Kim et al. show that DPP4 (dipeptidyl peptidase 4) was selectively expressed on the surface of senescent human diploid fibroblasts and that this enabled their preferential elimination.
Keywords: CD26, cell senescence, human diploid fibroblasts
Abstract
Senescent cell accumulation in aging tissues is linked to age-associated diseases and declining function, prompting efforts to eliminate them. Mass spectrometry analysis revealed that DPP4 (dipeptidyl peptidase 4) was selectively expressed on the surface of senescent, but not proliferating, human diploid fibroblasts. Importantly, the differential presence of DPP4 allowed flow cytometry-mediated isolation of senescent cells using anti-DPP4 antibodies. Moreover, antibody-dependent cell-mediated cytotoxicity (ADCC) assays revealed that the cell surface DPP4 preferentially sensitized senescent, but not dividing, fibroblasts to cytotoxicity by natural killer cells. In sum, the selective expression of DPP4 on the surface of senescent cells enables their preferential elimination.
Cell senescence is a state of terminal growth arrest triggered by stress signals such as critically short telomeres, oxidative damage, oncogene activation, and hypoxia (Kuilman et al. 2010). Compared with proliferating cells, senescent cells exhibit an enlarged morphology, distinct metabolic and gene expression patterns, and increased activity of a neutral β-galactosidase (Crowe et al. 2014). They also display a senescence-associated secretory phenotype (SASP) characterized by the production and secretion of proinflammatory factors, angiogenic factors, and matrix metalloproteases that alter tissue function by promoting angiogenesis, attracting immune cells, and remodeling the extracellular matrix (Coppé et al. 2010).
Cell senescence has a range of complex effects on physiology and disease processes. Among its beneficial effects, senescent cells promote tissue morphogenesis and wound healing and suppress fibrosis and tumorigenesis in young organisms (Prieur and Peeper 2008; Muñoz-Espín et al. 2013; Storer et al. 2013; Demaria et al. 2014; Muñoz-Espín and Serrano 2014). On the other hand, extensive detrimental effects have been documented for senescent cells accumulating in tissues of older organisms, as they trigger or exacerbate age-related diseases such as cancer, cataracts, arthritis, and atherosclerosis (Baker et al. 2008, 2011; Prieur and Peeper 2008; Muñoz-Espín and Serrano 2014; van Deursen 2014).
The accumulation of senescent cells in aging tissues, as investigated in a genetic mouse model in which senescent cells were selectively eliminated, was found to promote aging-associated declines and diseases. Indeed, the removal of senescent cells from aging mouse tissues—achieved by triggering apoptotic death of cells that expressed the senescence protein p16—enhanced longevity and promoted healthy life span associated with a reduction in tumorigenesis and extended function of the renal, cardiovascular, muscular, and adipose systems (Baker et al. 2011, 2016).
With increasing evidence that senescent cells adversely influence aging-associated declines and diseases, a major goal in the field is to design interventions that recognize and eradicate senescent cells selectively. Senescent cells differ greatly from proliferating cells in the patterns of expressed proteins, including those on the cell surface that can serve as markers and therapeutic targets. Thus, we set out to identify cell surface proteins uniquely present in senescent cells. This strategy is similar to that used to eliminate cancer cells selectively (Rasmussen and Ditzel 2009). Using mass spectrometry (MS) analysis, we identified DPP4 (dipeptidyl peptidase 4; also known as CD26) as a surface protein that was strikingly more abundant in senescent cells. Importantly, senescent cells were preferentially eliminated by antibody-dependent cell-mediated cytotoxicity (ADCC), as the presence of DPP4 on their surface rendered them suitable targets for destruction by natural killer (NK) cells recognizing an anti-DPP4 antibody.
Results and Discussion
Identification of DPP4 as a novel senescent cell surface marker
To find novel surface markers selectively present on the plasma membrane of senescent cells, we used a well-characterized model of cellular senescence: proliferating WI-38 human diploid fibroblasts (HDFs; at population doubling level [PDL] 23) compared with those that had become senescent (PDL59) following extended culture. Senescent HDFs displayed a characteristic flattened and enlarged morphology and exhibited elevated senescence-associated β-galactosidase (SA-β-gal) activity, a distinctive trait of cellular senescence (Fig. 1A). Analysis of the incorporation of 3H-thymidine or BrdU further indicated that senescent cells had significantly lower proliferating activity (Fig. 1A). We fractionated membrane-associated proteins and cell surface-associated proteins as described (Materials and Methods) and surveyed them by MS analysis (see Supplemental Table S1 for complete MS data set). We focused on the proteins that were more abundant among membrane proteins (a fraction that included endoplasmic reticulum, mitochondria, and other intracellular membrane structures) and among cell surface proteins in senescent compared with proliferating fibroblasts. Among the 118 protein candidates at the overlap of the two groups (Fig. 1B), the top 15 proteins and the PSM (peptide spectrum match) count in proliferating relative to senescent cells are listed in Figure 1C. The leading candidate on this list was DPP4 (CD26). DPP4 peptides detected by MS analysis are indicated (Supplemental Fig. S1A).
Figure 1.
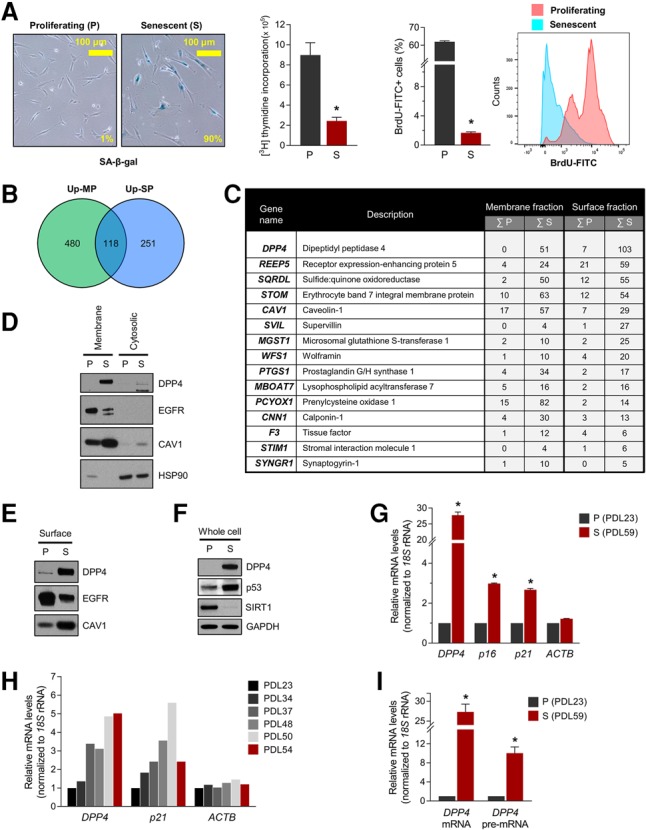
Identification of DPP4 as a novel senescent cell surface marker protein. (A, left) Detection of the senescent marker SA-β-gal in proliferating (P; PDL23) and senescent (S; PDL59) WI-38 HDFs; the percentages of blue cells are indicated. (Middle) Measurement of 3H-thymidine and BrdU incorporation in proliferating and senescent WI-38 cells. (Right) Flow cytometry analysis of BrdU-FITC-positive proliferating and senescent cells. (B) Venn diagram summarizing the MS analysis; the numbers of proteins more abundant in cell membrane preparations (Up-MP; green) and cell surface preparations (Up-SP; blue) from senescent cells relative to proliferating cells are indicated. (C) The top 15 proteins from the intersection in B. The numbers indicate the sums of the PSM from two experiments. (D–F) Western blot analysis of DPP4 levels in membrane and cytosolic lysates (D), surface proteins (E), and whole-cell lysates (F). (HSP90) Cytosolic protein marker; (CAV1 and EGFR) membrane protein markers; (SIRT1) protein marker of proliferating cells; (p53) protein marker of senescent cells; (GAPDH) loading control protein. (G–I) Steady-state levels of DPP4 mRNA and DPP4 pre-mRNA quantified by RT-qPCR analysis. Total RNA was prepared from proliferating and senescent cells (G,I) or cells at PDLs between PDL23 and PDL54 (H). Senescent markers p21 mRNA and p16 mRNAs were included as positive controls, and ACTB mRNA was used as a negative control. mRNA levels were normalized to 18S rRNA levels in each sample; mRNAs in PDL23 cells were set as 1 in G–I. The graphs in A, G, and I represent the means ± SEM from three independent experiments. (*) P-value < 0.05.
To validate these differences in DPP4 levels, we fractionated the membrane, surface-associated, and cytosolic fractions of proliferating and senescent WI-38 HDFs. Western blot analysis (Fig. 1D) revealed that DPP4 levels were robustly elevated in the membrane fraction of senescent fibroblasts but not proliferating fibroblasts or cytosolic fractions (Fig. 1D); membrane-associated proteins EGFR and Caveolin-1 (CAV1) as well as cytosolic protein HSP90 were included to monitor the cell fractionation. DPP4 was also more highly expressed on the cell surface and in whole-cell lysates (Fig. 1E,F) of senescent relative to proliferating WI-38 cells. Proteins present on the cell membrane (EGFR and CAV1), proteins showing altered levels with senescence (EGFR, CAV1, SIRT1, and p53), and a loading control (GAPDH) were also assessed.
We investigated the mechanism that led to the rise in DPP4 levels in senescent cells. RNA isolation from proliferating and senescent cells followed by RT-qPCR analysis revealed that DPP4 mRNA was markedly higher in senescent cells (Fig. 1G); senescent markers p16 (CDKN2A) mRNA and p21 (CDKN1A) mRNA were also elevated (Fig. 1G). DPP4 mRNA levels increased gradually with advancing PDLs (Fig. 1H). We quantified DPP4 pre-mRNA levels as a surrogate measure of de novo DPP4 mRNA transcription (Fig. 1I); the strong increase in DPP4 pre-mRNA levels in senescent cells suggested that increased DPP4 gene transcription was a major mechanism leading to DPP4 increase with senescence.
To test whether DPP4 increased more generally in senescent cells, we triggered senescence by exposing proliferating WI-38 and IMR-90 HDFs to ionizing radiation (IR); 10 d later, SA-β-gal activity was selectively elevated in IR-treated cells. Western blot and RT-qPCR analyses indicated that DPP4 and DPP4 mRNA (Supplemental Fig. S1B,C) were up-regulated in IR-induced senescent HDFs, although more modestly than in HDFs undergoing replicative senescence (Fig. 1F,G). We further tested whether DPP4 levels were elevated in other models of senescence by exposing human umbilical vein endothelial cells (HUVECs) and human aortic endothelial cells (HAECs) to 4 Gy of IR to elicit senescence; 14 d later, RT-qPCR analysis revealed significantly elevated DPP4 mRNA in senescent (IR-treated) compared with proliferating (untreated) HUVECs and HAECs (Supplemental Fig. S2A,B). Another trigger of senescence, treatment with doxorubicin (Dox), also led to a rise in DPP4 mRNA abundance in WI-38 cells (Supplemental Fig. S2C). Moreover, oncogene-induced senescence (OIS) in mouse embryonic fibroblasts (MEFs) expressing the oncogene HRASG12V via viral transduction also revealed a robust rise in DPP4 levels (Supplemental Fig. S2D). Together, these data indicate that DPP4 levels were highly and broadly elevated in senescent cells relative to proliferating cells at least in part by transcriptional induction of DPP4 mRNA levels and that DPP4 localized on the surface of the plasma membrane.
Contribution to senescence by DPP4
Given the rise in DPP4 levels in senescent cells, we asked whether DPP4 itself might help promote cellular senescence. To test this possibility, we overexpressed DPP4 in proliferating WI-38 cells by infection with a lentivirus that expressed DPP4-Myc using control viral particles that expressed only the Myc tag. After selection of infected cells in puromycin for 20 d, protein lysates were prepared, and Western blot analysis was used to assess senescence markers. As shown, markers p16 and p21 were elevated while SIRT1 levels declined in cells overexpressing DPP4 (Fig. 2A), supporting the notion that DPP4 promoted cell senescence. In addition, overexpression of DPP4 in WI-38 cells elevated SA-β-gal activity (Fig. 2B) and decreased 3H-thymidine incorporation (Fig. 2C).
Figure 2.
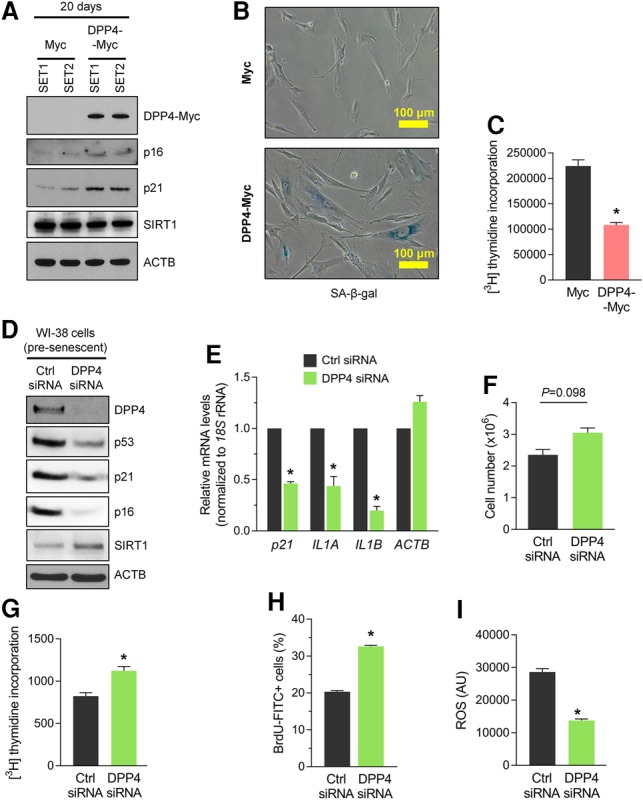
DPP4 contributes to the senescence program. (A) Proliferating WI-38 cells were infected with lentiviral particles expressing either Myc tag alone or DPP4-Myc. After 20 d of puromycin selection, cells were harvested, and whole-cell lysates were prepared for Western blot analysis to examine the levels of proteins DPP4-Myc (using anti-DPP4 antibody), p16, p21, and SIRT1 and loading control ACTB (β-Actin); endogenous DPP4 was undetectable. (B,C) Detection of the senescent marker SA-β-gal (B) and measurement of 3H-thymidine incorporation (C) in the populations described in A. (D–I) Seventy-two hours after transfecting presenescent WI-38 cells (PDL39–PDL45) with control (Ctrl) siRNA or DPP4 siRNA, the levels of DPP4, p53, p21, p16, SIRT1, and ACTB in whole-cell lysates were assessed by Western blot analysis (D); the steady-state levels of p21, IL1A, IL1B, and ACTB mRNAs were calculated by RT-qPCR analysis and normalized to 18S rRNA levels (E); cell numbers were quantified (F); 3H-thymidine (G) and BrdU (H) incorporation was measured; and reactive oxygen species (ROS) production was measured (I). The graphs in C and E–I represent the means ± SEM from three independent experiments. (*) P-value < 0.05.
We further tested whether DPP4 contributed to eliciting senescence by silencing DPP4 in presenescent WI-38 cells (approximately PDL39–PDL45) and assessing senescence markers. As shown, the robust silencing of DPP4 by 72 h after transfection of siRNA (Supplemental Material) led to marked reductions in the levels of p53, p21, and p16 and increased SIRT1 levels, as determined by Western blot analysis (Fig. 2D). Likewise, RT-qPCR analysis revealed that the levels of senescence markers p21 mRNA, IL1A mRNA, and IL1B mRNA were significantly reduced in the DPP4 siRNA group (Fig. 2E). Additionally, DPP4 silencing increased the number of cells in the population (Fig. 2F) and promoted incorporation of 3H-thymidine and BrdU (Fig. 2G,H), further indicating that DPP4 contributed to the growth suppression characteristic of senescence. Interestingly, DPP4 silencing reduced the production of reactive oxygen species (ROS) in presenescent cells (Fig. 2I). Taken together, our results indicate that DPP4 enhances fibroblast senescence.
Specific selection of senescent cells using cell surface DPP4
Given that DPP4 was identified as a cell surface protein in senescent cells (Fig. 1B,C), we used confocal microscopy to further investigate this localization. Proliferating and senescent WI-38 HDFs were fixed but not permeabilized in order to detect only proteins present on the outside of the plasma membrane. As shown in Figure 3A, DPP4 signal (red) was virtually undetectable in proliferating cells but was highly abundant in senescent cultures and was found throughout the cell surface. In light of these results, we tested whether DPP4 might be a suitable selection marker for senescent cells. We used two mouse antibodies, anti-DPP4-PE and (control) mIgG-PE, to label proliferating and senescent WI-38 HDFs without fixation or permeabilization to analyze cell surface markers. Labeled samples were analyzed by flow cytometry to assess the presence of DPP4 on the cell surface. MFI (mean fluorescence intensity) analysis revealed that DPP4-labeled cells were enriched eightfold in senescent compared with proliferating WI-38 HDFs (Fig. 3B). Moreover, the number of DPP4-positive cells identified by flow cytometry analysis using DPP4-PE was only 6.05% in proliferating cells but rose to 67.9% in senescent cells (Fig. 3C). In sum, DPP4 levels are markedly elevated on the surface of senescent fibroblasts and serve as a suitable marker for the specific isolation of senescent cells.
Figure 3.
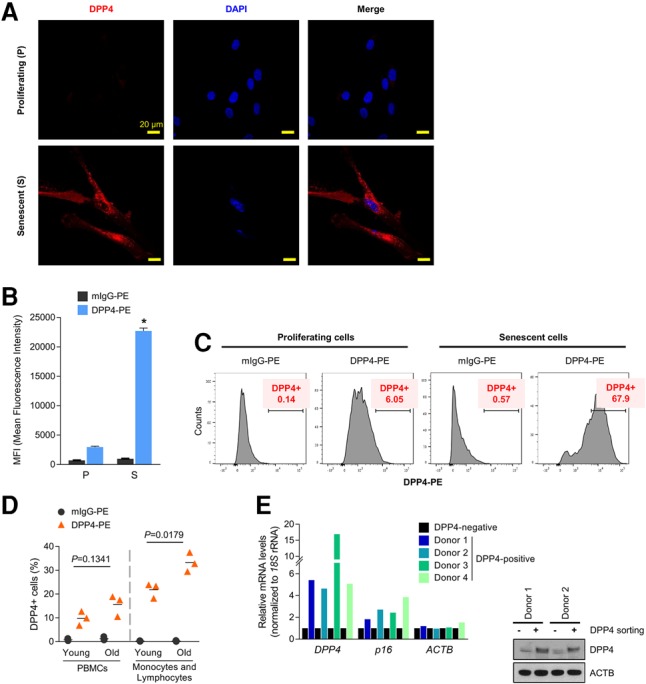
DPP4 is more highly expressed in senescent cells and localizes on the cell surface. (A) Proliferating (P) or senescent (S) WI-38 HDFs were fixed with methanol, and the endogenous DPP4 protein was detected by confocal microscopy. (B,C) WI-38 (proliferating and senescent) HDFs were harvested and incubated with either anti-DPP4-PE or control mIgG-PE antibodies. (B) DPP4-positive cells were analyzed by flow cytometry, and the MFI of DPP4-PE in proliferating and senescent cells was quantified. The graph represents the means ± SEM. from three independent experiments. (*) P-value < 0.05. (C) Histograms from the flow analysis representing control isotype (left) or DPP4 (right) in proliferating and senescent cells; the percentages of DPP4-PE-positive cells are indicated. The experiment shown is representative of three independent experiments. (D) Human peripheral blood mononuclear cells (PBMCs) obtained from young (27- to 36-yr-old) and old (78- to 88-yr-old) donors were analyzed by flow cytometry using antibodies that recognized DPP4-PE (▴) or mouse IgG-PE (•). The graph shows the percentage of DPP4-PE-positive cells. (Left) PBMC populations. (Right) Monocyte and lymphocyte populations. Each data point represents a young (n = 3 total) or old (n = 3 total) subject; horizontal lines indicate the mean values. (E) Human PBMCs obtained from four donors (27, 27, 60, or 63 yr old) were sorted into DPP4-positive cells and DPP4-negative cells by MACS using a DPP4-PE antibody and anti-PE microbeads. The steady-state levels of DPP4, p16, and ACTB mRNAs were quantified by RT-qPCR analysis after normalization to 18S rRNA levels. Each mRNA in DPP4-negative cells was set as 1.
With advancing age, senescent cells accumulate in a variety of tissues, including blood. We thus asked whether DPP4-positive cells might be detected in peripheral blood mononuclear cells (PBMCs) from three healthy young individuals (27–36 yr of age) and three healthy old individuals (78–88 yr of age) (Materials and Methods). As shown in Figure 3D, the percentage of DPP4-positive cells (as determined by flow cytometry using the DPP4-PE antibody) was higher in “old” PBMCs than in “young” PBMCs (15.5% vs. 10%). Since most DPP4 signals came from monocytes and lymphocytes, we calculated the percentages of DPP4-positive monocytes and lymphocytes and found that these percentages remained significantly higher in the old donor group than the young donor group (33% vs. 21%); see Supplemental Figure S3 for contour plots of the individual donors. To determine whether DPP4-positive cells were senescent, PBMCs from four donors (ages 27, 27, 60, and 63 yr) were labeled with DPP4-PE and sorted using anti-PE microbeads by MACS (magnetic-activated cell sorting) analysis. After isolation of RNA from the affinity-purified cells, RT-qPCR analysis was used to assess the expression of senescence markers. As shown (Fig. 3E), DPP4-positive cells in each of the four donors expressed higher levels of DPP4 and DPP4 mRNA by Western blot and RT-qPCR analyses, respectively (to verify that the affinity pull-down was successful), and, importantly, all four samples also displayed higher levels of p16 mRNA, which encodes the major senescence-associated protein p16. The data obtained using PBMCs further support the notion that DPP4 also serves as a surface marker for the selection of senescent cells among a heterogeneous cell population.
Selective elimination of DPP4-positive senescent cells by ADCC assay
Our initial goal was to devise a method to eliminate senescent cells selectively using a differentially expressed surface protein. Thus, after establishing that DPP4 is located on the surface of the plasma membrane of senescent cells (Figs. 1, 3), we set out to test whether we could eliminate senescent cells selectively using an antibody directed at DPP4 and the ADCC assay. Originally developed for cancer therapy, the ADCC assay uses antibodies to recognize a specific antigen on the cell surface and guide NK cells to selectively destroy the antibody-labeled cells (Weiner 2015). We carried out ADCC analysis using increasing concentrations of anti-DPP4 antibody (up to 5 µg/mL) to bind the DPP4 surface marker and thereby label proliferating and senescent WI-38 fibroblasts. We then isolated NK cells from human PBMCs and added them to the WI-38 cultures, allowing the NK cells to destroy the cells labeled by anti-DPP4 antibody. Treatment with NK cells from eight different donors in the presence of rabbit or humanized anti-DPP4 antibodies (Fig. 4A) indicated that, in every case, senescent cells exhibited stronger reductions in cell viability, as low as ∼40% (as seen with “donor 8” of NK cells), compared with proliferating cells. As shown in Supplemental Figure S4, ADCC assay using a control rabbit IgG (rIgG) revealed that this antibody did not affect cell viability in proliferating or senescent cells.
Figure 4.
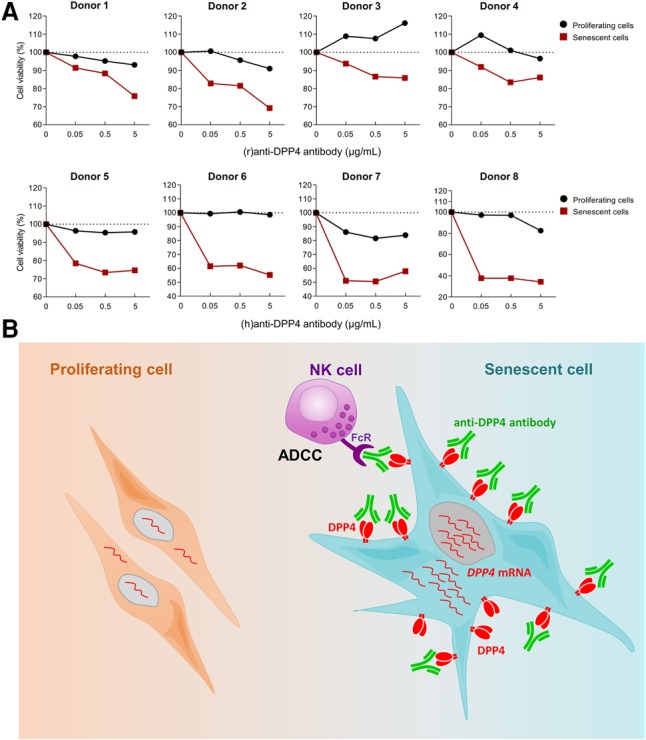
Eliminating DPP4-positive senescent cells by ADCC. (A) Proliferating and senescent WI-38 cells (5 × 104) were incubated without or with 0.05, 0.5, and 5 µg/mL anti-DPP4 antibody and either 2.5 × 105 NK cells [for rabbit (r)anti-DPP4] or 1.25 × 106 or 2.5 × 106 NK cells [for humanized (h)anti-DPP4 antibody] per well for 4 h. After removing NK cells, WI-38 cells were incubated for another 18 h, and cell viability was measured by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay. Cell numbers were compared with those that received no anti-DPP4 antibody. Donors 1–4 were incubated with (r)anti-DPP4, and donors 5–8 were incubated with (h)anti-DPP4. (B) Proposed model. (Left) In proliferating cells, DPP4 is expressed at low levels or is undetectable. (Right) In senescent cells, DPP4 mRNA levels increase transcriptionally, leading to increased production of DPP4, which localizes as a dimer on the cell surface, exposed to the extracellular space. The abundance and localization of DPP4 in senescent cells enable the selective elimination of senescent cells by ADCC.
Targeting DPP4 to eliminate senescent cells
In sum, we identified DPP4 as a protein robustly up-regulated on the plasma membrane of senescent cells. DPP4 levels increased via transcriptional induction of DPP4 mRNA in senescent cells. Whether this increase might be mediated via transcription factors HNF and/or STAT1α in fibroblasts, as shown previously in B lymphocytes and intestinal epithelial cells (Bauvois et al. 2000; Erickson et al. 2000; D'Angelo et al. 2010), remains to be tested. We further found that senescent cells can be selectively targeted by anti-DPP4 antibodies and eliminated. Accordingly, we propose a model (Fig. 4B) in which the highly abundant DPP4 on the exposed surface of a senescent cell allows it to be recognized and eliminated by anti-DPP4 antibody-directed NK-elicited cell death. These results underscore the usefulness of targeting for selective destruction proteins differentially present on the plasma membrane of senescent cells.
DPP4 is best known as a protease that inactivates two hormones named incretins: glucose-dependent insulinotropic peptide (GIP) and glucagon-like peptide-1 (GLP-1). Incretins trigger a rapid release of insulin from pancreatic β cells after a meal; since they suppress a sudden rise in blood glucose, they are particularly beneficial in diabetes (Wu et al. 2016). Although the significance of having elevated DPP4 in senescent cells is unclear, DPP4 might influence some of the changes in glucose homeostasis that occur with aging. For example, a rise in DPP4 levels from accumulating senescent cells could contribute to the impairment of glucose metabolism that leads to hyperglycemia in the elderly. In this regard, it will be interesting to test whether DPP4 inhibitors (e.g., sitagliptin) (Scott 2017), which enhance the so-called “incretin effect” and have been used to treat diabetes, recapitulate the enhanced proliferation and suppressed senescence seen after silencing DPP4 (Fig. 2).
DPP4 and senolytic therapy
Similarly, as incretin-independent actions of DPP4 are emerging, it will be important to study whether the health benefits of DPP4 inhibitors depend partly on their impact on senescent cells irrespective of DPP4-mediated inactivation of incretins. For example, DPP4 present on the plasma membrane of lymphocytes (which we found to be elevated with age) (Fig. 3D) associates with CAV1, a membrane protein that also rises with senescence, leading to the activation of IRAK-1 and NF-κB (Ohnuma et al. 2005, 2008). Likewise, DPP4 present on the membrane of antigen-presenting cells and inflammatory cells in adipose tissue promotes inflammation independent of its effects on insulin production (Zhong et al. 2013). These actions of DPP4 as well as its interaction with extracellular matrix components such as collagen and fibronectin (Löster et al. 1995; Zhong et al. 2013) might implicate DPP4 as a factor related to SASP traits, which are modulated by NF-κB and profoundly affect extracellular matrix remodeling.
SASP has been linked to the disease-enhancing impact of senescent cells that accumulate in aging tissues, since SASP factors (cytokines, growth factors, and MMPs) perturb tissue metabolism locally and systemically. Accordingly, senescent cell elimination via genetic manipulation improved age-related pathologies (Baker et al. 2008, 2011, 2016). To achieve the same goal, several drugs called “senolytics” have been identified that selectively destroy senescent cells. Senolytics ABT737 (Yosef et al. 2016) and ABT263 (Zhu et al. 2016) were BH3 mimetic inhibitors of anti-apoptotic proteins (Bcl-xL, Bcl-2, and Bcl-w) originally developed for cancer therapy, although inhibiting Bcl-xL was found to have severe side effects. Other senolytics, such as dasatinib and quercetin (Roos et al. 2016; Zhu et al. 2016), trigger apoptosis in subsets of senescent cells, while piperlongumine appears to work best in combination with other senolytics (Wang et al. 2016). In this context, the identification of DPP4 as a targetable senescence marker can complement interventions aimed at eliminating senescent cells.
In closing, DPP4 is overexpressed in cancers such as malignant pleural mesothelioma (MPM) and renal cell and colorectal carcinoma. A recent study found that DPP4 was more highly expressed on the plasma membrane of MPM than normal mesothelioma cells (Angevin et al. 2017). Importantly, in preclinical trials, MPM tumor growth was delayed by treatment with anti-DPP4 antibody, suggesting that it was inhibitory in both cultured cells and patients. Antibody modifications such as conjugation with a toxin might enhance the cytotoxic activity of the anti-DPP4 antibody on DPP4-bearing cells. In summary, our findings have identified the cell membrane-associated protein DPP4 senescence marker as a promising target of therapeutic intervention in conditions in which it is desirable to eliminate senescent cells.
Materials and methods
Cells, cell culture, IR, and SA-β-gal assay
The source and culture of HDFs WI-38 and IMR-90, MEFs, HUVECs, and HAECs are indicated in the Supplemental Material. Proliferating WI-38 HDFs were used at PDLs ranging between PDL18 and PDL23, and senescent cells were used after additional culture (PDL47–PDL55). SA-β-gal analysis and siRNA transfections using Lipofectamine 2000 (Invitrogen) are described (Supplemental Material). Human PBMCs were isolated from participants of the Baltimore Longitudinal Study of Aging (BLSA) (Ferrucci 2008) aged 27–36 yr old (young) or 78–88 yr old (old). Proliferating WI-38 cells (approximately PDL25) and IMR-90 cells (approximately PDL25) were rendered senescent by exposure to 10 Gy of IR; cells were harvested 10 d later. Proliferating WI-38 cells were transduced with lentiviruses that expressed either DPP4-Myc or Myc (GeneCopoeia, Inc.) and selected using 1 µg/mL puromycin for 20 d before harvest.
RNA isolation, reverse transcription, and quantitative real-time PCR
Total RNA was extracted using TriPure isolation reagent (Roche), and cDNA was synthesized using random hexamers and reverse transcriptase (Invitrogen) as described (Supplemental Material). Reactions for qPCR amplification contained SYBR Green master mix (Kapa Biosystems) and were performed using an Applied Biosystems 7300 instrument. The gene-specific primers used are listed in the Supplemental Material.
Protein analysis
The preparation and analysis of cell membrane and cell surface proteins by Western blotting and MS as well as the detection of DPP4 using immunofluorescence are explained in the Supplemental Material.
Flow cytometry of WI-38 cells and PBMCs and MACS
Proliferating and senescent WI-38 cells were counted using a TC20 cell counter (Bio-Rad), washed using FACS buffer (0.5% BSA in PBS), and seeded into 96-well plates. Dead cells were stained with Zombie Aqua fixable viability kit (BioLegend); after washing, human TruStain FcX (BioLegend) was added to block the Fc receptor, and cells were labeled with DPP4-PE (BD Biosciences) or mIgG-PE (BioLegend) for 15 min at 4°C in the dark. FACS analysis was performed on a Canto II flow cytometer (BD Biosciences) using FlowJo software (FlowJo version 10.2).
Human PBMCs were incubated with DPP4-PE (BD Biosciences) for 10 min at 4°C in the dark to label DPP4-expressing cells and then labeled with anti-PE magnetic microbeads (Miltenyi Biotec, Inc.) for 15 min at 4°C. Magnetically labeled cells were separated by MACS (Supplemental Material). Total RNA was extracted from DPP4-positive and DPP4-negative cells.
ADCC assay
Peripheral blood from participants of the BLSA (National Institute on Aging) and healthy volunteers was collected under Human Subject Protocol 2003054 and Tissue Procurement Protocol 2003-076. White blood cells were isolated by ACK lysis, and PBMCs were isolated using Ficoll (Ficoll-Paque Plus, GE Healthcare) gradients following the manufacturer's instructions. NK cells were isolated from PBMCs using a human NK cell isolation kit (Miltenyi Biotec, Inc.). Proliferating and senescent WI-38 cells growing on 24-well plates (5 × 104 cells per well) were incubated with different concentrations of control rIgG or with rabbit or humanized monoclonal antibodies recognizing 0.05, 0.5, and 5 µg/mL DPP4 for 15 min at 37°C. The isolated NK cells (2.5 × 105 cells per well for rabbit antibody and 1.25 × 106 or 2.5 × 106 for humanized antibody) were added at a ratio of 5:1 (proliferating) or 25:1 or 50:1 (senescent; NK cells:fibroblasts [proliferating or senescent]) and incubated for 4 h at 37°C. NK cells were removed by washing, fibroblasts were returned to the incubator, and, 18 h later, cell viability was analyzed using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay (Sigma).
Supplementary Material
Acknowledgments
We thank M.K. Evans and N. Noren Hooten for providing HUVECs and HAECs. This work was supported in part by the National Institute on Aging and National Cancer Institute Intramural Research Program and the National Institutes of Health. K.O. and C.M. were supported by Grant-in-Aid (S1311011), grant 150401-01, and Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research (KAKENHI) grants 15H04879, 15K15324, 16H05345, and 16K09878.
Footnotes
Supplemental material is available for this article.
Article published online ahead of print. Article and publication date are online at http://www.genesdev.org/cgi/doi/10.1101/gad.302570.117.
References
- Angevin E, Isambert N, Trillet-Lenoir V, You B, Alexandre J, Zalcman G, Vielh P, Farace F, Valleix F, Podoll T, et al. 2017. First-in-human phase 1 of YS110, a monoclonal antibody directed against CD26 in advanced CD26-expressing cancers. Br J Cancer 116: 1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Perez-Terzic C, Jin F, Pitel KS, Niederländer NJ, Jeganathan K, Yamada S, Reyes S, Rowe L, Hiddinga HJ, et al. 2008. Opposing roles for p16Ink4a and p19Arf in senescence and ageing caused by BubR1 insufficiency. Nat Cell Biol 10: 825–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. 2011. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479: 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, et al. 2016. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530: 184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauvois B, Djavaheri-Mergny M, Rouillard D, Dumont J, Wietzerbin J. 2000. Regulation of CD26/DPPIV gene expression by interferons and retinoic acid in tumor B cells. Oncogene 19: 265–272. [DOI] [PubMed] [Google Scholar]
- Coppé JP, Desprez PY, Krtolica A, Campisi J. 2010. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5: 99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe EP, Nacarelli T, Bitto A, Lerner C, Sell C, Torres C. 2014. Detecting senescence: methods and approaches. Methods Mol Biol 1170: 425–445. [DOI] [PubMed] [Google Scholar]
- D'Angelo A, Bluteau O, Garcia-Gonzalez MA, Gresh L, Doyen A, Garbay S, Robine S, Pontoglio M. 2010. Hepatocyte nuclear factor 1α and β control terminal differentiation and cell fate commitment in the gut epithelium. Development 137: 1573–1582. [DOI] [PubMed] [Google Scholar]
- Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dollé ME, et al. 2014. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 31: 722–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson RH, Lai RS, Kim YS. 2000. Role of hepatocyte nuclear factor 1α and 1β in the transcriptional regulation of human dipeptidyl peptidase IV during differentiation of Caco-2 cells. Biochem Biophys Res Commun 270: 235–239. [DOI] [PubMed] [Google Scholar]
- Ferrucci L. 2008. The Baltimore Longitudinal Study of Aging (BLSA): a 50-year-long journey and plans for the future. J Gerontol A Biol Sci Med Sci 63: 1416–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. 2010. The essence of senescence. Genes Dev 24: 2463–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löster K, Zeilinger K, Schuppan D, Reutter W. 1995. The cysteine-rich region of dipeptidyl peptidase IV (CD 26) is the collagen-binding site. Biochem Biophys Res Commun 217: 341–348. [DOI] [PubMed] [Google Scholar]
- Muñoz-Espin D, Serrano M. 2014. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol 15: 482–496. [DOI] [PubMed] [Google Scholar]
- Muñoz-Espín D, Cañamero M, Maraver A, Gómez-López G, Contreras J, Murillo-Cuesta S, Rodríguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M, et al. 2013. Programmed cell senescence during mammalian embryonic development. Cell 155: 1104–1118. [DOI] [PubMed] [Google Scholar]
- Ohnuma K, Yamochi T, Uchiyama M, Nishibashi K, Iwata S, Hosono O, Kawasaki H, Tanaka H, Dang NH, Morimoto C. 2005. CD26 mediates dissociation of Tollip and IRAK-1 from caveolin-1 and induces upregulation of CD86 on antigen-presenting cells. Mol Cell Biol 25: 7743–7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnuma K, Dang NH, Morimoto C. 2008. Revisiting an old acquaintance: CD26 and its molecular mechanisms in T cell function. Trends Immunol 29: 295–301. [DOI] [PubMed] [Google Scholar]
- Prieur A, Peeper DS. 2008. Cellular senescence in vivo: a barrier to tumorigenesis. Curr Opin Cell Biol 20: 150–155. [DOI] [PubMed] [Google Scholar]
- Rasmussen N, Ditzel HJ. 2009. Scanning the cell surface proteome of cancer cells and identification of metastasis-associated proteins using a subtractive immunization strategy. J Proteome Res 8: 5048–5059. [DOI] [PubMed] [Google Scholar]
- Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, Hagler M, Jurk D, Smith LA, Casaclang-Verzosa G, et al. 2016. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 15: 973–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LJ. 2017. Sitagliptin: a review in type 2 diabetes. Drugs 77: 209–224. [DOI] [PubMed] [Google Scholar]
- Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V, Yosef R, Pilpel N, Krizhanovsky V, Sharpe J, Keyes WM. 2013. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 155: 1119–1130. [DOI] [PubMed] [Google Scholar]
- van Deursen JM. 2014. The role of senescent cells in ageing. Nature 509: 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chang J, Liu X, Zhang X, Zhang S, Zhang X, Zhou D, Zheng G. 2016. Discovery of piperlongumine as a potential novel lead for the development of senolytic agents. Aging 8: 2915–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner GJ. 2015. Building better monoclonal antibody-based therapeutics. Nat Rev Cancer 15: 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Rayner CK, Horowitz M. 2016. Incretins. Handb Exp Pharmacol 233: 137–171. [DOI] [PubMed] [Google Scholar]
- Yosef R, Pilpel N, Tokarsky-Amiel R, Biran A, Ovadya Y, Cohen S, Vadai E, Dassa L, Shahar E, Condiotti R, et al. 2016. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat Commun 7: 11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Rao X, Deiuliis J, Braunstein Z, Narula V, Hazey J, Mikami D, Needleman B, Satoskar AR, Rajagopalan S. 2013. A potential role for dendritic cell/macrophage-expressing DPP4 in obesity-induced visceral inflammation. Diabetes 62: 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB, Pirtskhalava T, Giorgadze N, Johnson KO, Giles CB, et al. 2016. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 15: 428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


