Abstract
Purpose of the review
To summarize recent evidence supporting the use of reactive carbonyl species scavengers in the prevention and treatment of disease.
Recent findings
The newly developed 2-aminomethylphenol class of scavengers shows great promise in preclinical trials for a number of diverse conditions including neurodegenerative diseases and cardiovascular disease. In addition, new studies with the thiol-based and imidazole-based scavengers have found new applications outside of adjunctive therapy for chemotherapeutics.
Summary
Reactive oxygen species (ROS) generated by cells and tissues act as signaling molecules and as cytotoxic agents to defend against pathogens, but ROS also cause collateral damage to vital cellular components. The polyunsaturated fatty acyl chains of phospholipids in the cell membranes are particularly vulnerable to damaging peroxidation by ROS. Evidence suggests that the breakdown of these peroxidized lipids to reactive carbonyls species plays a critical role in many chronic diseases. Antioxidants that abrogate ROS-induced formation of reactive carbonyl species also abrogate normal ROS signaling and thus exert both beneficial and adverse functional effects. The use of scavengers of reactive dicarbonyl species represent an alternative therapeutic strategy to potentially mitigate the adverse effects of ROS without abrogating normal signaling by ROS. In this review, we focus on three classes of reactive carbonyl species scavengers: thiol-based scavengers (2-mercaptoethanesulfonate and amifostine), imidazole-based scavengers (carnosine and its analogs), and 2-aminomethylphenols-based scavengers (pyridoxamine, 2-hydroxybenzylamine, and 5’-O-pentyl-pyridoxamine) that are either undergoing pre-clinical studies, advancing to clinical trials, or are already in clinical use.
Keywords: Reactive Carbonyl Species, Lipid Aldehydes, Reactive Oxygen Species, Scavengers, Oxidative Stress, Post-translational modifications
Introduction
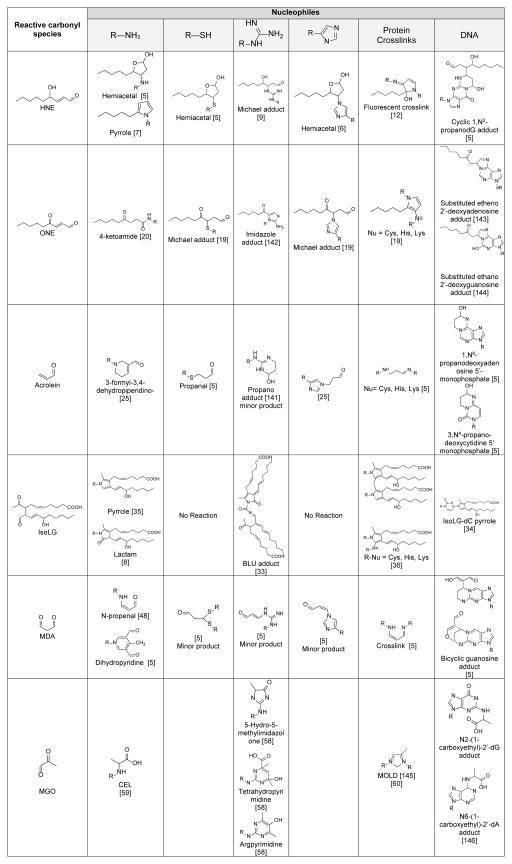
Reactive oxygen species (ROS) perform key signaling and defense functions within cells and tissues, but dysregulated ROS formation or catabolism contributes to disease processes by damaging cellular macromolecules and invoking unresolved inflammatory responses. Several ROS, such as the hydroxyl radical (HO−), drive peroxidation of polyunsaturated fatty acids (PUFAs) such as linolenic acid and arachidonic acid present as the acyl chain tails of membrane phospholipids. Non-enzymatic rearrangement and fragmentation of these phospholipid peroxides generates a broad spectrum of peroxidized lipids. Perhaps the most important class of these peroxidized lipids in terms of disease pathogenesis are the reactive carbonyl species (RCS), which react with cellular proteins, nucleic acids, and phospholipids to significantly impact enzymatic function and cell signaling. RCS generated by lipid peroxidation include mono aldehydes (e.g. hexanal), alkenals (e.g. acrolein), bifunctional alkenals, (e.g. 4-hydroxy-2-nonenal), 1,2-dicarbonyls (e.g. methylglyoxal), 1,3-dicarbonyls (e.g. malondialdehyde), and 1,4-dicarbonyls (e.g. isolevuglandins and 4-oxo-2-nonenal) (Table 1).
Table 1.
Reactive carbonyl species and their reactions with nucleophiles to form stable adducts
Increased levels of lipid-derived RCS have been well documented in vivo, but the question of whether reducing their formation or activity would provide significant health benefits remains unanswered. Lipid RCS appear to exert their adverse effects by modifying proteins, nucleic acids, and phosphatidylethanolamines, so that compounds that prevent these modification are the primary focus of current therapeutic development. Conceptually, RCS modification could exert biological effects either by loss of function or gain of function. Gain of function would be most likely, since the low level of RCS typically seen even in pathological settings is generally not consistent with what is needed to induce loss of function. For instance, while modification of lysines or cysteines in enzymatic active sites can cause loss of function, most cells possess significant spare enzymatic capacity. In order to meaningfully reduce function, a relatively high level of RCS is needed. Yet only 1 to 5% of total copies of an enzyme per cell typically appear to be modified by RCS, even under pathophysiological conditions. In contrast, RCS modifications that occur at relatively low RCS concentration (nM or very low μM) can lead to gain of function. For instance, RCS modification can create novel epitopes or pattern recognition molecules recognized by immune cells. Modification of only a few copies of an enzyme, receptor, structural protein, or other target such as phosphatidylethanolamine is generally needed to profoundly alter cellular responses. Fortunately, the existence of low levels of RCS makes scavenging by small molecules a potential therapeutic approach for treating chronic diseases.
Properties of individual reactive carbonyls species
In the past decade, a number of nucleophilic compounds with reasonable bioavailability and low toxicity have been developed for use as RCS scavengers. Table 2 illustrates the major scavenger classes that are currently in or under investigation for clinical use including: thiol-based scavengers (e.g. 2-mercaptoethanosulfate and amifostine); imidazole-based scavengers (e.g. carnosine); and the recently developed 2-aminomethylphenols (e.g. pyridoxamine, 5’-O-pentyl-pyridoxamine (PPM), and 2-hydroxybenzylamine (2-HOBA)). The guanidine-based class of scavengers, will not be discussed extensively in this review because the development of aminoguanidine (trade name pimagedine), the lead compound in this class, was discontinued when clinical trials failed to show efficacy and demonstrated mild toxicity [1, 2]. Nevertheless, another guanidine compound, metformin, is widely used in the treatment in diabetes. While metformin scavenges RCS in vitro [3], whether RCS scavenging actually mediates any of its beneficial effects in vivo has not been established. If so, then future studies may revive development of new guanidine-based scavengers.
Table 2.
Dicarbonyl scavengers and their in vivo applications
| Scavenger by class | Reaction rate | In vivo applications or studies |
|---|---|---|
| Thiol-based | ||
|
Acrolein = ONE > HNE |
|
|
Acrolein = ONE > HNE > > others | |
| Imidazole-based | ||
|
Acrolein = ONE > HNE > MGO > others |
|
| 2-aminomethylphenols | ||
|
IsoLG > ONE > MGO > Acrolein = MDA |
|
|
IsoLG > ONE > MGO > Acrolein = MDA |
|
|
IsoLG > ONE > MGO > Acrolein = MDA | |
Multiple classes of RCS scavengers are needed because individual RCS differ dramatically in their ability to modify specific functional groups. RCS scavengers based on these different functional groups can therefore also be used as experimental probes to assess contribution of particular RCS types to disease processes. We will therefore illustrate these differences in functional group reactivity using commonly studied representatives of various RCS types in the following section.
4-hydroxy-2-nonenal (HNE)
The α,β-unsaturated aldehyde 4-hydroxynonenal (HNE) is the most extensively studied RCS formed in vivo. All ω-6 PUFA can give rise to HNE and multiple mechanisms have been proposed for its formation [4]. The α,β-unsaturated aldehydes including HNE preferentially react with sulfhydryl groups of thiols to form Michael adducts [5]. In the case of HNE, the Michael adduct subsequently undergoes secondary reaction to form a cyclic hemiacetal (Table 1). HNE also forms Michael adducts with imidazoles [6] but at a slower rate than with thiols (Table 1). HNE can also react with primary amines to form both Michael and pyrrole adducts [7]. Such reactions are slow compared to other RCS that preferentially react with amines, such as the 4-ketoaldehydes. For instance, HNE reacts 100-fold slower than isolevuglandins (IsoLG) with bovine serum albumin [8]. HNE also reacts to some extent with arginine to form a Michael adduct [9]. In addition to amino acid residues, HNE also reacts with phosphatidylethanolamine to form Michael and pyrrole adducts [10], and with DNA bases to form exocyclic etheno-DNA adducts [11]. HNE crosslinks proteins, and at least one of the resulting crosslinked adducts is fluorescent [12].
Pharmacological thiol-based scavengers such as 2-mercaptoethanesulfonate (MESNA) and penicillamine, are highly effective for scavenging HNE. Because HNE forms Michael adducts with imidazoles and primary amines, carnosine and its analogs are also good scavengers [13–15]. 2-aminomethylphenols [16, 17] and aminoguanidine [18] are very poor HNE scavengers. These classes of scavengers will be explained in greater detail below.
4-oxo-2-nonenal (ONE)
4-oxo-2-nonenal (ONE) is an α,β-unsaturated, 1,4-dicarbonyl that is generated from similar precursors as HNE, but the presence of the 4-keto group of ONE in place of the 4-hydroxyl group of HNE dramatically alters ONE’s biochemical properties and reactivity. For instance, even after ONE reacts with thiols to form a Michael adduct, the remaining 4-ketoaldehyde functional group can still react with primary amines to form pyrrole adducts [19]. This secondary reaction is one of several potential mechanisms whereby ONE can generate crosslinks, making ONE a very potent crosslinking compound [19]. Even in the absence of thiols, ONE can rapidly react with primary amines to form a highly stable ketoamide adduct [20] (Table 1). Thus ONE is potentially one of the most damaging RCS formed in vivo.
Because ONE is highly reactive to thiols and amines, it may be scavenged by thiols, imidazoles, and 2-aminomethylphenols. Because an ONE-thiol adduct retains the capacity to react with primary amines, scavengers that combine two functional groups (e.g. amifostine or carnosine) or that form stable, non-reactive adducts with ONE (e.g. 2-aminomethylphenols) are more effective than mono-functional endogenous scavengers such as gluthathione.
Acrolein
The biochemistry and biological activity of acrolein has been extensively reviewed [5, 21]. Important sources of acrolein in vivo include the oxidation of polyamines, amino acids, carbohydrates, and glycerol. Cigarette smoke is also a major source of acrolein [21].
Acrolein reacts non-enzymatically with sulfhydryl groups of cellular thiol compounds (e.g. glutathione) to form Michael adducts (Table 1) at a rate about 100-fold faster than HNE [5]. In cells and tissues, the reaction of acrolein with glutathione is also catalyzed by several different glutathione transferases and this reaction appears to be an important mechanisms for detoxifying acrolein [22]. Not surprisingly, this reaction can significantly deplete glutathione levels when cells are exposed to sufficiently high acrolein concentrations, leading to cell death [23]. In addition to its reaction with the thiol groups of cysteine, acrolein reacts with the imidazole group of histidine and with primary amines, but at a much slower rate [5]. Acrolein can also slowly react with the guanidine group of arginine and of guanosine [21, 24]. Reaction of acrolein with primary amines forms an Nε-(3-formyl-3,4-dehydropiperidino) adduct (FDP) [25] while reaction with arginine and nucleic acids forms cyclic hydroxyl-N-propano adducts [5] (Table 1). Acrolein is equipotent with HNE in crosslinking proteins, and significantly more potent than hexanal or malondialdehyde (MDA) [26]. The biological activity of acrolein has been primarily attributed to its modification of thiols, either via depletion of glutathione to invoke toxic effects or the modification of active site cysteines such as in phosphatases to inhibit their activity [5].
Pharmacological thiols such as MESNA have been developed for therapeutic use as acrolein scavengers [27]. Imidazole based scavengers such as carnosine also react with acrolein, and acrolein-carnosine adducts can be detected in urine at slightly lower concentrations than hydroxyalkenal adducts [28]. 2-aminomethylphenols are relatively poor acrolein scavengers.
Isolevuglandins (IsoLGs)
Isolevulandins (IsoLGs), sometimes referred to as isoketals, are a large family of 4-ketoaldehyde regio- and stereo-isomers initially characterized by Bob Salomon’s group at Case Western Reserve University in the 1980s [29–31]. Subsequent work by both the Salomon group and that of L. Jackson Robert II’s group at Vanderbilt University further characterized these products and their reaction with cellular nucleophiles [8, 32]. IsoLGs are members of the prostaglandin/isoprostane family of eicosanoids that are generated enzymatically by cyclooxygenases and non-enzymatically by lipid peroxidation. IsoLGs react extremely rapidly with primary amines [8], but more slowly with guanidine groups like arginine [33] or with the nucleic acids [34]. IsoLGs react poorly or not at all with thiols or imidazoles. Their hyper-reactivity with primary amines arise from the ability of the initial imine adduct to undergo a secondary reaction of the 4-keto group with the nitrogen to form an irreversible pyrrole adduct [35] (Table 1). These pyrrole adducts easily oxidize in the presence of molecular oxygen to form lactam and hydroxylactam adducts [25], as well as pyrrole-pyrrole dimers and trimers [36]. The proclivity of IsoLGs to crosslink proteins even at very low concentrations set them apart from other RCS.
IsoLG has been shown to exert potent inflammatory effects, particularly in activating endothelial cells [37], macrophages [38], and dendritic cells [39]. In particular, dendritic cells take up proteins modified by IsoLGs, present these neo-antigens to T cells, which activates them, leading to hypertension and vascular disease [39, 40]. Reactions of IsoLG with proteins may also drive development of arrhythmias by modification of ion channels [41–43] and amyloid forming proteins [44]. IsoLG modification can block degradation of proteins by the proteasomal pathway [45] and induce endoplasmic reticulum stress [37]. The signaling pathways that mediate the inflammatory effects of IsoLGs are poorly understood, although the Receptor for Advanced Glycation Endproducts (RAGE) mediates some of the biological effects of IsoLG-modified PE [38]. IsoLG lactam adducts resemble tethered prostaglandins, but to what extent prostanoid receptors contribute to their biological effects is poorly understood [38, 46].
Only 2-aminomethylphenols can effectively scavenge IsoLGs. Because IsoLGs specifically react with primary amines, thiol-based compounds like 2-mercaptoethanosulfate are not effective IsoLG scavengers. Therefore, if 2-aminomethylphenols are effective when other scavenger classes are not, this provides evidence for the contribution of IsoLG or other 1,4-dicarbonyls to disease pathogenesis.
Malondialdehyde (MDA)
The 1,3-dicarbonyl malondialdehyde (MDA) is one of the most abundant lipid peroxidation product, formed by non-enzymatic peroxidation of PUFA [47, 5]. MDA also forms enzymatically through thromboxane synthetase from prostaglandin H2, which generates MDA at a 1:1 ratio with thromboxane A2. MDA is a common biomarker of lipid peroxidation because its reaction with thiobarbituric acid forms an intensely colored chromogen (Thiobarbituric acid reactive substances, or TBARS assay).
Although MDA is generated abundantly, it reacts slowly compared to many other RCS. For instance, when 100 μM HNE and MDA are reacted with 1 mM bovine serum albumin, about 75% of the HNE has reacted in 1 hour compared to less than 50% of MDA has reacted in 5 hours [5]. At physiological pH, the reaction of MDA with thiols such as glutathione is trivial. MDA reacts with primary amines such as lysine to form a Schiff base which rearranges to a propenal adduct [48]. This may react further with other lysine residues of the same or different protein molecule to form crosslinks (Table 1). In addition, multiple MDA molecules can condense on primary amines to form dihydropyridine products. MDA also reacts with deooxyguanosine and deoxyadenosine to form stable adducts, with the predominant form under physiological conditions being the pyrimidopurinone M1G [49] Of interest, an N-substituted form of MDA, adenine propenal, was recently described to form from the oxidation of adenosine [50], and is more reactive with lysine than unsubstituted MDA.
Because of its preferential reaction with primary amines, the optimal scavenger class for MDA appear to be the 2-aminomethylphenols [51]. Reduced TBARS levels in vivo have been reported with administration of thiol-based scavengers such as MESNA [52, 53] or carnosine [54, 55]. However, these studies used the TBARS assay without further characterization by high performance liquid chromatography, so whether the measured RCS were actually MDA or some other RCS is unknown.
Methylglyoxal (MGO)
The biochemistry and biological activities of the 1,2-dicarbonyl MGO has been reviewed recently [56, 57]. MGO is generated by the oxidation of carbohydrates, fatty acids, and amino acids and catabolized by glyoxlase I. Formation of MGO is particularly prevalent in diabetes due to the high levels of plasma glucose. Initial characterization of the reaction of MGO with various amino acids showed that MGO slowly reacts with the α-amine of amino acids, but reacts faster with histidine, cysteine, or lysine, and much faster with arginine [58]. However, the overall reaction rate of MGO with protein is relatively slow. When radiolabeled MGO was reacted with bovine serum albumin, about 50% of MGO reacted after 6 hours, and complete MGO reaction was achieved in 2 days [58]. In the reaction of MGO with bovine serum albumin, only arginine residues showed high levels of modification [58]. MGO reacts with guanidines such as arginine to form 5-methylimidazol-4-one (MG-H1), tetrahydropyrimidine, and argpyrimidine (Table 1) [58]. MGO reacts with lysine to form the highly stable adduct N-carboxy-ethyl-lysine (CEL) as well as an imidazolium cross-link [59, 60]. MGO rapidly reacts with thiols such as cysteine to form hemithioacetal adducts, but this reaction is highly reversible, so no stable product is formed unless further reaction with other nucleophiles occurs. Increased levels of MG-H1 and CEL protein adducts have been detected in numerous diseases including diabetes, atherosclerosis, and kidney disease, and are especially increased in conditions where glyoxalase expression is reduced [61].
Modification of bovine serum albumin by MGO in vitro could be inhibited by co-incubation with other amines, with the best inhibition given by aminoguanidine, then arginine, and then lysine [58]. Other studies showed such modification could also be inhibited by cysteine and penicillamine, but not by N-acetylcysteine, suggesting that the thiols required an amine for efficacy 58]. Co-incubation with carnosine also protected ovalbumin from modification by MGO [62]. 2-aminomethylphenols effectively scavenge MGO [63], forming an imidazolium crosslinked product analogous to that formed with lysine [63].
Hexanal
Oxidation of ω-6 PUFAs results in the generation of hexanal, a volatile saturated mono-aldehyde that is relatively unreactive compared to alkenals and dicarbonyls. Given its ease of measurement, hexanal levels have been used as a measure of lipid peroxidation in a number of human diseases [64–67]. Hexanal reacts with primary amines such as lysine or phosphatidylethanolamine to form Schiff base adducts that are highly reversible. Therefore, adding even 10-fold excess of various amino acids to hexanal forms very little stable adducts and does not significantly reduce hexanal concentration [68]. Sulfhydryl containing compounds such as glutathione and alpha-lipoic acid and most imidazole compounds also do not to react to an appreciable extent with hexanal [68]. Given its relatively poor reactivity with cellular nucleophiles and its catabolism by ALDH3A1 [69], there has been little impetus for the development of hexanal scavengers.
Reactive Carbonyl Species scavengers ameliorate disease
To be effective in vivo, scavengers must outcompete sensitive cellular nucleophiles for reaction with RCS. To achieve greater reaction rates than endogenous nucleophiles, RCS scavengers utilize additional functional groups in proximity to the primary nucleophile which catalyze rate-limiting reactions or participate in forming stable and irreversible final products. Even with reaction rates enhanced 1000-fold, scavengers need to be in μM concentrations to effectively compete with endogenous nucleophiles, which are present in tissues at mM concentrations. This requires scavengers to have sufficient bioavailability and partitioning into tissues to achieve such concentrations without also manifesting toxicity. Aminoguanidine failed in clinical trials due to toxicity and poor efficacy. For the remainder of this review, we will focus on the best studied pharmacological RCS scavengers from three classes: thiols (MESNA and amifostine), imidazoles (carnosine), and 2-aminomethylphenols (pyridoxamine, pentylpyridoxamine, and 2-hydroxybenzylamine) that have shown effectiveness in ameliorating disease in animal models and humans (Table 2). Studies using these first generation scavengers suggests that RCS scavenging prevents and/or reverses disease and that further development of these approaches is warranted.
Thiol Based Scavengers
Although endogenous thiols such as glutathione, lipoic acid, or N-acetylcysteine have been used a dietary supplements, their already high levels in healthy humans warranted development of pharmaceutical thiols with higher reactivity. MESNA and amifostine (via its active metabolite WR-1065) react with acrolein at significantly faster rates than does glutathione [70]. Both MESNA and amifostine are used extensively in human clinical conditions and will therefore be discussed in detail. However, it is worthwhile noting that other thiol-based medications such as penicillamine are quite competent RCS scavengers (at least in vitro). To what extent the ability of these other drugs to scavenge RCS contribute to therapeutic effects needs more extensive evaluation.
2-mercaptoethanesulfonate (MESNA)
MESNA (trade names include Uromitexan or Mesnex) has been used extensively since the 1990s as an adjunctive treatment with chemotherapeutic agents like cyclophosphamides and ifosfamide that generate acrolein as a byproduct and thereby incite urotoxicity [71]. Screening a series of thiol containing compounds for their ability to scavenge acrolein and to reduce urotoxicity in cyclophosphamide-treated rats identified MESNA as a highly efficacious scavenger [71]. An initial trial in 1986 showed that co-administration of MESNA with ifosfamide to patients with advanced non-small cell lung cancer significantly reduced ifosfamide-induced hemorrhagic cystitis [72]. A subsequent placebo-controlled, double-blind study showed that co-administration of MESNA with ifosfamide markedly reduced micturition pain and hematuria compared to placebo [73]. MESNA administration to patients receiving cyclophosphamide prior to bone marrow transplant also reduced overall rates of hematuria, discomfort and bladder spasms, and urinary tract infections [74].
Animal and clinical trials support co-administration of MESNA with other therapeutic agents where RCS-mediated toxicity is an adverse effect. Initial studies in rats showed that MESNA co-administration with the chemotherapeutic agent doxorubicin markedly decreased doxorubicin-induced protein modification and tumor necrosis factor (TNF) increases [75]. A subsequent clinical trial confirmed the ability of MESNA to blunt plasma TNF increases induced by doxorubicin treatment of patients with breast cancer or non-Hodgkin lymphoma [76]. MESNA also reduced the nephrotoxicity associated with use of contrast media in radiographic procedures in randomized control trials [77]. Cisplatin is a widely used chemotherapeutic agent that increases RCS levels and co-administration of MESNA with cisplatin reduced RCS levels and ovarian damage in rats [52].
Animal studies suggest the utility of MESNA in clinical conditions unrelated to chemotherapeutics. For instance, administration of MESNA attenuates RCS-mediated gastric mucosal damage and inflammatory cytokine levels [78]. Similarly, in a mouse model of ulcerative colitis, MESNA alone or co-administered with omega-3 PUFAs markedly attenuates intestinal damage [79]. Acetaminophen-induced liver injury is associated with significant formation of acrolein, and treatment of mice with MESNA significantly reduces acetaminophen-induced liver injury and acrolein adduct formation [80]. Traumatic brain injury is associated with extremely high levels of RCS and MESNA treatment markedly reduced RCS levels and protected brain tissue in rats subjected to traumatic brain injury [81]. Clinical trials are needed to confirm the efficacy of MESNA against these same conditions in humans.
Amifostine
Amifostine (trade name Ethyol) is a prodrug that is hydrolyzed to its active metabolite, WR-1065, by alkaline phosphatases. The reactivity of acrolein with WR-1065 is 3x faster than with MESNA [70]. In addition to scavenging acrolein and related RCS, WR-1065 may also act by inducing cellular anoxia and by scavenging free radicals [82].
Amifostine is used adjunctively with radiation therapy where xerostomia and mucositis limit the dose of radiation used. A recent meta-analysis of seventeen clinical trials including 1167 patients found that amifostine significantly reduces severe mucositis (relative risk 0.72), xerostomia (relative risk 0.7), and dysphagia (relative risk 0.39) in patients receiving radiotherapy [83].
Although amifostine or WR-1065 should be excellent candidates for RCS scavenging in clinical conditions besides cancer, surprisingly few studies have been reported. In a rat model of Parkinson’s disease, WR-1065 was found to reduce RCS levels and protect against development of motor imbalance [84]. Amifostin also reduced ischemia/reperfusion injury in rabbits subjected to temporary occlusion of the descending thoracic aorta [85]. Hopefully, the efficacy of amifostine in these clinical conditions will be further examined.
Imidazole based scavengers
Like thiols, imidazoles participate in Michael addition reaction to target α,β-unsaturated carbonyls. Unlike thiols, imidazoles do not under undergo oxidation. This greater stability makes imidazole-based scavengers as effective as thiol-based scavengers despite their lower reaction rates for α,β-unsaturated carbonyls, especially when additional nucleophilic groups are included with the imidazoles. Carnosine is the best studied imidazole-based scavenger and will be discussed below. It is worth noting that many anti-fungal agents and losartan contain imidazole moieties so their potential to act as scavengers warrants more evaluation.
Carnosine
Carnosine is an endogenous dipeptide (β-alanyl-L-histidine) highly concentrated (~10 mM) in the brain and muscles [86]. Levels of carnosine are dramatically reduced in patients with diabetes mellitus [87, 88] and with Alzheimer’s disease [89], and modestly reduced in vegetarians, females, and elderly populations [90, 91]. In addition to RCS scavenging, carnosine acts as a physiological pH buffer, a hydroxyl radical scavenger, a redox active metal chelator, a stimulator of nitric oxide synthesis, and activator of carbonic anhydrase [92]
Comparison of carnosine scavenging of HNE, methylglyoxal, glyoxal, and MDA in vitro showed that carnosine was most effective at scavenging HNE [93]. In vivo evidence for the ability of carnosine to scavenge RCS include detection of HNE modified carnosine in the urine of Zucker fatty rats [94] and detection of acrolein-carnosine adducts in human urine [95].
Rodent data supports the efficacy of carnosine in diabetes, cardiovascular disease, and ischemia/reperfusion injury. In db/db mice (a model of type 2 diabetes), 4 weeks of carnosine treatment reduced proteinurea and vascular permeability compared to vehicle treatment [96]. Intraperitoneal injection of 100 mg/kg carnosine into db/db mice also enhanced rate of wound healing. In STZ-induced diabetic mice, 1 g/l carnosine given in drinking water markedly reduced RCS levels in liver and kidney, resulting in reduced fasting plasma glucose and increased plasma insulin levels [97]. In a STZ-induced diabetic nephropathy rat model, treatment with carnosine reduced glucosuria, HbA1c levels, cataract formation, albumin excretion, and preserved podocyte number [98]. Carnosine-containing eye drops significantly lowered levels of glycated lens proteins and delayed progression of cataracts in STZ-treated rats [99].
Treatment of atherosclerotic prone ApoE−/− mice with octyl-D-carnosine reduced atherosclerotic lesion formation [100]. Proteins modified by acrolein and HNE were decreased in these animals while RCS-modified carnosine was increased in urine [100]. Administration of octyl-D-carnosine for 12 to 20 weeks to STZ-induced diabetic apoE−/− mice stabilized plaque phenotypes and attenuated the size and necrotic area of atherosclerotic lesions [101, 102].
Carnosine injection prior to permanent middle cerebral artery occlusion ameliorated ischemic brain damage in C57BL6 mice [103]. Interestingly, carnosine analogs where the α-amine and imidazole are modified (e.g. N-acetyl carnosine and anserine) failed to exert protection [103]. In a rat model of neonatal hypoxic injury, pretreatment with carnosine significantly reduced RCS levels in response to ligation [104]. This pre-treatment decreased infarct size and apoptosis in the brain, and improved performance on the Morris water maze test, demonstrating the potential of carnosine to protect against ischemic brain injury [104]. Administration of carnosine even 6 to 9 hours after the onset of ischemia was shown effective in both transient and permanent cerebral artery occlusion [105]. A recent meta-analysis of studies on the efficacy of carnosine in animal models of ischemic stroke concluded that a clear dose-effect of carnosine in reducing infarct volume with diminished effects if administration occurred 6 hours after ischemia [106].
Besides diabetes, atherosclerosis, and ischemic injury, carnosine has shown efficacy in other conditions of oxidative injury. Carnosine attenuated microglia activation and cortical neuron apoptosis in a rat model of experimental subarachnoid hemorrhage [107]. Co-administration of carnosine with doxorubicin normalized blood pressure, improved cardiac and stroke indices, and reduced cardiac muscle damage in rabbits with doxorubicin-induced congestive heart failure [108].
Given the efficacy of carnosine in animals, it is surprising how few large clinical trials have been conducted with carnosine supplementation. A number of pilot studies have shown therapeutic potential for diabetes, cardiovascular and neurodegenerative diseases. Carnosine supplementation (2 g/day) for 12 weeks improved glucose tolerance and insulin sensitivity in overweight and obese individuals [109]. Carnosine supplementation for six months also improved exercise tolerance in patients with stable chronic heart failure [110]. A pilot study in 2008 reported carnosine supplementation significantly improved neurological symptoms of Parkinson’s disease and reduced plasma RCS modified proteins [111]. A pilot study with 52 patients diagnosed with moderate probable Alzheimer’s disease and treated with the acetylcholinesterase inhibitor donepezil reported that the group that received carnosine (along with an antioxidant cocktail that included vitamin E, vitamin C, beta-caroteine, selenium, L-cysteine, and other compounds) for 6 months showed improvements in their Mini-Mental Status Exam II scores while the group that continued to receive only donepezil treatment maintained similar scores [112]. Despite the potential of carnosine supplementation in these pilot trials, no follow-up studies to confirm these effects have been reported for Parkinson’s or Alzheimer’s diseases.
In terms of other conditions, the application of N-acetyl-carnosine (an effective pro-drug for carnosine) in lubricant eyedrops [113] was efficacious for the prevention and treatment of visual impairment and vision disability [114]. Oral supplementation with carnosine for 4 weeks reduced pressure ulcer in institutionalized long-term care patients [115]. In 2013, a randomized double blind placebo controlled trial in 24 veterans suffering from Gulf War illness used escalating doses of carnosine from 500 to 1500 mg per day over 12 weeks found no improvement in most endpoints such as fatigue and pain scores but improvement in a cognitive task, the WAIS-R digit symbol substitution test [116]. Based on these results, carnosine appears to have untapped potential, but large scale clinical trials are clearly needed.
2-Aminomethylphenols
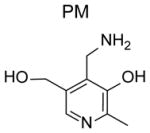
The use of 2-aminomethylphenols as dicarbonyl scavengers was first discovered in studies utilizing pyridoxamine (PM), one of six vitamin B6 vitamers. As a primary amine with excellent bioavailability and low toxicity [117], PM was a rational choice for testing its ability to scavenge MGO and other glucose derived RCS in circulation. Supplementation is needed for effective scavenging of MGO by PM in plasma because PM is rapidly converted within cells into pyridoxal phosphate, an enzymatic co-factor that lacks scavenging capacity. Administration of PM protected against complications of diabetes (see below). At that time little attention was paid to whether the structural characteristics of PM enhanced its RCS reactivity compared to other bioavailable primary amines. Appreciation for this aspect of PM arose from studies examining its efficacy against IsoLG. Because of the extreme reactivity of 4-ketoaldehydes, we recognized that finding an effective scavenger for this class of RCS might be difficult, as it requires a compound with similar reactivity but a much higher biological concentration than lysine, or a similar biological concentration but a much faster reactivity for 4-ketoaldehydes compared to lysine. With the former possibility more likely, we screened a number of biologically available small molecule primary amines that included PM, glycine and aminoguanidine for their ability to block IsoLG modification of radiolabeled lysine. To our great surprise, equimolar PM almost completely suppressed the reaction of lysine with IsoLG, while equimolar glycine or aminoguanidine had only trivial effects [118].
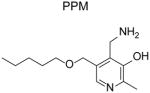
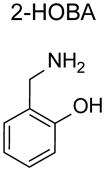
Structure-activity relationship studies performed with a model dicarbonyl, 4-oxopentanal unraveled the mechanism underlying the surprisingly potent activity of PM. PM reacted with 4-ketoaldehydes 2000 times faster did lysine, with the 2-aminomethylphenol moiety of PM being responsible for this high reactivity [118]. For instance, 2-hydroxybenzylamine (2-HOBA or salicylamine) and 5’-O-pentyl-pyridoxamine (PPM) show similar reactivity for 4-ketoaldehydes as PM [16, 118]. In contrast, the methoxy derivative of 2-HOBA (2-methoxy-benzylamine) and the regioisomer of 2-HOBA that has the hydroxyl group at the 4-position (4-hydroxybenzylamine) have very similar reactivity as lysine for 4-ketoaldehydes [119]. Related compounds that lack the aromatic ring (i.e. aminopropanol) show only modestly increased reactivity for 4-ketoaldehdyes compared to lysine [118].
To analyze the selectivity of PM for IsoLG versus other RCS, we compared the reactivity of PM with MGO (a 1,2-dicarbonyl) and with HNE (an α,β-unsaturated carbonyl) [118]. PM showed no increased reactivity compared to lysine for HNE. PM showed increased reactivity compared to lysine for MGO, but this enhanced reactivity was at least an order of magnitude less than what was seen with 1,4-dicarbonyls. Other investigators also found that the reaction rate of PM for compounds that were 1,2-dicarbonyls such as glyoxal, 2,3-butadione, and methyl pyruvate were several orders of magnitude slower at reacting with PM than 1,4-dicarbonyls such o-phthaladehyde and benzoquinone [120]. To reduce RCS modification of ubiquitin by 50%, an 8.8 molar excess of PM is required for HNE, a 9.3 molar excess for MGO, and a 1.31 molar excess is need for MDA [93]. Other 2-aminomethylphenols have not been studied for selectivity as exhaustively as PM, but in general show similar selectivity for 1,4-dicarbonyls [16]. An exception is PPM (as well as 5’-O-hexanyl-PM and 5’-O-heptyl-PM), which shows enhanced reactivity compared to lysine for 1,2-dicarbonyls and 1,4-dicarbonyls. We postulate that the hydrophobic microenvironment created by the alkyl tail of PPM favors the phenolic (rather than the zwitterionic) form that is required for scavenging 1,2-dicarbonyls.
The overall hydrophobicity of 2-HOBA and PPM relative to PM markedly increases their penetrance into cells or lipoproteins. Better penetrance may enhance their ability to inhibit formation of IsoLG adducts compared to PM, even though all three scavengers act equally well in aqueous conditions. In platelets, 2-HOBA and PPM markedly reduce levels of IsoLG-modified proteins, while PM is significantly less effective. Additionally, 2-HOBA and PPM convey greater protection against oxidant induced cell death than PM [16].
In summary, 2-aminomethylphenols serve as excellent scavengers for 1,4-dicarbonyls, while other classes of scavengers lack this capacity. Therefore, 2-aminomethylphenols may prove to be the first choice of scavenger for any dicarbonyl species, with MESNA or related thiols the first choices for α,β-unsaturated carbonyl species. Combination of the two classes might be particularly effective in ameliorating disease, although no studies to date have tested such combinations.
Pyridoxamine (Pyridorin)
Historically, the primary focus of animal and clinical trials with PM (trade name Pyridorin) have focused on diabetes and its complications including renal disease, cardiovascular disease, and retinopathy. An early study in STZ-induced diabetic rats showed that PM treatment for 28 weeks markedly inhibited renal disease, as shown by reduced albuminuria and plasma creatinine compared to placebo [121]. Subsequent studies performed in KK-Ay/Ta mice given PM or placebo for 12 weeks showed that PM treatment markedly protected kidney function as indicated by a lower urinary albumin/creatinine ratio [122]. PM also reduced fasting plasma insulin levels as well as decreased renal N-carboxy-methyl-lysine (CML), nitrotyrosine, and TGFβ1 levels [122]. PM treatment of db/db mice reduced kidney hypertrophy, proteinuria, mesangial expansion, podocyte loss [123] and the development of glucose intolerance [124]. In an ischemia/reperfusion model of acute kidney injury, pre-treatment of PM prior to nephrectomy reduced fibrosis and injury score [125]. PM pretreatment also reduced serum creatinine levels at 28 day post nephrectomy.
Another serious complication of diabetes is cardiovascular disease, which is driven in part by elevated levels of cholesterol and triglycerides that result in oxidative modification of lipoproteins. In an initial study with PM supplementation in STZ-treated rats, PM inhibited increases in plasma triglycerides and total cholesterol, while aminoguanidine failed to do so [121]. Since both PM and aminoguanidine reduced CML and CEL equally, the greater efficacy of PM on plasma lipids may be attributed to its ability to scavenge 1,4-dicarbonyls in addition to 1,2-dicarbonyls like MGO. A number of studies have since replicated the finding that PM reduces cholesterol and triglyceride levels. For instance, PM significantly reduced SCAP and SREBP-1c expression and decreased plasma cholesterol and intramuscular triglycerides compared to control when mice were fed a high-fructose diet [126]. In diet-induced obese mice, PM supplementation for 18 weeks inhibited weight gain without altering food intake, and significantly reduced accumulation of triglyceride droplets and macrophages in liver [124]. In these same studies, PM also reduced fasting plasma glucose and insulin levels [124]. PM supplementation also inhibited atherosclerotic lesion development in atherosclerosis-prone apoE−/− mice that were treated with STZ [127]. In another study using STZ-induced diabetic mice with experimental myocardial infarction, treatment with PM reduced total cholesterol, triglyceride, and LDL-cholesterol levels, and increased HDL cholesterol levels [128]. Importantly, PM significantly improved cardiac ejection fraction [128]. Further, PM supplementation reduced vascular calcification in other studies with STZ-treated rats fed a high fat diet and warfarin [129]. Beyond atherosclerosis, PM may have other beneficial effects on vascular functions that deteriorate with age, as PM supplementation for five months enhanced myocardial contractile function in 20-month old rats, compared to untreated aged matched controls [130].
PM supplementation has shown efficacy in a number of other animal models of disease. In a model of macular degeneration, PM reduced levels of retinal IsoLG-modified protein and protected against morphological changes in photoreceptor mitochondria [131]. Similarly, PM reduced the severity of endogenous uveitis present in mice immunized with the retinal protein K2 peptide and adjuvant [132]. In mice subjected to radiation treatment, PM supplementation markedly inhibited apoptosis in the intestinal tract [133] and increased survival [134].
The results of several randomized control phase 2 trials for PM (Pyridoxin) have been published. The first report combined the results of two 24-week studies with identical inclusion criteria where a total of 202 patients with overt nephropathy and diabetes (Type 1 or Type 2) were enrolled and randomized to receive PM or placebo [135]. All patients in these two Phase 2 studies received placebo or PM in addition to standard-of-care. In one study, patients received 50 mg of PM twice daily. In the other, patients were escalated up to 250 mg PM twice daily. Over the course of the trials, PM treatment prevented the accumulation of advanced glycation endproducts, indicating that the doses of PM were effective for RCS scavenging. While analysis of the two trials individually did not yield significant clinical improvements, analysis of merged data showed a significant decrease in serum creatinine for the groups receiving PM (0.11 ± 0.34 mg/dL) compared to placebo (0.21 ± 0.55 mg/dL) [135]. In addition, urinary TGF-β1 was also decreased with PM treatment with no changes in urinary albumin excretion [135].
A subsequent randomized controlled trial examined the effects of PM in 317 Type 2 diabetic patients with overt proteinuria (>1200 mg/g) that were being treated with the maximum recommended daily doses of blood pressure medications. These patients were randomized to placebo or PM (150 mg twice daily versus 300 mg twice daily) for 1 year. After treatment, there were no significant difference between groups in the primary end-points of the study measuring changes in renal function. However, when patients were stratified by tertile for baseline serum creatinine in a subsequent subgroup analysis, this analysis showed that for patients that started in the lowest tertile, treatment with PM did significantly reduce increases in serum creatinine levels (p=.048) and improved glomerular filtration rate [136]. NephroGenex felt that these data were sufficiently compelling to initiate a Phase 3 trial with PM that ended enrollment in March of 2016 (clinicaltrials.gov). The results of this trial have not yet been published.
2-hydroxybenzylamine and 5’-O-pentyl-pyridoxamine
Other 2-aminomethylphenols that have undergone in vivo testing are 2-hydroxybenzylamine (2-HOBA, also known as salicylamine) and to a lesser extent 5’-O-pentyl-pyridoxamine (PPM). By virtue of their greater lipophilicity and cellular scavenging than PM, 2-HOBA and PPM are anticipated to have greater efficacy than PM. Pharmacokinetic studies with 2-HOBA in mice demonstrated decent oral bioavailability (estimated at 38%) and partitioning from plasma into major tissues including brain and liver [137]. The half-life of 2-HOBA is approximately 62 minutes. A dose of 1 g/L in drinking water has been widely used for rodent studies, yielding tissue concentrations of 10 to 30 μmol/kg. 3 g/L in drinking water is considered the limit for safety, as 10 g/L in drinking water has shown reductions in water consumption and mild toxicity in mice.
2-HOBA has shown efficacy in ameliorating disease in several animal models that display increased protein modification by IsoLG and related dicarbonyls. Continuous treatment with 2-HOBA in humanized ApoE4 (hApoE4) mice, a model of Alzheimer’s disease, beginning at 3 months of age significantly inhibited memory loss that is normally seen at 12–14 months of age in these mice [138]. 2-HOBA also reduced IsoLG adducts and protected against memory loss in two mouse models of epilepsy [139].
Besides preserving working memory, 2-HOBA may also increase lifespan. The nematode worm C. elegans (with an average adult lifespan of less than 3 weeks) is often used as a rapid in vivo model of aging. In N2 wild-type adult worms treated with 2-HOBA, median lifespan is increased 56% [140]. The precise mechanisms whereby 2-HOBA increases longevity need to be determined. However, 2-HOBA treatment fails to extend lifespan in worms where the gene for sirtuin SIR-2.1 is knocked out, suggesting that RCS modification of this deacetylase might be a major factor decreasing longevity [140]. Future studies are needed to determine if 2-HOBA and other 2-aminomethylphenols such as PPM will increase lifespan in mammals.
In addition to effects on age-related diseases, 2-HOBA and PPM also provide protection against vascular disease. In C57BL6 mice infused with angiotensin II (AngII), a mouse model of hypertension, levels of IsoLG-modified proteins markedly increased in heart and aorta [39]. Administration of 2-HOBA blocked this increase, and more importantly, prevented the rise in blood pressure normally induce by AngII infusion [39]. Administration of PPM also inhibited AngII-induced hypertension, but non-scavenging analogs such as N-methyl-2-HOBA and 4-HOBA had no effect [39]. Administration of 2-HOBA markedly diminished collagen deposition and T cell infiltration into kidneys of AngII treated mice, and also prevented proteinuria [39]. Similar effects of 2-HOBA are seen in DOCA-salt induced hypertension [39]. One mechanism underlying IsoLG induced hypertension appears to be that IsoLG-modification of self-peptides generates neo-antigens for dendritic cells to present to CD8+ T cells, triggering their activation [39]. Accumulation of T cells into the kidney then leads to disease.
The relationship between lipid peroxidation, RCS, hypertension, and aortic stiffening, has been examined using transgenic mice that overexpress the p22 subunit of NADPH oxidase, resulting in markedly higher generation of superoxide [40]. Interestingly, in these mice aortic stiffening proceeded onset of hypertension, and elimination of T cells (by crossing with Rag-1−/− mice) eliminated both aortic stiffening and hypertension, suggesting that T cell response to IsoLG-generated neo-antigens might also play a role in aortic stiffening [40]. Transgenic p22 mice show significant increases in IsoLG-modified proteins and incubating aortic homogenates from these mice with dendritic cells was sufficient to induce proliferation of T cell co-cultured with the dendritic cells. Treatment of the p22 transgenic mice for five months with either tempol (which directly inactivates ROS) or 2-HOBA markedly reduced aortic stiffening and hypertension [40].
The results of these animal studies support the notion that RCS scavenging with 2-HOBA, and presumably other 2-aminomethylphenols like PPM, may be a novel treatment for chronic diseases including neurodegenerative diseases and vascular diseases. Clearly, clinical trials of 2-HOBA and PPM are needed to test this hypothesis. Approval for the first studies of 2-HOBA in humans was recently granted and these studies have begun enrollment.
Summary
Scavenging of RCS has shown significant effects in many animal models of disease, which support the notion that RCS generation contributes to pathogenesis of many diseases and that scavenging RCS might have clinical benefit. Nevertheless, only a few RCS scavengers such as the thiol-based scavengers MESNA and amifostine have become widely used, and these are primarily used as adjunctive therapies for chemotherapy. Because several major species of RCS are poorly scavenged by thiol-based scavengers, there is a clear need to develop other classes of RCS scavengers. There is also a need to test RCS scavengers individually or in combination in clinical trials directed at additional diseases or clinical conditions. Current RCS scavengers under development include carnosine analogs and 2-aminomethylphenols, but next generation compounds in these classes need to be developed that have better pharmacokinetic parameters and safety profiles. Only then will it be possible to fully evaluate the potential of RCS scavenger to improve human health and ameliorate chronic diseases.
Acknowledgments
We thank Dr. Venkataraman Amarnath for his review of the manuscript and helpful suggestions. Dr. Davies and Zhang received funding from the National Heart, Lung, and Blood Institute grant HL116263-01A1.
List of Abbreviations
- ACR
acrolein
- AngII
angiotensin II
- BSA
bovine serum albumin
- CEL
N-carboxy-ethyl-lysine
- CML
N-carboxy-methyl-lysine
- FDP
Nε-(3-formyl-3,4-dehydropiperidino)
- HNE
4-hydroxynonenal
- 2-HOBA
2-hydroxybenzylamine or salicylamine
- HO•−
Hydroxyl radical
- IsoLG
isolevuglandin
- MDA
malondialdehyde
- MESNA
2-mercaptoethanesulfonate
- MG-H1
5-methylimidazol-4-one
- MGO
methylglycoxal
- ONE
4-oxo-2-nonenal
- PE
phosphatidylethanolamine
- PM
pyridoxamine
- PPM
5’-O-pentyl-pyridoxamine
- PUFA
polyunsaturated fatty acids
- RAGE
Receptor for Advanced Glycation Endproducts
- STZ
streptozotocin
- TBARS
thiobarbituric acid reactive substances
- TNF
tumor necrosis factor
Footnotes
Disclosure: Dr. Davies is co-inventor on U.S. patent #7705054 for use of 2-aminomethylphenols as isolevuglandin scavengers for disorders involving oxidative injury.
Human and Animal Rights: All reported studies/experiments with human and animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines.
References
- 1.Bolton WK, Cattran DC, Williams ME, Adler SG, Appel GB, Cartwright K, et al. Randomized Trial of an Inhibitor of Formation of Advanced Glycation End Products in Diabetic Nephropathy. American Journal of Nephrology. 2004;24(1):32–40. doi: 10.1159/000075627. [DOI] [PubMed] [Google Scholar]
- 2.Freedman BI, Wuerth J-P, Cartwright K, Bain RP, Dippe S, Hershon K, et al. Design and Baseline Characteristics for the Aminoguanidine Clinical Trial in Overt Type 2 Diabetic Nephropathy (ACTION II) Controlled Clinical Trials. 1999;20(5):493–510. doi: 10.1016/s0197-2456(99)00024-0. doi: http://dx.doi.org/10.1016/S0197-2456(99)00024-0. [DOI] [PubMed] [Google Scholar]
- 3.Brown BE, Mahroof FM, Cook NL, van Reyk DM, Davies MJ. Hydrazine compounds inhibit glycation of low-density lipoproteins and prevent the in vitro formation of model foam cells from glycolaldehyde-modified low-density lipoproteins. Diabetologia. 2006;49(4):775–83. doi: 10.1007/s00125-006-0137-3. [DOI] [PubMed] [Google Scholar]
- 4.Liu W, Porter NA, Schneider C, Brash AR, Yin H. Formation of 4-hydroxynonenal from cardiolipin oxidation: Intramolecular peroxyl radical addition and decomposition. Free Radic Biol Med. 2011;50(1):166–78. doi: 10.1016/j.freeradbiomed.2010.10.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11(1):81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 6.Uchida K, Stadtman ER. Modification of histidine residues in proteins by reaction with 4-hydroxynonenal. Proc Natl Acad Sci U S A. 1992;89(10):4544–8. doi: 10.1073/pnas.89.10.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sayre LM, Arora PK, Iyer RS, Salomon RG. Pyrrole formation from 4-hydroxynonenal and primary amines. Chem Res Toxicol. 1993;6(1):19–22. doi: 10.1021/tx00031a002. [DOI] [PubMed] [Google Scholar]
- 8.Brame CJ, Salomon RG, Morrow JD, Roberts LJ., 2nd Identification of extremely reactive gamma-ketoaldehydes (isolevuglandins) as products of the isoprostane pathway and characterization of their lysyl protein adducts. J Biol Chem. 1999;274(19):13139–46. doi: 10.1074/jbc.274.19.13139. [DOI] [PubMed] [Google Scholar]
- 9.Zhao J, Chen J, Zhu H, Xiong YL. Mass spectrometric evidence of malonaldehyde and 4-hydroxynonenal adductions to radical-scavenging soy peptides. J Agric Food Chem. 2012;60(38):9727–36. doi: 10.1021/jf3026277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guichardant M, Taibi-Tronche P, Fay LB, Lagarde M. Covalent modifications of aminophospholipids by 4-hydroxynonenal. Free Radic Biol Med. 1998;25(9):1049–56. doi: 10.1016/s0891-5849(98)00149-x. [DOI] [PubMed] [Google Scholar]
- 11.Linhart KB, Glassen K, Peccerella T, Waldherr R, Linhart H, Bartsch H, et al. The generation of carcinogenic etheno-DNA adducts in the liver of patients with nonalcoholic fatty liver disease. Hepatobiliary Surg Nutr. 2015;4(2):117–23. doi: 10.3978/j.issn.2304-3881.2015.01.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Itakura K, Osawa T, Uchida K. Structure of a Fluorescent Compound Formed from 4-Hydroxy-2-nonenal and N(alpha)-Hippuryllysine: A Model for Fluorophores Derived from Protein Modifications by Lipid Peroxidation. J Org Chem. 1998;63(1):185–7. doi: 10.1021/jo971239+. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Xu G, Sayre LM. Carnosine Inhibits (E)-4-Hydroxy-2-nonenal-Induced Protein Cross- Linking: Structural Characterization of Carnosine HNE Adducts1. Chemical Research in Toxicology. 2003;16(12):1589–97. doi: 10.1021/tx034160a. [DOI] [PubMed] [Google Scholar]
- 14.Marchette LD, Wang H, Li F, Babizhayev MA, Kasus-Jacobi A. Carcinine Has 4- Hydroxynonenal Scavenging Property and Neuroprotective Effect in Mouse Retina. Investigative Ophthalmology & Visual Science. 2012;53(7):3572–83. doi: 10.1167/iovs.11-9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menini S, Iacobini C, Ricci C, Scipioni A, Fantauzzi CB, Giaccari A, et al. D-carnosine octylester attenuates atherosclerosis and renal disease in ApoE null mice fed a Western diet through reduction of carbonyl stress and inflammation. British Journal of Pharmacology. 2012;166(4):1344–56. doi: 10.1111/j.1476-5381.2012.01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies SS, Brantley EJ, Voziyan PA, Amarnath V, Zagol-Ikapitte I, Boutaud O, et al. Pyridoxamine analogues scavenge lipid-derived gamma-ketoaldehydes and protect against H2O2-mediated cytotoxicity. Biochemistry. 2006;45(51):15756–67. doi: 10.1021/bi061860g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17•.Amarnath V, Amarnath K. Scavenging 4-Oxo-2-nonenal. Chemical Research in Toxicology. 2015;28(10):1888–90. doi: 10.1021/acs.chemrestox.5b00301. Describes novel pathway by which 2-aminomethylphenols react with ONE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18•.Vidal N, Cavaille JP, Graziani F, Robin M, Ouari O, Pietri S, et al. High throughput assay for evaluation of reactive carbonyl scavenging capacity. Redox Biology. 2014;2:590–8. doi: 10.1016/j.redox.2014.01.016. doi: http://dx.doi.org/10.1016/j.redox.2014.01.016. Method of rapidly assessing lipid dicarbonyl reactivities and scavengers by use of fluorescent labeling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang WH, Liu J, Xu G, Yuan Q, Sayre LM. Model studies on protein side chain modification by 4-oxo-2-nonenal. Chem Res Toxicol. 2003;16(4):512–23. doi: 10.1021/tx020105a. [DOI] [PubMed] [Google Scholar]
- 20.Galligan JJ, Rose KL, Beavers WN, Hill S, Tallman KA, Tansey WP, et al. Stable histone adduction by 4-oxo-2-nonenal: a potential link between oxidative stress and epigenetics. J Am Chem Soc. 2014;136(34):11864–6. doi: 10.1021/ja503604t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevens JF, Maier CS. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Molecular nutrition & food research. 2008;52(1):7–25. doi: 10.1002/mnfr.200700412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y, Yang Y, Trent MB, He N, Lick SD, Zimniak P, et al. Glutathione-S-transferase A4-4 modulates oxidative stress in endothelium: possible role in human atherosclerosis. Atherosclerosis. 2004;173(2):211–21. doi: 10.1016/j.atherosclerosis.2003.12.023. doi: http://dx.doi.org/10.1016/j.atherosclerosis.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 23.Kehrer JP, Biswal SS. The Molecular Effects of Acrolein. Toxicological Sciences. 2000;57(1):6–15. doi: 10.1093/toxsci/57.1.6. [DOI] [PubMed] [Google Scholar]
- 24.Zemski Berry KA, Murphy RC. Characterization of Acrolein-Glycerophosphoethanolamine Lipid Adducts Using Electrospray Mass Spectrometry. Chemical research in toxicology. 2007;20(9):1342–51. doi: 10.1021/tx700102n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uchida K, Kanematsu M, Sakai K, Matsuda T, Hattori N, Mizuno Y, et al. Protein-bound acrolein: potential markers for oxidative stress. Proc Natl Acad Sci U S A. 1998;95(9):4882–7. doi: 10.1073/pnas.95.9.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCall MR, Tang JY, Bielicki JK, Forte TM. Inhibition of Lecithin-Cholesterol Acyltransferase and Modification of HDL Apolipoproteins by Aldehydes. Arteriosclerosis, Thrombosis, and Vascular Biology. 1995;15(10):1599–606. doi: 10.1161/01.atv.15.10.1599. [DOI] [PubMed] [Google Scholar]
- 27.Zhu Q, Sun Z, Jiang Y, Chen F, Wang M. Acrolein scavengers: Reactivity, mechanism and impact on health. Molecular Nutrition & Food Research. 2011;55(9):1375–90. doi: 10.1002/mnfr.201100149. [DOI] [PubMed] [Google Scholar]
- 28.Bispo VS, de Arruda Campos IP, Di Mascio P, Medeiros MHG. Structural Elucidation of a Carnosine-Acrolein Adduct and its Quantification in Human Urine Samples. Scientific Reports. 2016;6:19348. doi: 10.1038/srep19348. http://www.nature.com/articles/srep19348#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salomon RG, Miller DB. Levuglandins: isolation, characterization, and total synthesis of new secoprostanoid products from prostaglandin endoperoxides. Adv Prostaglandin Thromboxane Leukot Res. 1985;15:323–6. [PubMed] [Google Scholar]
- 30.Salomon RG, Jirousek MR, Ghosh S, Sharma RB. Prostaglandin endoperoxides 21. Covalent binding of levuglandin E2 with proteins. Prostaglandins. 1987;34(5):643–56. doi: 10.1016/0090-6980(87)90289-9. [DOI] [PubMed] [Google Scholar]
- 31.Salomon RG, Subbanagounder G, Singh U, O'Neil J, Hoff HF. Oxidation of low-density lipoproteins produces levuglandin-protein adducts. Chem Res Toxicol. 1997;10(7):750–9. doi: 10.1021/tx970016b. [DOI] [PubMed] [Google Scholar]
- 32.Boutaud O, Brame CJ, Salomon RG, Roberts LJ, 2nd, Oates JA. Characterization of the lysyl adducts formed from prostaglandin H2 via the levuglandin pathway. Biochemistry. 1999;38(29):9389–96. doi: 10.1021/bi990470+. [DOI] [PubMed] [Google Scholar]
- 33.Zagol-Ikapitte I, Bernoud-Hubac N, Amarnath V, Roberts LJ, 2nd, Boutaud O, Oates JA. Characterization of bis(levuglandinyl) urea derivatives as products of the reaction between prostaglandin H2 and arginine. Biochemistry. 2004;43(18):5503–10. doi: 10.1021/bi049842r. [DOI] [PubMed] [Google Scholar]
- 34.Carrier EJ, Amarnath V, Oates JA, Boutaud O. Characterization of covalent adducts of nucleosides and DNA formed by reaction with levuglandin. Biochemistry. 2009;48(45):10775–81. doi: 10.1021/bi9015132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DiFranco E, Subbanagounder G, Kim S, Murthi K, Taneda S, Monnier VM, et al. Formation and stability of pyrrole adducts in the reaction of levuglandin E2 with proteins. Chem Res Toxicol. 1995;8(1):61–7. doi: 10.1021/tx00043a008. [DOI] [PubMed] [Google Scholar]
- 36•.Bi W, Jang GF, Zhang L, Crabb JW, Laird J, Linetsky M, et al. Molecular Structures of Isolevuglandin-Protein Cross-Links. Chem Res Toxicol. 2016;29(10):1628–40. doi: 10.1021/acs.chemrestox.6b00141. Determination of the IsoLG crosslink structures and their dependence on protein sequence and other factors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo L, Chen Z, Cox BE, Amarnath V, Epand RF, Epand RM, et al. Phosphatidylethanolamines modified by gamma-ketoaldehyde (gammaKA) induce endoplasmic reticulum stress and endothelial activation. J Biol Chem. 2011;286(20):18170–80. doi: 10.1074/jbc.M110.213470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38•.Guo L, Chen Z, Amarnath V, Yancey PG, Van Lenten BJ, Savage JR, et al. Isolevuglandin-type lipid aldehydes induce the inflammatory response of macrophages by modifying phosphatidylethanolamines and activating the receptor for advanced glycation endproducts. Antioxid Redox Signal. 2015;22(18):1633–45. doi: 10.1089/ars.2014.6078. Identifies IsoLG-PE as a mediator of macrophage activation via the RAGE receptor, and therefore a likely target of RCS scavenging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39••.Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, et al. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest. 2014;124(10):4642–56. doi: 10.1172/JCI74084. Revealed a novel mechanisms by which IsoLG generation of neo-epitopes mediated hypertension and that 2-aminomethylphenols blocked development of hypertension. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40•.Wu J, Saleh MA, Kirabo A, Itani HA, Montaniel KR, Xiao L, et al. Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J Clin Invest. 2016;126(4):1607. doi: 10.1172/JCI87425. Pre-clinical study demonstrating that generation of IsoLG by NADPH oxidase contributes to aortic stiffening and hypertension and that 2-hydroxybenzylamine prevents this. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakajima T, Davies SS, Matafonova E, Potet F, Amarnath V, Tallman KA, et al. Selective gamma-ketoaldehyde scavengers protect Nav1.5 from oxidant-induced inactivation. J Mol Cell Cardiol. 2010;48(2):352–9. doi: 10.1016/j.yjmcc.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boyden PA, Davies SS, Viswanathan PC, Amarnath V, Balser JR, Roberts LJ., 2nd Potential role of isoketals formed via the isoprostane pathway of lipid peroxidation in ischemic arrhythmias. J Cardiovasc Pharmacol. 2007;50(5):480–6. doi: 10.1097/FJC.0b013e31815a0564. [DOI] [PubMed] [Google Scholar]
- 43.Fukuda K, Davies SS, Nakajima T, Ong BH, Kupershmidt S, Fessel J, et al. Oxidative mediated lipid peroxidation recapitulates proarrhythmic effects on cardiac sodium channels. Circ Res. 2005;97(12):1262–9. doi: 10.1161/01.RES.0000195844.31466.e9. [DOI] [PubMed] [Google Scholar]
- 44•.Sidorova TN, Yermalitskaya LV, Mace LC, Wells KS, Boutaud O, Prinsen JK, et al. Reactive gamma-ketoaldehydes promote protein misfolding and preamyloid oligomer formation in rapidly-activated atrial cells. J Mol Cell Cardiol. 2015;79:295–302. doi: 10.1016/j.yjmcc.2014.11.013. Demonstration of 4-ketoaldehydes promoting the formation of preamyloid oligomers and the protective effects of 2-hydroxybenzylamine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davies SS, Amarnath V, Montine KS, Bernoud-Hubac N, Boutaud O, Montine TJ, et al. Effects of reactive gamma-ketoaldehydes formed by the isoprostane pathway (isoketals) and cyclooxygenase pathway (levuglandins) on proteasome function. FASEB J. 2002;16(7):715–7. doi: 10.1096/fj.01-0696fje. [DOI] [PubMed] [Google Scholar]
- 46.Foreman D, Levison BS, Miller DB, Salomon RG. Anhydrolevuglandin D2 inhibits the uterotonic activity of prostaglandins F2 alpha and D2. Prostaglandins. 1988;35(1):115–22. doi: 10.1016/0090-6980(88)90279-1. [DOI] [PubMed] [Google Scholar]
- 47.Pryor WA, Stanley JP. Letter: A suggested mechanism for the production of malonaldehyde during the autoxidation of polyunsaturated fatty acids. Nonenzymatic production of prostaglandin endoperoxides during autoxidation. J Org Chem. 1975;40(24):3615–7. doi: 10.1021/jo00912a038. [DOI] [PubMed] [Google Scholar]
- 48.Chio KS, Tappel AL. Synthesis and characterization of the fluorescent products derived from malonaldehyde and amino acids. Biochemistry. 1969;8(7):2821–6. doi: 10.1021/bi00835a019. [DOI] [PubMed] [Google Scholar]
- 49.Marnett LJ. Chemistry and biology of DNA damage by malondialdehyde. IARC Sci Publ. 1999;(150):17–27. [PubMed] [Google Scholar]
- 50.Shuck SC, Wauchope OR, Rose KL, Kingsley PJ, Rouzer CA, Shell SM, et al. Protein Modification by Adenine Propenal. Chemical Research in Toxicology. 2014;27(10):1732–42. doi: 10.1021/tx500218g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zagol-Ikapite I, Sosa IR, Oram D, Judd A, Amarnath K, Amarnath V, et al. Modification of platelet proteins by malondialdehyde: prevention by dicarbonyl scavengers. Journal of Lipid Research. 2015;56(11):2196–205. doi: 10.1194/jlr.P063271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li X, Yang S, Lv X, Sun H, Weng J, Liang Y, et al. The mechanism of mesna in protection from cisplatin-induced ovarian damage in female rats. J Gynecol Oncol. 2013;24(2):177–85. doi: 10.3802/jgo.2013.24.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kabasakal L, Şehirli AÖ, Çetinel Ş, Cikler E, Gedik N, Şener G. Mesna (2-mercaptoethane sulfonate) prevents ischemia/reperfusion induced renal oxidative damage in rats. Life Sciences. 2004;75(19):2329–40. doi: 10.1016/j.lfs.2004.04.029. doi: http://dx.doi.org/10.1016/j.lfs.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 54.Aydin AF, Kusku-Kiraz Z, Dogru-Abbasoglu S, Uysal M. Effect of carnosine treatment on oxidative stress in serum, apoB-containing lipoproteins fraction and erythrocytes of aged rats. Pharmacol Rep. 2010;62(4):733–9. doi: 10.1016/s1734-1140(10)70331-5. [DOI] [PubMed] [Google Scholar]
- 55.Nagasawa T, Yonekura T, Nishizawa N, Kitts DD. In vitro and in vivo inhibition of muscle lipid and protein oxidation by carnosine. Mol Cell Biochem. 2001;225(1):29–34. doi: 10.1023/a:1012256521840. [DOI] [PubMed] [Google Scholar]
- 56•.Rabbani N, Xue M, Thornalley Paul J. Methylglyoxal-induced dicarbonyl stress in aging and disease: first steps towards glyoxalase 1-based treatments. Clinical Science. 2016;130(19):1677–96. doi: 10.1042/cs20160025. Excellent review describing the formation, metabolism, and consequences of dicarbonyl stress in various diseases and emerging dicarbonyl-targeting therapeutics. [DOI] [PubMed] [Google Scholar]
- 57.Allaman I, Bélanger M, Magistretti PJ. Methylglyoxal, the dark side of glycolysis. Frontiers in Neuroscience. 2015;9:23. doi: 10.3389/fnins.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lo TW, Westwood ME, McLellan AC, Selwood T, Thornalley PJ. Binding and modification of proteins by methylglyoxal under physiological conditions. A kinetic and mechanistic study with N alpha-acetylarginine, N alpha-acetylcysteine, and N alpha-acetyllysine, and bovine serum albumin. J Biol Chem. 1994;269(51):32299–305. [PubMed] [Google Scholar]
- 59.Ahmed MU, Brinkmann Frye E, Degenhardt TP, Thorpe SR, Baynes JW. N-epsilon-(carboxyethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. Biochemical Journal. 1997;324(Pt 2):565–70. doi: 10.1042/bj3240565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brinkmann E, Wells-Knecht KJ, Thorpe SR, Baynes JW. Characterization of an imidazolium compound formed by reaction of methylglyoxal and N-alpha-hippuryllysine. J Chem Soc Perkin Trans. 1995;22:2817–8. [Google Scholar]
- 61.Rabbani N, Xue M, Thornalley PJ. Dicarbonyls and glyoxalase in disease mechanisms and clinical therapeutics. Glycoconjugate journal. 2016;33:513–25. doi: 10.1007/s10719-016-9705-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hipkiss AR, Chana H. Carnosine protects proteins against methylglyoxal-mediated modifications. Biochem Biophys Res Commun. 1998;248(1):28–32. doi: 10.1006/bbrc.1998.8806. [DOI] [PubMed] [Google Scholar]
- 63.Nagaraj RH, Sarkar P, Mally A, Biemel KM, Lederer MO, Padayatti PS. Effect of pyridoxamine on chemical modification of proteins by carbonyls in diabetic rats: characterization of a major product from the reaction of pyridoxamine and methylglyoxal. Archives of Biochemistry and Biophysics. 2002;402(1):110–9. doi: 10.1016/S0003-9861(02)00067-X. doi: http://dx.doi.org/10.1016/S0003-9861(02)00067-X. [DOI] [PubMed] [Google Scholar]
- 64.Fuchs P, Loeseken C, Schubert JK, Miekisch W. Breath gas aldehydes as biomarkers of lung cancer. International Journal of Cancer. 2010;126(11):2663–70. doi: 10.1002/ijc.24970. [DOI] [PubMed] [Google Scholar]
- 65.Alhamdani MSS, Al-Kassir AHAM, Jaleel NA, Hmood AM, Ali HM. Elevated Levels of Alkanals, Alkenals and 4-HO-Alkenals in Plasma of Hemodialysis Patients. American Journal of Nephrology. 2006;26(3):299–303. doi: 10.1159/000094305. [DOI] [PubMed] [Google Scholar]
- 66.Corradi M, Pignatti P, Manini P, Andreoli R, Goldoni M, Poppa M, et al. Comparison between exhaled and sputum oxidative stress biomarkers in chronic airway inflammation. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2004;24(6):1011–7. doi: 10.1183/09031936.04.00002404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jalali M, Zare Sakhvidi MJ, Bahrami A, Berijani N, Mahjub H. Oxidative Stress Biomarkers in Exhaled Breath of Workers Exposed to Crystalline Silica Dust by SPME-GC-MS. J Res Health Sci. 2016;16(3):153–61. [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou S, Decker EA. Ability of amino acids, dipeptides, polyamines, and sulfhydryls to quench hexanal, a saturated aldehydic lipid oxidation product. J Agric Food Chem. 1999;47(5):1932–6. doi: 10.1021/jf980939s. [DOI] [PubMed] [Google Scholar]
- 69.Townsend AJ, Leone-Kabler S, Haynes RL, Wu Y, Szweda L, Bunting KD. Selective protection by stably transfected human ALDH3A1 (but not human ALDH1A1) against toxicity of aliphatic aldehydes in V79 cells. Chemico-Biological Interactions. 2001;130–132:261–73. doi: 10.1016/s0009-2797(00)00270-2. http://dx.doi.org/10.1016/S0009-2797(00)00270-2. [DOI] [PubMed] [Google Scholar]
- 70.Tacka KA, Dabrowiak JC, Goodisman J, Souid A-K. Kinetic Analysis of the Reactions of 4-Hydroperoxycyclophosphamide and Acrolein With Glutathione, Mesna, and Wr-1065. Drug Metabolism and Disposition. 2002;30(8):875–82. doi: 10.1124/dmd.30.8.875. [DOI] [PubMed] [Google Scholar]
- 71.Brock N, Pohl J. The development of mesna for regional detoxification. Cancer Treat Rev. 1983;10(Suppl A):33–43. doi: 10.1016/s0305-7372(83)80005-x. [DOI] [PubMed] [Google Scholar]
- 72.SAKURAI M, SAIJO N, SHINKAI T, EGUCHI K, SASAKI Y, TAMURA T, et al. The Protective Effect of 2-Mercapto-Ethane Sulfonate (MESNA) on Hemorrhagic Cystitis Induced by High-Dose Ifosfamide Treatment Tested by a Randomized Crossover Trial. Japanese Journal of Clinical Oncology. 1986;16(2):153–6. doi: 10.1093/oxfordjournals.jjco.a039132. [DOI] [PubMed] [Google Scholar]
- 73.Fukuoka M, Negoro S, Masuda N, Furuse K, Kawahara M, Kodama N, et al. Placebo-controlled double-blind comparative study on the preventive efficacy of mesna against ifosfamide-induced urinary disorders. J Cancer Res Clin Oncol. 1991;117(5):473–8. doi: 10.1007/BF01612769. [DOI] [PubMed] [Google Scholar]
- 74.Vose JM, Reed EC, Pippert GC, Anderson JR, Bierman PJ, Kessinger A, et al. Mesna compared with continuous bladder irrigation as uroprotection during high-dose chemotherapy and transplantation: a randomized trial. J Clin Oncol. 1993;11(7):1306–10. doi: 10.1200/JCO.1993.11.7.1306. [DOI] [PubMed] [Google Scholar]
- 75.Aluise CD, Miriyala S, Noel T, Sultana R, Jungsuwadee P, Taylor TJ, et al. 2-Mercaptoethane sulfonate prevents doxorubicin-induced plasma protein oxidation and TNF-α release: Implications for the reactive oxygen species-mediated mechanisms of chemobrain. Free Radical Biology and Medicine. 2011;50(11):1630–8. doi: 10.1016/j.freeradbiomed.2011.03.009. doi: http://dx.doi.org/10.1016/j.freeradbiomed.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 76••.Hayslip J, Dressler EV, Weiss H, Taylor TJ, Chambers M, Noel T, et al. Plasma TNF-α and Soluble TNF Receptor Levels after Doxorubicin with or without Co-Administration of Mesna- A Randomized, Cross-Over Clinical Study. PLoS ONE. 2015;10(4):e0124988. doi: 10.1371/journal.pone.0124988. First clinical demonstration of MESNA inhibiting inflammation when co-administered with redox-active chemotherapeutic doxorubicin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ludwig U, Riedel MK, Backes M, Imhof A, Muche R, Keller F. MESNA (sodium 2-mercaptoethanesulfonate) for prevention of contrast medium-induced nephrotoxicity - controlled trial. Clin Nephrol. 2011;75(4):302–8. doi: 10.5414/cn106651. [DOI] [PubMed] [Google Scholar]
- 78.Amirshahrokhi K, Khalili A-R. Gastroprotective effect of 2-mercaptoethane sulfonate against acute gastric mucosal damage induced by ethanol. International Immunopharmacology. 2016;34:183–8. doi: 10.1016/j.intimp.2016.03.006. doi: http://dx.doi.org/10.1016/j.intimp.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 79.Triantafyllidis I, Poutahidis T, Taitzoglou I, Kesisoglou I, Lazaridis C, Botsios D. Treatment with Mesna and n-3 polyunsaturated fatty acids ameliorates experimental ulcerative colitis in rats. International Journal of Experimental Pathology. 2015;96(6):433–43. doi: 10.1111/iep.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arai T, Koyama R, Yuasa M, Kitamura D, Mizuta R. Acrolein, a highly toxic aldehyde generated under oxidative stress in vivo, aggravates the mouse liver damage after acetaminophen overdose. Biomedical Research. 2014;35(6):389–95. doi: 10.2220/biomedres.35.389. [DOI] [PubMed] [Google Scholar]
- 81.Yilmaz ER, Kertmen H, Gürer B, Kanat MA, Arikok AT, Ergüder BI, et al. The protective effect of 2-mercaptoethane sulfonate (MESNA) against traumatic brain injury in rats. Acta Neurochirurgica. 2013;155(1):141–9. doi: 10.1007/s00701-012-1501-3. [DOI] [PubMed] [Google Scholar]
- 82.Kouvaris JR, Kouloulias VE, Vlahos LJ. Amifostine: The First Selective-Target and Broad-Spectrum Radioprotector. The Oncologist. 2007;12(6):738–47. doi: 10.1634/theoncologist.12-6-738. [DOI] [PubMed] [Google Scholar]
- 83.Gu J, Zhu S, Li X, Wu H, Li Y, Hua F. Effect of Amifostine in Head and Neck Cancer Patients Treated with Radiotherapy: A Systematic Review and Meta-Analysis Based on Randomized Controlled Trials. PLoS ONE. 2014;9(5):e95968. doi: 10.1371/journal.pone.0095968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kheradmand A, Nayebi AM, Jorjani M, Khalifeh S, Haddadi R. Effects of WR1065 on 6-hydroxydopamine-induced motor imbalance: Possible involvement of oxidative stress and inflammatory cytokines. Neuroscience Letters. 2016;627:7–12. doi: 10.1016/j.neulet.2016.05.040. doi: http://dx.doi.org/10.1016/j.neulet.2016.05.040. [DOI] [PubMed] [Google Scholar]
- 85.Chronidou F, Apostolakis E, Papapostolou I, Grintzalis K, Georgiou CD, Koletsis EN, et al. Beneficial effect of the oxygen free radical scavenger amifostine (WR-2721) on spinal cord ischemia/reperfusion injury in rabbits. Journal of Cardiothoracic Surgery. 2009;4(1):1–12. doi: 10.1186/1749-8090-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Quinn PJ, Boldyrev AA, Formazuyk VE. Carnosine: its properties, functions and potential therapeutic applications. Mol Aspects Med. 1992;13(5):379–444. doi: 10.1016/0098-2997(92)90006-l. [DOI] [PubMed] [Google Scholar]
- 87.Aerts L, Van Assche FA. Low taurine, gamma-aminobutyric acid and carnosine levels in plasma of diabetic pregnant rats: consequences for the offspring. J Perinat Med. 2001;29(1):81–4. doi: 10.1515/JPM.2001.012. [DOI] [PubMed] [Google Scholar]
- 88.Gualano B, Everaert I, Stegen S, Artioli GG, Taes Y, Roschel H, et al. Reduced muscle carnosine content in type 2, but not in type 1 diabetic patients. Amino Acids. 2012;43(1):21–4. doi: 10.1007/s00726-011-1165-y. [DOI] [PubMed] [Google Scholar]
- 89.Fonteh AN, Harrington RJ, Tsai A, Liao P, Harrington MG. Free amino acid and dipeptide changes in the body fluids from Alzheimer's disease subjects. Amino Acids. 2007;32(2):213–24. doi: 10.1007/s00726-006-0409-8. [DOI] [PubMed] [Google Scholar]
- 90.Harris RC, Wise JA, Price KA, Kim HJ, Kim CK, Sale C. Determinants of muscle carnosine content. Amino Acids. 2012;43(1):5–12. doi: 10.1007/s00726-012-1233-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Everaert I, Mooyaart A, Baguet A, Zutinic A, Baelde H, Achten E, et al. Vegetarianism, female gender and increasing age, but not CNDP1 genotype, are associated with reduced muscle carnosine levels in humans. Amino Acids. 2011;40(4):1221–9. doi: 10.1007/s00726-010-0749-2. [DOI] [PubMed] [Google Scholar]
- 92.Hipkiss AR. Carnosine and its possible roles in nutrition and health. Adv Food Nutr Res. 2009;57:87–154. doi: 10.1016/S1043-4526(09)57003-9. [DOI] [PubMed] [Google Scholar]
- 93•.Colzani M, De Maddis D, Casali G, Carini M, Vistoli G, Aldini G. Reactivity, Selectivity, and Reaction Mechanisms of Aminoguanidine, Hydralazine, Pyridoxamine, and Carnosine as Sequestering Agents of Reactive Carbonyl Species: A Comparative Study. ChemMedChem. 2016 doi: 10.1002/cmdc.201500552. Systematic analyses of four carbonyl scavengers in their abilities to quench HNE, MDA, MGO and glyoxal which offers insight into improving scavenger design. [DOI] [PubMed] [Google Scholar]
- 94.Orioli M, Aldini G, Benfatto MC, Facino RM, Carini M. HNE Michael adducts to histidine and histidine-containing peptides as biomarkers of lipid-derived carbonyl stress in urines: LC-MS/MS profiling in Zucker obese rats. Analytical chemistry. 2007;79(23):9174–84. doi: 10.1021/ac7016184. [DOI] [PubMed] [Google Scholar]
- 95•.Regazzoni L, de Courten B, Garzon D, Altomare A, Marinello C, Jakubova M, et al. A carnosine intervention study in overweight human volunteers: bioavailability and reactive carbonyl species sequestering effect. Sci Rep. 2016;6:27224. doi: 10.1038/srep27224. Interventional study with carnosine that shows urinary excretion of carnosine-acrolein adducts depends on individual diversity of carnosine intake, metabolism, and basal RCS production. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peters V, Schmitt CP, Zschocke J, Gross ML, Brismar K, Forsberg E. Carnosine treatment largely prevents alterations of renal carnosine metabolism in diabetic mice. Amino Acids. 2012;42(6):2411–6. doi: 10.1007/s00726-011-1046-4. [DOI] [PubMed] [Google Scholar]
- 97.Lee YT, Hsu CC, Lin MH, Liu KS, Yin MC. Histidine and carnosine delay diabetic deterioration in mice and protect human low density lipoprotein against oxidation and glycation. Eur J Pharmacol. 2005;513(1–2):145–50. doi: 10.1016/j.ejphar.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 98.Peters V, Riedl E, Braunagel M, Hoger S, Hauske S, Pfister F, et al. Carnosine treatment in combination with ACE inhibition in diabetic rats. Regul Pept. 2014;194–195:36–40. doi: 10.1016/j.regpep.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 99.Yan H, Guo Y, Zhang J, Ding Z, Ha W, Harding JJ. Effect of carnosine, aminoguanidine, and aspirin drops on the prevention of cataracts in diabetic rats. Mol Vis. 2008;14:2282–91. [PMC free article] [PubMed] [Google Scholar]
- 100.Barski OA, Xie Z, Baba SP, Sithu SD, Agarwal A, Cai J, et al. Dietary carnosine prevents early atherosclerotic lesion formation in apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol. 2013;33(6):1162–70. doi: 10.1161/ATVBAHA.112.300572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101•.Menini S, Iacobini C, Ricci C, Blasetti Fantauzzi C, Pugliese G. Protection from diabetes-induced atherosclerosis and renal disease by D-carnosine-octylester: effects of early vs late inhibition of advanced glycation end-products in Apoe-null mice. Diabetologia. 2015;58(4):845–53. doi: 10.1007/s00125-014-3467-6. Valuable pre-clinical study showing carnosine both inhibits early AGE formation and protects mice from diabetes-induced vascular and renal disease. [DOI] [PubMed] [Google Scholar]
- 102.Menini S, Iacobini C, Ricci C, Scipioni A, Blasetti Fantauzzi C, Giaccari A, et al. D-Carnosine octylester attenuates atherosclerosis and renal disease in ApoE null mice fed a Western diet through reduction of carbonyl stress and inflammation. Br J Pharmacol. 2012;166(4):1344–56. doi: 10.1111/j.1476-5381.2012.01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Min J, Senut MC, Rajanikant K, Greenberg E, Bandagi R, Zemke D, et al. Differential neuroprotective effects of carnosine, anserine, and N-acetyl carnosine against permanent focal ischemia. J Neurosci Res. 2008;86(13):2984–91. doi: 10.1002/jnr.21744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang X, Song L, Cheng X, Yang Y, Luan B, Jia L, et al. Carnosine pretreatment protects against hypoxia-ischemia brain damage in the neonatal rat model. Eur J Pharmacol. 2011;667(1–3):202–7. doi: 10.1016/j.ejphar.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 105.Bae ON, Serfozo K, Baek SH, Lee KY, Dorrance A, Rumbeiha W, et al. Safety and efficacy evaluation of carnosine, an endogenous neuroprotective agent for ischemic stroke. Stroke. 2013;44(1):205–12. doi: 10.1161/STROKEAHA.112.673954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106•.Davis CK, Laud PJ, Bahor Z, Rajanikant GK, Majid A. Systematic review and stratified meta-analysis of the efficacy of carnosine in animal models of ischemic stroke. J Cereb Blood Flow Metab. 2016 doi: 10.1177/0271678X16658302. Systematic review of effects of carnosine administration in animal models of ischemic stroke. [DOI] [PMC free article] [PubMed]
- 107.Zhang ZY, Sun BL, Yang MF, Li DW, Fang J, Zhang S. Carnosine attenuates early brain injury through its antioxidative and anti-apoptotic effects in a rat experimental subarachnoid hemorrhage model. Cell Mol Neurobiol. 2015;35(2):147–57. doi: 10.1007/s10571-014-0106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zieba R, Wagrowska-Danilewicz M. Influence of carnosine on the cardiotoxicity of doxorubicin in rabbits. Pol J Pharmacol. 2003;55(6):1079–87. [PubMed] [Google Scholar]
- 109•.de Courten B, Jakubova M, de Courten MP, Kukurova IJ, Vallova S, Krumpolec P, et al. Effects of carnosine supplementation on glucose metabolism: Pilot clinical trial. Obesity. 2016;24(5):1027–34. doi: 10.1002/oby.21434. Interventional pilot study showing that carnosine supplementation improved insulin sensitivity and glucose handling in obese and overweight individuals. [DOI] [PubMed] [Google Scholar]
- 110.Lombardi C, Carubelli V, Lazzarini V, Vizzardi E, Bordonali T, Ciccarese C, et al. Effects of oral administration of orodispersible levo-carnosine on quality of life and exercise performance in patients with chronic heart failure. Nutrition. 2015;31(1):72–8. doi: 10.1016/j.nut.2014.04.021. doi: http://dx.doi.org/10.1016/j.nut.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 111.Boldyrev A, Fedorova T, Stepanova M, Dobrotvorskaya I, Kozlova E, Boldanova N, et al. Carnosine [corrected] increases efficiency of DOPA therapy of Parkinson's disease: a pilot study. Rejuvenation Res. 2008;11(4):821–7. doi: 10.1089/rej.2008.0716. [DOI] [PubMed] [Google Scholar]
- 112.Cornelli U. Treatment of Alzheimer's disease with a cholinesterase inhibitor combined with antioxidants. Neurodegener Dis. 2010;7(1–3):193–202. doi: 10.1159/000295663. [DOI] [PubMed] [Google Scholar]
- 113.Babizhayev MA, Yermakova VN, Sakina NL, Evstigneeva RP, Rozhkova EA, Zheltukhina GA. N alpha-acetylcarnosine is a prodrug of L-carnosine in ophthalmic application as antioxidant. Clin Chim Acta. 1996;254(1):1–21. doi: 10.1016/0009-8981(96)06356-5. [DOI] [PubMed] [Google Scholar]
- 114.Babizhayev MA, Micans P, Guiotto A, Kasus-Jacobi A. N-acetylcarnosine lubricant eyedrops possess all-in-one universal antioxidant protective effects of L-carnosine in aqueous and lipid membrane environments, aldehyde scavenging, and transglycation activities inherent to cataracts: a clinical study of the new vision-saving drug N-acetylcarnosine eyedrop therapy in a database population of over 50,500 patients. Am J Ther. 2009;16(6):517–33. doi: 10.1097/MJT.0b013e318195e327. [DOI] [PubMed] [Google Scholar]
- 115.Sakae K, Agata T, Kamide R, Yanagisawa H. Effects of L-carnosine and its zinc complex (Polaprezinc) on pressure ulcer healing. Nutr Clin Pract. 2013;28(5):609–16. doi: 10.1177/0884533613493333. [DOI] [PubMed] [Google Scholar]
- 116.Baraniuk JN, El-Amin S, Corey R, Rayhan R, Timbol C. Carnosine treatment for gulf war illness: a randomized controlled trial. Glob J Health Sci. 2013;5(3):69–81. doi: 10.5539/gjhs.v5n3p69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sullivan D, Peterson R, Mujer C, Gad S. A 7-day intravenous toxicity study and neurotoxicity assessment of pyridorin in Sprague-Dawley rats. Human & Experimental Toxicology. 2016 doi: 10.1177/0960327116661023. [DOI] [PubMed] [Google Scholar]
- 118.Amarnath V, Amarnath K, Amarnath K, Davies S, Roberts LJ., 2nd Pyridoxamine: an extremely potent scavenger of 1,4-dicarbonyls. Chem Res Toxicol. 2004;17(3):410–5. doi: 10.1021/tx0300535. [DOI] [PubMed] [Google Scholar]
- 119.Zagol-Ikapitte I, Amarnath V, Bala M, Roberts LJ, 2nd, Oates JA, Boutaud O. Characterization of scavengers of gamma-ketoaldehydes that do not inhibit prostaglandin biosynthesis. Chem Res Toxicol. 2010;23(1):240–50. doi: 10.1021/tx900407a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chipinda I, Mbiya W, Adigun RA, Morakinyo MK, Law BF, Simoyi RH, et al. Pyridoxylamine reactivity kinetics as an amine based nucleophile for screening electrophilic dermal sensitizers. Toxicology. 2014;315:102–9. doi: 10.1016/j.tox.2013.11.009. doi: http://dx.doi.org/10.1016/j.tox.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Degenhardt TP, Alderson NL, Arrington DD, Beattie RJ, Basgen JM, Steffes MW, et al. Pyridoxamine inhibits early renal disease and dyslipidemia in the streptozotocin-diabetic rat. Kidney Int. 2002;61(3):939–50. doi: 10.1046/j.1523-1755.2002.00207.x. [DOI] [PubMed] [Google Scholar]
- 122.Tanimoto M, Gohda T, Kaneko S, Hagiwara S, Murakoshi M, Aoki T, et al. Effect of pyridoxamine (K-163), an inhibitor of advanced glycation end products, on type 2 diabetic nephropathy in KK-Ay/Ta mice. Metabolism. 2007;56(2):160–7. doi: 10.1016/j.metabol.2006.08.026. doi: http://dx.doi.org/10.1016/j.metabol.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 123.Liu R, Zhong Y, Li X, Chen H, Jim B, Zhou M-M, et al. Role of Transcription Factor Acetylation in Diabetic Kidney Disease. Diabetes. 2014;63(7):2440–53. doi: 10.2337/db13-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124•.Maessen DE, Brouwers O, Gaens KH, Wouters K, Cleutjens JP, Janssen BJ, et al. Delayed Intervention With Pyridoxamine Improves Metabolic Function and Prevents Adipose Tissue Inflammation and Insulin Resistance in High-Fat Diet–Induced Obese Mice. Diabetes. 2016;65(4):956–66. doi: 10.2337/db15-1390. Provocative pre-clinical study reporting that PM protects against high fat induced body weight gain. [DOI] [PubMed] [Google Scholar]
- 125•.Skrypnyk NI, Voziyan P, Yang H, de Caestecker CR, Theberge M-C, Drouin M, et al. Pyridoxamine reduces postinjury fibrosis and improves functional recovery after acute kidney injury. American Journal of Physiology - Renal Physiology. 2016;311(2):F268–F77. doi: 10.1152/ajprenal.00056.2016. First pre-clinical report of PM reducing short and long term injury and improving renal function after ischemia-reperfusion acute kidney injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mastrocola R, Nigro D, Chiazza F, Medana C, Dal Bello F, Boccuzzi G, et al. Fructose-derived advanced glycation end-products drive lipogenesis and skeletal muscle reprogramming via SREBP-1c dysregulation in mice. Free Radical Biology and Medicine. 2016;91:224–35. doi: 10.1016/j.freeradbiomed.2015.12.022. doi: http://dx.doi.org/10.1016/j.freeradbiomed.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 127.Watson AMD, Soro-Paavonen A, Sheehy K, Li J, Calkin AC, Koitka A, et al. Delayed intervention with AGE inhibitors attenuates the progression of diabetes-accelerated atherosclerosis in diabetic apolipoprotein E knockout mice. Diabetologia. 2011;54(3):681–9. doi: 10.1007/s00125-010-2000-9. [DOI] [PubMed] [Google Scholar]
- 128.Cao W, Chen J, Chen Y, Chen S, Chen X, Huang H, et al. Advanced glycation end products induced immune maturation of dendritic cells controls heart failure through NF-κB signaling pathway. Archives of Biochemistry and Biophysics. 2015;580:112–20. doi: 10.1016/j.abb.2015.07.003. doi: http://dx.doi.org/10.1016/j.abb.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 129•.Brodeur MR, Bouvet C, Bouchard S, Moreau S, Leblond J, deBlois D, et al. Reduction of Advanced-Glycation End Products Levels and Inhibition of RAGE Signaling Decreases Rat Vascular Calcification Induced by Diabetes. PLoS ONE. 2014;9(1):e85922. doi: 10.1371/journal.pone.0085922. Pre-clinical study with PM showing efficacy to reduce diabetes-induced calcification in arteries in rats. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang C-H, Wu E-T, Wu M-S, Tsai M-S, Ko Y-H, Chang R-W, et al. Pyridoxamine protects against mechanical defects in cardiac ageing in rats: studies on load dependence of myocardial relaxation. Experimental Physiology. 2014;99(11):1488–98. doi: 10.1113/expphysiol.2014.082008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Charvet CD, Saadane A, Wang M, Salomon RG, Brunengraber H, Turko IV, et al. Pretreatment with Pyridoxamine Mitigates Isolevuglandin-associated Retinal Effects in Mice Exposed to Bright Light. Journal of Biological Chemistry. 2013;288(41):29267–80. doi: 10.1074/jbc.M113.498832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dong Z, Iwata D, Kitaichi N, Takeuchi M, Sato M, Endo N, et al. Amelioration of experimental autoimmune uveoretinitis by inhibition of glyceraldehyde-derived advanced glycation end-product formation. Journal of Leukocyte Biology. 2014;96(6):1077–85. doi: 10.1189/jlb.3A0513-288RRR. [DOI] [PubMed] [Google Scholar]
- 133.Thotala D, Chetyrkin S, Hudson B, Hallahan D, Voziyan P, Yazlovitskaya E. Pyridoxamine protects intestinal epithelium from ionizing radiation-induced apoptosis. Free Radic Biol Med. 2009;47(6):779–85. doi: 10.1016/j.freeradbiomed.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yazlovitskaya EM, Voziyan PA, Manavalan T, Yarbrough WG, Ivanova AV. Cellular oxidative stress response mediates radiosensitivity in Fus1-deficient mice. Cell Death Dis. 2015;6:e1652. doi: 10.1038/cddis.2014.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Williams ME, Bolton WK, Khalifah RG, Degenhardt TP, Schotzinger RJ, McGill JB. Effects of Pyridoxamine in Combined Phase 2 Studies of Patients with Type 1 and Type 2 Diabetes and Overt Nephropathy. American Journal of Nephrology. 2007;27(6):605–14. doi: 10.1159/000108104. [DOI] [PubMed] [Google Scholar]
- 136.Lewis EJ, Greene T, Spitalewiz S, Blumenthal S, Berl T, Hunsicker LG, et al. Pyridorin in Type 2 Diabetic Nephropathy. Journal of the American Society of Nephrology. 2012;23(1):131–6. doi: 10.1681/asn.2011030272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zagol-Ikapitte I, Matafonova E, Amarnath V, Bodine CL, Boutaud O, Tirona RG, et al. Determination of the Pharmacokinetics and Oral Bioavailability of Salicylamine, a Potent γ-Ketoaldehyde Scavenger, by LC/MS/MS. Pharmaceutics. 2010;2(1):18–29. doi: 10.3390/pharmaceutics2010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Davies SS, Bodine C, Matafonova E, Pantazides BG, Bernoud-Hubac N, Harrison FE, et al. Treatment with a gamma-ketoaldehyde scavenger prevents working memory deficits in hApoE4 mice. J Alzheimers Dis. 2011;27(1):49–59. doi: 10.3233/JAD-2011-102118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139•.Pearson JN, Warren E, Liang L-P, Roberts LJ, Ii, Patel M. Scavenging of highly reactive gamma-ketoaldehydes attenuates cognitive dysfunction associated with epileptogenesis. Neurobiology of Disease. 2017;98:88–99. doi: 10.1016/j.nbd.2016.11.011. doi: http://dx.doi.org/10.1016/j.nbd.2016.11.011. Preclinical study showing 2-hydroxybenzylamine reduces IsoLG adducts and blocks seizure-associated memory impairment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140•.Nguyen TT, Caito SW, Zackert WE, West JD, Zhu S, Aschner M, et al. Scavengers of reactive gamma-ketoaldehydes extend Caenorhabditis elegans lifespan and healthspan through protein-level interactions with SIR-2.1 and ETS-7. Aging (Albany NY) 2016;8(8):1759–80. doi: 10.18632/aging.101011. Provocative study showing 2-hydroxybenzylamine prolongs lifespans of C. elegans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lambert C, Li J, Jonscher K, Yang TC, Reigan P, Quintana M, et al. Acrolein inhibits cytokine gene expression by alkylating cysteine and arginine residues in the NF-kappaB1 DNA binding domain. J Biol Chem. 2007;282(27):19666–75. doi: 10.1074/jbc.M611527200. [DOI] [PubMed] [Google Scholar]
- 142.Oe T, Lee SH, Silva Elipe MV, Arison BH, Blair IA. A novel lipid hydroperoxide-derived modification to arginine. Chem Res Toxicol. 2003;16(12):1598–605. doi: 10.1021/tx034178l. [DOI] [PubMed] [Google Scholar]
- 143.Lee SH, Rindgen D, Bible RH, Jr, Hajdu E, Blair IA. Characterization of 2'-deoxyadenosine adducts derived from 4-oxo-2-nonenal, a novel product of lipid peroxidation. Chem Res Toxicol. 2000;13(7):565–74. doi: 10.1021/tx000057z. [DOI] [PubMed] [Google Scholar]
- 144.Rindgen D, Nakajima M, Wehrli S, Xu K, Blair IA. Covalent modifications to 2'-deoxyguanosine by 4-oxo-2-nonenal, a novel product of lipid peroxidation. Chem Res Toxicol. 1999;12(12):1195–204. doi: 10.1021/tx990034o. [DOI] [PubMed] [Google Scholar]
- 145.Brinkmann E, Wells-Knecht KJ, Thorpe SR, Baynes JW. Characterization of an imidazolium compound formed by reaction of methylglyoxal and Nα-hippuryllysine. J Chem Soc Perkin Trans. 1995;1:2. doi: 10.1039/P19950002817. [DOI] [Google Scholar]
- 146.Frischmann M, Bidmon C, Angerer J, Pischetsrieder M. Identification of DNA adducts of methylglyoxal. Chem Res Toxicol. 2005;18(10):1586–92. doi: 10.1021/tx0501278. [DOI] [PubMed] [Google Scholar]