INTRODUCTION
More than 8 million patients with chest pain and/or anginal equivalent symptoms present to emergency departments (ED) each year, accounting for the second, most common cause of ED visits for adults.1 Cardiovascular emergencies account for approximately 10% of all ED visits.1,2 ED clinicians are required to rapidly differentiate between life-threatening conditions and non–life-threatening ones and accurately determine which course of treatment will result in optimal patient outcomes.3 Cardiac monitoring strategies, which include 12-lead electrocardiography (ECG) and bedside monitors, enable clinicians to detect arrhythmias, myocardial ischemia, and QT-interval measurements in real time.
Cardiac monitoring was first introduced nearly 60 years ago for critically ill patients, but today is used increasingly to monitor ED patients with a variety of conditions. Early monitoring focused on heart rate measurement and fatal arrhythmia detection.4 Today, monitoring has expanded to include diagnoses of complex arrhythmias, acute myocardial ischemia, and pharmacologically induced prolonged QT intervals.5 Emergency nurses are often the first care providers to evaluate patients presenting to the ED; therefore, they are pivotal in determining the urgency of initiating cardiac monitoring for risk stratification of patients arriving in the ED. Emergency nurses require ongoing education and training on equipment because cardiac monitoring technologies are evolving rapidly to meet the demands of complex, patient care. This paper describes current cardiac monitoring practices in an ED setting, with a primary focus on arrhythmia, myocardial ischemia, and QT-interval monitoring.
Cardiac monitoring is a useful, noninvasive diagnostic tool to monitor the wide array of patient conditions in the ED. To assist clinicians in determining which patients need monitoring, experts in electrocardiology and cardiac monitoring convened to develop practice standards for hospital ECG monitoring.4,6 These practice standards encompass all areas of hospital cardiac monitoring, including arrhythmia, myocardial ischemia, and QT interval monitoring. Guidelines reflect expert opinions based on clinical experience and research; however, data for best practices for hospital cardiac monitoring are limited.6 Conditions common to the ED setting and implications for cardiac monitoring are discussed; each is categorized by the rating system below, which was developed by the American College of Cardiology Emergency Cardiac Care Committee for cardiac monitoring.4
Class I: Cardiac monitoring is indicated in most, if not all, patients in this group.
Class II: Cardiac monitoring may be beneficial to some patients but not considered essential for all patients.
Class III: Cardiac monitoring is not indicated because a patient’s risk of a serious event is so low that monitoring has no therapeutic monitoring benefit.
ARRHYTHMIA MONITORING IN THE EMERGENCY DEPARTMENT
Arrhythmias frequently reflect underlying diseases and comorbidities, and are detected by clinicians as well as computer algorithms in cardiac monitors, which are set to trigger an alarm when a life-threatening arrhythmia is detected.7 Box 1 lists conditions for which arrhythmia monitoring may be beneficial (class II) or unnecessary (class III).4,6 However, ED patients at significant risk for immediate fatal arrhythmias, such as ventricular fibrillation (Fig. 1) or asystole, should receive continuous cardiac monitoring (class I).
Box 1. Recommendations for cardiac arrhythmia monitoring in the emergency department.
Class I conditions
Patients resuscitated from cardiac arrest.
Patients in the early phase of acute coronary syndrome.
Patients with newly diagnosed high-risk coronary lesions.
Patients after cardiac surgery.
Patients after implantation of automatic defibrillator or pacemaker lead who are pacemaker dependent.
Patients with temporary or transcutaneous pacemaker.
Patients with AV block (Wenckebach, Mobitz II, complete block, new-onset bundle branch block in the setting of myocardial infarction).
Patients with arrhythmia complicating Wolff–Parkinson–White syndrome with rapid conduction over an accessory pathway.
Patients with drug-induced long-QT syndrome.
Patients with acute heart failure, pulmonary edema.
Patients with major trauma, acute respiratory failure, sepsis, shock, pulmonary embolus, major noncardiac surgery, drug overdose, or other indications for intensive care.
Patients who require conscious sedation or anesthesia for diagnostic/therapeutic procedures.
Patients with any hemodynamically unstable arrhythmia.
Patients with syncope owing to underlying heart condition.
Pediatric patients diagnosed with any arrhythmia.
Class II conditions
Patients with subacute heart failure.
Patients with do not resuscitate orders.
Class III conditions
Patients with chronic, rate-controlled atrial fibrillation.
Obstetric patients, unless heart disease is present.
Adapted from Drew BJ, Califf RM, Funk M, et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation 2004;110(17):2721–46; and Drew BJ, Funk M. Practice standards for ECG monitoring in hospital settings: executive summary and guide for implementation. Crit Care Nurs Clin North Am 2006;18(2):157–68.
Fig. 1.
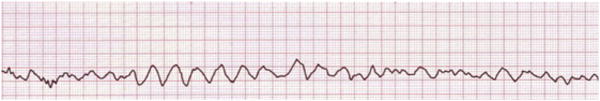
Example of ventricular fibrillation, a class I condition in arrhythmia monitoring.
Cardiac Arrest
Patients resuscitated from cardiac arrest should be monitored continuously for arrhythmias in the ED because they are at high risk for recurring arrhythmias. A cardiac monitor/defibrillator should be attached to the patient on arrival in the ED to ascertain underlying rhythm and monitoring should continue until the cause of the event is known, and/or until an implantable defibrillator is in place.4 Patients recovering from cardiac arrest should also have continuing cardiac monitoring if required to leave the ED for diagnostic or therapeutic procedures or are transported to the intensive care unit for admission.6
Acute Coronary Syndrome
Accelerated diagnostic protocols have been developed to discern low-risk from high-risk patients presenting to the ED with chest pain.1 Risk stratification for patients with suspected acute coronary syndrome (ACS) includes prompt arrhythmia and ischemia monitoring on ED arrival, serial 12-lead ECG acquisition, and cardiac biomarker testing over a 6- to 12-hour period.1,4 Patients with negative results are deemed low risk and receive a confirmatory study, for example, exercise and treadmill testing, before discharge.1 Alternatively, high-risk patients with acute myocardial infarction who undergo early reperfusion therapy in the prehospital period or in the ED are at risk for malignant reperfusion arrhythmias. These patients should receive uninterrupted, arrhythmia monitoring during both interhospital and intrahospital transport.4 Arrhythmia monitoring is also indicated for patients with newly diagnosed, critical left main coronary artery disease and other high-risk coronary lesions who are candidates for urgent revascularization. Patients with unstable angina should undergo cardiac monitoring until infarction is ruled out and signs that transient ECG changes and symptoms are absent.4
Recently, Winkler and colleagues8 studied 278 patients diagnosed with ACS to determine the potential benefits of ST-segment monitoring in the ED; they found the incidence of ventricular arrhythmias (premature ventricular contractions, nonsustained ventricular tachycardia, and malignant arrhythmias) over the first 24 hours of hospitalization to be lower than studies conducted before the reperfusion era in the late 1980s.
Heart Failure and/or Pulmonary Edema
Continuous arrhythmia monitoring is recommended for patients with signs and symptoms of heart failure and/or pulmonary edema. Acute heart failure is a major risk factor for atrial and ventricular arrhythmias, and some therapies such as intravenous positive inotropic drugs have significant proarrhythmic properties.4
Atrioventricular Block
Patients who present to the ED with palpitations, syncope, dizziness, or lightheadedness may be experiencing an atrioventricular (AV) block; ongoing arrhythmia monitoring is indicated for patients with Mobitz I or Mobitz II (second-degree AV block), complete heart block (third-degree AV block), or new-onset, bundle branch block in the setting of acute myocardial infarction.4 ECG monitoring should continue until the block resolves or definitive therapy (a permanent pacemaker) is implemented.
After Cardiac Surgery
Patients presenting to the ED after cardiac surgery should be monitored for arrhythmias because they are at risk for developing ventricular tachycardia or fibrillation, AV block, and sinus node dysfunction.4 Moreover, the incidence of postoperative atrial fibrillation is 32% after coronary artery bypass surgery, 64% after combined bypass and mitral valve replacement surgery, 49% after combined bypass and aortic valve replacement, and 11% after heart transplantation.9,10 Emergency nurses should consider that the onset of atrial fibrillation typically occurs on the second to fourth postoperative days, and that a majority of these patients are asymptomatic.4,11
Syncope
Patients presenting to the ED with syncope of truly unknown origin should receive arrhythmia monitoring because heart disease is a major predictor of death and/or fatal arrhythmia in syncopal patients.4 Patients should be monitored until an arrhythmic cause has been ruled out by electrophysiologic testing or other evaluation is completed.
ISCHEMIA MONITORING IN THE EMERGENCY DEPARTMENT
Coronary heart disease is the leading cause of death in the United States and becoming the most frequent cause of death worldwide.12,13 Coronary heart disease is characterized by stable and unstable periods, the latter reflects progression of occlusion in a coronary artery and manifests as ACS. ACS is a spectrum of time-sensitive clinical syndromes that includes (1) unstable angina, (2) non–ST-segment elevation myocardial infarction, and (3) ST-segment elevation myocardial infarction.14 Prompt diagnosis and effective early management of ACS in the ED are imperative because numerous clinical trials have established that a more aggressive approach to the treatment of myocardial ischemia improves patient outcomes.4,14,15 If left undetected, ACS results in devastating outcomes such as myocardial infarction, heart failure, and death.15–17 Strategies to prevent infarction or reduce infarct size rely heavily on the ability of clinicians to identify myocardial ischemia in the ED. Cardiac monitoring enables clinicians to identify rapidly patients who require urgent intervention from those with benign conditions who can be discharged home.1,14,18,19
The 12-Lead Electrocardiogram
To date, the 12-lead ECG remains the gold standard used for initial screening, identifying, and evaluating patients with chest pain and anginal equivalents.20 The ECG is the most widely used initial diagnostic test because it is ubiquitous, noninvasive, and inexpensive, and provides vital information about cardiac rhythm, presence of arrhythmias, myocardial ischemia/infarction, and other abnormalities.15,21,22 The American College of Cardiology, The American Heart Association, and The European Society of Cardiology recommend that all patients who present to the ED with chest pain have a 12-lead ECG recorded within 10 minutes of arrival.23–26 This recommendation is based on the premise that longer delays are associated with worse outcomes, and ST-segment pattern recognition shortens the delay between the first medical contact and life-saving reperfusion therapies.15,23,27
The standard 12-lead ECG uses 10 electrodes to record the electrical activity of the heart. Six precordial leads are placed across the precordium in anatomically specific locations and 3 limb leads may be placed either (1) on the distal limbs, the preferred placement, for standard, resting 12-lead ECG acquisition, or (2) where the limbs attach to the torso (Mason-Likar) for continuous ECG monitoring, such as exercise testing (Fig. 2).28 Using different limb lead placements produces a similar, but not identical, 12-lead ECG; different limb lead placement between serial ECGs may result in waveform morphology changes that computer algorithms falsely interpret as myocardial ischemia.4,6 Therefore, emergency nurses should receive ongoing training and education about the importance of correct and standardized electrode placement for accurate cardiac monitoring.29
Fig. 2.
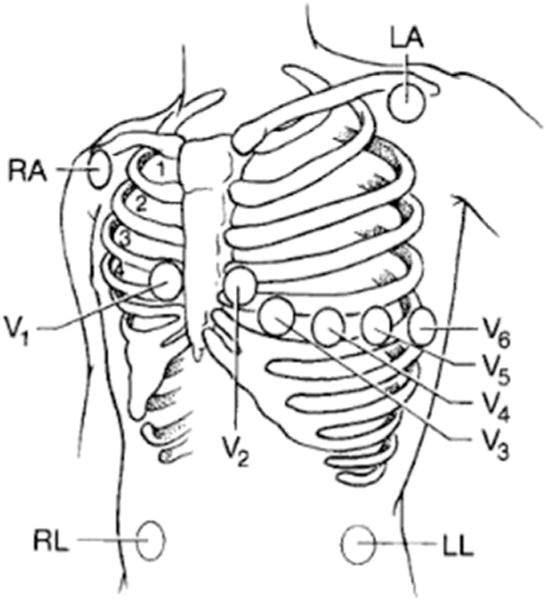
Mason-Likar electrode placement for continuous 12-lead electrocardiographic monitoring. LA, left arm; LL, left leg; RA, right arm; RL, right leg. (From Zegre Hemsey J. Optimizing pre-hospital electrocardiography to improve the early diagnosis of acute coronary syndrome. San Francisco (CA): University of California; 2011; with permission.)
Electrocardiographic Signs of Ischemia
Changes in the intracellular action potential in myocardial ischemia, injury, and infarction result in changes in ECG waveforms. ECG changes indicating ischemia (Fig. 3) include ST-elevation, ST-depression, or T-wave inversion and occur before myocardial infarction, providing the ability to intervene to restore blood flow before myocardial cell death ensues. The presence of acute ischemic changes on the initial ECG, often conducted at presentation to the ED, is associated with a higher risk of cardiac events.30 Acute ischemic changes are unpredictable and dynamic in nature, which suggests that a single snap-shot 12-lead ECG is inadequate, and continuous or serial ECG monitoring is superior diagnostically.20,31–33 The presence of ischemic signs may advise the ED clinicians to activate the catheterization laboratory for patients suffering from acute myocardial infarction; this extends into the prehospital period where paramedics may analyze ECGs acquired in the ambulance and activate the catheterization laboratory before hospital arrival.34
Fig. 3.
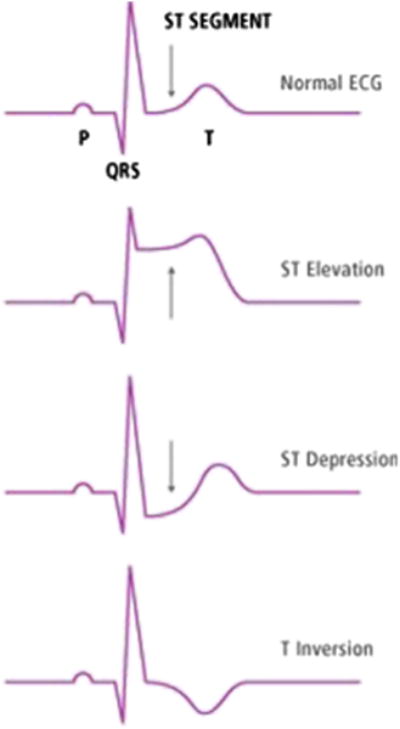
Changes in the electrocardiograph (ECG) in the ST-segment indicative of myocardial ischemia and infarction. (From Zegre Hemsey J. Optimizing pre-hospital electrocardiography to improve the early diagnosis of acute coronary syndrome. San Francisco (CA): University of California; 2011; with permission.)
Universal ECG criteria for myocardial infarction were developed to increase the sensitivity and specificity of the ECG by recognizing gender, age, and lead differences.35 Electrocardiographic criteria (in the absence of left ventricular hypertrophy and left bundle branch block) include:
ST elevation at the J-point in 2 contiguous leads with the cutpoints: 0.2 mV or greater in men 40 years or older; 0.25 mV or greater in men less than 40 years, or 0.15 mV or greater in women in leads V2 to V3 and/or 0.1 mV or greater in all other leads.
ST-depression and T-wave changes defined by new horizontal or down-sloping ST depression 0.05 mV or greater in 2 contiguous leads and/or T inversion 0.1 mV or greater in 2 contiguous leads with prominent R wave or R/S ratio of greater than 1.
Emergency nurses or technicians typically obtain the initial ECG in the ED, which is interpreted by an emergency physician or cardiologist. Because of the decreasing mentoring of physicians to learn ECG interpretation, there has been a recent call for the training of cardiovascular nurse practitioners to interpret ECG.36 This is an important consideration in the ED setting, where clinicians rely on interdisciplinary teamwork to manage the large array of patients with cardiac complaints.
Serial Electrocardiographic Monitoring
Serial ECG acquisition is recommended when the initial ECG is nondiagnostic, but patient signs or symptoms are consistent with acute myocardial infarction.27 Current guidelines specifically recommend the initial ECGs be repeated at 5- to 10-minute intervals if the initial ECG is not diagnostic but the patient remains symptomatic and a high clinical suspicion for ACS persists.37 Prior studies suggest that serial ECG recordings enhance the diagnostic sensitivity for ACS as compared with abnormalities on a single tracing and offer the opportunity for clinicians to observe changes between tracings that may be more difficult to interpret individually, enabling detection of evolving changes of ischemia that, by nature, are dynamic and unpredictable.31 Serial ECG comparisons should be made using the same recording technique because differences in waveform morphology have been observed between differing electrode configurations (eg, standard vs Mason-Likar placement).6
Continuous ST-Segment Monitoring in the Emergency Department
The American College of Cardiology/The American Heart Association guidelines recommend continuous bedside ST-segment monitoring for patients with a nondiagnostic initial ECG as an alternative to serial 12-lead ECGs.38 Patients with ACS are the highest priority for ST-segment monitoring until they remain event free for 12 to 24 hours. Practice standards for ECG monitoring recommend continuous ST-segment monitoring in (class I) patients for 8 to 12 hours in combination with serum biomarker testing to determine treatment priority in ED patients with suspected ACS or at risk for ACS.4,18,39 Conversely, patients with left ventricular hypertrophy, left bundle branch block, ventricular pacing, and those with intermittent right bundle branch block may not necessarily benefit from ST-segment monitoring (class III) because these conditions present secondary ST/T-wave abnormalities that confound interpretation and may trigger false ST-segment alarms.40
ST-segment analysis software became available on bedside monitors in the mid 1980s, and is widely available in the United States.39 However, surveys suggest that ST-segment monitoring software is not activated routinely by nurses because of lack of education, generations of numerous false alarms, and difficulty of use.4,41 Moreover, the standard 12-lead ECG is not always convenient for continuous monitoring,4 especially in the ED where patients are on gurneys, moved frequently to accommodate patient flow, and transported for diagnostic testing. Improvements to the user interface may help this situation.
Studies examining the value of ST-segment monitoring in the ED are limited. Fesmire and colleagues32 examined the usefulness of ST-segment monitoring with serial ECG acquisition in 1000 patients with chest pain in the ED. Serial ECGs with ST-segment monitoring had greater sensitivity (68% vs 55%; P<.0001) and specificity (99 vs 97; P<.01) for detecting ACS compared with the initial “snapshot” ECG acquired on ED arrival. More recently, Bovino and colleagues18 examined the value of bedside continuous ST-segment monitoring 163 patients with risk-stratified chest pain in the ED at intermediate risk for ACS. Investigators found no difference in detection of ischemia/infarction, time to diagnosis, or 30-day adverse events among patients before and after ST-segment monitoring implementation.18 Further research is required to establish evidence-based guidelines for ST-segment monitoring in the ED setting.
Reduced Lead Sets
Reduced lead set technology enables synthesis of a 12-lead ECG from a small number of leads/electrodes, which makes continuous cardiac monitoring more feasible in the ED.4 The EASI 5-lead system (Philips IntelliVue, Andover, MA) was the first derived 12-lead ECG and was developed by Dower42 in 1988; it mathematically derives an ECG from 4 recording electrodes and a reference electrode (Fig. 4). Importantly, the derived 12-lead ECG is comparable with the standard 12-lead ECG for diagnosis of acute myocardial ischemia and wide complex QRS tachycardias, each of which requires multiple leads for accurate interpretation.4,43,44
Fig. 4.
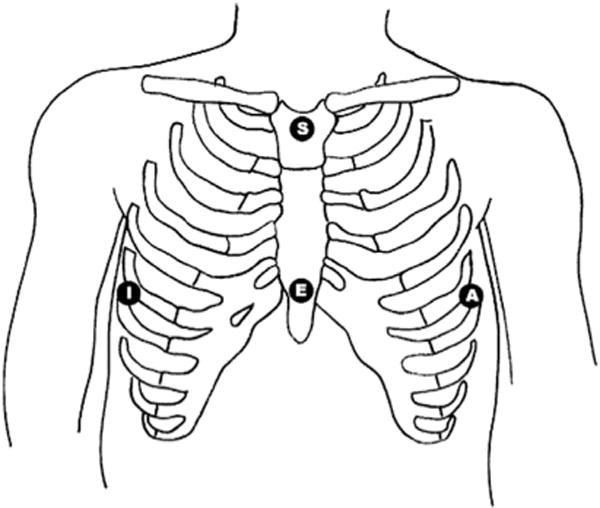
EASI lead system electrode placement. The fifth ground electrode (not shown) may be placed anywhere on the body. (From Sejersten M, Pahlm O, Pettersson J, et al. Comparison of EASI-derived 12-lead electrocardiograms versus paramedic-acquired 12-lead electrocardiograms using Mason-Likar limb lead configuration in patients with chest pain. J Electrocardiology 2006;39(1):13–21.)
Fig. 5 shows another commonly used 5-lead configuration for cardiac monitoring in the ED. Four limb electrodes and a fifth chest electrode can be placed in any of the standard precordial (V1–V6) positions; the chest electrode allows for recording of a true V lead, which may enhance the accuracy for arrhythmia detection.4 However, this derived lead configuration is not sensitive for detection of myocardial ischemia.
Fig. 5.
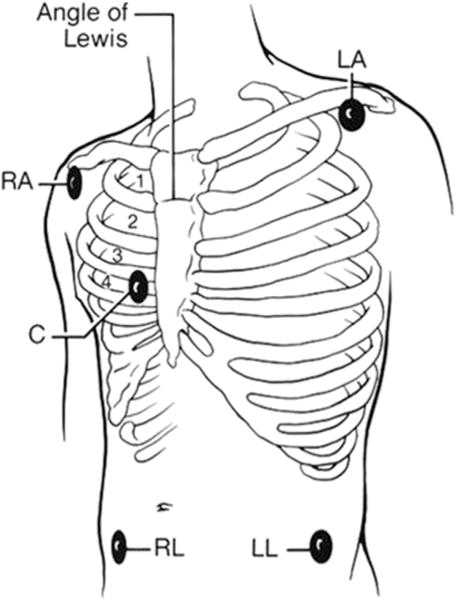
Standard 5-electrode lead system in the emergency department. LA, left arm; LL, left leg; RA, right arm; RL, right leg. (From Drew BJ, Califf RM, Funk M, et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation 2004;110(17):2721–46.)
QT INTERVAL MONITORING
Acute lengthening of the QT interval, an indirect measure of ventricular repolarization, can be observed in multiple clinical situations and is associated with syncope and sudden death from torsade de pointes (TdP).4 TdP is a malignant polymorphic ventricular tachycardia that resembles ventricular fibrillation; it may self-terminate or progress to cardiac arrest and sudden cardiac death (Fig. 6).4,7,45 Typical features of TdP include (1) changes in amplitude and morphology of QRS complexes around the isoelectric line and (2) drug-induced TdP episodes that begin with a short-long-short pattern of R-R cycles consisting of a short premature ventricular complex followed by a compensatory pause and another premature ventricular complex.46 A normal corrected QT (QTc) for women is less than 0.46 seconds and for men is less than 0.45 seconds; a QTc of greater than 0.50 seconds in either gender positively correlates with a higher risk for TdP. The duration of a QTc is a reliable indicator of risk of cardiac events; therefore, patients with long QT syndrome and associated ventricular arrhythmias should receive QT monitoring in the ED (class I recommendation).4,45
Fig. 6.
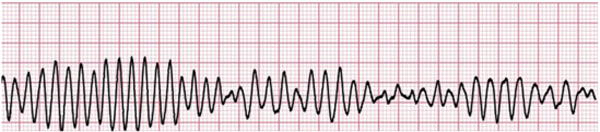
Torsade de pointes is a lethal arrhythmia.
The importance of QT monitoring in the ED cannot be underestimated because ED patients are uniquely at risk for developing TdP owing to their vast array of chief complaints and high acuity.45 Specific patient characteristics are associated with development of TdP (Box 2) and should be considered during triage and risk stratification.45
Box 2. Characteristics of patients at risk for developing torsade de pointes.
Women
Elderly
Heart disease
Acute neurologic events
Bradyarrhythmias with long pauses
Electrolyte disturbances (hypomagnesia, hypokalemia)
Malnutrition
Polypharmacy
Genetics (long QT syndrome, family history of sudden cardiac death, syncope)
Renal/hepatic dysfunction
Adapted from Pickham D, Drew BJ. QT/QTc interval monitoring in the emergency department. J Emerg Nurs 2008;34(5):428–34; with permission.
Practice guidelines recommend measuring patients’ QT/QTc interval at baseline and documenting repeat measures at least once every 8 hours.4,45 Patients in the ED may initially require QT/QTc monitoring more frequently, especially if receiving medications (Box 3) known to prolong the QT interval.45 Therefore, it is important for emergency nurses to acquire the knowledge, skill, and judgment to tailor QT/QTc monitoring to individual patient needs.45 Specific indications for QT interval monitoring of ED patients are listed in Box 4. Practice guidelines recommend that QT monitoring should continue until (1) the culprit drug is discontinued and/or decreased and no further prolonged QTc is noted, (2) no further QT-related arrhythmias occur, (3) definitive therapy (permanent pacemaker) is established, and/or (4) electrolyte disorder has been corrected.4,6,40
Box 3. Medications raising patient-risk of torsade de pointes.
| Generic Name | Clinical Use |
|---|---|
| Amiodarone | Antiarrhythmic |
| Amiodarone | Anticancer |
| Azithromycin | Antibiotic |
| Chlorpromazine | Antipsychotic/antiemetic |
| Ciprofloxacin | Antibiotic |
| Cocaine | Local anesthetic |
| Disopyramide | Antiarrhythmic |
| Dofetilide | Antiarrhythmic |
| Dronedarone | Antiarrhythmic |
| Droperidol | Antipsychotic/antiemetic |
| Erythromycin | Antibiotic |
| Flecainide | Antiarrhythmic |
| Haloperidol | Antipsychotic |
| Ibutilide | Antiarrhythmic |
| Levofloxacin | Antibiotic |
| Methadone | Opioid agonist |
| Ondansetron | Antiemetic |
| Quinidine | Antiarrhythmic |
| Sotalol | Antiarrhythmic |
| Thioridazine | Antipsychotic |
Data from Arizona CERT. Available at: www.qtdrugs.org. Accessed December 14, 2015.
Box 4. Indications for QT interval monitoring.
Patients started on antiarrhythmic drug known to cause torsade de pointe (disopyramide, dofetilide, ibutilide, procainamide quinidine, sotalol).
Patients who overdose from proarrhythmic agents.
Patients with new onset bradyarrhythmias (complete heart block, long sinus pauses).
Patients with severe hypokalemia or hypomagnesemia.
Adapted from Pickham D, Drew BJ. QT/QTc interval monitoring in the emergency department. J Emerg Nurs 2008;34(5):429; with permission.
Emergency nurses are in a unique position to identify and measure the QT interval on 12-lead ECGs acquired in the ED; they can be empowered with the knowledge of applying correction formulas to determine the QTc, which accounts for the influence of heart rate.45 Moreover, ED nurses can learn to interpret and document QT measurements when provided by the standard 12-lead ECG machine to optimize management of patients at possible risk for TdP.
SUMMARY
The ED is a fast-paced, dynamic, and chaotic setting that requires quick and accurate decision making to distinguish high-acuity patients. The priority for emergency clinicians is to recognize and stabilize patients with emergent cardiovascular conditions that include, but are not limited to, myocardial ischemia/infarction and potentially life-threatening arrhythmias. Cardiac monitoring is one of the most commonly used diagnostic practices in the ED, and emergency nurses are poised to use valuable information revealed in ECG waveforms for early triage and risk stratification. Future research is needed to drive evidenced-based monitoring practices specific to patients in the ED.
KEY POINTS.
Emergency department (ED) care demands rapid, accurate diagnosis and stabilization of patients with time-sensitive conditions such as acute myocardial infarction and arrhythmia.
Cardiac monitoring strategies in the ED include standard 12-lead electrocardiography and bedside monitoring for arrhythmias and myocardial ischemia.
Electrocardiographic signs of myocardial ischemia drive clinical decision making such as the activation of cardiac catheterization of patients with ST-elevation myocardial infarction.
Footnotes
Disclosures: Nothing to disclose (J.K. Zègre-Hemsey, M.G. Carey); Philips Healthcare (J.L. Garvey).
References
- 1.Amsterdam EA, Kirk JD, Bluemke DA, et al. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation. 2010;122(17):1756–76. doi: 10.1161/CIR.0b013e3181ec61df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. National Hospital Ambulatory Medical Care Survey: 2011 emergency department summary. Vol. 386. Atlanta (GA): Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 3.O’Connor RE, Al Ali AS, Brady WJ, et al. Part 9: acute coronary syndromes: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 Suppl 2):S483–500. doi: 10.1161/CIR.0000000000000263. [DOI] [PubMed] [Google Scholar]
- 4.Drew BJ, Califf RM, Funk M, et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation. 2004;110(17):2721–46. doi: 10.1161/01.CIR.0000145144.56673.59. [DOI] [PubMed] [Google Scholar]
- 5.Drew BJ, Harris P, Zegre-Hemsey JK, et al. Insights into the problem of alarm fatigue with physiologic monitor devices: a comprehensive observational study of consecutive intensive care unit patients. PLoS One. 2014;9(10):e110274. doi: 10.1371/journal.pone.0110274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drew BJ, Funk M. Practice standards for ECG monitoring in hospital settings: executive summary and guide for implementation. Crit Care Nurs Clin North Am. 2006;18(2):157–68. doi: 10.1016/j.ccell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Priori SG, Blomstrom-Lundqvist C, Mazzanti A, et al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC) endorsed by: association for European Paediatric and Congenital Cardiology (AEPC) Eur Heart J. 2015;36(41):2793–867. doi: 10.1093/eurheartj/ehv316. [DOI] [PubMed] [Google Scholar]
- 8.Winkler C, Funk M, Schindler DM, et al. Arrhythmias in patients with acute coronary syndrome in the first 24 hours of hospitalization. Heart Lung. 2013;42(6):422–7. doi: 10.1016/j.hrtlng.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Creswell LL, Schuessler RB, Rosenbloom M, et al. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg. 1993;56(3):539–49. doi: 10.1016/0003-4975(93)90894-n. [DOI] [PubMed] [Google Scholar]
- 10.Thoren E, Hellgren L, Stahle E. High incidence of atrial fibrillation after coronary surgery. Interact Cardiovasc Thorac Surg. 2016;22(2):176–80. doi: 10.1093/icvts/ivv326. [DOI] [PubMed] [Google Scholar]
- 11.Funk M, Richards SB, Desjardins J, et al. Incidence, timing, symptoms, and risk factors for atrial fibrillation after cardiac surgery. Am J Crit Care. 2003;12(5):424–33. [quiz: 434–5] [PubMed] [Google Scholar]
- 12.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 13.Steg PG, James SK, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33(20):2569–619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 14.Body R. Emergent diagnosis of acute coronary syndromes: today’s challenges and tomorrow’s possibilities. Resuscitation. 2008;78(1):13–20. doi: 10.1016/j.resuscitation.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Antman EM, Hand M, Armstrong PW, et al. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the Canadian Cardiovascular Society endorsed by the American Academy of Family Physicians: 2007 writing group to review new evidence and update the ACC/AHA 2004 Guidelines for the management of patients with ST-elevation myocardial infarction, writing on behalf of the 2004 writing committee. Circulation. 2008;117(2):296–329. doi: 10.1161/CIRCULATIONAHA.107.188209. [DOI] [PubMed] [Google Scholar]
- 16.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):e362–425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 18.Bovino LR, Funk M, Pelter MM, et al. The value of continuous ST-segment monitoring in the emergency department. Adv Emerg Nurs J. 2015;37(4):290–300. doi: 10.1097/TME.0000000000000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhuiya FA, Pitts SR, McCaig LF. Emergency department visits for chest pain and abdominal pain: United States 1999–2008. NCHS Data Brief. 2010;(43):1–8. [PubMed] [Google Scholar]
- 20.Kudenchuk PJ, Maynard C, Cobb LA, et al. Utility of the prehospital electrocardiogram in diagnosing acute coronary syndromes: the Myocardial Infarction Triage and Intervention (MITI) Project. J Am Coll Cardiol. 1998;32(1):17–27. doi: 10.1016/s0735-1097(98)00175-2. [DOI] [PubMed] [Google Scholar]
- 21.Zegre-Hemsey J, Sommargren CE, Drew BJ. Initial ECG acquisition within 10 minutes of arrival at the emergency department in persons with chest pain: time and gender differences. J Emerg Nurs. 2011;37(1):109–12. doi: 10.1016/j.jen.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savonitto S, Ardissino D, Granger CB, et al. Prognostic value of the admission electrocardiogram in acute coronary syndromes. JAMA. 1999;281(8):707–13. doi: 10.1001/jama.281.8.707. [DOI] [PubMed] [Google Scholar]
- 23.Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non ST-Elevation Myocardial Infarction): developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation. 2007;116(7):e148–304. doi: 10.1161/CIRCULATIONAHA.107.181940. [DOI] [PubMed] [Google Scholar]
- 24.Bassand JP, Hamm CW, Ardissino D, et al. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J. 2007;28(13):1598–660. doi: 10.1093/eurheartj/ehm161. [DOI] [PubMed] [Google Scholar]
- 25.Graff L, Palmer AC, Lamonica P, et al. Triage of patients for a rapid (5-minute) electrocardiogram: a rule based on presenting chief complaints. Ann Emerg Med. 2000;36(6):554–60. doi: 10.1067/mem.2000.111057. [DOI] [PubMed] [Google Scholar]
- 26.Glickman SW, Shofer FS, Wu MC, et al. Development and validation of a prioritization rule for obtaining an immediate 12-lead electrocardiogram in the emergency department to identify ST-elevation myocardial infarction. Am Heart J. 2012;163(3):372–82. doi: 10.1016/j.ahj.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 27.Ayer A, Terkelsen CJ. Difficult ECGs in STEMI: lessons learned from serial sampling of pre-and in-hospital ECGs. J Electrocardiol. 2014;47(4):448–58. doi: 10.1016/j.jelectrocard.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Adams MG, Drew BJ. Body position effects on the ECG: implication for ischemia monitoring. J Electrocardiol. 1997;30(4):285–91. doi: 10.1016/s0022-0736(97)80040-4. [DOI] [PubMed] [Google Scholar]
- 29.Schijvenaars BJ, van Herpen G, Kors JA. Intraindividual variability in electrocardiograms. J Electrocardiol. 2008;41(3):190–6. doi: 10.1016/j.jelectrocard.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 30.Zegre Hemsey JK, Dracup K, Fleischmann KE, et al. Prehospital electrocardiographic manifestations of acute myocardial ischemia independently predict adverse hospital outcomes. J Emerg Med. 2013;44(5):955–61. doi: 10.1016/j.jemermed.2012.07.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zegre Hemsey JK, Dracup K, Fleischmann K, et al. Prehospital 12-lead ST-segment monitoring improves the early diagnosis of acute coronary syndrome. J Electrocardiol. 2012;45(3):266–71. doi: 10.1016/j.jelectrocard.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fesmire FM, Percy RF, Bardoner JB, et al. Usefulness of automated serial 12-lead ECG monitoring during the initial emergency department evaluation of patients with chest pain. Ann Emerg Med. 1998;31(1):3–11. doi: 10.1016/s0196-0644(98)70274-4. [DOI] [PubMed] [Google Scholar]
- 33.Fu GY, Joseph AJ, Antalis G. Application of continuous ST-segment monitoring in the detection of silent myocardial ischemia. Ann Emerg Med. 1994;23(5):1113–5. doi: 10.1016/s0196-0644(94)70111-3. [DOI] [PubMed] [Google Scholar]
- 34.Garvey JL, Monk L, Granger CB, et al. Rates of cardiac catheterization cancelation for ST-segment elevation myocardial infarction after activation by emergency medical services or emergency physicians: results from the North Carolina Catheterization Laboratory Activation Registry. Circulation. 2012;125(2):308–13. doi: 10.1161/CIRCULATIONAHA.110.007039. [DOI] [PubMed] [Google Scholar]
- 35.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60(16):1581–98. doi: 10.1016/j.jacc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Drew BJ, Dracup K, Childers R, et al. Finding ECG readers in clinical practice: is it time to change the paradigm? J Am Coll Cardiol. 2014;64(5):528. doi: 10.1016/j.jacc.2014.01.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kushner FG, Hand M, Smith SC, Jr, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54(23):2205–41. doi: 10.1016/j.jacc.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 38.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130(25):2354–94. doi: 10.1161/CIR.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 39.Drew BJ, Krucoff MW. Multilead ST-segment monitoring in patients with acute coronary syndromes: a consensus statement for healthcare professionals. ST-segment monitoring practice guideline International Working Group. Am J Crit Care. 1999;8(6):372–86. [quiz: 387–8] [PubMed] [Google Scholar]
- 40.Funk M, Winkler CG, May JL, et al. Unnecessary arrhythmia monitoring and underutilization of ischemia and QT interval monitoring in current clinical practice: baseline results of the Practical Use of the Latest Standards for Electrocardiography trial. J Electrocardiol. 2010;43(6):542–7. doi: 10.1016/j.jelectrocard.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patton JA, Funk M. Survey of use of ST-segment monitoring in patients with acute coronary syndromes. Am J Crit Care. 2001;10(1):23–32. [quiz: 33–4] [PubMed] [Google Scholar]
- 42.Dower GE, Yakush A, Nazzal SB, et al. Deriving the 12-lead electrocardiogram from four (EASI) electrodes. J Electrocardiol. 1988;21(Suppl):S182–7. doi: 10.1016/0022-0736(88)90090-8. [DOI] [PubMed] [Google Scholar]
- 43.Drew BJ, Adams MG, Pelter MM, et al. ST segment monitoring with a derived 12-lead electrocardiogram is superior to routine cardiac care unit monitoring. Am J Crit Care. 1996;5(3):198–206. [PubMed] [Google Scholar]
- 44.Drew BJ, Pelter MM, Wung SF, et al. Accuracy of the EASI 12-lead electrocardiogram compared to the standard 12-lead electrocardiogram for diagnosing multiple cardiac abnormalities. J Electrocardiol. 1999;32(Suppl):38–47. doi: 10.1016/s0022-0736(99)90033-x. [DOI] [PubMed] [Google Scholar]
- 45.Pickham D, Drew BJ. QT/QTc interval monitoring in the emergency department. J Emerg Nurs. 2008;34(5):428–34. doi: 10.1016/j.jen.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 46.Drew BJ, Ackerman MJ, Funk M, et al. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121(8):1047–60. doi: 10.1161/CIRCULATIONAHA.109.192704. [DOI] [PMC free article] [PubMed] [Google Scholar]


