Abstract
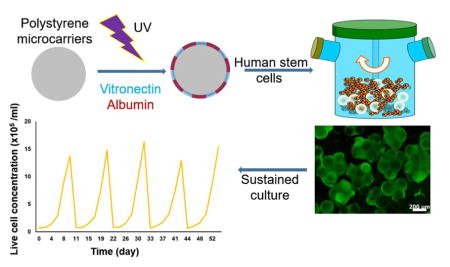
The development of platforms for the expansion and directed differentiation of human pluripotent stem cells (hPSCs) in large quantities under xeno-free conditions is a key step toward the realization of envisioned stem cell-based therapies. Microcarrier bioreactors afford great surface-to-volume ratio, scalability and customization with typical densities of 106–107 cells/ml or higher. In this study, a simple and inexpensive method was established for generating microcarriers without animal-derived components. While coating polystyrene beads with vitronectin alone did not support the culture of hPSCs in stirred suspension, the inclusion of recombinant human serum albumin and UV irradiation led to enhanced seeding efficiency and retention while cells grew more than 20-fold per passage for multiple successive passages and without loss of cell pluripotency. Human PSCs expanded on microcarriers were coaxed to tri-lineage differentiation demonstrating that this system can be used for the self-renewal and specification of hPSCs to therapeutically relevant cell types. Such systems will be critical for the envisioned use of stem cells in regenerative medicine and drug discovery.
Keywords: Xeno-free microcarriers, human pluripotent stem cells, bioreactor culture, vitronectin
Graphical Abstract
INTRODUCTION
Human pluripotent stem cells (hPSCs) are a promising source of cellular products for engineering tissues and reconstituting the function of damaged organs. Realization of this potential however relies on the development of processes and practices for the large scale generation of therapeutically useful hPSC progeny. Such processes should be robust, reproducible and of manageable cost. Stirred-suspension bioreactors (SSBs)1 are commonly used for the mammalian cell culture-based generation of biologics and cell therapy products. Thus, the adoption to a commercial production setting of cultivation schemes utilizing SSBs for the expansion and differentiation of hPSCs is deemed to be linked to fewer hurdles.
SSBs afford flexibility for the culture of cells as aggregates, after encapsulation or on microcarriers. Microcarrier cultures provide a high surface area-to-volume ratio allowing for more efficient utilization of media and factors while issues such as limited oxygen and nutrient transfer associated with the formation of large aggregates are less pronounced. Human PSCs have been successfully expanded and differentiated to definitive endoderm, cardiomyocytes and neural progenitor cells in stirred-suspension microcarrier vessels2–4.
The culture of hPSCs on microcarriers typically requires coating the beads with animal-derived matrices such as Matrigel2, 4–6 or collagen7 precluding the clinical use of these cells. While various substrates such as vitronectin8–10 and laminin11 have been proposed for the 2D culture of stem cells, translation of their use to 3D systems with agitation is challenging at best. In spinner flask cultures, beads with synthetic vitronectin peptides conjugated on their surface failed to retain human stem cells requiring additional processing and the inclusion of poly-L-lysine for enhancing cell adhesion and subsequent growth12.
Peptide conjugation based on a simple amidation reaction allow the customization of the microcarrier surface to better serve the needs of specific hPSC lines. However, the amidation reaction is typically characterized by low efficiency and requires optimization depending on the peptide(s) under consideration. This increases the time, labor and overall cost associated with the functionalization of the microcarriers and the bioprocess.
Toward addressing these concerns, we established a method for the xeno-free culture of hPSCs on microcarriers in stirred suspension. Beads coated with recombinant truncated human vitronectin (VN), which is routinely used for the stem cell maintenance in dishes, did not provide sufficient support for the adhesion and proliferation of hPSCs in xeno-free E8 medium. The inclusion of recombinant human serum albumin (HSA) with UV irradiation, as well as medium modifications resulted in higher seeding efficiency, successful growth of hPSCs and maintenance of their pluripotency over multiple successive passages. Thus, a simple and inexpensive approach is described for the scalable expansion and directed specification of stem cells in SSBs opening avenues for the bioprocessing of hPSC products for regenerative medicine.
MATERIALS AND METHODS
Human pluripotent stem cell culture
Human ESCs (H9 (WA09); passages 30–50) were obtained from the WiCell Research Institute (Madison, WI). Cells cultured in dishes coated with Matrigel (BD Biosciences, San Jose, CA) or recombinant human vitronectin, truncated (VN; cat. no. A14700, Life Technologies, Grand Island, NY) and in TeSR-E8 medium (E8; Stem Cell Technologies, Vancouver, BC) were maintained in 5% CO2/95% air at 37 °C. Medium was replaced every day, and the cells were passaged every 5–6 days by enzymatic dissociation with dispase (Life Technologies, Grand Island, NY) for cells cultured on Matrigel or with Gentle Cell Dissociation reagent (Stem Cell Technologies).
Viable cells were counted in a hemocytometer or using a TC-20 counter (Bio-Rad, Hercules, CA) after Trypan Blue staining (Sigma-Aldrich, St. Louis, MO). Alternatively, cells were stained with 20 μg/ml fluorescein diacetate (FDA-live cells; Sigma-Aldrich) in PBS for 5 min and after being washed twice with PBS, they were analyzed by fluorescence microscopy or flow cytometry.
Lactate dehydrogenase (LDH) activity was determined in culture samples with a LDH cytotoxicity detection assay (Roche, Indianapolis, IN) according to the manufacturer’s instructions2, 13.
Microcarrier preparation
Microcarriers (Plastic (cat. no. P102-1521), or Plastic Plus (cat. no. PP102-1521) as stated; SoloHill, Ann Arbor, MI) were equilibrated in PBS and autoclaved. After the microcarriers were cooled down to room temperature, 200 μL of 0.5 mg/mL VN per 0.5 g of microcarriers was added to 2 ml of bead-PBS suspension and mixed well for 1 hr. For albumin coating, 2 ml of 0.01 g/ml human serum albumin (HSA; cat. no. A9731, Sigma-Aldrich) in PBS was added subsequently and the beads were incubated for another 30 min. The supernatant was removed carefully, the microcarriers were transferred to 35-mm petri dishes. For UV irradiation, beads were gently shaken under a UV lamp (8W, 254 nm; VWR, Randor, PA) for 40 minutes. The beads received illumination at 1.3 mW as measured by a power meter and photodiode sensor (Thorlabs Inc., Newton, NJ). Following treatment, the microcarriers were washed with E8 media before cell seeding.
Microcarrier seeding and passaging
Human ESC colonies were incubated with 10 μM ROCK inhibitor (Y-27632; Enzo Life Sciences, Farmingdale, NY) for 1 hour and then dispersed into single cells using Accutase (Innovative Cell Technologies, San Diego, CA). Ten million cells were incubated with 0.5 g microcarriers in 10-cm petri dishes with 8 ml of E8 medium supplemented with 10 μM ROCK inhibitor. The dishes were shaken periodically (~15 min) for 3 hrs and after overnight incubation, the microcarriers were transferred to 125-ml spinner flasks (Pro-Culture, Corning, Elmira, NY) with up to 50 ml of fresh E8 medium. Pluronic F68 was added at 0.02% as stated. The agitation rate was set at 45 rpm and the culture was maintained at 37 °C and 5% CO2/95% air. After the first day, the medium was replaced by medium without Y-27632. Subsequent medium changes were performed at 75% volume every day. The cultures were sampled every other day for determination of cell number, viability and LDH activity.
Before passaging, cell-laden microcarriers were pretreated with 10 μM of ROCK inhibitor for 1 hr and then were washed once with DMEM/F12. Upon incubation with Accutase for 15 min and gentle trituration the cells were collected by passing the suspension through a 40 μm mesh strainer (BD Biosciences, San Jose, CA).
RT-PCR and quantitative PCR
Total RNA was isolated using Trizol reagent (Life Technologies, Carlsbad, CA) according to the manufacturer’s instructions. Reverse transcription was performed using the ImPromII reverse transcriptase (Promega, Madison, WI) as previously described2. Quantitative PCR (qPCR) was performed on a Step One™ real-time PCR system (Life Technologies, Grand Island, NY) using the DyNAmo™ SYBR Green qPCR Kit (Thermo Scientific, Waltham, MA). All reactions were run in triplicates. Amplification specificity was verified by the melting curve method. Relative gene expression was normalized to the endogenous β-actin (ACTB) expression. All reactions were run in triplicates. Primer sequences are shown in Table 1.
Table 1.
Primers used for qPCR assays in this study (shown in a 5′-to-3′ orientation).
| Gene | Forward primer | Reverse primer | Amplicon size (bp) |
|---|---|---|---|
| NANOG | AGATGCCTCACACGGAGACT | ACACAGCTGGGTGGAAAGAGA | 194 |
| FOXA2 | GAAGATGGAAGGGCACGA | CACGTACGACGACATGTTCA | 193 |
| SOX17 | CTTTCATGGTGTGGGCTAAGG | GTACTTGTAGTTGGGGTGGTCCT | 191 |
| PAX6 | AACAGACACAGCCCTCACAAAC | CGGGAACTTGAACTGGAACTGAC | 275 |
| ISL1 | GCGGAGTGTAATCAGTATTTGGA | CACTCGATGTGATACACCTTGG | 184 |
| KDR | CCAGAAGTAAAAGTAATCCCAGATG | CTTTAAAAGTTCTGCTTCCTCACTG | 246 |
| TUBB3 | TGTACTCCTTCCTGCTGGACTT | CCCCAACTCTCACTATGTGGAT | 270 |
| NES | CAGCGTTGGAACAGAGGTTG | GGGAATTGCAGCTCCAGCTT | 289 |
| SOX2 | TTTGATCCTGATTCCAGTTTGC | CAGCTCCGTCTCCATCATGT | 226 |
| POU5F1 | AAGCTGGAGAAGGAGAAGCTG | AATAGAACCCCCAGGGTGAG | 158 |
| GSC | AACCTCTTCCAGGAGACCAAGT | GACGACGTCTTGTTCCACTTCT | 182 |
| CXCR4 | ACTTTGGGAACTTCCTATGCAA | GCAAAGATGAAGTCGGGAATA | 217 |
Flow cytometry
Cells detached from the beads were fixed in a 4% paraformaldehyde solution (Sigma-Aldrich) for 10 min, washed with PBS and permeabilized with Cytonin (Trevigen, Gaithersburg, MD) for 1 hr. Then, the samples were blocked with 3% normal donkey serum (NDS; Jackson Immunoresearch Laboratories, West Grove, PA) for 20 min and incubated for 1 hr at room temperature with primary antibodies: rabbit anti-OCT4 (Santa Cruz Biotechnology, Santa Cruz, CA) and mouse anti-SSEA4 (AbCam, Cambridge, MA). After three washes with 1% NDS, cells were incubated with appropriate DyLight secondary antibodies (Jackson Immunoresearch Laboratories) for 1 hr at room temperature. After three more washes with PBS, the samples were analyzed with an Attune flow cytometer (Life Technologies). Cells were identified as positive for a particular antigen if their emitted fluorescence level was higher than 99% of that of isotype control samples.
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde (Sigma) in PBS, permeabilized/blocked in PBS with 0.1% Triton X-100 (Mallinckrodt Baker, Phillipsburg, NJ) and 1% bovine serum albumin (BSA; Sigma) for 30 min and incubated overnight at 4 °C with primary antibodies: Mouse anti-SSEA4 (AbCam, Cambridge, MA) and rabbit anti-OCT4 (SantaCruz Biotechnology Inc., Santa Cruz, CA), goat anti-SOX17 (R&D Systems, Minneapolis, MN), rabbit anti-FOXA2 (Cell Signaling Technology, Danvers, MA), rabbit anti-MOX1 (Novus Biologicals, Littleton, CO), mouse anti-KDR (PE-conjugated, R&D Systems), rabbit anti-β-Tubulin III (TUJ1, Sigma, St. Louis, MO), mouse anti-Nestin (R&D Systems). After three washes with PBS, cells were incubated with corresponding DyLight secondary antibodies (Jackson Immunoresearch Laboratories) for 1 h at room temperature. Nuclear DNA was stained with DAPI (Vectashield, Vector Laboratories, Burlingame, CA).
Fourier transform infrared spectroscopy (FTIR) and absorbance study on treatment petri dishes
Petri dishes having the same polystyrene surface as plastic microcarriers were coated with 20 μL of 0.5 mg/ml vitronectin solution in 200 μL PBS or PBS only (control) for at least 1 hr at room temperature. Subsequently, 200 μL of 0.01 g/ml HSA solution (in PBS) or PBS alone was added for 30 min. Dishes were placed on a shaker and irradiated with UV for 40 min. The dishes were analyzed with a FTIR spectrometer (Jasco, Easton, MD).
In addition, supernatant from these samples was collected and scanned for determination of each absorbance between 200 nm and 830 nm with a spectrophotometer (Eppendorf, Hauppauge, NY).
The data were imported in MATLAB (Mathworks Inc., Natick, MA) for visualization.
Enzyme-linked immunosorbent assay (ELISA)
Beads subjected to different treatments were washed twice with PBS, re-suspended in 100 μL PBS, and transferred to a 96-well plate. A standard curve was prepared with serially diluted vitronectin solutions. After overnight incubation, the samples were removed, the plates were washed three times with PBS, and blocked with 100 μL of 5% BSA in PBS at 4°C overnight. Two more washings with PBS were carried out before 100 μL of primary anti-vitronectin antibody (Abcam) at 1:10,000 dilution was added to each well. After further incubation overnight at 4 °C, the plate was washed 4 times with PBS and 100 μL horseradish peroxidase (HRP) – conjugated antibody (Jackson ImmunoResearch Laboratories) were added at 37 °C for 1 hr. The plates were washed again four times with PBS and 100 μL 3,3′,5,′5-tetramethylbenzidine (TMB) (ThermoFisher Scientific, Waltham, MA) was added for 15–30 minutes. One hundred μL of 1 M HCl was added to stop the reaction and 100 μL from each well were transferred to a new 96-well plate and read at 450 nm with a SpectraMax microplate reader (Molecular Devices, Sunnyvale, CA). The standard curve was fitted to a 4-parameter logistic equation.
Karyotyping
Cells harvested from microcarriers after five passages were replated on T-25 flasks and al-lowed to grow until about 50% confluence. G-banding analysis was performed (Tufts Cytogenetics Laboratory, Boston, MA).
Embryoid body (EB) formation
Single hPSCs harvested from VN-HSA-UV microcarriers were induced embryoid body (EB) formation. Harvested EBs were transferred to Petri dishes and maintained in Dulbecco’s modified Eagle’s medium/Ham’s F12 medium (DMEM/F12) (Life Technologies), supplemented with 20% FBS (PAA Laboratories, Dartmouth, MA). Medium was replenished every 2 days until analysis of the EBs.
Definitive endoderm, mesoderm, and neuroectoderm differentiation
Cells harvested from microcarriers were replated on Matrigel-coated dishes. For bioreactor differentiation, the medium used for expansion of hPSCs was exchanged with differentiation medium keeping the total working volume constant. Differentiation to definitive endoderm, mesoderm and neuroectoderm were performed according to previous reports [158, 159, 181, 182] as detailed below. Cells were viewed on a Leica DM IL LED microscope connected to a Leica DFC3000G camera (Leica Microsystems, Buffalo Grove, IL).
Definitive endoderm (DE) differentiation of hESCs harvested from microcarriers coated with vitronectin and HSA and irradiated with UV (VN+HSA+UV) was performed as previously described [159]. Briefly, after 5 passages, cells on microcarriers were disassociated into single cells with Accutase, re-plated and maintained on dishes. Differentiation was carried out in RPMI (GIBCO, Grand Island, NY) supplemented with 100 ng/ml activin A (R&D Systems) from days 0 to 4 and various amounts of Knockout Serum Replacer (KSR; GIBCO, Grand Island, NY) as follows: no KSR on day 1, 0.2% KSR on day 2, and 2% KSR on days 3–4.
Mesoderm differentiation was carried out in RPMI medium supplemented with activin A, BMP4 and KSR for 5 days: On day 1, RPMI was supplemented with 100 ng/ml activin A. On days 2 and 3, RPMI was supplemented with 0.2% KSR, 10 ng/ml activin A and 10 ng/ml BMP4 (R&D Systems). On days 4 and 5, RPMI was supplemented with 2% KSR, 10 ng/ml activin A and 10 ng/ml BMP4.
Neuroectoderm differentiation was initiated with neural induction medium (NIM): DMEM/F12:Neurobasal medium (1:1), 1×N2 supplement and 1×B27 supplement without vitamin A (all from GIBCO)). After 24 hrs, cells were passaged using collagenase IV (GIBCO) and seeded in low-attachment dishes (BD Biosciences) to form EBs, which were cultured in NIM for 4 days with daily medium change. The medium was subsequently switched to neural proliferation medium (NPM: DMEM/F12:Neurobasal(1:1), 0.5×N2 supplement, 0.5×B27 supplement, 2 mM Glutamax and 20 ng/mL FGF2 (R&D Systems)) for another 3 days with daily medium change. After that, EBs were replated on Matrigel-coated dishes and cultured for another 2 days before characterization.
Statistical Analysis
Data are expressed as mean ± st.dev. unless stated otherwise. ANOVA and the post hoc Tukey test were performed using Minitab (Minitab Inc, State College, PA) or MATLAB. P values less than 0.05 were considered as significant.
RESULTS
Treating microcarriers with vitronectin, HSA, and UV irradiation
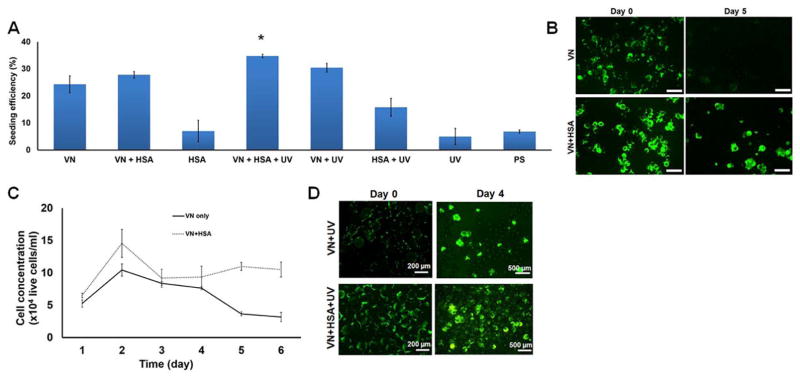
We set out to develop a xeno-free microcarrier system for the culture of hPSCs. For this purpose, polystyrene microcarriers were coated with recombinant human vitronectin (VN), which is used routinely as a substrate for the culture of hPSCs in dishes. Approximately 24.3±3.1% of the hPSCs seeded statically attached (seeding efficiency) on the bead surface (Fig. 1A). However, cells detached from beads (Fig. 1B) and the cell concentration declined over 5 days of culture in spinner flasks (Fig. 1C).
Figure 1.
Culture of hPSCs on microcarriers. (A) Human PSC seeding efficiency on microcarriers which underwent different treatment. Values are shown as mean±st.dev. from at least 3 independent experiments (* p<0.05 vs. all other treatments). VN: vitronectin; HSA: human serum albumin, UV: ultraviolet irradiation, PS: polystyrene (i.e. no treatment). (B) Human H9 hESCs are shown on VN− or VN+HSA-microcarriers on days 0 and 5 of culture. Cells were incubated with fluorescein diacetate for visualization. Bars: 500 μm. (C) Cell concentration vs. time for hPSCs culture on VN− (solid line) and VN+HSA-beads (dashed line). (D) Human PSC attachment on VN+UV and VN+HSA+UV microcarriers before (day 0) and after 4 days of culture in stirred suspension.
Because the culture medium is low in protein, we decided to include recombinant human serum albumin (HSA) maintaining the xeno-free environment. To that end, VN-coated beads where subsequently incubated with varying amounts of HSA. Treatment with 10 mg/ml HSA resulted in similar seeding efficiency (27.8±1.3%, p=0.15; Fig. 1A) as for microcarriers coated with VN but exhibited better growth (Figs. 1B–C). More importantly, cells were retained on VN+HSA microcarriers in spinner flask cultures. It should be noted that only 7±4% of the hPSCs adhered to beads coated only with HSA. This was expected given that albumin passivates surfaces against cell adhesion. The seeding efficiency on plain polystyrene beads was 6.8±0.6%.
Despite retention of the cells on VN+HSA beads, only 1.6-fold growth was observed after 5 days in spinner flasks and viability ranged between 50–70% under agitation conditions normally employed in hPSC cultures with Matrigel-coated or peptide-decorated beads2, 12. Moreover, the seeding efficiency on VN+HSA microcarriers was lower compared to those featuring Matrigel or peptides on their surface. These issues prompted us to look into ways for further improvement. UV irradiation is commonly utilized for patterning surfaces for cell adhesion. UV treatment of polymer surfaces induces the formation of −COOH groups, which promote the adhesion of cells and the adsorption of proteins14–16. In addition, UV modulates the conformation of proteins potentially altering the surface adhesiveness for cells. We thus irradiated VN- (VN+UV) and VN+HSA-coated microcarriers (VN+HSA+UV) with UV and determined their capacity for retaining hPSCs after seeding.
Indeed, VN+HSA+UV microcarriers exhibited the highest seeding efficiency (34.8±0.7%) which was significantly different than all other conditions (p<0.05) including for VN+UV beads (30.5±1.6%, p=0.013 vs. VN+HSA+UV). Moreover, VN+UV microcarriers were only sparsely occupied with cells after 4 days in spinner flask culture compared to VN+HSA+UV microcarriers (Fig. 1D). These findings suggest that the HSA in conjunction with UV irradiation extends the attachment of hPSCs on VN-coated microcarriers under agitation. Beads coated with HSA alone and treated with or without UV were suboptimal for cell attachment (Fig. 1A). Of note, hPSCs exhibited poor seeding on irradiated polystyrene beads (UV) (5±3%).
Characterization of the VN and HSA coatings on UV irradiated microcarriers
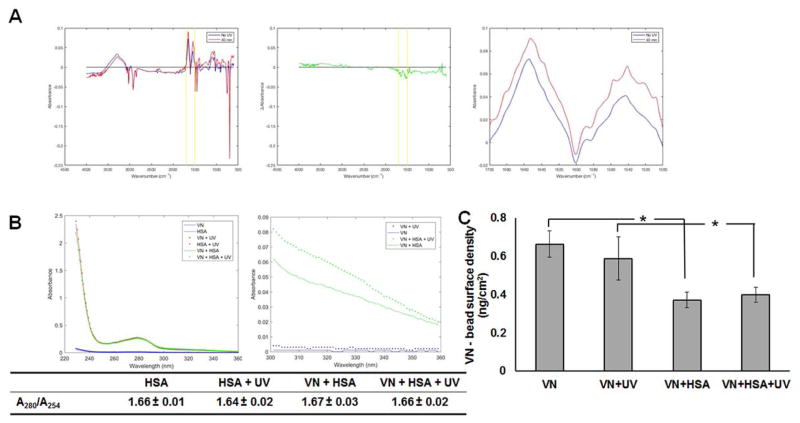
Next, we performed a series of analyses to better understand why the VN+HSA+UV microcarriers supported higher seeding efficiency and longer maintenance of the hPSCs in stirred-suspension. Fourier transform infrared (FTIR) spectroscopic analysis would unveil distinct structures or bonds formed on VN-coated beads after the addition of HSA and treatment with UV, because UV irradiation causes complex chemical modifications to polymer surfaces and proteins. When looking at the spectra for VN+HSA and VN+HSA+UV specimens, there is a significant difference in the 1500–1700 cm−1 range (Fig. 2A). The peak near 1637 cm−1 corresponds to the presence of intermolecular β-sheets17 typically resulting from protein aggregation18. The data thus suggest that UV may promote protein aggregation and the formation of intermolecular β-sheets. These sheets may support stem cell attachment and growth on microcarriers, similar to β-sheets among self-complementary oligopeptides forming stable matrices that favor cell attachment19.
Figure 2.
Characterization of engineered microcarriers. (A) FTIR analysis of VN+HSA microcarriers without UV treatment (no UV) or treated with UV for 40 minutes. Shown from left to right: the raw spectra, the spectrum representing the difference between the two spectra, and the zoomed in view of the raw spectra in the range of 1500–1700 cm−1 (indicated with yellow lines in the other two plots). (B) Absorbance data shown for different bead treatments as indicated. The plot on the right shows the data in the 300–360 nm range to better illustrate the differences in absorbance. (C) ELISA results are shown as mean±st.dev. from at least 3 independent experiments.
We also examined the absorption spectra of HSA and VN with or without UV treatment (Fig. 2B) for changes in HSA conformation as reported20. While there was no appreciable absorbance in VN samples (VN, VN+UV), those containing HSA (VN+HSA, VN+HSA+UV) displayed a characteristic local minimum at 254 nm (A254) and a peak at 280 nm (A280). The ratio A280/A254 serves as an indicator of UV-induced protein (particularly of the aromatic amino acids) degradation21. The A280/A254 values were similar for samples containing HSA regardless of UV irradiation suggesting that there was no detectable damage to the protein (Fig. 2B). However, there was a noticeable difference in the right tail of the spectra (300–360 nm; Fig. 2B) which was previously associated with albumin aggregation20 corroborating our FTIR observations. This increase in absorbance can be quantified by the difference in absorbance at 320 nm between irradiated and non-irradiated samples (AUV320-Ao320). This difference for the VN samples was 0.001 vs. 0.025 for VN+HSA samples (p<0.05).
The amount of surface VN displayed by microcarriers after different treatments was determined with ELISA (Fig. 2C). Vitronectin decreased by almost 40% (p<0.01) to 0.373±0.04 ng/cm2 after coating VN beads with HSA suggesting that albumin may be displacing or blocking some of the bound VN. UV irradiation alone (i.e. VN vs. VN+UV, and VN+HSA vs. VN+HSA+UV) did not result in considerable differences in VN content.
Taken together, the data suggest that UV treatment leads to aggregation of HSA and formation of intermolecular β-sheets which have been shown to support cell adhesion22, despite the decrease in detectable VN on the microcarriers.
Human PSC expansion on VN+HSA+UV microcarriers: Effect of surface charge and the inclusion of pluronic F68
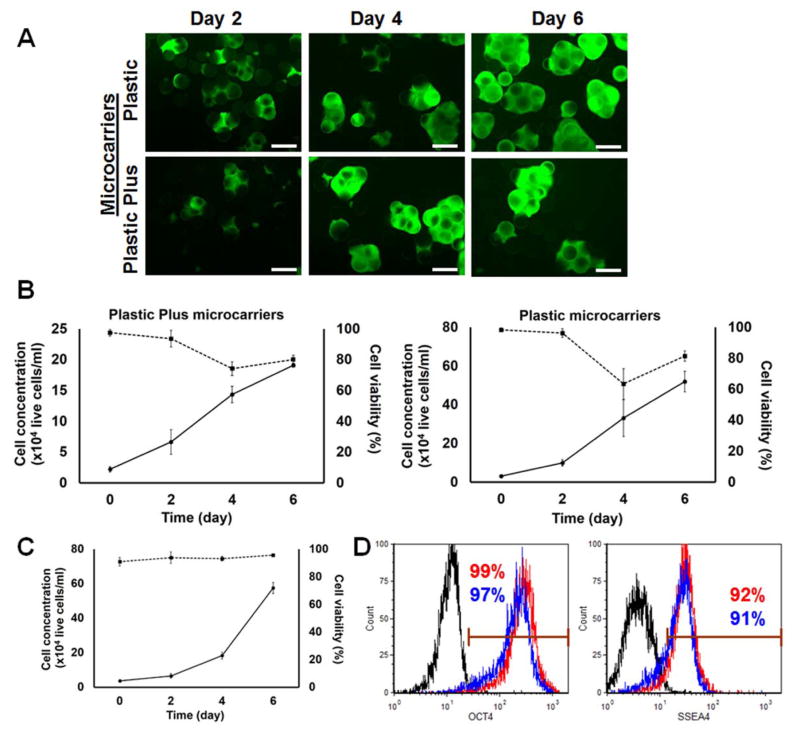
The bead surface charge has been purported to influence cell adhesion. To that end, we used two types of microcarriers with different net charge to prepare VN+HSA+UV beads as described above: polystyrene microcarriers with neutral (SoloHill Plastic microcarriers) and positive charge (SoloHill Plastic Plus microcarriers). All microcarriers were treated identically, seeded with hPSCs and transferred to spinner flasks (Fig. 3A). VN+HSA+UV beads with originally neutral charge exhibited a seeding efficiency (34.8±0.7%; see above) that was greater than for microcarriers with positive charge (23.3%±4.0%). Cells on the beads with neutral charge also exhibited a significantly higher fold-increase (16.2; p<0.05) compared to cultures on microcarriers with positive charge (8.7-fold) after 6 days of culture in E8 medium (Fig. 3B). Thus, the VN+HSA+UV treatment results in a higher net enhancement of neutrally-rather than positively-charged beads.
Figure 3.
Cells on VN+HSA+UV beads with neutral or positive surface charge cultured in stirred suspension are shown (A) at different culture times (FDA staining; bars: 200 μm) along (B) with their concentration (solid lines) and viability (dashed lines). (C) Concentration (solid line) and viability (dashed line) of hESCs cultured on VN+HSA+UV beads with neutral charge in stirred-suspension with E8 medium supplemented with pluronic F68. (D) Their expression of OCT4 and SSEA4 is also shown. Red curves: E8 supplemented with 0.02% F68, blue curves: E8 (no F68), Black curve: isotype control.
During these experiments we noted a dip in the viability of cells maintained in E8 medium (from >95% to 65–75% by day 4). Such decrease was not evident for hPSCs cultured on Matrigel-coated microcarriers in suspension with mTeSR1 medium, which is a defined but not xeno-free medium. A key difference here is the lack in the E8 medium of pluronic F68, which is a non-ionic surfactant used as a shear-protectant additive.
Supplementation of the E8 medium with 0.02% F68 resulted in higher viability than in cultures without F68 during 6 days of culture in spinner flask (Fig. 3C). More importantly, over 90% of the cells were OCT4+ and SSEA4+ (Fig. 3D).
Multi-passage suspension culture of hESCs seeded on VN+HSA+UV microcarriers
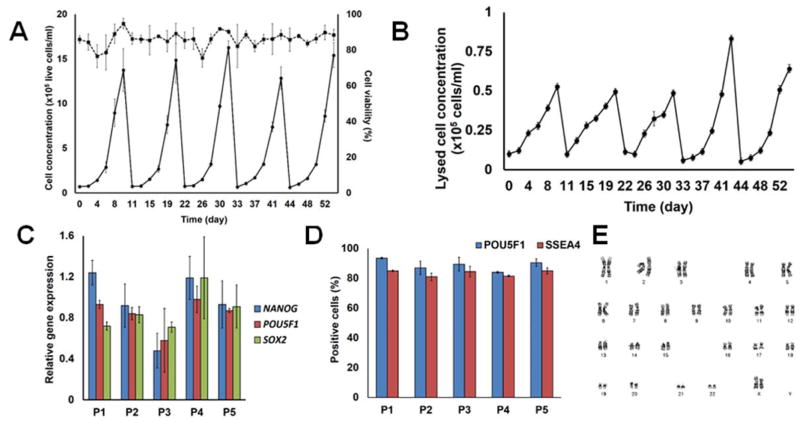
Human PSCs seeded on VN+HSA+UV beads proliferated without reduction in pluripotency marker expression in single-passage spinner flask cultures but whether a similar performance could be achieved over multiple passages was unclear. For this purpose, H9 hESCs were cultured on microcarriers successively for at least five passages. Moreover, the duration of each passage was lengthened to 10–11 days to reach even higher cell densities. Cell proliferation was consistent among passages with an average increase of 20.5±2.0-fold to concentrations of 1.3–1.6x106 cells/ml (Fig. 4A). Occasionally, there was increased variability in cell viability most likely due to adaptation of the cells to stirred-suspension conditions (first passage) or because of cell harvesting and re-seeding between passages. Based on determination of the released LDH activity (Fig. 4B) that is representative of cell lysis, the lysed cells were about 3% of the live cells although excursions to 6% were noted (e.g. on passage 4, Fig. 4B). At the end of each passage, the expression was determined by qPCR analysis of pluripotency genes such as NANOG, POU5F1 (OCT4) and SSEA4 (Fig. 4C).This was found to be consistently high and comparable to the expression in cells maintained on dishes. When analyzed by flow cytometry, over 80% of the cells were OCT4+ and SSEA4+ during the 5 passages (Fig. 4D). Taken together, these data suggest that hPSCs can be propagated on VN+HSA+UV microcarriers in a stirred-suspension vessel without loss of pluripotency marker expression for more than 50 days.
Figure 4.
Human ESCs (H9) expanded on VN+HSA+UV microcarriers in stirred suspension for multiple successive passages. (A) Cell growth (solid line), viability (dashed line) and (B) LDH activity profiles are shown. (C) Expression of pluripotency genes from the first (P1) to the last passage (P5) determined by qPCR. (D) Flow cytometric analysis of the fractions of OCT4+ and SSEA4+ hESCs. (E) Results of the karyotypic analysis of hESCs after 5 passages in microcarrier cultures.
These cells were also karyotypically normal based on G-banding analysis (Fig. 4E) and retained their potential for multi-lineage differentiation. To this end, cells harvested after 5 successive passages were plated on non-adhesive dishes where embryoid bodies (EBs) formed with spontaneous differentiation. Transcripts of genes characteristic of the three germ layers were detected: definitive endoderm (SOX17, FOXA2)23–24, mesoderm (ISL1) 25–26 and ectoderm (PAX6, NES)27 (Fig. 5A).
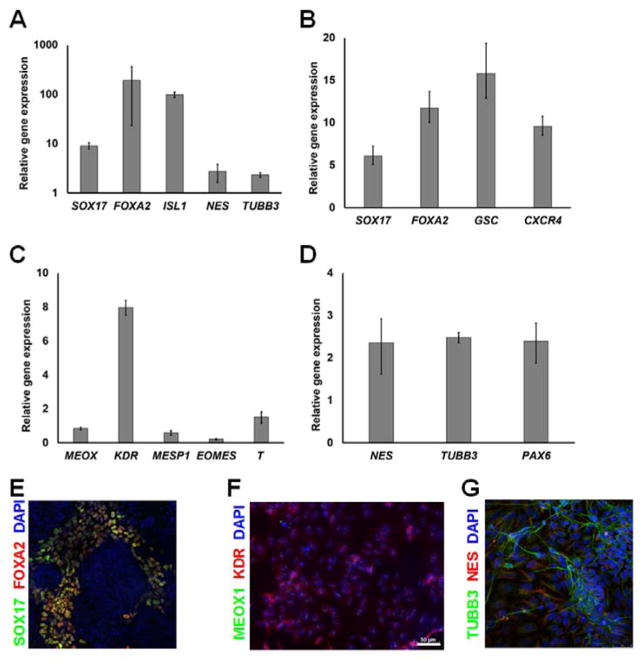
Figure 5.
Embryoid body (EB) formation and multilineage differentiation for hPSCs expanded in VN+HSA+UV microcarrier cultures for 5 continuous passages. (A) Cells within EBs displayed markers of DE, MS and NE analyzed by qPCR. Expression was normalized to that of undifferentiated cells with ACTB as the housekeeping gene. Quantitative PCR analysis of hPSCs expanded for 5 passages in microcarrier cultures and subjected to directed differentiation toward (B) DE, (C) MS and (D) NE and the corresponding immunocytochemistry results (E–G). The expression results in (B–D) was normalized to that of hPSCs cultured in basal media without the differentiation factors.
In addition to EB culture, hPSCs expanded over multiple passages on VN+HSA+UV beads were also transferred to Matrigel-coated dishes and directed to differentiation along definitive endoderm (DE), mesoderm (MS) and neuroectoderm (NE) fates as described2, 27–29. Commitment was assessed by the expression of relevant genes such as (DE) SOX17, FOXA2, GSC and CXCR4, (MS) KDR, ISL1 and MEOX1, (NE) TUBB3, PAX6 and NES by qPCR (Fig. 5B–D) and immunocytochemistry (Fig. 5E–G). Cells treated with basal media lacking differentiation factors served as controls.
These findings show that VN+HSA+UV microcarriers support the multi-passage expansion of hPSCs without loss of their potential for tri-lineage differentiation.
DISCUSSION
The development of scalable cultivation systems for hPSC expansion and differentiation under xeno-free conditions will be critical for enabling the realization of stem cell-based therapies. Despite the significant progress made in recent years, the culture of stem cells is characterized by great variability in its outcomes, complexity and high costs. In this study, a simple, fast and low-cost method is described for the customization of microcarriers with xeno-free components for the large-scale propagation of hPSCs. Human PSCs on beads featuring VN and HSA and treated with UV, proliferate in defined medium over multiple passages without loss of their viability and pluripotency. This approach of microcarrier customization does not entail reactions (e.g. amidation) for the conjugation of matrix molecules or peptides to the bead surface12. Such schemes typically exhibit variable efficiency and require substantial optimization.
The complete VN molecule and VN-derived peptides featuring the integrin-binding arginine–glycine–aspartic acid (RGD) motif 30, facilitate stem cell adhesion8–9, 31 in 2D surfaces. Yet, information about the potential of VN peptides to promote hPSC adhesion in 3D is limited. A VN decapeptide supports the maintenance of hPSCs in dishes8 but not in stirred-suspension microcarrier cultures without the use of poly-L-lysine12, which strengthens cell adhesion. Similarly, here cells readily came off beads coated with VN even though hPSCs are regularly maintained on VN-coated dishes. These observations are illustrative of the challenges (e.g. due to agitation-induced shear, surface curvature etc.) associated with the translation of the use of 2D substrates to 3D hPSC cultures as well as the development of novel such matrices. The inclusion of HSA, which was initially motivated by the low protein content of the medium, improved hPSC retention on VN+HSA beads but growth was suboptimal. However, when these carriers were irradiated with UV, cells were retained in stirred suspension and proliferated at a rate similar to cells cultured on Matrigel-coated microcarriers. Polystyrene beads coated with the full-length human plasma VN supported the growth of hESCs under static conditions32 noting that the reported VN coating density (450 ng/cm2) was higher than our ELISA results (~0.4 ng/cm2). The reasons for this difference in density are unclear but the use of different VN molecules (full-size vs. a truncated form used here) and culture modes (static vs. stirred suspension here) and the inclusion of HSA and UV treatment can be contributing factors.
Although the addition of HSA was prompted by the low protein content of the medium, sub-sequent UV irradiation caused the formation of intermolecular β-sheets from protein aggregation. UVC radiation is known to cause conformation changes and aggregation of human albumin20. Furthermore, self-complementary oligopeptides forming β-sheets facilitate the formation of a stable matrix supporting cell attachment19, 22. It is very likely that the same structures promote the adhesion of hPSCs on VN+HSA+UV beads, despite the reduction (vs. the VN+HSA beads) of the surface density of VN detected with ELISA. A study is currently underway to gain a better understanding of how the coating structure enhances the performance of microcarriers for hPSC culture.
The microcarrier surface charge is a parameter affecting the effectiveness of cell attachment. Positively-charged beads have been reported to perform better in terms of hPSC attachment and growth compared to microcarriers with neutral or negative surface charge33. This could be presumably because the cell glycocalyx has a net negative charge aiding the attachment to carriers with opposite charge. However, differences in cell attachment and growth diminish on beads with dissimilar surface charges after the application of extracellular matrix molecules (e.g. Matrigel). We observed that hPSCs had a higher seeding efficiency and level of growth on beads with neutral rather than positive charge and despite the carrier coating with VN and HSA. This could be due to the low surface density of VN that may not be sufficiently high to suppress the effects of charge. Conversely, the electrical charge may affect the UV-induced conformational changes of HSA (and its potential interaction with VN), thereby influencing cell attachment and growth.
It should also be noted that based on our findings, the supplementation of the medium with pluronic F68 significantly improved cell yield in stirred suspension microcarrier cultures. F68 is commonly used as a shear protectant agent and to prevent foaming in cell bioprocessing and thus its inclusion may not be critical in media used primarily for static culture (although some commercially available stem cell media contain F68 or Tween 20). The addition of F68 to the culture medium resulted in significantly improved and consistent hPSC viability and yield aligned with the use of the pluronic in typical bioreactor operation.
While this work was under review, results were reported on hiPSC propagation using VN-coated microcarriers with E8 medium34. Implementing a design-of-experiment methodology the authors demonstrated that a seeding density of 55×103 cells/cm2 or 20×106 cells total (1 g of microcarriers or 360 cm2) and a stirring rate of 44 rpm lead to a maximum (cell yield) of 3.5–4 cells (1.4×106 cells/ml) after 10 days of 50-ml spinner flask culture per cell seeded on the VN-coated microcarriers. In line with our findings, these results support the use of E8 medium and VN coating for the microcarrier culture of hPSCs. We employed a similar seeding density (55.6×103 cells/cm2) and agitation rate (45 rpm) as we have shown previously2, 12. Over multiple successive 11-day passages, the average peak density was approximately 1.5×106 live cells/ml per passage. This translates to a cell yield of 7.5 given that the starting amount of beads in our experiments was 0.5 g (180 cm2) per vessel. This difference in cell yield could be due to the fact that we supplemented the E8 medium with pluronic F68 thereby providing additional protection of the hPSCs from shear, and the engineering of the microcarriers with the inclusion of HSA and UV processing.
CONCLUSIONS
The present study demonstrates a simple and inexpensive approach for engineering xeno-free microcarriers intended for long-term scalable culture of hPSCs in SSBs. In conjunction with the inclusion of pluronic F68 in the medium, hPSCs on these microcarriers grew over multiple passages without adverse effects on their viability, growth and pluripotency. The defined, xeno-free microcarrier culture system described here is expected to facilitate the establishment of hPSC bioprocesses for the manufacturing of therapeutically relevant cell types.
Acknowledgments
Funding support has been provided by the National Institutes of Health (NHLBI, R01HL103709) and the National Science Foundation (CBET-1547785) to EST.
References
- 1.Kehoe DE, Jing D, Lock LT, Tzanakakis EM. Scalable Stirred-suspension Bioreactor Culture of Human Pluripotent Stem Cells. Tissue Eng Part A. 2010;16(2):405–21. doi: 10.1089/ten.TEA.2009.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lock LT, Tzanakakis ES. Expansion and differentiation of human embryonic stem cells to endoderm progeny in a microcarrier stirred-suspension culture. Tissue Eng Part A. 2009;15(8):2051–63. doi: 10.1089/ten.tea.2008.0455. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lecina M, Ting S, Choo A, Reuveny S, Oh S. Scalable platform for human embryonic stem cell differentiation to cardiomyocytes in suspended microcarrier cultures. Tissue engineering. Part C, Methods. 2010;16(6):1609–19. doi: 10.1089/ten.TEC.2010.0104. [DOI] [PubMed] [Google Scholar]
- 4.Bardy J, Chen AK, Lim YM, Wu S, Wei S, Weiping H, Chan K, Reuveny S, Oh SK. Microcarrier suspension cultures for high-density expansion and differentiation of human pluripotent stem cells to neural progenitor cells. Tissue engineering. Part C, Methods. 2013;19(2):166–80. doi: 10.1089/ten.TEC.2012.0146. [DOI] [PubMed] [Google Scholar]
- 5.Nie Y, Bergendahl V, Hei DJ, Jones JM, Palecek SP. Scalable culture and cryopreservation of human embryonic stem cells on microcarriers. Biotechnology progress. 2009;25(1):20–31. doi: 10.1002/btpr.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh SK, Chen AK, Mok Y, Chen X, Lim UM, Chin A, Choo AB, Reuveny S. Long-term microcarrier suspension cultures of human embryonic stem cells. Stem cell research. 2009;2(3):219–30. doi: 10.1016/j.scr.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Fernandes AM, Marinho PA, Sartore RC, Paulsen BS, Mariante RM, Castilho LR, Rehen SK. Successful scale-up of human embryonic stem cell production in a stirred microcarrier culture system. Braz J Med Biol Res. 2009;42(6):515–22. doi: 10.1590/s0100-879x2009000600007. S0100-879X2009000600007 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Melkoumian Z, Weber JL, Weber DM, Fadeev AG, Zhou Y, Dolley-Sonneville P, Yang J, Qiu L, Priest CA, Shogbon C, Martin AW, Nelson J, West P, Beltzer JP, Pal S, Brandenberger R. Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nature biotechnology. 2010;28(6):606–10. doi: 10.1038/nbt.1629. [DOI] [PubMed] [Google Scholar]
- 9.Braam SR, Zeinstra L, Litjens S, Ward-van Oostwaard D, van den Brink S, van Laake L, Lebrin F, Kats P, Hochstenbach R, Passier R, Sonnenberg A, Mummery CL. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alphavbeta5 integrin. Stem Cells. 2008;26(9):2257–65. doi: 10.1634/stemcells.2008-0291. [DOI] [PubMed] [Google Scholar]
- 10.Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, Smuga-Otto K, Howden SE, Diol NR, Propson NE, Wagner R, Lee GO, Antosiewicz-Bourget J, Teng JM, Thomson JA. Chemically defined conditions for human iPSC derivation and culture. Nature methods. 2011;8(5):424–9. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodin S, Domogatskaya A, Strom S, Hansson EM, Chien KR, Inzunza J, Hovatta O, Tryggvason K. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nature biotechnology. 2010;28(6):611–5. doi: 10.1038/nbt.1620. nbt.1620 [pii]. [DOI] [PubMed] [Google Scholar]
- 12.Fan Y, Hsiung M, Cheng C, Tzanakakis ES. Facile engineering of xeno-free microcarriers for the scalable cultivation of human pluripotent stem cells in stirred suspension. Tissue Eng Part A. 2014;20(3–4):588–99. doi: 10.1089/ten.TEA.2013.0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kehoe DE, Lock LT, Parikh A, Tzanakakis ES. Propagation of Embryonic Stem Cells in Stirred Suspension without Serum. Biotechnology progress. 2008;24(6):1342–52. doi: 10.1002/btpr.57. [DOI] [PubMed] [Google Scholar]
- 14.Lee JE, Park JC, Hwang YS, Kim JK, Kim JG, Sub H. Characterization of UV-irradiated dense/porous collagen membranes: morphology, enzymatic degradation, and mechanical properties. Yonsei medical journal. 2001;42(2):172–9. doi: 10.3349/ymj.2001.42.2.172. [DOI] [PubMed] [Google Scholar]
- 15.Yamazoe H, Uemura T, Tanabe T. Facile cell patterning on an albumin-coated surface. Langmuir: the ACS journal of surfaces and colloids. 2008;24(16):8402–4. doi: 10.1021/la801221r. [DOI] [PubMed] [Google Scholar]
- 16.Welle A, Horn S, Schimmelpfeng J, Kalka D. Photo-chemically patterned polymer surfaces for controlled PC-12 adhesion and neurite guidance. J Neurosci Methods. 2005;142(2):243–50. doi: 10.1016/j.jneumeth.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Kong J, Yu S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta biochimica et biophysica Sinica. 2007;39(8):549–559. doi: 10.1111/j.1745-7270.2007.00320.x. [DOI] [PubMed] [Google Scholar]
- 18.Lu R, Li W-W, Katzir A, Raichlin Y, Yu H-Q, Mizaikoff B. Probing the secondary structure of bovine serum albumin during heat-induced denaturation using mid-infrared fiberoptic sensors. Analyst. 2015;140(3):765–770. doi: 10.1039/c4an01495b. [DOI] [PubMed] [Google Scholar]
- 19.Zhang S, Holmes TC, DiPersio CM, Hynes RO, Su X, Rich A. Self-complementary oligopeptide matrices support mammalian cell attachment. Biomaterials. 1995;16(18):1385–1393. doi: 10.1016/0142-9612(95)96874-y. [DOI] [PubMed] [Google Scholar]
- 20.Michnik A, Michalik K, Drzazga Z. Effect of UVC radiation on conformational restructuring of human serum albumin. J Photochem Photobiol B. 2008;90(3):170–8. doi: 10.1016/j.jphotobiol.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Artyukhov VG, Pantyavin AA, Vashanov GA. Vacuum-UV-Radiation-Induced Structural-Functional Changes in Serum Albumin Molecules. Journal of Applied Spectroscopy. 68(2):291–298. doi: 10.1023/a:1019224404819. [DOI] [Google Scholar]
- 22.Holmes TC, de Lacalle S, Su X, Liu G, Rich A, Zhang S. Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds. Proc Natl Acad Sci U S A. 2000;97(12):6728–33. doi: 10.1073/pnas.97.12.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ang SL, Wierda A, Wong D, Stevens KA, Cascio S, Rossant J, Zaret KS. The formation and maintenance of the definitive endoderm lineage in the mouse: involvement of HNF3/forkhead proteins. Development. 1993;119(4):1301–15. doi: 10.1242/dev.119.4.1301. [DOI] [PubMed] [Google Scholar]
- 24.Sasaki H, Hogan BL. Differential expression of multiple fork head related genes during gastrulation and axial pattern formation in the mouse embryo. Development. 1993;118(1):47–59. doi: 10.1242/dev.118.1.47. [DOI] [PubMed] [Google Scholar]
- 25.Lin L, Cui L, Zhou W, Dufort D, Zhang X, Cai CL, Bu L, Yang L, Martin J, Kemler R, Rosenfeld MG, Chen J, Evans SM. Beta-catenin directly regulates Islet1 expression in cardiovascular progenitors and is required for multiple aspects of cardiogenesis. Proc Natl Acad Sci U S A. 2007;104(22):9313–8. doi: 10.1073/pnas.0700923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petropoulos H, Gianakopoulos PJ, Ridgeway AG, Skerjanc IS. Disruption of Meox or Gli activity ablates skeletal myogenesis in P19 cells. The Journal of biological chemistry. 2004;279(23):23874–81. doi: 10.1074/jbc.M312612200. [DOI] [PubMed] [Google Scholar]
- 27.Nat R, Nilbratt M, Narkilahti S, Winblad B, Hovatta O, Nordberg A. Neurogenic neuroepithelial and radial glial cells generated from six human embryonic stem cell lines in serum-free suspension and adherent cultures. Glia. 2007;55(4):385–99. doi: 10.1002/glia.20463. [DOI] [PubMed] [Google Scholar]
- 28.Jing D. Investigation of the cardiogenic differentiation of human pluripotent stem cells in static cultures and stirred-suspension bioreactors. State University of New York; Buffalo, United States -- New York: 2010. 3423476. [Google Scholar]
- 29.Wu J, Tzanakakis ES. Contribution of Stochastic Partitioning at Human Embryonic Stem Cell Division to NANOG Heterogeneity. PloS one. 2012;7(11):e50715. doi: 10.1371/journal.pone.0050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238(4826):491–7. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 31.Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK. Feeder-free growth of undifferentiated human embryonic stem cells. Nature biotechnology. 2001;19(10):971–4. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 32.Heng BC, Li J, Chen AK, Reuveny S, Cool SM, Birch WR, Oh SK. Translating human embryonic stem cells from 2-dimensional to 3-dimensional cultures in a defined medium on laminin- and vitronectin-coated surfaces. Stem Cells Dev. 2012;21(10):1701–15. doi: 10.1089/scd.2011.0509. [DOI] [PubMed] [Google Scholar]
- 33.Chen AK, Chen X, Choo AB, Reuveny S, Oh SK. Critical microcarrier properties affecting the expansion of undifferentiated human embryonic stem cells. Stem cell research. 2011;7(2):97–111. doi: 10.1016/j.scr.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Badenes SM, Fernandes TG, Cordeiro CS, Boucher S, Kuninger D, Vemuri MC, Diogo MM, Cabral JM. Defined Essential 8 Medium and Vitronectin Efficiently Support Scalable Xeno-Free Expansion of Human Induced Pluripotent Stem Cells in Stirred Microcarrier Culture Systems. PloS one. 2016;11(3):e0151264. doi: 10.1371/journal.pone.0151264. [DOI] [PMC free article] [PubMed] [Google Scholar]