Abstract
Background
Preclinical and postmortem studies have implicated the metabotropic glutamate receptor 5 (mGluR5) in the pathophysiology of major depressive disorder (MDD). The goal of the present study was to determine the role of mGluR5 in a large group of individuals with MDD compared to healthy controls (HC) in vivo with [18F]FPEB and positron emission tomography (PET). Furthermore, we sought to determine the role glutamate plays on mGluR5 availability in MDD.
Methods
Sixty-five participants (30 MDD and 35 HC) completed [18F]FPEB PET to estimate the primary outcome measure - mGluR5 volume of distribution (VT), and the secondary outcome measure - mGluR5 distribution volume ratio (DVR). A subgroup of 39 participants (16 MDD and 23 HC) completed proton magnetic resonance spectroscopy (1H MRS) to estimate anterior cingulate (ACC) glutamate, glutamine, and Glx (glutamate + glutamine) levels relative to creatine (Cr).
Results
No significant between-group differences were observed in mGluR5 VT or DVR. Compared to HC, individuals with MDD had higher ACC glutamate, glutamine, and Glx levels. Importantly, the ACC mGluR5 DVR negatively correlated with glutamate/Cr and Glx/Cr levels.
Conclusions
In this novel in vivo examination, we show an inverse relationship between mGluR5 availability and glutamate levels. These data highlight the need to further investigate the role of glutamatergic system in depression.
Keywords: MDD, mGluR5, PET, [18F]FPEB, 1H MRS, glutamate
Introduction
Major depressive disorder (MDD) is highly prevalent and disabling, with poorly understood neurobiology and with available treatment options that require weeks to months to exert full therapeutic benefit (1). Better understanding of the pathophysiology of MDD may facilitate the development of novel effective rapid acting therapeutics. Accumulating evidence strongly implicates altered glutamate neurotransmission in the pathophysiology of MDD, and suggests that glutamate modulation may induce rapid relief of depressive symptoms in treatment refractory patients (2). The metabotropic glutamate receptor 5 (mGluR5) is a key component of the glutamatergic system, and has been thought to play a critical role in the pathophysiology of depression (3, 4). In this report, we used the high affinity PET radiotracer [18F]FPEB to compare in vivo mGluR5 availability in individuals with MDD and healthy controls. In addition, we collected 1H MRS data to investigate the relationship between mGluR5 availability and cortical glutamate level.
Chronic stress and depression are associated with alterations in glutamate (the major excitatory neurotransmitter) transmission, including an increase in extracellular glutamate, which could lead to excitotoxicity and impaired synaptic integrity, contributing to the brain abnormalities observed in MDD (6). Glutamate neurotransmission is regulated by ionotropic and G-protein coupled metabotropic GluR, which are divided into 3 groups: group I (mGluR1 and 5), group II (mGluR2 and 3) and group III (mGluR4, 6, 7, 8)(5). The group I mGluRs couple to phospholipase C and stimulate cyclic AMP formation and arachidonic acid release (7); modulating synaptic transmission, neuronal excitability, gene expression and neuroplasticity. The mGluR5s are mostly located postsynaptically and on glial cells in the perisynaptic space (8–10), and have highest density in the hippocampus, intermediate in the caudate/putamen, cerebral cortex, and thalamus, and lowest in the cerebellum (11, 12).
Preclinical studies have repeatedly implicated mGluR5 impairment in the pathophysiology of depression (13). However, the relationship between mGluR5 changes and depressive-like behavior is complex and not fully understood. Animal models of depression showed reductions in mGluR5 protein and density in various brain regions (14, 15). Similarly, mGluR5 knockout mice exhibited depressive-like behavior (16) and chronic, but not acute, antidepressant treatment increased mGluR5 expression (17), raising the possibility that therapeutic effects might be mediated by mGluR5 regulation.
In humans, postmortem examination of mGluR5 density in MDD has revealed regional variability in findings. One study showed lower mGluR5 protein levels in the cerebellum of 14 MDD patients as compared to 14 controls (18). More recently, a postmortem study of tissue from ventral anterior cingulate (BA24) failed to demonstrate significant alterations in mGluR5 density in12 individuals with MDD as compared to 12 controls (19), suggesting mGluR5 alterations may not be present in MDD. However, a different study examining mGluR5 levels postmortem and in vivo showed lower monomer mGluR5 protein levels in 15 individuals with MDD as compared to 15 comparison individuals in parts of the PFC (BA9) (20). The same group used [11C]ABP688 PET to provide the first in vivo evidence of lower mGluR5 availability in 11 individuals with MDD in a current depressive episode as compared to 11 controls (20). Distribution volume ratio (DVR) with cerebellar activity as the reference region was used as the outcome measure. The authors reported 10–20% lower mGluR5 availability in several brain regions, including the thalamus, anterior cingulate cortex (ACC), anterior insula, lateral prefrontal cortex, the temporal and parietal lobes, as well as precentral, inferior prefrontal and lateral prefrontal gyri (20). However, an evaluation of mGluR5 availability in elderly depression (n=16) as compared to elderly controls using [11C]ABP688 and PET did not detect significant between-group differences in any of the regions assessed via distribution volume (VT) or DVR outcome measures (21). Thus, the extent of mGluR5 involvement in living individuals with MDD remains inconclusive. Of notes, similar pattern was observed in post-mortem studies showing decreased astrocytes in younger MDD, but not in older patients (22).
This study was designed to conclusively determine whether there is a difference in mGluR5 density in MDD with an adequately powered sample of medication-free MDD individuals. Considering that there is no human brain region devoid of mGluR5 in humans (23–26), we studied VT (an absolute measure) as our main outcome measure. Given previous study examined changes in mGluR5 availability using DVR, we also examined DVR (a relative measure) as outcome measure for quantification of mGluR5 availability. The radiotracer we used was 3-[18F]fluoro-5-[(pyridin-3-yl)ethynyl]benzonitrile ([18F]FPEB, KD = 0.11±0.04 nM – 0.15±0.02 nM(25)). Test-retest for the outcome measure VT during bolus plus infusion administration was −2 – −6% when using arterial blood and 0 – 4% when using venous blood. Furthermore, both mean arterial and mean venous radiotracer concentrations reach equilibrium by 90 mins (time of data collection) and our work shows 1% difference between arterial and venous quantifications, suggesting arterial line is not required for outcome measure calculation. Therefore, this tracer is excellent for use in psychiatric populations (27). To assess whether increased cortical levels of glutamate are associated with decreased mGluR5 density, we measured glutamate levels in the ACC of a subgroup of subjects using 1H MRS. A postmortem study reported elevated tissue glutamate levels in depression in the anterior cingulate cortex (ACC)(28) and occipital cortex detected elevated glutamate levels in individuals with MDD (29). There is, however, also MRS literature showing lower ACC glutamate and glutamate+glutamine (GLX) levels in individuals with depression (30, 31), while other studies found reduced ACC glutamine in MDD, but not glutamate (32). We hypothesized that individuals with MDD would have lower mGluR5 availability based on the previous report. We also hypothesized a negative correlation between ACC mGluR5 availability and ACC glutamate levels, consistent with the hypothesis that increased glutamate leads to excitotoxicity.
Methods and Materials
Subjects
The Institutional Review Board (IRB) and safety committees approved all study procedures. All subjects provided written informed consent prior to participation. Thirty medication-free individuals with MDD and 35 healthy controls (HC) completed the PET study, and 16 MDD and 23 HC of those successfully completed 1H MRS scans within 1–2 days of PET. Participants underwent physical, neurological and psychological examination to rule out any major medical or neurological illness, and to confirm diagnosis. Electrocardiography, complete blood counts, serum chemistries, thyroid function test, liver function test, urinalysis and urine toxicology screening, and plasma pregnancy tests (for women) were performed during screening. Urine toxicology and pregnancy tests were repeated prior to each scan. Study criteria included, age 18–65 years-old, MDD diagnosis with current major depressive episode (MDD group) or no psychiatric disorder (HC group) as confirmed by a Structured Clinical Interview for DSM-IV (33), no active suicidal ideation, no lifetime history of bipolar disorder or schizophrenia, no diagnosis of alcohol or substance abuse (past 6 months) or dependence (past 12 months) except for nicotine, no positive urine toxicology, medication free for at least 2 weeks, no history of loss of consciousness > 5 minutes.
Considering previous reports of lower mGluR5 binding in smokers (34), tobacco smokers and nonsmokers were matched across groups. Tobacco smokers were defined as those who had smoked at least 10 cigarettes daily for at least 1 year, and who had, urine cotinine levels >100ng/mL and carbon monoxide levels >11 ppm. Nonsmokers were defined as having smoked <40 cigarettes in lifetime, and having negligible urine cotinine (<100ng/mL) and carbon monoxide (<8ppm) levels. Plasma cotinine levels of >150ng/mL for smokers and plasma of <15ng/mL for nonsmokers were preferred, but not available to collect for some subjects due to technical issues (e.g., clotting).
Magnetic resonance imaging (MRI) and spectroscopy (MRS)
MRI scans were collected using a 3T scanner (Trio, Siemens Medical Systems, Erlangen, Germany) for registration with PET and region-of-interest (ROI) definition. MRI scans were performed within 1–2 weeks of PET scan. A 3D T1-weighted gradient-echo (MPRAGE) with 1 mm3 isometric resolution (FA=7°, TE=3.34 ms, TI=1100 ms, and TR=2500 ms) was used.
Proton MRS scans were obtained with PRESS localization (TE/TR = 68/2500 ms) from a 3×3×3 cm3 voxel centered in front of the genu of the corpus callosum on a 4T magnet (Bruker Instruments, Billerica, MA). Subjects lay supine in the scanner for about 1h. Using software written in MATLAB (The Mathworks, Inc.), each FID was line-broadened with a −2 Hz/6 Hz Lorentzian-Gaussian conversion, zero-filled to 16K points, and Fourier-transformed. The spectra were phased and fitted with in-house spectral fitting software using a basis set that contained glutamate, glutamine, aspartate, myo-inositol, scyllo-inositol, choline, and creatine for quantification. Metabolites were quantified relative to tissue creatine (Cr). Glutamate + glutamine (Glx) was included to facilitate interpretation in the context of previous literature (30, 35).
Brain [18F]FPEB PET scanning
[18F]FPEB was synthesized using a previously published method (27). PET scans were acquired on a High Resolution Research Tomograph (HRRT) scanner (Siemens/CTI, Knoxville, TN), with spatial resolution (full width at half maximum) of 2–3 mm. All of the PET scans were acquired in the afternoon as we and others previously showed diurnal variation in mGluR5 availability (36, 37). A radial artery catheter was inserted at the wrist area to measure the metabolite-corrected input function. In the cases where arterial line placement failed (i.e., in 12 HC and 13 MDD), a venous catheter was inserted to measure metabolite-corrected input function. We previously showed excellent correlation between arterial and venous concentrations of [18F]FPEB (27) when using constant infusion of tracer (Cvenous = 1.01 * Carterial, r2=0.95). The test/retest variability of VT from this method was 12%. A venous catheter was inserted at the antecubital area on the different side for intravenous administration of [18F]FPEB.
Before the scan, an optical motion-tracking tool was fastened to the subject’s head. A 6-min transmission scan was obtained for attenuation correction. [18F]FPEB was administered as bolus plus infusion (B/I) for 120 minutes with a Kbol of 190 min (27, 38). Equilibrium was reached at 60 min and outcome data were calculated from 90–120 min scan time. The injected dose was 153.7± 40 MBq for the HC group and 155.3± 42 MBq for the MDD group (n = 30) at the time of injection. The injected mass was 0.37 ± 0.22 μg for the HC group and 0.33 ± 0.19 μg for the MDD group. There were no significant between-group differences in the injected dose or mass. Dynamic scan data were reconstructed with corrections for attenuation, normalization, randoms, scatter, deadtime, and motion (Vicra, NDI System Waterloo, Ontario), using the ordered-subset expectation maximization (OSEM)-based MOLAR algorithm (39).
The PET images were coregistered to each subject’s T1-weighted MR images using a six-parameter mutual information algorithm (FLIRT, FSL 3.2, Analysis Group, FMRIB, Oxford, UK), which was then coregistered to the MR template by nonlinear transformation using the Bioimagesuite software (version 2.5; http://www.bioimagesuite.com). Regions of interest (ROIs) from the AAL (Anatomical Automatic Labeling for SPM2) template were used. [18F]FPEB VT was estimated by the equilibrium analysis method using blood data (38). DVR was calculated as VTtarget/VTreference, using the cerebellum as reference for comparability to previous reports (20, 34).
Statistical analysis
SPSS Statistics V21 (IBM Corporation, NY, USA) was used for the study analyses. Prior to conducting each analysis, the distribution of outcome measures was examined using normality histograms, Skewness, and Kurtosis measures. Transformations and non-parametric tests were used as necessary. Demographics, and clinical characteristics were compared using t-test and chi-square.
We used t-test to compare PET and MRS measures between MDD and HC, as well as between smokers and non-smokers. Considering that smoking status had a significant effect on caudate mGluR5, we constructed a general linear model examine the effect of diagnosis with smoking status as covariate. To determine the relationship between ACC PET and MRS measures, we conducted both Pearson and Spearman correlation analyses. Exploratory analyses, with correction for multiple testing, examined the relationship between PET/MRS measures and the severity of illness using the length of depressive episode, and current severity of symptoms as measured by Beck Depression Inventory (40). All tests were 2-tailed with significance set at p ≤ 0.05. The study has 80% power to detect an effect size of d = 0.7 for the mGluR5 differences and d = 0.9 for the glutamate differences. False discovery rate (FDR) was implemented for multiple comparison correction.
Results
Demographics and clinical characteristics are detailed in Table 1. There were no significant between-group differences in age, smoking status, or sex. The average length of current episode and severity of depressive symptoms is consistent with a population with moderate course of illness and severity. In the MDD group, four subjects had comorbid posttraumatic stress disorder and two subjects had comorbid obsessive compulsive disorder.
Table 1.
Demographic and Clinical Characteristics.
| MDD (N=30) | HC (N=35) | ||
|---|---|---|---|
|
| |||
| Mean ± SEM | Mean ± SEM | p | |
| Age (y) | 36.7 ± 2.4 | 35.7 ± 2.0 | 0.75 |
| Female (N; %) | 21 (70%) | 18 (51%) | 0.13 |
| Smokers (N; %) | 10 (33%) | 13 (37%) | 0.75 |
| BDI | 25.1 ± 1.6 | 1.4 ± 0.4 | < 0.01 |
| Length of episode (d) | 358 ± 89 | ||
| Comorbidity | 4 PTSD, 2 OCD | none | |
Abbreviations: MDD: major depressive disorder; y: years; N: number; d: days; BDI, Beck depression inventory; PTSD: post traumatic stress disorder, OCD: obsessive compulsive disorder.
MDD vs. HC
We did not detect significant differences in mGluR5 availability between MDD and HC, using either mGluR5 VT or DVR (Table 2). Furthermore, we did not detect significant effect of input function (arterial versus venous) on mGluR5 quantification, nor differences in the receptor availability in the subgroups that participated in the MRS scanning. In the caudate, smokers have numerically lower levels of mGluR5 VT (smokers: 23 ±1.6; non-smokers: 29 ±1.4; t = 2.3; p = 0.02) and DVR (smokers: 2.4 ±0.05; non-smokers: 2.2 ±0.1; t = 2.6; p = 0.01). However, these differences did not survive correction for multiple comparisons (corrected p > 0.05). Caudate mGluR5 (VT and DVR) did not differ between MDD and HC, with smoking status as covariate. Other ROIs showed no significant mGluR5 binding between smokers and non-smokers (all p values > 0.05).
Table 2.
Metabotropic Glutamate Receptor 5 (mGluR5) Availability and Anterior Cingulate Glutamate Level
| ROI | HC Mean (N=35) | HC SEM | MDD Mean (N=30) | MDD SEM | t | p | FDRa |
|---|---|---|---|---|---|---|---|
| mGluR5 VT | |||||||
| Amygdala | 25.74 | 0.96 | 26.43 | 1.19 | −0.45 | 0.65 | 0.74 |
| Anterior Insula | 30.24 | 1.00 | 31.74 | 1.37 | −0.90 | 0.37 | 0.59 |
| Caudate | 25.40 | 1.12 | 26.62 | 1.35 | −0.71 | 0.48 | 0.68 |
| Ant Cingulate | 29.33 | 1.00 | 31.23 | 1.29 | −1.18 | 0.24 | 0.52 |
| Post Cingulate | 19.87 | 0.76 | 20.01 | 0.92 | −0.12 | 0.90 | 0.92 |
| Frontal Cortex | 23.49 | 0.84 | 25.63 | 1.09 | −1.57 | 0.12 | 0.46 |
| Hippocampus | 22.76 | 0.95 | 23.39 | 1.06 | −0.44 | 0.66 | 0.74 |
| Occipital Cortex | 24.01 | 0.84 | 25.79 | 1.08 | −1.32 | 0.19 | 0.51 |
| Parietal Cortex | 23.97 | 0.84 | 26.30 | 1.12 | −1.68 | 0.10 | 0.46 |
| Posterior Insula | 27.54 | 0.98 | 29.17 | 1.29 | −1.02 | 0.31 | 0.56 |
| Putamen | 27.32 | 0.96 | 29.40 | 1.21 | −1.36 | 0.18 | 0.51 |
| Temporal Cortex | 27.18 | 0.95 | 28.96 | 1.22 | −1.16 | 0.25 | 0.52 |
| Thalamus | 18.87 | 0.72 | 19.49 | 0.95 | −0.53 | 0.60 | 0.74 |
| Cerebellum | 10.50 | 0.38 | 11.54 | 0.49 | −1.70 | 0.10 | 0.46 |
| mGluR5 DVR | |||||||
| Amygdala | 2.48 | 0.06 | 2.31 | 0.07 | 1.79 | 0.08 | 0.46 |
| Anterior Insula | 2.91 | 0.06 | 2.78 | 0.07 | 1.41 | 0.16 | 0.51 |
| Caudate | 2.44 | 0.08 | 2.32 | 0.07 | 1.18 | 0.24 | 0.52 |
| Ant Cingulate | 2.82 | 0.06 | 2.74 | 0.07 | 0.87 | 0.39 | 0.59 |
| Post Cingulate | 1.93 | 0.07 | 1.77 | 0.07 | 1.62 | 0.11 | 0.46 |
| Frontal Cortex | 2.25 | 0.05 | 2.24 | 0.05 | 0.14 | 0.89 | 0.92 |
| Hippocampus | 2.18 | 0.06 | 2.05 | 0.06 | 1.58 | 0.12 | 0.46 |
| Occipital Cortex | 2.31 | 0.05 | 2.26 | 0.05 | 0.67 | 0.50 | 0.68 |
| Parietal Cortex | 2.30 | 0.05 | 2.30 | 0.05 | 0.10 | 0.92 | 0.92 |
| Posterior Insula | 2.65 | 0.06 | 2.56 | 0.07 | 1.01 | 0.31 | 0.56 |
| Putamen | 2.63 | 0.06 | 2.58 | 0.06 | 0.63 | 0.53 | 0.68 |
| Temporal Cortex | 2.61 | 0.05 | 2.54 | 0.06 | 0.95 | 0.35 | 0.59 |
| Thalamus | 1.81 | 0.05 | 1.70 | 0.05 | 1.56 | 0.12 | 0.46 |
| ACC Metabolites | (N=23) | (N=16) | |||||
| Glutamate | 0.59 | 0.04 | 0.78 | 0.07 | −2.28 | 0.03 | 0.03 |
| Glutamine | 0.27 | 0.03 | 0.46 | 0.07 | −2.50 | 0.02 | 0.03 |
| Glx | 0.87 | 0.06 | 1.24 | 0.11 | −3.12 | 0.003 | 0.009 |
Notes:
False Discover Rate (FDR) corrected p values;
Abbreviations: VT, volume of distribution; DVR, distribution volume ratio; MDD, major depressive disorder; HC, healthy control; ACC, anterior cingulate cortex
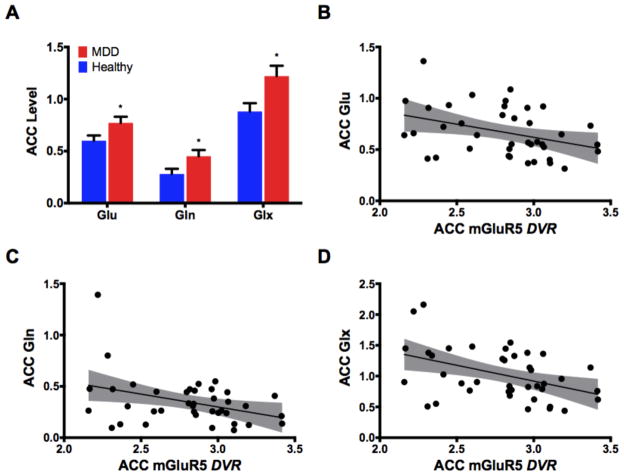
The MDD group exhibited higher ACC glutamate, glutamine, and Glx levels (Fig. 1A; Table 2). To rule out the possibility that the higher Glx, Glu, Gln levels are due to reduction in Cr, we have conducted a secondary analysis in which NAA or Cho were used as internal references. Glx/NAA, Glx/Cho, Gln/NAA, and Gln/Cho were significantly higher in MDD (all p values < 0.05), but non-significant higher levels of Glu/NAA (p = 0.07) and Glu/Cho (p = 0.12). In addition, we compared Cr between groups using Cho as internal reference. This analysis showed no significant Cr/Cho difference between MDD and HC (p = 0.17). Further we examined gray matter volume as additional internal reference but found no Cr differences between groups (p = 0.15).
Figure 1. Glutamate level in the anterior cingulate cortex (ACC).
(A) The levels of glutamate (Glu), glutamine (Gln), and glutamate+glutamine (Glx) in the ACC of patients with MDD (red) compared to healthy (blue). (B–D) Scatter plots of the correlations between metabotropic glutamate receptor 5 (mGluR5) distribution volume ratio (DVR) in the whole group and ACC levels of glutamate (B), glutamine (C), and Glx (D). Abbreviations: (A) *, p ≤ 0.05, Bars/Error-bars: Mean/SEM; (B–D) shaded areas are the 95% confidence band of the best-fit line.
Smoking status did not differ between MDD and HC in the MRS sample (p = 0.5). ACC Glx, glutamate, and glutamine did not differ between smokers and non-smokers (all p values > 0.2).
ACC mGluR5 vs. Glutamate
In the whole sample, ACC mGluR5 VT did not correlate with ACC glutamate (r = − 0.01; p = 0.95; corrected p = 0.95), glutamine (r = 0.09; p = 0.58; corrected p = 0.87), or Glx (r = 0.02; p = 0.89; corrected p = 0.95). Using Pearson correlation, ACC mGluR5 DVR was negatively correlated with ACC Glx (r = − 0.43; p = 0.005; corrected p = 0.03), but correlated with ACC glutamate (r = − 0.37; p = 0.02; corrected p = 0.07) and glutamine (r = − 0.29; p = 0.07; corrected p = 0.14) at trend only (Fig. 1B–D). Using non-parametric Spearman’s correlation, ACC mGluR5 DVR was negatively correlated with ACC Glx (r = − 0.44; p = 0.005; corrected p = 0.015), glutamate (r = − 0.39; p = 0.01; corrected p = 0.015), and glutamine (r = − 0.33; p = 0.04; corrected p = 0.04). Using one-tailed tests did not change the results (data not shown).
Post-hoc exploratory Pearson analysis showed the following correlation in the MDD group between ACC mGluR5 DVR and Glx (r = − 0.54; p = 0.02; corrected p = 0.06), glutamate (r = − 0.42; p = 0.10; corrected p = 0.15) or glutamine (r = − 0.34; p = 0.19; corrected p = 0.19). Using Spearman’s correlation, the following correlations were found in the MDD group between ACC mGluR5 DVR and Glx (r = − 0.62; p = 0.005; corrected p = 0.03), glutamate (r = − 0.46; p = 0.07; corrected p = 0.07) or glutamine (r = − 0.51; p = 0.04; corrected p = 0.06). ACC mGluR5 DVR did not correlate with glutamate measures in healthy controls (all p values > 0.3). There were no correlations between mGluR5 VT and glutamate measures in either group (all p values > 0.4). Given the hypothesis that increases in Glu are associated with decreased receptor number, we examined whether a one-tailed test would change the significance. However, using one-tailed test did not change the results, except for the Spearman’s correlation between ACC mGluR5 DVR and each of glutamate and glutamine which became significant (corrected p < 0.05).
Depression Severity vs. Glutamate and mGluR5
Examining glutamate levels in the MDD group, we found a trend for positive correlation between current depressive severity, as measured by BDI, and ACC glutamine (r = 0.58; uncorrected p = 0.02; corrected p = 0.057) and Glx (r = 0.56; uncorrected p = 0.02; corrected p = 0.057); but no correlation with glutamate (r = 0.34; uncorrected p = 0.20; corrected p = 0.40). There were no significant correlations between BDI and any of the mGluR5 measures, or between the length of depressive episode and any of the glutamate or mGluR5 measures.
Discussion
We conducted an adequately powered study of mGluR5 and glutamate levels in unmedicated individuals with MDD. We did not detect significantly lower mGluR5 availability in the MDD group using VT or DVR as the outcome measures, consistent with 1 of 2 previous PET studies and with postmortem data. However, we detected higher ACC glutamate levels in the MDD compared to HC group, suggesting an alteration in glutamatergic system specific to MDD. Importantly, we report for the first time a negative association between glutamate levels and mGluR5 availability in vivo in the ACC.
In our large sample, we did not detect any differences in mGluR5 VT associated with diagnosis of MDD in any of the regions assessed. Given one prior work examining mGluR5 availability in vivo in MDD had employed the DVR as their outcome measure (with cerebellum as reference), we examined mGluR5 DVR outcome as well. Similarly to the VT outcome, we did not detect significant differences based on the MDD diagnosis and thus replicated one of two previous preliminary reports (21). Differences between Deschwanden et al. (20) and our study include the larger sample size in our study with MDD and HC subjects closely matched by demographic variables, including tobacco smoking dependence, and well controlled time of day for scanning procedures. We (36), and others (37), previously showed diurnal variability in mGluR5 availability, and thus if this factor was not controlled for by Deschwanden et al., this could contribute to the observed between groups differences. Furthermore, the differences in radoligands themselves might have contributed to the variability in findings, although a previous study using [11C]ABP688 in elderly depression did not detect between group differences (21). Both of the radioligands bind to the negative allosteric site on mGluR5 and are displaceable by MPEP suggesting similar properties (41), there are differences in nondisplaceable binding and affinity of the radiotracers, with [18F]FPEB exhibiting estimated 3–5 fold higher BPND values and greater affinity for the NAM site on mGluR5 (0.15±0.02 nM vs. 1.7±0.2 nM/L) (36, 42). Severity of depressive symptoms did not appear to be different between the studies. Although it would be tempting to state that the in vivo and postmortem evaluation in Deschwanden et al. resulted in parallel findings, the postmortem sample consisted mostly of individuals who died by suicide and had tested positive on the toxicology report, whereas the in vivo sample appears to lack suicidal ideation and none of the subjects abused drugs. Nonetheless, the above novel studies provide invaluable information on the potential role of the glutamatergic system in depressive symptomatology.
In our MRS data, we detected higher ACC glutamate/Cr levels in the MDD group. Prior MRS studies have reported lower, higher, or no differences in cortical glutamate in MDD (30), and in the ACC specifically, prior studies have shown lower or no differences in glutamate level in MDD compared to control (35). However, postmortem glutamate quantification shows higher glutamate levels in the ACC in the MDD as compared to the HC group (28). Sample characteristics, including medication status and severity of depression, and differences in glutamate quantification may have contributed to the inconsistency in the literature. Previously reported glutamate abnormalities have been primarily related to more refractory forms of depression (43–48). In the current study, depressive severity positively correlated with ACC glutamine and ACC Glx at a trend level. To the extent that total glutamate level might reflect underlying neurotransmission dysregulation, the latter findings would be consistent with preclinical models relating depression to glutamate excitotoxicity (6).
Consistent with our prediction, ACC mGluR5 availability correlated negatively with glutamate level in the total sample, and at trend in the MDD group, supporting the hypothesis of mGluR5 internalization in the presence of increased glutamate (23, 49). However, the significant correlation was observed with the secondary outcome measure only (DVR) and would not survive multiple comparisons, somewhat limiting generalizability of the findings. Further, this correlation is likely not due to a direct competition between glutamate and the radioligand. In our study, the radioligand is a negative allosteric modulator (NAM) that binds to a site in the transmembrane domain of the receptor and not to the N-terminal orthosteric site where glutamate binds. Consistent with their different sites of binding, glutamate does not directly impact the binding of [18F]FPEB or its homolog [11C]ABP688 in a membrane preparation, but it does lead to receptor internalization (36). However, in vitro studies have established that FPEB and ABP688 bind to mGluR5 at the plasma membrane but are not sufficiently membrane permeable to access internalized receptors (50). Thus, in our study, it is likely that we are measuring receptor availability at the plasma membrane and we are unlikely measuring a direct competition between the radioligand and endogenous neurotransmitter. The lack of a significant correlation between mGluR5 availability and MRS measures in the HC group might be surprising at first given the equivalent mGluR5 levels between the HC and MDD groups. However, we observed a dysregulation in glutamate levels in the MDD group only, suggesting glutamatergic dysregulation is specific to that group. Thus, if glutamate levels are normally regulated in the HC group, there might be less of a dispersion of this variable and thus lack of an observable correlation, which in the MDD group might be indicative of glutamate excitotoxicity.
Together, our data do not suggest the presence of lower mGluR5 availability in depression. However, this is not to conclude that treatments targeting mGluR5 have no benefit to individuals with MDD given they may aid with regulation of glutamate levels. For example, mGluR5 antagonists and NAMs have shown rapid antidepressant-like effects (13, 51), while chronic treatment with traditional antidepressants has shown reduction in mGluR5 signaling (52). A hypothesis for mGluR5 antagonist-induced antidepressant effects is that mGluR5 normalizes glutamate neurotransmission in MDD. Consistent with this hypothesis, mGluR5 antagonists inhibit glutamate release (53) and mGluR5 NAMs downregulate excessive glutamate transmission (54).
Limitations of this study include a relatively small cohort of MRS participants and the use of tissue creatine as an internal reference. However, previous work has shown no differences in tissue creatine in depression (55). Another limitation is the single (cube) acquisition of the MRS ACC region does not allow for a 100% overlap with the anatomical ACC. Our study criteria, requiring medication-free participants and excluding patients with active suicidal ideation, may have led to a patient cohort with depression that is not very chronic, severe, or refractory (see Table 1). However, analysis of our sample shows that all of the individuals studied were depressed for months to years, and none were in their first depressive episode. While we acquired both VT and DVR PET measures of mGluR5 availability, all study findings were related to DVR, which render the interpretation of these findings more complex. Strengths of the study include a large PET sample, state-of-the-art imaging modalities, a well-characterized medication-free patient cohort, and a multimodal PET/MRS approach.
To conclude, we did not detect lower mGluR5 availability in the MDD as compared to HC individuals. However, by combining MRS and PET data, we show a first in vivo evidence of glutamatergic influence on mGluR5 density. This work suggests mGluR5 may be a treatment site to reduce the burden of MDD through regulation of glutamate.
Supplementary Material
Table S1. Volumes of regions of interest
Acknowledgments
We thank the study participants for their invaluable contribution. We thank the staff at the Yale University PET Center for their help with radiotracer syntheses and related analyses, as well as imaging the subjects. Support provided by K01 MH092681 (Esterlis), Women’s Health Research at Yale (P50DA03394; Esterlis), K23 MH101498 (Abdallah), Yale Center for Clinical Investigation (UL1RR024139; Esterlis, Abdallah, Carson, Sanacora), Nancy Taylor Foundation (Esterlis); DANA Foundation (Esterlis); U.S. Department of Veterans Affairs via its support of the VA National Center for PTSD (Abdallah, Esterlis, Sanacora); K05 DA022413 (Javitch), and R01 MH054137 (Javitch). This publication was also made possible by CTSA Grant Number UL1 TR000142 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
Financial Disclosures
C.G.A. has served as a consultant and/or on advisory boards for Genentech and Janssen. J.H. was a full-time employee of UCB Pharma at the time this study was conducted; he is now a full-time employee of Denali Therapeutics Inc. G.S. has served as a consultant for Allergan, Alkermes, AstraZeneca, Avanier Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly and Company, Hoffman La-Roche, Janssen, Merck and Company, Navigen, Naurex, Noven Pharmaceuticals, Servier Pharmaceuticals, Taisho Pharmaceuticals, Takeda, Teva and Vistagen Therapeutics. G.S. has also received additional research contracts from AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Company, Johnson and Johnson, Hoffman La-Roche, Merck and Company, Naurex and Servier over the last 24 months. Free medication was provided to G.S. for an NIH-sponsored study by Sanofi-Aventis. FG is an employee of Novartis Pharma AG. All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mathers C, Fat DM, Boerma J. The global burden of disease: 2004 update. World Health Organization; 2008. [Google Scholar]
- 2.Abdallah CG, Sanacora G, Duman RS, Krystal JH. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med. 2015;66:509–523. doi: 10.1146/annurev-med-053013-062946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wieronska JM, Pilc A. Metabotropic glutamate receptors in the tripartite synapse as a target for new psychotropic drugs. Neurochem Int. 2009;55:85–97. doi: 10.1016/j.neuint.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 4.Krystal JH, Mathew SJ, D’Souza DC, Garakani A, Gunduz-Bruce H, Charney DS. Potential psychiatric applications of metabotropic glutamate receptor agonists and antagonists. CNS Drugs. 2010;24:669–693. doi: 10.2165/11533230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Niciu MJ, Ionescu DF, Richards EM, Zarate CA., Jr Glutamate and its receptors in the pathophysiology and treatment of major depressive disorder. J Neural Transm (Vienna) 2014;121:907–924. doi: 10.1007/s00702-013-1130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2012;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aramori I, Nakanishi S. Signal transduction and pharmacological characteristics of a metabotropic glutamate receptor, mGluR1, in transfected CHO cells. Neuron. 1992;8:757–765. doi: 10.1016/0896-6273(92)90096-v. [DOI] [PubMed] [Google Scholar]
- 8.Lavialle M, Aumann G, Anlauf E, Prols F, Arpin M, Derouiche A. Structural plasticity of perisynaptic astrocyte processes involves ezrin and metabotropic glutamate receptors. Proc Natl Acad Sci U S A. 2011;108:12915–12919. doi: 10.1073/pnas.1100957108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lujan R, Roberts JD, Shigemoto R, Ohishi H, Somogyi P. Differential plasma membrane distribution of metabotropic glutamate receptors mGluR1 alpha, mGluR2 and mGluR5, relative to neurotransmitter release sites. J Chem Neuroanat. 1997;13:219–241. doi: 10.1016/s0891-0618(97)00051-3. [DOI] [PubMed] [Google Scholar]
- 10.Lujan R, Nusser Z, Roberts JD, Shigemoto R, Somogyi P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur J Neurosci. 1996;8:1488–1500. doi: 10.1111/j.1460-9568.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- 11.Daggett LP, Sacaan AI, Akong M, Rao SP, Hess SD, Liaw C, et al. Molecular and functional characterization of recombinant human metabotropic glutamate receptor subtype 5. Neuropharmacology. 1995;34:871–886. doi: 10.1016/0028-3908(95)00085-k. [DOI] [PubMed] [Google Scholar]
- 12.Ohnuma T, Augood SJ, Arai H, McKenna PJ, Emson PC. Expression of the human excitatory amino acid transporter 2 and metabotropic glutamate receptors 3 and 5 in the prefrontal cortex from normal individuals and patients with schizophrenia. Brain Res Mol Brain Res. 1998;56:207–217. doi: 10.1016/s0169-328x(98)00063-1. [DOI] [PubMed] [Google Scholar]
- 13.Palucha A, Pilc A. Metabotropic glutamate receptor ligands as possible anxiolytic and antidepressant drugs. Pharmacol Ther. 2007;115:116–147. doi: 10.1016/j.pharmthera.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Kovacevic T, Skelin I, Minuzzi L, Rosa-Neto P, Diksic M. Reduced metabotropic glutamate receptor 5 in the Flinders Sensitive Line of rats, an animal model of depression: an autoradiographic study. Brain Res Bull. 2012;87:406–412. doi: 10.1016/j.brainresbull.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wieronska JM, Branski P, Szewczyk B, Palucha A, Papp M, Gruca P, et al. Changes in the expression of metabotropic glutamate receptor 5 (mGluR5) in the rat hippocampus in an animal model of depression. Pol J Pharmacol. 2001;53:659–662. [PubMed] [Google Scholar]
- 16.Shin S, Kwon O, Kang JI, Kwon S, Oh S, Choi J, et al. mGluR5 in the nucleus accumbens is critical for promoting resilience to chronic stress. Nat Neurosci. 2015;18:1017–1024. doi: 10.1038/nn.4028. [DOI] [PubMed] [Google Scholar]
- 17.Smialowska M, Szewczyk B, Branski P, Wieronska JM, Palucha A, Bajkowska M, et al. Effect of chronic imipramine or electroconvulsive shock on the expression of mGluR1a and mGluR5a immunoreactivity in rat brain hippocampus. Neuropharmacology. 2002;42:1016–1023. doi: 10.1016/s0028-3908(02)00062-x. [DOI] [PubMed] [Google Scholar]
- 18.Fatemi SH, Folsom TD, Rooney RJ, Thuras PD. mRNA and protein expression for novel GABAA receptors theta and rho2 are altered in schizophrenia and mood disorders; relevance to FMRP-mGluR5 signaling pathway. Translational psychiatry. 2013;3:e271. doi: 10.1038/tp.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matosin N, Fernandez-Enright F, Frank E, Deng C, Wong J, Huang XF, et al. Metabotropic glutamate receptor mGluR2/3 and mGluR5 binding in the anterior cingulate cortex in psychotic and nonpsychotic depression, bipolar disorder and schizophrenia: implications for novel mGluR-based therapeutics. Journal of psychiatry & neuroscience : JPN. 2014;39:407–416. doi: 10.1503/jpn.130242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deschwanden A, Karolewicz B, Feyissa AM, Treyer V, Ametamey SM, Johayem A, et al. Reduced metabotropic glutamate receptor 5 density in major depression determined by [(11)C]ABP688 PET and postmortem study. Am J Psychiatry. 2011;168:727–734. doi: 10.1176/appi.ajp.2011.09111607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeLorenzo C, Sovago J, Gardus J, Xu J, Yang J, Behrje R, et al. Characterization of brain mGluR5 binding in a pilot study of late-life major depressive disorder using positron emission tomography and [(1)(1)C]ABP688. Translational psychiatry. 2015;5:e693. doi: 10.1038/tp.2015.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanacora G, Banasr M. From pathophysiology to novel antidepressant drugs: glial contributions to the pathology and treatment of mood disorders. Biol Psychiatry. 2013;73:1172–1179. doi: 10.1016/j.biopsych.2013.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeLorenzo C, DellaGioia N, Bloch M, Sanacora G, Nabulsi N, Abdallah C, et al. In Vivo Ketamine-Induced Changes in [(11)C]ABP688 Binding to Metabotropic Glutamate Receptor Subtype 5. Biol Psychiatry. 2015;77:266–275. doi: 10.1016/j.biopsych.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kagedal M, Cselenyi Z, Nyberg S, Raboisson P, Stahle L, Stenkrona P, et al. A positron emission tomography study in healthy volunteers to estimate mGluR5 receptor occupancy of AZD2066 - estimating occupancy in the absence of a reference region. Neuroimage. 2013;82:160–169. doi: 10.1016/j.neuroimage.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Patel S, Hamill TG, Connolly B, Jagoda E, Li W, Gibson RE. Species differences in mGluR5 binding sites in mammalian central nervous system determined using in vitro binding with [18F]F-PEB. Nucl Med Biol. 2007;34:1009–1017. doi: 10.1016/j.nucmedbio.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Wong DF, Waterhouse R, Kuwabara H, Kim J, Brasic JR, Chamroonrat W, et al. 18F-FPEB, a PET radiopharmaceutical for quantifying metabotropic glutamate 5 receptors: a first-in-human study of radiochemical safety, biokinetics, and radiation dosimetry. J Nucl Med. 2013;54:388–396. doi: 10.2967/jnumed.112.107995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park E, Sullivan JM, Planeta B, Gallezot JD, Lim K, Lin SF, et al. Test-retest reproducibility of the metabotropic glutamate receptor 5 ligand [(1)(8)F]FPEB with bolus plus constant infusion in humans. Eur J Nucl Med Mol Imaging. 2015;42:1530–1541. doi: 10.1007/s00259-015-3094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biological Psychiatry. 2007;62:1310–1316. doi: 10.1016/j.biopsych.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 29.Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, et al. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry. 2004;61:705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- 30.Luykx JJ, Laban KG, van den Heuvel MP, Boks MP, Mandl RC, Kahn RS, et al. Region and state specific glutamate downregulation in major depressive disorder: a meta-analysis of (1)H-MRS findings. Neurosci Biobehav Rev. 2012;36:198–205. doi: 10.1016/j.neubiorev.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 31.Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- 32.Walter M, Henning A, Grimm S, Schulte RF, Beck J, Dydak U, et al. The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch Gen Psychiatry. 2009;66:478–486. doi: 10.1001/archgenpsychiatry.2009.39. [DOI] [PubMed] [Google Scholar]
- 33.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders: Patient Edition (SCIDI/P. Version 2.0. Biometric Research, New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- 34.Akkus F, Ametamey SM, Treyer V, Burger C, Johayem A, Umbricht D, et al. Marked global reduction in mGluR5 receptor binding in smokers and ex-smokers determined by [11C]ABP688 positron emission tomography. Proc Natl Acad Sci U S A. 2013;110:737–742. doi: 10.1073/pnas.1210984110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuksel C, Ongur D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry. 2010;68:785–794. doi: 10.1016/j.biopsych.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeLorenzo C, Gallezot JD, Gardus J, Yang J, Planeta B, Nabulsi N, et al. In vivo variation in same-day estimates of metabotropic glutamate receptor subtype 5 binding using [11C]ABP688 and [18F]FPEB. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2016 doi: 10.1177/0271678X16673646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elmenhorst D, Mertens K, Kroll T, Oskamp A, Ermert J, Elmenhorst EM, et al. Circadian variation of metabotropic glutamate receptor 5 availability in the rat brain. J Sleep Res. 2016;25:754–761. doi: 10.1111/jsr.12432. [DOI] [PubMed] [Google Scholar]
- 38.Sullivan JM, Lim K, Labaree D, Lin SF, McCarthy TJ, Seibyl JP, et al. Kinetic analysis of the metabotropic glutamate subtype 5 tracer [(18)F]FPEB in bolus and bolus-plus-constant-infusion studies in humans. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013;33:532–541. doi: 10.1038/jcbfm.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carson RE, Barker WC, Liow J-S, Johnson CA. Nuclear Science Symposium Conference Record, 2003 IEEE. IEEE; 2003. Design of a motion-compensation OSEM list-mode algorithm for resolution-recovery reconstruction for the HRRT; pp. 3281–3285. [Google Scholar]
- 40.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 41.Wang JQ, Tueckmantel W, Zhu A, Pellegrino D, Brownell AL. Synthesis and preliminary biological evaluation of 3-[(18)F]fluoro-5-(2-pyridinylethynyl)benzonitrile as a PET radiotracer for imaging metabotropic glutamate receptor subtype 5. Synapse. 2007;61:951–961. doi: 10.1002/syn.20445. [DOI] [PubMed] [Google Scholar]
- 42.Ametamey SM, Kessler LJ, Honer M, Wyss MT, Buck A, Hintermann S, et al. Radiosynthesis and preclinical evaluation of 11C-ABP688 as a probe for imaging the metabotropic glutamate receptor subtype 5. J Nucl Med. 2006;47:698–705. [PubMed] [Google Scholar]
- 43.Zhang C, Li Z, Wu Z, Chen J, Wang Z, Peng D, et al. A study of N-methyl-D-aspartate receptor gene (GRIN2B) variants as predictors of treatment-resistant major depression. Psychopharmacology (Berl) 2013 doi: 10.1007/s00213-013-3297-0. [DOI] [PubMed] [Google Scholar]
- 44.Merkl A, Schubert F, Quante A, Luborzewski A, Brakemeier EL, Grimm S, et al. Abnormal cingulate and prefrontal cortical neurochemistry in major depression after electroconvulsive therapy. Biol Psychiatry. 2011;69:772–779. doi: 10.1016/j.biopsych.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 45.Grimm S, Luborzewski A, Schubert F, Merkl A, Kronenberg G, Colla M, et al. Region-specific glutamate changes in patients with unipolar depression. J Psychiatr Res. 2012;46:1059–1065. doi: 10.1016/j.jpsychires.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 46.Portella MJ, de Diego-Adelino J, Gomez-Anson B, Morgan-Ferrando R, Vives Y, Puigdemont D, et al. Ventromedial prefrontal spectroscopic abnormalities over the course of depression: a comparison among first episode, remitted recurrent and chronic patients. J Psychiatr Res. 2011;45:427–434. doi: 10.1016/j.jpsychires.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 47.de Diego-Adelino J, Portella MJ, Gomez-Anson B, Lopez-Moruelo O, Serra-Blasco M, Vives Y, et al. Hippocampal abnormalities of glutamate/glutamine, Nacetylaspartate and choline in patients with depression are related to past illness burden. Journal of psychiatry & neuroscience : JPN. 2013;38:107–116. doi: 10.1503/jpn.110185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang J, Narr KL, Woods RP, Phillips OR, Alger JR, Espinoza RT. Glutamate normalization with ECT treatment response in major depression. Mol Psychiatry. 2013;18:268–270. doi: 10.1038/mp.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyake N, Skinbjerg M, Easwaramoorthy B, Kumar D, Girgis RR, Xu X, et al. Imaging changes in glutamate transmission in vivo with the metabotropic glutamate receptor 5 tracer [11C] ABP688 and N-acetylcysteine challenge. Biol Psychiatry. 2011;69:822–824. doi: 10.1016/j.biopsych.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 50.Lin x, Donthamsetti P, Skinbjerg M, Slifstein M, Abi-Dargham A, Javijtch J. mGluR5 Workshop. Yale University; 2015. FPEB and ABP688 cannot accesss internalized mGluR5 receptors. [Google Scholar]
- 51.Tatarczynska E, Klodzinska A, Chojnacka-Wojcik E, Palucha A, Gasparini F, Kuhn R, et al. Potential anxiolytic- and antidepressant-like effects of MPEP, a potent, selective and systemically active mGlu5 receptor antagonist. Br J Pharmacol. 2001;132:1423–1430. doi: 10.1038/sj.bjp.0703923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Witkin JM, Marek GJ, Johnson BG, Schoepp DD. Metabotropic glutamate receptors in the control of mood disorders. CNS Neurol Disord Drug Targets. 2007;6:87–100. doi: 10.2174/187152707780363302. [DOI] [PubMed] [Google Scholar]
- 53.Thomas LS, Jane DE, Harris JR, Croucher MJ. Metabotropic glutamate autoreceptors of the mGlu(5) subtype positively modulate neuronal glutamate release in the rat forebrain in vitro. Neuropharmacology. 2000;39:1554–1566. doi: 10.1016/s0028-3908(99)00223-3. [DOI] [PubMed] [Google Scholar]
- 54.Morin N, Morissette M, Gregoire L, Gomez-Mancilla B, Gasparini F, Di Paolo T. Chronic treatment with MPEP, an mGlu5 receptor antagonist, normalizes basal ganglia glutamate neurotransmission in L-DOPA-treated parkinsonian monkeys. Neuropharmacology. 2013;73:216–231. doi: 10.1016/j.neuropharm.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 55.Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, et al. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56:1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Volumes of regions of interest