Background
Soil-transmitted helminth (STH = Ascaris lumbricoides, Trichuris trichiura and hookworms) infections are a major public health problem in many developing countries (Savioli et al, 2002). Worm infections can cause a wide range of pathologic conditions (Awasthi S et al, 2003), including reduced growth rate, deficiencies in cognitive and learning processes, impaired micronutrient status and iron deficiency anaemia (Crompton DWT, 1992; Crompton DWT & Nesheim MC, 2002; Oberhelman RA et al, 1998). STH infections are among the most common communicable diseases in school-age children world-wide and tend to occur at highest prevalence and intensity in this age group (Awasthi S et al, 2003; Crompton DWT, 1999; Savioli et al, 2002).
In the summer of 2002, the World Food Programme (WFP) approached the World Health Organization (WHO), the Afghan Ministry of Education (MoE), and the Afghan Ministry of Health (MoH) to launch an inter-agency deworming campaign in Afghanistan.
Afghanistan is a landlocked country in south-central Asia. Neighbouring countries are Pakistan in the south and south-east, Iran in the west, Turkmenistan, Uzbekistan and Tajikistan in the north, and China in the north-east. The capital is Kabul (approximately 3,000,000 inhabitants). Country’s surface is 647,500 square km in size; overall population (July 2002) is 27,755,775.
Despite the absence of recent epidemiological data, STH infections are known to be present among Afghan population. An epidemiological study carried out in 1978 in 4 villages located in 4 different areas of the country showed the public health relevance of A. lumbricoides infection; moreover, in 2 of the 4 villages the infection intensity was extremely severe (Buck et al., 1978). A more recent publication ranked STH among the most common diseases affecting Afghan refugees seeking asylum in New Zealand (New Zealand Ministry of Health, 2001).
However, in order to assess the distribution, and to classify the regional endemic level of STH infections for appropriate planning of deworming interventions at community level, the decision was made to undertake a baseline survey.
Materials and methods
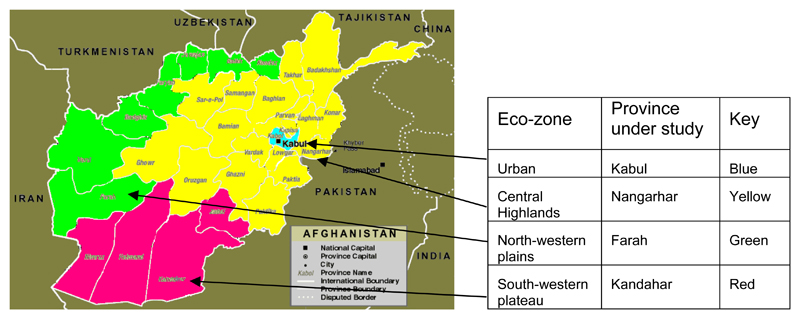
The survey was carried out in February and March 2003 in four Provinces of Afghanistan, each representative of an ecologically homogeneous area (eco-zone) of the country (Figure 1):
Kabul City in Kabul Province, the fast-growing capital of Afghanistan, whose inhabitants have more than doubled from 1,300,000 in 2001 to 3,000,000 in 2003; as metropolitan sample of Urban ecological areas.
Nangarhar Province, as sample of the Central highlands ecological area, an extension of the Himalayan mountain chain, which includes the Hindu-Kush mountain range.
Farah Province, as sample of North-western plains ecological area, the major agricultural area of the country.
Kandahar Province, as sample of the South-western plateau ecological area, consisting primarily of desert and semi-desert landscape and including the Rigestan desert.
Figure 1.
Map of Afghanistan showing eco-zones and provinces under study
In each eco-zone, one province was randomly selected from among those provinces with WFP school feeding schools (schools covered by WFP school feeding interventions) that were operating in February/early March (this restricted the choice in Kabul to the special winter school accelerated classes run in selected schools; in the Central Highlands and North-western plains eco-zones, eligible provinces were those with “hot climate” districts in which schools operate throughout winter months). For each one of the identified four Provinces, one district was randomly chosen. Within each district five primary schools were randomly selected among those that had a large enough school population to cover the required sample size (i.e. 50 children).
Approximately 50 children per school were enrolled in the survey. In total, 1001 stool samples were examined. Table 1 shows the distribution and gender balance of children enrolled in our survey in each province. The age range of the children was between 8 and 15 years, the bulk of the sample consisting of 9 to 12 year olds; 55.3% of the students in the sample were male
Table 1. Distribution and gender balance of children enrolled in the survey.
| Province | % Male (n) | % Female (n) | % of the whole sample |
|---|---|---|---|
| Kabul | 31.8% (76) | 68.2% (163) | 23.9% (239) |
| Nangarhar | 32.8% (84) | 67.2% (172) | 25.5% (256) |
| Farah | 59.4% (148) | 40.6% (101) | 24.9% (249) |
| Kandahar | 95.7% (246) | 4.3% (11) | 25.7% (257) |
| TOTAL | 55.3% (554) | 44.7% (447) | 100% (1001) |
Schools were visited in the morning for stool sample collection. Each child was given a clean plastic screw-lid container and asked to provide a fresh stool specimen. Per day, a maximum of two schools were visited.
Faecal specimens were analysed in the afternoon at the nearest WHO Office/MoH Laboratory. Parasitological examination to assess prevalence and intensity of STH infection was performed using a single, 41.7-mg, Kato-Katz stool examination (WHO 1991), and was usually performed within 60 minutes from preparation. The number of eggs of each STH species was calculated per slide and per gram of faeces (epg).
In addition to parasitological data, other data was collected. This included personal data (age, sex); nutritional data (weight, height, haemoglobin concentration - on fresh whole blood sample, collected by finger prick, analysed by digital haemoglobinometer HemoCue AB, Angelholm, Sweden).
The Afghan Ministry of Health and the Afghan Ministry of Education were involved in the planning and the implementation of the survey and gave their approval to the investigation methods. The objectives of the survey were explained to the schoolmasters, and their consent obtained.
Following the rule “no survey without service”, all children enrolled in the survey received one tablet of Albendazole 400mg (anthelminthic drug). Furthermore, teachers and staff involved in organizing the data collection at school level were also provided with free tablets.
After the parasitological examination, each child was classified as infected or not infected. If infected, he or she was subsequently classified according to the intensity of each STH infection, measured as eggs per gram of faeces (epg), and known to be an indirect measure of the worm burden. The thresholds used were those recommended by WHO (World Health Organization, 2002).
The following information was subsequently calculated for each school:
the prevalence of infection with each STH species
the prevalence of moderate-heavy intensity infection with each STH species
the cumulative prevalence of STH infection (the prevalence of infection with at least one STH)
the cumulative prevalence of moderate-heavy intensity STH infection (the prevalence of moderate-heavy intensity infection with at least one STH)
Data from each school (not showed here) were merged together with those of the other schools in the same district in order to calculate data at district level and consequently at province level and for each of the four eco-zones.
Each eco-zone was therefore classified combining data on the cumulative prevalence of STH infections and on the cumulative prevalence of moderate-heavy intensity infections, following the WHO guidelines (World Health Organization, 2002)
Results
The prevalence and intensity of each species in each of the provinces included in the survey is shown in table 2. The table also shows the cumulative prevalence of STH infections and the cumulative prevalence of moderate-heavy STH infections.
Table 2. Prevalence and intensity of STH infections.
| Kabul | Nangarhar | Farah | Kandahar | TOTAL | |
|---|---|---|---|---|---|
| Sample size | 239 | 256 | 249 | 257 | 1001 |
|
Ascaris lumbricoides Prevalence of infection (n) |
57% (137) | 27% (69) | 43% (106) | 37% (96) | 41% (408) |
|
Ascaris lumbricoides Prevalence of mod-heavy int. infection (n) |
26% (62) | 3% (8) | 6% (16) | 3% (8) | 9% (94) |
|
Trichuris trichiura Prevalence of infection (n) |
13% (31) | 6% (16) | 13% (32) | 8% (20) | 10% (99) |
|
Trichuris trichiura Prevalence of mod-heavy int. infection (n) |
0.8% (2) | 0.4% (1) | 0.8% (2) | ----- | 0.5% (5) |
|
Hookworms Prevalence of infection (n) |
----- | 3% (7) | ----- | ----- | 0.7% (7) |
|
Hookworms Prevalence of mod-heavy int. infection (n) |
----- | ----- | ----- | ----- | ----- |
|
Any STH Cumulative Prevalence of infection (n) |
62% (148) | 35% (89) | 50% (124) | 43% (110) | 47% (471) |
|
Any STH Cumulative Prevalence of moderate-heavy Intensity infection (n) |
26% (63) | 3% (8) | 7% (18) | 3% (8) | 10% (97) |
Only 40 children (4%) were found to have anaemia (haemoglobin concentration <11 g/dl); of these 4 (0.4%) had severe anaemia (haemoglobin concentration <7 g/dl). Only two cases of anaemia, one of which severe, were found to be associated with hookworm infection.
Discussion
About half (47%) of the schoolchildren population under study appeared to be infected with at least one STH. Ascaris lumbricoides was the most widespread STH, affecting 41% of the population under study, followed by Trichuris trichiura (10%) and hookworms (0.7%).
About 10% of the sample presented infection with moderate-severe intensity, which is a well-known cause of severe morbidity (Awasthi S et al, 2003). Among the subjects with moderate-heavy intensity, 97% (94/97) were infected with A. lumbricoides, while only 5% (5/97) were infected with T. trichiura. No moderate-heavy intensity infection due to hookworms was recorded.
The prevalence of stool samples with polyparasitism (co-occurrence of more than one STH infection in the same individual) was 4.3% (43/1001) (Table 3). Only 0.2% (2/1001) of the sample presented with moderate-heavy intensity polyparasitic STH infections. All polyparasitic infections were double infections; 42 cases of polyparasitism out of 43 were due to A. lumbricoides-T. trichiura co-infection.
Table 3. Polyparasitic infections.
| Province | Prevalence of polyparasitic infection (n) | Prevalence of mod-heavy intensity polyparasitic infection (n) |
|---|---|---|
| Kabul (239 samples) | 8.4% (20) | 0.4% (1) |
| Nangarhar (256 samples) | 1.2% (3) | 0.4% (1) |
| Farah (249 samples) | 5.6% (14) | ----- |
| Kandahar (257 samples) | 2.3% (6) | ----- |
| TOTAL (1001 samples) | 4.3% (43) | 0.2% (2) |
Kabul resulted to be the province where soil-transmitted helminthiasis poses the most severe health burden. 62% of the schoolchildren enrolled in this province resulted to be infected with at least one STH (cumulative prevalence of STH infection), and 26% presented STH infection with moderate-heavy intensity (cumulative prevalence of moderate-heavy intensity STH infection), which is a well known cause of severe morbidity. In the three other provinces under study, the cumulative prevalence of STH infection ranged from 35% of Nangarhar province to 43% of Kandahar province to 50% of Farah province. In all these provinces, the cumulative prevalence of moderate-heavy intensity STH infection was well below 10% (Nangarhar: 3%; Kandahar: 3%; Farah: 7%).
The classification of the endemic level of a community – also called community diagnosis – is a key-point of the WHO strategy for prevention and control of soil-transmitted helminthiasis (World Health Organization, 2002). Data generated from school-based surveys can be used to classify the whole ecological area where schools are located, as data collected from children attending schools are generally representative of the situation in the community (Montresor A et al, 1998).
On the basis of this classification, the optimal frequency of treatment of school age children can be determined, as well as the urgency for other control measures (World Health Organization, 2002).
According to the data generated by our survey, the four eco-zones of Afghanistan have been classified as follows:
Urban: category I (high prevalence or high intensity)
Central highlands: category II (moderate prevalence and low intensity)
North-western plains: category II (moderate prevalence and low intensity)
South-western plateau: category II (moderate prevalence and low intensity)
Conclusion
We conclude that all the eco areas of Afghanistan should be considered for inclusion in a national programme of mass drug distribution according to the WHO policy (World Health Organization, 2002).
The capital city of Afghanistan emerges from this survey as the most suitable environment for high rates of transmission of STH. In discordance with what was observed in other studies (Scolari C et al, 2000; Singh B & Cox-Singh, 2001), prevalence and intensity of infection appears to be higher in urban environments than in rural ones, probably as a result of a conglomerate of bad sanitation related to war events (which affected Kabul much more than smaller towns or rural settings), a high incidence of precarious livelihoods among the large population of refugees and internally displaced persons, and the specific climate and soil conditions which provide a suitable habitat for soil-transmitted helminths. Ascariasis results to be the most widespread infection in all provinces, followed by trichuriasis. Hookworm infection was found only in Nangarhar province, however the prevalence of infection was low (3%), and no infection of moderate-heavy intensity was detected.
The co-occurrence of polyparasitism appears to be in Afghanistan less common than observed in other countries across the world (Booth et al, 1998; Chunge et al, 1995; Tchuem Tchuente et al, 2003).
According to our survey, anaemia does not seem to be a relevant problem in Afghanistan and iron supplementation is not required among primary school-age children.
Gender imbalance among children enrolled in the survey can be attributed to the randomization of sampling, as in some cases girl-only or male-only schools were selected. However, also inequalities in school enrolment and as a consequence the need to implement measures aimed at the provision of treatment to non-enrolled children should be taken into account (Montresor A et al, 2001).
Based on the findings of the baseline survey and in accordance with the standard WHO recommendations on suitable treatment intensity for category I and II areas, the decision was made to implement one round of deworming treatment with Mebendazole 500mg in all eco-zones and to treat two times the major urban areas (Kabul, Heart, Kandahar, Mazar-e-Sharif) The drug distribution will be embedded in a health awareness sensitisation campaign (radio spots, leaflets, posters, banners, cascade training of teachers, MoE and MoH personnel at provincial and district level) targeting caretakers and community leaders, apart from primary school-age children, to ensure sustainability and change in behaviour, attitude and knowledge. The campaign is to be implemented in March 2004, using official and non-formal primary schools throughout the country as drug distribution points to reach the entire primary school-age population, including non-enrolled children.
Acknowledgement
The authors are very grateful to the staff of the Office of the World Health Organization Representative in Kabul, especially to Dr Said Salah Youssouf, former WR-Afghanistan, Dr Yon Fleerackers, Epidemiologist, and Mr Ahmad Khalid Janbaz, Data Manager; and to the staff of the Afghanistan Country Office of the World Food Programme, especially to Mr Mujeeb Haidery, Staff Assistant.
Special thanks are due to Mr Abdul Saboor, Mr Mohamed Mussa and Mr Naqibullah for their invaluable help, skill and suggestion.
We also would like to express our gratitude to all the staff of the Afghan Ministry of Health and the Afghan Ministry of Education.
Contributor Information
Mahdi Ramsan, Public Health Laboratory Ivo de Carneri, P.O. Box 122, Chake Chake, Pemba Island, Zanzibar (Tanzania).
Albis-Francesco Gabrielli, Programme for Communicable Diseases in Complex Emergencies, CDS/CPE/CE, World Health Organization, Geneva (Switzerland).
Dorjgochoo Tsogzolmaa, Office of the World Health Organization Representative, Kabul (Afghanistan).
Bai Bojang, World Food Programme Country Office, Kabul (Afghanistan).
Craig Naumann, World Food Programme Country Office, Kabul (Afghanistan).
Mohammed Hanif Khoshal, Afghan Ministry of Health, Kabul (Afghanistan).
Riadh Ben-Ismail, World Health Organization Regional Office for Eastern Mediterranean, WHO/EMRO, Cairo, Egypt.
Máire Connolly, Programme for Communicable Diseases in Complex Emergencies, CDS/CPE/CE, World Health Organization, Geneva (Switzerland).
Antonio Montresor, World Health Organization, Hanoi (Vietnam).
Lorenzo Savioli, Strategy Development and Monitoring for Parasitic Diseases and Vector Control, CDS/CPE/PVC, World Health Organization, Geneva (Switzerland).
References
- Awasthi S, Bundy DAP, Savioli L. Helminthic infections. Clinical review. BMJ. 2003;327:431–3. doi: 10.1136/bmj.327.7412.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth M, Bundy DAP, Albonico M, Chwaya HM, Alawi KS, Savioli L. Associations among multiple geohelminth species in schoolchildren from Pemba Island. Parasitology. 1998;116:85–93. doi: 10.1017/s003118209700190x. [DOI] [PubMed] [Google Scholar]
- Buck AA, et al. Epidemiology of poly-parasitism. I. Occurrence, Frequency and Distribution of multiple infections in rural communities in Chad, Peru, Afghanistan, and Zaire. Tropenmed Parasit. 1978;29:61–70. [PubMed] [Google Scholar]
- Chunge RN, Karumba N, Ouma JH, Thiongo FW, Sturrock RF, Butterworth AE. Polyparasitism in two rural communities with endemic Schistosoma mansoni infection in Machakos District, Kenya. Journal of Tropical Medicine and Hygiene. 1995;98:440–444. [PubMed] [Google Scholar]
- Crompton DWT. Ascaris and childhood malnutrition. Trans R SocTrop Med Hyg. 1992;86:577–579. doi: 10.1016/0035-9203(92)90133-w. [DOI] [PubMed] [Google Scholar]
- Crompton DWT. How much human helminthiasis is there in the world? JParasitol. 1999;85(3):397–403. [PubMed] [Google Scholar]
- Crompton DWT, Nesheim MC. Nutritional Impact of Intestinal Helminthiasis during the Human Life Cycle. Annu Rev Nutr. 2002;22:35–59. doi: 10.1146/annurev.nutr.22.120501.134539. [DOI] [PubMed] [Google Scholar]
- Montresor A, Crompton DWT, Bundy DAP, Hall A, Savioli L. Guidelines for the Evaluation of Soil-transmitted helminthiasis and Schistosomiasis at community level. World Health Organization; Geneva: 1998. [Google Scholar]
- Montresor A, Ramsan M, Chwaya HM, Ameir H, Foum A, Albonico M, Gyorkos TW, Savioli L. Extending anthelminthic coverage to non-enrolled school-age children using a simple and low-cost method. Trop Med Int Health. 2001;6(7):535–537. doi: 10.1046/j.1365-3156.2001.00750.x. [DOI] [PubMed] [Google Scholar]
- New Zealand Ministry of Health. Refugee Health Care: a Handbook for Health Professionals. 2001 Nov [Google Scholar]
- Oberhelman RA, Guerrero ES, Fernandez ML, Silio M, Mercado D, Cominskey N, Ihenacho G, Mera R. Correlations between intestinal parasitosis, physical growth, and psychomotor development among infants and children from rural Nicaragua. Am J Trop Med Hyg. 1998;58(4):470–5. doi: 10.4269/ajtmh.1998.58.470. [DOI] [PubMed] [Google Scholar]
- Scolari C, Torti C, Beltrame A, Matteelli A, Castelli F, Gulletta M, Ribas M, Morana S, Urbani C. Prevalence and distribution of soil-transmitted helminth (STH) infections in urban and indigenous schoolchildren in Ortigueira, State of Paranà, Brasil: implications for control. Tropical Medicine and International Health. 2000;5(4):302–307. doi: 10.1046/j.1365-3156.2000.00549.x. [DOI] [PubMed] [Google Scholar]
- Savioli L, Stansfield S, Bundy DA, Mitchell A, Bhatia R, Engels D, Montresor A, Neira M, Shein M. Schistosomiasis and soil-transmitted helminth infections: forging control efforts. Trans R Soc Trop Med Hyg. 2002;96(6):577–9. doi: 10.1016/s0035-9203(02)90316-0. Erratum in: Trans R Soc Trop Med Hyg, 97(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Cox-Singh J. Parasites that cause problems in Malaysia: soil-transmitted helminths and malaria parasites. Trends in Parasitology. 2001;17(12):597–600. doi: 10.1016/s1471-4922(01)02148-1. [DOI] [PubMed] [Google Scholar]
- Tchuem Tchuente L-A, Behnke JM, Gilbert FS, Southgate VR, Vercruysse J. Polyparasitism with Schistosoma haematobium and soil-transmitted helminth infections among school children in Loum, Cameroon. Trop Med Int Health. 2003;8(11):975–986. doi: 10.1046/j.1360-2276.2003.01120.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Basic Laboratory Methods in Medical Parasitology. World Health Organization; Geneva: 1991. Faecal specimens; pp. 25–27. [Google Scholar]
- World Health Organization. Prevention and Control of Schistosomiasis and Soil-Transmitted Helminths. Geneva: 2002. 2002. [PubMed] [Google Scholar]