Abstract
Inflammation is a key pathogenic factor in age-related macular degeneration (AMD). However, the clinical importance of combining anti-VEGF agents and topical NSAIDs to reduce inflammation remains unclear. In this study, we systematically reviewed clinical trials comparing combined treatment versus anti-VEGF alone in AMD patients. We quantified treatment effects via meta-analysis. The pooled weighted mean difference (WMD, -0.91, 95%CI: -1.39 to -0.42, P = 0.0003) demonstrates that combined treatment may reduce required anti-VEGF injection number, probably by means of decreasing central retina thickness (CRT) (WMD = -22.9, 95% CI: -41.20 to -4.59, P = 0.01). The best corrected visual acuity (BCVA) did not change significantly between these two groups (WMD = - 0.01, 95%CI: -0.23 to 0.20, P = 0.90). Topical NSAIDs slightly increased the incidence of foreign body sensation (Odds Ratio [OR] = 2.63, 95%Cl: 1.06 to 6.52, P = 0.76). Combining topical NSAIDs and anti-VEGF agents may provide a new strategy for AMD treatment.
Introduction
Age-related macular degeneration (AMD) is the third leading cause of blindness worldwide, after cataracts and glaucoma [1] The central vision lost as a result of AMD is irreversible and permanent. AMD produces suffering for the patient and presents a heavy financial burden both to the family of the patient and to society as well. Various factors are involved in the pathogenesis of neovascular AMD (nAMD, an AMD subtype), including aging, oxidative stress, genetic factors, inflammation, and increased vascular endothelial growth factor (VEGF). It appears that VEGF is important in the pathogenesis of AMD. Anti-VEGF treatments, including Ranibizumab, Bevacizumab, and Aflibercept, have shown significant benefit in AMD.
However, some patients respond slowly or poorly to anti-VEGF treatment, suggesting that other pathogenic factors should also be considered as treatment targets. Since various studies demonstrate the role of inflammation in the pathogenesis of neovascularization, it is compelling to consider anti-inflammatories as adjunctive therapy [2][3][4]. Unlike intraocular steroids, which carry with them substantial side-effects [5], topical nonsteroidal anti-inflammatory agents (NSAIDs) offer simple, safe, and effective means to reduce inflammation through prevention of prostaglandin synthesis via cyclooxygenase inhibition.
There has been considerable evidence for the effectiveness of topical NSAIDs in preventing cystoid macular edema after cataract surgery [6]. However, the literature is mixed regarding the effect of NSAIDs in nAMD. Several studies have compared the number of anti-VEGF intravitreal injections needed, best corrected visual acuity (BCVA), central retina thickness (CRT) and side effects following anti-VEGF treatment combined with or without NSAIDs [7][8][9][10]. However, the sample sizes were small, casting doubt on the validity of the studies. Here, we perform a meta-analysis of clinical trials, summarizing the effects of combining topical NSAIDs and anti-VEGF in nAMD patients.
Methods
This study was performed according PRISMA checklist guidelines, and is registered in PROSPERO, number CRD42016039935.
Literature search
A systematic literature review was performed to identify relevant articles comparing anti-VEGF agents combined with topical NSAIDS and anti-VEGF alone for the treatment of nAMD from inception to December 2016. Two independent reviewers (SL, AH), searched electronic databases including PubMed, EMBASE and the Cochrane Central Register of Controlled Trials. Following key words were used: “anti-VEGF OR ranibizumab OR bevacizumab OR Aflibercept OR Lucentis OR Avastin OR Eylea” AND “macular degeneration OR AMD OR ARMD” AND “anti-inflammatory agents non-steroidal OR NSAIDs OR bromfenac OR diclofenac OR ketorolac OR nepafenac”. No language restrictions were set for the search. References of included studies and major reviews were searched manually for additional eligible studies. Grey articles were searched from the OpenGrey website and Google Scholar. After excluding duplications derived from different sources, article titles and abstracts were evaluated by two independent researchers to exclude case reports, cross-sectional studies, retrospective studies and unrelated articles. The full texts of remaining articles were examined for eligibility. Disagreements were settled by consensus or a third senior reviewer (XD) if needed.
The following including criteria were used to check study eligibility: (1) Design: prospective RCT or quasi-RCT; (2) Patients: treated or naïve wet AMD requiring anti-VEGF therapy; (3) Intervention and control: anti-VEGF combined with topical NSAIDS (study group) versus anti-VEGF alone (control group); (4) Outcomes: at least one of the following: injection number of anti-VEGF, best corrected visual acuity (BCVA), and central retinal thickness (CRT); (5) Other: a minimum follow-up of 6 months. Studies were excluded if any of the following conditions existed: (1) retrospective cohort study, case control study, or cross sectional study; (2) studies with inadequate information for calculating data on outcomes; (3) duplicated report; (4) study with a sample size smaller than 10 eyes in each arm; (6) mean observation period shorter than 6 months. If multiple publications on the overlapping population were available, the most comprehensive one was included in the meta-analysis. If multiple studies by the same team derived from different patients were available, all of them were deemed eligible.
Data extraction
Data were extracted from each study by two independent reviewers (SL, AH) with a standardized form, including first author name, publication time, patient and study characteristics, follow-up duration, sample size, type of anti-VEGF, method of anti-VEGF treatment, type of NSAID, method of NSAID treatment, injection number of anti-VEGF, BCVA at the end point, CRT at the end point, number of patients with adverse effects of NSAID treatment. All BCVA values were transferred into the log minimum angle of resolution (LogMAR) for analysis. Additional data and information were requested and obtained from authors through email contacts if data or information were not explicitly presented in the articles. Any discrepancies in data extraction were resolved via re-assessment and discussion with the third reviewer (XD).
Evaluation of risk of bias
Risk of bias of each included study was evaluated using the Cochrane risk of bias tool, as described in the Cochrane systematic review of interventions manuals. This tool evaluated the risk of bias in seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias. The risk of bias in each domain was labeled as low, unclear or high risk for each study by two authors (SL and AH) independently. Disagreements were resolved as described above.
Statistical analysis
Meta-analysis was performed using RevMan (version5.2.3). Weight mean differences (WMD) were used for continuous variables, and odds ratios (OR) were used for dichotomous variables. Pooling estimates and their 95% confidential intervals (95%CI) were calculated. The random-effects model was used to calculate pooling estimates when significant inter-study heterogeneity existed. Otherwise, the fixed-effects model was adopted. Forest plots were produced for a clearer visualization. Statistical heterogeneity among studies was examined using the Cochran Q test, with a P < 0.10 indicating significant heterogeneity. Heterogeneity among studies also was quantified using the I2 statistic, which represents the percentage of the total variation among studies. An I2 value of 25%-50%, 50%-75%, > 75% was defined as low, moderate, and severe inter-study heterogeneity, respectively. Analyses were stratified by study design, types of topical NSAIDs, types of anti-VEGF agents, and follow-up time. One-way sensitivity analyses were performed by recombining the studies after deleting one study at a time sequentially. Potential publication bias was assessed by examining the symmetry of funnel plots and Egger’s test if more than 10 studies were included.
Results
Literature search
The search process is illustrated as a flow diagram in Fig 1. Initially, a total of 105 articles were retrieved from databases. No related grey articles were found. After removing 34 duplicates, the titles and abstracts of 71 potential relevant articles were reviewed. Among the 71 papers, 59 were excluded because of un-relevant topics. Full texts of the remaining 12 publications were assessed in their entirety. Six were excluded as they were case reports or case series (two), editorials or comments (one), or retrospective studies (three). The remaining six studies, including two quasi-RCTs and four RCTs, were included in this meta-analysis.
Fig 1. Flow diagram for literature searching and inclusion.
Characteristics of included studies
Details of the features of included studies are shown in Table 1. Four RCTs and two quasi-RCTs were included in the mata-analysis, involving a total of 278 patients (142 in study group and 136 in control group). All studies were published between 2012 and 2015. Two studies were conducted in Italy, two in Poland, and the other two in the United States and Japan, respectively. Each study compared the BCVA and/or central retinal thickness (CRT) folloiwng combined treatment with topical NSAIDs and anti-VEGF or anti-VEGF alone in nAMD patients. Five papers reported the injection number of anti-VEGF needed with or without topical NSAIDs. One of these did not provide the standard deviation and was therefore excluded meta-analysis. Follow up duration for all studies were between 6 months to 12 months. Of the 278 eyes, 172 (62%) in four trials received ranibizumab as the anti-VEGF agent, 54 eyes (19%) in one trial received aflibercept, and 52 eyes (19%) in another trial received bevacizumab as anti-VEGF agent. Three Studies looked at treatment with anti-VEGF using the 3 + PRN protocol, two studies used the 4 + PRN protocol and one used the 1 + PRN protocol. Bromfenac was used in four studies, including 89 eyes (63%). Ketorolac was employed in two trials, including 53 eyes (37%). Adverse effects of topical NSAIDs were assessed in three trials.
Table 1. Baseline characteristics of the included studies.
| Study | Design | Country | Patients | No. of patients | Mean age | Anti-VEGF | NSAIDs | Follow-up | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Flaxel et al., 2012[10] | RCT | United States | New or recurrent exudative or neovascular AMD | 20/10 | 85.5/77.5 | Ranibizumab (4+PRN) | Bromfenac (1 drop twice daily for 12 months) | 12 | BCVA, CRT |
| Gomi et al., 2012[8] | RCT | Japan | nAMD with lesions smaller than 2 disk diameters | 16/22 | 75/74.4 | Ranibizumab (1+PRN) | Bromfenac (1 drop twice daily for 6 months) | 6 | CVA, CRT, No.of injection |
| Russo et al., 2013[11] | RCT | Italy | New neovascular AMD | 28/26 | 76/77.8 | Ranibizumab (3+PRN) | Ketorolac (1 drop three times a day for 6 months) | 6 | CVA, CRT, No.of injection |
| Wyględowska-Promieńska et al., 2014[12] | Quasi-RCT | Poland | Exudative AMD | 26/26 | 72.4/72.3 | Bevacizumab (3+PRN) | Bromfenac (1 drop twice daily for 3 months) | 8 | CVA, CRT, No.of injection |
| Semeraro et al., 2015[13] | RCT | Italy | Naïve eyes affected by neovascular AMD | 25/25 | 76.3/77.2 | Ranibizumab (3+PRN) | Ketorolac (1 drop three times a day for 12 months) | 12 | CVA, CRT, No.of injection |
| Wyględowska-Promieńska et al., 2015[14] | Quasi-RCT | Poland | Exudative AMD | 27/27 | 72.3/72.8 | Aflibercept (4+PRN) | Bromfenac (1 drop twice daily for 3 months) | 8 | BCVA, CRT |
Risk of bias of the included studies
Study quality was assessed using the Cochrane Risk of Bias Tool for RCTs, which classify the involved trials to low risk, unclear risk and high risk (Table 2).
Table 2. Assessment of risk of bias of the included studies.
| Domain | Flaxel et al. 2012 | Gomi et al. 2012 | Russo et al. 2013 | Wyględowska-Promieńska et al., 2014 | Semeraro et al. 2015 | Wyględowska-Promieńska et al. 2015 |
|---|---|---|---|---|---|---|
| Random sequence generation | Low risk | Low risk | Low risk | High risk | Low risk | High risk |
| Allocation concealment | Unclear | Unclear | Unclear | High risk | Unclear | High risk |
| Blinding for visual acuity | ||||||
| Participants and personnel | High risk | Low risk | High risk | High risk | High risk | High risk |
| Outcome assessment | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Blinding for other outcomes | ||||||
| Participants and personnel | High risk | Low risk | High risk | High risk | High risk | High risk |
| Outcome assessment | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Incomplete outcome data | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Selective reporting | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Other bias | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
Results of meta-analysis
Four studies compared the mean injection numbers between treatment and control group. Pooling results showed that combined topical NSAIDs with anti-VEGF was associated with fewer anti-VEGF injections (WMD = -0.91, 95%CI: -1.39 to -0.42, P = 0.0003, Fig 2A). Because severe heterogeneity among studies was observed (P = 0.008, I2 = 75%), the random effect model was adopted. Subgroup studies were assessed according to type of topical NSAID, anti-VEGF, and duration of follow-up (Fig 2B). Regardless of anti-VEGF agent used, combined treatment decreased the number of anti-VEGF treatments required. This trend is more significant with follow-up duration greater than 6 months. However, only bromfenec demonstrated a statistically-significant reduction of anti-VEGF injection number.
Fig 2. Forest plot showing the weighted mean difference of required anti-VEGF injections, comparing combined treatment and anti-VEGF alone.
A. Pooled data computed using the random effects model. B. Data was grouped by type of NSAIDs (bromfenac and ketorolac), type of anti-VEGF (ranibizumab and bevacizumab) and follow-up duration (6 months and greater than 6 months).
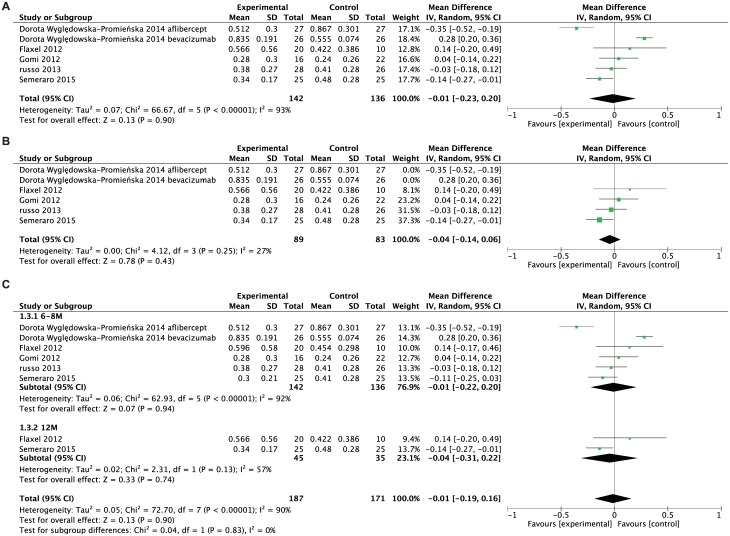
To evaluate the clinical treatment effect of the combined strategy, we analyzed the possibility of improving visual acuity in these two groups. The mean BCVA (logMAR) at final follow-up in the combined treatment group and anti-VEGF alone group were not statistically significant (Fig 3A), with the WMD = - 0.01, 95%CI: -0.23 to 0.20, P = 0.90. Since we detected severe heterogeneities among studies (P < 0.00001, I2 = 93%), a subgroup analysis was performed to identify the source of heterogeneity. The BCVAs from two quasi-RCTs were strongly different from other studies in the forest plot. Therefore, the two quasi-RCTs were excluded from the analysis owing to differences in study design. After removing quasi-RCTs, the heterogeneity decreased but yet failed to detect significant change (P = 0.25, I2 = 27%, Fig 3B). A grouping was also examined with respect to follow-up duration. This also failed to show any difference between the two groups (Fig 3C).
Fig 3. Forest plot showing weighted mean difference of BCVA comparing combined treatment and anti-VEGF alone.
A. Pooled data computed the BCVA at the end point between study and control group by using the random effects model. B. Pooled data showed the results did not change after removing two quasi-RCTs. C. Data was grouped by follow-up duration (6–8 months and 12 months).
In addition to BCVA, CRT is crucial to assessment of treatment effectiveness. Fig 4 shows mean CRT at final follow-up in study group and control group. Combining topical NSAIDs with anti-VEGF may reduce the CRT significantly (followed up from 6 months to 12 months), with WMD of -22.9, 95% CI: -41.20 to -4.59, P = 0.01. Heterogeneity between studies was 62% (I2). Therefore, the random effect model was used.
Fig 4. Forest plot showing weighted mean difference of CRT comparing combined treatment and anti-VEGF alone using the random effects model.
The adverse effects of topical NSAIDs in both groups were analyzed. Common adverse effects include abnormal sensation, stinging, pain, itchy eye, headache, sore eyelid, foreign body sensation, viral conjunctivitis, dry eye, eye strain, light sensitivity and floaters. Of these, only foreign body sensation significantly increased with topical NSAIDs (odds ratio [OR] = 2.63, 95%Cl: 1.06 to 6.52, P = 0.76, Fig 5). Heterogeneity between studies was 0% (I2) in a fixed-effects model. Pooled estimates of other adverse effects are summarized in Table 3.
Fig 5. Forest plot showing the odds ratio of foreign body sensation after combined treatment or anti-VEGF alone using a fixed-effects model.
Table 3. Pooling estimates of adverse effect of NSAIDs.
| Side effect | OR | 95%Cl | P | I2(Fixed) | Studies |
|---|---|---|---|---|---|
| Abnormal sensation | 1.01 | 0.41–2.46 | 0.95 | 0% | 3 |
| Stinging | 1.90 | 0.93–3.88 | 0.90 | 0% | 3 |
| Pain | 0.67 | 0.27–1.70 | 0.67 | 0% | 3 |
| Itchy eye | 1.00 | 0.45–2.20 | 0.46 | 0% | 3 |
| Headache | 0.86 | 0.38–1.93 | 0.78 | 0% | 3 |
| Sore eyelid | 0.68 | 0.18–2.53 | 0.82 | 0% | 3 |
| Foreign body sensation | 2.63 | 1.06–6.52 | 0.76 | 0% | 3 |
| Viral conjunctivitis | 0.31 | 0.06–1.66 | 0.86 | 0% | 3 |
| Dry eye | 2.84 | 0.12–64.87 | 0.93 | 0% | 3 |
| Eye strain | 1.37 | 0.56–3.36 | 0.97 | 0% | 3 |
| Light sensitivity | 0.64 | 0.29–1.45 | 0.58 | 0% | 3 |
| Floaters | 0.90 | 0.43–1.90 | 0.50 | 0% | 3 |
A sensitivity analysis was performed by omitting one study at a time and re-examining remaining data. No study had a significant effect on pooled data (not shown). As only six studies were included in the meta-analysis, potential publication bias was not examined.
Discussion
Anti-VEGF is currently the first line therapy for nAMD. Recently, however, increasing attention is being paid to alternative strategies, including combination with anti-inflammatory agents. Topical NSAIDs can achieve effective concentrations in the vitreous body with limited side-effects [15]. Although conflicting results have been reported in early retrospective studies, recent clinical trials demonstrated a beneficial trend of combining topical NSAIDs and anti-VEGF compared with anti-VEGF alone. Because of small sample sizes and mild measured effects, these studies failed to demonstrate statistical significance. Prior to carrying out a large-scale clinical trial, a meta-analysis pooling data from multiple studies may provide important insights into combination therapy.
In our meta-analysis, we included four well-designed RCTs and two quasi-RCTs, focusing on number of anti-VEGF treatment required, BCVA, CRT following treatment, and side-effects of topical NSAIDs. We found that addition of topical NSAIDs leads to a mild but stable reduction in the number of required anti-VEGF injections. This suggests use of NSAIDs may strengthen or maintain the effect of anti-VEGF. This effect is more pronounced at one-year follow up. Though topical NSAIDs show no effect on BCVA, we clearly observed a benefit on decreasing central retinal thickness. This suggests that topical NSAIDs may aid in decreasing retinal edema and sub-retinal fluid absorption. Only bromfenec but not ketorolac demonstrated a statistically-significant reduction of anti-VEGF injection number. More clinical trials may need to conclude the effect of ketorolac in decreasing the required anti-VEGF treatment. Mechanism of bromfenec and ketorolac are slightly different. Bromfenac is more selective to COX-2 while ketorolac being more selective to COX-1, which may be associated with the different therapeutic effects. [16].
A key strength of this meta-analysis is that all including studies are clinical trials. Although some are pilot studies, all are well-designed. However, several limitations should be taken in account. First, all included studies have small number of participants, lowering the power of the analysis. Nevertheless, the evidence level of this meta-analysis is higher compared with single studies. Second, the number of included studies is too small to carry out publication bias testing. In the study, related unpublished results were not found. An update of this meta-analysis may be necessary as further RCTs are published. Two of the included studies are quasi-RCTs, which do not have a trusted randomization process. In this analysis, we used a sensitivity test and found that excluding any study did not affect the final result. Finally, follow-up duration of all included studies is up to one year. Longer term studies are needed to establish the effect of sustained combination treatment.
Conclusion
Combining topical NSAIDs with intravitreal anti-VEGF results in a small but statistically significant reduction in required anti-VEGF injections and central retinal thickness. BCVA was not improved significantly. No additional side effects were observed apart from foreign body sensation. Combining topical NSAIDs and anti-VEGF agents may serve as a new strategy in AMD treatment. Conclusions drawn from this meta-analysis should be interpreted cautiously owing to the limited number of participants in included studies.
Supporting information
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Friedman DS, O’Colmain BJ, Muñoz B, Tomany SC, McCarty C, de Jong PTVM, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol Chic Ill 1960. 2004. April;122(4):564–72. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Wang VM, Chan C-C. The role of anti-inflammatory agents in age-related macular degeneration (AMD) treatment. Eye. 2011. February;25(2):127–39. doi: 10.1038/eye.2010.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshinaga N, Arimura N, Otsuka H, Kawahara K, Hashiguchi T, Maruyama I, et al. NSAIDs inhibit neovascularization of choroid through HO-1-dependent pathway. Lab Invest. 2011. September;91(9):1277–90. doi: 10.1038/labinvest.2011.101 [DOI] [PubMed] [Google Scholar]
- 4.Augustin AJ, Kirchhof J. Inflammation and the pathogenesis of age-related macular degeneration. Expert Opin Ther Targets. 2009. June;13(6):641–51. doi: 10.1517/14728220902942322 [DOI] [PubMed] [Google Scholar]
- 5.Gillies MC, Simpson JM, Billson FA, et al. Safety of an intravitreal injection of triamcinolone: Results from a randomized clinical trial. Arch Ophthalmol. 2004. March 1;122(3):336–40. doi: 10.1001/archopht.122.3.336 [DOI] [PubMed] [Google Scholar]
- 6.Warren KA, Bahrani H, Fox JE. NSAIDs in combination therapy for the treatment of chronic pseudophakic cystoid macular edema. Retina Phila Pa. 2010. February;30(2):260–6. [DOI] [PubMed] [Google Scholar]
- 7.Zweifel SA, Engelbert M, Khan S, Freund KB. Retrospective review of the efficacy of topical bromfenac (0.09%) as an adjunctive therapy for patients with neovascular age-related macular degeneration. Retina Phila Pa. 2009. December;29(10):1527–31. [DOI] [PubMed] [Google Scholar]
- 8.Gomi F, Sawa M, Tsujikawa M, Nishida K. Topical bromfenac as an adjunctive treatment with intravitreal ranibizumab for exudative age-related macular degeneration. Retina Phila Pa. 2012. October;32(9):1804–10. [DOI] [PubMed] [Google Scholar]
- 9.Chen E, Benz MS, Fish RH, Brown DM, Wong TP, Kim RY, et al. Use of nepafenac (Nevanac®) in combination with intravitreal anti-VEGF agents in the treatment of recalcitrant exudative macular degeneration requiring monthly injections. Clin Ophthalmol Auckl NZ. 2010;4:1249–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flaxel C, Schain MB, Hamon SC, Francis PJ. Prospective randomized controlled trial of combination ranibizumab (Lucentis) and bromfenac (Xibrom) for neovascular age-related macular degeneration: a pilot study. Retina Phila Pa. 2012. March;32(3):417–23. [DOI] [PubMed] [Google Scholar]
- 11.Russo A, Costagliola C, Delcassi L, Romano MR, Semeraro F. A randomised controlled trial of ranibizumab with and without ketorolac eyedrops for exudative age-related macular degeneration. Br J Ophthalmol. 2013. October;97(10):1273–6. doi: 10.1136/bjophthalmol-2013-303417 [DOI] [PubMed] [Google Scholar]
- 12.Wyględowska-Promieńska D, Piotrowska-Gwóźdź A, Piotrowska-Seweryn A, Mazur-Piotrowska G, Rokicki W. Combination of bevacizumab and bromfenac therapy in age-related macular degeneration: a pilot study. Med Sci Monit Int Med J Exp Clin Res. 2014;20:1168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Semeraro F, Russo A, Delcassi L, Romano MR, Rinaldi M, Chiosi F, et al. TREATMENT OF EXUDATIVE AGE-RELATED MACULAR DEGENERATION WITH RANIBIZUMAB COMBINED WITH KETOROLAC EYEDROPS OR PHOTODYNAMIC THERAPY. Retina Phila Pa. 2015. August;35(8):1547–54. [DOI] [PubMed] [Google Scholar]
- 14.Wyględowska-Promieńska D, Piotrowska-Gwóźdź A, Piotrowska-Seweryn A, Mazur-Piotrowska G. Combination of Aflibercept and Bromfenac Therapy in Age-Related Macular Degeneration: A Pilot Study Aflibercept and Bromfenac in AMD. Med Sci Monit Int Med J Exp Clin Res. 2015;21:3906–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baklayan GA, Patterson HM, Song CK, Gow JA, McNamara TR. 24-hour evaluation of the ocular distribution of (14)C-labeled bromfenac following topical instillation into the eyes of New Zealand White rabbits. J Ocul Pharmacol Ther Off J Assoc Ocul Pharmacol Ther. 2008. August;24(4):392–8. [DOI] [PubMed] [Google Scholar]
- 16.Waterbury LD, Silliman D, Jolas T. Comparison of cyclooxygenase inhibitory activity and ocular anti-inflammatory effects of ketorolac tromethamine and bromfenac sodium. Curr Med Res Opin. 2006;22(6):1133–1140. doi: 10.1185/030079906X112471 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.