Abstract
Histone H3 lysine 9 (H3K9) methylation is a hallmark of heterochromatin. H3K9 demethylation is crucial in mouse sex determination; The H3K9 demethylase Jmjd1a deficiency leads to increased H3K9 methylation at the Sry locus in embryonic gonads, thereby compromising Sry expression and causing male-to-female sex reversal. We hypothesized that the H3K9 methylation level at the Sry locus is finely tuned by the balance in activities between the H3K9 demethylase Jmjd1a and an unidentified H3K9 methyltransferase to ensure correct Sry expression. Here we identified the GLP/G9a H3K9 methyltransferase complex as the enzyme catalyzing H3K9 methylation at the Sry locus. Based on this finding, we tried to rescue the sex-reversal phenotype of Jmjd1a-deficient mice by modulating GLP/G9a complex activity. A heterozygous GLP mutation rescued the sex-reversal phenotype of Jmjd1a-deficient mice by restoring Sry expression. The administration of a chemical inhibitor of GLP/G9a enzyme into Jmjd1a-deficient embryos also successfully rescued sex reversal. Our study not only reveals the molecular mechanism underlying the tuning of Sry expression but also provides proof on the principle of therapeutic strategies based on the pharmacological modulation of epigenetic balance.
Author summary
In eukaryotes, DNA wraps an octamer of the core histones. Covalent modifications on the histones have diverse biological functions including transcriptional regulation. Histone H3 lysine 9 (H3K9) methylation is a hallmark of transcriptionally silenced chromatin. In mammals, the sex-determining gene Sry initiates testis differentiation in embryonic gonads. Sry expression in gonads is fine-tuned in both space and time. Here, we demonstrated that fine-tuning of Sry expression is achieved by the balance in activities between H3K9 demethylase and H3K9 methyltransferase. We found that the GLP/G9a complex is the enzyme catalyzing H3K9 methylation of Sry. Based on this finding, we tried to rescue the sex-reversal phenotype of the mutant mice by modulating the H3K9 methylation balance of Sry. We succeeded by modulating the H3K9 methylation balance not only with a genetic approach but also with a chemical approach using an inhibitor of GLP/G9a enzyme. Aberrant histone methylation levels are associated with diseases, including cancer, and intellectual disability. Our study provides proof for the principle of therapeutic strategies based on the pharmacological modulation of histone methylation balance.
Introduction
Covalent modifications of histone tails are epigenetic marks that play roles in many nuclear processes. Among them, methylation of histone H3 lysine 9 (H3K9) is a hallmark of transcriptionally silenced heterochromatin. Various types of H3K9 methyltransferases (“writers”) and demethylases (“erasers”) have been discovered in mammals. Considering that these H3K9 methylation “writers” and “erasers” are expressed in a cell-type-specific and developmental-stage-specific manner, H3K9 methylation levels are regulated not statically but dynamically during development [1]. In this situation, a specific combination of H3K9 methylation “writer” and “eraser” may antagonistically tune the expression levels of their target genes.
We previously demonstrated that H3K9 demethylation plays an indispensable role in mouse sex development [2]. XY mice lacking Jmjd1a (also called Kdm3a), an “eraser” for H3K9 methylation, showed male-to-female sex reversal. Jmjd1a demethylates H3K9 of the sex-determining gene Sry in sexually undifferentiated gonads at embryonic day 11.5 (E11.5), thereby activating Sry transcription. Jmjd1a deficiency induced a decrease, but not a delay of Sry expression in the developing gonads. We found a significant increase of dimethylated H3K9 (H3K9me2) at the Sry locus in embryonic gonads at E11.5 [2], suggesting the existence of an H3K9me2 “writer” that catalyzes H3K9 methylation at the Sry locus.
Several lines of evidence suggest that aberrant histone methylation levels are associated with diseases, including cancer, and intellectual disability [1]. Therefore, normalizing histone modification levels by manipulating the activity of the corresponding modifier is proposed as a potentially powerful therapeutic strategy [3]. Therefore, we speculated that manipulation of the activity of the H3K9me2 “writer” responsible for H3K9 methylation at the Sry locus normalizes Sry expression in the mice lacking the H3K9me2 “eraser” Jmjd1a.
In this study, we identified the H3K9 methyltransferase GLP/G9a complex as the enzyme responsible for H3K9 methylation at the Sry locus. Based on this finding, we aimed to rescue the aberrant sex development of Jmjd1a-deficient mice by modulating the activity of the GLP/G9a complex. The GLP heterozygous mutation rescued not only H3K9 hypermethylation at the Sry locus but also the perturbed Sry expression in Jmjd1a-deficient embryos. Strikingly, the sex-reversal phenotype in Jmjd1a-deficient mice was completely rescued by the GLP heterozygous mutation. We also aimed to rescue the phenotype by artificially manipulating the activity of the GLP/G9a complex. The administration of the GLP/G9a complex inhibitor into Jmjd1a-deficient embryos at a specific developmental time point rescued the aberrant sex differentiation of these mice. Our studies provide a novel strategy by which diseases attributed to the dysfunction of an epigenetic “eraser” can be rescued by blocking the activity of the corresponding epigenetic “writer.”
Results
Co-expression of GLP/G9a H3K9 complex with Jmjd1a in Sry-expressing pre-Sertoli cells
In mammals, a number of enzymes possess intrinsic H3K9 methyltransferase activities, such as Suv39h1 [4], Suv39h2 [5], Eset [6], G9a [7], and GLP [8]. Among them, G9a (also called Ehmt2/Kmt1c) and GLP (also called Ehmt1/Kmt1d) form a stoichiometric heterodimer complex [9–11]. Jmjd1a deficiency resulted in the increase of H3K9me2, but not trimethylated H3K9me3 in the developing gonads at E11.5, suggesting that the enzyme counteracting Jmjd1a-mediated H3K9 demethylation produces H3K9me2 (Fig 1A and 1B, S1 Fig). We previously demonstrated that the global level of H3K9me2 in developing mouse embryos was dominantly catalyzed by the GLP/G9a complex [10]. Taking these findings together, the GLP/G9a complex was the strongest candidate for an enzyme that counteracts Jmjd1a-mediated H3K9 demethylation in the developing gonads.
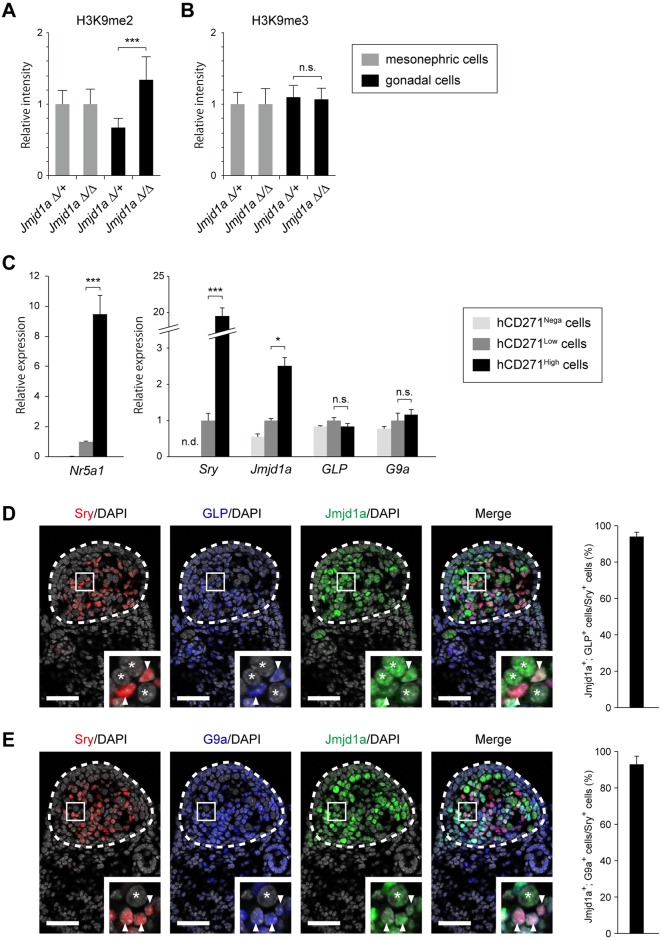
Fig 1. Expression of GLP/G9a H3K9 methyltransferase complex in XY embryonic gonads at E11.5.
(A, B) Quantitative comparison of the immunofluorescence intensities of H3K9me2 (A) and H3K9me3 (B). Representative staining profiles are shown in S1 Fig. The intensities of H3K9 methylation of Jmjd1aΔ/+ mesonephric cells were defined as 1. Data are presented as mean ± SD. *** P < 0.001; n.s., not significant. (C) Relative mRNA expression profiles of Jmjd1a, GLP, and G9a in gonadal somatic cell populations. Gonadal somatic cells were prepared from dissociated gonads from E11.5 Nr5a1-hCD271-tg embryos and fractionated according to the expression levels of hCD271 by FACS (S2 Fig). Each fraction was subjected to mRNA expression analysis by RT-qPCR. The endogenous Nr5a1 expression level was strictly correlated with the expression levels of hCD271 (left). Sry and Jmjd1a transcripts were substantially enriched in the hCD271-high population whereas GLP/G9a transcripts were detected in each population (right). mRNA expression levels in the hCD271-low population were defined as 1. Data are presented as mean ± SD. * P < 0.05, *** P < 0.001; n.s., not significant. (D, E) Triple immunofluorescence analyses for GLP (D) and G9a (E), counterstained with anti-Jmjd1a and anti-Sry in the center regions of E11.5 gonads. Enlarged boxes indicate co-expression of GLP (D) and G9a (E) with Jmjd1a in Sry-expressing pre-Sertoli cells (arrowheads). Asterisks represent germ cells. The population of the cells containing both signals of GLP (or G9a) and Jmjd1a among the Sry-expressing pre-Sertoli cells is presented at right. More than 200 cells per embryo (n = 3) were examined. Data are presented as mean ± SD. Scale bar, 50 μm.
Somatic cells of E11.5 embryonic gonads contain subpopulations with high and low expression levels of an orphan nuclear receptor, Nr5a1 (also called Sf-1/Ad4BP) [12]. Because a previous study demonstrated that the Nr5a1-high population contains Sry-expressing pre-Sertoli cells [13], we examined mRNA expression levels of GLP/G9a in this population (Fig 1C). We had established a transgenic mouse line Nr5a1-hCD271-tg, in which the human cell surface marker CD271 (also called LNGFR) is expressed depending on the Nr5a1 promoter [2] [14]. We prepared a single cell suspension from the gonads/mesonephroi of E11.5 Nr5a1-hCD271-tg embryos and then fractionated it according to the expression level of hCD271 by fluorescence-activated cell sorting (FACS) (S2 Fig). The hCD271-negative population contained mesonephric cells and germ cells [2]. As expected, quantitative RT-PCR (RT-qPCR) analysis demonstrated that the endogenous Nr5a1 expression levels were high and low in hCD271-high and -low populations, respectively (Fig 1C, left). Concordant with the previous study [13], Sry transcript was substantially enriched in the hCD271-high population (Fig 1C, right). Jmjd1a transcript was also significantly enriched in the hCD271-high population. GLP and G9a transcripts were detected in all populations at similar levels, suggesting the ubiquitous expression of GLP/G9a complex in the developing gonads (Fig 1C, right). To address whether GLP/G9a complex and Jmjd1a were co-expressed in Sry-expressing pre-Sertoli cells, we performed triple immunostaining analyses of E11.5 embryonic gonads with antibodies against GLP/G9a, Jmjd1a, and Sry. As shown in Fig 1D and 1E, GLP/G9a complex was expressed in Sry-expressing pre-Sertoli cells. Furthermore, Sry- and GLP/G9a complex-positive cells contained robust signals for Jmjd1a (Fig 1D and 1E). Cells containing both signals of GLP (or G9a) and Jmjd1a among the Sry-expressing pre-Sertoli cells ware calculated. As summarized in Fig 1D and 1E (right panels), almost all Sry-positive cells contained the signals of both GLP/G9a complex and Jmjd1a. We therefore concluded that GLP/G9a H3K9 complex and Jmjd1a are actually co-expressed in Sry-expressing pre-Sertoli cells.
GLP/G9a complex-mediated H3K9 methylation counteracts Jmjd1a-mediated H3K9 demethylation in gonadal somatic cells
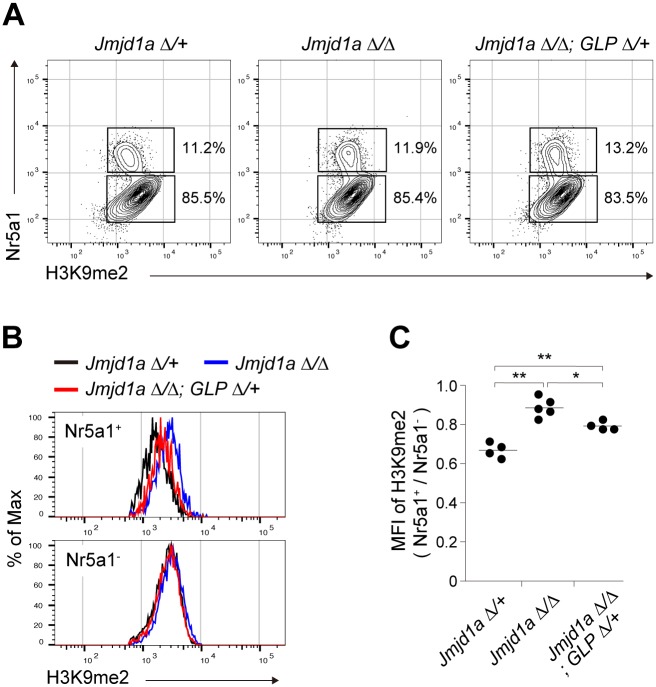
We previously demonstrated that GLP is a limiting factor that controls the stability of the GLP/G9a heterodimer complex. In addition, the heterodimer formation of GLP/G9a was shown to be essential for H3K9 methylation in vivo [10] [15]. Thus, we first examined whether a GLP mutation might rescue the increased H3K9me2 levels in Jmjd1a-deficient mice. A homozygous GLP mutation in mice leads to embryonic lethality around E9.5 [10]. We therefore generated mice heterozygous for the GLP mutation (GLPΔ; lacking the coding sequences for the catalytic SET domain) combined with a Jmjd1a-null (Jmjd1aΔ/Δ) background. Embryonic gonads/mesonephroi at E11.5 were stained with antibodies against H3K9me2 and Nr5a1. The H3K9 methylation levels of Nr5a1-positive gonadal somatic cells were compared by FACS analysis (Fig 2A). Jmjd1a deficiency resulted in an increased H3K9me2 level in Nr5a1-positive gonadal somatic cells, indicating the substantial contribution of Jmjd1a to H3K9 demethylation (Fig 2B and 2C). Notably, introduction of the GLP mutation into the Jmjd1aΔ/Δ background significantly reduced the H3K9me2 level in gonadal somatic cells (Fig 2B and 2C). Thus, we concluded that the GLP/G9a complex counteracts Jmjd1a-mediated H3K9 demethylation in the developing gonads in the sex-determining period.
Fig 2. GLP/G9a complex-mediated H3K9 methylation counteracts Jmjd1a-mediated H3K9 demethylation in gonadal somatic cells.
(A) Flow cytometric analyses of E11.5 gonadal somatic cells for quantifying the H3K9 methylation levels. Cells were prepared from gonads with mesonephroi and then co-stained with antibodies against H3K9me2 and Nr5a1. Upper and lower boxes indicate the populations of Nr5a1-positive gonadal somatic cells and Nr5a1-negative mesonephric cells, respectively. (B) Representative histogram analyses for H3K9me2 levels of Nr5a1-positive gonadal somatic cells (upper) and Nr5a1-negative mesonephric cells (lower). (C) Statistical analysis of H3K9me2 levels of gonadal somatic cells of the indicated genotypes. Median fluorescence intensity (MFI) values for H3K9me2 of Nr5a1-positive gonadal somatic cells were normalized to those of Nr5a1-negative cells, and then plotted (n = 4–5). * P < 0.05; ** P < 0.01.
GLP/G9a complex catalyzes H3K9 dimethylation at the Sry locus
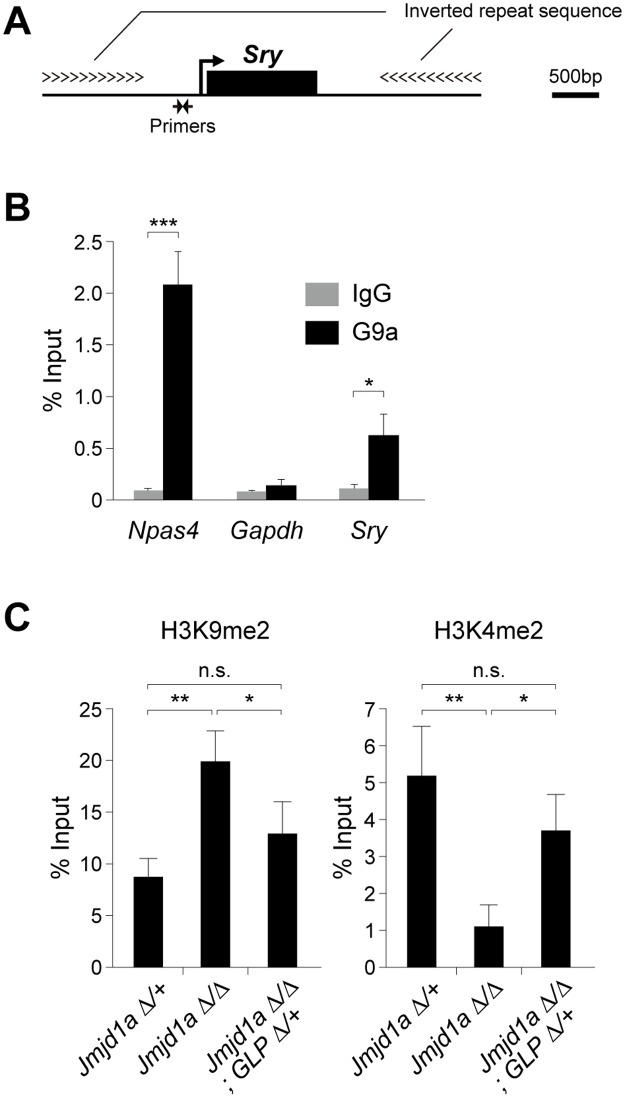
To examine the possible counteracting role of the GLP/G9a complex on Jmjd1a-mediated H3K9 demethylation at single gene level, especially at the Sry locus, chromatin immunoprecipitation (ChIP) analyses were performed. We previously demonstrated that Jmjd1a is enriched at the linear promoter region of Sry in E11.5 gonadal somatic cells [2] (Fig 3A). We purified gonadal somatic cells from E11.5 XY Nr5a1-hCD271-tg embryos and then subjected these cells to ChIP-qPCR analyses. As shown in Fig 3B, we found that G9a is also accumulated in the linear promoter region of Sry. We used Npas4 as a positive control locus, that had been identified as one of the target loci of G9a [16]. We therefore concluded that H3K9 methyltransferase GLP/G9a complex and H3K9 demethylase Jmjd1a both target the Sry locus in embryonic gonadal somatic cells in the sex-determining period.
Fig 3. GLP/G9a complex catalyzes H3K9 dimethylation at the Sry locus.
(A) Diagram of the Sry locus and primer location of the linear promoter region of Sry. (B) ChIP-qPCR analysis for G9a at the linear promoter region of Sry. Gonadal somatic cells were purified from E11.5 XY Nr5a1-hCD271-tg embryos, pooled, cross-linked, and then introduced into ChIP-qPCR analysis. We used Npas4, that had been identified as one of the target loci of G9a, as a positive control locus [16]. Data are presented as mean ± SD. * P < 0.05; *** P < 0.001. (C) ChIP-qPCR analyses for H3K9me2 (left) and H3K4me2 (right) at the Sry locus. Gonadal somatic cells of the indicated genotypes were purified according to the method described in S3 Fig, pooled for each genotype (2 to 4 embryos) and then subjected to native ChIP analysis. ChIP experiment was performed independently twice and gave similar results. Data are presented as mean ± SD. * P < 0.05; ** P < 0.01; n.s., not significant.
We next aimed to elucidate the impact of the GLP mutation on the H3K9me2 level of the Sry locus. Gonadal somatic cells were immunomagnetically purified from XY Jmjd1aΔ/Δ, GLPΔ/+, and Nr5a1-CD271-tg embryos for ChIP analysis (the experimental scheme is shown in S3 Fig). Importantly, the numbers of purified cells were similar among XY Jmjd1aΔ/+-, XY Jmjd1aΔ/Δ-, and XY Jmjd1aΔ/Δ;GLPΔ/+ gonads, indicating that these mutations did not affect gonadal somatic cell numbers (S3 Fig). Purified gonadal somatic cells were then subjected to native ChIP-qPCR analyses. Consistent with our previous study, Jmjd1a deficiency resulted in an increase of H3K9me2 at the Sry locus in E11.5 gonadal somatic cells, compared with the level in control cells (Fig 3C). Importantly, the increased level of H3K9me2 at the Sry locus was significantly rescued by the GLP mutation (Fig 3C). We also demonstrated the inverse relationship between H3K9me2 and H3K4me2 at the Sry locus. The latter is an epigenetic mark for transcriptionally activated chromatin (Fig 3C). Taking these findings together, the GLP/G9a complex is the bona fide enzyme responsible for H3K9 methylation at the Sry locus in E11.5 gonadal somatic cells.
Jmjd1a- and GLP/G9a complex-mediated expression tuning is confined to Sry within the Y chromosome genes
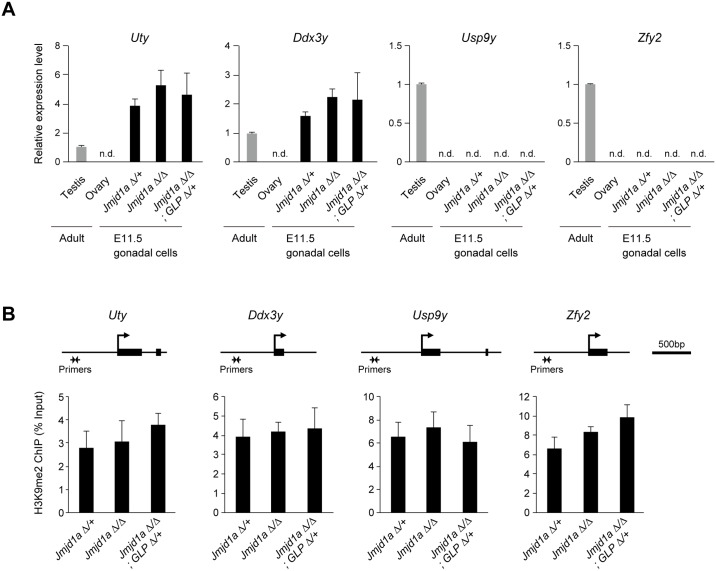
We next elucidated whether the Jmjd1a mutation and/or Jmjd1a/GLP compound mutations may also induce transcriptional perturbation of Y chromosome genes other than Sry. Gonadal somatic cells were immunomagnetically purified from E11.5 embryos and then subjected to mRNA expression analysis. As shown in Fig 4A, we could not detect significant differences in the mRNA expression levels of Sry-neighboring genes, Uty, Ddx3y, Usp9y, and Zfy2, between control and mutant gonads. Accordingly, our previous microarray analysis showed that the expression levels of Y chromosome genes other than Sry were not affected by Jmjd1a deficiency [2]. Next, we evaluated the H3K9me2 levels of Uty, Ddx3y, Usp9y, and Zfy2 by ChIP-qPCR analysis using purified E11.5 gonadal somatic cells (Fig 4B). Again, we could not detect significant differences in the H3K9me2 levels of these genes between control and mutant gonadal somatic cells. Taking these results together, Jmjd1a- and GLP/G9a complex-mediated expression tuning is highly confined to the Sry locus and is not extended to other genes on Y chromosome.
Fig 4. Jmjd1a- and GLP/G9a complex-mediated expression tuning is not extended to other genes on the Y chromosome.
(A) E11.5 XY gonads of the indicated genotypes were dissected and subjected to mRNA expression analysis. We examined the expression levels of Sry-neighboring genes (Uty, Ddx3y, Usp9y, and Zfy2), which are located on the short arm of the Y chromosome. There was no significant difference in the expression levels of these genes between control and mutant gonads. mRNA expression levels in the adult testis (3 months) were defined as 1. All data are presented as mean ± SD. (B) ChIP-qPCR analyses for H3K9me2 at the Uty, Ddx3y, Usp9y, and Zfy2 loci. Primer locations are shown at the top. There was no significant difference of the H3K9me2 levels between control and mutant gonads. All data are presented as mean ± SD.
It is known that H3K4me3 and H3K9ac are enriched at the linear promoter region of Sry in E11.5 gonadal somatic cells [13]. To address whether Jmjd1a and/or Jmjd1a/GLP compound mutations may influence these modifications, we performed ChIP-qPCR analysis using purified E11.5 gonadal somatic cells. As shown in S4 Fig, neither Jmjd1a nor Jmjd1a/GLP compound mutations induced significant alterations of H3K4me3 and H3K9ac at the Sry locus. It is also known that CpG sequences of the linear promoter region of Sry are hypomethylated in embryonic gonads at the time of Sry expression [17]. To address whether Jmjd1a deficiency may influence DNA methylation of Sry promoter, we fractionated E11.5 gonadal somatic cells carrying the Nr5a1-hCD271 transgene into hCD271-high and -low populations by FACS and introduced them into bisulfite sequence analysis. In control gonads, the DNA methylation level of Sry promoter was significantly low in the hCD271-high population compared to that of the hCD271-low population in E11.5 embryonic gonads (S4 Fig). These results are in accordance with a previous study [13]. We next compared DNA methylation levels of the Sry promoter of the hCD271-high population between Jmjd1aΔ/+ and Jmjd1aΔ/Δ littermates. However, we could not find significant difference levels (S4 Fig). We therefore concluded that Jmjd1a mutation did not induce a significant alteration of DNA methylation at the Sry locus.
The GLP mutation rescues the reduced expression of Sry in Jmjd1a-deficient embryos
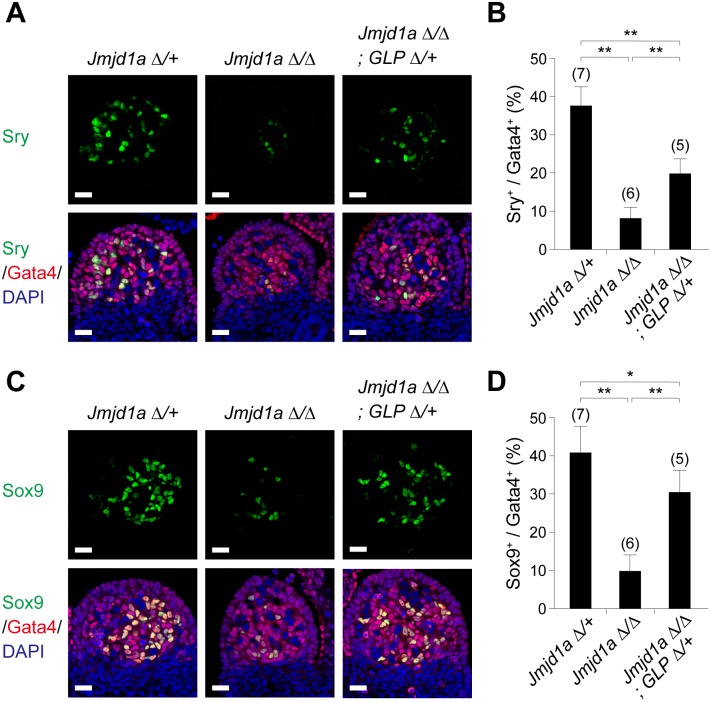
Sry activation is the first event in mammalian sex differentiation. In mice, sufficient and temporally accurate expression of Sry in sexually undifferentiated gonads at E11.5 is critical for triggering the testis-determining pathway [18]. To address whether the GLP mutation also rescues the perturbed expression of Sry in Jmjd1a-deficient gonads, we examined Sry expression by co-immunofluorescence analysis for Sry and Gata4, a marker of gonadal somatic cells. As shown in Fig 5A and 5B, the number of Sry-positive cells was reduced to approximately 25% in XY Jmjd1aΔ/Δ gonads at E11.5. The number of Sry-positive cells was significantly, although not completely, rescued in XY Jmjd1aΔ/Δ;GLPΔ/+ littermates, indicating that the GLP/G9a complex and Jmjd1a antagonistically tune Sry expression in E11.5 gonadal somatic cells. We also performed expression analysis of Sox9, a downstream target of Sry, in E11.5 gonads (Fig 5C and 5D). The number of Sox9-positive cells was also increased by the GLP mutation. Interestingly, the GLP mutation had a more profound effect on the increase of Sox9-positive cells compared with that of Sry-positive cells, presumably reflecting the activation of a non-cell-autonomous pathway of Sox9 expression [19].
Fig 5. The GLP mutation rescues the reduced expression of Sry in Jmjd1a-deficient embryos.
(A, C) Co-immunostaining profiles of Sry (A) and Sox9 (C) with a gonadal somatic cell marker, Gata4, in the center regions of E11.5 gonads of the indicated genotypes. Scale bar, 20 μm. (B, D) The ratios of cells positive for Sry (B) and Sox9 (D) to Gata4-positive cells in E11.5 gonads of the indicated genotypes. Numbers of examined animals are shown above the bars. All data are presented as mean ± SD. * P < 0.05; ** P < 0.01.
The GLP mutation rescues abnormal sex differentiation of XY Jmjd1a-deficient embryos
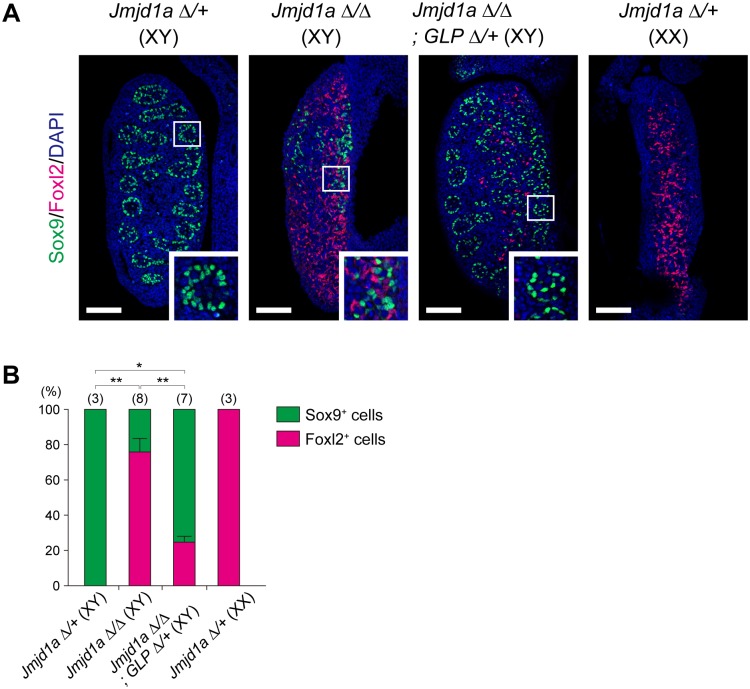
To examine embryonic gonadal cell differentiation just after sex determination, we investigated the expression of the testicular Sertoli cell marker Sox9 and the ovarian somatic cell marker Foxl2 in XY Jmjd1aΔ/Δ- and XY Jmjd1aΔ/Δ;GLPΔ/+ embryonic gonads at E13.5 (Fig 6A). Control XY gonads had Sox9-positive cells and did not contain Foxl2-positive cells. Furthermore, multiple tubule-like structures were found in control XY gonads. On the other hand, Jmjd1a-deficient XY gonads were ovotestes containing not only Sox9- but also Foxl2-positive cells and had no tubule-like structures. As shown in Fig 6A and 6B, the GLP mutation restored the number of Sox9-positive cells and testicular tubule formation, both of which were perturbed by Jmjd1a deficiency, indicating that the GLP mutation successfully rescued gonadal sex differentiation of Jmjd1a-deficient embryos.
Fig 6. The GLP mutation rescues abnormal sex differentiation of XY Jmjd1a-deficient embryos.
(A) Evaluation of sex differentiation of E13.5 embryonic gonads by immunofluorescence analysis. Sox9 and Foxl2 mark testicular Sertoli and ovarian somatic cells, respectively. The enlarged box indicates that the GLP mutation rescued the tubule-like structure that was absent in XY Jmjd1aΔ/Δ gonads. Scale bar, 200 μm. (B) Quantification of Sox9- and Foxl2-positive cells in E13.5 gonads of the indicated genitypes. Numbers of embryos examined are shown above the bars. Data are presented as mean ± SD. * P < 0.05; ** P < 0.01.
GLP and G9a form a heterodimer, which is essential for H3K9 methylation in vivo. We thus also performed epistatic analyses between G9a and Jmjd1a in mouse sex development. Notably, a G9a heterozygous mutation did not rescue the sex-reversal phenotype of XY Jmjd1a-deficient embryos (S5 Fig). Our previous studies indicated that GLP, but not G9a, is a limiting factor controlling the amount of GLP/G9a holoenzyme complex [10]. Consistent with this, we found that the GLP heterozygous mutation induced a significant reduction of G9a protein in embryonic gonads (S6 Fig). Thus, the decreased level of H3K9me2 associated with the GLP heterozygous mutation may be attributable to the reduction in the amount of the GLP/G9a complex.
We had previously established GLP-tg mice [20]. In this line, cDNA for Flag-tagged GLP was inserted in the Rosa26 locus and was designed to be expressed ubiquitously depending on an artificial CAG promoter [20]. To learn whether the overexpression of GLP affects sex determination, we compared the expression levels of Nr5a1, Sry, and Sox9 in gonads of GLP-tg XY embryos at E11.5. As shown in S7 Fig, although the amount of GLP transcript was actually elevated in XY GLP-tg gonads of E11.5 embryos, those of Nr5a1, Sry, and Sox9 transcripts were indistinguishable between XY control and XY GLP-tg gonads (S7 Fig). A possible explanation is that unidentified limiting factor(s) other than GLP may be required for the GLP/G9a complex to exert its function in the developing gonads.
The GLP mutation rescues XY sex reversal in Jmjd1a-deficient adult mice
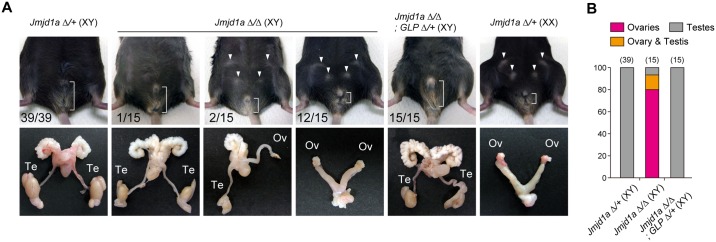
We finally verified the impact of the GLP mutation on the sex-reversal phenotype of Jmjd1a-deficient mice in adults. Consistent with our previous study [2], XY mice lacking Jmjd1a alone were frequently sex-reversed; for example, analysis of the external genitalia revealed that of 15 XY Jmjd1aΔ/Δ animals, one carried male-type genitalia, two carried intersex-type genitalia with a micropenis and well-developed mammary glands, and another carried typical female-type genitalia (Fig 7A). In addition, analysis of the internal genitalia demonstrated that one exhibited bilateral testes, two exhibited a testis and an ovary, and another had two ovaries. In contrast, all XY Jmjd1aΔ/Δ;GLPΔ/+ littermates exhibited male-type external genitalia with bilateral testes (Fig 7B). Taking these findings together, we conclude that the GLP mutation completely rescued the adult sex-reversal phenotype of Jmjd1a-deficient XY mice.
Fig 7. The GLP mutation rescues XY sex reversal in Jmjd1a-deficient adult mice.
(A) External genitalia (upper) and gonads and genital tracts (lower) of 3-months-old mice of the indicated genotypes. Arrowheads represent mammary glands. The distance between anus and penis or vagina is indicated. Frequencies are presented in the lower left corner. Te, testis; Ov, ovary. (B) Frequency analysis of abnormal sex differentiation of 3-months-old mice, determined by examining internal genitalia. Numbers of animals examined are shown above the bars.
Embryonic administration of the GLP/G9a inhibitor UNC0642 reverses aberrant sex development of Jmjd1a-deficient mice
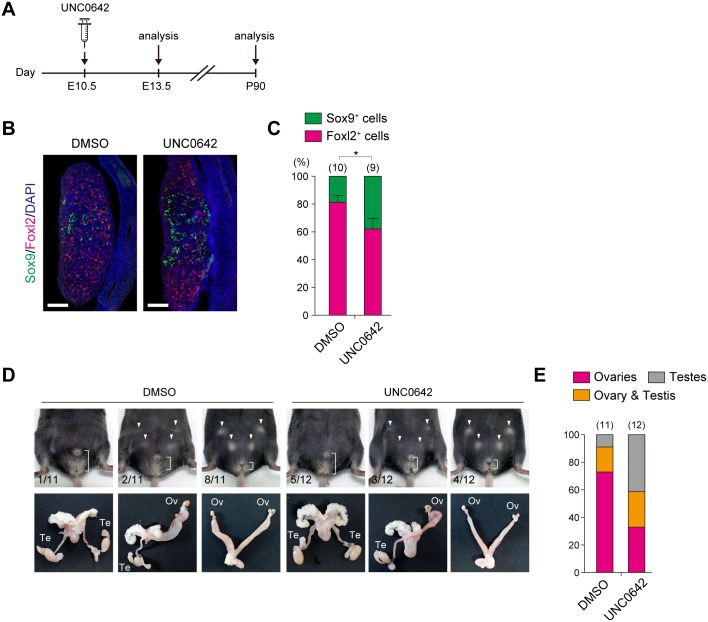
Our genetic experiments revealed that modulation of H3K9 methyltransferase activity of the GLP/G9a complex might be therapeutically effective for rescuing the aberrant sex development of Jmjd1a-deficient mice. We next aimed to rescue the phenotype in a different way using UNC0642, a chemical inhibitor of the GLP/G9a complex, which was recently developed by Liu et al. [21]. The experimental scheme of UNC0642 administration to Jmjd1a-deficient embryos is shown in Fig 8A. Briefly, 0.5 mg of UNC0642 was intraperitoneally injected once into pregnant females carrying E10.5 Jmjd1a-deficient embryos, and then the gonadal differentiation of the embryos was examined. As shown in Fig 8B and 8C, UNC0642 administration to Jmjd1a-deficient embryos resulted in a significant increase in the number of Sox9-positive male somatic cells at E13.5, while solvent only did not (compare with Fig 6B). This indicates that UNC0642 partially, but significantly, rescued the gonadal sex differentiation of Jmjd1a-deficient embryos after sex determination. We next investigated the impact of the embryonic administration of UNC0642 on the sex development of Jmjd1a-deficient mice by examining the external and internal genitalia of adult mice (Fig 8D). Although partially or completely sex-reversed mice were still found in the UNC0642-administered Jmjd1a-deficient mice, five out of 12 UNC0642-administered animals carried bilateral testes. In contrast, only one out of 11 animals in the solvent-injected control group exhibited bilateral testes (Fig 8D and 8E). Taking these findings together, we conclude that administration of UNC0642 into E10.5 embryos successfully rescued the subsequent sex development of Jmjd1a-deficient mice.
Fig 8. Embryonic administration of the GLP/G9a inhibitor UNC0642 rescues aberrant sex development of Jmjd1a-deficient mice.
(A) Experimental scheme of UNC0642 treatment. 0.5 mg of UNC0642 was intraperitoneally injected into pregnant females carrying E10.5 Jmjd1a-deficient embryos, and the subsequent gonadal differentiation of E13.5 embryos (B) and 3-months-old adults (D) was examined. (B) Immunofluorescence analysis of sex differentiation of E13.5 embryonic gonads using antibodies against Sox9 and Foxl2. Scale bar, 200 μm. (C) Quantification of Sox9- and Foxl2-positive cells in E13.5 gonads. Numbers of embryos examined are shown above the bars. Data are presented as mean ± SD. * P < 0.05. (D) External genitalia (upper) and gonads and genital tracts (lower) of UNC0642-treated (right) and solvent-treated (left) XY Jmjd1a-deficient animals. Arrowheads represent mammary glands. The distance between anus and penis or vagina is indicated. Frequencies are presented in the lower left corner. Te, testis; Ov, ovary. (E) Frequency analysis of abnormal sex differentiation of 3-months-old mice, determined by examining the internal genitalia. Numbers of animals examined are shown above the bars.
Discussion
Here, we identified GLP/G9a H3K9 methyltransferase complex as an enzyme counteracting Jmjd1a-mediated H3K9 demethylation at the Sry locus in gonadal somatic cells. To our knowledge, this is the first study to identify the set of histone methyltransferase and demethylase that in combination account for stage- and cell-type-specific gene regulation in mammalian development.
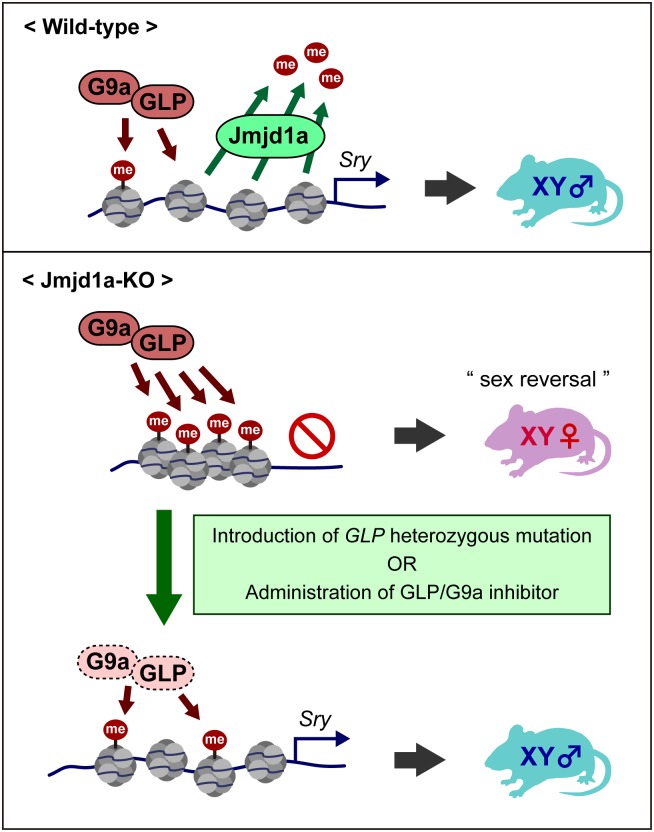
Our data show that the molecular balance of the GLP/G9a complex and Jmjd1a is a critical factor for the tuning of Sry expression (Fig 9). We previously showed that G9a and GLP are expressed in almost all adult tissues in mice [9, 10]. On the other hand, previous studies demonstrated that Jmjd1a is expressed in a tissue- and developmental stage-specific manner [22–24]. Considering that the expression of Sry is suppressed in almost all adult tissues in mice, this Sry silencing might be explained, at least in part, by the robust H3K9 methylation of the GLP/G9a complex and the absence of H3K9 demethylase in these tissues. We previously demonstrated the temporally specific expression of Jmjd1a in embryonic gonadal somatic cells, which reaches a plateau around E11.5 [2]. In addition, we demonstrated the cell-type-specific expression of Jmjd1a in this study, as Jmjd1a transcript was substantially enriched in the Nr5a1-high population of E11.5 embryonic gonads (Fig 1C). High levels of Jmjd1a expression may overcome GLP/G9a complex-mediated H3K9 methylation, thereby inducing Sry expression in the pre-Sertoli cells.
Fig 9. Fine-tuning of Sry expression is achieved by the balance in activities between H3K9 demethylase Jmjd1a and H3K9 methyltransferase GLP/G9a complex.
In wild-type embryonic gonads, Jmjd1a removes H3K9 methylation marks, which were deposited by GLP/G9a complex, from the Sry locus, thereby ensuring Sry activation. In Jmjd1a-deficient embryonic gonads, the absence of Jmjd1a results in the GLP/G9a complex-mediated H3K9 hypermethylation at the Sry locus, thereby compromising Sry expression and causing male-to-female sex reversal. Normalization of the H3K9 methylation balance of the Sry locus by a genetic or a pharmacological approach rescues the aberrant sex development of Jmjd1a-deficient mice by restoring Sry expression.
Although the GLP mutation significantly rescued the perturbed Sry expression in Jmjd1a-deficient embryonic gonads, the Sry-positive cell population in Jmjd1aΔ/Δ;GLPΔ/+ gonads remained approximately half of that detected in control gonads (Fig 5B). Therefore, it is a surprising finding that the GLP mutation completely rescued the sex reversal of Jmjd1a-deficient XY mice in adults (Fig 7). The GLP mutation reduces global H3K9me2 levels of Jmjd1a-deficient gonadal somatic cells (Fig 2). Thus, it is also possible that GLP/G9a complex has a role in the sex differentiation pathway, independent of Sry regulation. In this regard, the GLP mutation may inhibit the ovarian development pathway in Jmjd1a-deficient gonads. Although we could not rule out this possibility, it seems likely that the restored Sry expression is a primary cause for the rescue of the sex reversal of Jmjd1a-deficient XY mice. Because there is a certain threshold level for Sry expression to induce the male pathway [18], it is conceivable that Sry expression substantially exceeds the threshold level as a result of the GLP mutation in the Jmjd1a-deficient background, thereby conferring profound effects on the subsequent male pathway.
Eset is known as an enzyme responsible for tri-methylation of H3K9 [6]. A previous study demonstrated that Eset is expressed in embryonic gonads [25]. Accordingly, we also confirmed the expression of Eset in E11.5 XY gonadal somatic cells by RT-qPCR analysis [2]. We introduced the Eset heterozygous mutation into the Jmjd1a-mutant background, and then the sex development of XY Jmjd1aΔ/Δ; EsetΔ/+ embryos was examined (S8 Fig). Consequently, we could not find any profound effect of the Eset mutation on the perturbed sex development of Jmjd1a-deficient mice (S8 Fig). Our previous study indicated that the H3K9me3 level of Sry was unchanged by Jmjd1a deficiency in E11.5 embryonic gonads. Altogether, it seems likely that Jmjd1a-mediated H3K9 demethylation does not counteract Eset-mediated H3K9 tri-methylation, at least in the Sry locus.
Previous studies revealed the enrichment of H3K4me3/H3K9ac and that low levels of CpG methylation are characteristic in the Sry promoter region of gonadal somatic cells during the sex-determining period [17] [13]. In this study, we demonstrated that Jmjd1a deficiency and/or the increased level of H3K9me2 did not affect H3K4me3/H3K9ac and CpG methylation levels of the Sry locus (S4 Fig). On the other hand, it seems likely that H3K9me2 and H3K4me2 marks are exclusively deposited mutually and these marks exert antagonistic functions for transcription, at least in the Sry locus. Jmjd1a deficiency and/or the increased levels of H3K9me2 resulted in the decrease of H3K4me2 of this locus, concomitantly with the suppression of Sry [2], also shown in Fig 3. The fact that Jmjd1a deficiency result in the loss of H3K4me2, but not H3K4me3, in the Sry locus warrants further discussion. It is one possible explanation that Jmjd1a or Jmjd1a-mediated H3K9 hypomethylation may prevent the accession of specific enzyme(s) responsible for H3K4me2 demethylation.
We have demonstrated that just a single administration of GLP/G9a inhibitor to E10.5 embryos significantly rescues the aberrant sex development of Jmjd1a-deficient mice. Mutation, silencing, or downregulation of histone methylation “erasers” was found in several types of cancer [26]. Our experiments suggest a new therapeutic strategy, in which diseases arising from the dysfunction of an epigenetic “eraser” can be rescued by blocking the activity of the counteracting epigenetic “writer.”
Materials and methods
Ethics statement
Animal experiments were performed under the animal ethical guidelines of Tokushima University and Kyoto University. The Ethics Committee of Tokushima University for Animal Research (Approval number: 14108) and the Animal Experimentation Committee of Kyoto University (Approval number: A12-6-2) approved this study.
Animals
Mouse lines of GLPΔ/+, G9aΔ/+, Jmjd1aΔ/+, and Nr5a1-hCD271-tg were sequentially backcrossed to C57BL/6J, and then the F5 or later generation was used. Since the sex reversal frequencies of Jmjd1a-deficient mice were dependent on the origin of the Y chromosome [2], we used mice carrying a Y chromosome of CBA origin in this study. However, we only used mice carrying a Y chromosome of B6 origin in the experiments shown in S5 Fig.
Antibodies
Guinea-pig polyclonal antibodies against Sry were generated by the immunization of bacterially expressed 6xHis-tagged Sry (residues 82–395, NP_035694). Additional antibodies used in this study were as follows: goat anti-Gata4 (Santa Cruz, C-20), rabbit anti-Sox9 (Millipore, AB5535), goat anti-Foxl2 (Abcam, ab-5096), mouse anti-LNGFR (Miltenyi Biotec), rabbit anti-Jmjd1a [2], mouse anti-G9a (Perseus Proteomics, 8620A), mouse anti-GLP (Perseus Proteomics, B0422), rabbit anti-G9a (CST, #3306), mouse anti-H3K9me2 [27], mouse anti-H3K4me2 [27], mouse anti-H3K4me3 [27], mouse anti-H3K9ac [27], and rabbit anti-Nr5a1 (a gift from Dr. K. Morohashi).
Histology and immunohistochemistry
Tissues were fixed in either Bouin’s solution or 4% paraformaldehyde, embedded in paraffin, and cut into 4-μm sections. For histological analysis, sections were stained with hematoxylin/eosin or hematoxylin/PAS. For immunohistochemistry, sections were deparaffinized and rehydrated, and then autocleaved at 105°C for 5 min in 10 mM citric acid buffer (pH 6.0). To quench endogenous peroxidase, the sections were treated with 0.3% (v/v) hydrogen peroxide. After blocking with TBS containing 2% skim milk and 0.1% Triton-X100 at room temperature for 1 h, sections were incubated with primary antibodies overnight at 4°C. For fluorescence staining, the sections were incubated with Alexa-conjugated secondary antibodies (Life Technologies) at room temperature for 1 h and counterstained with DAPI. The sections were mounted in Vectashield (Vector) and observed with a confocal laser scanning microscope (LSM700, Carl Zeiss).
Flow cytometry and cell sorting
Isolated gonads and mesonephroi from E11.5 embryos were digested with Accutase (Nacalai) to obtain a single cell suspension. For flow cytometric analysis, cells were fixed with 2% paraformaldehyde (PFA) in PBS for 10 min, permeabilized with ice-cold ethanol for 20 min, and blocked with 0.5% skim milk in PBS for 1 h. They were then stained with primary antibodies overnight at 4°C and subsequently incubated with Alexa-conjugated secondary antibodies (Life Technologies) for 1 h at room temperature. Data were collected using FACSCanto 2 (BD Bioscience) and analyzed with FlowJo software (TreeStar). For FACS sorting, cells were stained with FITC-labeled anti-LNGFR and sorted based on fluorescence intensity using FACS Aria 2 (BD Bioscience) as shown in S2 Fig.
ChIP analysis
The experimental scheme for ChIP analysis is shown in S3 Fig. Briefly, two-cell embryos were prepared by in vitro fertilization using sperm derived from Jmjd1aΔ/+;GLPΔ/+;Nr5a1-hCD271-tg males and eggs derived from Jmjd1aΔ/+ females, and then transferred to pseudopregnant recipients. Gonadal somatic cells were purified from embryos that had developed to tail somite stage 17–19, as described previously [2, 14]. For native ChIP analysis of histone modifications, purified cells were pooled (n = 2–4 per genotype) and subjected to ChIP analysis following a protocol described previously [15], with minor modifications. Briefly, cells were suspended in 5 μl of 0.3 M sucrose-containing buffer 1 (60 mM KCl, 15 mM NaCl, 5 mM MgCl2, 0.1 mM EGTA, 0.5 mM dithiothreitol, 0.1 mM PMSF, 3.6 ng/ml aprotinin, 15 mM Tris–HCl, pH 7.5) and lysed by the addition of 5 μl of 0.3 M sucrose-containing buffer 1 with 0.8% NP40 on ice for 10 min. After the addition of 80 μl of 1.2 M sucrose-containing buffer 1, the chromatin fraction was collected as pellets by centrifugation. These pellets were digested with micrococcal nuclease (MNase) (0.02–0.05 U, Takara) in 10 μl of MNase digestion buffer (0.32 M sucrose, 4 mM MgCl2, 1 mM CaCl2, 0.1 mM PMSF, 50 mM Tris–HCl, pH 7.5), using Thermo Mixer (Eppendorf) at 37°C and 1000 rpm for 15 min, and then digestion was stopped with EDTA. Supernatant was obtained by centrifugation and incubated with anti-H3K9me2-, anti-H3K9ac-, anti-H3K4me2-, or anti-H3K4me3-coated magnetic beads (Dynabeads Protein G, Invitrogen) in 50 μl of incubation buffer (50 mM NaCl, 5 mM EDTA, 0.1% NP40, 0.1 mM PMSF, 20 mM Tris–HCl, pH 7.5), at 4°C for 2 h. DNA was extracted from the immune complexes according to the standard protocol and then analyzed by real-time PCR using primers specific for Y chromosome genes (Sry, Uty, Ddx3y, Usp9y, and Zfy2). For cross-link ChIP analysis of G9a, purified gonadal somatic cells from 20 embryos (approximately 8 × 105 cells) were pooled and combined with 5 × 106 cells of female mouse embryonic fibroblasts, cross-linked with 25 mM DSG (Thermo Fisher Scientific) and 1% formaldehyde, and applied to ChIP analysis with rabbit anti-G9a antibody following a protocol described previously [2].
Bisulfite sequencing analysis
Genomic DNA was isolated using the All DNA/RNA Micro kit (QIAGEN). Genomic DNA was treated with sodium bisulfite using the MethylEasy Xceed Rapid DNA Bisulfite Modification Kit (Human Genetic Signatures) following the manufacturer’s instructions. The bisulfite-treated DNA was PCR-amplified using the primer pair 5′-TTTATATTGGGTTATAGAGTTAGAATAGAT-3′ and 5′-CCAAAATATACTTATAACAAAAATTTTAAT-3′. PCR products were subcloned into the pGEM-T Easy vector (Promega) and sequenced.
Primers
The primer sets used in ChIP-qPCR analysis were as follows: Sry linear prom.-f (5′-TGGTCAGTGGCTTTTAGCTCT-3′) and Sry linear prom.-r (5′-AGATGTGATGCAAAGAGAAACA-3′) for Sry, Npas4 ChIP F (5′-CTATGGCCATTTCAGCACCG-3′) and Npas4 ChIP R (5′-AGCTGTTCGACGTCCTGAAG-3′) for Naps4, Gapdh ChIP F (5′-TTGCTTAGGCCTTCCTTCTTC-3′) and Gapdh ChIP R (5′-CATCACCTGGCCTACAGGATA-3′) for Gapdh, ChIP-Uty-F (5′-CCTTTGTGAGGGACTGTTCA-3′) and ChIP-Uty-R (5′-CCACTCAACCACATCAAACC-3′) for Uty, ChIP-Ddx3y-F (5′-ACAATTCCACAACCCAAGGT-3′) and ChIP-Ddx3y-R (5′-AGGTTTCAGCCCACTCATTT-3′) for Ddx3y, ChIP-Usp9y-F (5′-AAGGGACACACAGTTCTCCA-3′) and ChIP-Usp9y-R (5′- CTTGTGAGAAGGGACTGAGG-3′) for Usp9y, ChIP-Zfy2-F (5′- AGGCAGTCTTAGATGCGAAA-3′) and ChIP-Zfy2-R (5′- TCCTGACTCACAACAACAGC-3′) for Zfy2. The primer sets used in RT-qPCR analysis were as follows: Gapdh RT-PCR F (5′-ATGAATACGGCTACAGCAACAGG-3′) and Gapdh RT-PCR R (5′-CTCTTGCTCAGTGTCCTTGCTG-3′) for Gapdh, Ad4BP-e2-F (5′-TTGTCGACTGGGCACGAAGGTGCAT-3′) and Ad4BP-e2-R (5′-GCAGCTCGCTCCAACAGTTCTGCAG-3′) for Nr5a1, Sry-5-SD (5′-TACCTACTTACTAACAGCTGACATCAC-3′) and Sry-3-SD (5′-TGTCATGAGACTGCCAACCACAGGG-3′) for Sry, TSGA-EX 21F (5′-ACTCCAGAGGATCGGAAATATGGGACC-3′) and TSGA-EX 21R (5′-GGGAATTCCCACATAAACCATGACATTGGC-3′) for Jmjd1a, GLP-RT-1B (5′-AACCCAACCTTGTGCCTGTGCGAG-3′) and GLP-RT-2 (5′-CGAGCTGCTCCCCAGCCTGAATCAG-3′) for GLP, G9a-RT-1B (5′-ACCCCAACATCATCCCTGTCCGGG-3′) and G9a-RT-2 (5′-GTCCCAGAATCGGTCACCGTAGTC-3′) for G9a, Sox9-RT-F (5′-AGGAAGCTGGCAGACCAGTA-3′) and Sox9-RT-R (5′-CGTTCTTCACCGACTTCCTC-3′) for Sox9, Uty-RT-F (5′-AAGGCGCTTTGTGGATTAGA-3′) and Uty-RT-R (5′-CTGATTCCACTTTTCCTTCAGC-3′) for Uty, Ddx3y-RT-F (5′- TTGGTCTTGACCTGAAATCATCA-3′) and Ddx3y-RT-R (5′- GCTTCCCTCTGGAATCACGA-3′) for Ddx3y, Usp9y-RT-F (5′- CTTGGTCCCAAATTGCAAGC-3′) and Usp9y-RT-R (5′- TCGGATGGCTTCTTGTCTTG-3′) for Usp9y, Zfy2-Rt-F (5′- GCTTAAGACCTCCAGCAAAAG-3′) and Zfy2-Rt-R (5′- CCGGTCTCTGGCTTTAATGT-3′) for Zfy2. The primer sets used for genotyping were as follows: GLP-6570F (5′-CTGTCCAGTTCCCGATTTTCAAGACTGC-3′) and GLP-5936R (5′-GTCCCACTGGCCACACTGGCAATTC-3′) for detection of the GLPΔ allele; TSGA-G1475R (5′-GAACTGCACCATTAGCTGTCACTTCC-3′), TSGA-1980F (5′-CATGCAGTGAAAGATGCAGTTGCTA-3′), and TSGA-6410F-NheSac (5′-CTAAATATCAAGGCTAGCGAGCTCG-3′) for detection of the Jmjd1aΔ allele; Sf1-1741F (5′-CACAGACCAGGGCAATCCCAAGCCA-3′) and pMACS-LI 2264R (5′-GTCGGAGAACGTCACGCTGTCCAG-3′) for Nr5a1-hCD271-tg; Rbmy1a1-F (5′-AATATGCCAAGAGGAGAGCCGGCGTCTTCC-3′) and Rbmy1a1-R2 (intron) (5′-CCAAGTTGTTGTGGCATTTGGACATC-3′) for detection of the Y chromosome; and GE28R (5′-GCTCCAGGGCGATGGCCTCCGCTGAATGC-3′), GI27-2F (5′-CGGGACAGGGTTTCTCTGTGTAGTCC-3′), and GI-25F (5′-CTGCACGCTGCCTAGATGGAGCATG-3′) for detection of the G9aΔ allele.
GLP/G9a inhibitor UNC0642
Pregnant females at E10.5 were administered 0.5 mg of UNC0642 (Tocris) dissolved in 30 μl of DMSO and mixed with 17.5 μl of ethanol, 52.5 μl of castor oil, and 100 μl of PBS.
Generation of Eset-mutant mice using a CRISPR/Cas9 system
Eset-mutant mice were produced by electroporating Cas9 mRNA and gRNA into mouse zygotes according to a protocol published recently [28]. Briefly, 400 ng/μl Cas9 mRNA and 100 ng/μl of each gRNA targeting the genomic sequences of Eset (shown in S8 Fig) were introduced into zygotes (C57BL/6J × C57BL/6J) by electroporation using Genome Editor GEB15 (BEX, Tokyo, Japan). The electroporation conditions were four pulses of 30 V (3 ms ON + 97 ms OFF). The surviving two-cell-stage embryos were transferred to the oviducts of pseudopregnant females. Genotyping of the generated mice was performed using the primer pair 5′-CCCTGGCTGTCCTAGAACTCAC-3′ and 5′-AGGGTTCATTCAGGCTACAAAG-3′.
Statistics
One-way analysis of variance (one-way ANOVA) and Tukey’s honestly significant difference test were used for statistical analysis.
Supporting information
Embryonic gonads at E11.5 were immunostained with antibodies against H3K9me2 (A) or H3K9me3 (B). Gonadal somatic cells were marked with anti-Gata4 antibodies. G, gonads; M, mesonephroi. Scale bar, 50 μm.
(PDF)
(PDF)
(A) Schematic illustration of the purification of gonadal somatic cells for ChIP analysis. Two-cell embryos were prepared by in vitro fertilization using sperm derived from Jmjd1aΔ/+;GLP Δ/+;Nr5a1-hCD271-tg males and oocytes derived from Jmjd1aΔ/+ females and were then transferred to pseudopregnant recipients. After in utero development, the embryos were collected at tail somite stages 17–19. After genotyping analysis, gonadal somatic cells were labeled with anti-hCD271 antibody and then purified through affinity columns. Cells corresponding to two to four embryos of each genotype were pooled and then subjected to ChIP analysis. (B) Numbers of purified gonadal somatic cells at tail somite stages 17–19 of the indicated genotypes. The numbers of gonadal somatic cells were consistent regardless of the genotypes. Numbers of examined embryos are shown above the bars.
(PDF)
(A) Gonadal somatic cells of the indicated genotypes were purified according to the method described in S3 Fig, pooled for each genotype (2 to 4 embryos), and then subjected to ChIP-qPCR analyses for H3K4me3 (left) and H3K9ac (right). There was no significant difference of the modification levels between control and mutant gonads. (B) DNA methylation levels of the linear promoter region of Sry were quantified by bisulfite sequence analysis. hCD271-tagged gonadal somatic cells were fractionated into hCD271-high (Nr5a1-high) and hCD271-low (Nr5a1-low) populations as shown in S2 Fig. In control gonads, Sry-expressing cells were enriched predominantly in the hCD271-high population (Fig 1E). Analyzed CpG sequences of the Sry promoter region are presented at the top. The CpG positions are indicated relative to the start codon. (C) Summary of CpG methylation levels of the Sry promoter region. In a comparison of the DNA methylation levels in hCD271-high populations, we could not find significant levels for the difference between Jmjd1aΔ/+ and Jmjd1aΔ/Δ littermates. P values were obtained using the Mann–Whitney U-test.
(PDF)
(A) Immunofluorescence analysis with antibodies against Sox9 and Foxl2 on E13.5 embryonic gonadal sections of the indicated genotypes. Scale bar, 50 μm. (B) Quantification of Sox9- and Foxl2-positive cells in E13.5 gonads. Numbers of examined embryos are shown above the bars. Data are presented as mean ± SD. ** P < 0.01; n.s., not significant.
(PDF)
Gonads/mesonephroi of E11.5 XY embryos were stained with antibodies against GLP (A) and G9a (B), in combination with anti-Nr5a1 antibodies. (left) Representative data of flow-cytometric dot-blot analysis of the indicated proteins. (right) Plots of median fluorescence intensity (MFI) values for the indicated proteins in Nr5a1-positive gonadal somatic cells. *** P < 0.001.
(PDF)
We had previously established GLP-tg mice that carry an extra copy of GLP cDNA in the Rosa26 locus [20]. In this line, the exogenous GLP cDNA is expressed ubiquitously by CAG promoter. Although GLP mRNA was actually overexpressed in the gonads of XY GLP-tg embryos at E11.5, mRNA levels of Nr5a1, Sry and Sox9 were not affected.
(PDF)
(A) Comparison of Eset genomic sequences between wild-type and mutant alleles, generated by genome editing with the CRISPR/Cas9 system. We intended to disrupt the exon8 encoding TUDOR domain of Eset. Two guide RNAs (gRNAs), corresponding to a sequence within intron 7 and a sequence nearly at the 3’ end of exon 8, were introduced with Cas9 mRNA into fertilized eggs of C57BL/6 mice. Dashes represent deleted sequences in the mutant allele. Capital and lower-case letters represent exonic and intronic sequences, respectively. (B) Genotyping for the Eset mutant allele by PCR. Location of the primers is indicated in (A). (C) Phenotype analysis of the Eset-mutant mice established in this study. No Eset homozygous mutant embryos were found among 34 embryos derived from the mating of Eset heterozygous mutant mice, indicating Eset homozygous mutant embryos died and were absorbed by E13.5. (D) Evaluation of the gonadal sex differentiation of E13.5 XY Jmjd1aΔ/Δ; EsetΔ/+ embryos by immunofluorescence analysis for Sox9 and Foxl2. (E) The ratios of Sox9- and Foxl2-positive cells of the indicated genotypes are summarized. Eset heterozygous mutation did not affect the sex development of Jmjd1a-deficient mice. Numbers of embryos examined are shown above the bars. Data are presented as mean ± SD. *** P < 0.001; n.s., not significant.
(PDF)
Acknowledgments
We are grateful to Toru Nakano and Peter Koopman for critical advice on the manuscript. We thank Hiroshi Kimura and Ken-ichirou Morohashi for providing antibodies against modified histones and Nr5a1, respectively. We also thank Enago for the English language review. We are especially grateful to the members of the Tachibana laboratory for technical support.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by KAKENHI from the Japan Society for the Promotion of Science (https://www.jsps.go.jp/) Grant Numbers 26250037 (MT), 16H01218 (MT), 16H01409 (MT), 17H06424 (MT), 16K21196 (SKu), and 16K18492 (NO); Funding Program for Next Generation World-Leading Researchers (http://www.cao.go.jp/) (MT); Takeda Science Foundation (http://www.takeda-sci.or.jp/) (MT); the NOVARTIS Foundation (http://japanfoundation.novartis.org/) (SKu); and a Promotion of Science Cooperative Research Grant of the Institute for Enzyme Research, Joint Usage/Research Center, Tokushima University (HM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Greer EL, Shi Y. Histone methylation: a dynamic mark in health, disease and inheritance. Nature reviews Genetics. 2012;13(5):343–57. Epub 2012/04/05. doi: 10.1038/nrg3173 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuroki S, Matoba S, Akiyoshi M, Matsumura Y, Miyachi H, Mise N, et al. Epigenetic regulation of mouse sex determination by the histone demethylase Jmjd1a. Science. 2013;341(6150):1106–9. Epub 2013/09/07. doi: 10.1126/science.1239864 . [DOI] [PubMed] [Google Scholar]
- 3.Arrowsmith CH, Bountra C, Fish PV, Lee K, Schapira M. Epigenetic protein families: a new frontier for drug discovery. Nat Rev Drug Discov. 2012;11(5):384–400. doi: 10.1038/nrd3674 . [DOI] [PubMed] [Google Scholar]
- 4.Rea S, Eisenhaber F, O'Carroll D, Strahl BD, Sun ZW, Schmid M, et al. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature. 2000;406(6796):593–9. doi: 10.1038/35020506 . [DOI] [PubMed] [Google Scholar]
- 5.O'Carroll D, Scherthan H, Peters AH, Opravil S, Haynes AR, Laible G, et al. Isolation and characterization of Suv39h2, a second histone H3 methyltransferase gene that displays testis-specific expression. Mol Cell Biol. 2000;20(24):9423–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang L, Xia L, Wu DY, Wang H, Chansky HA, Schubach WH, et al. Molecular cloning of ESET, a novel histone H3-specific methyltransferase that interacts with ERG transcription factor. Oncogene. 2002;21(1):148–52. doi: 10.1038/sj.onc.1204998 . [DOI] [PubMed] [Google Scholar]
- 7.Tachibana M, Sugimoto K, Fukushima T, Shinkai Y. Set domain-containing protein, G9a, is a novel lysine-preferring mammalian histone methyltransferase with hyperactivity and specific selectivity to lysines 9 and 27 of histone H3. J Biol Chem. 2001;276(27):25309–17. Epub 2001/04/24. doi: 10.1074/jbc.M101914200 . [DOI] [PubMed] [Google Scholar]
- 8.Ogawa H, Ishiguro K, Gaubatz S, Livingston DM, Nakatani Y. A complex with chromatin modifiers that occupies E2F- and Myc-responsive genes in G0 cells. Science. 2002;296(5570):1132–6. doi: 10.1126/science.1069861 . [DOI] [PubMed] [Google Scholar]
- 9.Tachibana M, Sugimoto K, Nozaki M, Ueda J, Ohta T, Ohki M, et al. G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev. 2002;16(14):1779–91. Epub 2002/07/20. doi: 10.1101/gad.989402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tachibana M, Ueda J, Fukuda M, Takeda N, Ohta T, Iwanari H, et al. Histone methyltransferases G9a and GLP form heteromeric complexes and are both crucial for methylation of euchromatin at H3-K9. Genes Dev. 2005;19(7):815–26. Epub 2005/03/19. doi: 10.1101/gad.1284005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ueda J, Tachibana M, Ikura T, Shinkai Y. Zinc finger protein Wiz links G9a/GLP histone methyltransferases to the co-repressor molecule CtBP. J Biol Chem. 2006;281(29):20120–8. Epub 2006/05/17. doi: 10.1074/jbc.M603087200 . [DOI] [PubMed] [Google Scholar]
- 12.Morohashi K, Honda S, Inomata Y, Handa H, Omura T. A common trans-acting factor, Ad4-binding protein, to the promoters of steroidogenic P-450s. J Biol Chem. 1992;267(25):17913–9. Epub 1992/09/05. . [PubMed] [Google Scholar]
- 13.Gierl MS, Gruhn WH, von Seggern A, Maltry N, Niehrs C. GADD45G functions in male sex determination by promoting p38 signaling and Sry expression. Dev Cell. 2012;23(5):1032–42. Epub 2012/10/30. doi: 10.1016/j.devcel.2012.09.014 . [DOI] [PubMed] [Google Scholar]
- 14.Kuroki S, Akiyoshi M, Ideguchi K, Kitano S, Miyachi H, Hirose M, et al. Development of a general-purpose method for cell purification using Cre/loxP-mediated recombination. Genesis. 2015;53(6):387–93. doi: 10.1002/dvg.22863 . [DOI] [PubMed] [Google Scholar]
- 15.Tachibana M, Matsumura Y, Fukuda M, Kimura H, Shinkai Y. G9a/GLP complexes independently mediate H3K9 and DNA methylation to silence transcription. The EMBO journal. 2008;27(20):2681–90. Epub 2008/09/27. doi: 10.1038/emboj.2008.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mozzetta C, Pontis J, Fritsch L, Robin P, Portoso M, Proux C, et al. The histone H3 lysine 9 methyltransferases G9a and GLP regulate polycomb repressive complex 2-mediated gene silencing. Mol Cell. 2014;53(2):277–89. doi: 10.1016/j.molcel.2013.12.005 . [DOI] [PubMed] [Google Scholar]
- 17.Nishino K, Hattori N, Tanaka S, Shiota K. DNA methylation-mediated control of Sry gene expression in mouse gonadal development. The Journal of biological chemistry. 2004;279(21):22306–13. Epub 2004/02/24. doi: 10.1074/jbc.M309513200 . [DOI] [PubMed] [Google Scholar]
- 18.Kashimada K, Koopman P. Sry: the master switch in mammalian sex determination. Development. 2010;137(23):3921–30. Epub 2010/11/11. doi: 10.1242/dev.048983 . [DOI] [PubMed] [Google Scholar]
- 19.Wilhelm D, Martinson F, Bradford S, Wilson MJ, Combes AN, Beverdam A, et al. Sertoli cell differentiation is induced both cell-autonomously and through prostaglandin signaling during mammalian sex determination. Dev Biol. 2005;287(1):111–24. Epub 2005/09/28. doi: 10.1016/j.ydbio.2005.08.039 . [DOI] [PubMed] [Google Scholar]
- 20.Deguchi K, Nagamatsu G, Miyachi H, Kato Y, Morita S, Kimura H, et al. Posttranscriptional Regulation of Histone Lysine Methyltransferase GLP in Embryonic Male Mouse Germ Cells. Biology of reproduction. 2013;88(2):36 Epub 2013/01/04. doi: 10.1095/biolreprod.112.103572 . [DOI] [PubMed] [Google Scholar]
- 21.Liu F, Barsyte-Lovejoy D, Li F, Xiong Y, Korboukh V, Huang XP, et al. Discovery of an in vivo chemical probe of the lysine methyltransferases G9a and GLP. J Med Chem. 2013;56(21):8931–42. doi: 10.1021/jm401480r [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoog C, Schalling M, Grunder-Brundell E, Daneholt B. Analysis of a murine male germ cell-specific transcript that encodes a putative zinc finger protein. Molecular reproduction and development. 1991;30(3):173–81. Epub 1991/11/01. doi: 10.1002/mrd.1080300302 . [DOI] [PubMed] [Google Scholar]
- 23.Okada Y, Scott G, Ray MK, Mishina Y, Zhang Y. Histone demethylase JHDM2A is critical for Tnp1 and Prm1 transcription and spermatogenesis. Nature. 2007;450(7166):119–23. Epub 2007/10/19. doi: 10.1038/nature06236 . [DOI] [PubMed] [Google Scholar]
- 24.Tachibana M, Nozaki M, Takeda N, Shinkai Y. Functional dynamics of H3K9 methylation during meiotic prophase progression. The EMBO journal. 2007;26(14):3346–59. Epub 2007/06/30. doi: 10.1038/sj.emboj.7601767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jameson SA, Natarajan A, Cool J, DeFalco T, Maatouk DM, Mork L, et al. Temporal transcriptional profiling of somatic and germ cells reveals biased lineage priming of sexual fate in the fetal mouse gonad. PLoS Genet. 2012;8(3):e1002575 doi: 10.1371/journal.pgen.1002575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hojfeldt JW, Agger K, Helin K. Histone lysine demethylases as targets for anticancer therapy. Nat Rev Drug Discov. 2013;12(12):917–30. doi: 10.1038/nrd4154 . [DOI] [PubMed] [Google Scholar]
- 27.Kimura H, Hayashi-Takanaka Y, Goto Y, Takizawa N, Nozaki N. The organization of histone H3 modifications as revealed by a panel of specific monoclonal antibodies. Cell Struct Funct. 2008;33(1):61–73. . [DOI] [PubMed] [Google Scholar]
- 28.Hashimoto M, Takemoto T. Electroporation enables the efficient mRNA delivery into the mouse zygotes and facilitates CRISPR/Cas9-based genome editing. Sci Rep. 2015;5:11315 doi: 10.1038/srep11315 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Embryonic gonads at E11.5 were immunostained with antibodies against H3K9me2 (A) or H3K9me3 (B). Gonadal somatic cells were marked with anti-Gata4 antibodies. G, gonads; M, mesonephroi. Scale bar, 50 μm.
(PDF)
(PDF)
(A) Schematic illustration of the purification of gonadal somatic cells for ChIP analysis. Two-cell embryos were prepared by in vitro fertilization using sperm derived from Jmjd1aΔ/+;GLP Δ/+;Nr5a1-hCD271-tg males and oocytes derived from Jmjd1aΔ/+ females and were then transferred to pseudopregnant recipients. After in utero development, the embryos were collected at tail somite stages 17–19. After genotyping analysis, gonadal somatic cells were labeled with anti-hCD271 antibody and then purified through affinity columns. Cells corresponding to two to four embryos of each genotype were pooled and then subjected to ChIP analysis. (B) Numbers of purified gonadal somatic cells at tail somite stages 17–19 of the indicated genotypes. The numbers of gonadal somatic cells were consistent regardless of the genotypes. Numbers of examined embryos are shown above the bars.
(PDF)
(A) Gonadal somatic cells of the indicated genotypes were purified according to the method described in S3 Fig, pooled for each genotype (2 to 4 embryos), and then subjected to ChIP-qPCR analyses for H3K4me3 (left) and H3K9ac (right). There was no significant difference of the modification levels between control and mutant gonads. (B) DNA methylation levels of the linear promoter region of Sry were quantified by bisulfite sequence analysis. hCD271-tagged gonadal somatic cells were fractionated into hCD271-high (Nr5a1-high) and hCD271-low (Nr5a1-low) populations as shown in S2 Fig. In control gonads, Sry-expressing cells were enriched predominantly in the hCD271-high population (Fig 1E). Analyzed CpG sequences of the Sry promoter region are presented at the top. The CpG positions are indicated relative to the start codon. (C) Summary of CpG methylation levels of the Sry promoter region. In a comparison of the DNA methylation levels in hCD271-high populations, we could not find significant levels for the difference between Jmjd1aΔ/+ and Jmjd1aΔ/Δ littermates. P values were obtained using the Mann–Whitney U-test.
(PDF)
(A) Immunofluorescence analysis with antibodies against Sox9 and Foxl2 on E13.5 embryonic gonadal sections of the indicated genotypes. Scale bar, 50 μm. (B) Quantification of Sox9- and Foxl2-positive cells in E13.5 gonads. Numbers of examined embryos are shown above the bars. Data are presented as mean ± SD. ** P < 0.01; n.s., not significant.
(PDF)
Gonads/mesonephroi of E11.5 XY embryos were stained with antibodies against GLP (A) and G9a (B), in combination with anti-Nr5a1 antibodies. (left) Representative data of flow-cytometric dot-blot analysis of the indicated proteins. (right) Plots of median fluorescence intensity (MFI) values for the indicated proteins in Nr5a1-positive gonadal somatic cells. *** P < 0.001.
(PDF)
We had previously established GLP-tg mice that carry an extra copy of GLP cDNA in the Rosa26 locus [20]. In this line, the exogenous GLP cDNA is expressed ubiquitously by CAG promoter. Although GLP mRNA was actually overexpressed in the gonads of XY GLP-tg embryos at E11.5, mRNA levels of Nr5a1, Sry and Sox9 were not affected.
(PDF)
(A) Comparison of Eset genomic sequences between wild-type and mutant alleles, generated by genome editing with the CRISPR/Cas9 system. We intended to disrupt the exon8 encoding TUDOR domain of Eset. Two guide RNAs (gRNAs), corresponding to a sequence within intron 7 and a sequence nearly at the 3’ end of exon 8, were introduced with Cas9 mRNA into fertilized eggs of C57BL/6 mice. Dashes represent deleted sequences in the mutant allele. Capital and lower-case letters represent exonic and intronic sequences, respectively. (B) Genotyping for the Eset mutant allele by PCR. Location of the primers is indicated in (A). (C) Phenotype analysis of the Eset-mutant mice established in this study. No Eset homozygous mutant embryos were found among 34 embryos derived from the mating of Eset heterozygous mutant mice, indicating Eset homozygous mutant embryos died and were absorbed by E13.5. (D) Evaluation of the gonadal sex differentiation of E13.5 XY Jmjd1aΔ/Δ; EsetΔ/+ embryos by immunofluorescence analysis for Sox9 and Foxl2. (E) The ratios of Sox9- and Foxl2-positive cells of the indicated genotypes are summarized. Eset heterozygous mutation did not affect the sex development of Jmjd1a-deficient mice. Numbers of embryos examined are shown above the bars. Data are presented as mean ± SD. *** P < 0.001; n.s., not significant.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.