suppression within the first 12 months of therapy as patients with high viral loads and those on protease inhibitor-based regimen may not suppress by 6 months yet nonetheless by 12 months.
All statistical analyses were performed by using Stata software (version 13.1; StataCorp, College Station, Texas, USA).
Results
Study population characteristics
Of 29 884 individuals, 25 860 were excluded from analyses as follows: 916 followed-up in two African cohorts, 1520 who were infected through a route other than injecting drug use or sexual intercourse (157 haemophilia, 44 transfusion, 184 other and 1135 unknown), 9716 who never initiated cART, 5347 who were ART experienced at cART initiation, 4009 who did not achieve viral suppression within the first 6 months of therapy, 691 as CD4+ T-cell count and/or HIV-RNA measurements at cART initiation were not available, 485 who had a viral load at cART initiation less than 1000 copies/ml, 2680 for whom seroconversion date was above 12 months after the last negative test and 496 as CD4+ T-cell count within 12 months from seroconverters in the absence of ART was not available.
Of 4024 individuals included in the analyses, 294 (7.3%) were classified as rapid progressors, who were more likely than nonrapid progressors to be women (17.7 vs. 11.2%), infected through sex between men and women (30.9 vs. 18.2%), migrants originating from sub-Saharan Africa (12.6 vs. 3.9%) and, when they subsequently started cART, did so at with higher log10 HIV-RNA levels (5.1 vs. 4.8) and lower CD4+ T-cell counts (164 vs. 350) (Table 1).
Table 1.
Sociodemographic and clinical characteristics at start of combination antiretroviral therapy for 4024 individuals by precombination antiretroviral therapy progressor status.
| Nonrapid progressors | Rapid progressors | P value | |
| 3730 (92.7) | 294 (7.3) | ||
| Sex | 0.001 | ||
| Men | 3312 (88.8) | 242 (82.3) | |
| Women | 418 (11.2) | 52 (17.7) | |
| Age at cART (years) | |||
| Median (IQRa) | 36 (30; 42) | 35 (29; 42) | 0.66 |
| <30 | 928 (24.9) | 74 (25.2) | 0.89 |
| 30–39 | 1501 (40.2) | 122 (41.5) | |
| 40–49 | 887 (23.8) | 64 (21.8) | |
| ≥50 | 414 (11.1) | 34 (11.6) | |
| Transmission category | <0.001 | ||
| Sex between men | 2887 (77.4) | 187 (63.6) | |
| Sex between men and women | 679 (18.2) | 91 (31.0) | |
| IDU | 164 (4.4) | 16 (5.4) | |
| Geographical origin | <0.001 | ||
| Non sub-Saharan Africa | 2729 (73.2) | 195 (66.3) | |
| Migrants from sub-Saharan Africa | 145 (3.9) | 37 (12.6) | |
| Unknown | 856 (22.9) | 62 (21.1) | |
| Ethnic group | 0.001 | ||
| White | 1800 (48.3) | 118 (40.1) | |
| Black | 111 (2.9) | 17 (5.8) | |
| Other | 45 (1.2) | 8 (2.7) | |
| Unknown | 1774 (47.6) | 151 (51.4) | |
| Higher education ever attained | 0.001 | ||
| Preprimary or primary education | 88 (2.4) | 13 (4.4) | |
| Secondary education | 527 (14.1) | 45 (15.3) | |
| Postsecondary education | 503 (13.5) | 18 (6.1) | |
| Unknown | 2612 (70.0) | 218 (74.2) | |
| Acute infectionb | 0.11 | ||
| No | 2504 (67.1) | 184 (62.6) | |
| Yes | 1226 (32.9) | 110 (37.4) | |
| Date of SC [median (IQRa)] | 05 March (00 December; 08 June) | 05 August (02 March; 08 September) | 0.04 |
| Date of cART initiation [median (IQRa)] | 08 February (03 March; 10 July) | 06 September (02 November; 09 June) | <0.001 |
| cART based on | 0.48 | ||
| NNRTI | 1656 (44.4) | 120 (40.8) | |
| PI | 1544 (41.4) | 128 (43.5) | |
| 3 class/other | 530 (14.2) | 46 (15.7) | |
| CD4+ T-cell counts at cART (cells/μl) | |||
| Median (IQRa) | 350 (270; 473) | 164 (120; 196) | <0.001 |
| <200 | 276 (7.4) | 234 (79.6) | <0.001 |
| 200–350 | 1602 (42.9) | 53 (18.0) | |
| >350 | 1852 (49.7) | 7 (2.4) | |
| Log10 HIV-RNA at cART initiation | |||
| Median (IQRa) | 4.8 (4.3–5.3) | 5.1 (4.6–5.7) | <0.001 |
| <4 | 502 (13.5) | 26 (8.8) | <0.001 |
| 4–5 | 1728 (46.3) | 102 (34.7) | |
| >5 | 1500 (40.2) | 166 (56.5) | |
| AIDS diagnosis at cART | 0.005 | ||
| No | 3589 (96.2) | 272 (92.5) | |
| Yes | 137 (3.7) | 22 (7.5) | |
| Unknown | 4 (0.1) | 0 | |
| Hepatitis C virus antibodies at cART | 0.93 | ||
| No | 2013 (54.0) | 160 (54.4) | |
| Yes | 163 (4.4) | 14 (4.8) | |
| Unknown | 1554 (41.6) | 120 (40.8) | |
| Hepatitis B surface antigen | 0.47 | ||
| No | 1949 (52.2) | 159 (54.1) | |
| Yes | 73 (2.0) | 3 (1.0) | |
| Unknown | 1708 (45.8) | 132 (44.9) | |
| Time from SC to cART initiation (months) | |||
| Median (IQRa) | 16 (4; 38) | 6 (3; 9) | <0.001 |
| <6 | 1118 (30.0) | 142 (48.3) | <0.001 |
| 6–12 | 507 (13.6) | 106 (36.0) | |
| ≥12 | 2105 (56.4) | 46 (15.7) |
cART, combination antiretroviral therapy; IQR, interquartile range; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; SC, seroconversion.
bAcute infection is defined as having laboratory evidence of acute seroconversion or an HIV test interval of less than 30 days.
Trends in CD4+ T-cell counts after combination antiretroviral therapy initiation
A total of 40 893 CD4+ T-cell count measurements were available post-cART initiation, 3451 among rapid progressors and 37 442 among nonrapid progressors. The median number of CD4+ T-cell counts measurements per individual was 10 [interquartile range (IQR): 6; 16] for rapid progressors and 9 (IQR: 5; 14) for nonrapid progressors with a median interval of 2.8 (IQR: 2.3; 3.5) and 2.9 (2.2; 3.6) months between consecutive determinations for rapid and non-rapid progressors, respectively.
Median CD4+ T-cell count profiles for rapid and nonrapid progressors, overall and among individuals who started cART with CD4+ T-cell counts less than 200 cells/μl, are shown in Fig. 1. Unadjusted and adjusted trends in CD4+ T-cell counts after cART initiation from the piecewise linear mixed-effects model at 0–1, 1–18 and more than 18 months are given in Table 2. After initial adjustment for sex, risk group, geographical origin, age and log10 HIV-RNA level at cART initiation, rapid progressors had faster initial and long-term CD4+ T-cell increases than nonrapid progressors: differences in mean CD4+ increase/month (square root scale) were 1.34 (95% CI: 0.95; 1.72, P < 0.001) in 0–1 months, 0.03 (−0.001; 0.05, P = 0.063) in 1–18 months and 0.04 (95% CI: 0.02; 0.06, P < 0.001) in 18–60 months. Applying the postestimation adjustment procedure [26] to compare rapid and nonrapid progressors with the same underlying CD4+ T-cell counts at cART initiation, we found that rapid progressors experienced a faster CD4+ T-cell increase than nonrapid progressors in 0–1 months (1.82, 95% CI: 1.61; 2.04, P < 0.001), which reversed to slightly slower increases in months 1–18 (−0.05, −0.06; −0.03, P < 0.001) and no significant differences in months 18–60 (−0.003, 95% CI: −0.01; 0.01, P = 0.62) (Table 2).
Fig. 1.
Observed CD4+ T-cell counts in rapid and nonrapid progressors.
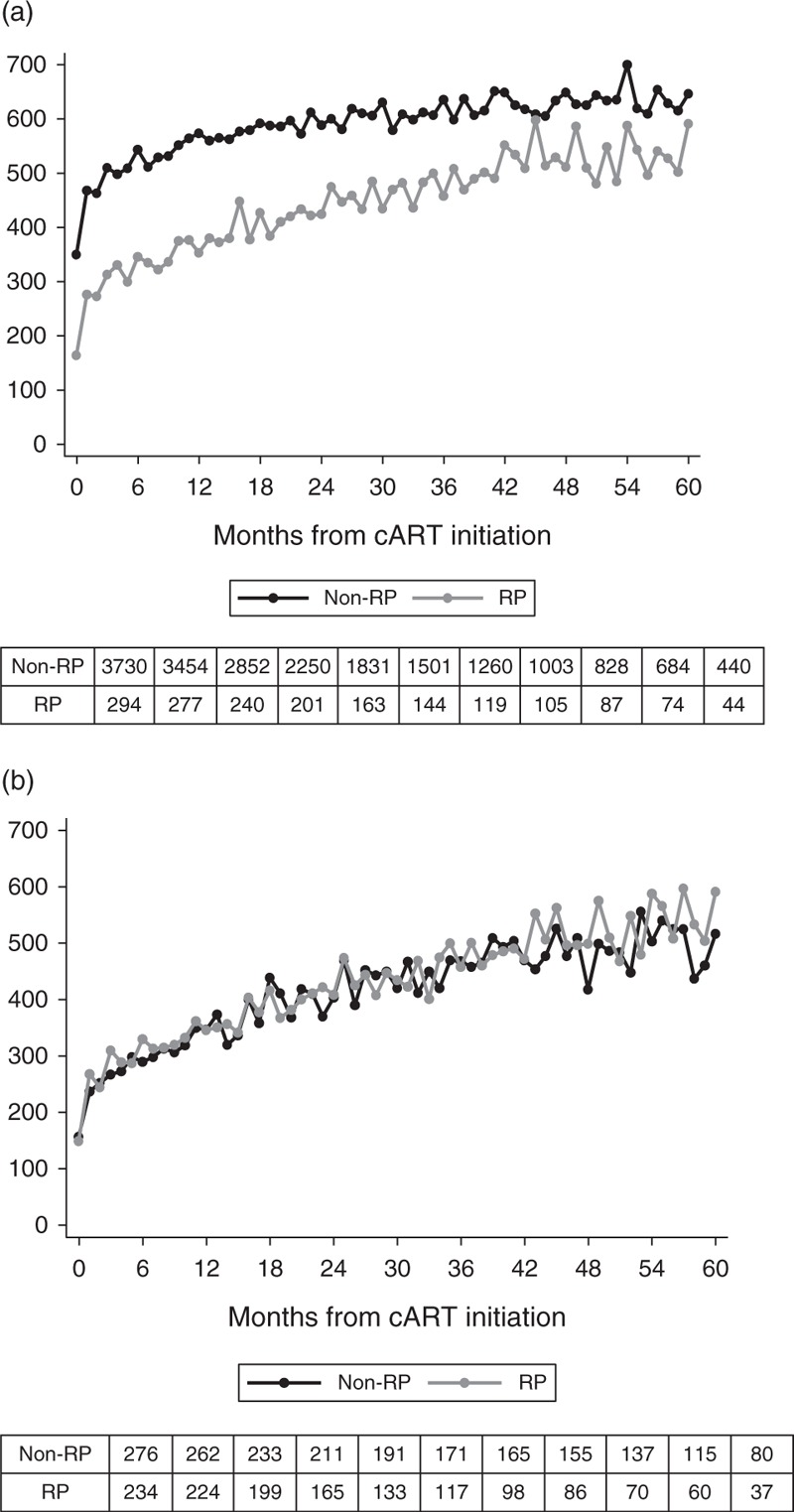
(a) All patients. (b) Patients starting combination antiretroviral therapy with CD4+ T-cell counts less than 200 cells/μl.
Table 2.
Immunological outcome following combination antiretroviral therapy according to the rapid progressors status from linear mixed-effects model in the univariate and multivariate analyses.
| Months from cART initiation | Unadjusted analyses | Adjusted analyses | ||||||
| Model 1a | Model 1 + postestimation adjustment procedureb | |||||||
| Mean (95% CI) increase in CD4+ T-cell count per month (square root scale) | Difference (95% CI) in mean increase in CD4+ T-cell count per month (square root scale) | P value | Difference (95% CI) in mean increase in CD4+ T-cell count per month (square root scale) | P value | Difference (95% CI) in mean increase in CD4+ T-cell count per month (square root scale) | P value | ||
| Nonrapid progressors | Rapid progressors | Rapid vs. nonrapid progressors | ||||||
| 0–1 | 2.94 (2.84; 3.05) | 4.55 (4.17; 4.92) | 1.60 (1.21; 1.99) | <0.001 | 1.34 (0.95; 1.72) | <0.001 | 1.82 (1.61; 2.04) | <0.001 |
| 1–18 | 0.17 (0.16; 0.17) | 0.20 (0.17; 0.22) | 0.03 (0.004; 0.06) | 0.024 | 0.03 (−0.001; 0.05) | 0.063 | −0.05 (−0.06; −0.03) | <0.001 |
| 18–60 | 0.04 (0.03; 0.04) | 0.07 (0.06; 0.09) | 0.04 (0.02; 0.05) | <0.001 | 0.04 (0.02; 0.06) | <0.001 | −0.003 (−0.01; 0.01) | 0.62 |
CI, confidence interval; cART, combination antiretroviral therapy.
aFrom an initial piecewise linear mixed model including rapid progressors status, sex, age at cART initiation, risk group (MSM, sex between men and women, IDUs), geographical origin (migrants from sub-Saharan Africa, non-sub-Saharan Africa, unknown) used as a proxy of subtype and log10 HIV-RNA levels at cART initiation.
bAfter the postestimation adjustment procedure to provide an estimate of the difference for patients with the same underlying CD4+ T-cell count at cART initiation.
Figure 2a depicts the evolution of CD4+ T-cell counts for rapid and nonrapid progressors MSM of 30 years of age, of non-sub-Saharan African origin who started cART at 5 log10 HIV-1 RNA. It shows how rapid progressors, in spite of steeper initial CD4+ T-cell counts increases, fail to reach the threshold of 500 cells/μl until month 36. Figure 2b illustrates the same changes comparing rapid and nonrapid progressors who start cART at CD4+ T-cell count of 180 cells/μl. Figure 2b also shows rapid progressors having steeper initial CD4+ T-cell count increases but given that nonrapid progressors are now ‘forced’ to start cART at the same CD4+ T-cell count, rapid progressors maintain higher values to month 60.
Fig. 2.
Predicted CD4+ T-cell counts in rapid and nonrapid progressors.
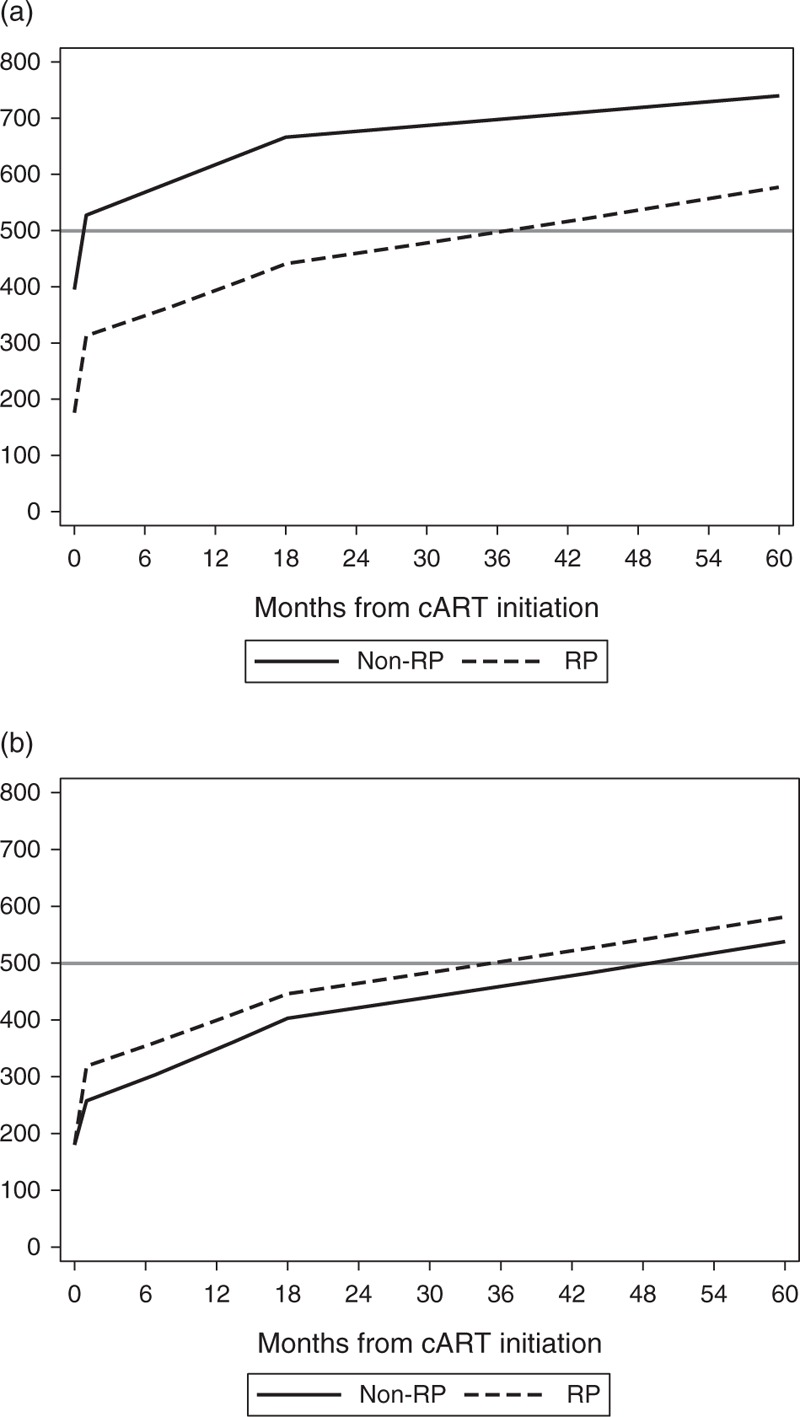
(a) From an initial piecewise linear mixed model (men, MSM, non-SSA, 30 years old at start of cART and 5 log10 HIV-RNA level at cART initiation). (b) After the postestimation adjustment procedure (men, MSM, non-SSA, 30 years old at start of cART, 5 log10 HIV-RNA level at cART initiation and an underlying CD4+ T-cell count at cART initiation of 180 cells/μl). cART, combination antiretroviral RP, rapid progressors; SSA, sub-Saharan Africa.
Analyses using 100 and 350 cells/μl as CD4+ T-cell thresholds to define rapid progressors led to results consistent with those of the main analyses although differences in mean CD4+ increase/month between rapid and nonrapid progressors were attenuated when considering 350 cells/μl as the CD4+ T-cell threshold. Similar results were also obtained when the first 6 months from seroconverters were used to classify patients as rapid and nonrapid progressors, when analyses were restricted to individuals who started cART after year 2000. Restricting analyses to individuals who initiated cART with CD4+ T-cell counts less than 200 cells/μl yielded results similar to those obtained when using an adjustment procedure [26]. Allowing patients to achieve viral suppression within the first 12 months of therapy yielded results consistent with those of the main analyses.
CD4+ T-cell count restoration (≥ 500 cells/μl) following suppressive combination antiretroviral therapy
Among 4024 eligible patients, a total of 3092, 1379 and 484 were available for CD4+ T-cell analyses at 12, 36 and 60 months, respectively. Patients with missing data were more likely than persons with available measurements to be younger at cART initiation, infected through injecting drug use and to have started cART in more recent years with higher CD4+ T-cell counts. The percentage of patients experiencing optimal restoration of CD4+ T-cell counts of at least 500 cells/μl was significantly lower among rapid than nonrapid progressors at months 12 (29.2 vs. 62.5%) and 36 (47.1 vs. 72.4%) but no differences were found at month 60 (70.4 vs. 71.8%). After initial adjustment for sex, risk group, geographical origin, age and log10 HIV-RNA level at cART initiation, rapid progressors remained less likely to achieve counts of at least 500 cells/μl at months 12 (OR: 0.21, 95% CI: 0.13; 0.36, P < 0.001) and 36 (OR: 0.30, 95% CI: 0.11; 0.87, P = 0.03), a difference that disappeared at month 60 (OR: 0.79, 95% CI: 0.30; 2.09, P = 0.63). Additional adjustment for CD4+ T-cell counts at cART initiation (<100, 100–199, ≥200), however, showed no differences between rapid and nonrapid progressors at 12 (OR: 0.86, 95% CI: 0.61; 1.20, P = 0.36), 36 (OR: 0.90, 95% CI: 0.38; 2.17, P = 0.82) or 60 months (OR: 1.56, 95% CI: 0.55; 4.46, P = 0.40) (Table 3). Similar results were obtained when using 600 as a cutoff to define optimal CD4+ T-cell restoration (data not shown).
Table 3.
CD4+ T-cell count restoration at 12, 36 and 60 months from combination antiretroviral therapy initiation.
| N | N (%) CD4+ cell count ≥500 | Unadjusted analyses | Adjusted analyses | ||||||
| Model 1a | Model 1 + CD4+ at cART initiationb | ||||||||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | ||||
| 12 months | Nonrapid progressors | 2852 | 1782 (62.5) | 1.00 | 1.00 | 1.00 | |||
| Rapid progressors | 240 | 70 (29.2) | 0.25 (0.14; 0.42) | <0.001 | 0.21 (0.13; 0.36) | <0.001 | 0.86 (0.61; 1.20) | 0.36 | |
| 36 months | Nonrapid progressors | 1260 | 912 (72.4) | 1.00 | 1.00 | 1.00 | |||
| Rapid progressors | 119 | 56 (47.1) | 0.34 (0.11; 1.05) | 0.06 | 0.30 (0.11; 0.87) | 0.03 | 0.90 (0.38; 2.17) | 0.82 | |
| 60 months | Nonrapid progressors | 440 | 316 (71.8) | 1.00 | 1.00 | 1.00 | |||
| Rapid progressors | 44 | 31 (70.4) | 0.94 (0.37; 2.36) | 0.89 | 0.79 (0.30; 2.09) | 0.63 | 1.56 (0.55; 4.46) | 0.40 | |
CI, confidence interval; cART, combination antiretroviral therapy; OR, odds ratio.
aAdjusted for sex, age at cART initiation, risk group (MSM, sex between men and women, IDUs), geographical origin (migrants from sub-Saharan Africa, non-sub-Saharan Africa, unknown) used as a proxy of subtype and log10 HIV-RNA levels at cART initiation.
bAdjusted for sex, age at cART initiation, risk group (MSM, sex between men and women, IDUs), geographical origin (sub-Saharan Africa, non-sub-Saharan Africa, unknown) used as a proxy of subtype, log10 HIV-RNA levels at cART initiation and CD4+ T cells at cART initiation (<100, 100–199, ≥200 cells/μl).
Analyses defining an optimal CD4+ T-cell restoration as achieving CD4+ T-cell counts of at least 600 cells/μl led to results consistent with those of the main analyses. In addition, performing the other procedures of sensitivity analyses mentioned earlier yielded results consistent with those of the main analysis (data not shown).
Discussion
Rapid progression prior to cART initiation hinders optimal CD4+ T-cell recovery once HIV-1 suppressive response to cART is achieved. Although rapid progressors experience faster initial increases in CD4+ T-cells than nonrapid progressors, they are less likely to achieve CD4+ T-cell counts of at least 500 cells/μl during the first 36 months after cART. These outcomes are largely explained by their lower CD4+ T-cell counts at cART initiation.
Our current work builds on previous work conducted by the CASCADE Collaboration showing that individuals with a faster rate of CD4+ T-cell loss precART tended to experience faster immune reconstitution once they started cART, independently of baseline CD4+ T-cell count and plasma HIV-RNA value [12]. We show that rapid progressors who achieve suppressive response to cART experience delays in achieving optimal CD4+ count recovery during the first 36 months after cART, which puts them at a higher risk of developing immunodeficiency-related complications. That this may happen in a nonnegligible proportion of the population is of concern. In our study, 7.3% of HIV-1 seroconverters fulfilled the criteria of rapid progression, a similar percentage to that previously reported in other studies [2,5,10], although study populations were not strictly comparable.
When comparing the outcomes of these rapid progressors with patients whose progression was not rapid, but whose CD4+ T-cell count at cART was similar, the differences in CD4+ T-cell responses largely disappeared. Nevertheless, steeper CD4+ T-cell responses were still experienced by the rapid progressors suggesting that a prior faster decline may allow mounting enhanced responses. It could be that the rapid loss of CD4+ T cells from the peripheral blood observed in rapid progressors is secondary to cell redistribution in lymph nodes in a higher proportion than in nonrapid progressors, as well as to cellular death. Thus, recovery is bound to be more rapid once HIV viral replication is suppressed and thus the cause of the redistribution is eliminated [27,28]. This suggests that not only the absolute CD4+ T-cell value but also the time to reach that value influences responses to cART in virally suppressed individuals; cART may elicit better CD4+ T-cell responses in a time window before deep immunological damage has been caused.
These data confirm the potency of current cART regimes in their ability to reduce viremia and thus facilitate subsequent immune recovery of CD4+ T cells, even among those who had experienced rapid progression to low CD4+ count prior to starting cART. However, the similar dynamics in the recovery of CD4+ T cells after the first month of therapy prevents rapid progressors from fully recovering from their profound initial loss of cells.
This study has some limitations. First, the high proportion of patients with missing data made it difficult to assess CD4+ T-cell count restoration at 12, 36 and, especially, 60 months after cART initiation. However, as missingness appeared not related to status of the rapid progressors, there is no reason to believe that this should influence our results. Second, in 3.7% of seroconverters (1.7% in rapid progressors and 3.8% in nonrapid progressors), seroconversion date was estimated as the date of seroconversion illness, which has been associated with faster disease progression [29]. However, in sensitivity analyses excluding these patients, results were consistent with those of the main analyses. Third, although our work is based on HIV-1 seroconverters who are unlikely to be comparable with the general HIV-infected population in a number of ways, it has been recently shown that there are no major differences in HIV disease progression between seroconverters and seroprevalent individuals [30] suggesting that our results are generalizable to the HIV-positive population.
Our findings have implications for public health policy, clinical management and basic science research. Ideally, cART should be started as soon as possible after HIV-1 diagnosis regardless of the CD4 T-cell count (START study: http://www.niaid.nih.gov/news/newsreleases/Archive/2011/Pages/START.aspx).
However, in clinical settings wherein cART is not widely available, our results would support strategies that promote frequent testing to reduce the proportion of patients initiating cART at low CD4+ T-cell counts, which is largely secondary to delayed HIV-1 diagnoses. For those testing early, we suggest frequent CD4+ T-cell count monitoring close to the time of HIV diagnoses to establish the rapid progressors phenotype in order to avoid unnecessary CD4+ T-cell count decay among rapid progressors. Finally, elucidating the immunopathological bases of rapid progression should help to improve individual clinical outcome and limit its impact in the global HIV-1 pandemics.
Acknowledgements
CASCADE Steering Committee: Julia Del Amo (Chair), Laurence Meyer (Vice Chair), Heiner C. Bucher, Geneviève Chêne, Osamah Hamouda, Deenan Pillay, Maria Prins, Magda Rosinska, Caroline Sabin, Giota Touloumi.
CASCADE Co-Ordinating Centre: Kholoud Porter (Project Leader), Ashley Olson, Andrea Cartier, Lorraine Fradette, Sarah Walker, Abdel Babiker.
CASCADE Clinical Advisory Board: Heiner C. Bucher, Andrea De Luca, Martin Fisher, Roberto Muga.
CASCADE Collaborators: Australia: PHAEDRA cohort (Tony Kelleher, David Cooper, Robert Finlayson, Mark Bloch); Sydney AIDS Prospective Study and Sydney Primary HIV Infection cohort (Tony Kelleher, Tim Ramacciotti, Linda Gelgor, David Cooper, Don Smith); Austria: Austrian HIV Cohort Study (Robert Zangerle); Canada: South Alberta Clinic (John Gill); Estonia Tartu Ülikool (Irja Lutsar); France: ANRS CO3 Aquitaine cohort (Geneviève Chêne, Francois Dabis, Rodolphe Thiebaut); ANRS CO4 French Hospital Database (Dominique Costagliola, Marguerite Guiguet); Lyon Primary Infection cohort (Philippe Vanhems); French ANRS CO6 PRIMO cohort (Marie-Laure Chaix, Jade Ghosn); ANRS CO2 SEROCO cohort (Laurence Meyer, Faroudy Boufassa); Germany: German HIV-1 seroconverter cohort (Osamah Hamouda, Claudia Kücherer, Barbara Bartmeyer); Greece: AMACS (Anastasia Antoniadou, Georgios Chrysos, Georgios L. Daikos); Greek Haemophilia cohort (Giota Touloumi, Nikos Pantazis, Olga Katsarou); Italy: Italian Seroconversion Study (Giovanni Rezza, Maria Dorrucci); ICONA cohort (Antonella d’Arminio Monforte, Andrea De Luca); Netherlands: Amsterdam Cohort Studies among homosexual men and drug users (Maria Prins, Ronald Geskus, Jannie van der Helm, Hanneke Schuitemaker); Norway: Oslo and Ulleval Hospital cohorts (Mette Sannes, Oddbjorn Brubakk, Anne-Marte Bakken Kran); Poland: National Institute of Hygiene (Magdalena Rosinska); Spain: Badalona IDU Hospital cohort (Roberto Muga, Jordi Tor); Barcelona IDU Cohort (Patricia Garcia de Olalla, Joan Cayla); CoRIS-scv (Julia del Amo, Santiago Moreno, Susana Monge); Madrid cohort (Julia Del Amo, Jorge del Romero); Valencia IDU cohort (Santiago Pérez-Hoyos); Sweden: Swedish InfCare HIV Cohort, Sweden (Anders Sönnerborg); Switzerland: Swiss HIV Cohort Study (Heiner C. Bucher, Huldrych Günthard, Martin Rickenbach); Ukraine: Perinatal Prevention of AIDS Initiative (Ruslan Malyuta); United Kingdom: Public Health England (Gary Murphy); UK Register of HIV Seroconverters (Kholoud Porter, Anne Johnson, Andrew Phillips, Abdel Babiker); University College London (Deenan Pillay); African cohorts: Genital Shedding Study (US: Charles Morrison; Family Health International, Robert Salata, Case Western Reserve University, Uganda: Roy Mugerwa, Makerere University, Zimbabwe: Tsungai Chipato, University of Zimbabwe); International AIDS Vaccine Initiative (IAVI) Early Infections Cohort (Kenya, Rwanda, South Africa, Uganda, Zambia: Pauli N. Amornkul, IAVI, USA; Jill Gilmour, IAVI, UK; Anatoli Kamali, Uganda Virus Research Institute/Medical Research Council Uganda; Etienne Karita, Projet San Francisco, Rwanda).
EuroCoord Executive Board: Fiona Burns, University College London, UK; Geneviève Chêne, University of Bordeaux, France; Dominique Costagliola (Scientific Coordinator), Institut National de la Santé et de la Recherche Médicale, France; Carlo Giaquinto, Fondazione PENTA, Italy; Jesper Grarup, Region Hovedstaden, Denmark; Ole Kirk, Region Hovedstaden, Denmark; Laurence Meyer, Institut National de la Santé et de la Recherche Médicale, France; Heather Bailey, University College London, UK; Alain Volny Anne, European AIDS Treatment Group, France; Alex Panteleev, St. Petersburg City AIDS Centre, Russian Federation; Andrew Phillips, University College London, UK, Kholoud Porter, University College London, UK; Claire Thorne, University College London, UK.
EuroCoord Council of Partners: Jean-Pierre Aboulker, Institut National de la Santé et de la Recherche Médicale, France; Jan Albert, Karolinska Institute, Sweden; Silvia Asandi, Romanian Angel Appeal Foundation, Romania; Geneviève Chêne, University of Bordeaux, France; Dominique Costagliola (Chair), INSERM, France; Antonella d’Arminio Monforte, ICoNA Foundation, Italy; Stéphane De Wit, St. Pierre University Hospital, Belgium; Peter Reiss, Stichting HIV Monitoring, Netherlands; Julia Del Amo, Instituto de Salud Carlos III, Spain; José Gatell, Fundació Privada Clínic per a la Recerca Bíomèdica, Spain; Carlo Giaquinto, Fondazione PENTA, Italy; Osamah Hamouda, Robert Koch Institut, Germany; Igor Karpov, University of Minsk, Belarus; Bruno Ledergerber, University of Zurich, Switzerland; Jens Lundgren, Region Hovedstaden, Denmark; Ruslan Malyuta, Perinatal Prevention of AIDS Initiative, Ukraine; Claus Møller, Cadpeople A/S, Denmark; Kholoud Porter, University College London, United Kingdom; Maria Prins, Academic Medical Centre, Netherlands; Aza Rakhmanova, St. Petersburg City AIDS Centre, Russian Federation; Jürgen Rockstroh, University of Bonn, Germany; Magda Rosinska, National Institute of Public Health, National Institute of Hygiene, Poland; Manjinder Sandhu, Genome Research Limited; Claire Thorne, University College London, UK; Giota Touloumi, National and Kapodistrian University of Athens, Greece; Alain Volny Anne, European AIDS Treatment Group, France.
EuroCoord External Advisory Board: David Cooper, University of New South Wales, Australia; Nikos Dedes, Positive Voice, Greece; Kevin Fenton, Public Health England, USA; David Pizzuti, Gilead Sciences, USA; Marco Vitoria, World Health Organisation, Switzerland.
EuroCoord Secretariat: Silvia Faggion, Fondazione PENTA, Italy; Lorraine Fradette, University College London, UK; Richard Frost, University College London, UK; Andrea Cartier, University College London, UK; Dorthe Raben, Region Hovedstaden, Denmark; Christine Schwimmer, University of Bordeaux, France; Martin Scott, UCL European Research & Innovation Office, UK.
Ethics: Austrian HIV Cohort Study: Ethik-Kommission der Medizinischen Universität Wien, Medizinische Universität Graz – Ethikkommission, Ethikkommission der Medizinischen Universität Innsbruck, Ethikkommission des Landes Oberösterreich, Ethikkommission für das Bundesland Salzburg; PHAEDRA cohort: St Vincent's Hospital, Human Research Ethics Committee; Southern Alberta Clinic Cohort: Conjoint Health Research Ethics Board of the Faculties of Medicine, Nursing and Kinesiology, University of Calgary; Aquitaine Cohort: Commission Nationale de l’Informatique et des Libertés; French Hospital Database: Commission nationale de l’informatique et des libertés CNIL; French PRIMO Cohort: Comite Consultatif de Protection des Personnes dans la Recherché Biomedicale; SEROCO Cohort: Commission Nationale de l’Informatique et des Libertés (CNIL); German HIV-1 Seroconverter Study: Charité, University Medicine Berlin; AMACS: Bioethics & Deontology Committee of Athens University Medical School and the National Organization of Medicines; Greek Haemophilia Cohort: Bioethics & Deontology Committee of Athens University Medical School and the National Organization of Medicines; ICoNA cohort: San Paolo Hospital Ethic Committee; Italian Seroconversion Study: Comitato etico dell’Istituto Superiore di Sanità; Amsterdam Cohort Studies in Homosexual Men and IDUs: Academic Medical Centre, University of Amsterdam; Oslo and Ulleval Hospital Cohorts: Regional komite for medisinsk forskningsetikk – Øst- Norge (REK 1); Badalona IDU Hospital Cohort: Comité Etico de Investigación Clínica del Hospital Universitari Germans Trias i Pujol; CoRIS-scv: Comité Etico de Investigación Clínica de La Rioja; Madrid Cohort: Ethics Committee of Universidad Miguel Hernandez de Elche; Valencia IDU Cohort: Comité Etico de Investigación Clínica del Hospital Dr Peset-Valencia; Swiss HIV Cohort Study: Kantonale Ethikkommission, spezialisierte Unter-kommission Innere Medizin, Ethikkommission beider Basel, Kantonale Ethikkommission Bern, Comité départemental d’éthique de médecine et médecine communautaire, Commission d’éthique de la recherche clinique, Université de Lausanne, Comitato etico cantonale, Ethikkommission des Kantons St.Gallen; UK Register of HIV Seroconverters: South Birmigham REC; Early Infection Cohorts: Kenya Medical Research Institute, Kenyatta National Hospital, Uganda Virus Research Institute Science and Ethics Committee, Uganda National Council for Science and Technology, Uganda Virus Research Institute Science and Ethics Committee, Uganda National Council for Science and Technology, University of Zambia Research Ethics Committee, Emory IRB, National Ethics Committee of Rwanda, University of Cape Town Research Ethics Committee, University of Kwazulu Natal Nelson R Mandela School of Medicine; Genital Shedding Study Cohort: University Hospitals of Cleveland, IRB for Human Investigation (CWRU), AIDS Research Committee (ARC), STD/AIDS Control Programme, Uganda Ministry of Health, Committee on Human Research (CHR), Office of Research Administration (UCSF), Biomedical Research & Training Institute (BRTI) – Zimbabwe, Institutional Review Office, Fred Hutchinson Cancer Research Center, Medical Research Council of Zimbabwe (MRCZ).
Funding: This work was supported by the European Union Seventh Framework Programme (FP7/2007–2013) under EuroCoord grant agreement n° 260694.
Authors’ contributions: All authors were involved in the setting up of the cohort and conceptualized the design. J.M.-P., J.B., J. D., J.D.A. and I.J. asked the research question presented in this manuscript. All authors were involved in data collection. I.J. was responsible for statistical analyses and was supervised by N.P. I.J. and J.D.A. wrote the first draft of the article. All authors were involved in interpretation of the data and commented interim drafts. All authors have reviewed the final manuscript.
I.J. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the analysis.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Olson A, Meyer L, Prins M, Thiebaut R, Gurdasani D, Guiquet M, et al. CASCADE Collaboration in EuroCoord. An evaluation of HIV elite controller definitions within a large seroconverter cohort study. PLoS One 2014; 9:e86719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olson AD, Guiguet M, Zangerle R, Gill J, Perez-Hoyos S, Lodi S, et al. CASCADE Collaboration in EuroCoord. Evaluation of rapid progressors in HIV infection as an extreme phenotype. J Acquir Immune Defic Syndr 2014; 67:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pereyra F, Addo MM, Kaufmann DE, Liu Y, Miura T, Rathod A, et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis 2008; 197:563–571. [DOI] [PubMed] [Google Scholar]
- 4.International HIV Controllers Study. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 2010; 330:1551–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rotger M, Dalmau J, Rauch A, McLaren P, Bosinger SE, Martinez R, et al. Comparative transcriptomics of extreme phenotypes of human HIV-1 infection and SIV infection in sooty mangabey and rhesus macaque. J Clin Invest 2011; 12:2391–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalmau J, Rotger M, Erkizia I, Rauch A, Reche P, Pino M, et al. CoRP Study Group. Highly pathogenic adapted HIV-1 strains limit host immunity and dictate rapid disease progression. AIDS 2014; 28:1261–1272. [DOI] [PubMed] [Google Scholar]
- 7.Dalmau J, Puertas MC, Azuara M, Mariño A, Frahm N, Mothe B, et al. Contribution of immunological and virological factors to extremely severe primary HIV type 1 infection. Clin Infect Dis 2009; 48:229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markowitz M, Mohri H, Mehandru S, Shet A, Berry L, Kalyanaraman R, et al. Infection with multidrug resistant, dual-tropic HIV-1 and rapid progression to AIDS: a case report. Lancet 2005; 365:1031–1038. [DOI] [PubMed] [Google Scholar]
- 9.Casado C, Colombo S, Rauch A, Martinez R, Gunthard HF, Garcia S, et al. Host and viral genetic correlates of clinical definitions of HIV-1 disease progression. PLoS One 2010; 5:e11079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muñoz A, Wang MC, Bass S, Taylor JM, Kingsley LA, Chmiel JS, et al. Acquired immunodeficiency syndrome (AIDS)-free time after human immunodeficiency virus type 1 (HIV-1) seroconversion in homosexual men. Multicenter AIDS Cohort Study Group. Am J Epidemiol 1989; 130:530–539. [DOI] [PubMed] [Google Scholar]
- 11.Negredo E, Massanella M, Puig J, Perez-Alvarez N, Gallego-Escuredo JM, Villarroya J, et al. Nadir CD4 T cell count as predictor and high CD4 T cell intrinsic apoptosis as final mechanism of poor CD4 T cell recovery in virologically suppressed HIV-infected patients: clinical implications. Clin Infect Dis 2010; 50:1300–1308. [DOI] [PubMed] [Google Scholar]
- 12.Mussini C, Cossarizza A, Sabin C, Babiker A, De Luca A, Bucher HC, et al. CASCADE Collaboration. Decline of CD4+ T-cell count before start of therapy and immunological response to treatment in antiretroviral-naïve individuals. AIDS 2011; 25:1041–1049. [DOI] [PubMed] [Google Scholar]
- 13.Gazzola L, Tincati C, Bellistri GM, Monforte AD, Marchetti G. The absence of CD4+ T cell count recovery despite receipt of virologically suppressive highly active antiretroviral therapy: clinical risk, immunological gaps, and therapeutic options. Clin Infect Dis 2009; 48:328–337. [DOI] [PubMed] [Google Scholar]
- 14.Kelley CF, Kitchen CM, Hunt PW, Rodriguez B, Hecht FM, Kitahata M, et al. Incomplete peripheral CD4+ cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clin Infect Dis 2009; 48:787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piketty C, Castiel P, Belec L, Batisse D, Si Mohamed A, Gilguin J, et al. Discrepant responses to triple combination antiretroviral therapy in advanced HIV disease. AIDS 1998; 12:745–750. [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann GR, Furrer H, Ledergerber B, Perrin L, Opravil M, Vernazza P, et al. Swiss HIV Cohort Study. Characteristics, determinants, and clinical relevance of CD4 T cell recovery to <500 cells/microL in HIV type 1-infected individuals receiving potent antiretroviral therapy. Clin Infect Dis 2005; 41:361–372. [DOI] [PubMed] [Google Scholar]
- 17.Baker JV, Peng G, Rapkin J, Krason D, Reilly C, Cavert WP, et al. Terry Beirn Community Programs for Clinical Research on AIDS (CPCRA). Poor initial CD4+ recovery with antiretroviral therapy prolongs immune depletion and increases risk for AIDS and non-AIDS diseases. J Acquir Immune Defic Syndr 2008; 48:541–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pacheco YM, Jarrin I, Del Amo J, Moreno S, Iribarren JA, Viciana P, et al. CoRIS-MD. Risk factors, CD4 long-term evolution and mortality of HIV-infected patients who persistently maintain low CD4 counts, despite virological response to HAART. Curr HIV Res 2009; 7:612–619. [DOI] [PubMed] [Google Scholar]
- 19.Piketty C, Weiss L, Thomas F, Mohamed AS, Belec L, Kazatchkine MD. Long-term clinical outcome of human immunodeficiency virus-infected patients with discordant immunologic and virologic response to a protease inhibitor-containing regimen. J Infect Dis 2001; 183:1328–1335. [DOI] [PubMed] [Google Scholar]
- 20.Tan R, Westfall AO, Willig JH, Mugavero MJ, Saag MS, Kaslow RA, et al. Clinical outcome of HIV-infected antiretroviral-naïve patients with discordant immunologic and virologic responses to highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2008; 47:553–558. [DOI] [PubMed] [Google Scholar]
- 21.Massanella M, Negredo E, Pérez-Alvarez N, Puig J, Ruiz-Hernandez R, Bofill M, et al. CD4 T-cell hyperactivation and susceptibility to cell death determine poor CD4 T-cell recovery during suppressive HAART. AIDS 2010; 24:959–968. [DOI] [PubMed] [Google Scholar]
- 22.Engsig FN, Zangerle R, Katsarou O, Dabis F, Reiss P, Gill J, et al. Antiretroviral Therapy Cohort Collaboration (ART-CC) and the Collaboration of Observational HIV Epidemiological Research Europe (COHERE) in EuroCoord. Long-term mortality in HIV-positive individuals virally suppressed for >3 years with incomplete CD4 recovery. Clin Infect Dis 2014; 58:1312–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gutierrez F, Padilla S, Masiá M, Iribarren JA, Moreno S, Viciana P, et al. CoRIS-MD. Clinical outcome of HIV-infected patients with sustained virologic response to antirretroviral therapy: long-term follow-up of a multicenter cohort. PloS One 2006; 1:e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Wolf F, Sabin C, Kirk O, Thorne C, Chene G, Porter K. Developing a multidisciplinary network for clinical research on HIV infection: the EuroCoord experience. Clin Invest 2012; 2:255–264. [Google Scholar]
- 25.Pantazis N, Morrison C, Amornkul PN, Lewden C, Salata RA, Minga A, et al. CASCADE Collaboration in EuroCoord and ANRS 1220 Primo-CI Study Group. Differences in HIV natural history among African and non-African seroconverters in Europe and seroconverters in sub-Saharan Africa. PLoS One 2012; 7:e32369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrison L, Dunn DT, Green H, Copas AJ. Modelling the association between patient characteristics and the change over time in a disease measure using observational cohort data. Stat Med 2009; 28:3260–3275. [DOI] [PubMed] [Google Scholar]
- 27.Bucy RP, Hockett RD, Derdeyn CA, Saag MS, Squires K, Sillers M, et al. Initial increase in blood CD4(+) lymphocytes after HIV antiretroviral therapy reflects redistribution from lymphoid tissues. J Clin Invest 1999; 103:1391–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aiuti F, Mezzaroma I. Failure to reconstitute CD4+ T-cells despite suppression of HIV replication under HAART. AIDS Rev 2006; 8:88–97. [PubMed] [Google Scholar]
- 29.Lindback S, Brostrom C, Karlsson A, Gaines H. Does symptomatic primary HIV-1 infection accelerate progression to CDC stage IV disease, CD4 count below 200 x 10(6)/l, AIDS, and death from AIDS?. BMJ 1994; 309:1535–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lodi S, Phillips A, Touloumi G, Pantazis N, Bucher HC, Babiker A, et al. CD4 decline in seroconverter and seroprevalent individuals in the precombination of antiretroviral therapy era. AIDS 2010; 24:2697–2704. [DOI] [PubMed] [Google Scholar]


