Abstract
Introduction
The etiology of Tolosa-Hunt Syndrome (THS) is still unknown. The initial standard magnetic resonance imaging (MRI) may not be sufficient for diagnosis, so dynamic contrast-enhanced MRI may be necessary to demonstrate the presence of lesions.
Methods
Seven patients diagnosed with THS according to the International Headache Society criteria (beta version) were included into the study. Patients were assessed in terms of type, age, symptoms and findings, accompanying disease, localization of the determined lesion, response to treatment, and clinical progress. The “Tolosa-Hunt protocol” was applied in all patients, and the cavernous sinuses, orbital apices, and orbits were evaluated. The parameters used for the patients were as follows: Turbospin echo T1 and T2 weighted sequences on the axial plane, turbospin echo fat-saturated T2 weighted sequence on the coronal plane, turbospin echo T2 weighted sequence on the sagittal plane, spin echo fat-saturated T1 sequences repeated on the axial and coronal planes followed by intravenous administration of gadolinium. In all sequences the slice thickness was 3 mm.
Results
Four of seven cases diagnosed with THS were males, and the average age of the patients was 45.7±18.1 years (range 25–69 years). A follow-up MRI in patient 5 after three months showed decreased signal intensity and enhancement of the affected cavernous sinus.
Conclusion
Conventional MRI may be insufficient to show the granulomatous inflammation, and an MRI method referred to as the Tolosa-Hunt protocol should be applied to those who are thought to have THS.
Keywords: Diagnosis, dynamic contrast MRI, Tolosa-Hunt protocol, Tolosa-Hunt syndrome
INTRODUCTION
Tolosa-Hunt Syndrome (THS) is a rare disorder characterized by periorbital or hemicranial pain, ipsilateral oculomotor paralysis, and prompt response to steroids. Clinical and pathological findings were first defined by Tolosa in 1954, and Hunt added new cases at 1961 (1,2). The etiology of THS is still unknown. Its primary symptoms include idiopathic granulomatous inflammation of the cavernous sinus, superior orbital fissure, and/or the apex of the orbit (3,4). The incidence has been estimated at one case per million (5). THS may present at any age, from the first through the eighth decade, and both sexes are affected equally (6,7,8). Spontaneous remissions and relapses may occur. Recent studies have reported recurrence in 21%–40% of the patients months or even years after remission. Recurrence was generally reported on the same side (4,6). Conventional magnetic resonance imaging (MRI) methods sometimes fall short of showing the granulomatous lesion in the cavernous sinus. Because dynamic MRI can show the space and soft tissues in cavernous sinus better, it should be used in cases with an initial diagnosis of THS (8,9).
Cases with a diagnosis of painful ophthalmoplegia who are found to have cranial nerve paralysis or paralyses should be evaluated in terms of THS because recurrence may be observed in these cases because they do not receive steroid treatment (4). The aim of this study is to show the importance of an MRI protocol in the diagnosis of Tolosa-Hunt Syndrome.
METHODS
Seven patients admitted to the Department of Neurology of Ondokuz Mayıs University Hospital and diagnosed with THS according to the Headache Classification Committee of the International Headache Society criteria (beta version) were included into the study. All patients signed the informed consent form. Authors declared that the research was conducted according to the principles of the World Medical Association Declaration of Helsinki “Ethical Principles for Medical Research Involving Human Subjects” (amended in October 2013).
Patients were assessed in terms of type, age, symptoms and findings, accompanying disease, localization of the determined lesion, response to the treatment, and clinical progress. Complete blood count, serum biochemistry, erythrocyte sedimentation rate, thyroid function tests, antinuclear antibody, lupus erythematosus preparation, antineutrophil cytoplasmic antibody, serum protein electrophoresis, test for Lyme disease and HIV, cranial MRI examinations, and computerized tomography (CT) angiography were done in every patient, and lumbar puncture was performed in four patients.
The “Tolosa-Hunt protocol”
In all patients, MRI studies were performed in a 1.5 Tesla MRI unit (Symphony, Siemens). The cavernous sinuses, orbital apices, and orbits were evaluated. The parameters used for the patients were as follows: Turbospin echo T2 weighted sequences (T2 w) (TR: 3000, TE:74, flip angle: 150), spin echo T1 w sequence (TR:473, TE:7.7, flip angle: 90) on the axial plane, turbospin echo fat-saturated T2 w sequence (TR: 3300, TE:74, flip angle: 150) on the coronal plane, and turbospin echo T2 w sequence (TR: 6030, TE:103, flip angle: 150) on the sagittal plane. Spin echo fat-saturated T1 sequences were repeated on the axial and coronal planes followed by intravenous administration of gadolinium (0.1 mL/kg). In all sequences, the slice thickness was 3 mm. Follow-up MRI was performed after 2–4 months.
Statistical Analysis
Collected data were analyzed through both descriptive (mean, standard deviation, median, frequency, rate, and minimum and maximum). Statistical analyses were performed using the Statistical Software Package version 16.0 (SPSS Inc.; Chicago, IL, USA).
RESULTS
Four of seven cases diagnosed with THS were males. The mean age of the patients was 45.7±18.1 years (range 25–69 years). The clinical and imaging findings are summarized in Table 1. Cerebrospinal fluid examinations were performed in four patients, and all were unremarkable. Methylprednisolone (1 g) was administered to all patients by the intravenous route for five days, and the pain level was significantly reduced in all patients. Prednisolone (60 mg/day) was given by the oral route, and the dose was tapered down over six weeks. A follow-up MRI performed after three months in patient 5 showed decreased signal intensity and enhancement of the affected cavernous sinus (Figure 1, 2).
Table 1.
Clinical and radiological features of the patients
| Patient no | Sex | Age | Side | Conventional MRI | Contrast-enhanced MRI (Tolosa-Hunt Protocol) | Cranial nerve deficits | Recurrence | Cranial nerve deficits in ipsilateral or contralateral | Follow-up |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 50 | Right | Normal | Infiltration in right superior orbital fissure | III | 3 months | Ipsilateral III, VI | 23 months |
| 2 | Female | 64 | Left | Normal | Infiltration in left cavernous sinus | III, IV, VI, V1 | 1 month | Ipsilateral II, III, VI | 22 months |
| 3 | Female | 46 | Right | Normal | Infiltration in right cavernous sinus | III | 4 months | Ipsilateral III | 44 months |
| 4 | Male | 46 | Right | Normal | Infiltration in right cavernous sinus | IV | 4 months | Contralateral VI | 65 months |
| 5 | Female | 20 | Right | Normal | Infiltration in right cavernous sinus and superior orbital fissure | VI | 6 months | Ipsilateral IV, VI | 19 months |
| 6 | Male | 25 | Right | Normal | Infiltration in left cavernous sinus | III, VI | - | _ | 9 months |
| 7 | Male | 69 | Right | Normal | Infiltration in right cavernous sinus | III | - | _ | 11 months |
MRI: magnetic resonance imaging
Figure 1.
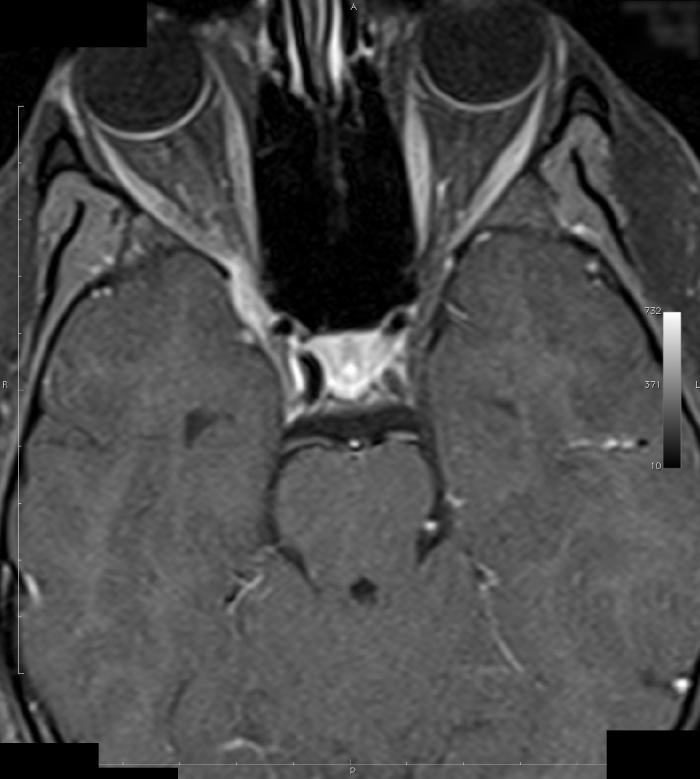
Patient 5 (before corticosteroid treatment): Post-gadolinium spin echo T1 weighted (TR/TE/FA:473/7.7/90) axial MRI
Figure 2.
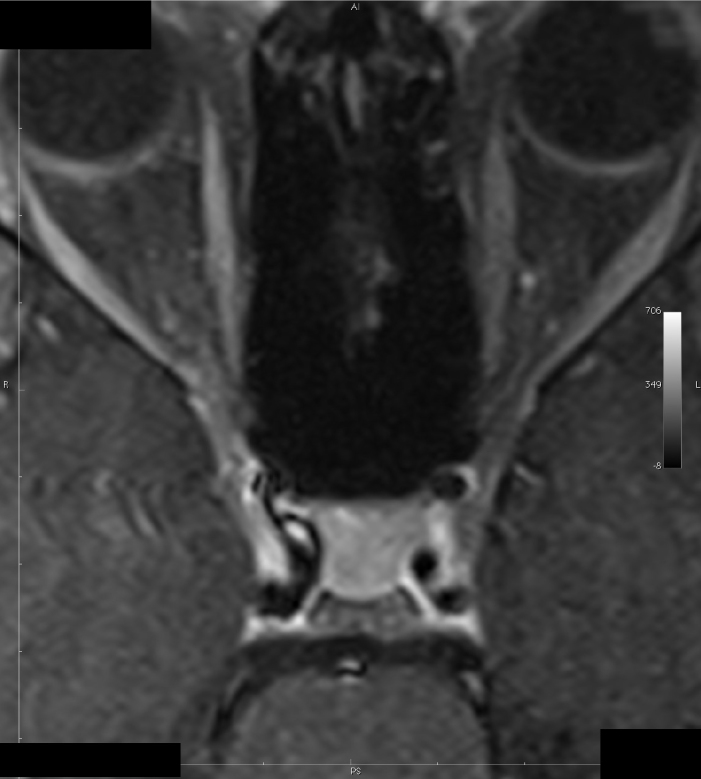
Patient 5 (after corticosteroid treatment): Post-gadolinium spin echo T1 weighted (TR/TE/FA:473/7.7/90) axial MRI
Of the five cases that were found to have recurrence, three patients had been misdiagnosed and thus did not receive steroid treatment and two patients did not use maintenance treatment. Recurrence occurred on the same side in four of the cases, while it occurred on the opposite side in one of the patients, and except for one patient, involvement of different nerves was found in case of recurrence. Contralateral cranial nerve paralysis developed in patient 4 at four months after the first attack (Table 1).
DISCUSSION
Among our cases with a diagnosis of Tolosa-Hunt Syndrome, there were patients in early and advanced ages. The youngest case was 25 years old, while the oldest case was 69 years old. Their follow-up periods varied between 9 months and 65 months. A total of 12 attacks were observed in seven cases, and in the attacks, the most affected cranial nerves were the ophthalmic branch of the fifth, sixth, fourth cranial nerve and the optical nerve.
Case number 2 had used antibiotherapy with a diagnosis of cavernous sinus infection and was assessed again a month later with a diagnosis of painful ophthalmoplegia. Lumbar puncture showed no features, and MRI performed according to the Tolosa-Hunt protocol showed a lesion in the left cavernous sinus. After correct diagnoses were made in cases that were reevaluated (lumbar punction, Tolosa-Hunt protocol, thorax CT, brain neck angiography, vasculitis, and rheumatic blood tests) after relapse, oral steroid treatment was started and maintenance treatment was extended to 5–6 months.
Routine conventional MRIs of all the cases were normal, while contrasted dynamic MRI showed cavernous sinus lesions in six cases and cavernous out-of-sinus lesions in one patient. Contrast involvement was found in the identified lesions of our cases. In addition, in the first attack, the right cavernous sinus was involved in five of the patients, while the left cavernous sinus was involved in two of the patients. The smallest lesion in the cavernous sinus was 4×5 mm, and the largest was 6×18 mm.
In our cases, the period between the onset of headache and the cranial nerve being affected varied between 1 and 14 days, and a decrease was seen in the severity of the headache following the cranial nerve paralysis. When steroid therapy was started, headache was found to disappear completely within the first 72 hours.
Although recurrence in THS patients generally occurred at the same side, it might occasionally be seen on the contralateral side. Contralateral cranial nerve paralysis developed in patient 4 at four months after the first attack. This condition might be explained by the high invasion potential of the non-specific granulomatous inflammation located in the cavernous sinus, superior orbital fissure, or orbital apex. Bilateral involvement is rare in recurrence (6). Patient 4, who had isolated trochlear nerve paralysis, is the fifth case reported in the English literature. Because the trochlear nerve lies in close vicinity to the oculomotor nerve at the lateral wall of the cavernous sinus, these two cranial nerves are generally involved together.
Performing orbital venography is not mandatory for the diagnosis of THS. On the other hand, other causes of painful ophthalmoplegia, including lymphoma, menengioma, craniopharyngioma, paracellar epidermoid cyst, caroticocavernous fistule, thrombosis of the cavernous sinus, giant cell arteritis, systemic lupus erythematosus, diabetic ophthalmoplegia, and ophthalmoplegic migraine, should be excluded by the appropriate methods (10,11,12,13). Also, meningioma and diabetic ophthalmoplegia do not respond to steroid treatment. Orbital myositis, thyroid ophthalmopathy presenting with isolated trochlear nerve paralysis, cycsticercosis, multiple sclerosis, Lyme disease, and herpes zoster ophthalmicus should also be excluded in these patients (14,15,16,17).
A classical MRI finding in THS is the enlargement of the cavernous sinus. The granulomatous lesion seems isointense in T1 sequences and iso-hypointense in T2 sequences and shows significant contrast involvement after the intravenous injection of the contrast material, but MRI might also be normal in many patients who have the clinical characteristics of THS (18,19,20). We observed in our patients that if the MRI was normal or if the lesions in the MRI were small, recurrence was rare.
Standard MR techniques were 5–6 mm, and dense section examinations were 3–4 mm. Lesions may not have been seen in patients with a diagnosis of THS because they might have been small. In a series of THS cases reported by Jain et al., a case with a granulomatous lesion of 3×3 mm was reported (21).
This condition may be explained by the failure of the conventional MRI techniques for the differentiation of the abnormal soft tissue lesions in the venous spaces of the cavernous sinus (9,22,23). Dynamic contrast enhances the probability of defining the intracavernous lesions (24).
An angiographic procedure is necessary for the diagnosis of THS and to exclude aneurysms of the intracavernous carotid artery and posterior cerebral artery (6). All CT angiographies in our cases were normal.
Idiopathic orbital pseudotumor is characterized by the presence of non-granulomatous inflammation in the eye without a known systemic or local reason. Sudden pain, eyelid swelling and redness, diplopia, and decrease in visual acuity may be seen in acute forms (25). If lacrimal gland involvement is not significant in MRI, it is possible to misdiagnose the condition as THS. Also, THS may be a variant of the orbital pseudotumor, and these two diseases may be thought of as the different presentations of the same pathology (24). Periorbital pain due to THS responds quickly and dramatically to corticosteroids, but it should not be forgotten that a good response to corticosteroids may also be observed in lymphoma, vasculitis, and paracellar epidermoid tumors (6,18).
Diabetic ophthalmoplegia, giant cell arteritis, and ophthalmoplegic migraine are the most challenging conditions for the clinician from a differential diagnosis point of view. The healing of the cranial nerve paralysis within 3 months is almost a rule for diabetic ophthalmoplegia (26,27). Absence of response to corticosteroids in diabetic ophthalmoplegia is a differential property. Because the giant cell arteritis may cause a significant diagnostic problem among older THS patients, pathological studies may be required for these patients (6,28). Erythrocyte sedimentation rate may be increased in both conditions. Ophthalmoplegic migraine is typically seen among children or young adults (29,30,31). If there is a rapid progression in the neurological findings, there is no response to steroids, and the lesions in the MRI do not heal within 3 months, malignant diseases should be suspected.
Because Tolosa-Hunt Syndrome rarely causes sequelae and because it shows a course with a good prognosis in general and limits itself, it does not receive much attention. Because most of the cases are not diagnosed or are misdiagnosed, the frequency of THS in the literature has been reported to be low.
Cranial MRI with contrast enhancement is the cornerstone of THS diagnosis because the biopsy for definitive diagnosis of THS is technically very difficult and is only used very rarely. On the other hand, it should be noted in the diagnostic criteria for THS that conventional MRI may be insufficient to show the granulomatous inflammation. The MRI method referred to as the Tolosa-Hunt protocol should be defined better, and the importance of dynamic MRI should be emphasized.
Footnotes
Ethics Committee Approval: Authors declared that the research was conducted according to the principles of the World Medical Association Declaration of Helsinki “Ethical Principles for Medical Research Involving Human Subjects” (amended in October 2013).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - Ç.K.A., T.Ö.; Design - Ç.K.A., T.Ö.; Supervision - Ç.K.A., T.Ö.; Resource - Ç.K.A., T.Ö.; Materials Ç.K.A.; Data Collection and/or Processing - Ç.K.A.; Analysis and/or Interpretation - Ç.K.A., T.Ö.; Literature Search - Ç.K.A., T.Ö.; Writing - Ç.K.A., T.Ö.; Critical Reviews - T.Ö.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support..
REFERENCES
- 1.Tolosa E. Periarteritic lesions of the carotid siphon with the clinical features of a carotid infraclinoid aneurysm. J Neurol Neurosurg Psychiatry. 1954;17:300–302. doi: 10.1136/jnnp.17.4.300. https://doi.org/10.1136/jnnp.17.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunt WE, Meagher JN, Lefever HE, Zeman W. Painful opthalmoplegia: its relation to indolent inflammation of the carvernous sinus. Neurology. 1961;11:56–62. doi: 10.1212/wnl.11.1.56. https://doi.org/10.1212/WNL.11.1.56. [DOI] [PubMed] [Google Scholar]
- 3.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version) Cephalalgia. 2013;33:629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 4.Gladstone JP, Dodick DW. Painful ophtalmoplegia: overview with a focus on Tolosa-Hunt syndrome. Curr Pain Headache Rep. 2004;8:321–329. doi: 10.1007/s11916-004-0016-x. https://doi.org/10.1007/s11916-004-0016-x. [DOI] [PubMed] [Google Scholar]
- 5.Iaconetta G, Stella L, Esposito M, Cappabianca P. Tolosa-Hunt syndrome extending in the cerebello-pontine angle. Cephalalgia. 2005;25:746. doi: 10.1111/j.1468-2982.2005.00924.x. https://doi.org/10.1111/j.1468-2982.2005.00924.x. [DOI] [PubMed] [Google Scholar]
- 6.Kline LB, Hoyt WF. The Tolosa-Hunt syndrome. J Neurol Neurosurg Psychiatry. 2001;71:577–582. doi: 10.1136/jnnp.71.5.577. https://doi.org/10.1136/jnnp.71.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colnaghi S, Versino M, Marchioni E, Pichiecchio A, Bastianello S, Cosi V, Nappi G. ICHD-II diagnostic criteria for Tolosa-Hunt syndrome in idiopathic inflammatory syndromes of the orbit and/or the cavernous sinus. Cephalalgia. 2008;28:577–584. doi: 10.1111/j.1468-2982.2008.01569.x. https://doi.org/10.1111/j.1468-2982.2008.01569.x. [DOI] [PubMed] [Google Scholar]
- 8.Shindler KS. Tolosa-Hunt syndrome. www.uptodate.com Section ed: Brazis PW, 2012 UpToDate.
- 9.Gencer M, Türkoğlu R, Çetinkaya Y, Tutkavul K, Yüksel G, Tireli H. Tolosa-Hunt Syndrome: clinical and radiological follow-up. Arch Neuropsychiatry. 2002;39:108–112. [Google Scholar]
- 10.Attout H, Rahmeh F, Ziegler F. Cavernous sinus lymphoma mimicking Tolosa-Hunt syndrome. Rev Med Interne. 2000;21:795–798. doi: 10.1016/s0248-8663(00)00226-5. https://doi.org/10.1016/S0248-8663(00)00226-5. [DOI] [PubMed] [Google Scholar]
- 11.Esmaeli B, Ginsberg L, Goepfert H, Deavers M. Squamous cell carcinoma with perineural invasion presenting as a Tolosa-Hunt like syndrome: a potential pitfall in diagnosis. Ophthal Plast Reconstr Surg. 2000;16:450–452. doi: 10.1097/00002341-200011000-00009. https://doi.org/10.1097/00002341-200011000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Tatagiba M, Iaconetta G, Samii M. Epidermoid cyst of the cavernous sinus: clinical features, pathogenesis and treatment. Br J Neurosurg. 2000;14:571–575. doi: 10.1080/02688690050206747. https://doi.org/10.1080/02688690050206747. [DOI] [PubMed] [Google Scholar]
- 13.Calistri V, Mostardini C, Pantano P, Pierallini A, Colonnese C, Caramia F. Tolosa-Hunt syndrome in a patient with systemic lupus erythematosus. Eur Radiol. 2001;12:341–344. doi: 10.1007/s003300100960. https://doi.org/10.1007/s003300100960. [DOI] [PubMed] [Google Scholar]
- 14.Moorman CM, Elston JS. Acute orbital myositis. Eye. 1995;9:96–101. doi: 10.1038/eye.1995.15. https://doi.org/10.1038/eye.1995.15. [DOI] [PubMed] [Google Scholar]
- 15.Kau HC, Kao SC, Peng CH, Hsu WM, Tsai CC. Methylprednisolone pulse therapy in patient with isolated superior oblique myositis. Eye. 2005;20:1106–1109. doi: 10.1038/sj.eye.6702145. https://doi.org/10.1038/sj.eye.6702145. [DOI] [PubMed] [Google Scholar]
- 16.Moster ML, Bosley TM, Slavin ML, Rubin SE. Thyroid ophthalmopathy presenting as superior oblique paresis. J Neuroophthalmol. 1992;12:94–97. [PubMed] [Google Scholar]
- 17.Tsuda H, Ito T, Yoshioka M, Ishihara N, Sekine Y. Isolated trochlear nerve palsy in herpes zoster ophthalmicus. Interne Med. 2006;46:535–536. doi: 10.2169/internalmedicine.46.6373. https://doi.org/10.2169/internalmedicine.46.6373. [DOI] [PubMed] [Google Scholar]
- 18.Le Mantia L, Curone M, Rapoport AM, Bussone G. Tolosa Hunt syndrome: critical literature review based on IHS 2004 criteria. Cephalalgia. 2006;26:772–781. doi: 10.1111/j.1468-2982.2006.01115.x. https://doi.org/10.1111/j.1468-2982.2006.01115.x. [DOI] [PubMed] [Google Scholar]
- 19.Schuknecht B, Sturm V, Huisman TAGM, Landau K. Tolosa-Hunt syndrome: MR imaging features in 15 patients with 20 episodes of painful ophthalmoplegia. Eur J Radiol. 2009;69:445–453. doi: 10.1016/j.ejrad.2007.11.034. https://doi.org/10.1016/j.ejrad.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 20.Jimenez-Cabellero PE, Florensa J, Marsal-Alanso C, Alvarez-Tejerina A. Recurrent Tolosa-Hunt syndrome with normal neuroimaging. Rev Neurol. 2005;41:30–33. [PubMed] [Google Scholar]
- 21.Jain R, Sawhney S, Koul RL, Chand P. Tolosa-Hunt syndrome: MRI appearances. Journal of Medical Imaging and Radiation Oncology. 2008;52:447–451. doi: 10.1111/j.1440-1673.2008.01988.x. https://doi.org/10.1111/j.1440-1673.2008.01988.x. [DOI] [PubMed] [Google Scholar]
- 22.Pascual J, Cerezal L, Canga A, Alvarez de Arcaya A, Polo JM, Berciano J. Tolosa-Hunt syndrome: focus on MRI diagnosis. Cephalalgia. 1999;19:36–38. doi: 10.1177/0333102499019s2509. https://doi.org/10.1177/0333102499019S2509. [DOI] [PubMed] [Google Scholar]
- 23.de Arcaya AA, Cerezal L, Canga A, Polo JM, Berciano J, Pascual J. Neuroimaging diagnosis of Tolosa-Hunt syndrome: MRI contribution. Headache. 1999;39:321–325. doi: 10.1046/j.1526-4610.1999.3905321.x. https://doi.org/10.1046/j.1526-4610.1999.3905321.x. [DOI] [PubMed] [Google Scholar]
- 24.Schuknecht B, Sturm V, Huisman TAGM, Landau K. Tolosa-Hunt syndrome: MR imaging features in 15 patients with 20 episodes of painful ophthalmoplegia. Eur J Radiol. 2009;69:445–453. doi: 10.1016/j.ejrad.2007.11.034. https://doi.org/10.1016/j.ejrad.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 25.Weber AL, Romo LV, Sabates NR. Pseudotumor of the orbit. Clinical, pathologic, and radiologic evaluation. Imaging in ophthalmology II. Radiol Clin N Am. 1999;37:151–168. doi: 10.1016/s0033-8389(05)70084-1. https://doi.org/10.1016/S0033-8389(05)70084-1. [DOI] [PubMed] [Google Scholar]
- 26.Larson DL, Auchincloss JH. Multiple symmetric bilateral cranial nerve palsies in patients with unregulated diabetes mellitus: report of three cases. Ann Intern Med. 1950;85:265–271. doi: 10.1001/archinte.1950.00230080069003. https://doi.org/10.1001/archinte.1950.00230080069003. [DOI] [PubMed] [Google Scholar]
- 27.Eshbaugh CG, Siarkowski RM, Smith JL, Kline LB. Simultaneous, multiple cranial neuropathies in diabetes mellitus. J Neuroophtalmol. 1995;15:219–224. https://doi.org/10.1097/00041327-199512000-00004. [PubMed] [Google Scholar]
- 28.Barricks ME, Traviesa DB, Glaser JS, Levy IS. Ophtalmoplegia in cranial arteritis. Brain. 1977;100:209–221. doi: 10.1093/brain/100.2.209. https://doi.org/10.1093/brain/100.2.209. [DOI] [PubMed] [Google Scholar]
- 29.Mark AS, Blake P, Atlas SW, Ross M, Brown D, Kolsky M. Gd-DTPA enhancement of the cisternal portion of the oculomotor nerve on MR imaging. AJNR AJ Neuroradiol. 1992;13:1463–1470. [PMC free article] [PubMed] [Google Scholar]
- 30.Stommel EW, Ward TN, Harris RD. MRI findings in a case of ophtalmoplegic migraine. Headache. 1993;33:234–237. doi: 10.1111/j.1526-4610.1993.hed3305234.x. https://doi.org/10.1111/j.1526-4610.1993.hed3305234.x. [DOI] [PubMed] [Google Scholar]
- 31.Ostergaard JR, Moller HV, Christensen T. Recurrent ophtalmoplegia in childhood: diagnostic and etiologic considerations. Cephalalgia. 1996;17:70–73. doi: 10.1046/j.1468-2982.1996.1604276.x. [DOI] [PubMed] [Google Scholar]


