Abstract
Background
Osteomyelitis is one of the refractory diseases encountered in orthopedics, while Staphylococcus aureus (S. aureus) is the most common causative organism in osteomyelitis. However, the precise mechanisms underlying the bone loss caused by S. aureus infection have not been well defined. Here, we investigated the effect of S. aureus on osteoclast differentiation and the probable molecular mechanism.
Material/Methods
RAW 264.7 cells were treated for 5 days with live S. aureus, inactivated S. aureus, and S. aureus filtrate. Then, the formation of osteoclast-like cells and resorption pits was observed, and the expression of osteoclast-specific genes (TRAP, MMP-9, cathepsin K, CTR and Atp6v0d2) was detected by real-time PCR. Moreover, key proteins in the signaling pathway associated with osteoclast differentiation were detected with Western blot.
Results
The data showed that live S. aureus, inactivated S. aureus, and S. aureus filtrate induced osteoclast formation, promoted bone resorption, and increased the expression of osteoclast-specific genes in a dose-dependent manner in the absence RANKL. In addition, we found that the S. aureus-induced osteoclastogenesis was related to the degradation of IκB-α, phosphorylation of NF-κB p65, and increased expression of NFATc1. Thus, we used JSH-23 to inhibit NF-κB transcriptional activity. The effect of the S. aureus-induced osteoclastogenesis and the expression of osteoclast-specific genes and NFATc1 were inhibited, which indicated that the NF-κB signaling pathway plays a role in S. aureus-induced osteoclastogenesis.
Conclusions
This study demonstrated that S. aureus induces osteoclastogenesis through its cell wall compound and secretion of small soluble molecules, and the NF-κB signaling pathway plays a role in this process.
MeSH Keywords: NF-kappa B p52 Subunit, Osteoclasts, Osteomyelitis, Staphylococcus Aureus
Background
Osteomyelitis is an infectious disease of the bones, which is characterized by severe inflammation and progressive bone destruction [1]. With the increase of open fractures and orthopedic procedures, the incidence of osteomyelitis is significantly increasing [2]. However, due to lack of effective management, osteomyelitis causes serious morbidity and causes a great deal of physical and psychological damage to patients [3]. Staphylococcus aureus (S. aureus) is the most common causative organism in osteomyelitis [4,5], and accounts for approximately 80% of all osteomyelitis cases [6]. S. aureus infection usually leads to excessive bone destruction and results in bone defects [7,8], but the precise mechanisms underlying the bone loss caused by S. aureus infection are not well understood.
Bone is a dynamic organ, which is constantly remodeled by osteoblasts (mediating bone formation) and osteoclasts (mediating bone resorption) [9], and the balance between bone formation and bone resorption plays a major role in the maintenance of bone mineralization [10]. As to the mechanisms of bone loss caused by S. aureus infection, a large number of studies focused on the direct effects of S. aureus on bone formation [11]. It is clear that S. aureus reduces osteogenic differentiation from marrow mesenchymal stem cells [12] and induces cell apoptosis and death of osteoblasts [13,14]. Moreover, S. aureus inhibits osteoblast proliferation and bone mineralization by producing virulence factors: staphylococcal protein A, Panton-Valentine leukocidin, and coagulase [15].
However, with respect to bone resorption, the direct effects of S. aureus on osteoclasts have been poorly studied. Some studies have explored the direct effect of S. aureus capsular material [16–18] or its components (such as lipoteichoic acid [19,20], peptidoglycan [21,22]) on osteoclasts, but the results are controversial due to the different methods and osteoclast precursors used in the experiments. Moreover, as the most common pathogenic bacteria in osteomyelitis, S. aureus is able to synthesize a large number of virulence factors, except for the cytotoxic components of the cell wall, and the effects of S. aureus on osteoclasts are caused by the combination of these virulence factors. Therefore, we took RAW 264.7cell line as osteoclast precursors, which is internationally recognized and widely used in studies of osteoclast differentiation [23]. We treated RAW 264.7 cells with live S. aureus, inactivated S. aureus, and S. aureus filtrate to investigate the effects of S. aureus on osteoclastogenesis. In addition, we explored the underlying mechanism of S. aureus -induced osteoclastogenesis, and the results indicated that S. aureus promoted osteoclastogenesis through its cell wall compound and secretion of small soluble molecules, and we found that the NF-κB signaling pathway plays a role in this process.
Material and Methods
Chemicals
Penicillin-Streptomycin solution and high-glucose Dulbecco’s modified Eagle’s medium (DMEM) were purchased from Hyclone (USA); fetal bovine serum (FBS) was purchased from Gibco (USA); acid phosphatase leukocyte kit (TRAP, 387A) and methyl thiazolyl tetrazolium (MTT) were obtained from Sigma-Aldrich Co. (USA). JSH-23 (Cat. No. S7351) was from Selleck Chemicals; soluble receptor-activated nuclear factor κB ligand (RANKL) was obtained from R&D Systems (USA); and the GeneJET RNA purification kit was purchased from Thermo Scientific (USA). PrimeScript™ RT reagent kit with gDNA Eraser (Perfect Real Time) and SYBR® Premix Ex Taq™ II (Tli RNaseH Plus) were purchased from Takara Bio Co., Ltd. (Dalian, China). Oligonucleotide primer sets were synthesized by AuGCT DNA-SYN Biotechnology Synthesis Lab Co., Ltd. (Beijing, China). Immobilon Western Chemiluminescent HRP substrate and polyvinylidene difluoride (PVDF) membranes were purchased from Millipore (Germany). Antibodies against IκB-α, NF-κB p65, Akt, p38, ERK1/2, JNK, NFATc1, and GAPDH, or the phosphorylated form of NF-κB p65, Akt, p38, ERK1/2, JNK, and HRP-linked rabbit IgG antibody were purchased from Cell Signaling Technology (USA). IL-1a (Interleukin-1 alpha) mouse in vitro SimpleStep ELISA™ kit, Mouse TNF alpha SimpleStep ELISA® kit and IL-6 (Interleukin-6) mouse ELISA kit were purchased from Abcam (England).
S. aureus culture
The S. aureus (ATCC43300) culture was performed following the protocols reported in our previous study [15]. In brief, S. aureus was grown overnight in tryptic soy broth at 37°C with shaking, and then S. aureus was harvested by centrifugation and resuspended in phosphate-buffered saline (PBS). The tablet colony counting method was used to count the number of S. aureus in suspension. Inactivated S. aureus was prepared by exposing the counted live S. aureus to a temperature of 95°C for 20 min. The S. aureus filtrate was prepared by culturing live S. aureus in high-glucose Dulbecco’s modified Eagle’s medium in the upper compartment of a Transwell bicameral chamber with a 0.4-mm pore size polycarbonate filter (Corning, New York, USA), which permits small soluble molecules produced by S. aureus to penetrate into the lower compartment, and the concentration of S. aureus filtrate was roughly determined by BCA protein assay kit (Beyotime, Shanghai, China).
Cell culture
RAW 264.7 mouse monocytes/macrophage cell line (TIB-71; ATCC) was used as osteoclast precursors, the cells were grown in high-glucose DMEM, supplemented with 10% fetal bovine serum and 1% Penicillin-Streptomycin solution, and in a humidified atmosphere of 95% air and 5% CO2 at 37°C; the media were changed every 3 days.
Cell viability assay (MTT assay)
RAW 264.7 cells were plated in 96-well plates at a density of 5×103 cells/well with 10 replicates in each group and cultured overnight, and then the cells were treated with live S. aureus (multiplicity of infection (MOIs) of 5: 1, 10: 1, 20: 1 and 40: 1 CFU per cell), inactivated S. aureus (MOIs of 10: 1, 20: 1 and 40: 1 CFU per cell), S. aureus filtrate (concentrations of 5, 10 and 20 μg/ml), and equal volume of PBS (control) for 1, 3, and 5 days; the media and stimuli were changed every 3 days. Note that live S. aureus infected RAW264.7 cells for 24 h, and then were inactivated with sensitive antibiotics, and RAW264.7 cells were cultured to a predetermined time. After culturing at indicated days, the cells were added with 10 μl MTT solution and incubated at 37°C for 4 h in the dark. Then, the media were removed, and the plates were added with 100 μl dimethyl sulfoxide solution and shaken for 10 min. Subsequently, the optical density (OD) values of every well were detected with a microplate reader (Model 680, BIO-RAD, USA) at 570 nm.
In vitro osteoclastogenetic assays
To differentiate into osteoclasts, RAW 264.7 cells were seeded in 96-well plates at a density of 5×103 cells/well with 5 duplicates in each group and cultured overnight. The cells were treated with inactivated S. aureus (MOIs of 10: 1, 20: 1 and 40: 1 CFU per cell), S. aureus filtrate (concentrations of 5, 10, and 20 μg/ml), RANKL (100 ng/ml) and PBS (control) for 5 days; the media and stimuli were replaced every 3 days. For live S. aureus infection, the cells were cultured in DMEM with no antibiotics and stimulated with live S. aureus (MOIs of 5: 1, 10: 1, 20: 1, and 40: 1 CFU per cell) for 24 h. To verify the role of NF-κB, JSH-23 (20 μM) was added 1 h ahead of stimuli, and left for 24 h. After that, live S. aureus was removed with sensitive antibiotics, and the cells were cultured for another 4 days. After culturing for a total of 5 days, the cells were fixed and stained using a TRAP staining kit according to the manufacturer’s instructions. TRAP-positive and multi-nucleated (≥3 nuclei) cells in every well were counted as osteoclast-like cells.
Bone resorption assay
RAW 264.7 cells were seeded in 24-well Corning® Osteo Assay Surface Multiple Well Plates (Corning, USA) at a density of 5×104 cells/well with 4 duplicates in each group and cultured overnight. Then, the cells were treated with inactivated S. aureus (MOIs of 10: 1, 20: 1, and 40: 1 CFU per cell), S. aureus filtrate (concentrations of 5, 10 and 20 μg/ml), RANKL (100 ng/ml), and PBS (control) for 5 days; the media and stimuli were replaced every 3 days. For live S. aureus infection, the cells were cultured in DMEM with no antibiotics and stimulated with live S. aureus (MOIs of 5: 1, 10: 1, 20: 1 and 40: 1 CFU per cell) for 24 h. To verify the role of NF-κB, JSH-23 (20 μM) was added 1 h ahead of stimuli, and left for 24 h. After that, live S. aureus were removed with sensitive antibiotics, and the cells were cultured for another 4 days. After culturing for 5 days, the media and cells were removed, and the areas of resorption pits were photographed by an inverted microscope and analyzed with Scion image software (Scion Corp., USA).
Real-time quantitative reverse transcription polymerase chain reaction (RT-PCR)
RAW 264.7 cells were seeded in 6-well plates at a density of 1×105 cells/well and cultured overnight. Then, the cells were treated with inactivated S. aureus (MOIs of 10: 1, 20: 1, and 40: 1 CFU per cell), S. aureus filtrate (concentrations of 5, 10, and 20 μg/ml), RANKL (100 ng/ml) and PBS (control) for 5 days; the media and stimuli were replaced every 3 days. For live S. aureus infection, the cells were cultured in DMEM with no antibiotics and stimulated with live S. aureus (MOIs of 5: 1, 10: 1, 20: 1, and 40: 1 CFU per cell) for 24 h. After that, live S. aureus was removed with sensitive antibiotics, and the cells were cultured for another 4 days. After culturing at indicated days, the cells were harvested and the total RNA was isolated with the GeneJET RNA purification kit according to the manufacturer’s procedure, and the concentration of total RNA was measured with a microspectrophotometer (NanoDrop 2000, Thermo Scientific, USA). Total RNA of 500 ng was used to synthesize to cDNA by reverse transcription using PrimeScript™ RT reagent kit with gDNA Eraser. Real-time PCR was performed in CFX96 Real-time System (BIO-RAD, USA) with a SYBR® Premix Ex Taq™. Reactions were initiated by incubation at 94°C for 5 min, and PCR (94°C for 30 s, 60°C (58°C for GAPDH) for 34 s and 72°C for 30 s) was performed for 40 cycles; all reactions were performed in triplicate. Relative quantities of the tested genes were normalized to GAPDH mRNA, and the normalized data are expressed using the comparative 2−ΔΔCT method. The primer sets used in experiments are shown in Table 1.
Table 1.
Primer sequences used in this study.
| Target gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| TRAP | GCGACCATTGTTAGCCACATACG | CGTTGATGTCGCACAGAGGGAT |
| MMP9 | GCTGACTACGATAAGGACGGCA | GCGGCCCTCAAAGATGAACGG |
| Cathepsin K | AGCAGAACGGAGGCATTGACTC | TTTAGCTGCCTTTGCCGTGGC |
| Calcitonin receptor | TGGTGCGGCGGGATCCTATAAGT | AGCGTAGGCGTTGCTCGTCG |
| ATP6v0d2 | ACGGTGATGTCACAGCAGACGT | CCTCTGGATAGAGCCTGCCGCA |
| GAPDH | CCCAGAAGACTGTGGATGG | CAGATTGGGGGTAGGAACAC |
Western blot
RAW 264.7 cells were cultured in 25-cm2 cell culture flasks; when the cells covering the bottom of the flasks were about 80%, the culture media were changed, and the cells were cultured for another 1 h in the cell incubator. After that, the cells were stimulated with live S. aureus at a MOI of 20: 1 for 0, 5, 10, 20, 40, and 60 min. For NFATc1 measurement, RAW 264.7 cells were seeded in 6-well plates at a density of 1×106 cells/well and infected with live S. aureus at a MOI of 20: 1 for 24 h, after which, S. aureus was removed with sensitive antibiotics and the cells were cultured for 0, 1, and 2 days. To explore the relationship between NF-κB and NFATc1, JSH-23 was added 1 h ahead of live S. aureus, and left for 24 h. At the indicated time, the cells were harvested and lysed using high-efficiency RIPA tissue/cell lysis solution (Solarbio, Beijing, China). After centrifugation, the proteins in the supernatant were collected. Subsequently, protein concentration was measured by a BCA protein assay kit (Beyotime, Shanghai, China), and protein samples were mixed with the sample buffer and boiled for 5 min. After that, protein samples (20 μg) were loaded onto 8–12% polyacrylamide gels, transferred to PVDF membranes subsequently blocked with 5% skimmed milk or BSA TBS (0.05% Tween-20) for 1 hat room temperature, and then were probed with specific antibodies against total ERK1/2, p38, JNK, NF-κB p65, IκB-α, Akt, GAPDH, and NFATc1, or the phosphorylated form of ERK1/2, p38, JNK, NF-κB p65, and Akt. Detection was carried out using HRP-linked rabbit IgG antibody, followed by ECL Western blotting detection reagents, and the densitometer scans of the blots were performed using the Universal Hood 2 Electrophoresis Imaging Cabinet (Bio-Rad, USA).
Data analysis
Each experiment was repeated in triplicate and similar results were obtained. Results are expressed as the mean ±SD. SPSS 19.0 software was used for statistical analysis, and the statistical significance was determined by one-way analysis of variance (ANOVA) with LSD/Student-Newman-Keul test; and p<0.05 was considered statistically significant.
Results
Effects of live S. aureus, inactivated S. aureus, and S. aureus filtrate on cell viability
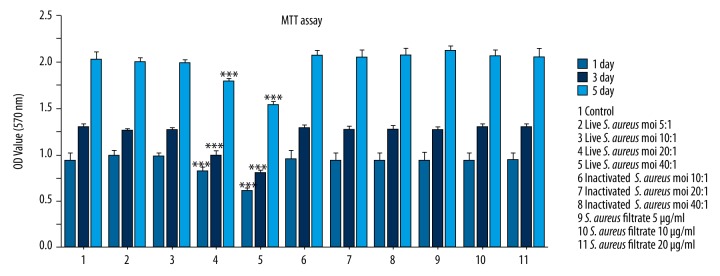
The cytotoxic effects of live S. aureus, inactivated S. aureus, and S. aureus filtrate on RAW 264.7 cells were measured by MTT assay. As shown in Figure 1, the MOIs of inactivated S. aureus and concentrations of S. aureus filtrate used in experiments and live S. aureus with MOIs of 5: 1 and 10: 1 CFU per cell showed no obvious cytotoxicity to RAW 264.7 cells. However, live S. aureus with MOIs of 20: 1 and 40: 1 CFU per cell inhibited the proliferation activity of RAW264.7 cells.
Figure 1.
Effects of live S. aureus, inactivated S. aureus, and S. aureus filtrate on the cell viability of RAW 264.7 cells. RAW 264.7 cells were cultured in 96-well culture plates and treated with live S. aureus (MOIs of 5: 1, 10: 1, 20: 1, and 40: 1 CFU per cell), inactivated S. aureus (MOIs of 10: 1, 20: 1, and 40: 1 CFU per cell), S. aureus filtrate (concentrations of 5, 10, and 20 μg/ml), and PBS (control). Cell proliferation was evaluated by MTT assay on days 1, 3, and 5. Data are shown as means ±SD, *** P<0.001, compared with control (PBS).
S. aureus induced the formation of osteoclast-like cells from RAW 264.7 cells
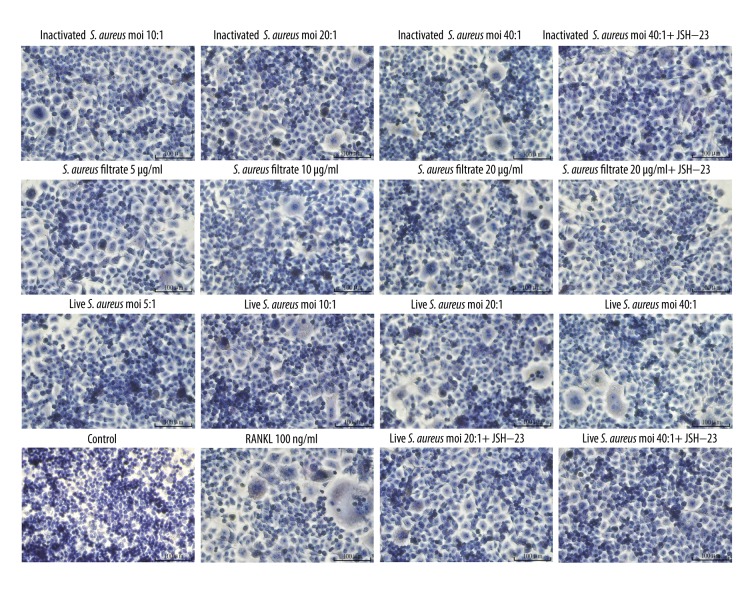
The excessively increased osteoclast activity is closely related to a variety of osteolytic diseases, such as rheumatoid arthritis, osteomyelitis, and periodontitis [24]. Therefore, we explored the effect of S. aureus on osteoclast formation through 3 aspects of live S. aureus, inactivated S. aureus, and S. aureus filtrate. As shown in Figures 2 and 3, after a 5-day culture, RANKL significantly promoted the formation of osteoclast-like cells. Live S. aureus, inactivated S. aureus, and S. aureus filtrate promoted the formation of osteoclast-like cells in a dose-dependent manner in the absence of RANKL. However, when pretreated with JSH-23, the formation of osteoclast-like cells induced by live S. aureus, inactivated S. aureus, and S. aureus filtrate was obviously decreased. Collectively, S. aureus induced the formation of osteoclast-like cells through its cell wall compound and secretion of small soluble molecules, but JSH-23 inhibited this induction effect.
Figure 2.
Effects of S. aureus on the osteoclast-like cell formation from RAW 264.7 cells. RAW 264.7 cells were cultured in 96-well culture plates and treated with live S. aureus (MOIs of 5: 1, 10: 1, 20: 1, and 40: 1 CFU per cell), inactivated S. aureus (MOIs of 10: 1, 20: 1, and 40: 1 CFU per cell), S. aureus filtrate (concentrations of 5, 10, and 20 μg/ml), JSH-23(20 μM), RANKL (100 ng/ml), and PBS (control) for 5 days. Then, the cells were stained for TRAP and photographed. The positive expression of TRAP showed as red particles in the cytoplasm, and the nuclei were stained blue. Images shown above were magnified 200 times. As shown in the images, with the increase of MOIs of live S. aureus and inactivated S. aureus, and the concentrations of S. aureus filtrate, the number of osteoclast-like cell (TRAP-positive and ≥3 nuclei) increased gradually. However, when treated with JSH-23, the formation of osteoclast-like cells induced by live S. aureus, inactivated S. aureus and S. aureus filtrate was obviously reduced.
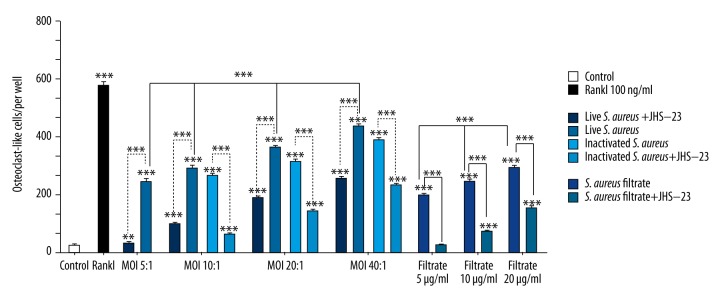
Figure 3.
Results of statistical analysis of osteoclast-like cells in each experimental group. RAW 264.7 cells were treated with live S. aureus, inactivated S. aureus, S. aureus filtrate, JSH-23, RANKL, and PBS for 5 days. Then, the cells were stained for TRAP, and the number of osteoclast-like cells (TRAP-positive and ≥3 nuclei) in every well was counted. Data are shown as means ±SD. One-way ANOVA was used to compared among groups. For the groups with statistical significance, LSD/Student-Newman-Keul test was used to compare the 2 groups, “*” just above the column indicated the comparison with the control group, while the other “*” indicates the comparison between the 2 groups, ** p<0.01, *** P<0.001.
S. aureus promoted the formation of resorption pits in the absence of RANKL
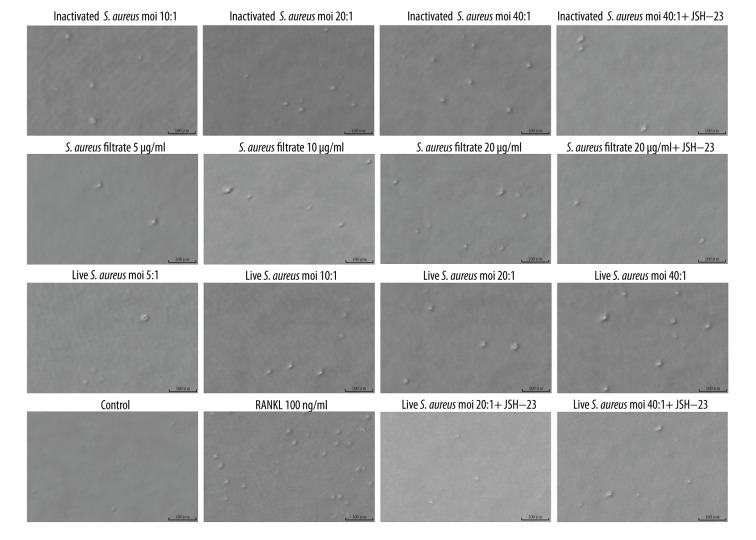
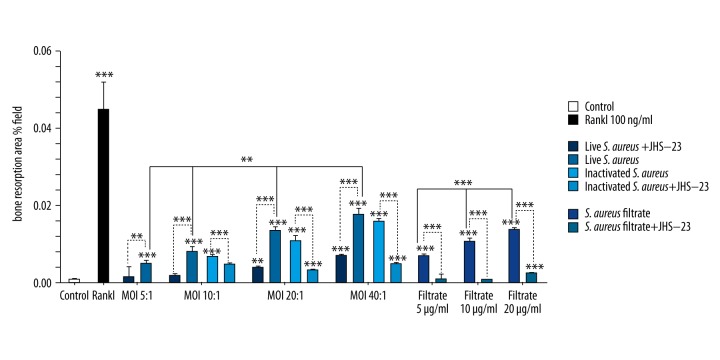
Since S. aureus promoted the formation of osteoclast-like cells derived from RAW 264.7 cells, and because bone resorption is the most important feature that undoubtedly identifies mature osteoclasts [25], we then evaluated the bone resorption activity of S. aureus-induced osteoclast-like cells from RAW 264.7 cells. As shown in Figures 4 and 5, after a 5-day culture, resorption pits were clearly observed in RANKL, live S. aureus, inactivated S. aureus, and S. aureus filtrate groups (Figure 4). The area of resorption pits induced by RANKL was the largest (Figures 4, 5); and live S. aureus, inactivated S. aureus, and S. aureus filtrate induced the formation of resorption pits in a dose-dependent manner in the absence of RANKL ((Figures 4, 5). However, when pretreated with JSH-23, the formation of resorption pits induced by live S. aureus, inactivated S. aureus, and S. aureus filtrate was obviously decreased (Figures 4, 5). Collectively, S. aureus promoted bone resorption through its cell wall compound and secretion of small soluble molecules, but JSH-23 inhibited this promotion effect.
Figure 4.
Effects of S. aureus on the bone resorption activity of osteoclasts derived from RAW 264.7 cells. RAW 264.7 cells were seeded in COAS plates and incubated with live S. aureus (MOIs of 5: 1, 10: 1, 20: 1, and 40: 1 CFU per cell), inactivated S. aureus (MOIs of 10: 1, 20: 1, and 40: 1 CFU per cell), S. aureus filtrate (concentrations of 5, 10, and 20 μg/ml), JSH-23(20 μM), RANKL (100 ng/ml), and PBS (control) for 5 days. After that, the cells were removed and resorption pits were observed and photographed under the same microscope. Images shown above were magnified 200 times. As shown in the images, with the increase of MOIs of live S. aureus and inactivated S. aureus, and the concentrations of S. aureus filtrate, the area and the number of resorption pits increased gradually. However, when treated with JSH-23, the area and the number of resorption pits induced by live S. aureus, inactivated S. aureus, and S. aureus filtrate were obviously reduced.
Figure 5.
Results of statistical analysis of resorption pits in each experimental group. RAW 264.7 cells were treated with live S. aureus, inactivated S. aureus, S. aureus filtrate, JSH-23, RANKL, and PBS for 5 days. Then, the cells were removed, and the resorption pits were photographed and analyzed with Scion image software. Data indicated the ratio of the cumulative area of resorption pits divided by the entire image area in the same field of view, and the data are shown as means ±SD. One-way ANOVA was used to compared among groups. For the groups with statistical significance, the LSD/Student-Newman-Keul test was used to compare between the 2 groups. “*” just above the column indicates the comparison with the control group, while other “*” indicates the comparison between the 2 groups, ** p<0.01, *** P<0.001.
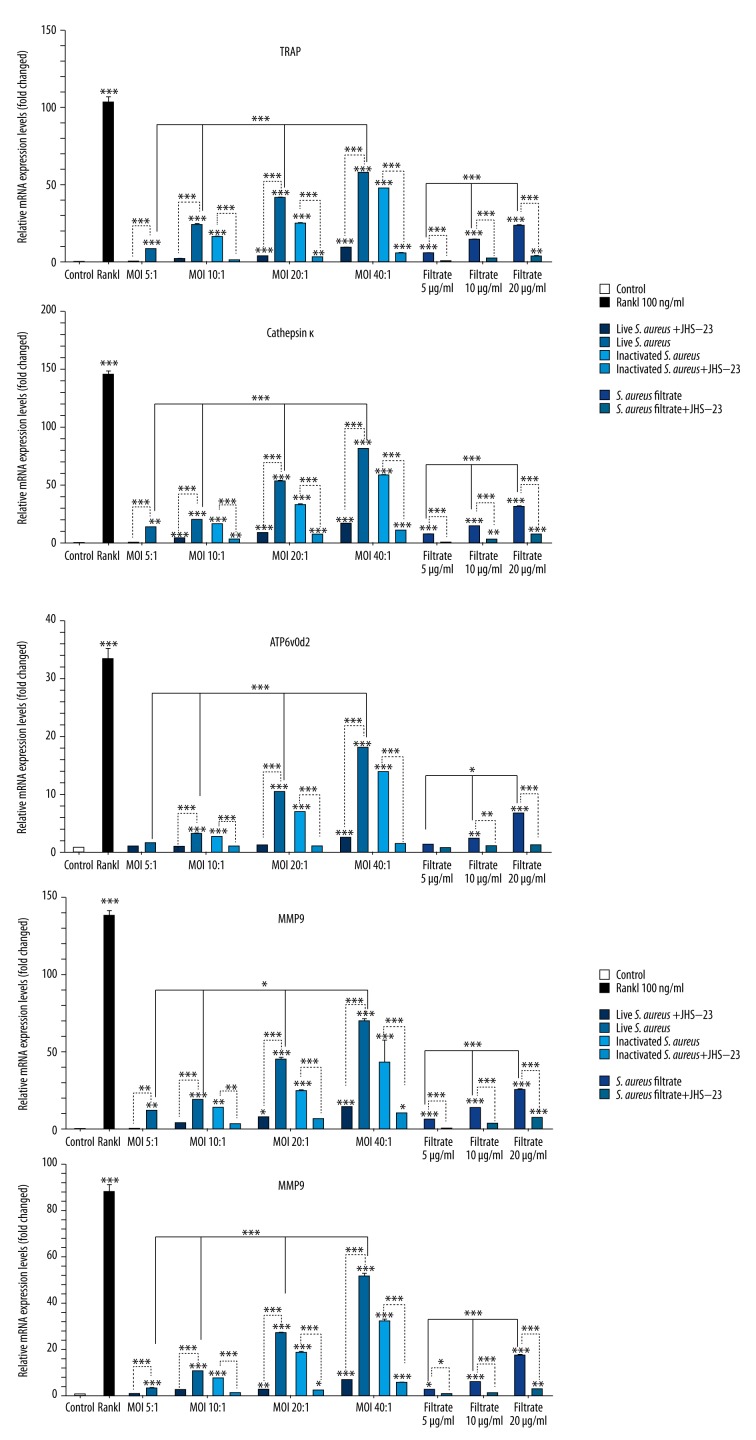
S. aureus increased the mRNA expression levels of osteoclast-specific genes in RAW 264.7 cells
Osteoclasts originated from hematopoietic cells of the monocyte/macrophage family [26] and RAW 264.7 cells have been shown to differentiate into mature osteoclasts when treated with RANKL [26,27]. Even in the absence of RANKL, LIGHT, IL-6 and TNF-α can induce RAW264.7 cells to differentiate into mature osteoclasts [28]. To verify the effect of S. aureus in promoting osteoclastogenesis in RAW264.7cells, we measured the mRNA expression levels of osteoclast-specific genes, such as TRAP, MMP-9, cathepsin K, CTR, and Atp6v0d2. As shown in Figure 6, RAW 264.7 cells themselves had the mRNA expression of TRAP, MMP-9, cathepsin K, CTR, and Atp6v0d2. Moreover, live S. aureus, inactivated S. aureus, and S. aureus filtrate increased the mRNA expression of osteoclast-specific genes in RAW 264.7 cells in a dose-dependent manner. However, when JSH-23 was added, the expression of osteoclast-specific genes induced by live S. aureus, inactivated S. aureus, and S. aureus filtrate were obviously decreased.
Figure 6.
Effects of S. aureus on the mRNA expression levels of osteoclast-specific genes. RAW 264.7 cells were cultured with live S. aureus (MOIs of 5: 1, 10: 1, 20: 1, and 40: 1 CFU per cell), inactivated S. aureus (MOIs of 10: 1, 20: 1, and 40: 1 CFU per cell), S. aureus filtrate (concentrations of 5, 10, and 20 μg/ml), RANKL (100 ng/ml), and PBS (control) for 5 days. The expression of osteoclast-specific genes, such as TRAP, MMP-9, Cathepsin K, CTR, and ATP6v0d2, was measured by real-time PCR, and the results were normalized to the expression of GAPDH. Data are shown as means ±SD. One-way ANOVA was used to compare differences among groups. For the groups with statistical significance, the LSD/Student-Newman-Keul test was used to compare the 2 groups. “*” just above the column indicates the comparison with the control group, while other “*” indicates the comparison between the 2 groups, * P<0.05, ** P<0.01, *** P<0.001.
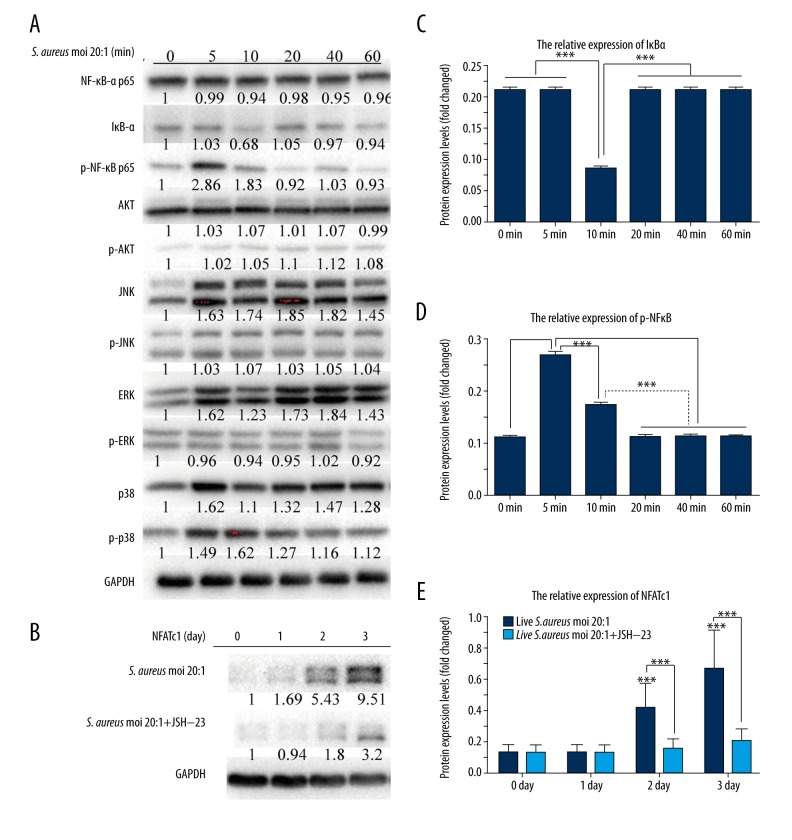
S. aureus induced osteoclast differentiation through the activation of NF-κB and the increased expression of NFATc1
It was reported that NF-κB [29], 3 mitogen-activated protein kinases (MAPKs, including p38, ERK and JNK) [30], and PI-3K/Akt [31] signaling pathways are involved in osteoclast differentiation. To elucidate the signaling pathways by which S. aureus promotes osteoclast differentiation, we measured the protein expression levels of these signaling pathways and NFATc1 in RAW 264.7 cells stimulated by live S. aureus. As shown in Figure 7, the degradation of IκBα occurred at 10 min after live S. aureus treatment (Figure 7A, 7C), while the phosphorylation of NF-κB p65 rose markedly at 5 and 10 min (Figure 7A, 7D). Moreover, proteins in the signaling pathways of Akt and MAPKs showed no obvious change when treated with live S. aureus (Figure 7A).
Figure 7.
Effects of S. aureus on the protein expression levels of NF-κB, MAPKs, AKT signaling pathways, and NFATc1. RAW 264.7 cells were treated with live S. aureus at a MOI of 20: 1 for the indicated times (0, 5, 10, 20, 40, and 60 min and 1, 2, and 3 days). Cells were lysed for Western blot analysis with specific antibodies against total ERK1/2, p38, JNK, NF-κB p65, IκB-α, Akt, GAPDH, and NFATc1, or the phosphorylated form of p38, ERK1/2, JNK, Akt, and NF-κB p65. Relative levels of these proteins were quantified by densitometric analysis and normalized to GAPDH. (A) and (B) shows the electrophoresis of the corresponding proteins. (C) Fold changes in IκB-α levels. (D) Fold changes in phospho-NFκB p65 levels. (E) Fold changes in NFATc1 levels. All experiments were performed at least 3 times. “*” just above the column indicates the comparison with 0 day, while other “*” indicates the comparison between the 2 groups, *** P<0.001.
The nuclear factor of activated T cells (NFATc1) is an important transcription factor in osteoclast differentiation; the induction of NFATc1 is a hallmark event in the cell terminal determination of osteoclasts [32,33]; and the presence of NFATc1 in osteoclast precursors could promote them to differentiate into mature osteoclasts in the absence of RANKL [33]. Thus, we examined the expression of NFATc1 in RAW 264.7 cells treated with live S. aureus for 0, 1, 2, and 3 days, and the results showed that S. aureus significantly promoted the expression of NFATc1 on days 2 and 3 (Figure 7B, 7E). However, when pretreated with JSH-23, the increased expression of NFATc1 was inhibited (Figure 7B, 7E). These findings suggest that S. aureus may induce osteoclast differentiation through the NF-κB signaling pathway, which subsequently activates NFATc1 and determined osteoclast differentiation.
Discussion
Mature osteoclasts are derived from hematopoietic cells of the monocyte/macrophage family [26]. Recent studies have found that macrophage-colony stimulating factor (M-CSF) and receptor activator of NF-κB ligand (RANKL) play an important role in the process of osteoclast differentiation. M-CSF promotes the survival of osteoclast precursors and osteoclasts [34,35], and induces RANK expression in osteoclast precursors [36], which combines with RANKL and initiates intracellular signaling pathways to regulate osteoclast differentiation [37]. In addition, RAW 264.7 cells express RANK and can differentiate into mature osteoclasts induced by RANKL [27], and therefore play an important role in studies of osteoclast formation and function in vitro [23].
Here, we comprehensively studied the effect of S. aureus on osteoclast differentiation, and found that S. aureus induced osteoclast differentiation and promoted bone resorption in a dose-dependent way, which was verified in live S. aureus, inactivated S. aureus, and S. aureus filtrate. This means that S. aureus promotes osteoclast differentiation through its cell wall compound and secretion of small soluble molecules. Our results are in agreement with the results of some scholars [17], but in contrast with the results of some other studies [38,39]. The main reason for these different results may be the difference in osteoclast precursors used in the experiments; they induce bone marrow cells with M-CSF and RANKL to obtain osteoclast precursors, but the osteoclast precursors they have obtained are early osteoclast precursors, committed osteoclast precursors, or mature osteoclasts, which have not been identified. In addition, the time of action and concentrations of M-CSF and RANKL undoubtedly affect the formation of osteoclast precursors. Live S. aureus acting on different stages of osteoclast precursors may yield different results. Here, we used RAW 264.7 cells as osteoclast precursors, which are widely used in studies of osteoclast formation and function in vitro, and this may be considered a more reasonable choice. Since bone is a dynamic organ, the balance between bone formation and bone resorption plays a major role in the maintenance of bone mineralization [10]. When we study the mechanisms of osteolytic diseases, we should not only study the effect of pathogenic factors on bone formation, but also explore the influence of pathogenic factors on bone resorption. When the effect of bone resorption is stronger than that of bone formation, bone destruction will arise. Our study found that the increase and activation of osteoclast differentiation induced by S. aureus infection is also an important cause of the formation of infectious bone defects, and the treatment methods of inhibiting osteoclast differentiation and activation may provide some help for the treatment of osteomyelitis.
In addition, we explored the molecular mechanism of S. aureus-induced osteoclast differentiation. RANKL, mainly derived from osteocytes [40], plays an important role in osteoclast differentiation. The binding of RANKL to RANK triggers a sequence of intracellular signal transduction pathways, including NF-κB and MAPKs, as well as PI-3K/AKT signaling pathways, which evokes the activation and autoamplification of NFATc1 and determines the terminal differentiation of osteoclasts. Moreover, NF-κB induces the initial activation of NFATc1 [33] and can induce osteoclast differentiation in the absence of RANKL [41]. In the present study, we found that the degradation of IκBα and phosphorylation of NF-κB in RAW 264.7 cells stimulated with S. aureus, and the changes of these 2 proteins have a certain relevance in time, but proteins in MAPKs and PI-3K/AKT signaling pathways showed no obvious change. We also found that the high expression of NFATc1 occurred on days 2 and 3 after S. aureus treatment. These results indicate that S. aureus activates NF-κB, which subsequently activates NFATc1 and promotes osteoclast differentiation in the absence of RANKL. Thus, we used JSH-23 to inhibit the activity of NF-κB and repeated some experiments. We found that the formation of osteoclast-like cells and resorption pits was decreased, and the expression of NFATc1 and osteoclast- specific genes, such as TRAP, MMP-9, Cathepsin K, CTR, and ATP6v0d2, were inhibited, which suggests that the activation of NF-κB plays a role in S. aureus-induced osteoclast differentiation. In addition, some researchers found that NF-κB plays an important role in the expression of inflammatory cytokines [42], and the NF-κB signaling pathway was activated in the process of S. aureus infecting osteoblasts. Combined with our experimental results, the evidence suggests that inhibitors of the NF-κB signaling pathway can be useful in the treatment of osteomyelitis. However, the mechanism by which S. aureus induces NF-κB activation needs additional research.
The autoamplification of NFATc1 in the final stage of osteoclast differentiation regulates the expression of many osteoclast-specific genes, such as TRAP [33], MMP-9 [43], cathepsin K, CTR, and Atp6v0d2 [44,45]. In our research, we found that the increased expression of NFATc1 occurred at 2 days and 3 days after S. aureus stimulation, and the mRNA expression levels of TRAP, MMP-9, Cathepsin K, and Atp6v0d2 in RAW 264.7 cells treated with S. aureus were increased in a dose-dependent manner. However, when added with JSH-23, an inhibitor of NF-κB activation, the expression of NFATc1 was inhibited, and the mRNA expression of osteoclast-specific genes was also decreased, which indicates that S. aureus activated NF-κB, which subsequently activated NFATc1 and promoted osteoclast differentiation.
Conclusions
Our results demonstrate that S. aureus promotes osteoclast differentiation and bone resorption through its cell wall compound and secretion of small soluble molecules, and the activation of NF-κB plays a major role in this process. Our research provides a molecular basis for understanding the pathogenesis of S. aureus-mediated osteomyelitis, which indicates that S. aureus infection not only inhibits bone formation, but also enhances bone resorption, and the formation of infectious bone defect is the effect of these 2 aspects.
Footnotes
Conflicts of interest
The authors have declared that no competing interests exist.
Source of support: This study was funded by the National Natural Sciences Foundation of China (No. 81472096)
References
- 1.Klosterhalfen B, Peters KM, Tons C, et al. Local and systemic inflammatory mediator release in patients with acute and chronic posttraumatic osteomyelitis. J Trauma. 1996;40(3):372–78. doi: 10.1097/00005373-199603000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Beck-Broichsitter BE, Smeets R, Heiland M. Current concepts in pathogenesis of acute and chronic osteomyelitis. Curr Opin Infect Dis. 2015;28(3):240–45. doi: 10.1097/QCO.0000000000000155. [DOI] [PubMed] [Google Scholar]
- 3.Grimbly C, Odenbach J, Vandermeer B, et al. Parenteral and oral antibiotic duration for treatment of pediatric osteomyelitis: A systematic review protocol. Syst Rev. 2013;2:92. doi: 10.1186/2046-4053-2-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jorge LS, Chueire AG, Rossit AR. Osteomyelitis: A current challenge. Braz J Infect Dis. 2010;14(3):310–15. [PubMed] [Google Scholar]
- 5.Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004;364(9431):369–79. doi: 10.1016/S0140-6736(04)16727-5. [DOI] [PubMed] [Google Scholar]
- 6.Labbe JL, Peres O, Leclair O, et al. Acute osteomyelitis in children: The pathogenesis revisited? Orthop Traumatol Surg Res. 2010;96(3):268–75. doi: 10.1016/j.otsr.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Parsons B, Strauss E. Surgical management of chronic osteomyelitis. Am J Surg. 2004;188(1A Suppl):57–66. doi: 10.1016/S0002-9610(03)00292-7. [DOI] [PubMed] [Google Scholar]
- 8.Giannoudis PV, Atkins R. Management of long-bone non-unions. Injury. 2007;38(Suppl 2):S1–2. doi: 10.1016/s0020-1383(07)80002-7. [DOI] [PubMed] [Google Scholar]
- 9.Feng X, McDonald JM. Disorders of bone remodeling. Ann Rev Pathol. 2011;6:121–45. doi: 10.1146/annurev-pathol-011110-130203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taichman RS. Blood and bone: Two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood. 2005;105(7):2631–39. doi: 10.1182/blood-2004-06-2480. [DOI] [PubMed] [Google Scholar]
- 11.Wright JA, Nair SP. Interaction of staphylococci with bone. Int J Med Microbiol. 2010;300(2–3):193–204. doi: 10.1016/j.ijmm.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiedler T, Salamon A, Adam S, et al. Impact of bacteria and bacterial components on osteogenic and adipogenic differentiation of adipose-derived mesenchymal stem cells. Exp Cell Res. 2013;319(18):2883–92. doi: 10.1016/j.yexcr.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 13.Rasigade JP, Trouillet-Assant S, Ferry T, et al. PSMs of hypervirulent Staphylococcus aureus act as intracellular toxins that kill infected osteoblasts. PLoS One. 2013;8(5):e63176. doi: 10.1371/journal.pone.0063176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuchscherr L, Heitmann V, Hussain M, et al. Staphylococcus aureus small-colony variants are adapted phenotypes for intracellular persistence. J Infect Dis. 2010;202(7):1031–40. doi: 10.1086/656047. [DOI] [PubMed] [Google Scholar]
- 15.Jin T, Zhu YL, Li J, et al. Staphylococcal protein A, Panton-Valentine leukocidin and coagulase aggravate the bone loss and bone destruction in osteomyelitis. Cell Physiol Biochem. 2013;32(2):322–33. doi: 10.1159/000354440. [DOI] [PubMed] [Google Scholar]
- 16.Meghji S, Crean SJ, Hill PA, et al. surface-associated protein from Staphylococcus aureus stimulates osteoclastogenesis: Possible role in S. aureus-induced bone pathology. Br J Rheumatol. 1998;37(10):1095–101. doi: 10.1093/rheumatology/37.10.1095. [DOI] [PubMed] [Google Scholar]
- 17.Lau YS, Wang W, Sabokbar A, et al. Staphylococcus aureus capsular material promotes osteoclast formation. Injury. 2006;37(Suppl 2):S41–48. doi: 10.1016/j.injury.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Nair S, Song Y, Meghji S, et al. Surface-associated proteins from Staphylococcus aureus demonstrate potent bone resorbing activity. J Bone Miner Res. 1995;10(5):726–34. doi: 10.1002/jbmr.5650100509. [DOI] [PubMed] [Google Scholar]
- 19.Yang J, Ryu YH, Yun CH, Han SH. Impaired osteoclastogenesis by staphylococcal lipoteichoic acid through Toll-like receptor 2 with partial involvement of MyD88. J Leukoc Biol. 2009;86(4):823–31. doi: 10.1189/jlb.0309206. [DOI] [PubMed] [Google Scholar]
- 20.Kim SN, Kim MH, Min YK, Kim SH. Licochalcone A inhibits the formation and bone resorptive activity of osteoclasts. Cell Biol Int. 2008;32(9):1064–72. doi: 10.1016/j.cellbi.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Takami M, Kim N, Rho J, Choi Y. Stimulation by toll-like receptors inhibits osteoclast differentiation. J Immunol. 2002;169(3):1516–23. doi: 10.4049/jimmunol.169.3.1516. [DOI] [PubMed] [Google Scholar]
- 22.Kishimoto T, Kaneko T, Ukai T, et al. Peptidoglycan and lipopolysaccharide synergistically enhance bone resorption and osteoclastogenesis. J Periodontal Res. 2012;47(4):446–54. doi: 10.1111/j.1600-0765.2011.01452.x. [DOI] [PubMed] [Google Scholar]
- 23.Collin-Osdoby P, Osdoby P. RANKL-mediated osteoclast formation from murine RAW 264.7 cells. Methods Mol Biol. 2012;816:187–202. doi: 10.1007/978-1-61779-415-5_13. [DOI] [PubMed] [Google Scholar]
- 24.Gyurko R, Shoji H, Battaglino RA, et al. Inducible nitric oxide synthase mediates bone development and P. gingivalis-induced alveolar bone loss. Bone. 2005;36(3):472–79. doi: 10.1016/j.bone.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Chambers TJ, Revell PA, Fuller K, Athanasou NA. Resorption of bone by isolated rabbit osteoclasts. J Cell Sci. 1984;66:383–99. doi: 10.1242/jcs.66.1.383. [DOI] [PubMed] [Google Scholar]
- 26.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 27.Suda T, Takahashi N, Udagawa N, et al. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20(3):345–57. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 28.Adamopoulos IE, Mellins ED. Alternative pathways of osteoclastogenesis in inflammatory arthritis. Nat Rev Rheumatol. 2015;11(3):189–94. doi: 10.1038/nrrheum.2014.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soysa NS, Alles N, Aoki K, Ohya K. osteoclast formation and differentiation: An overview. J Med Dent Sci. 2012;59(3):65–74. [PubMed] [Google Scholar]
- 30.Matsuzaki K, Udagawa N, Takahashi N, et al. Osteoclast differentiation factor (ODF) induces osteoclast-like cell formation in human peripheral blood mononuclear cell cultures. Biochem Biophys Res Commun. 1998;246(1):199–204. doi: 10.1006/bbrc.1998.8586. [DOI] [PubMed] [Google Scholar]
- 31.Moon JB, Kim JH, Kim K, et al. Akt induces osteoclast differentiation through regulating the GSK3beta/NFATc1 signaling cascade. J Immunol. 2012;188(1):163–69. doi: 10.4049/jimmunol.1101254. [DOI] [PubMed] [Google Scholar]
- 32.Asagiri M, Sato K, Usami T, et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med. 2005;202(9):1261–69. doi: 10.1084/jem.20051150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takayanagi H, Kim S, Koga T, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3(6):889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 34.Woo KM, Kim HM, Ko JS. Macrophage colony-stimulating factor promotes the survival of osteoclast precursors by up-regulating bcl-x(l) Exp Mol Med. 2002;34(5):340–46. doi: 10.1038/emm.2002.48. [DOI] [PubMed] [Google Scholar]
- 35.Fuller K, Owens JM, Jagger CJ, et al. Macrophage colony-stimulating factor stimulates survival and chemotactic behavior in isolated osteoclasts. J Exp Med. 1993;178(5):1733–44. doi: 10.1084/jem.178.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arai F, Miyamoto T, Ohneda O, et al. commitment and differentiation of osteoclast precursor cells by the sequential expression of c-fms and receptor activator of nuclear factor kappab (rank) receptors. J Exp Med. 1999;190(12):1741–54. doi: 10.1084/jem.190.12.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boyce BF. Advances in the regulation of osteoclasts and osteoclast functions. J Dent Res. 2013;92(10):860–67. doi: 10.1177/0022034513500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trouillet-Assant S, Gallet M, Nauroy P, et al. Dual impact of live Staphylococcus aureus on the osteoclast lineage, leading to increased bone resorption. J Infect Dis. 2015;211(4):571–81. doi: 10.1093/infdis/jiu386. [DOI] [PubMed] [Google Scholar]
- 39.Kassem A, Lindholm C, Lerner UH. Toll-like receptor 2 stimulation of osteoblasts mediates Staphylococcus aureus induced bone resorption and osteoclastogenesis through enhanced RANKL. PLoS One. 2016;11(6):e0156708. doi: 10.1371/journal.pone.0156708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakashima T, Hayashi M, Fukunaga T, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17(10):1231–34. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 41.Otero JE, Chen T, Zhang K, Abu-Amer Y. Constitutively active canonical NF-kappaB pathway induces severe bone loss in mice. PLoS One. 2012;7(6):e38694. doi: 10.1371/journal.pone.0038694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simmonds RE, Foxwell BM. Signalling, inflammation and arthritis: NF-kappaB and its relevance to arthritis and inflammation. Rheumatology. 2008;47(5):584–90. doi: 10.1093/rheumatology/kem298. [DOI] [PubMed] [Google Scholar]
- 43.Okada Y, Naka K, Kawamura K, et al. Localization of matrix metalloproteinase 9 (92-kilodalton gelatinase/type IV collagenase = gelatinase B) in osteoclasts: Implications for bone resorption. Lab Invest. 1995;72(3):311–22. [PubMed] [Google Scholar]
- 44.Kim Y, Sato K, Asagiri M, et al. Contribution of nuclear factor of activated T cells c1 to the transcriptional control of immunoreceptor osteoclast-associated receptor but not triggering receptor expressed by myeloid cells-2 during osteoclastogenesis. J Biol Chem. 2005;280(38):32905–13. doi: 10.1074/jbc.M505820200. [DOI] [PubMed] [Google Scholar]
- 45.Nakashima T, Takayanagi H. Osteoimmunology: Crosstalk between the immune and bone systems. J Clin Immunol. 2009;29(5):555–67. doi: 10.1007/s10875-009-9316-6. [DOI] [PubMed] [Google Scholar]