Abstract
Significant work has documented neuroplasticity in development, demonstrating that developmental pathways are shaped by experience. Plasticity is often discussed in terms of the results of differences in input; differences in brain structures, processes, or responses reflect differences in experience. In this paper, I discuss how developmental plasticity also effectively changes input into the system. That is, structures and processes change in response to input, and those changed structures and processes influence future inputs. For example, plasticity may change the pattern of eye movements to a stimulus, thereby changing which part of the scene becomes the input. Thus, plasticity is not only seen in the structures and processes that result from differences in experience, but also is seen in the changes in the input as those structures and processes adapt. The systematic study of the nature of experience, and how differences in experience shape learning, can contribute to our understanding of neuroplasticity in general.
Keywords: Experience, Plasticity, Input, Infant cognition, Learning to learn
1. Introduction
Development during infancy is characterized by a series of changes—the acquisition of new abilities, the refinement of old abilities, the integration of processes, and the reorganization of systems. The first postnatal year, for example, is characterized by the acquisition of independent sitting (e.g., Adolph & Robinson, 2015), a shift from processing faces in a piecemeal fashion to processing faces holistically (e.g., Schwarzer, Zauner, & Jovanovic, 2007), the coordination of visual exploration and reaching (e.g., von Hofsten, 2004), and the emergence of brain responses specific to face processing (e.g., de Haan, Johnson, & Halit, 2003). All these developmental changes reflect, to some extent, neuroplasticity. That is, the neuroanatomical structures and connections that support these abilities have developed and adapted in response to infants’ experiences.
Developmental outcomes reflect a cascade of events, however. Any given milestone or achievement reflects the specific experiences—biological or environmental—that have shaped physical, motor, and cognitive abilities at various points in developmental time. These changes in abilities then lead to different opportunities for new experiences (i.e., different inputs), that then further change the child’s developmental trajectory (see Masten & Cicchetti, 2010). Moreover, multiple different factors contribute to the achievement of a given milestone (see Thelen & Smith, 1994). Consider as an example an infant whose first spoken word is “dog.” Clearly, the infant’s exposure to English contributed to this being the child’s first spoken word. However, children’s first spoken words also reflect their developing abilities to perceive speech sounds in their “native” language, to articulate specific speech-related sounds, to learn associations between specific objects and specific word forms, as well as their experience with a particular language. Therefore, many experiences through the first year contribute to this milestone. For example, infants’ daily exposure to one or more language shapes their developing processing of speech sounds (Dietrich, Swingley, & Werker, 2007; Kuhl, Tsao, & Liu, 2003; Werker & Desjardins, 1995; Werker & Tees, 1984). In addition, infants’ early actions on objects—and the resulting influences on their visual object perception—may contribute to their learning of object names (Smith, 2013).
In this paper, I focus on how plasticity changes the inputs that an organism experiences. At least since the time of Dewey, there has been a recognition that input is an integral part of the experience of the world (Dewey, 1896). In the context of development, psychologists have long recognized the importance of input for development as well as how inputs change over development (Gibson, 1982, 1988; Piaget, 1954). However, despite the importance of input for understanding cognitive development, much of the work on cognitive development focuses on the outputs or products of development—changes in brain organization, strategies, skills, cognitive structures. When constructing programs of research to understand cognitive development, however, we should also consider how the input itself changes over development, and how those changes in the input contribute in important ways to subsequent development. These ideas have much in common with cascade approaches to understanding development (Bornstein, Hahn, & Wolke, 2013; Masten & Cicchetti, 2010), and one goal of this paper is to encourage researchers to think about developmental cascades broadly across domains, timeframes, and areas of development.
In this paper I will consider how differenct developmental outcomes (e.g., structures, processes, responses) translate to differences in the input, and those differences in the input further influence future developmental outcomes. Thus, here I will examine how differences in what information serves as input derive from variations in experience. This paper is organized in three sections. First, I discuss how different levels of variation in the input create different levels of variation in the products of development—i.e., plasticity. This discussion sets the stage for the second section, in which I provide two examples from research findings in two domains of how plasticity is revealed in the input. Finally, I conclude by providing a framework for understanding different ways in which input contributes to plasticity, and posit some goals for future research.
2. Levels of variation in the input and plasticity
There has long been a recognition between input and plasticity. Recognizing that there is considerably variation in the amount of overlap in the input across individuals, Greenough, Black, and Wallace (1987) distinguished between Experience Expectant Plasticity and Experience Dependent Plasticity. Some inputs are essentially universal—except under very extreme conditions, every human child experiences gravity, a caregiver, exposure to variations in temperature, and so on. These universal inputs lead to what has been referred to as Experience Expectant Plasticity (Greenough et al., 1987). Because there is little variation in the input, there is little variation in the structures, processes, and responses that develop, and it can appear that there is no plasticity. For example, there is a significant amount of consistency across individuals in the neuroanatomical organization and structures for representing visual and auditory information. In such cases it is tempting to conclude that those developmental products—e.g., the resulting brain organization—do not reflect plasticity at all. However, extreme examples illustrate that typical development reflects how the system responds to high level of overlap in the input—the lack of variation in the products of development reflects, at least in part, the lack of variation in the input.
Plasticity in these systems is observed when considering cases of extreme deprivation. The brain systems of children born blind or deaf, for example, do not receive the input that is experienced by the vast majority of developing brains. The resulting brain organization illustrates profound neuroplasticity; systems in infants born deaf or blind that would have processed information from the ears or eyes (if that input was available) adapt and develop to process other kinds of information (Bedny, Richardson, & Saxe, 2015; Finney, 2001; Finney et al., 2003; Ptito et al., 2012). The uniformity of the developing structure, therefore, reflects the overlap in the input.
Other inputs vary, resulting in differences in structures, processes, and skills, reflecting Experience Dependent Plasticity (Greenough et al., 1987). In these cases, different structures, processes, or skills emerge as a function of the differences in experience or inputs. For example, infants who have previously looked at and learned about dogs and cats will have a different context for learning about new dogs and cats than will infants who have not previously looked at and learned about dogs and cats. In this case, infants form different structures, processes, or representations that reflect different input.
These examples illustrate two extremes—experience expectant plasticity reflects situations in which there is high overlap in the input across individuals and experience dependent plasticity reflects situations in which there is little overlap in the input across individuals. However, there is significant variation in the amount of overlap individuals experience in a particular input, and that variation may have significant consequences for development. In some cases, the timing of the input differs, and thus input influences processing at different developmental time points. Consider the case of children who are born with dense central cataracts, and thus are essentially blind at birth, but have their vision restored during infancy. These infants do not receive patterned visual input until those cataracts are corrected; thus the timing of experience with patterned visual stimuli differs for infants born with and without cataracts. Children born with cataracts do not have any input about patterned visual stimuli—including faces—until vision is restored, whereas infants who are born sighted have patterned visual input, including faces, from birth. Thus, during this period of deprivation the input to the visual parts of the brain for a child with cataracts and a child with normal vision will have little if any overlap. Once the cataracts are removed and vision is restored, the child who was initially blind will have patterned vision. At this point, his or her input—at least in terms of exposure to patterned light—has much overlap with his or her sighted peers. However, although a child who was initially blind may have normal vision in many respects, the differences in input in the first weeks or months of postnatal life will result in this child processing visual stimuli differently than individuals born with typical visual abilities. Indeed, individuals who are born with dense central cataracts later (years after the cataracts are corrected) process faces differently than do individuals who were not blind at birth (Mondloch et al., 2013). Thus, the difference in the input before the cataracts were removed began a cascade of events that ultimately yielded differences in developmental outcome.
Variation in the amount of overlap of input is characteristic of typical developmental experiences. This can be differences in the timing of input. Variation in when children acquire the ability to walk, for example, changes the timing of a shift in the input into the visual system. The view from the upright, vertical walking posture is not available to infants who are unable to walk. Because all children (who learn how to walk) experience this shift in visual input, the input is in some sense universal. However, infants learn to walk at different ages, and thus the differences in input—and any resulting effect on the plasticity of the neural structures and systems that deal with the visual world—will occur at different developmental times.
Other kinds of differences in daily experience also can yield differences in the amount of overlap in input across individuals. Consider children’s exposure to faces in their daily lives. In some ways the input is highly overlapping—human infants receive exposure to human faces. However, there are differences in the input with respect to the particular kinds of faces that serve as input to the developing face processing mechanism. Infants in Shanghai see very few Caucasian faces, infants in Grinnell Iowa see very few Asian faces, and infants in San Francisco see a mix of Asian and Caucasian faces. All infants are exposed to faces, but the input into their developing face processing system varies in terms of the racial make up of the faces they see. We know that such differences in variation—at least with respect to face processing—matter. Gaither, Pauker, and Johnson (2012) observed that Asian, Caucasian, and Asian-Caucasian biracial infants in Los Angeles processed own- and other-race faces differently. Although the Asian infants and Caucasian infants were clearly distinct from one another, the Asian-Caucasian biracial infants—who had more variability in the input (at least in terms of race) in the face input they processed—fell somewhere in-between the other two groups.
Infants can also experience differences in the distribution of input that can yield differences in developmental outcome. For example, both infants of depressed mothers and infants whose mothers are not depressed presumably experience a range of emotional expression, but the proportion of sad emotional expression varies for infants whose mothers are and are not depressed (e.g., Cohn, Campbell, Matias, & Hopkins, 1990; Cohn, Matias, Tronick, Connell, & Lyons-Ruth, 1986). In fact, infants whose mothers suffer from post-partum depression have more difficulty recognizing emotional expressions than infants whose mothers are not depressed (Bornstein, Arterberry, Mash, & Manian, 2010; Hernandez-Reif, Field, Diego, Vera, & Pickens, 2006), presumably reflecting differences in the proportion of happy and sad facial expressions infants see. Importantly, these differences are subtle, and this example shows that differences emerge not only when infants experience different input, but even when they experience the same input with different frequencies. Such examples show how some of aspects of the developing system are highly plastic and sensitive to small, but important, variations in input.
3. Plasticity changes the input
The main point here, however, is that plasticity actually creates change in the input itself. The experience-plasticity relations often focus on input as static. In other words, given a specific input (e.g., a word from a specific language), plasticity is observed in changes in the organism’s output (e.g., the ability to make a phonemic distinction). As a result, there is a unidirectional nature to the way the input-plasticity relation is often described—differences in input (experience) cause different developmental outcomes, or evidence of plasticity. However, input, like development itself, is dynamic and changing. We can see this clearly in the fact that the input that an organism receives often depends on the nature of the organism. For example, only once infants have the structures to perceive color will color be part of the visual input. Similarly, once infants can grasp and pick up objects they have as input the texture and weight of those objects. But the input that an organism receives also reflects the plasticity that has occurred in response to previous input. New information and experiences will be filtered through processes and structures that have already adapted to previous input and experiences. Input can also be changed by plasticity. Consider the example of the cataract patients described earlier. Deprivation to patterned light disrupts their processing of faces much later (Le Grand, Mondloch, Maurer, & Brent, 2001, 2004). It is possible that these first weeks of visual input are a critical period for holistic face processing—that is, the lack of input with faces during these early weeks may mean that the visual system misses an important window of opportunity for holistic processing to be acquired. This short period of deprivation means that early brain organization is occurring in the absence of typical visual input. Without that visual input, the brain organization is changed subtly, altering the input into the visual system once vision is restored. Although the visual system may compensate and other visual processes are indistinguishable from never-blind individuals, the change in the input may mean that holistic face processing cannot develop. This is not an isolated example. Infants exposed to Asian faces process new Asian faces in a way that allows them to discriminate between individuals, whereas infants exposed to Caucasian faces process new Asian faces in a way that makes such discrimination difficult (Kelly, Quinn et al., 2007; Kelly et al., 2009). Infants exposed to only English become insensitive to aspects of speech that allow them to hear distinctions used in Hindi (Werker & Tees, 1984), whereas infants exposed to Hindi will be able to hear those distinctions. Children who experienced physical abuse required different sensory information to recognize facial expressions than did children who had not been physically abused (Pollak & Sinha, 2002). The point is that even when processing the same information, children who have adapted to different experiences will attend to and learn different information about that information, and thus the input to the system will be different.
In the following paragraphs, I will discuss two examples from the literature to illustrate this point. The first example is face processing, a domain where much is known across the lifespan. The second example is the effect of pet experience on infants’ processing of animal images. Although there is much less known about this second domain, there are clear parallels in the two domains thus illustrating the point that the relation between experience and input is not specific to a single domain.
3.1. Example 1: the case of face processing
Infants are precocious face processors. Newborn infants prefer faces and face-like stimuli to other stimuli (e.g., Johnson, Dziurawiec, Ellis, & Morton, 1991; Simion, Valenza, Macchi Cassia, Turati, & Umiltà, 2002), in the first postnatal weeks they rapidly learn familiar faces, such as their mother’s (Bartrip, Morton, & de Schonen, 2001; Bushnell, Sai, & Mullin, 1989), and by 4 months they can differentiate between novel individual faces (Fagan, 1977; Pascalis et al., 1998). But infants’ face processing reflects differences in experience. By 3–4 months of age, infants prefer faces of their own (parents’) race (Kelly et al., 2005; Kelly, Liu et al., 2007) and faces that are the same gender as their primary caregiver (Quinn, Yahr, Kuhn, Slater, & Pascalis, 2002), particularly when the faces are from their familiar race (Quinn et al., 2008). Between 4 and 9 months, infants’ face processing becomes specialized based on their experience. Whereas young infants can discriminate between individual faces of their own race, an unfamiliar race, or even monkeys, by 9 months infants better discriminate human faces of their own- (parents) race than other-race or monkey faces (Kelly et al., 2009; Kelly, Quinn et al., 2007; Pascalis et al., 2005; Scott & Monesson, 2009).
Clearly, therefore, these remarkable achievements in infants’ face processing reflect plasticity. Over extensive experience with particular faces, infants’ responses (i.e., preferences, discrimination) reflect differences in experience. What is not clear from the preceding description is how the input for face processing is also changed by plasticity. Specifically, not only does infants’ processing of faces reflect differences in the kinds of faces in their environment, but also there are differences as a function of how plasticity influences the input into the face processing system.
Fig. 1 illustrates the notion of “developing input,” and how that input changes as a function of plasticity. In this figure, circles represent infants’ experience, and the grey rectangles represent internal processes, brain structures, or representations. The ovals within the circles are specific experiences or instances. The curved lines represent developmental time. The first thing to note is that in the first postnatal weeks, infants are exposed to a relatively small number of faces (Jayaraman, Fausey, & Smith, 2015), and those faces tend to be similar. Indeed, research suggests that young infants’ face experience is limited in variability, and most infants experience faces that are the same race and gender of their primary caregiver (Rennels & Davis, 2008; Sugden et al., 2014).
Fig. 1.
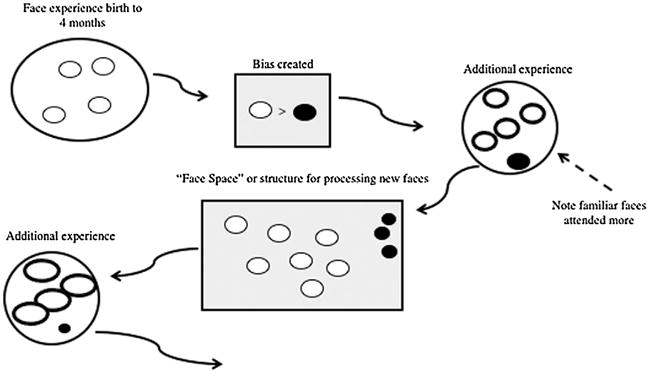
Schematic depiction of how processing of input shapes development over time. Circles represent experience; items of different color represent different types of experience and as items are more likely to receive attention they become bolded and bigger. Grey rectangles represent the internal structures, processes, or representations that develop as a function of the experience.
The first example of plasticity changing input is represented by the grey box labeled “bias created.” This box represents the fact that young infants prefer and look longer at faces that are familiar in terms of race or gender (Kelly et al., 2005; Kelly, Liu et al., 2007; Liu et al., 2015; Quinn et al., 2002), presumably reflecting this early experience. To be clear, this framework is neutral about the basis of this bias; it may reflect the fact that familiar faces are easier to process, are more emotionally satisfying or comforting, or some other factor. What is important for the present discussion is that when this bias emerges it has consequences for the infants’ face input.
The second circle, labeled “additional experience,” shows the effect of plasticity (in the form of a bias for familiar faces) on the input. The bias created by past experience determines which items will be attended—and thus will serve as input to the face processing systems as it continues to develop. Note that in the “additional experience” circle, faces similar to the biased face are bolded, indicating that infants will selectively prefer those faces, seek them out, and perhaps attend to those faces more deeply than the non-preferred faces. Two infants encountering the same set of faces, therefore, will focus different amounts of attention on the individual faces, attending more or more deeply to those for which they have a bias based on their previous face experience. As a result, the input into the face processing systems of these two infants will differ despite the fact they are looking at the same set of faces: infants may look more at and be more attentionally engaged when looking at the preferred faces. Of course, these biases are not static. They can change with additional experience with faces, and the strongest attentional effects would be predicted for infants who have had fairly consistent experience dominated by a single race. Indeed, Bar-Haim, Ziv, Lamy, and Hodes (2006) found that although African infants primarily exposed to African faces preferred African to Caucasian faces, this preference was reduced in African origin infants raised in a mixed-race community in Israel.
The consequences of differences in attention may be even more profound. Input from deeply attended faces may be different in quality than input from less deeply attended faces. Deeply attended faces may be encoded more strongly or with higher resolution. Markant, Oakes, and Amso (2016) used an inhibition of return (IOR) procedure to experimentally manipulate Caucasian 9-month-old infants’ attention to Caucasian and African American faces. In this task, infants were presented with Caucasian and African American faces on each trial, but the timing of between a cue and the onset of the stimuli cued them to attend to one of the faces. Infants whose attention was cued to African American faces showed evidence of having encoded and remembered African American faces but not Caucasian faces. Infants whose attention was cued to Caucasian faces showed the opposite pattern. Thus, the “other race effect” was shown in this case to be the “attended race effect,” raising the possibility that infants are more sensitive to their own familiar race (i.e., that of their parents) because they attend more deeply to those faces than to faces of other races. The point is that if infants engage attention more to own-race faces even more information about those faces would serve as input for the face processing system.
The effect of this characteristic of the input is illustrated in the “Face Space” box in Fig. 1. As shown here, the structure for processing, or “face space,” will vary as a function of differential processing of the more and less attended face stimuli. In Fig. 1, notice how the representations of or strategies for processing faces that are familiar (in terms of race or gender or some other feature) are larger, more central, and more numerous than the other faces (note: the different sizes and organization of “faces” in the middle grey box might reflect differences in the representations of faces, differences in strategies used for sampling information about those faces, or some other process related to face learning). Further, as a consequence of these differences in the face space box, in the final “additional experience” circle the familiar faces are even bigger, suggesting they are even more salient, more strongly attended to, easier to learn from, and so on.
Key to the notion of developing input—or input that is adapted in response to plasticity—is the underlying idea that infants are learning to learn, similar to how Adolph (2008), Adolph & Robinson (2015) describes infants’ emerging learning to learn how to locomote in the world. In the context of face processing, as infants encounter faces of a particular race, they visually investigate those faces, acquiring information about them and learning how to discriminate between different faces. Because infants’ experience is mostly with one race, they will learn to find the diagnostic features of faces of that race, and adopt a strategy of attending to those features. As a result of this learning to learn, infants become increasingly good at learning about and discriminating between individual faces of the familiar race. When they encounter faces of different races, their strategies may not be as effective, and they may be less sensitive to the differences between individuals of this less familiar race.
In fact, adults look differently at faces as a function both of their own experience and of the face race. Chinese adults and children look more at the noses and mouths of Asian faces than Caucasian faces (Fu, Hu, Wang, Quinn, & Lee, 2012; Hu, Wang, Fu, Quinn, & Lee, 2014). Similarly, European Caucasian adults look at the eyes of Caucasian faces and the mouths of Asian faces (Brielmann, Bülthoff, & Armann, 2014). By examining adults’ scanning of racially “pure” and racially ambiguous faces, Wang et al. (2015) demonstrated that different scanning patterns by Chinese adults reflect physiognomic differences between Caucasian and Asian faces, and are related to the ability of Chinese adults to recognize own race faces. Hills and Lewis (2011) showed that they could influence adult Caucasian participants’ recognition of African origin (American and UK) and Caucasian faces by cuing their attention to the most diagnostic region; the lower region of African origin faces and the upper region of Caucasian faces. The point is that there are indeed different diagnostic face regions for different races, and experience looking at and learning about faces of one race may help people learn how to learn about faces of that race that may not translate to learning about faces of other races.
If infants are indeed learning to learn, we predict that with increased experience processing faces, they would begin to focus on the most diagnostic features, shifting the input to the system toward those features. Lee and his colleagues have done extensive work on infants’ scanning of faces, comparing Chinese infants (in China) and Caucasian infants (in Canada) as they visually investigate Asian and Caucasian faces. The results of these studies indicate that over age Caucasian infants tend to increase their fixation of (and attention to) the eye regions of Caucasian faces (Wheeler et al., 2011; Xiao et al., 2013), and Chinese infants maintain their looking to the internal features of Chinese faces (Liu et al., 2011). These patterns are similar to how adults direct attention to Asian and Caucasian faces. Interestingly, infants do not show the same pattern when scanning other-race faces—that is, they do not appear to be sensitive to the diagnostic features of other races, nor do they simply scan other (unfamiliar) race faces in the same way the scan own-race faces. Caucasian infants decrease their looking to the noses of other-race Chinese faces (Xiao, Quinn, Pascalis, & Lee, 2014); the work with older viewers suggests the nose is diagnostic in Asian faces. Chinese infants decrease their looking to the internal features of other race Caucasian infants (Liu et al., 2011); this is not consistent with their focusing on the apparently diagnostic eyes of such faces.
What is clear is that the input differs depending on (1) the type of faces infants have been learning about, and (2) whether they are looking at own (familiar) or other (unfamiliar) race faces. There is not a universal “own-race” scanning pattern that emerges; instead, infants who have experience scanning Caucasian faces have learned to focus on the eyes of those faces, and infants who have experienced scanning Asian faces have learned to focus on the nose of those faces. Infants also do not use a single strategy for learning about both own- and other-race faces, demonstrating that the input to the system will differ depending on the particular face infants are looking at.
Do these patterns actually reflect infants’ adapting to the input and developing strategies to learn about faces from their own race? We do not yet have the data to address this question fully, although there is a hint that visual scanning patterns are related to learning. Amso, Fitzgerald, Davidow, Gilhooly, and Tottenham (2010) showed that infants’ scanning of faces was predictive of their recognition of a change from a happy to fearful facial expression. Similarly, Gaither et al. (2012) observed in 3-month-old monoracial Caucasian and Asian infants that infants’ scanning—that is, the number of transitions between the lower and upper half of the face—during habituation predicted their novelty preference in an own-race discrimination task, but not in an other-race discrimination task. Such results provide some support for the claim that differences in scanning reflect infants’ gradual recognition of the diagnostic features of the faces, and their shifting of attention to those diagnostic features.
3.2. Example 2: the case of pet experience
As a second example consider infants’ processing of images of animals as a function of their pet experience. Although much less research has examined this general topic, the existing work does illustrate how the input changes with differences in experience. As a starting point, there is evidence that infants, in general, are sensitive to the differences between images of dogs and cats. By 4 months, infants respond to the categorical distinction between dogs and cats (Oakes & Ribar, 2005; Quinn et al., 1993) and can distinguish between individual images of dogs and cats (Oakes, Kovack-Lesh, & Horst, 2009; Oakes & Ribar, 2005; Quinn et al., 1993). In addition, there is evidence that infants recognize the most diagnostic features of these animals. Mareschal, French, Quinn (2000) showed that the heads and faces of dogs and cats are the most informative for recognizing these categories. Subsequent work showed that young infants attend to and recognize differences in heads and features when categorizing cats and dogs (Quinn, Doran, Reiss, & Hoffman, 2009; Spencer & Quinn, 1997).
The work just described was conducted without considering pet experience, or with infants who did not have pet experience. However, other work has shown that infants’ experience with pets can influence infants’ processing of animal stimuli, much as infants’ face experience influences their processing of face stimuli. At 4 months of age, infants with pets show better discrimination of, memory for, and learning about images of cats and dogs than do infants who do not have pets (Kovack-Lesh, Horst, & Oakes, 2008; Kovack-Lesh, Oakes, & McMurray, 2012). Moreover, this difference between infants who do and do not have pets appears to reflect the kind of learning to learn as described for infants’ face processing.
Consider again Fig. 1. An infant with pets at home will have experience with pets. Thus, in this case, a bias may form simply that infants who have pets are more drawn to animal stimuli than are infants who do not have pets. As a result, those infants with pet experiences may have a stronger pull to attend to animal stimuli than infants without pet experience. Indeed, Hurley, Kovack-Lesh and Oakes (2010) found such a bias. Over a series of trials with either cats or dogs, 6-month-old infants who had pets at home were more engaged than were 6-month-old infants without pets. The infants with pets looked longer at and compared more images of dogs and cats than did infants without pets. Thus, difference in experience corresponded to a difference in the input; infants with pets had more and different input from animal images in this context than did infants without pets.
The kind of cascade illustrated in Fig. 1 would further suggest that this bias would contribute to the development of different strategies infants might use when learning about images of cats and dogs. Specifically, as discussed in the context of own- and other-race face processing, the prediction is that infants who have pet experience will have more experience visually inspecting and learning about cats and/or dogs. These infants then will have better learned how to learn about animal stimuli. In the context of thinking about how plasticity shapes the input, infants with pets—as a result of their experience with dogs and/or cats—would structure the input so that they would more effectively learn about new dogs and/or cats.
The results of several studies are consistent with these predictions. First, there appear to be differences in how infants with and without pets learn about images of animals during periods of attention. For example, Kovack-Lesh et al. (2008, 2012) found that infants who engaged in high levels of visual investigation (as indicated by glancing back and forth between two images of cats or dogs) categorized or formed memories of individual cats or dogs (depending on the task) only if they had previous pet experience. Similarly, Markant and Amso (2016) found that 4-month-old infants who engaged in a less sophisticated attentional strategy nevertheless learned about images of cats and dogs if they had pets at home. Together, these data suggest that infants who have experience with pets attend differently to images of cats and dogs than do infants who do not have experience with pets. In addition, the effect of attention on learning about dogs and cats was different for infants with and without pets. In other words, pet experience, like face processing experience, seems to lead to the biases that in turn lead to changes in attention, as depicted in Fig. 1.
Recall that infants’ face perception further reflects their experience in that the way they visually scan faces—and thus the input they get when they look at faces—differs depending on their own familiar race. Similar difference in infants’ scanning of animal stimuli are found for infants with and without pets. Infants who live with pets at home—and thus daily look at and visually examine dogs or cats—show different patterns of looking at images of dogs and cats in the lab than do infants who do not have pets at home. In particular, 4-month-old infants with pets looked more at the head and face regions of images of dogs and cats than did 4-month-old infants without pets (Hurley & Oakes, 2015; Kovack-Lesh et al., 2014). This head bias is particularly important because of the data described earlier demonstrating the head region is the most diagnostic region in images of dogs and cats (Mareschal et al., 2000). Thus, experience with pets shaped infants’ visual inspection to make them more attentive to informative regions—plasticity is reflected in the input. This changes the input that the visual system receives when infants look at cats or dogs, which may in turn impact the infants’ ability to learn about the cats or dogs. That is, infants have learned how to learn from their experience with pets. Indeed, although Markant and Amso (2016) found learning of head regions of images of cats and dogs both by infants with and without pets, they found that this learning was more robust in 4-month-old infants with pets than in 4-month-old infants who did not have pets.
Once again, we see that the strategies infants develop as a function of their experience actually shape the input the system receives in the future. Because infants who have pets at home look more at the heads and faces of images of animals, they will presumably attend more to these regions in their everyday life. As they acquire experience with animals, the input into the processing and representation of information about animals will contain more information about heads and faces than will the input of their peers who do not have pets at home. Thus, any future reorganization of these brain regions will reflect these differences in the input—even when infants were looking at and learning about precisely the same stimuli.
4. A framework for understanding how the input contributes to plasticity
In the preceding section, I described two examples illustrating how differences in experience shape the input received by developing systems. These examples show that the representations, processes, and structures that develop differently as a result of experience influencing future input. That is, plasticity influences the input. We not only see that brain organization is plastic and responsive to difference in experience, but also that the processes and strategies that are developed for acquiring information from the environment (e.g., determining the input) are also changed by plasticity. Moreover, the changes in the input reflect the infants’ learning to learn.
Fig. 2, which was taken from Oakes (2015) and adapted from Kovack-Lesh, McMurray, and Oakes (2014), provides a framework for understanding how these processes unfold over time, at least with respect to looking time. In this framework, the assumption is that looking time determines the input—what the infant looks at is what gets into the system (and provides the input for processing, representation, etc.). As illustrated in Fig. 2, infants’ looking at any point in time is determined by multiple factors and processes. Path 1 in Fig. 2 shows looking as determined by attentional processes—infants’ looking reflects their control over their attention, top-down and bottom up factors that determine where infants attend and so on (Johnson, 1990). These attentional processes become functional, or “come online” as the underlying neuroanatomical structures develop (Amso & Scerif, 2015). For example, orienting to a target and suppressing distractors relies on maturation of frontal brain regions, including the prefrontal cortex and frontal eye fields (Amso & Scerif, 2015). Focusing only on this pathway, therefore, might lead to the conclusion that changes in looking behavior—and the resulting input—largely reflects the development of neuroanatomical structures.
Fig. 2.
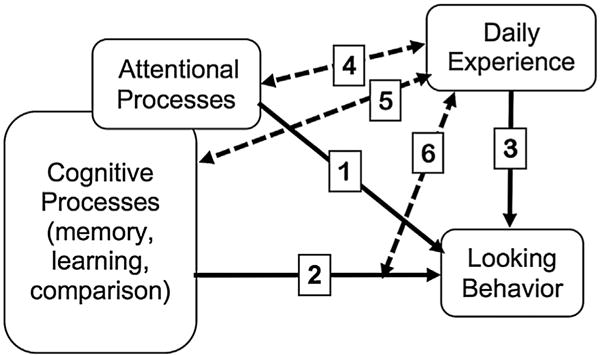
Conceptual framework for understanding infants’ looking behavior from Oakes, L. M. (2015). A biopsychosocial perspective on looking behavior in infancy. In S.D. Calkins (Ed.), Handbook of infant biopsychosocial development (Vol. 38, pp. 38–70). New York: Guilford Press. Reprinted with permission of Guilford Press, and adapted from “Four-month-old infants’ visual investigation of cats and dogs: Relations with pet experience and attentional strategy” by K.A. Kovack-Lesh, B. McMurray, & L. M. Oakes, Developmental Psychology, volume 50 (2014).
Pathway 2 recognizes that cognitive processes other than attentional processes also contribute to looking behavior. For example, the classic Sokolov comparator model of looking in infancy assumes that infants look while they are comparing a stored representation to the current stimulus. Long looking will result when the match is poor (for example, when no representation has yet been formed, or when the representation is weak), and looking will become shorter as the match is good. The duration of looking reflects how long it takes infants to gather information about the current stimulus, and to update the representation or memory of that stimulus. Infants look longer at novel stimuli because such stimuli take longer to represent than familiar stimuli (Colombo, 2002; Colombo et al., 2010; Hayne, 2004). Individual differences in looking reflect individual differences in speed of processing, rate of learning, and so on (Rose, Feldman, & Jankowski, 2002). These assumptions formed the foundation of early work on habituation of looking time.
Pathway 3 represents the direct effect that experience has on looking behavior. In addition to novelty effects due to in-the-moment learning about stimuli, infants’ looking reflects experience over a longer term—perhaps because stimuli that are closer to their experiences are easier to learn about, more comforting, or some other factor. This is the bias described in the previous sections that result in infants preferring faces that are the same races as their parents (Kelly et al., 2005; Kelly, Liu et al., 2007) and infants with pets look longer at images of cats and dogs than do infants without pets (Hurley et al., 2010). As illustrated in Fig. 1, these findings are consistent with infants’ developing biases to look at more familiar stimuli.
The important aspect of Fig. 2 for the present discussion, however, are the dotted pathways representing the interactive effect of the different components. In this paper, I have been focusing on Pathway 4, which depicts the relation between experience and attentional processes. It is important that the pathway has arrows on both ends, reflecting the fact that the interaction is bidirectional. As clear from the examples described here, experience shapes the way attention is deployed, and attention changes the input extracted from experience. Not only do infants prefer familiar own-race faces, the strategy they use to scan faces of familiar races differs from the strategy they use to scan faces of other races (Liu et al., 2011; Wheeler et al., 2011; Xiao et al., 2014). Similarly, infants with and without pets show different scanning strategies when looking at images of dogs can cats (Hurley & Oakes, 2015; Kovack-Lesh et al., 2014).
Moreover, it appears that the strategies infants use when looking at images in the lab reflect their previous learning to learn. When presented with animal images in the lab, infants with pets look more at the heads and faces than do infants without pets, the regions that are most diagnostic in differentiating dogs and cats (Mareschal et al., 2000). Over the first year, infants’ scanning of own-race faces comes to resemble that of adults scanning of those faces; their scanning of other-race faces also changes but not in ways that would lead to detection of more diagnostic features of those faces. Thus, in both examples given here, the scanning patterns associated with more experience should lead to input into the system that would produce more learning, more refined discrimination, and more diagnostic representations than the kind of scanning associated with less experience.
It is important that previous work in other domains has shown that differences in attentional strategies do determine what infants learn. Johnson, Slemmer, and Amso (2004) found that infants’ scanning of the classic rod-and-box display was related to whether or not they perceived the rod as connected or separate. More directly relevant, Amso et al. (2010) found that how infants scanned faces during habituation predicted whether they distinguished between faces of different emotional expressions. Similarly, in a different procedure Vanderwert et al. (2015) found that 7-month-old infants’ looking at the eyes of emotional expressions was related to their processing of those faces. Finally, young infants’ learning about cat and dog stimuli is refelcts an interaction between their attentional strategy and previous pet experience (Kovack-Lesh et al., 2008, 2012; Markant & Amso, 2016). An important goal for future research is to test these conclusions by directly examine the effects of different scanning patterns on infants’ learning, perhaps using a procedure like that used by Jankowski, Rose, and Feldman (2001) to induce poorer learners to more effectively sample information from a display.
5. Conclusion and suggestions for future directions
Plasticity is obviously understood in the context of experience, and how differences in experience correspond to dif-ference in the input to developing systems. The brain is plastic and adapts in the absence of typical experience, such as coordinated input from the two eyes. Similarly, the brain adapts and creates different organization in response to more subtle differences in experience, yielding structures and organizations that differ but not as dramatically as when a typical or expected experience is missing. In this paper, I argue that we gain deeper understanding by thinking about the input not as static, but rather as something that is dynamic and changing over time as a function of the plasticity reflecting earlier differences in input.
Of course, this discussion begs the questions of whether these ideas can be applied broadly to other domains and how we examine these proposals in research programs. Regarding the first question, it seems clear that the framework described here can be applied to a wide variety of domains. Indeed, work by Needham and her colleagues on how experience with sticky mittens is related to infants’ processing of visual objects, events, and faces (Libertus, Joh, & Needham, 2015; Libertus & Needham, 2011, 2014; Sommerville et al., 2005) fits this framework. This research suggests that infants’ effective picking up of objects—either “naturally” as a function of the development of motor skills or artificially through the use of sticky mittens—changes the input. For example, experience with sticky mittens can provide infants with direct input about how specific objects engage in an event (Rakison & Krogh, 2012). The point is that experience reaching changes the input, which may change the biases or strategies infants have when interacting with the visual world, which in turn changes the input for future processing.
It also should be clear that this framework makes predictions that can be addressed in research programs. As has been shown here, we can ask how infants’ sampling of the world—by visually scanning, reaching for objects, exploring surfaces, and so on—is shaped by their past experience. Learning to learn should be evident by changes in exploration strategies that are informed by past experience. Moreover, effective learning to learn should be evident by changes that result in qualitatively more informative or useful input—looking at the diagnostic regions of faces, reaching for the graspable part of a tool, testing an unfamiliar surfaces with one’s toes before walking onto that surface. Finally, we can ask whether adopting those more effective strategies actually results in superior or more effective learning.
Most importantly, this approach makes important developmental predictions that are tied to experience. As infants gain experience—either through exposure in everyday life (e.g., seeing many faces, living with a pet) or through the acquisition of a new skill (e.g., visually guided reaching, understanding the meanings of words)—those experiences will influence the plasticity in the development of systems, structures, and processes. However, that plasticity will also change the input in ways that will drive future plasticity. In this way, developmental change is a cascade of effects, in which adaptations that occur early in time will alter the way the child interacts with and gets input from the world at a later time, providing new and different opportunities for further adaptation.
Acknowledgments
Preparation of this manuscript was made possible by NIH grants R01 EY022525, R03 HD070651 and NSF grant BCS 0951580. I thank Karen Adolph, Steve Luck, and David Rakison for helpful comments on drafts of this paper and stimulating discussions about these issues.
References
- Adolph KE, Robinson SR. Motor development. Handbook of child psychology and developmental science. 2015:114–157. [Google Scholar]
- Adolph KE. Learning to move. Current Directions in Psychological Science. 2008;17:213–218. doi: 10.1111/j.1467-8721.2008.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amso D, Scerif G. The attentive brain: Insights from developmental cognitive neuroscience. Nature Reviews. 2015;16:606–619. doi: 10.1038/nrn4025. http://dx.doi.org/10.1038/nrn4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amso D, Fitzgerald M, Davidow J, Gilhooly T, Tottenham N. Visual exploration strategies and the development of infants’ facial emotion discrimination. Frontiers in Psychology. 2010;1:180. doi: 10.3389/fpsyg.2010.00180. http://dx.doi.org/10.3389/fpsyg.2010.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Ziv T, Lamy D, Hodes RM. Nature and nurture in own- race face processing. Psychological Science. 2006;17:159–163. doi: 10.1111/j.1467-9280.2006.01679.x. http://dx.doi.org/10.1111/j.1467-9280.2006.01679.x. [DOI] [PubMed] [Google Scholar]
- Bartrip J, Morton J, de Schonen S. Responses to mother’s face in 3-week to 5-month-old infants. British Journal of Developmental Psychology. 2001;19:219–232. http://dx.doi.org/10.1348/026151001166047. [Google Scholar]
- Bedny M, Richardson H, Saxe R. Visual cortex responds to spoken language in blind children. Journal of Neuroscience. 2015;35:11674–11681. doi: 10.1523/JNEUROSCI.0634-15.2015. http://dx.doi.org/10.1523/JNEUROSCI,0634-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH, Arterberry ME, Mash C, Manian N. Discrimination of facial expression by 5-month-old infants of nondepressed and clinically depressed mothers. Infant Behavior and Development. 2010;34:100–106. doi: 10.1016/j.infbeh.2010.10.002. http://dx.doi.org/10.1016/j.infbeh.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH, Hahn CS, Wolke D. Systems and cascades in cognitive development and academic achievement. Child Development. 2013;84:154–162. doi: 10.1111/j.1467-8624.2012.01849.x. http://dx.doi.org/10.1111/j.1467-8624.2012.01849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brielmann AA, Bülthoff I, Armann R. Looking at faces from different angles: Europeans fixate different features in Asian and Caucasian faces. Vision Research. 2014;100:105–112. doi: 10.1016/j.visres.2014.04.011. http://dx.doi.org/10.1016/j.visres.2014.04.011. [DOI] [PubMed] [Google Scholar]
- Bushnell IWR, Sai F, Mullin JT. Neonatal recognition of the mother’s face. British Journal of Developmental Psychology. 1989;7:3–15. http://dx.doi.org/10.1111/j.2044-835X.1989.tb00784.x. [Google Scholar]
- Cohn JF, Matias R, Tronick EZ, Connell D, Lyons-Ruth K. Face-to-face interactions of depressed mothers and their infants. New Directions for Child Development. 1986:31–45. doi: 10.1002/cd.23219863405. http://dx.doi.org/10.1002/cd.23219863405. [DOI] [PubMed]
- Cohn JF, Campbell SB, Matias R, Hopkins J. Face-to-face interactions of postpartum depressed and nondepressed mother-infant pairs at 2 months. Developmental Psychology. 1990;26:15–23. http://dx.doi.org/10.1037/0012-1649.26.1.15. [Google Scholar]
- Colombo J, Shaddy DJ, Anderson CJ, Gibson LJ. What habituates in infant visual habituation? A psychophysiological analysis. Infancy. 2010 doi: 10.1111/j.1532-7078.2009.00012.x. http://dx.doi.org/10.1111/j.1532-7078.2009.00012.x. [DOI] [PubMed]
- Colombo J. Infant attention grows up: The emergence of a developmental cognitive neuroscience perspective. Current Directions in Psychological Science. 2002;11:196–200. http://dx.doi.org/10.1111/1467-8721.00199. [Google Scholar]
- de Haan M, Johnson MH, Halit H. Development of face-sensitive event-related potentials during infancy: a review. International Journal of Psychophysiology. 2003;51:45–58. doi: 10.1016/s0167-8760(03)00152-1. pii. http://doi.org/S0167876003001521. [DOI] [PubMed] [Google Scholar]
- Dewey J. The reflex arc concept in psychology. The Psychological Review. 1896;3:357–370. [Google Scholar]
- Fagan JF. Infant recognition memory: Studies in forgetting. Child Development. 1977;48:68–78. doi: 10.1111/j.1467-8624.1977.tb04244.x. [DOI] [PubMed] [Google Scholar]
- Finney EM, Clementz BA, Hickok G, Dobkins KR. Visual stimuli activate auditory cortex in deaf subjects: Evidence from MEG. Neuroreport. 2003;14:1425–1427. doi: 10.1097/00001756-200308060-00004. http://dx.doi.org/10.1097/01.wnr.0000079894.11980.6a. [DOI] [PubMed] [Google Scholar]
- Finney EM. Visual stimuli activate auditory cortex in the deaf. Nature Neuroscience. 2001;4:1171. doi: 10.1038/nn763. http://dx.doi.org/10.1038/nn763. [DOI] [PubMed] [Google Scholar]
- Fu G, Hu CS, Wang Q, Quinn PC, Lee K. Adults scan own race and other race faces differently. Public Library of Science. 2012;7:e37688. doi: 10.1371/journal.pone.0037688. http://dx.doi.org/10.1371/journal.pone.0037688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaither SE, Pauker K, Johnson SP. Biracial and monoracial infant own-race face perception: An eye tracking study. Developmental Science. 2012;15:775–782. doi: 10.1111/j.1467-7687.2012.01170.x. http://dx.doi.org/10.1111/j.1467-7687.2012.01170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EJ. The concept of affordance in development: The renascence of functionalism. In: Collins WA, editor. The concept of development: The minnesota symposium on child psychology. Vol. 15. Book Section, Hillsdale, NJ: Erlbaum; 1982. pp. 55–81. [Google Scholar]
- Gibson EJ. Exploratory behavior in the development of perceiving, acting, and the acquiring of knowledge. Annual Review of Psychology. 1988;39:1–41. Book Section. [Google Scholar]
- Greenough WT, Black JE, Wallace CS. Experience and brain development. Child Development. 1987;58:539–559. [PubMed] [Google Scholar]
- Hayne H. Infant memory development: Implications for childhood amnesia. Developmental Review. 2004;24:33–73. http://dx.doi.org/10.1016/j.dr.2003.09.007. [Google Scholar]
- Hernandez-Reif M, Field T, Diego M, Vera Y, Pickens J. Happy faces are habituated more slowly by infants of depressed mothers. Infant Behavior and Development. 2006;29:131–135. doi: 10.1016/j.infbeh.2005.07.003. http://dx.doi.org/10.1016/j.infbeh.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Hills PJ, Lewis MB. Reducing the own-race bias in face recognition by attentional shift using fixation crosses preceding the lower half of a face. Visual Cognition. 2011;19:313–339. http://dx.doi.org/10.1080/13506285.2010.528250. [Google Scholar]
- Hu C, Wang Q, Fu G, Quinn PC, Lee K. Both children and adults scan faces of own and other races differently. Vision Research. 2014;102:1–10. doi: 10.1016/j.visres.2014.05.010. http://dx.doi.org/10.1016/j.visres.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley KB, Oakes LM. Experience and distribution of attention: Pet exposure and infants’ scanning of animal images. Journal of Cognition and Development. 2015;16:11–30. doi: 10.1080/15248372.2013.833922. http://dx.doi.org/10.1080/15248372.2013.833922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley KB, Kovack-Lesh KA, Oakes LM. The influence of pets on infants’ processing of cat and dog images. Infant Behavior and Development. 2010;33:619–628. doi: 10.1016/j.infbeh.2010.07.015. http://dx.doi.org/10.1016/j.infbeh.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski JJ, Rose SA, Feldman JF. Modifying the distribution of attention in infants. Child Development. 2001;72:339–351. doi: 10.1111/1467-8624.00282. [DOI] [PubMed] [Google Scholar]
- Jayaraman S, Fausey CM, Smith LB. The faces in infant-perspective scenes change over the first year of life. Public Library of Science. 2015;10:13–15. doi: 10.1371/journal.pone.0123780. http://dx.doi.org/10.1371/journal.pone.0123780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH, Dziurawiec S, Ellis H, Morton J. Newborns’ preferential tracking of face-like stimuli and its subsequent decline. Cognitive Psychology. 1991;40:1–19. doi: 10.1016/0010-0277(91)90045-6. http://dx.doi.org/10.1016/0010-0277(91)90045-6. [DOI] [PubMed] [Google Scholar]
- Johnson SP, Slemmer JA, Amso D. Where infants look determines how they see: Eye movements and object perception performance in 3-month-olds. Infancy. 2004;6:185–201. doi: 10.1207/s15327078in0602_3. http://dx.doi.org/10.1207/s15327078in0602_3. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Cortical maturation and the development of visual attention in early infancy. Journal of Cognitive Neuroscience. 1990;2:81–95. doi: 10.1162/jocn.1990.2.2.81. http://dx.doi.org/10.1162/jocn.1990.2.2.81. [DOI] [PubMed] [Google Scholar]
- Kelly DJ, Quinn PC, Slater AM, Lee K, Gibson A, Smith M, Pascalis O. Three-month-olds, but not newborns, prefer own-race faces. Developmental Science. 2005;8:F31–F36. doi: 10.1111/j.1467-7687.2005.0434a.x. http://dx.doi.org/10.1111/j.1467-7687.2005.0434a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DJ, Liu S, Ge L, Quinn PC, Slater AM, Lee K, Pascalis O. Cross-race preferences for same-race faces extend beyond the African versus Caucasian contrast in 3-month-old infants. Infancy. 2007;11:87–95. doi: 10.1080/15250000709336871. http://dx.doi.org/10.1080/15250000709336871.URL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DJ, Quinn PC, Slater AM, Lee K, Ge L, Pascalis O. The other-race effect develops during infancy: Evidence of perceptual narrowing. Psychological Science. 2007;18:1084–1089. doi: 10.1111/j.1467-9280.2007.02029.x. http://dx.doi.org/10.1111/j.1467-9280.2007.02029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DJ, Liu S, Lee K, Quinn PC, Pascalis O, Slater AM, Ge L. Development of the other-race effect during infancy: Evidence toward universality? Journal of Experimental Child Psychology. 2009;104:105–114. doi: 10.1016/j.jecp.2009.01.006. http://dx.doi.org/10.1016/j.jecp.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovack-Lesh KA, Horst JS, Oakes LM. The cat is out of the bag: The joint influence of previous experience and looking behavior on infant categorization. Infancy. 2008;13:285–307. http://dx.doi.org/10.1080/15250000802189428. [Google Scholar]
- Kovack-Lesh KA, Oakes LM, McMurray B. Contributions of attentional style and previous experience to 4-month-old infants’ categorization. Infancy. 2012;17:324–338. doi: 10.1111/j.1532-7078.2011.00073.x. http://dx.doi.org/10.1111/j.1532-7078.2011.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovack-Lesh KA, McMurray B, Oakes LM. Four-month-old infants’ visual investigation of cats and dogs: Relations with pet experience and attentional strategy. Developmental Psychology. 2014;50:402–413. doi: 10.1037/a0033195. http://dx.doi.org/10.1037/a0033195. [DOI] [PubMed] [Google Scholar]
- Kuhl P, Tsao F, Liu H. Foreign-language experience in infancy: effects of short-term exposure and social interaction on phonetic learning. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9096–9101. doi: 10.1073/pnas.1532872100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grand R, Mondloch CJ, Maurer D, Brent HP. Early visual experience and face processing. Nature. 2001;410:890. doi: 10.1038/35073749. [DOI] [PubMed] [Google Scholar]
- Le Grand R, Mondloch CJ, Maurer D, Brent HP. Impairment in holistic face processing following early visual deprivation. Psychological Science. 2004;15:762–768. doi: 10.1111/j.0956-7976.2004.00753.x. [DOI] [PubMed] [Google Scholar]
- Libertus K, Needham A. Reaching experience increases face preference in 3‘ Äêmonth‘ Äêold infants. Developmental Science. 2011;14:1355–1364. doi: 10.1111/j.1467-7687.2011.01084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libertus K, Needham A. Face preference in infancy and its relation to motor activity. International Journal of Behavioral Development. 2014;38:529–538. http://dx.doi.org/10.1177/0165025414535122. [Google Scholar]
- Libertus K, Joh AS, Needham A. Motor training at 3 months affects object exploration 12 months later. Developmental Science. 2015:1–9. doi: 10.1111/desc.12370. http://dx.doi.org/10.1111/desc.12370. [DOI] [PMC free article] [PubMed]
- Liu S, Quinn PC, Wheeler A, Xiao NG, Ge L, Lee K. Similarity and difference in the processing of same- and other-race faces as revealed by eye tracking in 4- to 9-month-olds. Journal of Experimental Child Psychology. 2011;108:180–189. doi: 10.1016/j.jecp.2010.06.008. http://dx.doi.org/10.1016/j.jecp.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Xiao NG, Quinn PC, Zhu D, Ge L, Pascalis O, Lee K. Asian infants show preference for own-race but not other-race female faces: The role of infant caregiving arrangements. Frontiers in Psychology. 2015;6:1–8. doi: 10.3389/fpsyg.2015.00593. http://dx.doi.org/10.3389/fpsyg.2015.00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mareschal D, French RM, Quinn PC. A connectionist account of asymmetric category learning in early infancy. Developmental Psychology. 2000;36:635–645. doi: 10.1037/0012-1649.36.5.635. http://dx.doi.org/10.1037//0012-1649.36.5.635. [DOI] [PubMed] [Google Scholar]
- Markant J, Amso D. The development of selective attention orienting is an agent of change in learning and memory efficacy. Infancy. 2016;21:154–176. doi: 10.1111/infa.12100. http://dx.doi.org/10.1111/infa.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markant J, Oakes LM, Amso D. Visual selective attention biases contribute to the other-race effect among 9-month-old infants. Developmental Psychobiology. 2016;58:355–365. doi: 10.1002/dev.21375. http://dx.doi.org/10.1002/dev.21375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten AS, Cicchetti D. Developmental cascades. Development and Psychopathology. 2010;22:491–495. doi: 10.1017/S0954579410000222. http://dx.doi.org/10.1017/S0954579410000222. [DOI] [PubMed] [Google Scholar]
- Mondloch CJ, Segalowitz SJ, Lewis TL, Dywan J, Le Grand R, Maurer D. The effect of early visual deprivation on the development of face detection. Developmental Science. 2013;16:728–742. doi: 10.1111/desc.12065. http://dx.doi.org/10.1111/desc.12065. [DOI] [PubMed] [Google Scholar]
- Oakes LM, Ribar RJ. A comparison of infants’ categorization in paired and successive presentation familiarization tasks. Infancy. 2005;7:85–98. doi: 10.1207/s15327078in0701_7. [DOI] [PubMed] [Google Scholar]
- Oakes LM, Kovack-Lesh KA, Horst JS. Two are better than one: Comparison influences infants’ visual recognition memory. Journal of Experimental Child Psychology. 2009;104:124–131. doi: 10.1016/j.jecp.2008.09.001. http://dx.doi.org/10.1016/j.jecp.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes LM. A biopsychosocial perspective on looking behavior in infancy. Handbook of infant biopsychosocial development 2015 [Google Scholar]
- Pascalis O, de Haan M, Nelson CA, de Schonen S. Long-term recognition memory for faces assessed by visual paired comparison in 3- and 6-month-old infants. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1998;24:249–260. doi: 10.1037//0278-7393.24.1.249. [DOI] [PubMed] [Google Scholar]
- Pascalis O, Scott LS, Kelly DJ, Shannon RW, Nicholson E, Coleman M, Nelson CA. Plasticity of face processing in infancy. PNAS proceedings of the National Academy of Sciences of the United States of America. 2005;102:5297–5300. doi: 10.1073/pnas.0406627102. http://dx.doi.org/10.1073/pnas.0406627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaget J. The construction of reality in the child. Book, Oxford, England: Basic Books; 1954. [Google Scholar]
- Pollak SD, Sinha P. Effects of early experience on children’s recognition of facial displays of emotion. Developmental Psychology. 2002;38:784–791. doi: 10.1037//0012-1649.38.5.784. http://dx.doi.org/10.1037/0012-1649.38.5.784. [DOI] [PubMed] [Google Scholar]
- Ptito M, Matteau I, Zhi Wang A, Paulson OB, Siebner HR, Kupers R. Crossmodal recruitment of the ventral visual stream in congenital blindness. Neural Plasticity. 2012 doi: 10.1155/2012/304045. http://dx.doi.org/10.1155/2012/304045. [DOI] [PMC free article] [PubMed]
- Quinn PC, Eimas PD, Rosenkrantz SL. Evidence for representations of perceptually similar natural categories by 3 and 4 month old infants. Perception. 1993;22:463–475. doi: 10.1068/p220463. http://dx.doi.org/10.1068/p220463. [DOI] [PubMed] [Google Scholar]
- Quinn PC, Yahr J, Kuhn A, Slater AM, Pascalis O. Representation of the gender of human faces by infants: A preference for female. Perception. 2002;31:1109–1121. doi: 10.1068/p3331. http://dx.doi.org/10.1068/p3331. [DOI] [PubMed] [Google Scholar]
- Quinn PC, Uttley L, Lee K, Gibson A, Smith M, Slater AM, Pascalis O. Infant preference for female faces occurs for same- but not other-race faces. Journal of Neuropsychology. 2008;2:15–26. doi: 10.1348/174866407x231029. 10.1348/174866407X231029. [DOI] [PubMed] [Google Scholar]
- Quinn PC, Doran MM, Reiss JE, Hoffman JE. Time course of visual attention in infant categorization of cats versus dogs: Evidence for a head bias as revealed through eye tracking. Child Development. 2009;80:151–161. doi: 10.1111/j.1467-8624.2008.01251.x. http://dx.doi.org/10.1111/j.1467-8624.2008.01251.x. [DOI] [PubMed] [Google Scholar]
- Rakison DH, Krogh L. Does causal action facilitate causal perception in infants younger than 6 months of age? Developmental Science. 2012;15:43–53. doi: 10.1111/j.1467-7687.2011.01096.x. http://dx.doi.org/10.1111/j.1467-7687.2011.01096.x. [DOI] [PubMed] [Google Scholar]
- Rennels JL, Davis RE. Facial experience during the first year. Infant Behavior and Development. 2008;31:665–678. doi: 10.1016/j.infbeh.2008.04.009. http://dx.doi.org/10.1016/j.infbeh.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. Processing speed in the 1 st year of life: A longitudinal study of preterm and full-term infants. Developmental Psychology. 2002;38:895–902. doi: 10.1037//0012-1649.38.6.895. [DOI] [PubMed] [Google Scholar]
- Schwarzer G, Zauner N, Jovanovic B. Evidence of a shift from featural to configural face processing in infancy. Developmental Science. 2007;10:452–463. doi: 10.1111/j.1467-7687.2007.00599.x. http://dx.doi.org/10.1111/j.1467-7687.2007.00599.x. [DOI] [PubMed] [Google Scholar]
- Scott LS, Monesson A. The origin of biases in face perception. Psychological Science. 2009;20:676–680. doi: 10.1111/j.1467-9280.2009.02348.x. http://dx.doi.org/10.1111/j.1467-9280.2009.02348.x. [DOI] [PubMed] [Google Scholar]
- Simion F, Valenza E, Macchi Cassia V, Turati C, Umiltà C. Newbornsà preference for up–down asymmetrical configurations. Developmental Science. 2002;5:427–434. [Google Scholar]
- Smith LB. It’s all connected: Pathways in visual object recognition and early noun learning. The American Psychologist. 2013;68:618–629. doi: 10.1037/a0034185. http://dx.doi.org/10.1037/a0034185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerville JA, Woodward AL, Needham A. Action experience alters 3-month-old infants’ perception of others’ actions. Cognitive Psychology. 2005;96:B1–B11. doi: 10.1016/j.cognition.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer J, Quinn PC. Heads you win, tails you lose: Evidence for young infants categorizing mammals by head and facial attributes. Early Development and Parenting. 1997;6:113–126. http://dx.doi.org/10.1002/(SICI)1099-0917(199709/12)6. [Google Scholar]
- Sugden NA, Mohamed-Ali MI, Moulson MC. I spy with my little eye: Typical, daily exposure to faces documented from a first-person infant perspective. Developmental Psychobiology. 2014;56:249–261. doi: 10.1002/dev.21183. http://dx.doi.org/10.1002/dev.21183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen E, Smith LB. A dynamic systems approach to the development of cognition and action. Book, Cambridge, MA: The MIT Press; 1994. [Google Scholar]
- Vanderwert RE, Westerlund A, Montoya L, Mccormick SA, Miguel HO, Nelson CA. Looking to the eyes influences the processing of emotion on face-sensitive event-related potentials in 7-month-old infants. Developmental Neurobiology. 2015;75:1154–1163. doi: 10.1002/dneu.22204. http://dx.doi.org/10.1002/dneu.22204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hofsten C. An action perspective on motor development. Trends in Cognitive Sciences. 2004;8:266–272. doi: 10.1016/j.tics.2004.04.002. http://dx.doi.org/10.1016/j.tics.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Wang Q, Xiao NG, Quinn PC, Hu CS, Qian M, Fu G, Lee K. Visual scanning and recognition of Chinese, Caucasian, and racially ambiguous faces: Contributions from bottom-up facial physiognomic information and top-down knowledge of racial categories. Vision Research. 2015;107:67–75. doi: 10.1016/j.visres.2014.10.032. http://dx.doi.org/10.1016/j.visres.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werker JF, Tees RC. Cross-language speech perception: evidence for perceptual reorganisation during the first year of life. Infant Behavior and Development. 1984;7:49–63. http://dx.doi.org/10.1016/S0163-6383(02)00113-3. [Google Scholar]
- Wheeler A, Anzures G, Quinn PC, Pascalis O, Omrin DS, Lee K. Caucasian infants scan own- and other-race faces differently. Public Library of Science. 2011;6:e18621. doi: 10.1371/journal.pone.0018621. http://dx.doi.org/10.1371/journal.pone.0018621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao WS, Xiao NG, Quinn PC, Anzures G, Lee K. Development of face scanning for own- and other-race faces in infancy. International Journal of Behavioral Development. 2013;37:100–105. doi: 10.1177/0165025412467584. http://dx.doi.org/10.1177/0165025412467584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao WS, Quinn PC, Pascalis O, Lee K. Own- and other-race face scanning in infants: Implications for perceptual narrowing. Developmental Psychobiology. 2014;56:262–273. doi: 10.1002/dev.21196. http://dx.doi.org/10.1002/dev.21196. [DOI] [PubMed] [Google Scholar]


