Abstract
Diabetes mellitus has become one of the biggest medical challenges affecting millions of people globally. Alternative treatments for diabetes are currently being intensively investigated to improve the treatment efficacy and life qualities for diabetic patients. Glucose-responsive insulin release (GRIR) systems have exhibited tremendous potential to improve the normal glycemic control and to reduce the incidence of hyperglycemia and hypoglycemia, which further reduces potential complications in diabetic patients. In a given GRIR drug formulation, accuracy, response time, and reversibility of the GRIR functions are three key features enabling potential seamless control of blood glucose level. Nevertheless, there is significant challenge preventing current GRIR formulations from achieving them. This review article analyzes the most updated literature and provides insights on the impact of GRIR mechanisms, and formulations on these key features, and the relevant in vitro and in vivo evaluation methods to test these functions.
Keywords: Glucose-responsive, Insulin, Mechanism, Formulation, Evaluation method
1. Introduction
Diabetes mellitus, a group of metabolic diseases, which is characterized by hyperglycemia as a result of defects in insulin secretion, insulin action, or both, has become one of the biggest medical challenges affecting millions of people globally [1,2]. For type 1 diabetes, subcutaneous insulin injection remains the primary route of treatment, where diabetic patients rely on self-monitoring of their blood glucose levels (BGLs) and multiple daily self-injections of insulin to keep their BGLs in the normal range. This is typically associated with pain and often inadequate glucose control [3–6]. Although non-invasive insulin therapy such as oral, nasal, buccal, transdermal, rectal and ocular delivery systems have been widely investigated [7–10], these methods in addition to subcutaneous injection are known as open-loop insulin release system. Since the glucose sensing and insulin release are not directly coupled, these methods still cannot tightly regulate the BGLs in patients [11,12]. Poor glucose control causes long-term complications, including cardiovascular disease, nephropathy, nerve damage, blindness, etc. [13]. Particularly, hypoglycemia resulting from an extra dosage of insulin can induce unconsciousness, brain damage and even death [14]. In order to improve the normal glycemic control, reduce the incidence of hyperglycemia and hypoglycemia, and the resulting complications in diabetic patients, there is an imperative need for closed-loop insulin release system to be developed.
A closed-loop insulin release system typically combines a glucose sensing element and a sensor-triggered insulin release element, and mimics the natural pancreatic function and to seamlessly release the demanded insulin in response to the body BGL. There are majorly two categories of closed-loop insulin release systems. One is based on electronic devices which integrate a subcutaneously implanted glucometer and an insulin pump with infusion catheter subcutaneously inserted to another body location. This type of device is commonly called artificial pancreas device system. Medtronic’s MiniMed 670G System is the first of its type that has been approved by U.S. Food and Drug Administration (FDA). The second category of closed-loop insulin release system is in the format of drug formulations that can be directly administered to the patients, such as through subcutaneous injections [5,15–17]. In these formulations, insulin is typically embedded in a matrix consisting of glucose-sensing elements. The matrix can undergo structural variation triggered by glucose concentration change, subsequently inducing a glucose-responsive insulin release (GRIR). When glucose concentration returns normal level, the matrix should be able to adjust itself to prevent further insulin release. The most notable example under this category is SmartInsulin® originally developed by Smart Cells, which was latterly acquired by Merck & Co. SmartInsulin® changes its drug name to “MK-2640” and is currently in early human trials at Merck.
This review article will focus on the second category, GRIR drug formulation, which becomes an emerging hot area for many research labs and companies. A successfully developed GRIR formulation is expected to achieve an immediate impact on the diabetic patent’s life through available administration routes. Despite review articles published over the past few years relating to GRIR systems [5,10,15,18], this article is among the few that analyzes the effect of GRIR mechanisms, and formulations on the accuracy, response time, and reversibility of the GRIR functions. These three features are the key that can make GRIR formulations to be as “smart” as a natural pancreas in regulating blood glucose. Nevertheless, there is significant challenge preventing current GRIR systems from achieving them [12,19,20]. To further examine the desirable smartness (high accuracy, fast response time, and high reversibility), this article provides insights on relevant in vitro and in vivo evaluation methods to be used and the rationales to do these tests.
2. Classic mechanisms for GRIR
To achieve GRIR, the systems are typically designed containing glucose sensing or glucose responsive module and insulin release module. There are three commonly used mechanisms to sense glucose, which are based on glucose oxidase, glucose-binding proteins (i.e., Con A), and glucose-binding molecules (i.e., phenylboronic acid). Typical insulin release mechanisms include matrix swelling, matrix disassembly or degradation, and glucose binding competition. These major glucose-sensing and insulin release mechanisms are illustrated in Fig. 1. For the final GRIR system to achieve reasonably good accuracy and response time, it requires the glucose-responsive module to accurately sense the glucose concentration, and the insulin release module to immediately release insulin or stop the release.
Fig. 1.
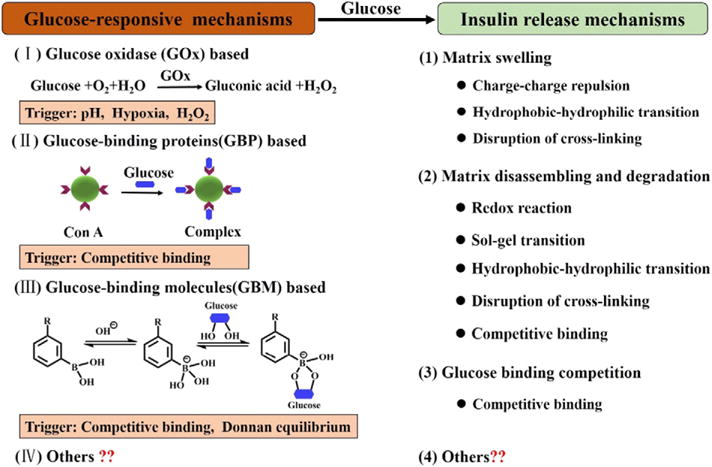
Schematic illustration of classic mechanisms to achieve GRIR, including both glucose-responsive mechanisms and insulin release mechanisms.
2.1. Glucose oxidase based GRIR mechanisms
Glucose oxidase enzyme (GOx) has been widely used as a sensing element for the quantification of blood glucose such as in a glucometer [21]. This is based on its catalytic conversion of glucose to gluconic acid by consuming oxygen (O2) and producing hydrogen peroxide (H2O2). Typical GRIR system was made by combing GOx with pH-sensitive matrixes (e.g., hydrogels [22–27], liposomes [28], polymersomes [29], and microcapsules [30]). The formation of gluconic acid results in a lowering of pH that further triggers the swelling of the pH-sensitive hydrogels, or disassembly of liposomes or microcapsules, and releases the insulin previously trapped within the matrixes.
The advantages of GOx based mechanism is that the catalytic reaction proceeds efficiently with a linear dependence on the glucose concentration. This feature makes this mechanism particularly suitable for accurate determination or sensing of glucose concentration [21], which is a desirable factor contributing to the accurate glucose response of the final GRIR system. Nevertheless, the GOx enzyme is also sensitive to environmental changes and can be potentially denatured when it is entrapped in or covalently linked to a polymeric matrix [15]. These scenarios can potentially reduce the accuracy and shorten the shelf-life of the resulting GRIR systems, which is an obvious disadvantage of GOx based mechanism. In addition, pH-responsive GOx based GRIR systems are limitedly studied in clinical trials, due to potential immunogenicity, and slow responsiveness (e.g., slow swelling and/or disassociation of the matrixes) especially at the physiological pH in vivo.
2.2. Glucose-binding protein based GRIR mechanisms
Glucose-binding proteins, mainly lectins, are natural carbohydrate binders, which can be used as biosensors in developing GRIR systems. The most commonly used lectins for GRIR is concanavalin A (Con A), which presents four binding sites having high affinity to D-glucose and D-glucosyl [31]. A GRIR action can be realized by high glucose concentration competing for the existing binding between polysaccharide and Con A. For example, glycosylated insulin and Con A can form a complex which is a typical glucose-binding protein based GRIR system [32–34]. SmartInsulin® is the most well-known example in this category consisting of the complex made between insulin-dextran conjugate and Con A [35]. When a high concentration of glucose was reached, it would replace the glycosylated insulin to bind Con A (break the glycosylated insulin-Con A complex), and the release of glycosylated insulin occurred. In addition, the binding between Con A and different polymers bearing saccharide residues was utilized to crosslink a hydrogel matrix wherein insulin was physically encapsulated [36–40]. High concentration glucose broke such crosslinking and led to the swelling of the hydrogel, promoting the release of entrapped insulin.
Con A, as a physical glucose-responsive binder, makes itself easily to be utilized for designing GRIR systems (as highlighted by SmartInsulin®). Nevertheless, Con A has inherent limitations including its toxicity, poor aqueous solubility and stability, and long response time [5,15]. The solubility, stability and glucose sensitivity of Con A have been improved through modification with hydrophilic polymers [34], but the immunogenicity resulting from Con A remains to be resolved.
2.3. Glucose-binding molecule based GRIR mechanisms
Phenylboronic acid (PBA) as a glucose-binding molecule can form a reversible covalent complex with polyol molecules such as glucose [41], PVA [42] and dextran [43] in aqueous solution, and is a promising glucose sensor. As shown in Fig. 1 (III), the PBA moieties have a pKa in the range of 8.2– 8.86 [41,44], and show two structural forms in equilibrium in aqueous solution: an uncharged form and a charged form. The uncharged form is relatively hydrophobic and the charged form is hydrophilic. With glucose in presence, the charged form of PBA can form a stable complex with glucose via reversible covalent bonding. This shifts the equilibrium toward the direction of increasing hydrophilic forms of phenylborate. PBA moieties were often incorporated into the crosslinked polymeric matrix where insulin was physically encapsulated [45–48]. At high glucose concentration, the equilibrium shifted and a higher negative charge density of the network resulted. This led to a swelling matrix via both Donnan equilibrium and charge-charge repulsion effects [49], and triggered the insulin release. In addition, PBA moieties were also used as cross-linkers for polyol molecules to construct multilayer particles [42,43,50–53] and core-shell particles [54–62]. In this case, the insulin release was obtained through disassembling and degradation of the microstructures triggered by the competition between polyol molecules and added free glucose in binding PBA moieties. Moreover, PBA-based GRIR systems consisted of PBA modified polymers and glycosylated insulins were also developed [63–65], where PBA modified polymers played the role of Con A as discussed in Section 2.2. Glycosylated insulin was released when glucose competitively bound PBA, replacing the existed PBA-glycosylated insulin binding. So far significant amount of PBA-based GRIR systems has been developed in the forms of macroscopic gels, micro/nanogels, nanoparticles, micelles, polymersomes, and microcapsules.
The advantages of PBA-based GRIR systems are the diversity of matrix composition, the variability of microstructure, and the adjustability of insulin release mechanism. Since PBA is a small glucose-binding molecule, it can be easily used for all kinds of chemical modifications for designing the GRIR systems. It should be noted that most PBA-based GRIR systems can only work in alkaline conditions but not at physiological pH because of the high pKa value of the PBA moiety. To enable glucose sensitivity at physiological pH, an electron-withdrawing group had been incorporated into the phenyl ring of PBA moiety or in the side chain of the PBA-containing polymer [44,48,64]. The idea was to tune the pKa of PBA to the physiological range. Nevertheless, a major problem with PBA is its low specificity to glucose, which affects the accuracy and response time for GRIR, particularly due to the conceivable interferences by other saccharides in blood such as the fructose, glycoproteins or glycolipids [19,66]. In addition, the safety and toxicity of PBA based system remain to be evaluated further.
2.4. Insulin release mechanisms in GRIR
In GRIR systems, the insulin or its derivative is typically entrapped, either physically or chemically, in a polymeric matrix. The matrix is a network of polymer chains integrated by physical or chemical interactions and can enable a variety of insulin release mechanisms including matrix swelling, matrix disassembling and degradation or glucose binding competition. Different mechanisms can truly affect the rate to release insulin and to stop its release.
For matrix swelling, such as in micro/nanogels, it can be triggered by charge-charge repulsion, hydrophobic-hydrophilic transition or disruption of the crosslinking. The behavior of insulin release is regulated by the matrix swelling and deswelling. A number of factors are critical for matrix swelling/deswelling kinetics, including matrix size, crosslinking density, and network homogeneity. For matrixes, possessing a core-shell structure or multilayer structure, they commonly achieve insulin release by the disassembling and degradation of microstructures, which can be triggered by a redox reaction, sol-gel transition, hydrophobic-hydrophilic transition, disruption of crosslinking, and competitive binding. The behavior of insulin release is regulated by the permeability of the shell or layer. The permeability can be turned by material compositions, the thicknesses of shell or layer, shapes, and sizes. For GRIR systems consisting of glycosylated insulin with Con A or PBA-modified polymers, they achieve insulin release directly through glucose binding competition.
In most of the cases, when insulin was released, it would result in the lowering of BGL. The closed-loop GRIR system had to rely on a lowered BGL to stop further insulin release which could cause the dangerous incidence of hypoglycemia. The ability for GRIR system to stop insulin release, however, is not straightforward to achieve and poses significant challenges in this field. No matter which type of insulin release mechanism is involved, when insulin is physically trapped within the matrix, there is a sustained insulin release even at hypoglycemic glucose concentrations. In addition, when the GRIR system experienced a high-glucose-to-low-glucose cycle, it is difficult for the system to return to its original status, resulting in a changed rate of basal insulin release. One potential strategy to overcome the issue of excessive insulin release (causing hypoglycemia) is to release glucagon to increase the BGL [10]. In fact, in a natural body system, both glucagon and insulin are involved in the feedback system to stabilize a normal BGL.
3. Progress beyond the classic mechanisms for GRIR
Recently, in order to improve glucose responsiveness of classic pH-responsive GOx-based GRIR systems at the physiological pH in vivo, Gu group reported an advanced hypoxia-responsive GOx-based GRIR system integrated into a painless microneedle-array patch, known as the Smart Insulin Patch [67]. This system has taken advantage of the local generation of hypoxia due to the consumption of oxygen in the GOx enzymatic reaction as a trigger that can more quickly cause structural variation of the matrix than pH triggers. The system consisted of hypoxia-sensitive hyaluronic acid (HS-HA) vesicles containing insulin and GOx. At high glucose concentration, the oxygen consumption in the GOx enzymatic reaction was increased, resulting in the local generation of hypoxia. This triggered the hydrophobic-to-hydrophilic transition of HS-HA, leading to a rapid insulin release. Gu group further designed the hypoxia and H2O2 dual-sensitive polymersome based vesicles, which can disassociate and subsequently release insulin triggered by hypoxia and H2O2 generated during GOx enzymatic reaction [68]. In another of their GRIR system, live β-cells were encapsulated and positioned into the microneedles to realize GRIR function [69]. Because of low glucose signal at the dermal environment, a glucose amplifying module was designed where α-amylase (AM) and glucoamylase (GA) were co-encapsulated with GOx into HS-HA vesicles. At relatively high glucose concentration, HS-HA vesicles were dissociated due to the enzymatically generated local hypoxia. Both AM and GA were released and promoted the α-amylose-to-glucose conversion to further amplify the glucose signal. This sufficient amount of glucose diffused into the encapsulated β-cells and induced the secretion of insulin that diffused out to the body to regulate BGLs. These cases from Gu group utilized hypoxia rather than a classic pH change following a typical GOx based enzymatic reaction. In addition, the glucose amplifying module plus encapsulated β-cells achieved a faster regulation of BGL as illustrated in type 1 diabetic mice [69]. The glucose amplifier is expected to contribute to the faster action of GRIR.
Additionally, Anderson group developed a PBA-modified insulin derivative showing the GRIR characteristics [70]. The PBA modified insulin was synthesized by covalent coupling an aliphatic moiety and a PBA moiety to insulin. The resulting insulin derivative bound to serum albumins or other hydrophobic components in serum at low BGL, but deattached from them and re-entered the serum at high BGL. Such attachment and de-attachment were driven by the changes of hydrophilicity and charge of PBA at different glucose concentrations. The PBA-modified insulin enabled a long-term GRIR activity and reduced the number of subcutaneous injections for adding insulin to the body. The in vivo results indicated that the PBA-modified insulin achieved a normal blood glucose control in diabetic mice and a reduced hypoglycemic index in healthy mice.
It is obvious that recent innovation in constructing GRIR systems are also based on classic glucose sensing mechanisms such as GOx and PBA, but by adding new elements such as enzymatically generated local hypoxia and/or H2O2, or albumin binding. One could expect that a fourth novel mechanism in either glucose sensing or insulin release, though currently unavailable (Fig. 1), will significantly expand the possibility of potential GRIR actions and advance the current field.
4. Formulations of GRIR system
For diabetic patients, subcutaneous infusion or injection of insulin formulations remains the major administration route. In addition, subcutaneous implantation of a graft sustaining a longer period of insulin release has also been considered feasible. Potential candidates of glucose responsive insulin for subcutaneous injection primarily include formulations based on micro/nanogels, core-shell particles, and microcapsules (as shown in Fig. 2). For majority GRIR systems, the rates of sensing glucose and releasing insulin depend on the diffusion coefficient of the two molecules within the formulation matrix and the size of the matrix [15,71]. So for a formulation of macroscopic size, it is reasonable that the response time will be longer compared to smaller-size formulations. This is the rationale for micro/nano-size particles being pursued as glucose-responsive formulations with fast response time. Each specific type of formulation (micro/nanogels, core-shell particles, or microcapsules) has its own uniqueness in improving the response time and accuracy and is discussed as follows.
Fig. 2.
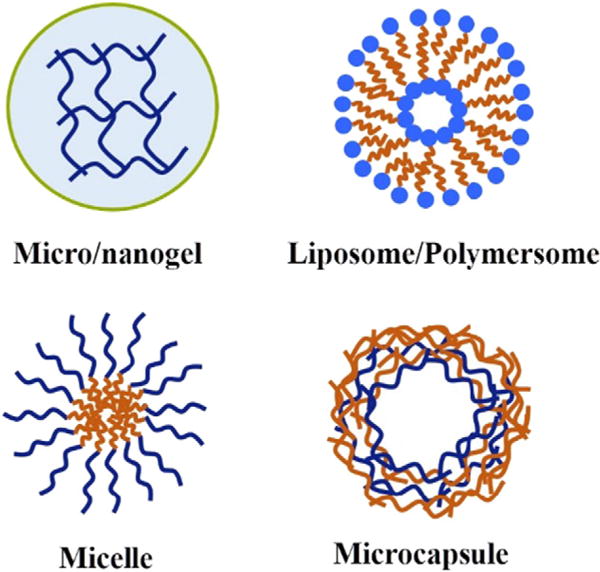
Schematic illustration of typical formulations of GRIR systems.
4.1. Micro/nanogels
Most glucose-responsive macroscopic hydrogels present slower response, such as in their size changes, to glucose concentration changes, and they are typically not fast enough to return to their original states once the glucose stress is released [66]. One of the easiest ways of achieving faster response is to reduce the dimension of hydrogels to micro/nanoscales. Thus, the micro/nanogel based GRIR systems have become a study of focus over the past few years.
Gu et al. recently developed GOx based microgels for GRIR [23]. The microgel matrix was based on chitosan crosslinked by Tripolyphosphate (TPP) and was used to entrap insulin and a GOx-containing nanogel particle. During the enzymatic conversion of glucose by GOx, the gluconic acid was generated, further protonated chitosan and subsequently expanded the microgels by >5-fold in volume. The swelling and dissociation of the microgels facilitated the fast release rate of insulin. The in vivo activity of microgels was evaluated by subcutaneously injecting the drug formulation to a type 1 diabetes mouse model. It was demonstrated that the incorporation of GOx into microgels achieved self-regulation of BGLs under hyperglycemic conditions.
Nie group developed a series of Con A based microgels for GRIR [40,72,73]. Typical insulin-loaded microgels were prepared by copolymerization of glucosyloxyethyl methacrylate (GEMA), methacrylated concanavalin A (Con A-E), and a pH sensitive monomer N-(2-(dimethylamino) ethyl)-methacrylamide (DMAEMA) in insulin solution [72]. The incorporation of Con A and DMAEMA enabled a glucose and pH dual-responsive system, which can sense the glucose concentration change and pH value change induced by the accumulation of acidic metabolites in diabetes. The in vitro insulin release study revealed that the microgels could quickly respond to the glucose concentration change in the medium due to the competitive binding between glucose and GEMA moiety with Con A, and that the insulin release behavior was also regulated by a small change in environmental pH. The kinetics study of insulin release indicated that insulin diffusion coefficient increased with the increase of glucose concentration and the decrease of pH.
The first reported PBA-based amphoteric microgels for GRIR was developed by Hoare et al. The microgel was prepared by copolymerization of N-isopropylacrylamide (NIPAAm), a cationic monomer N, N-dimethylaminoethylacrylate (DMAEA), and an anionic monomer acrylic acid (AA), with AA moieties subsequently modified by PBA [74]. Owing to the amphoteric character, this system exhibited a glucose-responsive swelling for up to 2-fold volume at physiological conditions and was used for insulin encapsulation and the followed triggered release. Since then, several groups have reported systems of glucose responsive microgels bearing PBA moieties for insulin release [75–77].
Based on the micro/nanosize nature of the structures, these systems are expected to show a shorter response time in realizing GRIR functions compared with macroscopic hydrogels. Nevertheless, the accuracy of these systems and their reversibility in response to glucose on a longer term basis, such as from one day to a few days (more relevant to clinical settings), have rarely been examined in majority literature and require further investigations, particularly in in vivo experiments.
4.2. Core-shell particles
The core-shell particles are of micro/nanosize and compose of one inner core and one outer shell completely enclosing the inner core, enabling a number of multi-faceted characteristics as drug release systems [78]. The core often plays the role of loading therapeutic molecules for delivery, and the shell governs the release kinetics of molecules loaded in the core. By turning composition, size and shape of the core-shell particles, both drug loading capacity, and release behavior can be controlled. Typical GRIR core-shell particles include micelles [56,57,60,79], liposomes [80–83] and polymersomes [84–86], and are expected to lead to high accuracy and fast response to environmental glucose concentration changes.
Tai et al. developed a pH-responsive polymersome formulation for GRIR by entrapping insulin and GOx into the pH-responsive polymersome assembled from a poly (ethylene glycol) (PEG) based amphiphilic block copolymer [29]. Glucose can passively cross the bilayer membrane of the polymersome and reach the GOx. The resulting enzymatic reaction caused a pH change, induced the hydrolysis of the pH-responsive polymersome, and triggered the insulin release. The in vivo study indicated that this formulation was highly effective in regulating blood glucose levels for a long period of time up to 5 days.
A Con A based liposome formulation for GRIR was developed by Karathanasis et al. The formulation was in a format of microparticle agglomerate which was constructed by crosslinking between Con A and glycosylated liposomes. The microparticle was about 23.9 μm in size, prevented macrophage uptake and clearance, and presented the GRIR function through pulmonary delivery in a rat model [87].
Recently, PBA-based micelles for GRIR have been increasingly investigated. Shi group reported on micelles that were self-assembled from amphiphilic block copolymers consisting of PEG as the outer hydrophilic shell and PBA-modified polymers as the inner hydrophobic core that could be cross-linked. GRIR function was achieved by swelling and disassembling or degradation of micelles in response to glucose concentration changes, and the nanosize of the micelles accelerated the insulin release at hyperglycemia levels under physiological conditions [56,57,60,88].
While the potential of core–shell particles for GRIR has been implicated, there are several challenges remained. Majority core-shell particles involve passive loading of insulin into the core region which is low in encapsulation efficiency (e.g., approximately 2.5– 29% depending on formulations). The reversibility of the GRIR behavior of these particles also requires further examination in animal studies.
4.3. Microcapsules
Hollow polyelectrolyte multilayer capsules (“microcapsules”) have received more attention as drug delivery systems based on their large capacity to contain therapeutic molecules. The fabrication processes are typically in aqueous media under mild conditions and are friendly to protein drugs. Compared with core-shell particles, microcapsules consist of a shell only. This shell is typically made of alternated cationic and anionic polyelectrolytes on a removable template using a layer-by-layer (LbL) deposition technique. The hollow core presents a large capacity to therapeutic molecule either in aqueous solution or in its solid state. The microcapsules do not generally undergo abrupt volume transitions but realize drug release through controlling the shell/capsule permeability [71,89,90].
Microcapsules having a glucose-sensitive multilayer shell have recently been developed as microscopic vehicles for GRIR. A typical example was reported by Li group, where the shell of microcapsule was made of GOx and hemoglobin (Hb) using LbL technique with glutaraldehyde (GA) as a cross-linking agent. At high glucose concertation, the GOx based enzymatic reaction prevailed, which enhanced the permeability of the multilayer shell and facilitated the insulin release [30]. The shell of microcapsule can also be based on Con A, where the shell can be fabricated through LbL deposition of Con A and glycogen on the template of CaCO3 microparticle, followed by template removal [91]. In this way, the size and wall thickness of the microcapsule can be well controlled. The GRIR mechanism involves high concentration glucose competing with the prior crosslinking/binding between glycogen and Con A on the shell, leading to an enhanced permeability of the microcapsule, and triggered insulin release. The shell of microcapsules can be further based on PBA [43,51–53]. PBA-modified polymers can be used to construct microcapsule walls by their covalent binding with polyol compounds or their electrostatic binding with cationic polymers. The PBA based microcapsules display glucose-responsive permeability enhancement triggered by competitive binding or charge-charge repulsion in response to glucose concentration increase. This subsequently induces the insulin release.
While most of the current microcapsules for GRIR focused more on capsule design and in vitro demonstrations, future effort should be directed to pre-clinical animal based studies.
4.4. Implantable formulations
For implantation applications, however, there is limited formulation having been developed due to multiple challenges. An ideal implantable formulation would respond quickly to elevation in blood glucose, and meanwhile has even higher requirement of preventing insulin over-release which causes dangerous hypoglycemia since the implant contains a large dose of insulin supposed to last for a long period of time. In addition, the biocompatibility of the implantable formulation must be addressed. Implantable drug delivery systems face a common challenge of foreign body reaction (FBR) and concomitant inflammation, which results in fibrotic isolation of the grafts and a hindered absorption of released drug such as insulin [35]. Moreover, since the management of diabetes generally requires life-long therapy, the implantable formulation must be conveniently administered for patients and be easily removable or have appropriate degradation and clearance kinetics in vivo [92].
5. Evaluation of GRIR system
For a given GRIR system that has been developed, it is important to examine the accuracy, response time and reversibility of its GRIR behavior; this is the basis for this particular system to fill the clinical need for a smart insulin to seamlessly regulate the BGL variation. For a GRIR system releasing insulin derivatives or insulin-polymer conjugates, their bioactivity should also be evaluated. There are a series of evaluation methods ranging from in vitro assays to in vivo examinations of GRIR. These evaluation methods as well as the rationales in doing these studies are briefly discussed below. It should be noted that despite the availability of these methods, many reported formulations have not been comprehensively characterized or evaluated for their GRIR function, particularly in animal studies.
5.1. Bioactivity of the released insulin
Depending on the design of GRIR systems, the working insulin released could be either native or modified insulin. For example, native insulin was typically released when the insulin releasing mechanism involves matrix swelling, matrix disassembly or degradation. In the case of glucose binding competition induced insulin release, the working insulin released was typically an insulin modified with polymers, such as dextran-insulin conjugate, [93] or small molecules, such as succinyl amidophenyl glucopyranoside [32]. It is particularly important to examine the bioactivity of the modified insulin. Reports show that conjugating dextran to insulin loses 60–90% of native insulin bioactivity [93,94]. Thus, a significantly higher molar dose of conjugated insulin has to be delivered than unmodified native insulin in achieving comparable glucose suppressive effect. This potentially increases the production cost and poses a safety concern in the long run when the drug has to be repeatedly taken.
Since proteins generally require stabilized structures to contain their particular bioactivity [95,96], one quick method was to analyze potential conformation change between the released insulin and the native insulin using circular dichroism spectroscopy (CD) [97–99]. CD is able to rapidly evaluate the secondary structure, folding and binding properties of proteins [100,101]. The far-UV-CD band at 208 nm primarily arises from the α-helix structure, and 223 nm is characteristic for the β-sheet structure (Fig. 3A). The ratio of the mean residue ellipticities at 208 nm and 223 nm ([Θ]208/[Θ]223] can be used as a qualitative measure of overall conformation structure of insulin, which is 1.26 to 1.62 for native insulin at different concentrations [100,102]. The secondary structure of the released insulin can be evaluated qualitatively by comparing the CD spectral characteristic and the [Θ]208/[Θ]223 ratios with native insulin.
Fig. 3.
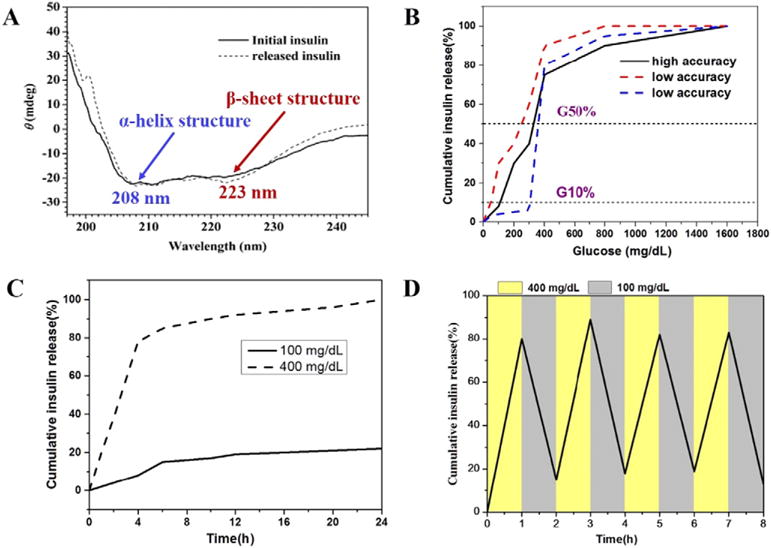
(A) UV-CD spectra of initially encapsulated insulin and released insulin (from ref. [62]). (B) Schematic glucose set-point curves. A formulation showing high accuracy of GRIR behavior typically has its G10% > 100 mg/dL and G50% > 350 mg/dL; (C) Schematic curves for kinetic studies. Time-dependent insulin release at different glucose concentration is shown; (D) Schematic curves for glucose cycling test.
To directly evaluate the biological activity of insulin, a cell-based assay is typically conducted, where the phosphorylation of protein kinase B (Akt) of the cells is quantified after the cells being incubated with the medium containing the released insulin [23,70,103]. This assay is based on an insulin-induced intracellular process. Once the insulin binds to the extracellular portion of α-subunits of the insulin receptor, it induces a conformational change of the insulin receptor, activates the kinase domain on the intracellular β-subunits and stimulates phosphorylation of Akt. Therefore, the quantification of Akt phosphorylation can show actual bioactivity of the released insulin.
It should be noted that to evaluate the bioactivity of the released insulin, one should not only determine the stability of the secondary structure of the released insulin but also test its bioactivity in interacting with cells, such as using the Akt phosphorylation assay. The test for actual bioactivity, however, has not been commonly involved in current literature.
5.2. In vitro GRIR tests (glucose set-point study, kinetic experiment, and cycling experiment)
There is a high demand for GRIR system to mimic the pancreatic cells and to seamlessly regulate BGLs. This requires the system having high glucose sensing accuracy and fast response to BGL change. A series of in vitro tests have been involved to access the accuracy, response time, and reversibility of GRIR system.
Glucose set-point (GSP) test is a method to evaluate the accuracy of insulin retention/release when GRIR system was challenged by a given glucose concentration. In a typical testing procedure [35,67], the insulin-loaded matrix is continuously incubated with a series of glucose concentration (e.g., 50, 100, 200, 400, 800, 1600 mg/dL) each for certain time duration e.g., 1 h) at 37 °C. The cumulative insulin released up to each of the challenge is quantified using a human insulin ELISA assay. A typical GSP curve shows the percentage of cumulative insulin released (Y-axis) after finishing the series glucose challenge up to a given concentration (X-axis), with the cumulative insulin released after finishing the highest concentration of glucose challenge (e.g., 1600 mg/dL) normalized as 100% (Fig. 3B). The two most relevant points on a GSP curve are (1) the point at insulin release onset, and (2) the point at which insulin was released most rapidly. The glucose concentration corresponding to 10% (G10%) insulin release and 50% insulin release (G50%) are chosen to characterize these two points, respectively [35]. Generally, G10% > 100 mg/dL and G50% > 350 mg/dL are preferred. By meeting this criterion the matrix will not over release insulin at hypoglycemic glucose level (<70 mg/dL), and only at high BGL do the insulin release rapidly, indicating that the system presents high accuracy to control the retention and release of insulin at BGLs of clinical relevance.
GSP study allows one to examine the accuracy of the system to control insulin retention/release at desired BGLs, but it does not capture the rate of GRIR (i.e., the response time) [61,69]. For this purpose, the insulin release kinetics is normally tested. In a typical testing procedure, insulin release from the GRIR matrix is monitored over time up to 24 h at 50, 100, 200, and 400 mg/dL glucose solution, respectively (Fig. 3C). At a given glucose concentration, the percentage of insulin released at different time points is calculated by normalizing the cumulative insulin released at that specific time point to the total amount of insulin-loaded. This is to validate the ability of GRIR formulation to release the basal amount of insulin (at the very slow rate) at the hypo-glycemic and normoglycemic level, and to rapidly release insulin at high glucose level.
Because of the capability to retain and release insulin at different BGLs, GRIR formulations are expected to reverse insulin release when glucose concentration decreases to hypoglycemic or normoglycemic level. Such reversibility is typically examined in a cycling study, where GRIR system is repeatedly challenged by alternating high and low glucose concentration and the corresponding insulin release is recorded [23,29]. In a typical procedure, the GRIR matrix can be repeatedly treated with 400 and 100 mg/dL glucose solution each for 1 h up to 8 cycles, and the released insulin is quantified over time (Fig. 4D). It should be emphasized that the diabetic patients may have complex variation in BGLs. Whether a GRIR formulation can sustain cycled glucose challenge and reverse insulin release becomes a prerequisite for solving this complex issue. Furthermore, the cycling test indicates the possibility for the formulation to sustain a long-term blood glucose control, which is highly demanded by the patients to reduce the inconvenience of drug administration.
Fig. 4.
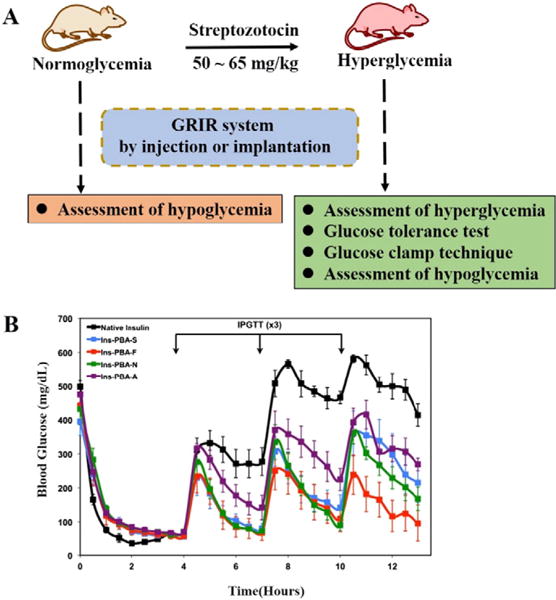
(A) Schematic illustration for in vivo GRIR tests including the assessment of hyperglycemia, glucose tolerance test, glucose clamp technique, and assessment of hypoglycemia. (B) In vivo screening of PBA-modified insulin derivatives in an STZ-induced diabetic mouse model. Insulin derivatives or native insulin were injected at the beginning of the experiment (0 h), with a series of IPGTTs performed at 4, 7, and 10 h following the insulin administration. Blood glucose from peripheral tail vein puncture was monitored throughout the study to evaluate GRIR activity (from ref. [70]).
It should be noted that glucose set-point study, kinetic experiment, and cycling experiment define the GRIR action from different angles and are desired characterization methods before a formulation moves to in vivo evaluation. Nevertheless, some of these tests, such as glucose set-point study, have been overlooked by many reported GRIR systems.
5.3. In vivo GRIR tests (assessment of hyperglycemia, glucose tolerance test, glucose clamp technique, and assessment of hypoglycemia)
A GRIR system can present good sensing accuracy, fast response and high reversibility in in vitro studies. Its suitability to become a promising drug candidate, however, requires further validation through in vivo animal testing. Commonly used in vivo GRIR tests include the assessment of hyperglycemia, glucose tolerance test, glucose clamp technique and assessment of hypoglycemia (Fig. 4A).
An STZ-induced animal model is typically involved to evaluate GRIR systems in vivo. Diabetes is induced in the mice or rats by intraperitoneal injection of streptozotocin (STZ; 50– 65 mg/kg) [29,61,104], a glucosamine–nitrosourea compound to damage pancreatic β cells resulting in hyperglycemia. BGLs of the diabetic animals are continuously monitored for several days by collecting the blood such as from the tail vein and testing using a glucometer. Animals with high BLGs (e.g., non-fasted BGLs above 250 mg/dL) are confirmed of their diabetic status, and randomly grouped and used for in vivo studies. To assess hyperglycemia (potential diabetes reversal), GRIR formulations are subcutaneously injected or implanted. BGLs of animal recipients are monitored periodically from minutes to hours after the administration. For animals continuously receiving GRIR formulations for a longer term (e.g., 2 weeks or above), a larger volume of blood samples can also be withdrawn from retro-orbital plexus or tail vein, and used for the determination of glycated albumin (GA). GA is one of the useful markers for the screening of diabetes in a routine medical examination, and typically provides a reliable method monitoring diabetic condition in the past two weeks. The quantitative ratio of glycated albumin over total albumin concentration can be analyzed by using the glycated albumin kit [24], and a lowered ratio over the treating period indicates an improved control of hyperglycemia.
In addition, to examine the insulin release feature in response to a BGL increase, an intraperitoneal injection glucose tolerance test (IPGTT) is typically conducted [70]. In this procedure, after injecting or implanting the GRIR formulations for several hours, a certain amount of glucose is intraperitoneally injected, and then BGLs of the animals are continuously monitored by glucometer (Fig. 4B). A successful GRIR formulation is expected to immediately respond to the glucose challenge, and gradually lower the abruptly increased BGL to normal range. The obtained BGL response curve can be compared with the one obtained from a healthy animal receiving the IPGTT to examine how closely the GRIR system is mimicking the natural pancreatic function.
To quantify the released insulin bioactivity by GRIR system in vivo, glucose clamp technique (hyperglycemic clamp technique) is generally conducted [105]. In a typical procedure, the animals are implanted with the GRIR formulations and kept fasting for 12 h, and then the plasma glucose concentration is acutely raised to 125 mg/dL above basal levels by a priming infusion of glucose with fast infusion rate in antecubital vein for 15 min, the pre-set hyperglycemic plateau (~220 mg/dL) is subsequently maintained by adjustment of glucose infusion rate at 5 min intervals for 2 h. During the hyperglycemic clamp study, the plasma insulin concentration (I) is determined. Since the plasma glucose concentration is held constant throughout the study, the glucose infusion rate (M) is an index of glucose metabolism. Therefore, the released insulin bioactivity can be evaluated by M/I ratio.
One significant advancement of GRIR system is its capability to prevent insulin release at low BGLs, and thus to lower the rate of hypoglycemic incidence. This can be evaluated by administrating the GRIR formulations to health animals and observing potential lowering of BGL (hypoglycemia) without conducting a glucose challenge.
It should be emphasized that the above discussed in vitro, and in vivo evaluations are highly important in determining the desired glucose responsive nature of a formulation and its suitableness to move to further preclinical or clinical studies. Nevertheless out of ~100 research papers surveyed in the field of GRIR systems, only ~80% of them conducted in vitro evaluations and ~25% of them reported in vivo studies.
6. Conclusions
GRIR systems have exhibited tremendous potential to overcome the limitations and drawbacks of conventional administration of insulin and to improve the treatment efficacy and life qualities for diabetic patients. Despite a variety of GRIR systems having been developed, there are still many challenges and questions remained to prevent a GRIR formulation from achieving clinical significance and entering the healthcare market. Major challenges include how to design GRIR systems to show (1) high accuracy on when to release insulin and when to stop insulin release in response to clinically relevant BGLs, (2) fast response to BGL change (e.g., an immediate insulin release or retention), and (3) high reversibility to stop excessive insulin release at hypo- and normo-glycemic conditions. Achieving all three desirable features will enable the development of novel GRIR systems with seamless control of BGLs similar to a natural pancreas. In addition, based on the controlled insulin release/retention function, GRIR systems are expected to continue the glucose sensing and insulin administration for a long term, which can drastically release the burden of patients in receiving repeated finger pricks and injections. This ultimate goal requires the future GRIR formulation to be non-toxic, biocompatible, and free from other long-term safety concerns.
Acknowledgments
This work was supported by the faculty start-up fund at Wayne State University, Chemical Engineering and Materials Science, the Juvenile Diabetes Research Foundation (1-SRA-2016-270-A-N), and the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (DP2DK111910).
References
- 1.A. American Diabetes. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32:S62–S67. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger PM. Insulin adherence behaviours and barriers in the multinational global attitudes of patients and physicians in insulin therapy study. Diabet Med. 2012;29:682–689. doi: 10.1111/j.1464-5491.2012.03605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veiseh O, Tang BC, Whitehead KA, Anderson DG, Langer R. Managing diabetes with nanomedicine: challenges and opportunities. Nat Rev Drug Discov. 2015;14:45–57. doi: 10.1038/nrd4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ravaine V, Ancla C, Catargi B. Chemically controlled closed-loop insulin delivery. J Control Release. 2008;132:2–11. doi: 10.1016/j.jconrel.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Veiseh O, Langer R. Diabetes a smart insulin patch. Nature <v >524. 2015:39–40. doi: 10.1038/524039a. [DOI] [PubMed] [Google Scholar]
- 7.Woo VC. New insulins and new aspects in insulin delivery. Can J Diabetes. 2015;39:335–343. doi: 10.1016/j.jcjd.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Heinemann L. Insulin pens and new ways of insulin delivery. Diabetes Technol Ther. 2015;17:S47–S52. doi: 10.1089/dia.2015.1506. [DOI] [PubMed] [Google Scholar]
- 9.Matteucci E, Giampietro O, Covolan V, Giustarini D, Fanti P, Rossi R. Insulin administration: present strategies and future directions for a noninvasive (possibly more physiological) delivery. Drug Des Devel Ther. 2015;9:3109–3118. doi: 10.2147/DDDT.S79322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mo R, Jiang T, Di J, Tai W, Gu Z. Emerging micro-and nanotechnology based synthetic approaches for insulin delivery. Chem Soc Rev. 2014;43:3595–3629. doi: 10.1039/c3cs60436e. [DOI] [PubMed] [Google Scholar]
- 11.Owens DR. New horizons - alternative routes for insulin therapy. Nat Rev Drug Discov. 2002;1:529–540. doi: 10.1038/nrd836. [DOI] [PubMed] [Google Scholar]
- 12.Bratlie KM, York RL, Invernale MA, Langer R, Anderson DG. Materials for diabetes therapeutics. Adv Healthc Mater. 2012;1:267–284. doi: 10.1002/adhm.201200037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muggeo M, Bonadonna RC, Zoppini G, Moghetti P, Bonora E, Verlato G, Brun E. Fasting plasma glucose variability predicts 10-year survival of type 2 diabetic patients - the Verona diabetes study. Diabetes Care. 2000;23:45–50. doi: 10.2337/diacare.23.1.45. [DOI] [PubMed] [Google Scholar]
- 14.McCoy RG, Van Houten HK, Ziegenfuss JY, Shah ND, Wermers RA, Smith SA. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care. 2012;35:1897–1901. doi: 10.2337/dc11-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Q, Wang L, Yu H, Wang J, Chen Z. Organization of glucose-responsive systems and their properties. Chem Rev. 2011;111:7855–7875. doi: 10.1021/cr200027j. [DOI] [PubMed] [Google Scholar]
- 16.Lu Y, Sun W, Gu Z. Stimuli-responsive nanomaterials for therapeutic protein delivery. J Control Release. 2014;194:1–19. doi: 10.1016/j.jconrel.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu J, Zhang Y, Bomba H, Gu Z. Stimuli-responsive delivery of therapeutics for diabetes treatment. Bioeng Transl Med. 2016;1:323–337. doi: 10.1002/btm2.10036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang YQ, Yu JC, Shen QD, Gu Z. Glucose-responsive synthetic closed-loop insulin delivery systems. Prog Chem. 2015;27:11–26. [Google Scholar]
- 19.Wu W, Zhou S. Responsive materials for self-regulated insulin delivery. Macromol Biosci. 2013;13:1464–1477. doi: 10.1002/mabi.201300120. [DOI] [PubMed] [Google Scholar]
- 20.Lu Y, Aimetti AA, Langer R, Gu Z. Bioresponsive materials. Nat Rev Mater. 2016;1:16075. [Google Scholar]
- 21.Yoo EH, Lee SY. Glucose biosensors: an overview of use in clinical practice. Sensors. 2010;10:4558–4576. doi: 10.3390/s100504558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishihara K, Kobayashi M, Ishimaru N, Shinohara I. Glucose-induced permeation control of insulin through a complex membrane consisting of immobilized glucose-oxidase and a poly(amine) Polym J (Jpn) 1984;16:625–631. [Google Scholar]
- 23.Gu Z, Dang TT, Ma M, Tang BC, Cheng H, Jiang S, Dong Y, Zhang Y, Anderson DG. Glucose-responsive microgels integrated with enzyme nanocapsules for closed-loop insulin delivery. ACS Nano. 2013;7:6758–6766. doi: 10.1021/nn401617u. [DOI] [PubMed] [Google Scholar]
- 24.Gu Z, Aimetti AA, Wang Q, Dang TT, Zhang Y, Veiseh O, Cheng H, Langer RS, Anderson DG. Injectable nano-network for glucose-mediated insulin delivery. ACS Nano. 2013;7:4194–4201. doi: 10.1021/nn400630x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farahani BV, Ghasemzaheh H, Afraz S. Intelligent semi-ipn chitosan-PEG-PAAm hydrogel for closed-loop insulin delivery and kinetic modeling. RSC Adv. 2016;6:26590–26598. [Google Scholar]
- 26.Li X, Fu M, Wu J, Zhang C, Deng X, Dhinakar A, Huang W, Qian H, Ge L. pH-sensitive peptide hydrogel for glucose-responsive insulin delivery. Acta Biomater. 2017 doi: 10.1016/j.actbio.2017.01.016. http://dx.doi.org/10.1016/j.actbio.2017.01.016. [DOI] [PubMed]
- 27.Di J, Yu J, Ye Y, Ranson D, Jindal A, Gu Z. Engineering synthetic insulin-secreting cells using hyaluronic acid microgels integrated with glucose-responsive nanoparticles. Cell Mol Bioeng. 2015;8:445–454. [Google Scholar]
- 28.Kim CK, Im EB, Lim SJ, Oh YK, Han SK. Development of glucose-triggered pH-sensitive liposomes for a potential insulin delivery. Int J Pharm. 1994;101:191–197. [Google Scholar]
- 29.Tai W, Mo R, Di J, Subramanian V, Gu X, Buse JB, Gu Z. Bio-inspired synthetic nanovesicles for glucose-responsive release of insulin. Biomacromolecules <v15. 2014:3495–3502. doi: 10.1021/bm500364a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qi W, Yan X, Juan L, Cui Y, Yang Y, Li J. Glucose-sensitive microcapsules from glutaraldehyde cross-linked hemoglobin and glucose oxidase. Biomacromolecules. 2009;10:1212–1216. doi: 10.1021/bm801502r. [DOI] [PubMed] [Google Scholar]
- 31.Brownlee M, Cerami A. Glucose-controlled insulin-delivery system: semisynthetic insulin bound to lectin. Science. 1979;206:1190–1191. doi: 10.1126/science.505005. [DOI] [PubMed] [Google Scholar]
- 32.Kim SW, Pai CM, Makino K, Seminoff LA, Holmberg DL, Gleeson JM, Wilson DE, Mack EJ. Self-regulated glycosylated insulin delivery. J Control Release. 1990;11:193–202. [Google Scholar]
- 33.Liu F, Song SC, Mix D, Baudys M, Kim SW. Glucose-induced release of glycosylpoly(ethylene glycol) insulin bound to a soluble conjugate of concanavalin A. Bioconjug Chem. 1997;8:664–672. doi: 10.1021/bc970128e. [DOI] [PubMed] [Google Scholar]
- 34.Kim JJ, Park K. Glucose-binding property of pegylated concanavalin. A, Pharm Res. 2001;18:794–799. doi: 10.1023/a:1011084312134. [DOI] [PubMed] [Google Scholar]
- 35.Zion TC. Glucose-responsive Materials for Self-regulated Insulin Delivery, Department of Chemical Engineering. Massachusetts Institute of Technology. 2004 http://hdl.handle.net/1721.1/28359.
- 36.Nakamae K, Miyata T, Jikihara A, Hoffman AS. Formation of poly(glucosyloxyethyl methacrylate)-concanavalin-A complex and its glucose-sensitivity. J Biomater Sci Polym Ed. 1994;6:79–90. doi: 10.1163/156856295x00779. [DOI] [PubMed] [Google Scholar]
- 37.Tanna S, Sahota TS, Sawicka K, Taylor MJ. The effect of degree of acrylic derivatisation on dextran and concanavalin A glucose-responsive materials for closed-loop insulin delivery. Biomaterials. 2006;27:4498–4507. doi: 10.1016/j.biomaterials.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 38.Tanna S, Taylor MJ, Sahota TS, Sawicka K. Glucose-responsive uv polymerised dextran-concanavalin A acrylic derivatised mixtures for closed-loop insulin delivery. Biomaterials. 2006;27:1586–1597. doi: 10.1016/j.biomaterials.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 39.Zhang R, Tang M, Bowyer A, Eisenthal R, Hubble J. Synthesis and characterization of a D-glucose sensitive hydrogel based on CM-dextran and concanavalin A. React Funct Polym. 2006;66:757–767. [Google Scholar]
- 40.Yin R, Han J, Zhang J, Nie J. Glucose-responsive composite microparticles based on chitosan, concanavalin A and dextran for insulin delivery. Colloids Surf B. 2010;76:483–488. doi: 10.1016/j.colsurfb.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 41.Ma R, Shi L. Phenylboronic acid-based glucose-responsive polymeric nanoparticles: synthesis and applications in drug delivery. Polym Chem. 2014;5:1503–1518. [Google Scholar]
- 42.Ding Z, Guan Y, Zhang Y, Zhu XX. Layer-by-layer multilayer films linked with reversible boronate ester bonds with glucose-sensitivity under physiological conditions. Soft Matter. 2009;5:2302–2309. [Google Scholar]
- 43.De Geest BG, Jonas AM, Demeester J, De Smedt SC. Glucose-responsive polyelectrolyte capsules. Langmuir. 2006;22:5070–5074. doi: 10.1021/la053368o. [DOI] [PubMed] [Google Scholar]
- 44.Matsumoto A, Ikeda S, Harada A, Kataoka K. Glucose-responsive polymer bearing a novel phenylborate derivative as a glucose-sensing moiety operating at physiological pH conditions. Biomacromolecules. 2003;4:1410–1416. doi: 10.1021/bm034139o. [DOI] [PubMed] [Google Scholar]
- 45.Kataoka K, Miyazaki H, Bunya M, Okano T, Sakurai Y. Totally synthetic polymer gels responding to external glucose concentration: their preparation and application to on-off regulation of insulin release. J Am Chem Soc. 1998;120:12694–12695. [Google Scholar]
- 46.Matsumoto A, Yamamoto K, Yoshida R, Kataoka K, Aoyagi T, Miyahara Y. A totally synthetic glucose responsive gel operating in physiological aqueous conditions. Chem Commun. 2010;46:2203–2205. doi: 10.1039/b920319b. [DOI] [PubMed] [Google Scholar]
- 47.Wu W, Mitra N, Yan ECY, Zhou S. Multifunctional hybrid nanogel for integration of optical glucose sensing and self-regulated insulin release at physiological pH. ACS Nano. 2010;4:4831–4839. doi: 10.1021/nn1008319. [DOI] [PubMed] [Google Scholar]
- 48.Matsumoto A, Ishii T, Nishida J, Matsumoto H, Kataoka K, Miyahara Y. A synthetic approach toward a self-regulated insulin delivery system. Angew Chem Int Ed Eng. 2012;51:2124–2128. doi: 10.1002/anie.201106252. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka T, Fillmore D, Sun ST, Nishio I, Swislow G, Shah A. Phase-transitions in ionic gels. Phys Rev Lett. 1980;45:1636–1639. [Google Scholar]
- 50.Zhang X, Guan Y, Zhang Y. Dynamically bonded layer-by-layer films for self-regulated insulin release. J Mater Chem. 2012;22:16299–16305. [Google Scholar]
- 51.Sato K, Kodama D, Endo Y, Yoshida K, Anzai J-i. Sugar-sensitive polyelectrolyte microcapsules containing insulin. Kobunshi Ronbunshu. 2010;67:544–548. [Google Scholar]
- 52.Levy T, Déjugnat C, Sukhorukov GB. Polymer microcapsules with carbohydrate-sensitive properties. Adv Funct Mater. 2008;18:1586–1594. [Google Scholar]
- 53.Wang B, Liu Z, Xu Y, Li Y, An T, Su Z, Peng B, Lin Y, Wang Q. Construction of glycoprotein multilayers using the layer-by-layer assembly technique. J Mater Chem. 2012;22:17954–17960. [Google Scholar]
- 54.Guo Q, Wu Z, Zhang X, Sun L, Li C. Phenylboronate-diol crosslinked glycopolymeric nanocarriers for insulin delivery at physiological pH. Soft Matter. 2014;10:911–920. doi: 10.1039/c3sm52485j. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y, Zhang X, Han Y, Cheng C, Li C. pH and glucose-sensitive glycopolymer nanoparticles based on phenylboronic acid for triggered release of insulin. Carbohydr Polym. 2012;89:124–131. doi: 10.1016/j.carbpol.2012.02.060. [DOI] [PubMed] [Google Scholar]
- 56.Ma R, Yang H, Li Z, Liu G, Sun X, Liu X, An Y, Shi L. Phenylboronic acid-based complex micelles with enhanced glucose-responsiveness at physiological pH by complexation with glycopolymer. Biomacromolecules. 2012;13:3409–3417. doi: 10.1021/bm3012715. [DOI] [PubMed] [Google Scholar]
- 57.Yang H, Sun X, Liu G, Ma R, Li Z, An Y, Shi L. Glucose-responsive complex micelles for self-regulated release of insulin under physiological conditions. Soft Matter. 2013;9:8589–8599. [Google Scholar]
- 58.Zhang YJ, Guan Y, Zhou SQ. Permeability control of glucose-sensitive nanoshells. Biomacromolecules. 2007;8:3842–3847. doi: 10.1021/bm700802p. [DOI] [PubMed] [Google Scholar]
- 59.Jin XJ, Zhang XG, Wu ZM, Teng DY, Zhang XJ, Wang YX, Wang Z, Li CX. Amphiphilic random glycopolymer based on phenylboronic acid: synthesis, characterization, and potential as glucose-sensitive matrix. Biomacromolecules. 2009;10:1337–1345. doi: 10.1021/bm8010006. [DOI] [PubMed] [Google Scholar]
- 60.Wang B, Ma R, Liu G, Li Y, Liu X, An Y, Shi L. Glucose-responsive micelles from self-assembly of poly(ethylene glycol)-b-poly(acrylic acid-co-acrylamidophenylboronic acid) and the controlled release of insulin. Langmuir. 2009;25:12522–12528. doi: 10.1021/la901776a. [DOI] [PubMed] [Google Scholar]
- 61.Guo HL, Li HM, Gao JT, Zhao GX, Ling LL, Wang B, Guo QQ, Gu Y, Li CX. Phenylboronic acid-based amphiphilic glycopolymeric nanocarriers for in vivo insulin delivery. Polym Chem. 2016;7:3189–3199. [Google Scholar]
- 62.Yao Y, Zhao L, Yang J, Yang J. Glucose-responsive vehicles containing phenylborate ester for controlled insulin release at neutral pH. Biomacromolecules. 2012;13:1837–1844. doi: 10.1021/bm3003286. [DOI] [PubMed] [Google Scholar]
- 63.Shiino D, Murata Y, Kataoka K, Koyama Y, Yokoyama M, Okano T, Sakurai Y. Preparation and characterization of a glucose-responsive insulin-releasing polymer device. Biomaterials. 1994;15:121–128. doi: 10.1016/0142-9612(94)90261-5. [DOI] [PubMed] [Google Scholar]
- 64.Shiino D, Murata Y, Kubo A, Kim YJ, Kataoka K, Koyama Y, Kikuchi A, Yokoyama M, Sakurai Y, Okano T. Amine containing phenylboronic acid gel for glucose-responsive insulin release under physiological pH. J Control Release. 1995;37:269–276. [Google Scholar]
- 65.Zhao Y, Trewyn BG, Slowing II, Lin VSY. Mesoporous silica nanoparticle-based double drug delivery system for glucose-responsive controlled release of insulin and cyclic amp. J Am Chem Soc. 2009;131:8398–8400. doi: 10.1021/ja901831u. [DOI] [PubMed] [Google Scholar]
- 66.Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev. 2012;64:49–60. doi: 10.1016/s0169-409x(01)00203-4. [DOI] [PubMed] [Google Scholar]
- 67.Yu JC, Zhang YQ, Ye YQ, DiSanto R, Sun WJ, Ranson D, Ligler FS, Buse JB, Gu Z. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc Natl Acad Sci U S A. 2015;112:8260–8265. doi: 10.1073/pnas.1505405112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu J, Qian C, Zhang Y, Cui Z, Zhu Y, Shen Q, Ligler FS, Buse JB, Gu Z. Hypoxia and H2O2 dual-sensitive vesicles for enhanced glucose-responsive insulin delivery. Nano Lett. 2017 doi: 10.1021/acs.nanolett.6b03848. http://dx.doi.org/10.1021/acs.nanolett.6b03848. [DOI] [PubMed]
- 69.Ye YQ, Yu JC, Wang C, Nguyen NY, Walker GM, Buse JB, Gu Z. Microneedles integrated with pancreatic cells and synthetic glucose-signal amplifiers for smart insulin delivery. Adv Mater. 2016;28:3115–3121. doi: 10.1002/adma.201506025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chou DHC, Webber MJ, Tang BC, Lin AB, Thapa LS, Deng D, Truong JV, Cortinas AB, Langer R, Anderson DG. Glucose-responsive insulin activity by covalent modification with aliphatic phenylboronic acid conjugates. Proc Natl Acad Sci U S A. 2015;112:2401–2406. doi: 10.1073/pnas.1424684112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bysell H, Mansson R, Hansson P, Malmsten M. Microgels and microcapsules in peptide and protein drug delivery. Adv Drug Deliv Rev. 2011;63:1172–1185. doi: 10.1016/j.addr.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 72.Yin R, Tong Z, Yang D, Nie J. Glucose and pH dual-responsive concanavalin A based microhydrogels for insulin delivery. Int J Biol Macromol. 2011;49:1137–1142. doi: 10.1016/j.ijbiomac.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 73.Yin RX, Wang KM, Han J, Nie J. Photo-crosslinked glucose-sensitive hydrogels based on methacrylate modified dextran-concanavalin A and PEG dimethacrylate. Carbohydr Polym. 2010;82:412–418. [Google Scholar]
- 74.Hoare T, Pelton R. Charge-switching, amphoteric glucose-responsive microgels with physiological swelling activity. Biomacromolecules. 2008;9:733–740. doi: 10.1021/bm701203r. [DOI] [PubMed] [Google Scholar]
- 75.Lapeyre V, Ancla C, Catargi B, Ravaine V. Glucose-responsive microgels with a core-shell structure. J Colloid Interface Sci. 2008;327:316–323. doi: 10.1016/j.jcis.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 76.Zhang XJ, Lu S, Gao CM, Chen C, Zhang X, Liu MZ. Highly stable and degradable multifunctional microgel for self-regulated insulin delivery under physiological conditions. Nanoscale. 2013;5:6498–6506. doi: 10.1039/c3nr00835e. [DOI] [PubMed] [Google Scholar]
- 77.Tang Z, Guan Y, Zhang Y. Contraction-type glucose-sensitive microgel functionalized with a 2-substituted phenylboronic acid ligand. Polym Chem. 2014;5:1782–1790. [Google Scholar]
- 78.Perez RA, Kim HW. Core-shell designed scaffolds for drug delivery and tissue engineering. Acta Biomater. 2015;21:2–19. doi: 10.1016/j.actbio.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 79.Zhang GH, Zhang X, Shen HY, Yang JJ, Yang J. Smarter glucose-sensitivity of polymeric micelles formed from phenylborate ester-co-pyrenylboronic ester for insulin delivery at physiological pH. RSC Adv. 2014;4:49964–49973. [Google Scholar]
- 80.Kim A, Yun MO, Oh YK, Ahn WS, Kim CK. Pharmacodynamics of insulin in polyethylene glycol-coated liposomes. Int J Pharm. 1999;180:75–81. doi: 10.1016/s0378-5173(98)00408-6. [DOI] [PubMed] [Google Scholar]
- 81.Jain SK, Amit KC, Chalasani KB, Jain AK, Chourasia MK, Jain A, Jain NK. Enzyme triggered pH sensitive liposomes for insulin delivery. J Drug Deliv Sci Tec. 2007;17:399–405. [Google Scholar]
- 82.Jo SM, Lee HY, Kim JC. Glucose-sensitivity of liposomes incorporating conjugates of glucose oxidase and poly(N-isopropylacrylamide-co-methacrylic acid-co-octadecylacrylate) Int J Biol Macromol. 2009;45:421–426. doi: 10.1016/j.ijbiomac.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 83.Dwivedi N, Arunagirinathan MA, Sharma S, Bellare J. Silica-coated liposomes for insulin delivery. J Nanomater. 2010:1–8. [Google Scholar]
- 84.Kim H, Kang YJ, Kang S, Kim KT. Monosaccharide-responsive release of insulin from polymersomes of polyboroxole block copolymers at neutral pH. J Am Chem Soc. 2012;134:4030–4033. doi: 10.1021/ja211728x. [DOI] [PubMed] [Google Scholar]
- 85.Alibolandi M, Alabdollah F, Sadeghi F, Mohammadi M, Abnous K, Ramezani M, Hadizadeh F. Dextran-b-poly(lactide-co-glycolide) polymersome for oral delivery of insulin: in vitro and in vivo evaluation. J Control Release. 2016;227:58–70. doi: 10.1016/j.jconrel.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 86.Hu X, Yu J, Qian C, Lu Y, Kahkoska AR, Xie Z, Jing X, Buse JB, Gu Z. H2O2-responsive vesicles integrated with transcutaneous patches for glucose-mediated insulin delivery. ACS Nano. 2017 doi: 10.1021/acsnano.6b06892. http://dx.doi.org/10.1021/acsnano.6b06892. [DOI] [PMC free article] [PubMed]
- 87.Karathanasis E, Bhavane R, Annapragada AV. Glucose-sensing pulmonary delivery of human insulin to the systemic circulation of rats. Int J Nanomedicine. 2007;2:501–513. [PMC free article] [PubMed] [Google Scholar]
- 88.Liu G, Yang H, Ma RJ, Shi LQ. Phenylboronic acid based glucose-responsive polymeric materials for insulin delivery and glucose monitoring. Acta Polym Sin. 2014;1161–1173 [Google Scholar]
- 89.Wang L, Liu Y, Zhang W, Chen X, Yang T, Ma G. Microspheres and microcapsules for protein delivery: strategies of drug activity retention. Curr Pharm Des. 2013;19:6340–6352. doi: 10.2174/1381612811319350010. [DOI] [PubMed] [Google Scholar]
- 90.Yoshida K, Hasebe Y, Takahashi S, Sato K, Anzai J. Layer-by-layer deposited nano- and micro-assemblies for insulin delivery: a review. Mater Sci Eng C. 2014;34:384–392. doi: 10.1016/j.msec.2013.09.045. [DOI] [PubMed] [Google Scholar]
- 91.Sato K, Kodama D, Endo Y, Anzai J. Preparation of insulin-containing microcapsules by a layer-by-layer deposition of concanavalin A and glycogen. J Nanosci Nanotechnol. 2009;9:386–390. doi: 10.1166/jnn.2009.j037. [DOI] [PubMed] [Google Scholar]
- 92.Webber MJ, Anderson DG. Smart approaches to glucose-responsive drug delivery. J Drug Target. 2015;23:651–655. doi: 10.3109/1061186X.2015.1055749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Baudyš M, Letourneur D, Liu F, Mix D, Jozefonvicz J, Kim SW. Extending insulin action in vivo by conjugation to carboxymethyl dextran. Bioconjug Chem. 1998;9:176–183. doi: 10.1021/bc970180a. [DOI] [PubMed] [Google Scholar]
- 94.Sakamoto Y, Akanuma Y, Kosaka K, Jeanrenaud B. Comparative effects of native insulin and insulin-dextran complexes on metabolism of adipose-tissue. Biochim Biophys Acta. 1977;498:102–113. doi: 10.1016/0304-4165(77)90091-5. [DOI] [PubMed] [Google Scholar]
- 95.Bhattacharya D, Basu S, Mandal PC. Uv radiation effects on flavocytochrome b(2) in dilute aqueous solution. J Photochem Photobiol B. 2000;59:54–63. doi: 10.1016/s1011-1344(00)00137-8. [DOI] [PubMed] [Google Scholar]
- 96.Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci. 2001;2:492–501. doi: 10.1038/35081564. [DOI] [PubMed] [Google Scholar]
- 97.Lee S, Kim K, Kumar TS, Lee J, Kim SK, Lee DY, Lee Y-k, Byun Y. Synthesis and biological properties of insulin–deoxycholic acid chemical conjugates. Bioconjug Chem. 2005;16:615–620. doi: 10.1021/bc049871e. [DOI] [PubMed] [Google Scholar]
- 98.Qi W, Yan X, Fei J, Wang A, Cui Y, Li J. Triggered release of insulin from glucose-sensitive enzyme multilayer shells. Biomaterials. 2009;30:2799–2806. doi: 10.1016/j.biomaterials.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 99.Di J, Price J, Gu X, Jiang X, Jing Y, Gu Z. Ultrasound-triggered regulation of blood glucose levels using injectable nano-network. Adv Healthc Mater. 2014;3:811–816. doi: 10.1002/adhm.201300490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pocker Y, Biswas SB. Conformational dynamics of insulin in solution - circular dichroic studies. Biochemistry (Mosc) 1980;19:5043–5049. doi: 10.1021/bi00563a017. [DOI] [PubMed] [Google Scholar]
- 101.Greenfield NJ. Using circular dichroism spectra to estimate protein secondary structure. Nat Protoc. 2007;1:2876–2890. doi: 10.1038/nprot.2006.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hinds K, Koh JJ, Joss L, Liu F, Baudyš M, Kim SW. Synthesis and characterization of poly(ethylene glycol)–insulin conjugates. Bioconjug Chem. 2000;11:195–201. doi: 10.1021/bc9901189. [DOI] [PubMed] [Google Scholar]
- 103.Delgado-Rivera R, Rosario-Melendez R, Yu WL, Uhrich KE. Biodegradable salicylate-based poly(anhydride-ester) microspheres for controlled insulin delivery. J Biomed Mater Res A. 2014;102:2736–2742. doi: 10.1002/jbm.a.34949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gordijo CR, Koulajian K, Shuhendler AJ, Bonifacio LD, Huang HY, Chiang S, Ozin GA, Giacca A, Wu XY. Nanotechnology-enabled closed loop insulin delivery device: in vitro and in vivo evaluation of glucose-regulated insulin release for diabetes control. Adv Funct Mater. 2011;21:73–82. [Google Scholar]
- 105.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Phys. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]


