Abstract
There is no coating technology currently available to prevent the notorious biofilm formation issue. Here, a potential solution to fully address this tough issue is reported by developing a super-antifouling coating. The use of zwitterionic hydrogel (a double-sided tape) and commercial superglue is combined and a durable and ultrarobust antifouling zwitterionic (DURA-Z) coating is created that can be easily and universally applied on common substrates. Commercial superglue mostly for binding hydrophobic materials is used to strongly immobilize the superhydrophilic DURA-Z coating through interpenetration. DURA-Z coating effectively solves several key challenges preventing the current antifouling coatings from practical use, including difficult fabrication, low efficacy, poor toughness, and durability. The fabricated DURA-Z coating retains antifouling property after 90 d of immersion in water, 50 d of buffer shearing, and 30 d of water flushing, and after repeated knife scratch and sandpaper abrasion under 570 kPa. The DURA-Z coating achieves a rarely reported long-term biofilm resistance to both Gram-positive and Gram-negative bacteria and fungi: it remains almost “zero” microbe adhesion after continuously challenged by more than 109 cells mL−1 culture medium for 30 d.
Keywords: antifouling, biofilm resistance, durability, zwitterions
There is no coating technology currently available that can prevent the notorious biofilm formation issue,[1,2] which induces infections[3] and impedes device function.[4] The state-of-the-art antibiofilm coatings are complex to fabricate and poor in performance due to instability in the working environment.[5] They usually started to accumulate bacteria after immediate contact and would be covered with dense biofilm usually within ≈7 d.[6,7] Here, we report the first potential solution to fully address this tough biofilm issue by developing an easily applied super-antifouling coating. Our coating was able to achieve almost “zero” bacteria adhesion even after one-month continuous contact with high concentration bacteria.
Our novel durable and ultrarobust antifouling zwitterionic (DURA-Z) coating combined the use of a zwitterionic hydrogel tape (a double-sided tape) and commercial superglue and can be easily fabricated and universally applied on common substrates of various shapes. Superhydrophilic zwitterionic polymer materials are known for their superior antifouling properties.[8] But they, like common hydrophilic coatings, drastically tend to dissolve in the aqueous environment resulting in poor coating durability.[9,10] The vulnerability to mechanical damages further prevents these hydrophilic coatings from being practically applied.[5,11] Superglue based on ethyl cyanoacrylate is one of the strongest linkages ever known for daily bonding projects and is known to mostly glue hydrophobic materials such as metal, plastic, or wood. It cannot stably bind to hydrophilic ones, e.g., a glass slide which has a water contact angle of ≈28°. Here we were able to immobilize a superhydrophilic DURA-Z gel network (water contact angle <2°) with the hydrophobic superglue, through a unique interpenetration mechanism. The obtained DURA-Z coating was superhydrophilic and retained antifouling property after various long-term durability tests in aqueous environments by incubating (90 d), shearing (50 d), and flushing (30 d) as well as mechanical damage tests by knife scratch and repeated sandpaper abrasion at 570 kPa. The pressure used in our abrasion test is more than 150 times higher than other similar tests for coating stability.[12] Remarkably, DURA-Z coating was able to achieve almost “zero” bacteria adhesion for at least one month when continuously incubated with highly concentrated bacteria or fungi (>109 cells mL−1) in culture media at both dynamic and static conditions. This remarkable antibiofilm performance has rarely been achieved. DURA-Z coating can be easily removed from the substrate and reapplied, which further extends the applicability of this coating strategy.
The formation of DURA-Z coating involves the combined use of a fabricated double-sided DURA-Z tape and superglue obtained from the hardware store. The DURA-Z tape was prepared by the in situ growing of a thin layer of zwitterionic polycarboxybetaine acrylamide (PCBAA, 3-((3-acrylamidopropyl)-dimethylammonio)propanoate) hydrogel on a commercially available polypropylene liner (Figure 1a, for detailed procedure, see the Supporting Information). The fabricated hydrogel tape was mechanically stronger than the pure zwitterionic PCBAA hydrogel and can withstand pulling, bending, wrenching, and rolling up (Figure 1b). This ensures ease of storage, transport, and mounting of the DURA-Z tape on substrates to be coated. The fabricated DURA-Z tape can be directly mounted as a coating layer without further treatment.
Figure 1.
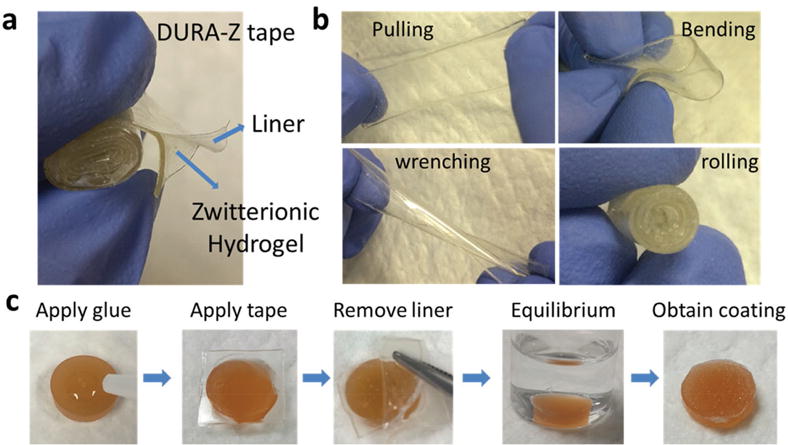
a) Structure of DURA-Z tape, b) DURA-Z tape under pulling, bending, wrenching, and rolling, and c) fabrication of DURA-Z coating on PU substrate.
The DURA-Z coating can be glued on a variety of substrates through a simple procedure (Figure 1c and Movie S1 (Supporting Information)). Briefly, a substrate was first cleaned, e.g., the polyurethane (PU) substrate was rinsed with alcohol and dried in air. Then the cyanoacrylate superglue was applied onto the dried substrate, followed by pressing the DURA-Z tape (hydrogel layer facing down) onto the superglue for a few seconds (100 μL superglue could cover ≈0.8 cm2 surface area). One hour was allowed to completely solidify the superglue. The liner on top of the hydrogel was removed and the glued coating was transferred to deionized (DI) water for equilibrium. 20 min later, the DURA-Z coating was polished either by a small shovel or tweezer, leaving the surface with a thin layer of coating.
The resulting DURA-Z coating showed completely different morphology compared to PU substrate or cyanoacrylate superglue surface under scanning electron microscope (SEM, Figure 2a) and atomic-force microscope (Figure S1, Supporting Information). SEM images on coating cross-section provided structural details of a surface hydrogel layer and a hydrogel/ glue interpenetrating layer (Figure 2a). The overall thickness of the coating (two layers) was measured to be 324 ± 13 μm. The thickness of the interpenetrating layer (187 ± 27 μm) was not changed much with varied fabricating parameters. Applying different volume of superglue did not induce changes in the interpenetrating thickness since the excessive amount of superglue would be squeezed out of the edge of DURA-Z tape. Applying higher compressive pressure to bind the DURA-Z tape to the substrate such as from ≈25 to ≈6000 Pa only resulted in a minor increase of interpenetrating thickness from 187 ± 27 to 196 ± 23 μm. The surface of the DURA-Z coating was characterized by IR spectroscopy and showed almost the same chemical composition as pure zwitterionic PCBAA hydrogel (Figure 2c, for detailed interpretation of IR spectra, see the Supporting Information). This implied the surface of the DURA-Z coating had superior antifouling property as good as a zwitterionic hydrogel.[8] Water contact angle (Figure 2b) indicated that the DURA-Z coating was superhydrophilic with great wettability (the water drop spread out quickly as demonstrated by Movie S1 in the Supporting Information), similar to pure PCBAA hydrogel surface.
Figure 2.
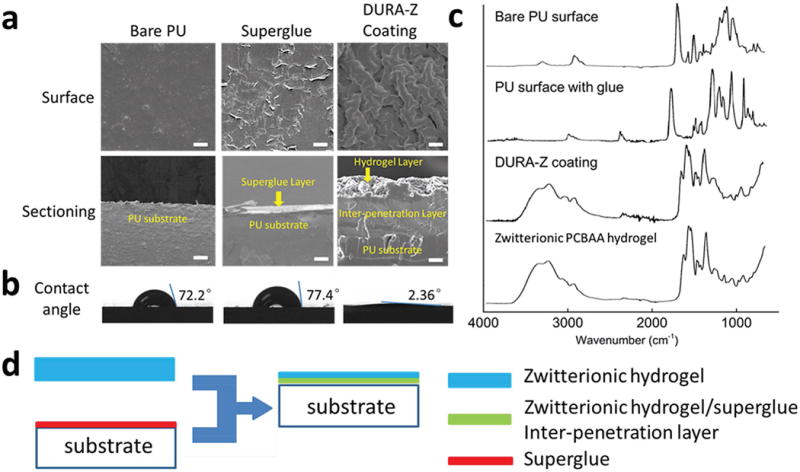
a) SEM imaging on the surface and sectioning of uncoated PU, superglue and DURA-Z coated substrates (scale bar = 100 μm), b) water contact angles on bare PU, superglue and DURA-Z coated substrates, c) IR spectra of bare PU surface, superglue coated PU surface, DURA-Z coated surface and zwitterionic PCBAA hydrogel surface, and d) illustration of the formation of DURA-Z coating.
Superhydrophilic polymer coatings were known to be unstable since polymers drastically tended to dissolve in water. Common hydrophilic coatings would fail within a few weeks in an aqueous environment.[5,6,9] We incubated the DURA-Z coating in DI water at room temperature for up to three months. The morphology of the coating was almost unchanged, and antifouling property (tested by human fibrinogen binding followed by enzyme-linked immunosorbent assay (ELISA) quantification of the absorbed protein, for detailed procedure, see the Supporting Information) was retained at the same level as freshly made coatings after the long-term incubation (Figure S2a, Supporting Information). The DURA-Z coating was further examined under various durability tests in aqueous environment, including (1) exposed to phosphate buffered saline (PBS) shearing (1500 rpm, 202G) at room temperature for 50 d (Figure S2b, Supporting Information), (2) exposed to PBS shearing (1500 rpm, 202G) at body temperature (37 °C) for 30 d (Figure 3a), and (3) subjected to continuous perpendicular water-flush at a flow rate of 42.8 mL s−1 for 30 d (Figure 3b). These challenging conditions did not change the morphology of the DURA-Z coating, and the anti-fouling property of the coating was well retained, indicated by the unchanged, significantly lowered human fibrinogen absorption on the coating, compared with uncoated PU (Figure 3a,b). In the working environment, the antifouling coating potentially experienced cycled dry/wet challenge. Many hydrophilic coatings can hardly retain antifouling function after being dried out and rehydrated.[13] The DURA-Z coating was challenged by dry/wet cycles under air flow and water, and showed complete rehydration (Figure S3a, Supporting Information) and the anti-fouling property was well-preserved even after 10 dry/wet cycles (Figure S3b, Supporting Information).
Figure 3.
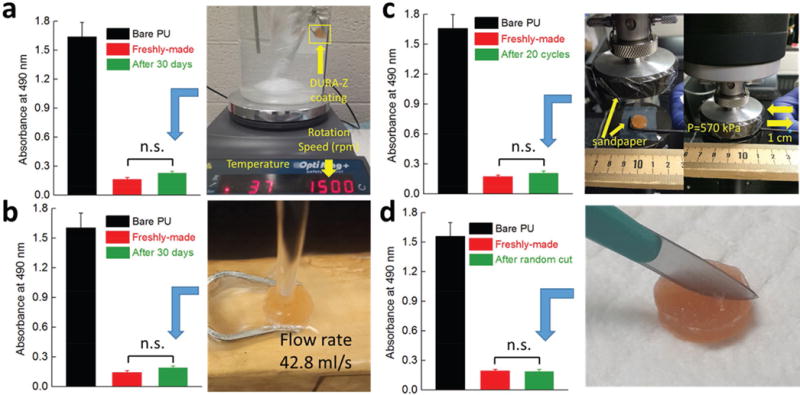
Antifouling property of DURA-Z coating after various durability and mechanical damage tests. a) 30 d exposure to PBS shearing under body temperature, b) 30 d exposure to perpendicular water flush, c) 20 cycles of abrasion test under 570 kPa, and d) random scratch by a scalpel. The antifouling property was evaluated by the resistance of human fibrinogen binding on the surface (absorbed protein) before and after the coating being challenged. All data are presented as mean of replicates (n = 3) ± standard deviation. Statistical analysis: unpaired, two-tailed t-test, n.s.: no significant difference at P > 0.05, meaning the great antifouling property was retained.
The DURA-Z coating was further subjected to mechanical damage tests. We used a sharp scalpel to randomly scratch the coating (Figure 3d and Movie S3 (Supporting Information)). The coating retained its small water contact angle (Movie S3, Supporting Information) and great antifouling property (Figure 3d). The resistance to sharp scratch was attributed to the self-healing capability of PCBAA hydrogel in physiological condition as previously reported.[14] Even with a deep incision when the DURA-Z coating layer was completely cut through and a permanent scratch was left on the substrate (blade thickness: 20–30 μm), the damaged DURA-Z coating layer was able to heal together in the water environment (Figure S4a,b, Supporting Information) and retain antifouling capability. We further examined the coating in an abrasion test, where a PU coupon with both sides coated with DURA-Z was placed between two stationary sandpapers (400 grit) and a pressure of 570 kPa was applied by a compressor (Figure 3c). The coupon was moved back and forth (displacement = 1 cm) for 20 circles and the coating was found to survive this abrasion test with great water wettability (Movie S4, Supporting Information), and unchanged great antifouling property (Figure 3c). It should be noted that the 570 kPa pressure used in our abrasion test is more than 150 times higher than other similar tests for coating stability.[12] In our sandpaper abrasion test, a PU coupon without DURA-Z coating applied cannot be moved by manpower under a pressure of 50 kPa (Movie S5, Supporting Information). The resistance to mechanical abrasion damage was attributed to the slippery surface property (low friction) of the DURA-Z coating. Applying DURA-Z coating reduced the friction index between PU coupon substrate and sand paper for more than 53% (see Figure S4c,d and friction index measurement procedure in the Supporting Information).
In biomedical applications where surface exposure to complex media, such as blood, was involved, the coating was required to resist the adhesion and activation of platelet, which further induced the thrombosis and blood clotting.[15] We challenged the DURA-Z coating with platelet rich plasma (PRP) from rat blood (for PRP collection procedure, see the Supporting Information) at 37 °C overnight and the platelet adhesion was visualized by SEM. The results indicated that the DURA-Z coating could effectively resist the adhesion of platelet (nearly “0” adhesion) in PRP, compare to the bare PU surface and the superglue coated surface (Figure S5, Supporting Information).
Microorganism attachment on surfaces leads to biofilm formation that causes medical device failure, infection, and marine fouling related fuel penalty. Hydrophilic polymer coatings, such as zwitterionic polymers, are nontoxic and environmentally friendly and have shown their effectiveness in resisting microorganism adhesion.[16] Nevertheless, due to the instability of hydrophilic polymers in an aqueous environment, these coatings were not able to maintain the resistance to microorganism adhesion for a longer period of time.[6,7] Here we expect the ultradurable and robust DURA-Z coating can greatly improve long-term microorganism resistance performance. To highlight this property, we utilized Escherichia coli (E. coli) as a model system to study the coating’s resistance to biofilm formation and quantified the bacterial adhesion by SEM. Our initial study has challenged the coating with dynamic bacterial culture condition. The DURA-Z coated PU substrate was placed in bacterial culture medium (Luria-Bertani (LB) broth) containing an extremely high number of bacteria (1.05 × 109 cells mL−1) at 37 °C for 30 d under continuous shaking (300 rpm). The culture medium was gently refreshed every 2 d, and the bacterial density within the refreshed medium was kept at 1.05 × 109 cells mL−1. After 30 d, bacteria on the substrate surface were fixed, dehydrated, vacuumed, and visualized under SEM (for detailed procedure, see the Supporting Information). Results indicated almost zero adhesion of bacteria on the zwitterionic DURA-Z coating, while biofilm had developed on the bare PU substrate and the superglue coated surface (Figure 4a).
Figure 4.
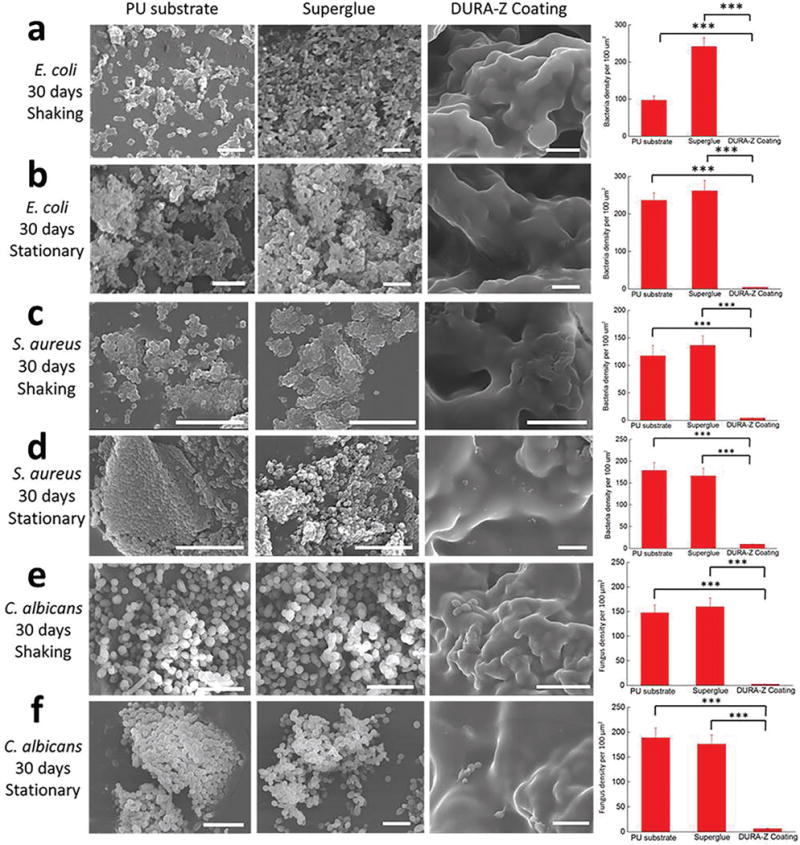
Representative SEM images of bacteria and fungi adhesion on bare PU, superglue, and DURA-Z coated PU substrates after 30 d of coculture with concentrated microbes and calculated microbe adhesion density for a) shaking condition with E. coli, b) stationary condition with E. coli, c) shaking condition with S. aureus, d) stationary condition with S. aureus, e) shaking conditions with C. albicans, and f) stationary condition with C. albicans. All data are presented as mean of biological replicates (n = 6) ± standard deviation. Statistical analysis: one-way analysis of variance (ANOVA) with Bonferroni multicomparison. ***: p < 0.0001. Scale bar = 10 μm.
We further challenged the DURA-Z coating with one of the harshest condition, where high-density bacteria (1.05 × 109 cells mL−1) were stationarily cultured with the substrate in LB broth at 37 °C and allowed to settle on the substrate surface through gravity. The culture medium was gently refreshed day-by-day, and the bacterial density within the refreshed medium was maintained at 1.05 × 109 cells mL−1. After 30 d, without any rinsing, only a small amount of bacteria was found to be scattered on DURA-Z coating (no biofilm forming) under SEM (Figure S6, Supporting Information). With 30 min of rinsing in PBS, these scattered bacteria were easily washed off from the DURA-Z coating (Figure 4b). By contrast, the biofilm (high-density bacteria adhered) formed on the bare PU substrate and the superglue coated substrate and remained to be firmly attached even after rinsing (Figure 4b).
In addition to the Gram-negative E. coli bacterium, we further tested the antibiofilm capability of the DURA-Z coating on Gram-positive bacterium (Staphylococcus aureus) and fungus (Candida albicans). The similar challenging condition (1.05 × 109 cells mL−1) was applied with S. aureus and C. albicans on the DURA-Z coating by both shaking and stationary methods for up to 30 d. Results indicated that the DURA-Z coating had strong resistance to the adhesion of both S. aureus (Figure 4c,d) and C. albicans (Figure 4e,f) after the long-term culture while uncoated surface had thick biofilm developed. It should be noted that long-term biofilm resistance was highly desirable to reduce infection and extend medical device lifetime but remained a challenge in the field.[2,17] We attribute the observed remarkable long-term biofilm resistance to the combined effect of ultralow fouling property and ultradurability of the zwitterionic DURA-Z coating.
The DURA-Z coating can be easily applied to a variety of substrates including stainless steel (Movie S2, Supporting Information), wood, and glass (Figure S7a, Supporting Information) by combining the use of DURA-Z tape and superglue. It should be noted that the glass substrate was pretreated with a thin layer of EVO-Stik glue before applying the coating due to the low affinity between cyanoacrylate superglue and glass. DURA-Z coatings obtained on these substrates were also found to be durable as indicated by unchanged great antifouling property after two months of incubation in water (Figure S8, Supporting Information). Even under PBS shearing (1500 rpm, 202G) at 37 °C for up to 30 d, the DURA-Z coating maintained its antifouling property on these different substrates (Figure S9, Supporting Information). They were also tested for E. coli bio-film resistance for 30 d using dynamic bacterial challenge condition. Almost zero bacteria adhesion was achieved on all tested DURA-Z coatings despite different types of substrates were coated (Figure S10, Supporting Information).
Practically speaking, it is frequently desirable that a coating layer can be fully removed from the substrate, followed by a new coating to be reapplied seamlessly.[18] This, however, can be hardly achieved with current chemical surface modification methods[19] without significantly damaging the original substrates. Here the DURA-Z coating binds with the substrate surface through commercial superglues which are dissolvable in organic solvents. Take the DURA-Z coated stainless steel, for example (Figure S7b, Supporting Information), upon 1 h incubation in acetone, the coating came off spontaneously without any damage to the substrate surface (Movie S6, Supporting Information). It is feasible to reapply a new DURA-Z coating on this same stainless steel substrate, and the new coating resisted protein binding as efficient as when it was first applied (Figure S7c, Supporting Information).
The application of DURA-Z tape is comparable to pasting wallpapers. The DURA-Z tapes are scalable in size and can be curved, bent, wrenched, and are tailorable to fit and coat substrates with complex surface shapes. We showed the SEM images for a bent DURA-Z coating on a corner using one piece of tape (Figure 5a) and the conjunction area between two pieces of tapes (Figure 5b). In order for the coating on complex surface shapes to retain good antifouling properties, the conjunction area between any two adjacent tapes should be well covered by the PCBAA hydrogel without the exposure of glue or substrate. This was achieved by the self-healing property of PCBAA hydrogel enabling the fusion between two adjacent DURA-Z coatings (Figure 5c). We tested the fusion of two adjacent pieces of DURA-Z coating on both PU and stainless steel substrates (Figure 5d). If the coating was freshly made, the two pieces fell apart after the superglue layer was dissolved in acetone. 48 h after making the coating and then placing it in acetone, the two pieces of DURA-Z fused into one. ELISA results indicated that up to three conjunction lines (four pieces of DURA-Z tapes) within ≈0.8 cm2 coated area did not compromise the antifouling property compared with the coating of the same area prepared using one piece of tape (Figure 5e).
Figure 5.
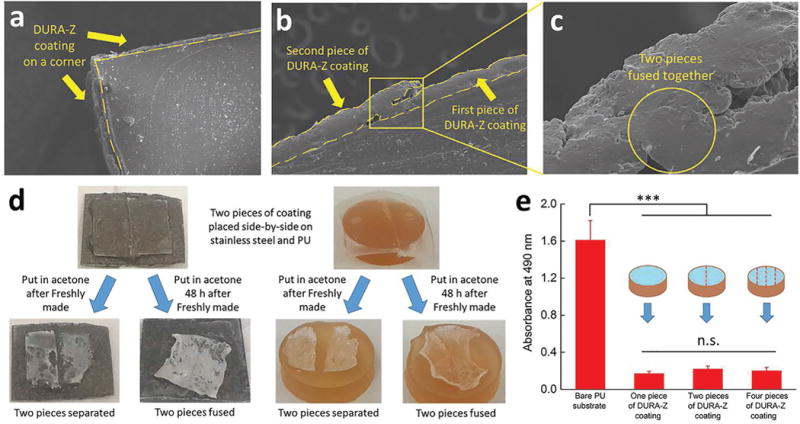
a) DURA-Z coating on a corner, b) SEM sectioning image of two adjacent pieces of DURA-Z coating, c) enlarged image of (b) on the fused area. d) Two pieces of adjacent DURA-Z coatings were placed in acetone after freshly made and 48 h after freshly made. e) Antifouling property of PU substrate with two and four pieces of adjacent DURA-Z coatings. All data are presented as mean of replicates (n = 3) ± standard deviation. Statistical analysis: one-way ANOVA with Bonferroni multicomparison. ***: p < 0.0001. n.s.: no significant difference, p > 0.5.
The key for the DURA-Z coating to achieve the ultradurability is because of the use of superglue to strongly bind the superhydrophilic polymer network (PCBAA hydrogel) and a hydrophobic substrate together. This is uncommon since commercial superglue is known to mostly bind hydrophobic substrates. Superglues are based on the rapid solidification of hydrophobic cyanoacrylate polymers, which have low affinity with hydrophilic materials such as zwitterionic polymers. In fact, we tried to glue linear zwitterionic PCBAA polymer powder and highly concentrated PCBAA/water solution on substrates using superglue, but the attached polymers quickly dissolved upon incubation in water and both obtained surfaces showed hydrophobic nature (Figure S11, Supporting Information). The success of using superglue to strongly bind the hydrophilic zwitterionic hydrogel layer (DURA-Z coating) is attributed to the unique interpenetration structure formed between these two (Figure 2a,d). It is expected that during the curing process, liquid cyanoacrylate monomers penetrated into the zwitterionic hydrogel network and were cross-linked. The entanglement between the DURA-Z and superglue networks immobilized the coating on the substrate for as long and strong as the superglue adhesive can last.
For biofilm resistance performance, DURA-Z coating is unprecedented. State-of-the-art antifouling coatings started to accumulate bacteria after just a few hours of contact and would be covered with dense biofilm usually within ≈7 d.[6,7] DURA-Z coating was able to achieve almost “zero” bacteria adhesion even after one-month continuous contact with high concentration bacteria and fungi. This coating technology is convenient to apply and can promisingly solve the biofilm threat, which deteriorates public health and impedes the function of most biomedical devices.
In summary, we developed an ultradurable and robust zwitterionic polymer network coating, namely DURA-Z, by combining the use of commercial superglue and the developed DURA-Z tapes. DURA-Z coating showed great stability and durability in a series of long-term challenges in aqueous environments as well as mechanical damage tests. With superior antifouling and durable property, the coating was able to achieve almost zero adhesion of microbes after one-month continuous coculture with both Gram-positive and Gram-negative bacteria and fungi at extremely high density. DURA-Z coating is applicable to substrates of various types and shapes to achieve long term durability and anti-fouling performance, and when needed, it can be easily removed and reapplied. This simple and inexpensive method will find applications as antifouling coatings in large, such as antibiofilm coatings, medical device coatings, and marine coatings.
Supplementary Material
Acknowledgments
This work was supported by the US National Science Foundation (DMR-1410853), the US National Institute of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health (DP2DK111910), and the faculty start-up fund at the Wayne State University, Chemical Engineering and Materials Science.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Swartjes JJTM, Das T, Sharifi S, Subbiahdoss G, Sharma PK, Krom BP, Busscher HJ, van der Mei HC. Adv Funct Mater. 2013;23:2843. [Google Scholar]
- 2.Lu Y, Yue ZG, Wang W, Cao ZQ. Front Chem Sci Eng. 2015;9:324. [Google Scholar]
- 3.a) Tamboto H, Vickery K, Deva AK. Plast Reconstr Surg. 2010;126:835. doi: 10.1097/PRS.0b013e3181e3b456. [DOI] [PubMed] [Google Scholar]; b) Francolini I, Donelli G. FEMS Immunol Med Microbiol. 2010;59:227. doi: 10.1111/j.1574-695X.2010.00665.x. [DOI] [PubMed] [Google Scholar]; c) Williams DL, Sinclair KD, Jeyapalina S, Bloebaum RD. J Biomed Mater Res, Part B. 2013;101b:1078. doi: 10.1002/jbm.b.32918. [DOI] [PubMed] [Google Scholar]
- 4.a) Zhao LZ, Wang HR, Huo KF, Cui LY, Zhang WR, Ni HW, Zhang YM, Wu ZF, Chu PK. Biomaterials. 2011;32:5706. doi: 10.1016/j.biomaterials.2011.04.040. [DOI] [PubMed] [Google Scholar]; b) van Heerden J, Turner M, Hoffmann D, Moolman J. J Plast Reconstr Aesthet Surg. 2009;62:610. doi: 10.1016/j.bjps.2007.09.044. [DOI] [PubMed] [Google Scholar]; c) Ding X, Yang C, Lim TP, Hsu LY, Engler AC, Hedrick JL, Yang YY. Biomaterials. 2012;33:6593. doi: 10.1016/j.biomaterials.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Wang W, Lu Y, Xie JB, Zhu H, Cao ZQ. Chem Commun. 2016;52:4671. doi: 10.1039/c6cc00109b. [DOI] [PubMed] [Google Scholar]
- 6.Cheng G, Li GZ, Xue H, Chen SF, Bryers JD, Jiang SY. Biomaterials. 2009;30:5234. doi: 10.1016/j.biomaterials.2009.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a) Zhao C, Zheng J. Biomacromolecules. 2011;12:4071. doi: 10.1021/bm2011455. [DOI] [PubMed] [Google Scholar]; b) Buskens P, Wouters M, Rentrop C, Vroon Z. J Coat Technol Res. 2013;10:29. [Google Scholar]; c) Cheng G, Zhang Z, Chen SF, Bryers JD, Jiang SY. Biomaterials. 2007;28:4192. doi: 10.1016/j.biomaterials.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.a) Jiang SY, Cao ZQ. Adv Mater. 2010;22:920. doi: 10.1002/adma.200901407. [DOI] [PubMed] [Google Scholar]; b) Zhang L, Cao ZQ, Bai T, Carr L, Ella-Menye JR, Irvin C, Ratner BD, Jiang SY. Nat Biotechnol. 2013;31:553. doi: 10.1038/nbt.2580. [DOI] [PubMed] [Google Scholar]; c) Carr LR, Xue H, Jiang SY. Biomaterials. 2011;32:961. doi: 10.1016/j.biomaterials.2010.09.067. [DOI] [PubMed] [Google Scholar]; d) He K, Duan H, Chen GY, Liu X, Yang W, Wang D. ACS Nano. 2015;9:9188. doi: 10.1021/acsnano.5b03791. [DOI] [PubMed] [Google Scholar]
- 9.Wang GZ, Wang LG, Lin WF, Wang Z, Zhang J, Ji FQ, Ma GL, Yuan ZF, Chen SF. ACS Appl Mater Interfaces. 2015;7:16938. doi: 10.1021/acsami.5b05162. [DOI] [PubMed] [Google Scholar]
- 10.Yang R, Gleason KK. Langmuir. 2012;28:12266. doi: 10.1021/la302059s. [DOI] [PubMed] [Google Scholar]
- 11.Holmes PF, Currie EPK, Thies JC, van der Mei HC, Busscher HJ, Norde W. J Biomed Mater Res, Part A. 2009;91a:824. doi: 10.1002/jbm.a.32285. [DOI] [PubMed] [Google Scholar]
- 12.Lu Y, Sathasivam S, Song JL, Crick CR, Carmalt CJ, Parkin IP. Science. 2015;347:1132. doi: 10.1126/science.aaa0946. [DOI] [PubMed] [Google Scholar]
- 13.a) Park JH, Lee GD, Ooshige H, Nishikata A, Tsuru T. Corros Sci. 2003;45:1881. [Google Scholar]; b) Qian B, Hou BR, Zheng M. Corros Sci. 2013;72:1. [Google Scholar]
- 14.Bai T, Liu SJ, Sun F, Sinclair A, Zhang L, Shao Q, Jiang SY. Biomaterials. 2014;35:3926. doi: 10.1016/j.biomaterials.2014.01.077. [DOI] [PubMed] [Google Scholar]
- 15.a) Meyer T, Robles-Carrillo L, Robson T, Langer F, Desai H, Davila M, Amaya M, Francis JL, Amirkhosravi A. J Thromb Haemostasis. 2009;7:171. doi: 10.1111/j.1538-7836.2008.03212.x. [DOI] [PubMed] [Google Scholar]; b) Radomski A, Jurasz P, Alonso-Escolano D, Drews M, Morandi M, Malinski T, Radomski MW. Br J Pharmacol. 2005;146:882. doi: 10.1038/sj.bjp.0706386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.a) Mahmud G, Huda S, Yang W, Kandere-Grzybowska K, Pilans D, Jiang SY, Grzybowski BA. Langmuir. 2011;27:10800. doi: 10.1021/la201066y. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Cheng G, Xue H, Li GZ, Jiang SY. Langmuir. 2010;26:10425. doi: 10.1021/la101542m. [DOI] [PubMed] [Google Scholar]; c) Zhang Z, Finlay JA, Wang LF, Gao Y, Callow JA, Callow ME, Jiang SY. Langmuir. 2009;25:13516. doi: 10.1021/la901957k. [DOI] [PubMed] [Google Scholar]; d) Fernandez ICS, van der Mei HC, Lochhead MJ, Grainger DW, Busscher HJ. Biomaterials. 2007;28:4105. doi: 10.1016/j.biomaterials.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 17.Costerton JW, Stewart PS, Greenberg EP. Science. 1999;284:1318. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 18.Owen SJ, Meier FS, Brombacher S, Volmer DA. Rapid Commun Mass Spectrom. 2003;17:2439. doi: 10.1002/rcm.1210. [DOI] [PubMed] [Google Scholar]
- 19.a) Yang WJ, Neoh KG, Kang ET, Teo SLM, Rittschof D. Prog Polym Sci. 2014;39:1017. [Google Scholar]; b) Goda T, Konno T, Takai M, Moro T, Ishihara K. Biomaterials. 2006;27:5151. doi: 10.1016/j.biomaterials.2006.05.046. [DOI] [PubMed] [Google Scholar]; c) Ganewatta MS, Miller KP, Singleton SP, Mehrpouya-Bahrami P, Chen YP, Yan Y, Nagarkatti M, Nagarkatti P, Decho AW, Tang CB. Biomacromolecules. 2015;16:3336. doi: 10.1021/acs.biomac.5b01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


