Abstract
Exome sequencing has readily enabled the discovery of the genetic mutations responsible for a wide range of diseases. This success has been particularly remarkable in the severe epilepsies and other neurodevelopmental diseases for which rare, often de novo, mutations play a significant role in disease risk. Despite significant progress, the high genetic heterogeneity of these disorders often requires large sample sizes to identify a critical mass of individuals with disease-causing mutations in a single gene. By pooling genetic findings across multiple studies, we have identified six individuals with severe developmental delay (6/6), refractory seizures (5/6), and similar dysmorphic features (3/6), each harboring a de novo mutation in PPP3CA. PPP3CA encodes the alpha isoform of a subunit of calcineurin. Calcineurin encodes a calcium- and calmodulin-dependent serine/threonine protein phosphatase that plays a role in a wide range of biological processes, including being a key regulator of synaptic vesicle recycling at nerve terminals. Five individuals with de novo PPP3CA mutations were identified among 4,760 trio probands with neurodevelopmental diseases; this is highly unlikely to occur by chance (p = 1.2 × 10−8) given the size and mutability of the gene. Additionally, a sixth individual with a de novo mutation in PPP3CA was connected to this study through GeneMatcher. Based on these findings, we securely implicate PPP3CA in early-onset refractory epilepsy and further support the emerging role for synaptic dysregulation in epilepsy.
Keywords: developmental and epileptic encephalopathy, epilepsy, de novo mutation, PPP3CA, calcineurin
Introduction
Epilepsies are a group of conditions in which recurrent seizures are the defining feature. Seizures arise from a dysregulation of excitatory and inhibitory networks in the brain. While the complex mechanisms of this electrical imbalance are not fully understood, genetic research has identified a large number of epilepsy risk-associated genes that are assumed to play a role in electrical homeostatic mechanisms in the brain.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 Studies of people with epilepsy and epilepsy mouse models implicate genes governing synaptic vesicle neurotransmitter release and reuptake, a cyclic process that controls the level of neurotransmitters in the synaptic cleft.14 In humans specifically, mutations in STXBP1 (MIM: 602926), STX1B (MIM: 601485), and DNM1 (MIM: 602377), each encoding proteins integrally involved in the synaptic vesicle cycle at the nerve terminal, have been securely associated with developmental and epileptic encephalopathies.1, 15, 16 Epileptic encephalopathies are severe disorders characterized by early-onset epilepsy and developmental delays with or without regression that may be worsened by ongoing epileptic activity.17 Additionally, there is growing evidence of causative de novo mutation in SNAP25 (MIM: 600322), as two case reports of individuals with neurodevelopmental diseases have been described.18, 19
We now implicate PPP3CA (MIM: 114105), which encodes a protein involved in synaptic vesicle recycling, in epilepsy and neurodevelopmental disorders, further strengthening the role of synaptic vesicle cycle dysregulation in epilepsy. We report six individuals, each with a de novo mutation in PPP3CA (GenBank: NM_000944.4); these likely pathogenic variants comprise four missense mutations, one which is recurrent, and a single nonsense mutation (Table 1). Five mutations were identified from a cohort of 4,760 individuals with a range of neurodevelopment disorders and a sixth case was linked to this study through the web-based tool GeneMatcher.20 The majority of individuals (5/6) harboring de novo mutations in PPP3CA have seizures and all have severe developmental delay. PPP3CA encodes an isoform of a subunit of the Ca2+ interacting serine/threonine phosphatase, calcineurin. In addition to a prominent role in the activation of T cells,21 calcineurin is responsible for the calcium-dependent dephosphorylation of dynamin-1 (encoded by DNM1), a critical process regulating endocytosis following depolarization at the nerve terminal.22 These findings show that PPP3CA plays a key role in intractable childhood epilepsy and neurodevelopmental disorders. They also illuminate a possible shared pathophysiology with DNM1-mediated epilepsy and further support a role for dysregulation of the synaptic vesicle recycling at the nerve terminal in epileptogenesis.
Table 1.
Individuals with PPP3CA Encephalopathy: De Novo PPP3CA Mutations and Clinical Features
| Individual 1 (EGI0251B1) | Individual 2 (lgsnd30299is1) | Individual 3 (isrl69xx3) | Individual 4 (T26323) | Individual 5 (Ambry Clinical WES) | Individual 6 (GeneMatcher – GeneDx) | |
|---|---|---|---|---|---|---|
| Mutation (GRCh37/hg19) | 4-101953430-G-A; NM_000944.4; c.1333C>T (p.Gln445Ter) | 4-102030220-T-C; NM_000944.4; c.275A>G (p.His92Arg) | 4-101953424-C-T; NM_000944.4; c.1339G>A (p.Ala447Thr) | 4-102004360-G-C; NM_000944.4; c.843C>G (p.His281Gln) | 4-102004359-C-T; NM_000944.4; c.844G>A (p.Glu282Lys) | 4-102004359-C-T; NM_000944.4; c.844G>A (p.Glu282Lys) |
| Age (gender) | 7 y (male) | 12.5 y (male) | 21.5 y (female) | 10 y (female) | 22 y (female) | 5.5 y (female) |
| Inheritance | de novo | de novo | de novo | de novo | de novo | de novo |
| Ethnicity | Korean, European | European | Ashkenazi, Sephardic | Maori, European New Zealander | Pakistani | Eastern European, Ashkenazi, Irish |
| Presenting Sz (offset) | 6 w: MF clonic and M | 3 m: FIAS | 3.5 y: GTCS | 3 m: ES (18 m) | 4 y: Ab (10 y) | N/A |
| Other Sz types (offset) | 5 m ES, T; 6 m F | 13 m ES (head drop-predominant), T with myoclonic eyelid flutter; 2 y 11 m drop seizures with jackknife flexion of torso & head propulsion to the floor from a seated position; 5 y SE, atypical Ab | T (age unknown) | 3 y FIAS (frontal lobe) | 5 y M (12 y); 8 y At, FM (12 y); 9 y GTCS, T (13 y) | N/A |
| Age of first developmental concerns | by 1 m | 3 m | 2 y | 3 m | 2 y | birth |
| Regression | no | 5 y with SE | 3.5 y | 12 m with worsening sz | no | no |
| Developmental Outcome | profound ID, NV, NA; cortical visual impairment; G-tube and tracheostomy | profound ID, NV, NA (could roll and sit prior to regression), poor visual tracking; | profound ID; limited vocabulary with short sentences (lots of perseverations); autistic features; ataxic gait progressed to wheelchair | profound ID; NA, (unable to sit independently); NV (babble only); G-tube; cortical visual impairment | severe ID, 5 words, unsteady gait; not toilet trained | severe ID, autistic features; walked at 23 m; single words |
| EEG | 5 m: hypsarrhythmia; 8 & 12 m: MFD, GSW; 4 & 5 y: hypsarrhythmia; | 5 m: L, R, & bilateral occipital spikes; 17 m: frequent MFD, and PS, abnormal background, burst suppression in sleep, modified hypsarrhythmia; 5 y: MFD, bursts of generalized fast activity, abnormal background | 3.5 y: MFD | 12 m: MFD, N background; 18 m: MFD, close to hypsarrhythmia in sleep; 2 y: N; 3 y: frequent frontal sharp and spikes; 9 y: frontal fast activity (beta persistent) and occasional frontal spikes | 8 y: abnormal background frequent GSW, PS and wave; 14 y: PSW 1-2 Hz bifrontal maximum | 20 m: MFD, abnormal background |
| MRI | 6 w: delayed myelination, especially orbitofrontal gyri and anterior temporal lobes; progressive bioccipital white matter loss; progressive bilateral T2 hyper intensity; low NAA to choline ratio | 6 m & 5 y: N | 5 & 10 y: N | 1 y, 2 y & 3 y: mild generalized prominence of subarachnoid spaces | 8 y & 9 y: mild cortical sulcal prominence & focal linear hyperintensity in the R centrum semiovale, consistent with a small developmental venous anomaly | 2.5 y: N |
| Epilepsy syndrome | early-onset epileptic encephalopathy | early-onset epileptic encephalopathy; Lennox-Gastaut syndrome | developmental & epileptic encephalopathy | West syndrome, 3 m (not recognized until 12 m), focal (frontal) epilepsy 3 y | developmental & epileptic encephalopathy | none (EEG done to rule out epileptic aphasia) |
| Neurological examination | severe hypotonia; severe opisthotonus in infancy | marked axial & peripheral hypotonia | spasticity | hypotonia, N growth parameters | mild hypotonia & hyporeflexia | N |
| AED response | refractory; some response to KD, VGB, PB, TPM, CBD; no response to LEV, RFM, ACTH, PRD | refractory; PB, ZNS, OCBZ, LEV (partial response), VPA, LTG (rash), methsuximide (rash), CZP, CLB, steroids, KD | refractory; VPA, TPM, CBZ, LTG, ZNS, prysoline, PB, OCBZ, felbatol, ETX, LEV, lacosamide; exacerbation on KD; partial response to VNS at 16 y | ES responsive to steroids and VGB; focal epilepsy refractory: VPA, TPM | refractory during childhood; seizure-free on VPA and LTG | NA |
| Dysmorphic features | none | mild hypertelorism | coarse facial features, thick lips and big tongue (not related to medication), hoarse voice, hypertelorism | none | hypertelorism, prominent nasal tip extending below the columella, extremely short philtrum, small hands and feet, short stature | single palmar crease R hand; micrognathia |
| Additional features | none | 17 m hyperkinetic choreoathetosis & dystonia of limbs | skin puffiness at hands & feet, hoarse voice | frequent laughing spells & rocking behaviors; bilateral talipes | cleft palate, R congenital talipes equinovarus, stereotypical repetitive & self-injurious behaviors | IUGR at 20 w gestation; born at 34 w; tracheostomy until 7 m due to airway difficulties; anxiety, aggression & self-injurious behaviors; stereotypic hand movements |
Abbreviations are as follows: Ab, absence seizure; At, atonic seizure; ACTH, adrenocorticotropic hormone; AZD, acetazolamide; CBD, cannabidiol; CBZ, carbamazepine; CLB, clobazam; CZP, clonazepam; EEG, electroencephalogram; ES, epileptic spasms; ETX, ethosuxamide; F, focal seizure; FD, focal epileptiform discharges; FM, focal motor seizure; G-tube, gastrostomy tube; GTCS, generalized tonic-clonic seizure; GSW, generalized spike and slow wave; ID, intellectual disability; IUGR, intrauterine growth retardation; KD, ketogenic diet; L, left; LEV, levetiracetam; LTG, lamotrigine; m, months; M, myoclonic seizure; MFD, multifocal epileptiform discharges; MRI, magnetic resonance imaging; N, normal; NA, nonambulatory; NV, nonverbal; OCBZ, Oxcarbazepine; PB, phenobarbitone; PRD, prednisolone; PS, polyspikes; R, right; RFM, rufinamide; Sz, seizure; SE, status epilepticus; T, tonic seizure; TPM, topiramate; VGB, vigabatrin; VPA, valproate; w, weeks; y, years; ZNS, zonisamide.
Subjects and Methods
Patient Populations
All individuals or, in the case of minors or those with intellectual disability, their parents or legal guardians gave informed consent for participation in this study. The study was performed according to the standards of the ethics committees and the institutional review boards at each center.
Epilepsy Genetics Initiative (EGI)
Data from 84 trios who had undergone clinical exome sequencing that did not yield a genetic diagnosis were deposited into the EGI repository after informed consent was obtained. Reanalysis at the Institute for Genomic Medicine identified one case subject (EGI0251B1) with a de novo PPP3CA variant.
Infantile Spasms/Lennox Gastaut Syndrome Cohort
Trio exomes for 356 individuals with epileptic encephalopathies were previously analyzed by the Epi4K and EuroEPINOMICS Consortia to identify de novo variants.1 A single case subject (lgsnd30299is1) with a de novo PPP3CA variant was identified.
Undiagnosed Disease Trios
This cohort consisted of a previously published cohort of 119 trios with undiagnosed genetic disorders. They had a range of phenotypes, including some with neurodevelopmental diseases.23 Individual isrl69xx3 had a de novo PPP3CA variant identified from this cohort.
Developmental and Epileptic Encephalopathies Targeted Sequencing Cohort
This cohort consisted of 508 individuals with a diverse range of developmental and epileptic encephalopathy phenotypes that were recruited from the epilepsy clinic through the practices of the investigators, and by referral for epilepsy genetics research internationally after informed consent. Each individual underwent phenotypic analysis with review of medical records, EEG, and MRI imaging when available and was classified into a specific epilepsy syndrome when possible. Targeted sequencing of PPP3CA was performed using single-molecule molecular inversion probes. Individual T26323 from this cohort had a de novo PPP3CA variant.
PPP3CA was selected for targeted sequencing based on the fact that it is found to be co-expressed with other genes known to cause developmental and epileptic encephalopathies (DEE) using the Web-based tool brain co-X.24 To prioritize genes for the targeted sequencing work performed in this study, we used a set of 42 genes where mutations are known to cause DEE (Table S1), 6 publicly available gene expression datasets that are specific to the human brain (Table S2), and an expression data cleaning algorithm (RUV)25 to prioritize 263 unique candidate genes where a de novo variant was identified by trio WES sequencing in a cohort of 356 individuals with a diagnosis of infantile spasms or Lennox-Gastaut syndrome.1 We note that an earlier version of this prioritization method has been previously described26 and did not prioritize PPP3CA, but it evaluated a much smaller list of 29 genes where mutations are known to cause DEE and only the Allen Human Brain Atlas Gene expression resource.27
Ambry Genetics
This cohort consisted of 3,693 individuals ascertained sequentially who underwent diagnostic WES sequencing through Ambry Genetics and were reported to have either developmental delay, intellectual disability, epilepsy, and/or autism. One individual with a de novo PPP3CA variant was identified through trio WES sequencing from this cohort.
Individual 6
This individual underwent diagnostic trio WES sequencing through GeneDx and was connected to our study through the clinician’s entry into GeneMatcher. The exact number of WES performed to ascertain this sixth case could not be obtained and thus was not included in the statistical analysis.
Sequencing Analysis
Two samples sequenced at the Institute for Genomic Medicine (IGM) (previously the Center for Human Genome Variation at Duke University) were collected as peripheral blood. Exome enrichment was done using the SureSelect Human All Exon - 65MB, or the SureSelect Human All Exon - 50MB (Agilent), depending on when the trio was sequenced but consistent within the trio at the time. Sequencing was performed in the Genomic Analysis Facility on an Illumina HiSeq 2000. Data for an additional case subject were transferred to the IGM from a clinical exome test performed at the UCLA clinical lab using the Agilent SureSelect Human All Exon 50 Mb XT kit and Illumina HiSeq 2500.
All data from these three trios were analyzed in the same way using our standard bioinformatics pipeline which has been previously described.1, 23 The pipeline employs GATK best practices, utilizing BWA 0.5.10,28 picard tools 1.59, and the Unified Genotyper from GATK 1.6-11.29, 30, 31 Reads are aligned to the hg19 reference sequence utilized in 1000 Genomes phase 2, which includes EBV-derived decoy sequences. Variant calls are functionally annotated with SnpEff 3.332 using Ensembl build 73. All samples are processed individually through the bioinformatics pipeline.
Variant calls are loaded into our internal mysql database AnnoDB and further analyzed using ATAV. De novo calls are made by comparing proband variant calls to parents and ensuring adequate coverage (10×) to ensure a true homozygous reference call. De novo calls are further filtered to be high quality, based on GQ (>20), MQ (>40), QD (>2), QUAL (>50).
One proband was identified through targeted sequencing of PPP3CA performed on a cohort of 508 individuals with developmental and epileptic encephalopathies of unknown cause despite previous testing for single-nucleotide and copy number variants prior to this study. A multiplex targeted capture strategy was used to target the coding exons and intron-exon boundaries (>5 bp) (Table S3). Single-molecule molecular inversion probes (smMIPS) were used as previously described, modified to have a 10-fold increase in the ratio of probe to genomic DNA (2,000:1).33, 34 A random five-nucleotide molecular tag within the probe allowed for distinction of genomic molecules and a high-confidence consensus call. Sequencing was performed on an Illumina HiSeq 2500 and mapping and processing were performed as previously described.35
Two additional individuals were identified through clinical trio exome sequencing performed by Ambry and GeneDx and matched through GeneMatcher.20 Genomic DNA extraction, exome library preparation, sequencing, bioinformatics filtering, and data analyses were conducted as previously described.36
Variant Prioritization
Variants were considered likely pathogenic if they arose de novo, affected the protein sequence, and were not observed in 13,122 internal or external control subjects (NHLBI Exome Sequencing Project Exome Variant Server, Genome Aggregation Database [gnomAD v.2.0], Exome Aggregation Consortium37 [ExAC v.2.0; 123,136 individuals], and the 1000 Genomes project38). Variants were validated by Sanger sequencing.
Statistical Testing
Testing for an excess of de novo variants was done by fitDNM.39 fitDNM incorporates gene size, mutation rate, and functional impact to test whether there are more de novo observations in the patient cohort than by chance. The p value was then corrected for multiple testing by the number of CCDS genes, 18,668. While one of the individuals did not have full exome sequencing performed, it is a more conservative calculation to correct for all genes even though they were not actually interrogated. The exact number of WES tests performed to ascertain the sixth case subject could not be obtained. The calculated p value is based on the first five case subjects.
Results
Due to the extremely high genetic heterogeneity of the epilepsies, many genes are initially reported in trio-sequencing studies to harbor only one or two individuals with a de novo mutation across the entire cohort. Depending on the number of trios studied and the size and mutability of the gene, the number of de novo mutations observed in a gene in a single study may not reach the threshold of significance required to robustly implicate the gene as pathogenic. However, it is not uncommon that a subset of these genes harboring de novo mutations will emerge as definitive epilepsy genes when studies are combined, allowing for larger cohorts to be evaluated.1, 7, 35 The same is now true for PPP3CA. In 2014, the Epi4K Consortium reported the identification of a de novo missense mutation in PPP3CA.1, 2 Motivated by the initial observation in the Epi4K cohort and evidence for co-expression of PPP3CA with genes previously associated with epileptic encephalopathy26 via brain-coX (Figure S1, Subjects and Methods),24 targeted sequencing of the gene using single-molecule molecular inversion probes was performed in 508 individuals with developmental and epileptic encephalopathies, and one additional case subject with a de novo PPP3CA mutation was identified. In 2015, another individual with a de novo missense mutation in PPP3CA was identified from exome sequencing of a cohort of individuals with severe congenital disorders.23 A fourth case subject was identified through the Epilepsy Genetics Initiative, a collaborative effort that seeks to identify novel epilepsy genes through aggregate re-analyses of clinically generated exome-sequence data from individuals who had not received a genetic diagnosis at the time of sequencing. A fifth case subject was identified through clinical exome sequencing at Ambry Genetics, and a sixth case subject was linked to this study through GeneMatcher.20 After compiling these observations, we used fitDNM to calculate the probability of observing five de novo mutations in PPP3CA by chance across all five cohorts consisting of a total of 4,760 case subjects with neurodevelopmental disease. Considering the site-based mutation rates, the size of the gene, and the size of the overall cohort, observing five de novo mutations with these predicted effects on the protein is not expected to occur by chance (uncorrected p = 1.2 × 10−8). This remains statistically significant when correcting for the 18,668 genes targeted in the Consensus Coding Sequence (CCDS v.14) (p = 2.161 × 10−4). While the number of exomes sequenced could not be obtained for the sixth case subject, this finding remains significant if the sixth case subject was found in a population smaller than 15,000. PPP3CA also shows extreme depletion of functional variation in the ExAC v.2.0 control population, a pattern consistent with the gene being under purifying selection and associated with severe disease. Of the observed variants in ExAC v.2.0, there is a depletion of variants that are predicted to be damaging: only eight missense variants have PolyPhen 2 HumVar scores40 greater than 0.95, and only one stop gain due to an indel and a single splice site variant are reported in 123,136 exomes. Based on the full variant profile of PPP3CA in ExAC v.2.0, the calculated residual variation intolerance score (RVIS) is −0.71 translating to being among the 20% most intolerant genes in the genome.41 PPP3CA has a genic constraint score of 3.89,42 further supporting the RVIS score that variation predicted to have an effect on protein function is extremely rare in a healthy genome. Given both the significant excess of de novo mutations found across our full cohort and the intolerance of functional variation in PPP3CA in the control population, we now securely implicate pathogenic variants in PPP3CA as a cause of developmental and epileptic encephalopathies.
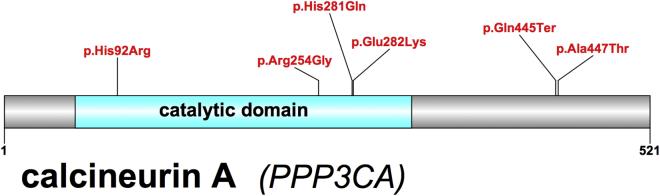
Of the six case subjects in total, one mutation is predicted to cause a premature stop codon in all Ensembl, RefSeq, and CCDS transcript annotations, while the other four mutations lead to missense changes, including one recurrent mutation (c.844G>A [p.Glu282Lys]; Table 1). Three of the four missense mutations fall within the protein phosphatase 2B (PP2B) catalytic domain (Figure 1). Recently, one additional de novo variant in the catalytic domain of calcineurin (c.760A>G [p.Arg254Gly]) was identified in a single individual reported to have an “abnormality of the nervous system” in the Deciphering Developmental Disorders Study.43 The catalytic domain is well conserved among the serine/threonine protein phosphatase family members (PP1, PP2A, PP2B, PP2C) and the low rate of mutation suggests that substitutions in this region may be deleterious.44 Furthermore, the active site is coordinated between three histidines (His92, His199, His281), two aspartic acids (Asp90, Asp118), and one asparagine (Asn150),45 and 4/6 case subjects have mutations at or adjacent to these amino acid positions.
Figure 1.
Calcineurin A (GenBank: NM_000944.4) Protein Structure with De Novo Mutations Mapped above the Image (Red)
The c.844G>A (p.Glu282Lys) is a recurrent mutation in our cohort. The c.760A>G (p.Arg254Gly) mutation was observed in an individual with an “abnormality of the nervous system” in the Deciphering Developmental Disorders study43 and has not been validated. The catalytic domain (amino acid positions 58–329) is highlighted in blue.
Clinically, all six case subjects presented with developmental delay (evident from birth to 2 years of age) which was noted by 3 months in four individuals (Table 1). Regression occurred in three children, with all individuals developing severe or profound ID. Three of the six individuals were non-verbal, two had single words, and one spoke in short sentences. Two had autistic features. Four individuals were non-ambulatory.
Epilepsy was present in 5/6 individuals. Three had seizure onset within 3 months of age and had epileptic spasms and focal seizures. In the remaining two case subjects, seizures started later (3.5 and 4 years). All individuals with epilepsy developed multiple seizure types including focal (4/5), epileptic spasms (3/5), tonic (3/5), myoclonic (2/5), generalized tonic-clonic (2/5), and atonic seizures (1/5). On EEG, multifocal epileptiform discharges were seen in all but one individual who had only generalized discharges. Seizures were refractory to antiepileptic drugs in 4/5 children.
The sixth individual (age 5.5) had no seizures, but an EEG performed for abnormal language development showed an abnormal background with multifocal epileptiform discharges (Table 1). Interestingly, individual 6 had the same c.844G>A (p.Glu282Lys) variant as individual 5 (age 22 years) who had the mildest epilepsy which resolved by 16 years. Individual 5 presented with absence seizures at 4 years followed by myoclonic seizures (5 years), atonic and focal seizures (8 years), and generalized tonic-clonic and tonic seizures (9 years).
Hypotonia was noted in four individuals and spasticity in one. In terms of dysmorphic features, hypertelorism was observed in 3/6; one of these individuals also had coarse facial features, thick lips, a large tongue, and a hoarse voice.
MRI was normal for 3/6 probands, with the other individuals having mild abnormalities of uncertain significance (Table 1).
Discussion
We present evidence that mutations of PPP3CA are associated with developmental and epileptic encephalopathies. PPP3CA encodes the protein calcineurin A. Calcineurin is a highly conserved heterodimer consisting of a catalytic subunit (calcineurin A) and a regulatory subunit (calcineurin B). Calcineurin A is responsible for the interaction of calcineurin with calmodulin.46, 47 Calcineurin in the brain plays an important role in synaptic transmission via regulation of the signaling response to calcium ions. Specifically it has been shown to suppress long-term potentiation,48 play an important role in synaptic plasticity through the regulation of retinoic acid,49 and regulate the activity of N-methyl-D-aspartate (NMDA) receptor channel gating.50
While the role of calcineurin in synaptic transmission is not fully understood, it is involved specifically with synaptic vesicle recycling, a critical process in neurotransmission. The synaptic vesicle cycle begins with cellular depolarization that occurs following the influx of calcium ions through voltage-gated calcium channels. With this depolarization, neurotransmitter is released into the synaptic cleft through a process of exocytosis. Endocytosis, also regulated by intracellular calcium levels, is the process by which these vesicles are recycled at the plasma membrane to enable refilling with neurotransmitters for the next cellular depolarization/neurotransmission event.14 Dynamin-1, encoded by DNM1, is a nerve terminal phosphoprotein with intrinsic guanosine triphosphatase (GTPase) that serves a critical role in endocytosis. During endocytosis, dephosphorylated dynamin molecules form tetramers that pinch off empty vesicles for recycling when they hydrolyze GTP.51 The activity of dynamin is regulated by calcineurin, the phosphatase that has been shown to dephosphorylate dynamin-1.22
Pathogenic variants in dynamin-1 are a known cause of developmental and epileptic encephalopathy that were first implicated when a spontaneous missense mutation was found in a mouse model of epilepsy.52 Several years after this discovery, disease-causing de novo mutations were identified in a cohort of individuals with epileptic encephalopathies.1 DNM1 mutations implicated in epilepsy reduce endocytosis.53 Based on the mechanistic link between calcineurin (encoded in part by PPP3CA) and dynamin-1 (encoded by DNM1), one possibility is that the mutations in PPP3CA may result in epilepsy by reducing DNM1-mediated endocytosis. Further supporting this conjecture, PPP3CA- and DNM1-mediated developmental and epileptic encephalopathies have overlapping clinical features. Both diseases are characterized by hypotonia, present with developmental delay, and lead to severe to profound impairment. Seizure onset is in the first year of life with infantile spasms in DNM1-related epilepsy, which were also seen in three of our six individuals with PPP3CA encephalopathy. In both diseases, individuals develop multiple seizure types and in the majority, the seizures are intractable.
In addition to DNM1, mutation in other genes involved in synaptic vesicle cycling include STXBP1, STX1B, and SNAP25 which have been implicated in epileptic encephalopathies.18, 19, 54, 55 STXBP1, SNAP25, and STX1B encode proteins that form part of the SNARE complex that mediates docking of synaptic vesicles in the process of exocytosis.54 The identification of pathogenic variants in PPP3CA adds to this growing body of evidence supporting the role of dysregulation of synaptic vesicle cycling in epilepsy pathophysiology.
Acknowledgments
We would like to thank all individuals and their families. We also thank individuals and groups who contributed exome sequence samples for analysis, including the Epi4K Consortium, the Epilepsy Phenome Genome Project (EPGP), the Epilepsy Genetics Initiative (EGI) - a signature program of Citizens United for Research in Epilepsy (CURE), EuroEPINOMICS-RES Consortium, and Dr. Vandana Shashi and Dr. Ann Bergin. C.T.M. is supported by postdoctoral fellowships from the Lennox-Gaustaut Syndrome Foundation and the American Epilepsy Society. This study was supported by the NIH National Institute of Neurological Disorders and Stroke (RO1 NS069605 to H.C.M.; U01NS077303; U01NS053998), the Health Research Council of New Zealand, Cure Kids New Zealand, the Australian National Health and Medical Research Council (APP1054618, APP1102971); Victorian State Government Operational Infrastructure Support, Australian Government NHMRC IRIISS funding, as well as additional sources that are listed in the Supplemental Data. K.L.H. and D.N.S. are full-time employees of Ambry Genetics, Inc. Exome sequencing is one of Ambry’s commercially available tests. D.B.G. is a founder and holds equity in Pairnomix and Praxis, and has research funding from Janssen, Gilead, Biogen, AstraZeneca, and UCB. I.E.S. received support from and/or has served as a paid consultant for UCB, Eisai, Athena Diagnostics, GlaxoSmithKline, Nutricia, Transgenomics, and Biocodex.
Published: September 21, 2017
Footnotes
Supplemental Data include one figure, three tables, and Supplemental Acknowledgments and can be found with this article online at http://dx.doi.org/10.1016/j.ajhg.2017.08.013.
Contributor Information
Heather C. Mefford, Email: hmefford@uw.edu.
Erin L. Heinzen, Email: eh2682@cumc.columbia.edu.
Web Resources
brain-coX, http://shiny.bioinf.wehi.edu.au/freytag.s/
CURE’s Epilepsy Genetics Initiative, https://www.cureepilepsy.org/egi/
GeneMatcher, https://genematcher.org/
NHLBI Exome Sequencing Project (ESP) Exome Variant Server, http://evs.gs.washington.edu/EVS/
OMIM, http://www.omim.org/
Supplemental Data
References
- 1.EuroEPINOMICS-RES Consortium. Epilepsy Phenome/Genome Project. Epi4K Consortium De novo mutations in synaptic transmission genes including DNM1 cause epileptic encephalopathies. Am. J. Hum. Genet. 2014;95:360–370. doi: 10.1016/j.ajhg.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Epi4K Consortium Epi4K: gene discovery in 4,000 genomes. Epilepsia. 2012;53:1457–1467. doi: 10.1111/j.1528-1167.2012.03511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epi4K Consortium. Epilepsy Phenome/Genome Project De novo mutations in epileptic encephalopathies. Nature. 2013;501:217–221. doi: 10.1038/nature12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suls A., Jaehn J.A., Kecskés A., Weber Y., Weckhuysen S., Craiu D.C., Siekierska A., Djémié T., Afrikanova T., Gormley P., EuroEPINOMICS RES Consortium De novo loss-of-function mutations in CHD2 cause a fever-sensitive myoclonic epileptic encephalopathy sharing features with Dravet syndrome. Am. J. Hum. Genet. 2013;93:967–975. doi: 10.1016/j.ajhg.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin H.C., Kim G.E., Pagnamenta A.T., Murakami Y., Carvill G.L., Meyer E., Copley R.R., Rimmer A., Barcia G., Fleming M.R., WGS500 Consortium Clinical whole-genome sequencing in severe early-onset epilepsy reveals new genes and improves molecular diagnosis. Hum. Mol. Genet. 2014;23:3200–3211. doi: 10.1093/hmg/ddu030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamdan F.F., Srour M., Capo-Chichi J.-M., Daoud H., Nassif C., Patry L., Massicotte C., Ambalavanan A., Spiegelman D., Diallo O. De novo mutations in moderate or severe intellectual disability. PLoS Genet. 2014;10:e1004772. doi: 10.1371/journal.pgen.1004772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carvill G.L., Weckhuysen S., McMahon J.M., Hartmann C., Møller R.S., Hjalgrim H., Cook J., Geraghty E., O’Roak B.J., Petrou S. GABRA1 and STXBP1: novel genetic causes of Dravet syndrome. Neurology. 2014;82:1245–1253. doi: 10.1212/WNL.0000000000000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Brien J.E., Meisler M.H. Sodium channel SCN8A (Nav1.6): properties and de novo mutations in epileptic encephalopathy and intellectual disability. Front. Genet. 2013;4:213. doi: 10.3389/fgene.2013.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lemke J.R., Hendrickx R., Geider K., Laube B., Schwake M., Harvey R.J., James V.M., Pepler A., Steiner I., Hörtnagel K. GRIN2B mutations in West syndrome and intellectual disability with focal epilepsy. Ann. Neurol. 2014;75:147–154. doi: 10.1002/ana.24073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michaud J.L., Lachance M., Hamdan F.F., Carmant L., Lortie A., Diadori P., Major P., Meijer I.A., Lemyre E., Cossette P. The genetic landscape of infantile spasms. Hum. Mol. Genet. 2014;23:4846–4858. doi: 10.1093/hmg/ddu199. [DOI] [PubMed] [Google Scholar]
- 11.Poduri A., Heinzen E.L., Chitsazzadeh V., Lasorsa F.M., Elhosary P.C., LaCoursiere C.M., Martin E., Yuskaitis C.J., Hill R.S., Atabay K.D. SLC25A22 is a novel gene for migrating partial seizures in infancy. Ann. Neurol. 2013;74:873–882. doi: 10.1002/ana.23998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staley K. Molecular mechanisms of epilepsy. Nat. Neurosci. 2015;18:367–372. doi: 10.1038/nn.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torkamani A., Bersell K., Jorge B.S., Bjork R.L., Jr., Friedman J.R., Bloss C.S., Cohen J., Gupta S., Naidu S., Vanoye C.G. De novo KCNB1 mutations in epileptic encephalopathy. Ann. Neurol. 2014;76:529–540. doi: 10.1002/ana.24263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heine M. Surface traffic in synaptic membranes. In: Kreutz M.R., Sala C., editors. Synaptic Plasticity. Springer Vienna; 2012. pp. 197–219. [Google Scholar]
- 15.Schubert J., Siekierska A., Langlois M., May P., Huneau C., Becker F., Muhle H., Suls A., Lemke J.R., de Kovel C.G.F., EuroEPINOMICS RES Consortium Mutations in STX1B, encoding a presynaptic protein, cause fever-associated epilepsy syndromes. Nat. Genet. 2014;46:1327–1332. doi: 10.1038/ng.3130. [DOI] [PubMed] [Google Scholar]
- 16.Saitsu H., Kato M., Mizuguchi T., Hamada K., Osaka H., Tohyama J., Uruno K., Kumada S., Nishiyama K., Nishimura A. De novo mutations in the gene encoding STXBP1 (MUNC18-1) cause early infantile epileptic encephalopathy. Nat. Genet. 2008;40:782–788. doi: 10.1038/ng.150. [DOI] [PubMed] [Google Scholar]
- 17.Scheffer I.E., Berkovic S., Capovilla G., Connolly M.B., French J., Guilhoto L., Hirsch E., Jain S., Mathern G.W., Moshé S.L. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:512–521. doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rohena L., Neidich J., Truitt Cho M., Gonzalez K.D., Tang S., Devinsky O., Chung W.K. Mutation in SNAP25 as a novel genetic cause of epilepsy and intellectual disability. Rare Dis. 2013;1:e26314. doi: 10.4161/rdis.26314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen X.-M., Selcen D., Brengman J., Engel A.G. Mutant SNAP25B causes myasthenia, cortical hyperexcitability, ataxia, and intellectual disability. Neurology. 2014;83:2247–2255. doi: 10.1212/WNL.0000000000001079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sobreira N., Schiettecatte F., Valle D., Hamosh A. GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum. Mutat. 2015;36:928–930. doi: 10.1002/humu.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rusnak F., Mertz P. Calcineurin: form and function. Physiol. Rev. 2000;80:1483–1521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- 22.Lai M.M., Hong J.J., Ruggiero A.M., Burnett P.E., Slepnev V.I., De Camilli P., Snyder S.H. The calcineurin-dynamin 1 complex as a calcium sensor for synaptic vesicle endocytosis. J. Biol. Chem. 1999;274:25963–25966. doi: 10.1074/jbc.274.37.25963. [DOI] [PubMed] [Google Scholar]
- 23.Zhu X., Petrovski S., Xie P., Ruzzo E.K., Lu Y.-F., McSweeney K.M., Ben-Zeev B., Nissenkorn A., Anikster Y., Oz-Levi D. Whole-exome sequencing in undiagnosed genetic diseases: interpreting 119 trios. Genet. Med. 2015;17:774–781. doi: 10.1038/gim.2014.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freytag S., Burgess R., Oliver K.L., Bahlo M. brain-coX: investigating and visualising gene co-expression in seven human brain transcriptomic datasets. Genome Med. 2017;9:55. doi: 10.1186/s13073-017-0444-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freytag S., Gagnon-Bartsch J., Speed T.P., Bahlo M. Systematic noise degrades gene co-expression signals but can be corrected. BMC Bioinformatics. 2015;16:309. doi: 10.1186/s12859-015-0745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliver K.L., Lukic V., Freytag S., Scheffer I.E., Berkovic S.F., Bahlo M. In silico prioritization based on coexpression can aid epileptic encephalopathy gene discovery. Neurol Genet. 2016;2:e51. doi: 10.1212/NXG.0000000000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hawrylycz M.J., Lein E.S., Guillozet-Bongaarts A.L., Shen E.H., Ng L., Miller J.A., van de Lagemaat L.N., Smith K.A., Ebbert A., Riley Z.L. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489:391–399. doi: 10.1038/nature11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H., Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26:589–595. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van der Auwera G.A., Carneiro M.O., Hartl C., Poplin R., del Angel G., Levy-Moonshine A., Jordan T., Shakir K., Roazen D., Thibault J. From FastQ data to high-confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr. Protoc. Bioinformatics. 2002;43 doi: 10.1002/0471250953.bi1110s43. 11.10.1-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DePristo M.A., Banks E., Poplin R., Garimella K.V., Maguire J.R., Hartl C., Philippakis A.A., del Angel G., Rivas M.A., Hanna M. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K., Kernytsky A., Garimella K., Altshuler D., Gabriel S., Daly M., DePristo M.A. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cingolani P., Platts A., Wang L., Coon M., Nguyen T., Wang L., Land S.J., Lu X., Ruden D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 2012;6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hiatt J.B., Pritchard C.C., Salipante S.J., O’Roak B.J., Shendure J. Single molecule molecular inversion probes for targeted, high-accuracy detection of low-frequency variation. Genome Res. 2013;23:843–854. doi: 10.1101/gr.147686.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Roak B.J., Vives L., Fu W., Egertson J.D., Stanaway I.B., Phelps I.G., Carvill G., Kumar A., Lee C., Ankenman K. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science. 2012;338:1619–1622. doi: 10.1126/science.1227764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Epi4K Consortium De novo mutations in SLC1A2 and CACNA1A are important causes of epileptic encephalopathies. Am. J. Hum. Genet. 2016;99:287–298. doi: 10.1016/j.ajhg.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farwell K.D., Shahmirzadi L., El-Khechen D., Powis Z., Chao E.C., Tippin Davis B., Baxter R.M., Zeng W., Mroske C., Parra M.C. Enhanced utility of family-centered diagnostic exome sequencing with inheritance model-based analysis: results from 500 unselected families with undiagnosed genetic conditions. Genet. Med. 2015;17:578–586. doi: 10.1038/gim.2014.154. [DOI] [PubMed] [Google Scholar]
- 37.Lek M., Karczewski K.J., Minikel E.V., Samocha K.E., Banks E., Fennell T., O’Donnell-Luria A.H., Ware J.S., Hill A.J., Cummings B.B., Exome Aggregation Consortium Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Auton A., Brooks L.D., Durbin R.M., Garrison E.P., Kang H.M., Korbel J.O., Marchini J.L., McCarthy S., McVean G.A., Abecasis G.R., 1000 Genomes Project Consortium A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang Y., Han Y., Petrovski S., Owzar K., Goldstein D.B., Allen A.S. Incorporating functional information in tests of excess de novo mutational load. Am. J. Hum. Genet. 2015;97:272–283. doi: 10.1016/j.ajhg.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adzhubei I., Jordan D.M., Sunyaev S.R. Predicting functional effect of human missense mutations using PolyPhen-2. Curr. Protoc. Hum. Genet. 2013;Chapter 7:20. doi: 10.1002/0471142905.hg0720s76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petrovski S., Wang Q., Heinzen E.L., Allen A.S., Goldstein D.B. Genic intolerance to functional variation and the interpretation of personal genomes. PLoS Genet. 2013;9:e1003709. doi: 10.1371/journal.pgen.1003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samocha K.E., Robinson E.B., Sanders S.J., Stevens C., Sabo A., McGrath L.M., Kosmicki J.A., Rehnström K., Mallick S., Kirby A. A framework for the interpretation of de novo mutation in human disease. Nat. Genet. 2014;46:944–950. doi: 10.1038/ng.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deciphering Developmental Disorders Study Prevalence and architecture of de novo mutations in developmental disorders. Nature. 2017;542:433–438. doi: 10.1038/nature21062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacKintosh R.W., Haycox G., Hardie D.G., Cohen P.T. Identification by molecular cloning of two cDNA sequences from the plant Brassica napus which are very similar to mammalian protein phosphatases-1 and -2A. FEBS Lett. 1990;276:156–160. doi: 10.1016/0014-5793(90)80531-m. [DOI] [PubMed] [Google Scholar]
- 45.Shi Y. Serine/threonine phosphatases: mechanism through structure. Cell. 2009;139:468–484. doi: 10.1016/j.cell.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 46.Aitken A., Klee C.B., Cohen P. The structure of the B subunit of calcineurin. Eur. J. Biochem. 1984;139:663–671. doi: 10.1111/j.1432-1033.1984.tb08055.x. [DOI] [PubMed] [Google Scholar]
- 47.Cohen, P.T.W., Chen, M.X., and Armstrong, C.G. (1996). Novel protein phosphatases that may participate in cell signaling. In Advances in Pharmacology (Academic Press), J.T. August, F. Murad, M.W. Anders, and J.C. Coyle, eds., pp. 67–89. [DOI] [PubMed]
- 48.Winder D.G., Mansuy I.M., Osman M., Moallem T.M., Kandel E.R. Genetic and pharmacological evidence for a novel, intermediate phase of long-term potentiation suppressed by calcineurin. Cell. 1998;92:25–37. doi: 10.1016/s0092-8674(00)80896-x. [DOI] [PubMed] [Google Scholar]
- 49.Arendt K.L., Zhang Z., Ganesan S., Hintze M., Shin M.M., Tang Y., Cho A., Graef I.A., Chen L. Calcineurin mediates homeostatic synaptic plasticity by regulating retinoic acid synthesis. Proc. Natl. Acad. Sci. USA. 2015;112:E5744–E5752. doi: 10.1073/pnas.1510239112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lieberman D.N., Mody I. Regulation of NMDA channel function by endogenous Ca(2+)-dependent phosphatase. Nature. 1994;369:235–239. doi: 10.1038/369235a0. [DOI] [PubMed] [Google Scholar]
- 51.Marks B., Stowell M.H.B., Vallis Y., Mills I.G., Gibson A., Hopkins C.R., McMahon H.T. GTPase activity of dynamin and resulting conformation change are essential for endocytosis. Nature. 2001;410:231–235. doi: 10.1038/35065645. [DOI] [PubMed] [Google Scholar]
- 52.Boumil R.M., Letts V.A., Roberts M.C., Lenz C., Mahaffey C.L., Zhang Z.W., Moser T., Frankel W.N. A missense mutation in a highly conserved alternate exon of dynamin-1 causes epilepsy in fitful mice. PLoS Genet. 2010;6:e1001046. doi: 10.1371/journal.pgen.1001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dhindsa R.S., Bradrick S.S., Yao X., Heinzen E.L., Petrovski S., Krueger B.J., Johnson M.R., Frankel W.N., Petrou S., Boumil R.M., Goldstein D.B. Epileptic encephalopathy-causing mutations in DNM1 impair synaptic vesicle endocytosis. Neurol Genet. 2015;1:e4. doi: 10.1212/01.NXG.0000464295.65736.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rickman C., Medine C.N., Bergmann A., Duncan R.R. Functionally and spatially distinct modes of munc18-syntaxin 1 interaction. J. Biol. Chem. 2007;282:12097–12103. doi: 10.1074/jbc.M700227200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stamberger H., Nikanorova M., Willemsen M.H., Accorsi P., Angriman M., Baier H., Benkel-Herrenbrueck I., Benoit V., Budetta M., Caliebe A. STXBP1 encephalopathy: A neurodevelopmental disorder including epilepsy. Neurology. 2016;86:954–962. doi: 10.1212/WNL.0000000000002457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.